Abstract
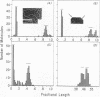
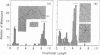
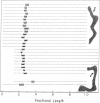
Digestion of mouse L cell mitochondrial DNA with EcoRI restriction endonuclease produces two linear duplex fragments comprising 86.3 ± 2.0% and 14.2 ± 1.0% of the circular genome length (16,000 ± 470 nucleotide pairs). Digestion of human HeLa cell mitochondrial DNA with EcoRI produces three linear duplex fragments comprising 49.2 ± 1.0%, 44.4 ± 0.9%, and 6.4 ± 0.4% of the circular genome length (16,590 ± 710 nucleotide pairs). These fragments are shown to be generated by cleavage in unique regions of the mouse and human mitochondrial DNAs. An electron microscopic analysis of partially replicated molecules cleaved by EcoRI establishes a unidirectional mode of DNA replication for L cell mitochondrial DNA. The origin for DNA replication is located on the larger EcoRI fragment at a position that is 1,890 ± 250 nucleotide pairs (11.8 ± 1.2% of the circular genome length) from the proximal restriction site. Replication proceeds unidirectionally away from this restriction site throughout the length of the larger EcoRI fragment. Analysis of L cell, D-loop mitochondrial DNA cleaved by EcoRI indicates that a unique sequence is synthesized in formation of the D-loop in these nonreplicating molecules. The origin of D-loop synthesis is located on the larger EcoRI fragment at a position 1,760 ± 180 nucleotide pairs (11.0 ± 1.1% of the circular genome length) from the proximal restriction site and is, therefore, the origin for unidirectional displacement replication.
Keywords: restriction enzyme, electron microscopy, unidirectional DNA synthesis
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Crawford L. V., Syrett C., Wilde A. The replication of polyoma DNA. J Gen Virol. 1973 Dec;21(3):515–521. doi: 10.1099/0022-1317-21-3-515. [DOI] [PubMed] [Google Scholar]
- Fareed G. C., Garon G. F., Salzman N. P. Origin and direction of simian virus 40 deoxyribonucleic acid replication. J Virol. 1972 Sep;10(3):484–491. doi: 10.1128/jvi.10.3.484-491.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedgpeth J., Goodman H. M., Boyer H. W. DNA nucleotide sequence restricted by the RI endonuclease. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3448–3452. doi: 10.1073/pnas.69.11.3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huberman J. A., Riggs A. D. On the mechanism of DNA replication in mammalian chromosomes. J Mol Biol. 1968 Mar 14;32(2):327–341. doi: 10.1016/0022-2836(68)90013-2. [DOI] [PubMed] [Google Scholar]
- Huberman J. A., Tsai A. Direction of DNA replication in mammalian cells. J Mol Biol. 1973 Mar 25;75(1):5–12. doi: 10.1016/0022-2836(73)90525-1. [DOI] [PubMed] [Google Scholar]
- Kasamatsu H., Vinograd J. Unidirectionality of replication in mouse mitochondrial DNA. Nat New Biol. 1973 Jan 24;241(108):103–105. doi: 10.1038/newbio241103a0. [DOI] [PubMed] [Google Scholar]
- Lee C. S., Davis R. W., Davidson N. A physical study by electron microscopy of the terminally reptitious, circularly permuted DNA from the coliphage particles of Escherichia coli 15. J Mol Biol. 1970 Feb 28;48(1):1–22. doi: 10.1016/0022-2836(70)90215-9. [DOI] [PubMed] [Google Scholar]
- Mertz J. E., Davis R. W. Cleavage of DNA by R 1 restriction endonuclease generates cohesive ends. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3370–3374. doi: 10.1073/pnas.69.11.3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow J. F., Berg P. Cleavage of Simian virus 40 DNA at a unique site by a bacterial restriction enzyme. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3365–3369. doi: 10.1073/pnas.69.11.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulder C., Delius H. Specificity of the break produced by restricting endonuclease R 1 in Simian virus 40 DNA, as revealed by partial denaturation mapping. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3215–3219. doi: 10.1073/pnas.69.11.3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robberson D. L., Clayton D. A. Pulse-labeled components in the replication of mitochondrial deoxyribonucleic acid. J Biol Chem. 1973 Jun 25;248(12):4512–4514. [PubMed] [Google Scholar]
- Robberson D. L., Clayton D. A. Replication of mitochondrial DNA in mouse L cells and their thymidine kinase - derivatives: displacement replication on a covalently-closed circular template. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3810–3814. doi: 10.1073/pnas.69.12.3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robberson D. L., Fried M. Sequence arrangements in clonal isolates of polyoma defective DNA. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3497–3501. doi: 10.1073/pnas.71.9.3497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robberson D. L., Kasamatsu H., Vinograd J. Replication of mitochondrial DNA. Circular replicative intermediates in mouse L cells. Proc Natl Acad Sci U S A. 1972 Mar;69(3):737–741. doi: 10.1073/pnas.69.3.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robberson D., Aloni Y., Attardi G. Electron microscopic visualization of mitochondrial RNA-DNA hybrids. J Mol Biol. 1971 Jan 28;55(2):267–270. doi: 10.1016/0022-2836(71)90196-3. [DOI] [PubMed] [Google Scholar]