Abstract
Wnt1 and Wnt3a secreted from the dorsal neural tube were previously shown to regulate a gene expression program in the dorsal otic vesicle that is necessary for vestibular morphogenesis (Riccomagno et al., 2005). Unexpectedly, Wnt1−/−; Wnt3a−/− embryos also displayed a pronounced defect in the outgrowth of the ventrally derived cochlear duct. To determine how Wnt signaling in the dorsal otocyst contributes to cochlear development we performed a series of genetic fate mapping experiments using two independent Wnt responsive driver strains (TopCreER and Gbx2CreER) that when crossed to inducible responder lines (RosalacZ or RosazsGreen) permanently labeled dorsomedial otic progenitors and their derivatives. Tamoxifen time course experiments revealed that most vestibular structures showed some degree of labeling when recombination was induced between E7.75 and E12.5, consistent with continuous Wnt signaling activity in this tissue. Remarkably, a population of Wnt responsive cells in the dorsal otocyst was also found to contribute to the sensory epithelium of the cochlear duct, including auditory hair and support cells. Similar results were observed with both TopCreER and Gbx2CreER strains. The ventral displacement of Wnt responsive cells followed a spatiotemporal sequence that initiated in the anterior otic cup at, or immediately prior to, the 17-somite stage (E9) and then spread progressively to the posterior pole of the otic vesicle by the 25-somite stage (E9.5). These lineage-tracing experiments identify the earliest known origin of auditory sensory progenitors within a population of Wnt responsive cells in the dorsomedial otic cup.
Keywords: cochlea, sensory progenitors, fate map, Wnt signaling, inner ear
Introduction
The mammalian inner ear is responsible for auditory and balance perception. Clusters of mechanosensory hair cells positioned at six strategic locations within the inner ear operate as sound and motion detectors that relay sensory information along associated nerve fibers to auditory and vestibular centers in the brain (Schwander et al., 2010; Cullen, 2012; Yu and Goodrich, 2014). One such sensory cluster, the organ of Corti, is a specialized auditory apparatus unique to mammals that comprises a single row of inner hair cells, three rows of outer hair cells and a variety of interspersed support cells that line the length of the cochlear duct. The approximately 16,000 auditory hair cells in the human ear discriminate sound frequencies according to a tonotopic map that forms from high to low along the basal to apical axis of the cochlear duct (Mann and Kelley, 2011). Irrevocable hair cell damage caused by various gene mutations, environmental exposures (loud noise, infectious agents, ototoxic drugs), as well as aging, may result in hearing impairment. Consequently, a detailed understanding of hair cell development continues to be warranted not only to expand on the molecular and cellular underpinnings by which this fascinating cell type forms, but to leverage this information for the purposeful design of novel regenerative medicine strategies in the treatment of hearing loss (Géléoc and Holt, 2014).
The sensory and nonsensory cells that make up the organ of Corti are derived from a common pool of epithelial progenitors that form along the medial wall of the elongating cochlear duct (Li et al., 1978; Corwin et al., 1993; Fekete et al., 1998; Jiang et al., 2013). Sox2 is the earliest known marker of this prosensory domain and is both necessary and sufficient for its induction (Kiernan et al., 2005; Pan et al., 2013). Multiple signaling pathways have been implicated in the specification, expansion and maintenance of the Sox2+ prosensory lineage, including Notch, Bmp, Fgf and Wnt (Pirvola et al., 2002; Daudet and Lewis, 2005; Brooker et al., 2006; Kiernan et al., 2006; Daudet et al., 2007; Ohyama et al., 2010; Pan et al., 2010; Yamamoto et al., 2011; Jacques et al., 2012; Pan et al., 2013; Ono et al., 2014). Between E12.5 and E14.5, sensory progenitors in the cochlear duct exit the cell-cycle in an apical-basal progression and then, over the course of several days, differentiate into hair and support cells following a reverse course that initiates at mid-basal levels (reviewed in Kelley 2006). Although progress has been made in understanding the molecular mechanisms that specify the prosensory domain and promote its differentiation into distinct auditory cell fates, less is known about the origin of the Sox2+ prosensory lineage at earlier stages of otic development.
