Abstract
To study intracellular glucose homeostasis, the glucose nanosensor FLIPglu-600µM, which undergoes changes in fluorescence resonance energy transfer (FRET) upon interaction with glucose, was expressed in four mammalian cell lines: COS-7, CHO, HEK293, and C2C12. Upon addition of extracellular glucose, the intracellular FRET ratio decreased rapidly as intracellular glucose increased. The kinetics were fast (τ =5 to 15 s) in COS and C2C12 cells and slow (τ =20 to 40 s) in HEK and CHO cells. Upon removal of extracellular glucose, the FRET ratio returned to its initial value at similar rates (τ = 15 to 40 s) in all cell types. In all cell types, the glucose uptake FRET signal was blocked by the glucose transporter (GLUTx) inhibitor cytochalasin B and was not affected by the Na/glucose transporter inhibitor phlorizin. Glucose clearance was inhibited by the glycolytic inhibitor iodoacetate. Using β-escin to permeabilize the cell, we found that the glucose gradient across the membrane was strongly dependent on the rates of glucose uptake versus glucose clearance. With 10 mM extracellular glucose and a high rate of glucose clearance, intracellular glucose level fell below 100 µM when glucose uptake rate was low, whereas it exceeded 0.5 mM when glucose uptake was high. Cells cultured in high glucose maintained lower basal intracellular glucose levels than cells cultured in low glucose, attributed to “reciprocal regulation” of glycolysis and gluconeogenesis. Basal glucose level also increased with elevated temperatures. Experiments performed with C2C12 cells demonstrated a shift from fast glucose uptake to slow glucose uptake in the absence of insulin during differentiation.
Keywords: FRET, Glucose nanosensor, Glucose uptake, Glucose metabolism, Iodoacetate, Cytochalasin B, β-escin
Introduction
Glucose, a main source of carbohydrate in food, provides energy to all cell types. During a meal, glucose taken up by liver cells is stored in glycogen granules and released between meals so that blood glucose remains around 5 mM. Whereas the uptake and release of glucose from the liver involves the glucose transporter GLUT2, most other cells use GLUT1. However, in muscle, glucose uptake is insulin-dependent and involves GLUT4, and in the brain, GLUT3 predominates (see [10] for review). In tissues such as the small intestine brush border and kidney proximal tubule in which glucose must be transported against its gradient (see [14] for review), the Na/glucose cotransporter plays a major role.
In theory, the level of intracellular glucose depends on the rates of uptake, efflux, and metabolism (gluconeogenesis and glucose consumption). Intracellular glucose is synthesized from non-sugar, substrates such as pyruvate and glucogenic amino acids or glycogen, and consumed via glycolysis, the pentose shunt or glycogen synthesis. In muscle and liver, glycogen synthesis plays a major role, but in most other cell types, glycolysis is the main source of glucose consumption. It is believed that transport of glucose via GLUTx transporters in and out of the cell is symmetrical and passive [2], thus, carrying glucose in both the inward and outward directions, although GLUT1 may exhibit some asymmetry [12]. Examples of GLUT-mediated glucose efflux have been suggested for small intestine brush border and kidney proximal tubule, where GLUT2 at the basolateral membrane transports glucose from the intracellular space into the blood. Similar efflux of glucose through GLUT2 is believed to accompany glucose release from liver cells (see [14] for review). However, the role of GLUT2 in this process has been questioned, as GLUT2 knockout mice showed normal glucose release [7].
To image and quantify changes in intracellular glucose, Fehr et al. [3] engineered a fluorescence resonance energy transfer (FRET)-based biosensor consisting of a bacterial periplasmic-binding protein, PBP, flanked by two green fluorescent protein variants, cyan fluorescent protein (CFP), and yellow fluorescent protein (YFP). PBPs form a large protein family [4], among which glucose/galactose-binding protein binds glucose and galactose with high affinity. This construct undergoes a decrease in FRET upon substrate binding and has been used successfully to estimate changes in intracellular sugar concentration. The first glucose sensor generated based on this construct had an affinity range between 0.02 and 1.5 µM, which is too sensitive to estimate the level of intracellular glucose. A mutant sensor, termed FLIPglu-600µM, with a binding constant of 0.6 mM was generated, which could be used to quantify intracellular glucose levels between 0.07 and 5.3 mM [3]. FLIPglu-600µM binds glucose and galactose with similar high affinity, but in the millimolar range, other sugars, such as ribose, and phosphorylated sugars, such as glucose-6-phospate, have no effect on the FRET ratio. When FLIPglu-600µM was expressed in COS-7 cells, Fehr et al. [3] demonstrated a fast rate of glucose uptake and clearance, with glucose metabolism responsible, in part, for intracellular glucose clearance. They estimated free intracellular glucose concentration to be half of that outside the cell [3].
We have used this technique to monitor the intracellular levels of glucose in real time in four different mammalian cell lines. We find that the rate of glucose uptake, which varies among cell types, has a profound effect on the level of intracellular free glucose. We demonstrate that intracellular glucose clearance is mediated primarily by glucose metabolism, such that glucose efflux does not play a significant role. Basal intracellular glucose levels are dynamically modulated by the chronic level of extracellular glucose and vary considerably depending on the rates of glucose uptake, glycolysis, and gluconeogenesis.
Materials and methods
Solutions and experimental techniques
The bath solution for cell imaging consisted of 140 NaCl, 5 KCl, 1.1 MgCl2, 2.5 CaCl2, 10 HEPES, with the pH adjusted to 7.2 with KOH. Glucose and pyruvate were added to this solution at various concentrations, and N-methyl-d-glucamine was added to maintain the solutions’ osmolarity. Solutions were perfused directly over the cells using a gravity-fed eight-way perfusion device (Warner Instruments, Hamden, CT, USA) with electrically controlled solenoids (The Lee Company, Westbrook, CT, USA). Input and output of solution volumes to the recording chamber (glass bottomed Petri dish) were equilibrated to maintain constant flow rates and pressures within the recording chamber. Iodoacetate (IAA), cytochalasin B, and β-escin were purchased from Sigma.
Molecular biology and cell culture
Complementary DNA (cDNA) for FLIPglu-600µM was generously provided by Dr. W.B. Frommer. This and mutant constructs were subcloned into the pcDNA3.1 amp mammalian expression vector (Invitrogen), which utilizes the cytomegalovirus promoter. HEK293, CHO, and COS-7 cells were transfected either with the calcium phosphate precipitation method [6] or lipofectamine 2000 (Invitrogen). Muscle-derived C2C12 cells could only be transfected with lipofectamine using 2 µg DNA plus 5 µl lipofectamine per 1.5 cc Petri dish. Expression of FLIPglu-600µM was sufficiently high after 24 h to perform FRET experiments. Cells were cultured in Dulbecco’s modification of Eagle’s medium, high (25 mM) or low (5.5 mM) glucose medium supplemented with 10% (v/v) fetal bovine serum (FBS), penicillin (100 U/ml), streptomycin (100 U/ml), and 2 mM glutamine, and divided once a week by treatment with trypsin. We monitored the glucose level with a “FreeStyle Flash meter” (Abbott, Alameda, CA, USA) in Petri dishes containing culture medium with 5.5 mM glucose. After 2 to 3 days of cell culture, the glucose level in the medium dropped below 2 mM and, after 4 days of culture, below the detection level (1 mM) in some cases. Thus, when we refer to low-glucose culture conditions, we mean that cells had been exposed for many hours to levels of glucose approximating 1 to 2 mM before FRET recording. For cell transfection with lipofectamine, the antibiotics were withdrawn from the culture medium 12 h before transfection. Differentiation of C2C12 cells into myotubes was carried out by switching the serum from 10% FBS to 2% horse serum. After 72 h, individual cells start to fuse and form long multinucleated myotubules that can be transfected using lipofectamine 2000 and used for FRET recordings.
