Abstract
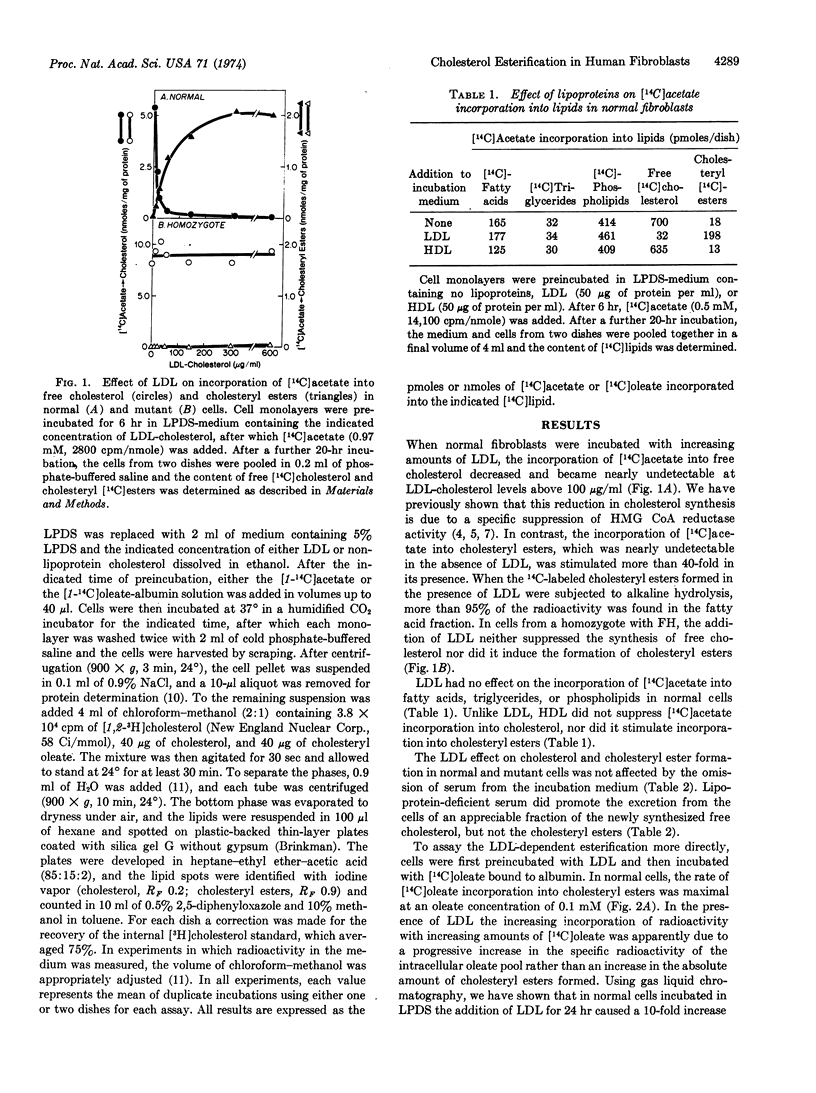
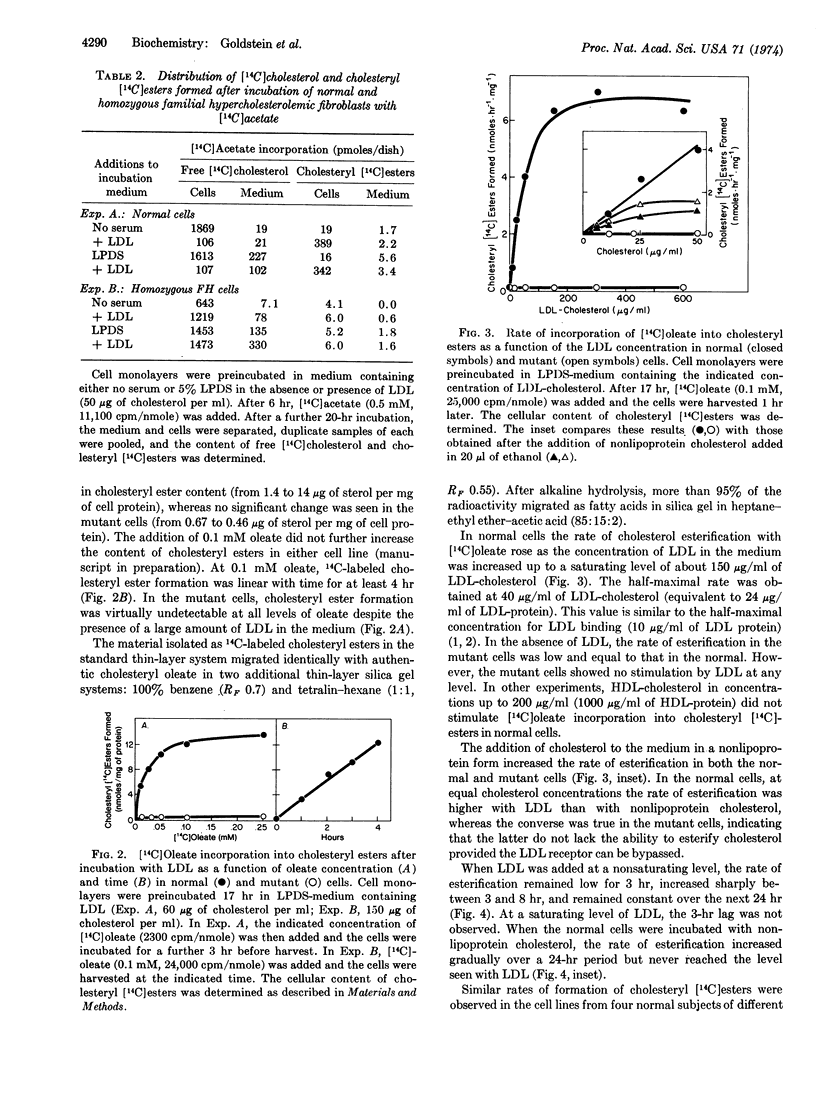
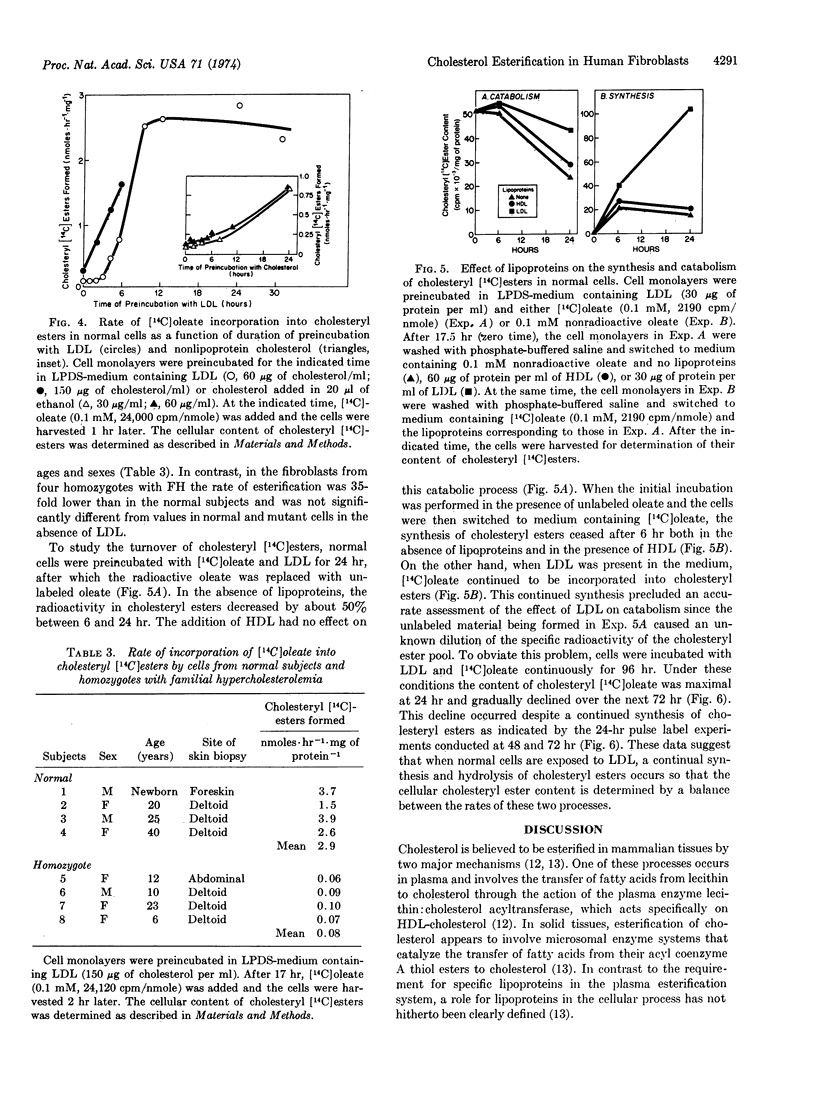
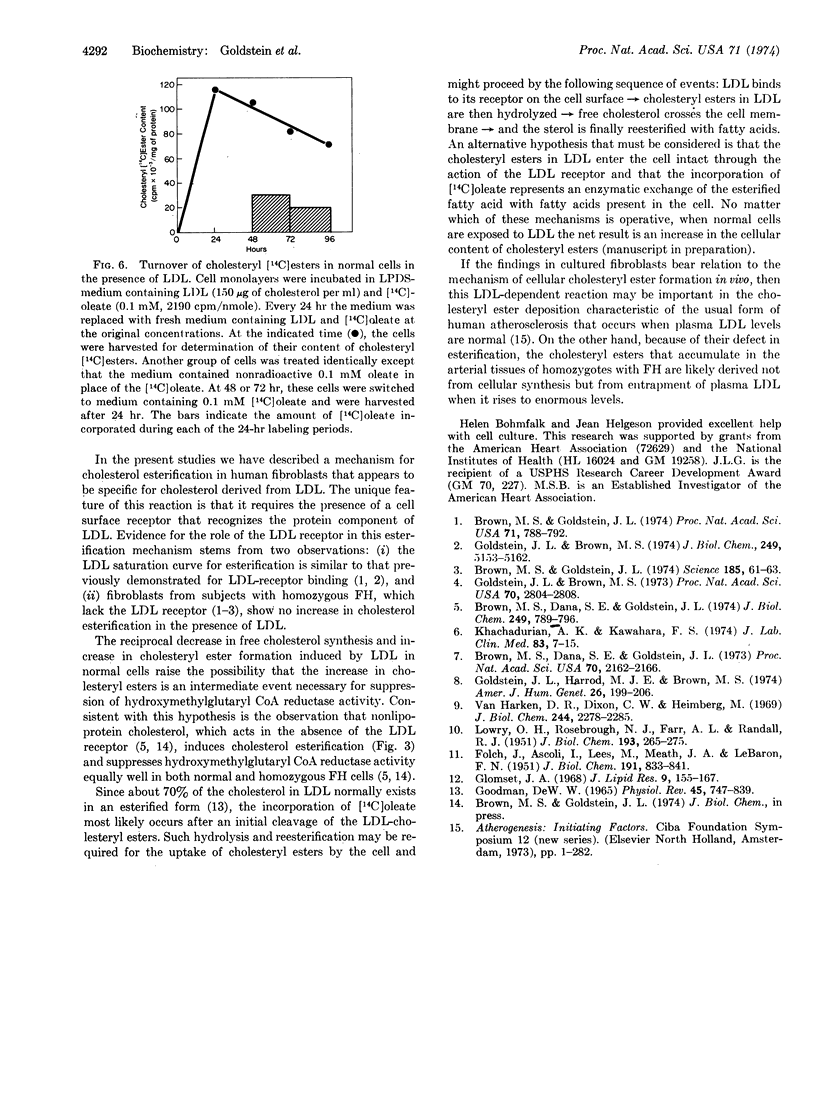
A new mechanism is described for the cellular esterification of cholesterol derived from extra-cellular lipoproteins. Incubation of monolayers of cultured fibroblasts from normal human subjects with low density lipoproteins led to a 30- to 40-fold increase in the rate of incorporation of either [14C]acetate or [14C]oleate into the fatty acid fraction of cholesteryl [14C]esters. This stimulation of cholesteryl ester formation by low density lipoproteins occurred despite the fact that endogenous synthesis of free cholesterol was completely suppressed by the lipoprotein. Thus, exogenous cholesterol contained in low density lipoproteins, rather than endogenously synthesized sterol, appeared to provide the cholesterol substrate for this cellular esterfication process. High density lipoproteins and the lipoprotein-deficient fraction of serum neither stimulated cholesteryl ester formation nor inhibited cholesterol synthesis. Both the low density lipoprotein-dependent increase in cholesterol esterification and decrease in free cholesterol synthesis required the interaction of the lipoprotein with its recently described cell surface receptor. Cells from homozygotes with familial hypercholesterolemia, which lack specific low density lipoprotein receptors, showed neither lipoprotein-dependent cholesterol esterification nor suppression of cholesterol synthesis. The reciprocal changes in free cholesterol synthesis and cholesteryl ester formation produced by low density lipoprotein-receptor interactions may play an important role in the regulation of the cholesterol content of mammalian cells.