The majority of cells that constitute the inner ear derive from the otic placode, an ectodermal thickening that forms adjacent to the hindbrain in response to Fgf, Wnt and Notch signals (Ladher et al., 2000; Phillips et al., 2001; Ladher et al., 2005; Ohyama et al., 2006; Jayasena et al., 2008; Chen and Streit, 2013; Sánchez-Guardado et al., 2014). Soon after its induction at E8.5 in the mouse, the otic placode invaginates to form the otic cup. Upon further growth and dissociation from the surface ectoderm, the tips of the cup fuse at the dorsal midline to form the otic vesicle (reviewed in Groves and Fekete, 2012). A small number of neural crest and neuroepithelial cells emerging from the dorsal neural tube also contribute to the inner ear, including sensory, nonsensory and neuronal lineages (Mayordomo et al., 1998; Streit, 2002; Freyer et al., 2011).
Lineage tracing experiments performed at the otic placode and vesicle stages in the frog demonstrated that sensory patches derive from multiple locations after extensive cell mixing (Kil and Collazo, 2001). In contrast, gene expression studies in the mouse and chicken suggest that markers of prospective utricular, saccular and cochlear sensory patches reveal a strong anteroventral bias by the otic vesicle stage (Morsli et al., 1998). Interestingly, a dynamic distribution of retinoic acid signaling was shown to specify cell fates along the anteroposterior axis of the otic cup, with low levels contributing to sensory and neuronal identities in the anterior compartment and higher levels promoting mostly nonsensory structures in the posterior region (Bok et al., 2011). Genetic fate mapping experiments of Ngn1 expressing cells in the mouse are consistent with these findings, and indicate that in some vestibular structures (utricle and saccule), hair cells and their innervating neurons derive from common progenitors in the anteroventral region of the otic vesicle (Raft et al., 2007). However, since auditory hair cells were not labeled in these experiments, the precise origin of this specific sensory lineage remains in question.
The Wnt/β-catenin signaling pathway is required for many facets of inner ear development, including otic placode induction, dorsal otic identity, vestibular morphogenesis, and the specification, proliferation and differentiation of prosensory progenitors (Ladher et al., 2000; Stevens et al., 2003; Riccomagno et al., 2005; Ohyama et al., 2006; Jacques et al., 2012; Rakowiecki and Epstein, 2013; Munnamalai and Fekete, 2013; Forristall et al., 2014; Shi et al., 2014). Previously, we showed that Wnt1 and Wnt3a secreted from the dorsal neural tube are required for the expression of several key dorsal otic determinants, including Dlx5, Dlx6 and Gbx2 (Riccomagno et al., 2005). Consequently, vestibular development was completely impaired in Wnt1−/−; Wnt3a−/− embryos. Yet, despite the normal appearance of ventral otic genes, cochlear morphogenesis was also compromised in these mutants. This result was surprising given our inability to detect Wnt signaling activity in the ventral otocyst during the initial stages of cochlear outgrowth. In an effort to reconcile these findings, we traced the fate of Wnt responsive cells in the dorsal otocyst and observed their contribution to the ventromedial wall of the cochlear duct. Thus, the failure to expand the Wnt responsive cell population in Wnt1−/−; Wnt3a−/− embryos likely explains the ventral defects in cochlear outgrowth (Riccomagno et al., 2005).
Several questions remain from this analysis. For instance, what is the definitive fate of the ventrally extending population of Wnt responsive cells? Do they contribute to some and/or all of the hair and support cells in the organ of Corti? When and how do these Wnt responsive cells move during otic development? How can one be certain that this ventral-ward displacement of dorsal otic progenitors is not simply an artifact of the TopCreER mouse line used in these studies?