Cell permeabilization
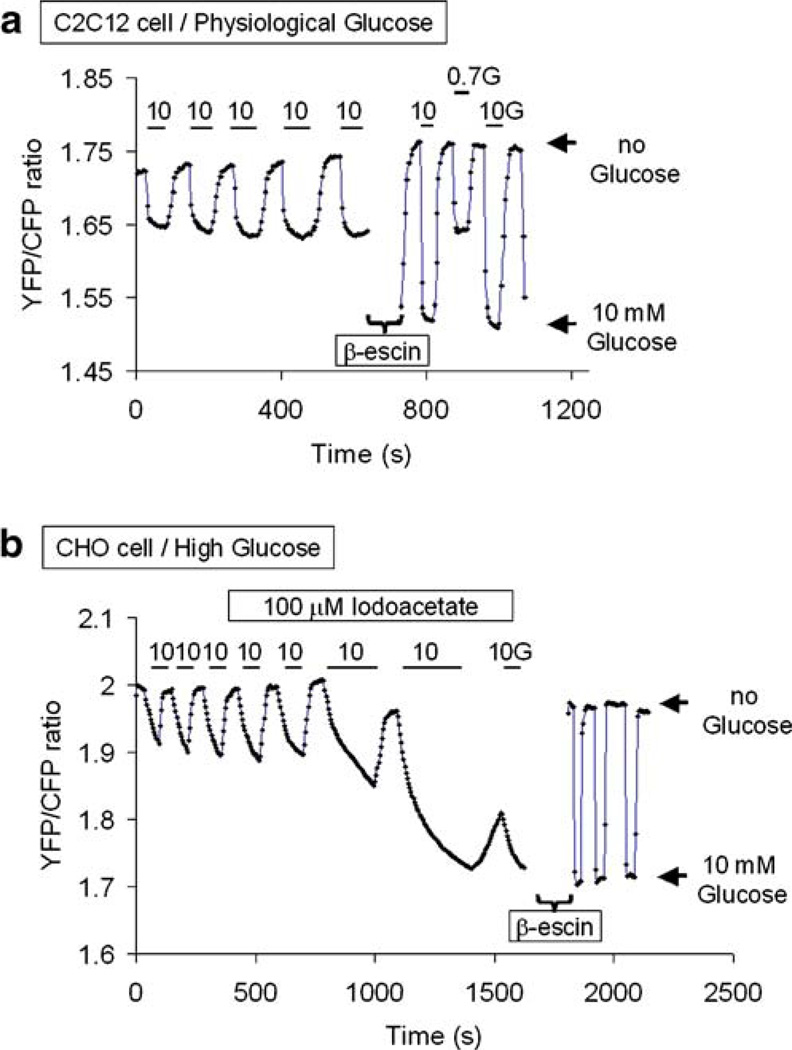
To estimate the intracellular glucose level and calibrate FLIPglu-600µM intracellularly, CHO and C2C12 cells previously transfected with the glucose sensor were permeabilized. We used β-escin (50–100 µM for 10–30 s) for cell membrane permeabilization experiments, in which the concentration and the time of application could be adjusted to retain enough glucose sensor inside the cell so that changes in FRET ratio could be measured accurately for over 10 min. Because of the slow sensor shift from the cell cytoplasm to the cell exterior and/or nucleus, our ΔR measurement in permeabilized cells may be an underestimate of the values measured in intact cells, but as pointed out in the results, the differences may not be very significant. We estimated that cell permeabilization was established when application and removal of outside glucose caused almost instantaneous change in FRET ratio. In addition, “complete” cell permeabilization could be directly visualized as the sensor penetrated the nucleus. For cell permeation, the bath solution was similar to that used for experimental recordings (see below), pH 7.2, and Ca2+ was omitted from the milieu. No adenosine triphosphate (ATP) was added. In these conditions, the FRET ratios values were sometimes shifted upward in permeabilized cells as compared to intact cells. The values obtained in the absence of glucose were then matched for permeabilized cells and intact cells after saturation of the FRET ratio (Fig. 5a). This adjustment was not necessary in the cells previously treated with IAA (Fig. 5b).
Fig. 5.
Estimate of intracellular glucose levels in cells permeabilized with β-escin. Data in a and b were recorded with a C2C12 cell and a CHO cell, respectively. In a, the C2C12 cell cultured in low glucose was treated with β-escin for 20 s. Unfortunately, in this case, the calibration traces were shifted upward by almost 0.2 units. We arbitrarily adjusted the traces so that the ratio measured in the absence of extracellular glucose would be similar before and after permeabilization. In b, the CHO cell cultured in high glucose had been exposed to IAA for 20 min before permeabilization with 100 µM β-escin applied for 30 s. The decrease in FRET ratio observed upon exposure to IAA indicates glucose accumulation. After permeabilization, revealed by the almost instantaneous change in FRET ratio with addition or removal of outside glucose, the ratio measured with 10 mM glucose coincided with the intracellular glucose level reached after prolonged exposure to IAA, and the ratio measured in the absence of external glucose coincided with the intracellular glucose level measured in the absence of glucose before IAA application. In this case, the calibration trace was not shifted in respect to the ratio measurement in the intact cell
FRET imaging
Images (16-bit) were acquired using a Nikon Eclipse TE300 microscope fitted with a ×60 (N.A. 1.4) oil immersion lens (Nikon) and equipped with a filter cube comprising a CFP bandpass excitation filter, 436/20b, together with a longpass dichroic mirror, 455DCLP (Chroma Technology Corp, Rockingham, VT, USA). Light-emitting diodes (LEDs, Lumileds, San Jose, CA, USA) were used as light sources: one emitting at 455±20 nm (royal blue) and the other emitting at 505±15 nm (cyan). LEDs and camera exposure were controlled by MetaFluor Imaging 6.1 software (Molecular Devices, Sunnyvale, CA, USA).
Ratiometric FRET measurements were performed by simultaneously monitoring CFP and YFP emissions of the sample when excited at the wavelengths for CFP (royal blue LED). The ratio between YFP and CFP emission were measured online in real time using MetaFlour Imaging software. For analysis, background light intensity was subtracted from the individual YFP and CFP emission. YFP and CFP images were acquired simultaneously using a Dual View image splitter (Optical Insights, Tucson, AZ, USA) equipped with a 505-nm long-pass dichroic filter to separate the CFP and YFP signals, a CFP emission filter (480/30), and a YFP emission filter (535/40). Superposition of the CFP and YFP images was carried out using the imaging software. Images were captured with a Cascade 512B digital camera (Photometrics, Tucson, AZ, USA). Exposure times were optimized in each case but varied between 300–500 ms and were recorded at a constant rate for each cell between 0.2–0.33 Hz. Many experiments lasted more than 1 h, leading to a slow drift in the FRET ratio baseline in some cases. In Figs. 4a, 5a and b, 8a and b, the drift was corrected using linear curve fitting; in Fig. 4b, the drift was corrected using an exponential curve fitting. No correction was carried out in Fig. 3a and b or Fig. 6.
Fig. 4.
Effects of varying glucose concentrations on FRET ratio. Data in a and b were recorded with a C2C12 cell and a CHO cell, respectively. Both were cultured in high glucose. The numbers above the traces indicate the concentration of glucose applied to the bath. The numbers below the traces are the values for the time constants for glucose uptake phase. c The fit to the data in a (upper trace) and in b (lower trace) using the equation Δ = Δmax * [G]/Kd + [G]. For the data in a, the estimated Kd was 1.5 mM and ΔRmax was 1.03. For the data in b, the estimated Kd was 7.2 mM and ΔRmax was 1.37. The two plots were normalized to their maximum ΔR
Fig. 8.
Effects of C2C12 cell differentiation on the rate of glucose uptake and clearance and intracellular glucose levels. The upper trace depicts the effect of prolonged exposure to IAA. Initially, addition of 10 mM glucose outside had no effect on the FRET ratio, but with time, the ratio begun to change, and ΔR increased gradually. Increase in ΔR was accompanied by an increase in intracellular glucose levels measured in the presence and absence of glucose outside. Upon removal of extracellular glucose, the level of intracellular glucose did not return to its initial value before application of IAA. The small ΔR measured with addition of 0.6 mM glucose outside (right of trace) reflects a low driving force for glucose and suggests that intracellular glucose concentration may be close to 600 µM. As already illustrated in Fig. 6, fitting the rates of glucose uptake and clearance (left-hand side and right-hand side, respectively in a, b and c) indicates that glucose accumulation due to glycolytic inhibition
Fig. 3.