Keywords: cholesterol synthesis, atherosclerosis, hyperlipidemia, low density lipoprotein receptors, hydroxymethylglutary-coenzyme A reductase
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown M. S., Dana S. E., Goldstein J. L. Regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity in cultured human fibroblasts. Comparison of cells from a normal subject and from a patient with homozygous familial hypercholesterolemia. J Biol Chem. 1974 Feb 10;249(3):789–796. [PubMed] [Google Scholar]
- Brown M. S., Dana S. E., Goldstein J. L. Regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity in human fibroblasts by lipoproteins. Proc Natl Acad Sci U S A. 1973 Jul;70(7):2162–2166. doi: 10.1073/pnas.70.7.2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. S., Goldstein J. L. Expression of the familial hypercholesterolemia gene in heterozygotes: mechanism for a dominant disorder in man. Science. 1974 Jul 5;185(4145):61–63. doi: 10.1126/science.185.4145.61. [DOI] [PubMed] [Google Scholar]
- Brown M. S., Goldstein J. L. Familial hypercholesterolemia: defective binding of lipoproteins to cultured fibroblasts associated with impaired regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity. Proc Natl Acad Sci U S A. 1974 Mar;71(3):788–792. doi: 10.1073/pnas.71.3.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOLCH J., ASCOLI I., LEES M., MEATH J. A., LeBARON N. Preparation of lipide extracts from brain tissue. J Biol Chem. 1951 Aug;191(2):833–841. [PubMed] [Google Scholar]
- Glomset J. A. The plasma lecithins:cholesterol acyltransferase reaction. J Lipid Res. 1968 Mar;9(2):155–167. [PubMed] [Google Scholar]
- Goldstein J. L., Brown M. S. Familial hypercholesterolemia: identification of a defect in the regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity associated with overproduction of cholesterol. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2804–2808. doi: 10.1073/pnas.70.10.2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein J. L., Harrod M. J., Brown M. S. Homozygous familial hypercholesterolemia: specificity of the biochemical defect in cultured cells and feasibility of prenatal detection. Am J Hum Genet. 1974 Mar;26(2):199–206. [PMC free article] [PubMed] [Google Scholar]
- Goodman D. S. Cholesterol ester metabolism. Physiol Rev. 1965 Oct;45(4):747–839. doi: 10.1152/physrev.1965.45.4.747. [DOI] [PubMed] [Google Scholar]
- Khachadurian A. K., Kawahara F. S. Cholesterol synthesis by cultured fibroblasts: decreased feedback inhibition in familial hypercholesterolemia. J Lab Clin Med. 1974 Jan;83(1):7–15. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Van Harken D. R., Dixon C. W., Heimberg M. Hepatic lipid metabolism in experimental diabetes. V. The effect of concentration of oleate on metabolism of triglycerides and on ketogenesis. J Biol Chem. 1969 May 10;244(9):2278–2285. [PubMed] [Google Scholar]