To address these outstanding questions, we performed a new series of genetic fate mapping experiments using two independent mouse lines (TopCreER and Gbx2CreER) that when crossed to an inducible reporter strain (RosalacZ or RosaZsGreen) permanently labeled dorsomedial otic progenitors and their derivatives. Tamoxifen time course experiments revealed that the majority of hair and support cells within the organ of Corti derive from a population of dorsomedial otic progenitors that become ventrally displaced between E8.5 and E9.5. Similar results were observed with both CreER lines. These and other results demonstrate that Wnt responsive progenitors in the dorsomedial otic cup are the earliest known origins of the cochlear sensory epithelium.
Materials and Methods
Animals
RosaZsGreen/+ (Ai6 line in Madisen et al., 2010) and RosalacZ/+ (Soriano, 1999) mouse lines were obtained from Jackson Labs (Bar Harbor, ME). ZsGreen is an extremely bright green fluorescent protein from Zoanthus species reef coral, which was knocked in to the Rosa 26 locus downstream of a floxed transcriptional stop cassette (Matz et al., 1999; Madisen et al., 2010). TopCreER and Gbx2CreER/+ mouse strains were described previously (Riccomagno et al., 2005; Chen et al., 2009). The production of Ngn1Cre and RosatdTomato/+ lines was described previously (Quiñones et al., 2010; Madisen et al., 2010).
Tamoxifen administration
Tamoxifen was dissolved in corn oil and administered to pregnant dams by oral gavage at a dose of 150 μg/g body weight (Sigma Aldrich St. Louis, MO).
Timed Matings
For time course experiments described in Fig. 6, matings were set up during the day between 10am and 4pm with the midpoint (1pm) corresponding to embryonic day 0 (E0) upon the identification of a vaginal plug. Tamoxifen was administered to pregnant dams at 8am on E7.75 of gestation and embryos were harvested the following day between 12pm and 6pm. For all other timed matings, noon of the day of vaginal plug detection corresponded to E0.5.
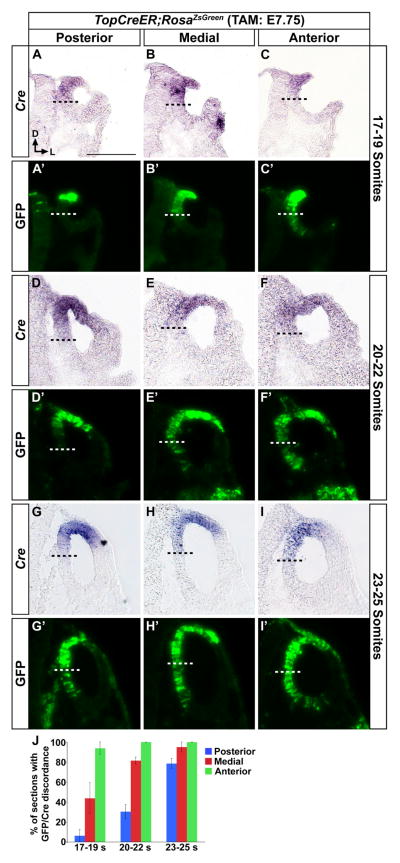
Figure 6. Wnt responsive cells are displaced ventrally over time from their dorsomedial origin in the otic cup/vesicle.
Transverse sections through the inner ears of TopCreER; RosaZsGreen embryos administered tamoxifen at E7.75 and collected at otic cup (E9.0, A–C), early closure (E9.25, D–F) and otic vesicle (E9.5, G–I) stages of ear development. Each section was stained for Cre expression by in situ hybridization. The panels marked with a prime (′) are the same sections as above imaged for GFP fluorescence (green). The Cre and GFP images were superimposed to position the ventral boundary of Cre expression (dashed black line) on the GFP panel (dashed white line). The first GFP cells to become ventrally displaced from their Cre expressing descendants – green cells falling below the dashed line – occurred in anterior regions of the otic cup (C–C′) and then progressed over time to include medial (E–E′, H–H′) and posterior (G–G′) regions of the otic vesicle. (J) Graphical representation of the percentage of sections at each stage and region demonstrating GFP discordance with Cre expression. Scale bar: 100 μm. Error bars represent s.e.m.