Effects of high and low glucose culture conditions. Data in a and b were obtained with C2C12 cells cultured in high (25 mM) and physiological (5 mM) glucose, respectively. a A typical decrease in FRET ratio observed with cells cultured in the presence of high glucose for more than 48 h. Data in b show a characteristic increase in FRET ratio observed with cells cultured in the presence of low glucose. Again, in this case, the change in FRET ratio occurred without any major change in the rate of glucose uptake or clearance
Fig. 6.
Glucose clearance in the presence of the glycolysis inhibitor iodoacetate, IAA. Data obtained with a CHO cell cultured in the presence of high glucose (25 mM). Addition of IAA caused a gradual decrease in FRET ratio over 20 to 30 min. This decrease resulted from intracellular glucose accumulation as illustrated in a, b and c (left-hand side plots) by the appearance of a second slow component (τ=1,500 to 2,000 s) during the phase of glucose loading. The first component corresponding to glucose uptake remained unchanged (a, b, and c, left-hand side plots). Intracellular glucose accumulation as a result of glycolysis inhibition is also illustrated by the gradual decrease in the rate of glucose clearance shown in a, b, and c (right-hand side plots)
In most cases, changes in FRET ratio measured upon the addition and removal of glucose could be fitted to a single exponential. However, a second slow component had to be added when fitting the rate of decrease measured after exposure to IAA an inhibitor of glycolysis. FRET ratio recovery after glucose removal was also fitted to a single exponential (Figs. 6a and 8a), except for long glucose exposures and/or IAA application, in which a slow decrease in basal glucose level sometimes preceded the fast phase of glucose clearance (Fig. 6b and c). In these cases, the first phase was excluded from the fit. We justify this procedure based on the observation that such a phase was either absent or very small under normal experimental conditions and at 35°C.
Experimental temperature
Most experiments were carried out at room temperature (25°C), but some experiments were carried at 35°C for comparison. In the latter experiments, the microscope’s stage and perfusion system were preheated to 35°C with a “dual automatic temperature controller” (Warner Instrument Corporation; Hamden, CT, USA). The Petri dish with the cells was then positioned on the stage of the microscope, and the perfusion was started. Data recording was started after 10 to 15 min when the temperature stabilized at 35°C in the Petri dish. Subsequently, the heater was turned off, and the temperature was allowed to reequilibrate to 25°C for 5 to 10 min, after which, data recording was resumed.
PCR analysis
To detect messenger RNA for the glucose transporters, GLUT1-4 primers were designed based on the analysis of the reference nucleotide sequences of human, mouse, and rat using the program Primer 3 (http://www.fokker.wi.mit.edu/primer3/input-040.htm). In doing so, the following constraints were used: 20 bp long, Tm>59, and generation of a fragment of at least 500 bp. Total RNA was isolated from the cell lines CHO, COS, HEK293, C2C12 differentiated, and undifferentiated, using the RNAeasy kit (Qiagen). cDNA was made using Invitrogen Superscript III First Strand Synthesis System and oligo dT priming. PCR was carried out using the following protocol: denaturation for 2 min at 94° then 40 cycles at 94° for 15 s, 55° for 30 s, and 72° for 1 min. Products were analyzed using Tris–borate buffer 1% agarose gels.
Results
Imaging of intracellular glucose in living cells
To image and quantify changes in intracellular glucose, we transfected HEK293, COS-7, CHO, and muscle-derived C2C12 cells with the low-affinity glucose sensor Flipglu-600µM. Figure 1 shows a representative experiment in a C2C12 cell, cultured in the presence of high (25 mM) extracellular glucose. The upper panel shows pseudocolor images of the FRET ratio at 5-s intervals upon glucose addition (above) and removal (below). The increase and decrease in intracellular glucose were complete within 15 to 20 s. With repeated short exposures to 10 mM extracellular glucose, the emission ratio YFP/CFP in the absence of glucose stabilized near 2.0 in this cell, and decreased to 1.75 during application of 10 mM extracellular glucose (upper trace), reflecting glucose entry into the cell. Upon removal of extracellular glucose, the ratio returned to its original value, indicating clearance of intracellular glucose. The corresponding emissions of YFP (middle trace) and CFP (lower trace) changed in opposite directions, as expected for FRET. In this case, the change in ratio, ΔR, was about 0.25. Changes in FRET ratio varied from less than 0.01 to 0.25, depending on the cell type and experimental conditions. The FRET ratio was lower at the cell periphery, corresponding to higher glucose, and higher in more central regions, except for the nucleus, from which the probe is excluded. This gradient pattern was observed in all cell types and is further illustrated in Fig. 2, which compares the global cytoplasmic change in FRET ratio with the FRET ratios from delineated regions of interest near the cell center and the cell periphery. Although the absolute FRET ratio values differed, their time courses were similar. When comparing data from cells expressing high versus low amounts of sensor, we noted in some instances a 5- to 10-s lag in glucose clearance in cells with a high sensor level, suggesting that, in these cases, the sensor’s glucose buffering capacity may have delayed glucose clearance.
Fig. 1.
Changes in FRET ratio in undifferentiated C2C12 cells. The upper panels show images of the FRET ratio in a cell transfected with the glucose sensor FLIPglu-600µM. Red indicates high ratio or low glucose, and green indicates low ratio or high glucose. Here, as for all transfected cells, the sensor was excluded from the nucleus. The ratio intensity was higher at the cell center near the nucleus. The ratio images were acquired every 5 s after the addition of glucose (a) and after glucose removal (b). Image acquisition started at the arrow placed over the trace of ratio measurements. The lower panels show the entire cell average FRET ratio (YFP/CFP; upper trace), YFP emission (middle trace), and CFP emission (lower trace). Each point represent one image acquired every 5 s. The bar on the top of the upper trace indicates the time of addition of 10 mM glucose to the perfusion milieu. Addition of glucose induced a fast decrease in ratio before reaching steady state, indicating a constant level of intracellular glucose. After the removal of external glucose, the ratio increased rapidly, returning to its initial level before the addition of external glucose. The concomitant decrease in YFP emission (middle trace) and increase in CFP emission (lower trace) demonstrates FRET between CFP and YFP
Fig. 2.
Glucose gradients in C2C12 cells. Average FRET ratios obtained for specific regions in a C2C12 cell exhibiting gradients of ratio intensity similar to Fig. 1. In a, traces 1 and 3 were obtained by defining two regions of interest, near the nucleus and at the cell periphery, respectively. Trace 2 was acquired by integrating the entire cell surface. Note that the CFP and YFP intensities were greater. In b, superposition of traces 1 and 3 indicates that the change in FRET ratio had similar time course in both cases, although the absolute level was higher at the cell center, suggesting lower level of intracellular glucose. The table summarizes these data
Correlation of FRET ratio changes with the rates of glucose uptake and clearance
Most cell cultures are carried out with high glucose (25 mM), which has been reported to downregulate the expression of GLUT1 [10] and upregulate enzymes in the glycolytic pathway [13]. We compared the rates of glucose uptake and clearance for the four cell types for media containing either high glucose (25 mM) or low glucose (<5 mM). As summarized in Table 1, when cultured in high glucose, COS-7 and C2C12 cells exhibited three- to fourfold faster glucose uptake rates than either HEK293 or CHO cells. When cultured in low glucose, the rates of glucose uptake remained similar in COS-7 and C2C12 cells but increased threefold in CHO cells. In C2C12 cells, the rate of glucose uptake increased modestly, but the difference was not statistically significant. These findings suggest that high glucose in the culture media modulates the expression of glucose transporters in CHO cells but not significantly in the other cell types. In addition, in HEK cells, the rate of glucose clearance increased under high-glucose culture conditions, consistent with upregulation of glycolytic enzymes.
Table 1.
Glucose uptake and clearance rate measurements with FLIPglu-600µM in cell lines
| Uptake rates | Clearance rates | |||||||
|---|---|---|---|---|---|---|---|---|
| Physiological glucose | High glucose | Physiological glucose | High glucose | |||||
| Cell type | τd1 | τd2 | τd1 | τd2 | τr1 | τr2 | τr1 | τr2 |
| HEK 293 | 29±7 (n=9) | 38±15 (n=11) | 42±9 (n=9) | 21±10 (n=11) | ||||
| CHO | 16.3±5 (n=11) | 49±13 (n=10) | 26±11 (n=11) | 28.5±12 (n=10) | ||||
| COS-7 | 12±2.5 (n=6) | 160 (n=2) | 16±2.5 (n=6) | 10±1.5 (n=6) | 150(n=2) | 13.5±5 (n=6) | ||
| C2C12 | 9±3 (n=18) | 25±6.5 |(n=8) | 13±7.5 (n=14) | 102±54 (n=12) | 175±7 (n=18) | 22.5±6 (n=14) | 320±150 (n=7) | |
Time constant values in seconds obtained from fitting the decrease and increase in FRET ratio observed upon addition and removal of 10 mM glucose to the bath solution. In most cases, a good fit could be achieved with a single exponential; however, when this was not satisfactory, a second component was introduced. The very slow component observed after prolonged exposure to IAA (τ =1,000 to 2,000 s) has not been included in this table and is reported in the appropriate result section.
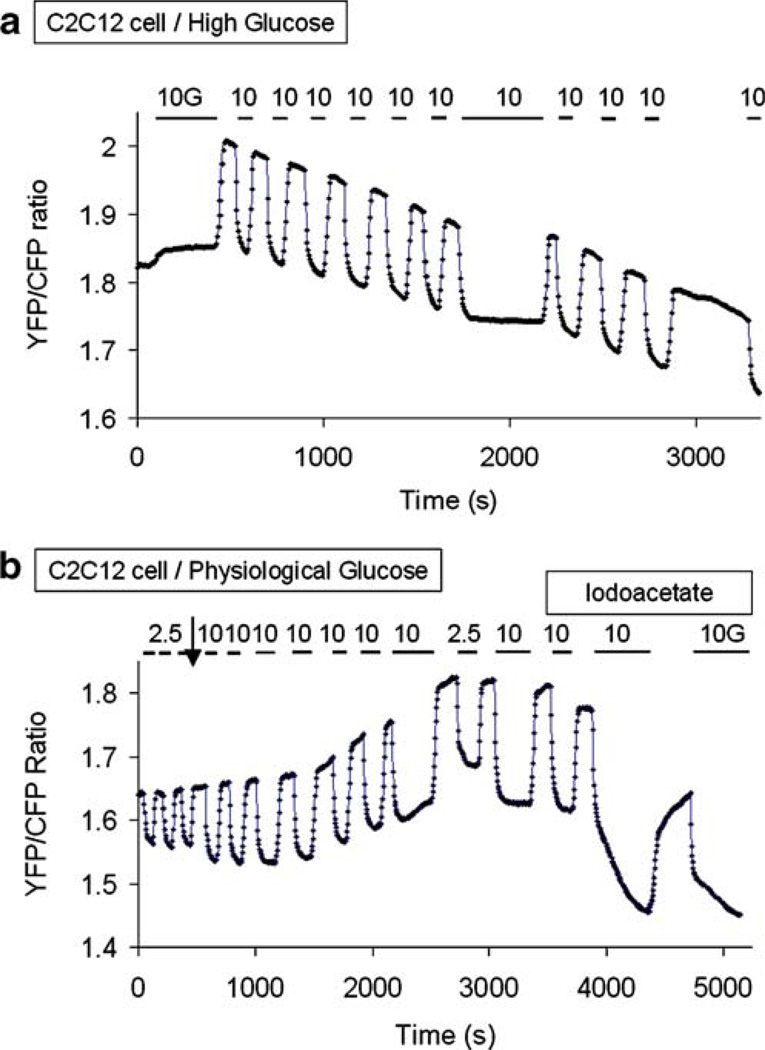
Effects on basal intracellular glucose of culturing cells in high versus low glucose
We also found that varying the glucose concentration in the culture media affected the basal intracellular glucose level. Figure 3 shows an example from a C2C12 cell cultured in high glucose until the beginning of the experiment. Under these conditions, the baseline FRET ratio in the absence of extracellular glucose was high, averaging 2.2±0.22 (n=9), and progressively decreased (i.e., intracellular glucose increased) when extracellular glucose was successively switched between 0 and 10 mM (Fig. 3a). Moreover, when extracellular glucose was removed for a prolonged period, as shown at end of trace in Fig. 3a, intracellular glucose continued to rise despite the absence of extracellular glucose, implying that gluconeogenesis (from either glycogen or non-sugar substrates) was stimulated by removal of extracellular glucose. In four cells, addition of insulin 5 to 10 min after the beginning of the experiment reversed the decay in FRET ratio, ruling out photobleaching as the cause (data not shown). In contrast, when cells were cultured in low glucose for more than 2 days and then kept in low extracellular glucose until the beginning of the experiment, the opposite effect was observed. The baseline FRET ratio was lower, indicating higher resting intracellular glucose, and averaged 1.66±0.13 (n=8), as compared to 2.2±0.22 with cells cultured in high glucose. Furthermore, as extracellular glucose was successively switched between 0 and 10 mM, intracellular glucose progressively decreased (Fig. 3b). In both cases, these effects, which occurred within 30 min, were not associated with any major changes in the rate of glucose uptake. However, with cells cultured in low glucose, the decrease in basal glucose level was associated with an increase in the rate of glucose clearance by almost twofold (data not shown). From these observations, we conclude that the resting intracellular glucose level is dynamically modulated by extracellular glucose and is lower when cells are cultured in high glucose and higher when cultured in low glucose. Thus, in cells cultured in high glucose, episodic removal of glucose causes a rise in basal intracellular glucose within 30 to 60 min, possibly caused by the stimulation of gluconeogenesis. In contrast, when cells are cultured in low glucose, episodic addition of extracellular glucose (10 mM or more) causes a decrease in basal intracellular glucose, possibly mediated via glycolysis activation and/or inhibition of gluconeogenesis (see “Discussion”).
In most of the subsequent figures, we subtracted the slow shifts in intracellular glucose baseline (see “Materials and methods” for details) to facilitate better visualization of the rates of glucose uptake and clearance in response to glucose pulses.
Relationship between extracellular and intracellular glucose concentrations during short glucose pulses
We next examined how the short-term effects of extracellular glucose concentration on the FRET ratio varied with cell type and culture conditions. Figure 4a shows recordings from a C2C12 cell cultured in high glucose, with rapid glucose uptake. When extracellular glucose was added at the concentrations indicated, the maximal decrease in the FRET ratio was large (~0.2) and reached saturation at around 10 mM extracellular glucose, with the half-maximal response at 1.5 mM. However, in a CHO cell cultured in high glucose (Fig. 4b), which demonstrated slow glucose uptake, the maximal change in FRET ratio measured at 20 mM extracellular glucose was smaller (~0.08) and saturated at extracellular glucose concentrations >20 mM, with the half-maximal response at ~7 mM. Thus, not surprisingly, the gradient between intracellular and extracellular glucose depends on the rate of glucose uptake or, more precisely, the balance between glucose uptake and glucose metabolism.
To relate the changes in FRET ratio to absolute glucose concentrations, we used β-escin to permeabilize the cell membrane. Figure 5a shows data from a C2C12 cell cultured in low (<5 mM) glucose, in which the basal glucose concentration is expected to be high because of gluconeogenesis (see Fig. 3b). After adding and removing 10 mM extracellular glucose several times to define the maximal change in FRET ratio and allow the ratio to reach near steady state (see Fig. 3b), the cell was permeabilized and rechallenged with 10 or 0.7 mM glucose. Assuming that the steady state FRET ratio values are not affected by permeabilization, this calibration indicates that intracellular glucose approached 1 mM during exposure to 10 mM extracellular glucose. This suggests that, with fast glucose uptake and active glucose metabolism, glucose metabolism creates a gradient between extracellular and intracellular glucose that allows for rapid glucose uptake under steady state conditions.
These steady state values for basal intracellular glucose are lower than that reported by Fehr et al. [3] who estimated that the intracellular level was approximately half of the extracellular glucose concentration such that, with 10 mM extracellular glucose, intracellular glucose would be approximately 5 mM. However, as shown in Fig. 3, the variation in intracellular glucose concentration responds dynamically to acute alterations in extracellular glucose concentration so that considerable variation is expected depending on the measurement conditions.