Whole mount cochlear preparations
Cochleae from E18.5 embryos were fixed in 4% paraformaldehyde for 3 hours at 4°C, washed in PBS, microdissected to expose the sensory epithelium, and incubated with a rabbit anti-myosin VIIa (1:500; Proteus Biosciences Inc) antibody, followed by a Cy3-conjugated goat anti-rabbit secondary antibody (1:200). GFP expression from the recombined RosaZsGreen/+ allele was detected by direct fluorescence using a Leica SP2 confocal microscope. The number of co-labeled hair cells in a 40 cell wide area at the base, midpoint and apex of the cochlear duct was counted from three to four embryos per stage of tamoxifen treatment. An unpaired t-test was performed to determine significant differences between regions.
X-gal staining
The heads of E18.5 embryos were bisected at the dorsal midline, the brains removed, and fixed in 4% paraformaldehyde for 1 hour at 4°C. Bisected heads were then incubated in X-gal staining solution at 37°C overnight according to Epstein et al (2000), and post fixed for 3 hours in 4% paraformaldehyde. The otic capsules were dissected, dehydrated in a graded methanol series, and cleared using a 1:2 solution of benzyl alcohol:benzyl benzoate. Cleared inner ears were imaged on a Leica MZ16 dissecting microscope.
Immunohistochemistry
The heads of E18.5 embryos were bisected and the brains removed, fixed in 4% paraformaldehyde for 3 hours, after which the otic capsule was dissected out, cryoprotected in 30% sucrose overnight, mounted in OCT embedding media (Sakura Finetek Torrence, CA) and snap frozen. Embryos were sectioned at 14 μm and stained with DAPI and the following antibodies: rabbit anti-MyosinVIIa (Proteus Biosciences Ramona, CA); and rabbit anti-Prox1 (Chemicon Billerica, MA) 1:500. Primary antibodies were detected with one of the following secondary antibodies: donkey anti-mouse IGG conjugated to Cy3 (Jackson ImmunoResearch West Grove, PA) or Alexa488 (Molecular Probes Eugene, OR); donkey anti-rabbit IGG conjugated to Cy3 or Alexa488.
In situ hybridization
Embryos were fixed in 4% paraformaldehyde for 1 hour, cryoprotected in 30% sucrose overnight, mounted in OCT embedding media (Sakura Finetek Torrence, CA) and snap frozen. Embryos were sectioned at 14 μm and imaged for ZsGreen fluorescence, followed by in situ hybridization for Cre expression (Brown and Epstein, 2011). For the experiments described in Fig. 6, at least 14 sections were analyzed from a minimum of four embryos per developmental stage.
EdU incorporation and quantification of mitotic index
EdU was dissolved in sterile H2O and administered to pregnant dams via intraperitoneal injection at a concentration of 50 μg/g of body weight, 1 hour prior to embryo harvest (Molecular Probes). Embryos were fixed in 4% paraformaldehyde at 4°C for 2 hours. EdU incorporation was detected at room temperature with the Click-iT® EdU Imaging Kit #C10339 (Molecular Probes). The staining protocol was optimized for frozen sections using the following modifications: 2 × 10′ PBS-Twen wash, 2 × 10′ 3% BSA incubation, 30′ incubation in the dark with Click-iT® reaction cocktail assembled in the recommended order immediately prior to application, 3% BSA wash, 2× PBS wash.
The mitotic index of GFP+ cells is presented as the average ratio of GFP+/EdU+ double-labeled cells divided by the total number of GFP+ cells (n=48–52 sections from six otic vesicles). The mitotic index of all DAPI+ cells is presented as the average ratio of EdU+ cells divided by the total number of DAPI+ cells (n=52 sections from six otic vesicles). The Cell Counter function in Image J (NIH) was utilized for all cell counts. An unpaired t-test was performed to determine the degree of significance.
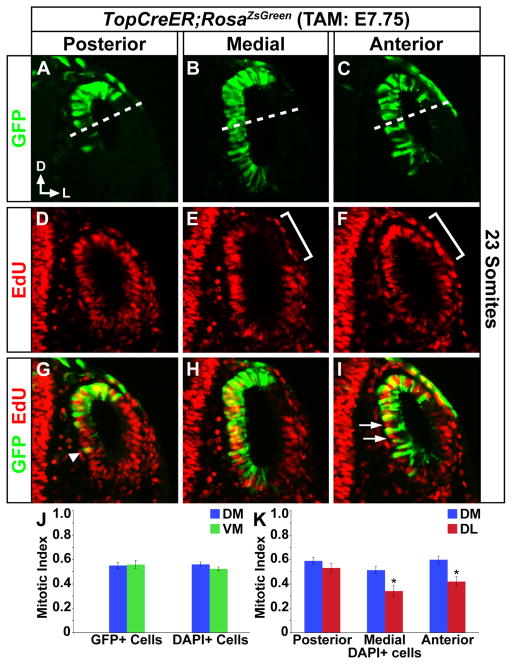
In reference to the results described in Fig. 7, the boundary separating the dorsomedial domain from the ventromedial domain and the dorsolateral domain from the ventrolateral domain (for counting purposes) was determined according to the following method. Upon measuring the extent of TopCre expression along the medial wall of the otic vesicle at 23 and 25 somites, it was determined that on average Cre is expressed along 38.8% and 40.5%, respectively, of the medial wall (23 somites: n=38 sections from four vesicles, SEM 0.9%; 25 somites: n=49 sections from four vesicles, SEM 0.9%). This measurement was then transposed onto EdU/GFP stained tissue and indicated as the expected boundary of Cre expression.
Figure 7. Proliferation of TopCreER; RosaZsGreen labeled otic progenitors.
Transverse sections through the inner ears of TopCreER; RosaZsGreen embryos exposed to tamoxifen at E7.75 and collected at E9.5 (23–25 somites). EdU was administered one hour prior to embryo harvest. (A–C) GFP (D–F) EdU (G–I) merged. (J–K) Bar graphs display mitotic indices of otic progenitors. Proliferation rate of GFP+ cells is equivalent in the dorsomedial (Cre+) (above dashed line) and ventromedial domains and does not differ from the rate for all DAPI+ cells. However, the mitotic index of otic progenitors is significantly increased on the dorsomedial (Wnt responsive) compared to dorsolateral (Wnt unresponsive) sides of the otic vesicle, particularly in medial (P=0.0045) and anterior regions (brackets in E, F; P=0.0012, unpaired Student’s t-test). The intercalation of proliferating cells (GFP−EdU+) between GFP+ progenitors may also contribute to their ventral displacement (arrows in I). GFP+ cells are occasionally observed several cell diameters from their nearest GFP+ neighbor (arrow head in G), consistent with migratory behavior. Abbreviations: DM, dorsomedial; VM, ventromedial; and DL, dorsolateral. Error bars represent s.e.m.
Results
Spatiotemporal distinctions in the fate of Wnt responsive cells in the inner ear
To determine the fate of Wnt responsive cells at distinct periods of otic development we made use of the TopCreER mouse line, which expresses the tamoxifen inducible Cre-ERT2 recombinase downstream of the Wnt responsive Top promoter (Indra et al., 1999; DasGupta and Fuchs, 1999; Riccomagno et al., 2005). TopCreER mRNA expression was confined to dorsomedial cells of the otic placode and cup at E8.5–E9.0 (12–19 somites), and to dorsal cells of the otic vesicle between E9.5 (20–25 somites) and E10.5 (35 somites), in a manner highly reminiscent of Topgal (Fig. 1A–E; and Riccomagno et al., 2005).