Role of metabolism in glucose clearance
Intracellular glucose clearance after the removal of extracellular glucose may result from glucose metabolism (the net balance between glucose consumption and gluconeogenesis) or efflux. To assess the role of these two processes, we examined the effects of the glycolytic inhibitor IAA (0.1 mM). At 0.1 mM, IAA selectively blocks the glycolytic enzyme glyceraldehyde-3-phosphate dehydrogenase, causing elevation of glucose 6-P and, thus, inhibition of hexokinase [11]. In addition to the inhibition of glucose metabolism to pyruvate, IAA blocks gluconeogenesis from non-sugar substrates such as pyruvate, glycerol, and glucogenic amino acids, although not glucose transformation into glycogen or vice versa. Whereas glyceraldehyde-3-phosphate dehydrogenase inhibition may occur immediately, sufficient accumulation of glucose-6-P to inhibit hexokinase activity takes more time (up to 30 min in our experience). Figure 5b shows results from a CHO cell cultured in high glucose and exposed repeatedly to 0 and 10 mM glucose before and after IAA. After metabolic poisoning with 0.1 mM IAA, intracellular glucose increased steadily during prolonged exposure to 10 mM glucose, and clearance gradually slowed when extracellular glucose was removed. This residual slow glucose clearance could be caused by either incomplete inhibition of glucose metabolism by IAA, glycogen synthesis, or reverse glucose transport. Ultimately, however, after 30 min or more exposure to IAA, the FRET ratio approached saturation with little recovery upon removal of extracellular glucose. At this stage, the effect of IAA was irreversible, and intracellular glucose remained high. Similar effects were observed with HEK cells, which, like CHO cells, exhibit slow kinetics of glucose uptake (Table 1). Thus, after prolonged IAA exposure, neither net glycogen synthesis nor reverse glucose transport were capable of reducing intracellular glucose. In contrast with COS cells and C2C12 cells with higher rates of glucose uptake, accumulation of intracellular glucose in response to IAA was less pronounced (Fig. 3b). With COS cells and C2C12 cells, the change in FRET ratio evoked by IAA and measured in the absence of extracellular glucose, averaged 0.13±0.02 versus 0.2±0.03 with HEK cells and CHO cells. We speculate that the lesser effect of IAA with COS cells and C2C12 cells was only apparent, resulting from the higher rate of glucose uptake generating higher basal intracellular glucose concentrations. Consistent with this hypothesis, the effect of IAA on basal intracellular glucose concentration was also less pronounced in CHO cells cultured in low glucose (which exhibited high rate of glucose uptake and high basal intracellular glucose) as compared to CHO cells cultured in high glucose (which exhibited low rate of glucose uptake and low basal intracellular glucose). Alternatively, IAA may have been less effective at inhibiting glycolysis, or the cells had more effective reverse glucose transport.
In the CHO cell shown in Fig. 5b, we estimated the level to which intracellular glucose accumulated during glycolytic inhibition with IAA by permeabilizing the cell membrane with β-escin once the FRET ratio had reached near saturation. The calibration indicates that, before IAA, the intracellular glucose level in the absence of extracellular glucose was below the level of detection by FLIPglu-600µM (~0.07 mM) and, thus, at or below the Km of hexokinase (0.05 mM). After prolonged IAA exposure in the presence of 10 mM glucose, however, the FRET ratio was comparable to that for 10 mM glucose after permeabilization. This implies that, with slow glucose uptake and active glucose metabolism, the metabolism creates a large gradient between extracellular and intracellular glucose.
As stated in “Materials and methods,” permeabilization with β-escin led to slow loss of the glucose sensor, so the FRET ratio calibration may have underestimated intracellular glucose concentration in intact cells. However, the difference may not be very significant, as in permeabilized cells, the zero glucose value was close to that measured at the beginning of the experiment in the absence of extracellular glucose, and the maximum value was close to that measured in the presence of 10 mM extracellular glucose after metabolism poisoning with IAA (Fig. 4a).
Figure 6 illustrates how IAA changed the relationship between rates of glucose uptake and clearance in a CHO cell cultured in high glucose. After each application of 10 mM extracellular glucose, uptake occurred at similar rates before and after exposure to IAA, but glucose clearance upon removal of extracellular glucose progressively slowed. This observation is consistent with the hypothesis that glucose clearance depends strongly on glucose utilization by glycolysis, more so than by net glycogen synthesis or reverse glucose transport. The time constant of glucose clearance before addition of IAA was 29 s, increased to 64 s after exposure to IAA for 5 to 10 min, and 182 s after 20 min exposure. This data clearly demonstrate uncoupling between the rate of glucose clearance, which slowed dramatically with IAA, and glucose uptake, which was unchanged. Furthermore, after prolonged exposure to IAA for more than 45 min, there was little glucose clearance upon removal of extracellular glucose (τ >1000 s), although the driving force for glucose efflux was maximal. Thus, after prolonged IAA exposure, reverse glucose efflux via GLUT1 or glucose metabolism into glycogen was not significant.
Whereas glucose clearance before IAA was well fitted by a monoexponential curve, the kinetics of glucose clearance after IAA became more complex. This may be related to the indirect inhibition of hexokinase by IAA, which depends on upstream accumulation of glucose-6-P [11]. Thus, when extracellular glucose was removed, gradual clearance of glucose-6-P by conversion to glycogen and other pathways may release the inhibition of hexokinase, stimulating metabolism to accelerate glucose clearance.
Finally, it was important to rule out the possibility that the dramatic decrease in FRET ratio evoked by IAA was not caused by a nonspecific effect on YFP or CFP emission. In few experiments with IAA, CFP increased, whereas YFP decreased as expected for FRET, but in most cases, CFP emission decreased slightly, which we attributed to photobleaching over the 30-min period required for IAA to exert its full effects. To address this issue, we mutated FLIPglu-600µM at position 236, D236A, to suppress glucose binding [3]. In 11 experiments, the FRET ratio decreased on average by less than 0.02 over a 20- to 30-min exposure to IAA. This change, which is about 1:20 of the ~0.2 decrease in FRET ratio observed with the wild-type glucose sensor upon addition of IAA, strengthens our hypothesis that IAA-induced change in ratio is caused by a large increase in intracellular glucose.
Effects of temperature on kinetics of glucose uptake and clearance and basal intracellular glucose level
Because most of our experiments were performed at 25°C (room temperature) for convenience, we investigated in nine different experiments how raising temperature to a physiological level (35°C) affected our results. We found that, as expected, the rates of glucose uptake and clearance both increased, with the time constants for glucose uptake and clearance decreasing from 10.2±0.8 s (n=19) to 6.3±0.5 s (n=21) and from 17.2±1.4 s (n=19) to 9.6±0.8 s (n=21), respectively. Figure 7b summarizes the data and it also shows that the slow decrease in basal glucose level, which occurs upon removal of extracellular glucose preceding the fast phase of glucose clearance, became almost undetectable at 35°C (compare the right upper and lower traces recorded at 35 and 25°C, respectively). This data strongly suggest that this initial phase is not an artifact because of the perfusion system but corresponds rather to a specific step in glucose clearance. The dramatic increase in this phase’s duration observed after IAA (Fig. 6b and c) suggests that glyceraldehyde-3-phosphate dehydrogenase (the target of IAA) is likely to play a role in this process.
Fig. 7.
Effects of temperature on the rates of glucose uptake and clearance and basal intracellular glucose levels from two different C2C12 cells cultured in high (25 mM). a A typical decrease and then increase in FRET ratio observed when the bath temperature was first set at 35°C and then lowered to 25°C. Note that basal intracellular glucose increased at 35°C and decreased at 25°C while the temperature was constant. Furthermore, these changes in FRET ratio occurred without any major change in the rates of glucose uptake or clearance. The table in a presents the rate constants for glucose uptake (τd) and clearance (τr) recorded during the changes in glucose concentration labeled 1 to 6. b Rates of glucose uptake and clearance measured at 35°C (upper traces) and 25°C (lower traces)
In addition to its effects on the rates of glucose uptake and clearance, temperature had profound effects on the basal level of intracellular glucose (Fig. 7a) that were similar in amplitude to those observed with cell culture in high and low glucose. As the temperature increased, basal glucose level also increased, although the rates of glucose uptake and clearance remained almost unchanged (Fig. 7a, table). This suggests that the glucose loading is not due to the increased glucose uptake or clearance but may reflect, instead, stimulation of gluconeogenesis at the expense of glycolysis by higher temperature. Conversely, as the temperature fell back to 25°C, the basal level of intracellular glucose decreased. It should be noted that the changes in FRET ratio observed after prolonged exposure to physiological temperature decreased in amplitude. Our data obtained with β-escin suggest that these small changes in amplitude reflect glucose sensor saturation as intracellular glucose exceeds 5 to 6 mM.