Figure 1. Dorsomedial restriction in the otic expression of TopCreER mRNA.
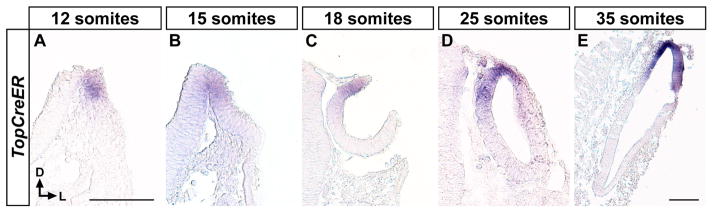
Transverse sections of whole mount stained embryos for TopCreER expression at distinct stages of inner ear development: A) early placode; B) placode; C) otic cup; D) otic vesicle; E) late otic vesicle. Somite stage of embryos is indicated. Scale Bar: 100 μm in (A) also applies to images in B–D.
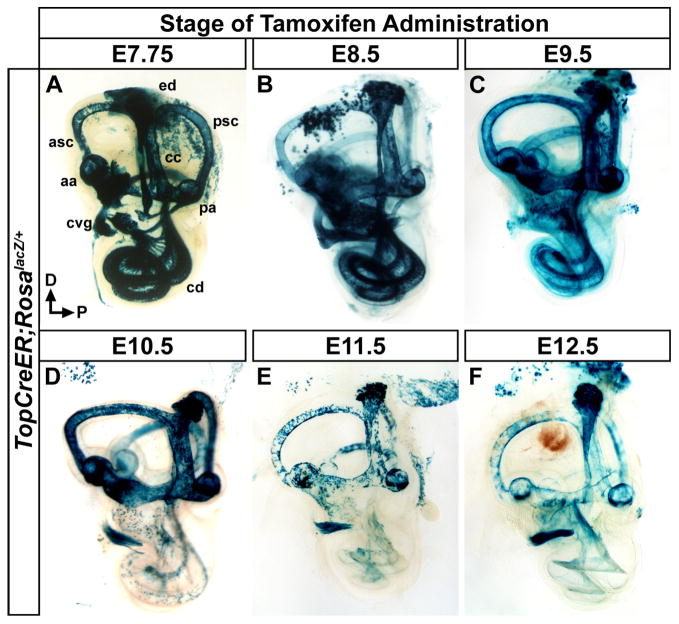
Previous studies demonstrated that Wnt responsive cells in the dorsomedial portion of the otic cup contributed not only to a variety of dorsally derived inner ear structures but also, unexpectedly, to ventral otic derivatives including the cochlea, saccule and cochlear vestibular ganglia (cvg) (Riccomagno et al., 2005). To determine the temporal window in which dorsally derived Wnt responsive cells contribute to vestibular and auditory tissues we performed a tamoxifen time course experiment. TopCreER males were crossed with RosalacZ females, an inducible reporter line that permanently activates lacZ expression in cells, and their descendants, after Cre mediated excision of an upstream transcriptional stop cassette (Soriano, 1999). Pregnant dams were administered a single dose of tamoxifen on a given day of gestation between E7.75 and E12.5. Embryos were then collected at E18.5 and stained with X-gal.
At each of the tamoxifen administration time points tested, the majority of dorsally derived vestibular structures, including the semicircular canals and associated cristae, common crus, endolymphatic duct and utricle showed some degree of X-gal staining (Fig. 2A–F). The anterior and posterior canals stained more uniformly when tamoxifen was administered at or prior to E10.5, compared to later stages when Wnt/β-catenin signaling activity becomes more restricted (Fig. 2A–F; Rakowiecki and Epstein, 2013). The lateral canal labeled sparsely throughout these experiments due to the limited expression of Cre on the dorsolateral side of the otocyst, the region from where progenitors of this canal originate (Morsli et al., 1999).
Figure 2. Temporal dynamics in the fate of Wnt responsive cells in the inner ear.