We have also carried out experiments with IAA at 35°C. While the spontaneous increase in basal glucose level at 35°C makes it difficult to estimate the contribution of IAA to intracellular glucose accumulation, our data indicate that after 15 min exposure to IAA, the changes in FRET ratio during extracellular glucose pulses became negligible (data not shown). These data are very similar to those observed at 25°C and suggest that intracellular glucose level reaches the maximum extracellular glucose concentration under these conditions.
Changes in glucose homeostasis induced by differentiation in C2C12 cells
We found that undifferentiated C2C12 cells had the highest rate of glucose uptake, whether cultured in low or high glucose. During muscle development, GLUT1 is downregulated, whereas GLUT4 is upregulated [5], which is essential for conferring insulin sensitivity to glucose uptake in muscle. Thus, as insulin increases after a meal, GLUT4 insertion into the plasma membrane is stimulated, and glucose uptake increases several-fold [1]; high GLUT1 activity would mask this effect. To investigate the effects of muscle differentiation on glucose homeostasis, we differentiated C2C12 cells and transfected them with FLIPglu-600µM. Differentiated cells exhibited a slow rate of glucose uptake, a high rate of clearance and a low intracellular level of glucose, similar to HEK cells or CHO cells cultured in high glucose. In five cells with measurable glucose uptake, the τ for glucose uptake averaged 69±13 s, whereas the τ for glucose clearance averaged 30±6 s, supporting the hypothesis that muscle differentiation induces GLUT1 downregulation. However, many other cells did not exhibit any change in FRET ratio upon addition and removal of extracellular glucose (Fig. 8). In these cases, exposure to 0.1 mM IAA caused the resting level of glucose to increase and subsequently respond to the 10 mM extracellular glucose pulses. We interpret these findings to indicate that before addition of IAA, the intracellular glucose concentration in differentiated C2C12 cells is below the level of detection by FLIPglu-600µM (70 µM), because of a slow rate of glucose uptake and high rate of metabolism. However, after IAA, inhibition of glucose metabolism allows glucose to accumulate in response to extracellular glucose, albeit with a slow time constant. When extracellular glucose is subsequently removed, intracellular glucose also slowly falls, perhaps, because of a combination of glycogen synthesis and relief of glucose-6-P mediated hexokinase inhibition.
Glucose uptake is mediated by a GLUT transporter, not Naglucose co-transport
Glucose is transported across cell membranes via either the uniporter GLUTx or the Na/glucose co-transporter [10, 14], which are blocked, respectively, by cytochalasin B (Cyto B, 20 µM) and phlorizin (100 µM) [2, 14]. In all four cell lines, the uptake of glucose was fully blocked by Cyto B (Fig. 9d) and was insensitive to phlorizin (τ =31.5±4.5 s and 37±5 s, with and without phlorizin, respectively), suggesting GLUT-facilitated glucose transport. PCR data (Fig. 10) further suggested that GLUT1 is the main glucose transporter in the four cell lines, consistent with the observation that GLUT1 is expressed at high levels in tumor cell lines [9]. Figure 9a illustrates the effects of Cyto B in a HEK cell cultured in high glucose (i.e., the slow glucose uptake phenotype, Table 1) and Fig. 9d in a C2C12 cell cultured in low glucose (i.e. the rapid glucose uptake phenotype). Although in all cell types, glucose entry in response to the addition of 10 mM extracellular glucose was completely blocked by prior addition of Cyto B (as in Fig. 9d), the situation was more complex when Cyto B was applied after the addition of 10 mM glucose. In HEK or CHO cells cultured in high glucose as in Fig. 9a, addition of Cyto B in the continued presence of 10 mM glucose caused intracellular glucose to decline at a rate similar to that observed upon removal of extracellular glucose in the absence of Cyto B (Fig. 9b versus c). This observation suggests that glucose metabolism rather than reverse glucose transport dominates glucose clearance in cells with a slow rate of glucose uptake (τ =59 s in the present experiment), consistent with the findings that IAA blocks almost completely glucose clearance in these cells. In contrast, in COS, C2C12, and CHO cells cultured in low glucose (Fig. 9d), in which glucose uptake was rapid (τ =12 s and see Table 1), the rate of glucose clearance upon addition of Cyto B in the presence of 10 mM glucose was considerably slower than when glucose was removed in the absence of Cyto B (Fig. 9e versus f). However, if extracellular glucose was removed, while Cyto B was still present (Fig. 9g), glucose clearance rate increased instantaneously and was similar to the rate observed upon removal of glucose in the absence of Cyto B (Fig. 9e versus g). The fact that partial inhibition of the GLUT transporter by Cyto B did not slow down glucose clearance when extracellular glucose was removed indicates that reverse glucose transport did not play a dominant role in the process underlying glucose clearance in these cells. In addition, these results suggest that when intracellular glucose is already high (i.e., after exposure to 10 mM extracellular glucose), GLUT-mediated glucose transport is not effectively blocked by Cyto B. This is consistent with the finding that intracellular glucose interferes with the ability of Cyto B to block GLUT by binding to an intracellular site [8]. In summary, although these findings indicate that GLUTx transporters mediate glucose uptake in these cell types, the contribution of reverse glucose transport to glucose clearance could not be clearly demonstrated. Furthermore, the experiments with IAA indicate, at least in cells with a slow glucose uptake phenotype (Figs. 5, 6, and 7), that glucose metabolism is the main mechanism responsible for glucose clearance.
Fig. 9.
Glucose uptake and clearance in the presence of the glucose transporter inhibitor cytochalasin B, Cyto B. a Data obtained with a HEK cell cultured in high glucose when exposed to Cyto B (20 µM) in the continuous presence of 10 mM glucose outside. The plots in b and c show the rate of glucose clearance after the removal of glucose (in the absence of Cyto B) and after the addition of Cyto B in the presence of 10 mM glucose. The closeness of the two τ suggests that, with cells exhibiting a slow glucose uptake phenotype, GLUTmediated glucose efflux has no role in glucose clearance. This trace also illustrates that the effect of Cyto B is readily reversible, as glucose entry resumed, albeit at a lower rate, 30 s after Cyto B removal. In d, data were obtained with a C2C12 cell cultured in low glucose, which exhibited a high rate of glucose uptake. In this case, Cyto B blocks completely glucose entry when applied before the addition of glucose outside. With prior loading of the cell with glucose, the rate of clearance was very much reduced as compared to that recorded after outside glucose removal (see plots e and f). However, the rate of clearance recovered almost completely under similar experimental conditions, when outside glucose was removed in the continuous presence of Cyto B (plot g). These data suggest that substantial glucose loading prevents, at least in part, GLUT inhibition by Cyto B
Fig. 10.
RTPCR analysis of GLUT 1 through 4 in CHO, COS, HEK293, and differentiated and undifferentiated C2C12 cell lines. CDNA isolated from the four cell lines indicate the presence of GLUT1 and GLUT3 in the four cell types. GLUT4 cDNA only became apparent when C2C12 cells were differentiated. HEK293 cells were probed with primers directed against human sequences. C2C12 cells were probed with primers directed against mouse sequences. Initial studies using human-, mouse-, and rat-derived sequence primers showed that GLUT1 and GLUT3 cDNA from CHO and COS cells could be detected by human sequence primers only. In these cells, the temperature for annealing was lowered from 55 to 53° to detect GLUT1-4
Discussion
We investigated the dynamics of intracellular glucose regulation in HEK293, COS-7, CHO, and differentiated or undifferentiated C2C12 cells using the glucose biosensor FLIPglu-600µM that undergoes changes in FRET upon binding glucose and is insensitive to phosphorylated glucose or glycolytic intermediates. Intracellular glucose concentration reflects the balance of glucose transport and metabolism. As expected, we found that GLUT transporters mediate glucose flux in all of these cell types, as glucose influx was completely blocked by cytochalasin B but not by phlorizin. The real-time PCR (RTPCR) data suggest that the dominant GLUT is GLUT1, consistent with previous reports, indicating that cell culture lines express elevated levels of GLUT1 [9]. Our most significant and novel findings, however, relate to the extent to which acute and chronic changes in extracellular glucose dynamically regulate intracellular glucose homeostasis by modulating both glucose transport and metabolism.
When CHO cells were chronically (>48 h) maintained in high glucose (25 mM) rather than low glucose (<5 mM), the rate of glucose uptake in response to acute changes in extracellular glucose decreased threefold, whereas the glucose clearance rate remained unchanged. This observation is consistent with biochemical data demonstrating that high glucose downregulates the expression of GLUT1 in cultured cells [10]. Also in HEK cells cultured in high glucose, the rate of glucose clearance increased, consistent with upregulation of glycolytic enzymes [13]. In the other cell types, steady-state rates of glucose uptake and clearance were not much affected by the extracellular glucose concentration in the culture medium (Table 1). However, in all of the cell types, the chronic level of extracellular glucose in the culture media modulated basal intracellular glucose concentration.
In all cell types cultured in high glucose, the basal intracellular glucose was low (i.e., the basal FRET ratio was high) when extracellular glucose was reduced and progressively increased over a period of 30–60 min when extracellular glucose was successively switched between 0 and 10 mM (Fig. 3a). This occurred in the absence of significant changes in the rates of glucose uptake or clearance (Fig. 3), quite different from the effect of IAA, where the overall increase in intracellular glucose was accompanied by a decrease in the rate of glucose clearance reflecting inhibition of glycolysis (Fig. 6). In addition, in the case of high-glucose culture media, the progressive increase in intracellular glucose, as extracellular glucose was successively alternated between 0 and 10 mM, could be reversed by insulin. In contrast, in cells cultured for >48 h in the presence of low glucose (<5 mM), basal intracellular glucose progressively decreased as extracellular glucose was successively alternated between 0 and 10 mM. In this case, it was often difficult to fit the rate of glucose uptake and clearance with a single exponential because of an additional phase of glucose clearance.
These findings can be explained by “reciprocal regulation” of glycolysis and gluconeogenesis during culture in chronically high versus low extracellular glucose. With chronically high extracellular glucose, it makes sense teleologically for glycolysis to be activated and gluconeogenesis suppressed so that intracellular glucose drops to a low level when extracellular glucose is acutely reduced. Conversely, during culture in chronically low glucose, glycolysis would turn off, and gluconeogenesis would be activated (assuming availability of alternative energy substrates), causing intracellular glucose to rise. Our FRET data in Fig. 3 support this hypothesis, as cells cultured in the presence of high glucose had a lower basal glucose level (as indicated by a higher basal FRET ratio) compared to cells cultured in low glucose. Moreover, when cells cultured in high glucose were subsequently exposed to alternating 0 and 10 mM glucose pulses, intracellular glucose progressively increased (Fig. 3a). This could only be because of the stimulation of gluconeogenesis, as even in the maintained absence of extracellular glucose, basal intracellular glucose rose continuously.
In cells cultured in low extracellular glucose concentration, on the other hand, the opposite effects were observed. Although, in our experiments, low glucose was lower than physiological glucose (5 mM), it likely represents a state in which glucose and alternative metabolic substrates, such as glycogen, endogenous fatty acids, and amino acids, are utilized in a balanced fashion. In this setting, basal intracellular glucose was higher (as indicated by a lower basal FRET ratio), consistent with inhibited glycolysis and activated gluconeogenesis. When glucose was transiently elevated to 10 mM (as in Fig. 3b), basal intracellular glucose decreased, consistent with activation of glycolysis and/or suppression of gluconeogenesis. As this transition was accompanied by a twofold increase in the rate of glucose clearance after removal of extracellular glucose, increased glycolysis is likely, at least in part, to be responsible for this phenomenon. The IAA-induced increase in intracellular glucose under these conditions (see Fig. 3b) provides additional evidence in favor of stimulation of glycolysis by transient elevation of extracellular glucose in cells chronically cultured in low glucose. To show that it was the elevation to 10 mM extracellular glucose that stimulates glycolysis, extracellular glucose was alternated between 0 and 2.5 mM, then between 0 and 10 mM and back. Basal glucose decreased only when extracellular glucose was elevated to 10 mM and increased when extracellular glucose was returned to 2.5 mM, consistent with 5 mM glucose being a threshold at which glucose clearance is stimulated. It should be pointed out, however, that such a decrease in basal intracellular glucose could be caused, in part, by gluconeogenesis inhibition.
In their publication, Fehr et al. [3] suggested that intracellular glucose was as much as half of the outside concentration, that is, with 10 mM outside, glucose inside would reach 5 mM. While some of our findings are consistent with this hypothesis, we found that basal intracellular glucose could vary dramatically, depending on the cell type and extracellular glucose concentration. For cells cultured in high glucose and in which glucose uptake was slow, intracellular glucose reached <10% of the extracellular glucose concentration and fell below the Km for hexokinase (0.05 mM) when extracellular glucose was removed (Fig. 5). Thus, glycolytic activity is robust enough to maintain a large transmembrane glucose gradient under these conditions. Accordingly, when glycolysis was inhibited with IAA, there was a dramatic increase in intracellular glucose, which approached the extracellular glucose concentration, as indicated by calibration with β-escin (Fig. 5b). Because the sensor saturates at glucose concentrations greater than 6 mM, it was not possible to estimate precisely the intracellular glucose level above this concentration. On the other hand, for cells with rapid glucose uptake, basal intracellular glucose was higher in the presence of 10 mM extracellular glucose but still in the range of 1 mM or less (Fig. 5a). Under these conditions, IAA caused a lesser increase in intracellular glucose compared to cells with a low rate of glucose uptake.
Finally, we investigated how the differentiation in C2C12 cells affects the rates of glucose uptake and clearance. From our PCR data, we found, as expected, that GLUT1 was downregulated, and insulin-dependent GLUT4 was upregulated during differentiation. This was associated with a dramatic decrease in glucose uptake in C2C12 cells in either the absence or presence of insulin. The lack of insulin response is also expected, as these experiments were conducted at room temperature, and insulin-mediated plasma membrane insertion of GLUT4 containing vesicles requires temperatures >32°C. Experiments with differentiated C2C12 cells at higher temperature are currently in progress. From the data in Fig. 8, it may be estimated that the rate of glucose clearance in differentiated C2C12 cells, in the absence of IAA, is high, similar to that observed in undifferentiated C2C12 cells. This may explain, in part, the very low intracellular glucose level observed with 10 mM glucose outside. This high rate of clearance may reflect the fact that tumor cells, unlike primary muscle cells, have a high rate of glucose metabolism. A slow rate of clearance in primary muscle cells may match a slow rate of uptake under unstimulated conditions, such that intracellular glucose may reach 300 to 500 µM. Our data in Fig. 8 show that during exposure to 0.1 mM IAA, the amplitude of the response to 10 mM extracellular glucose pulses increased as intracellular resting glucose level rose. This increase in amplitude of the change in FRET ratio was accompanied by a decrease in the rate of glucose clearance and an increase in the rate of glucose uptake. Similar, although lesser, increases in the rate of glucose uptake were observed in undifferentiated C2C12 cells exposed to IAA (Fig. 6). Inhibition of glucose metabolism by IAA provides a straightforward explanation for the decrease in the rate of glucose clearance, but because IAA will reduce glucose gradient across the cell membrane, the increase in rate of uptake cannot be explained by a change in driving force. Inhibition of GLUT transporter by intracellular ATP [3] provides an explanation for this phenomenon, whereby, a drop in intracellular ATP level after glucose metabolism inhibition may cause a gradual increase in the rate of glucose transport.
The phlorizin and Cyto B data support our hypothesis that the decrease in FRET ratio observed upon the addition of extracellular glucose results from glucose uptake via a glucose transporter, possibly GLUT1. Our data could not establish definitely, however, how much GLUT1-dependent reverse glucose transport contributes to intracellular glucose clearance after the removal of extracellular glucose. In cells with a slow glucose uptake rate phenotype, which are characterized by low levels of intracellular glucose, the rate of glucose clearance was unaffected by Cyto B. In contrast, glucose clearance slowed dramatically after glycolytic inhibition by IAA, suggesting that glycolytic was the main mechanism of glucose clearance, compared to reverse glucose transport or glucose consumption by glycogen synthesis or other metabolic pathways. On the other hand, in cells with high glucose uptake rates, IAA appeared to be less effective at increasing intracellular glucose and Cyto B could not accelerate glucose clearance efficiently when extracellular glucose was present (Fig. 9f), suggesting that either IAA was perhaps less effective at blocking glycolysis or that reverse glucose transport played a role. Additional experiments would have to be performed to assess, for instance, how the level of intracellular glucose regulates reverse glucose transport. Considering that GLUT1’s Km for intracellular glucose is 4.6 mM (at 20°C) [12], it is expected that reverse transport would play a role when intracellular glucose approaches 1 mM and is even greater than 6 mM after prolonged exposure to IAA. Finally, elevated intracellular glucose levels in cells with high rate of glucose uptake may explain the poor glucose clearance evoked by Cyto B in the presence of extracellular glucose (Fig. 9f), as elevated intracellular glucose prevents GLUT blocking by Cyto B [8]. Thus, maintained glucose influx because of partial inhibition of the glucose transporter by Cyto B would account for the slow rate of glucose clearance under these conditions, whereas more complete block of the glucose transporter by Cyto B in cells with slow rate of uptake and low intracellular glucose level would account for their fast rate of glucose clearance (Fig. 9c).
In summary, glucose homeostasis is dynamically modulated by coordinated alterations in glucose uptake and metabolism, depending on glucose availability, cell type, and state of differentiation. With cells cultured in high (25 mM) glucose, a high rate of glucose metabolism and a slow rate of glucose uptake typically yield intracellular glucose levels below the sensor detection level at 0.07 mM. With a high rate of glucose uptake, on the other hand, intracellular glucose level typically reaches 0.5–1 mM when extracellular glucose is 10 mM. With cells cultured in low (<5 mM) glucose, basal intracellular glucose levels are higher, potentially approaching the level of extracellular glucose when glycolysis is inhibited. In the four cell types studied here, a GLUT transporter, possibly GLUT1, mediate glucose uptake, and glucose metabolism plays the dominant role in intracellular glucose clearance.
Acknowledgment
The authors thank Dr. W. F. Frommer for his generous gift of the glucose sensor (FLIPGlu-600µM), Dr. L.-H. Xie for his constructive comments and Dr. P. Sdek for helping with C2C12 cell differentiation. This work was supported by NIH grants R37HL60025 and P01 HL071870, and Laubisch and Kawata Endowments to J.N.W.
Contributor Information
Scott A. John, UCLA Cardiovascular Research Laboratory, David Geffen School of Medicine at UCLA, Los Angeles, CA 90095, USA Department of Medicine (Cardiology), David Geffen School of Medicine at UCLA, Los Angeles, CA 90095, USA.
Michela Ottolia, UCLA Cardiovascular Research Laboratory, David Geffen School of Medicine at UCLA, Los Angeles, CA 90095, USA; Department of Physiology, David Geffen School of Medicine at UCLA, Los Angeles, CA 90095, USA.
James N. Weiss, UCLA Cardiovascular Research Laboratory, David Geffen School of Medicine at UCLA, Los Angeles, CA 90095, USA Department of Physiology, David Geffen School of Medicine at UCLA, Los Angeles, CA 90095, USA; Department of Medicine (Cardiology), David Geffen School of Medicine at UCLA, Los Angeles, CA 90095, USA.
Bernard Ribalet, Email: bribalet@mednet.ucla.edu, UCLA Cardiovascular Research Laboratory, David Geffen School of Medicine at UCLA, Los Angeles, CA 90095, USA; Department of Physiology, David Geffen School of Medicine at UCLA, Los Angeles, CA 90095, USA.
References
- 1.Bryant NJ, Govers R, James DE. Regulated transport of the glucose transporter GLUT4. Nat Rev Mol Cell Biol. 2002;3:267–277. doi: 10.1038/nrm782. [DOI] [PubMed] [Google Scholar]
- 2.Cloherty EK, Levine KB, Carruthers A. The red blood cell glucose presents multiple, nucleotide-sensitive sugar exit sites. Biochemistry. 2001;40:15549–15561. doi: 10.1021/bi015586w. [DOI] [PubMed] [Google Scholar]
- 3.Fehr M, Lalonde S, Lager I, Wolff MW, Frommer WB. In vivo imaging of the dynamics of glucose uptake in the cytosol of COS-7 cells by fluorescent nanosensors. J Biol Chem. 2003;278:19127–19133. doi: 10.1074/jbc.M301333200. [DOI] [PubMed] [Google Scholar]
- 4.Fukami-Kobayashi K, Tateno Y, Nishikawa K. Domain dislocation: a change of core structure in preiplasmic binding proteins in their evolutionary history. J Mol Biol. 1999;286:279–290. doi: 10.1006/jmbi.1998.2454. [DOI] [PubMed] [Google Scholar]
- 5.Gaster M, Handberg A, Beck-Nielsen H, Schroder HD. Glucose transporter expression in human skeletal muscle fibers. Am J Physiol Endocrinol Metab. 2000;279:E529–E538. doi: 10.1152/ajpendo.2000.279.3.E529. [DOI] [PubMed] [Google Scholar]
- 6.Graham FL, van der Eb AJ. Transformation of rat cells by DNA of human adenovirus 5. Virology. 1973;54:536–539. doi: 10.1016/0042-6822(73)90163-3. [DOI] [PubMed] [Google Scholar]
- 7.Guillam M-T, Burcelin R, Thorens B. Normal hepatic glucose production in the absence of GLUT2 reveals an alternative pathway for glucose release from hepatocytes. Proc Natl Acad Sci USA. 1998;95:12317–12321. doi: 10.1073/pnas.95.21.12317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Helgerson AL, Carruthers A. Equilibrium ligand binding to the human erythrocyte sugar transporter. J Biol Chem. 1987;262:5464–5475. [PubMed] [Google Scholar]
- 9.Hiraki Y, Garcia de Herreros A, Birnbaum MJ. Transformation stimulated transporter gene expression in the absence of protein kinase C. Proc Natl Acad Sci USA. 1989;86:8252–8256. doi: 10.1073/pnas.86.21.8252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klip A, Tsakiridis T, Marette A, Ortiz PA. Regulation of expression of glucose transporters by glucose: a review of studies in vivo and in cell cultures. FASEB J. 1994;8:43–53. doi: 10.1096/fasebj.8.1.8299889. [DOI] [PubMed] [Google Scholar]
- 11.Liu X, Kim CS, Kurbanov FT, Honzatko RB, Fromm HJ. Dual mechanism for glucose 6-phosphate inhibition of human brain hexokinase. J Biol Chem. 1999;274:31155–31159. doi: 10.1074/jbc.274.44.31155. [DOI] [PubMed] [Google Scholar]
- 12.Lowe AG, Walmsley AR. The kinetics of glucose transport in human red blood cells. BBA. 1986;857:146–154. doi: 10.1016/0005-2736(86)90342-1. [DOI] [PubMed] [Google Scholar]
- 13.Roche E, Assimacopoulos-Jeannet F, Witters LA, Perruchoud B, Yaney G, Corkey B, Asfari M, Prentki M. Induction by glucose of genes coding for glycolytic enzymes in a pancreatic β cell line (INS-1) J Biol Chem. 1997;272:3091–3098. doi: 10.1074/jbc.272.5.3091. [DOI] [PubMed] [Google Scholar]
- 14.Wright EM, Hirayama BA, Loo DF. Active sugar transport in health and disease. J Internal Med. 2007;261:32–43. doi: 10.1111/j.1365-2796.2006.01746.x. [DOI] [PubMed] [Google Scholar]