Whole mount views of X-gal stained inner ears (E18.5) exposed to tamoxifen at different developmental time points to induce TopCreER dependent activation of RosalacZ. (A–C) The inner ears of TopCreER; RosalacZ embryos receiving tamoxifen between E7.75 and E9.5 showed a high degree of X-gal staining in most dorsally derived vestibular structures including, the anterior and posterior semicircular canals (asc, psc) and associated ampullae (aa, pa), endolymphatic duct (ed), and common crus (cc). Ample staining was also observed in the ventrally derived cochlear duct (cd) and cochleovestibular ganglion (cvg). (D) Tamoxifen administration at E10.5 saw continued X-gal staining in vestibular structures, yet labeling in the cochlear duct was dramatically reduced. (E–F) TopCreER; RosalacZ embryos exposed to tamoxifen at E11.5 and E12.5 showed consistently patchy X-gal staining in vestibular structures due to reduced TopCreER expression at these stages. The cochlear duct was devoid of X-gal staining and sparse labeling was observed in the spiral ganglia.
X-gal staining in ventral otic derivatives, including the cochlear duct and spiral ganglia, was only observed in embryos administered tamoxifen between E7.75 to E9.5 (Fig. 2A–C), but not thereafter except for a scattering of cells in the vicinity of the spiral ganglia (Fig. 2D–F). The negligible amount of X-gal staining in the cochlea at E12.5 likely reflects the reduced sensitivity of the TopCreER transgene to Wnt signaling during later stages of cochlear morphogenesis, since other studies have demonstrated a requirement for Wnt/β-catenin in the proliferation and specification of auditory hair cell progenitors between E12.5 and E14.5 (Jacques et al., 2012; Shi et al., 2014). These fate-mapping experiments confirm previous reports that vestibular and auditory components of the inner ear derive from Wnt responsive cells in the dorsal otic cup and further reveal the temporal window during which TopCreER actively marks these lineages.
Auditory hair and support cells derive from Wnt responsive progenitors in the dorsomedial otic cup
Our data indicate that a population of Wnt responsive cells in the dorsomedial region of the otic cup contributes to the cochlear duct. To determine the definitive fates of these cells, we performed co-labeling experiments on inner ears isolated from TopCreER; RosaZsGreen/+ embryos exposed to a single dose of tamoxifen between E8.5 and E11.5 and harvested at E18.5. At this stage, most cells in the organ of Corti have acquired their terminal identities, which are readily distinguished by their location, morphology, and expression of cell-type specific markers.
Serial sections cut in the transverse plane through the cochlear duct of TopCreER; RosaZsGreen/+ embryos were imaged for GFP, to detect Wnt responsive cells, and either atypical Myosin VIIa (MyoVIIa), a marker of inner and outer hair cells, or the Prox1 homeoprotein, a support cell marker that labels pillar and Dieters’ cells (Hasson et al., 1997; Bermingham-McDonough et al., 2006). Extensive co-labeling was observed between GFP and MyoVIIa as well as GFP and Prox1 when tamoxifen was administered between E8.5 and E9.5 (Fig. 3A, B, E, F). GFP+ cells were also detected in regions adjacent to the organ of Corti, including the greater epithelial ridge, Hensen’s cells, Claudius’ cells, and spiral ganglia. Co-labeling of hair, pillar and Deiters’ cells was scantly observed upon tamoxifen administration at E10.5, whereas Hensen’s and Claudius’ cells still showed some GFP staining (Fig. 3C, G). No GFP staining was detected in the cochlea when recombination was induced at E11.5, with the exception of a few cells in the vicinity of the spiral ganglia (Fig. 3D, H). These findings indicate that a significant portion of the medial wall of the cochlear duct, including hair and support cells in the organ of Corti derives, at least in part, from a population of Wnt responsive cells in the dorsomedial otic cup.
Figure 3. Hair and support cells in the organ of Corti derive from Wnt responsive progenitors.
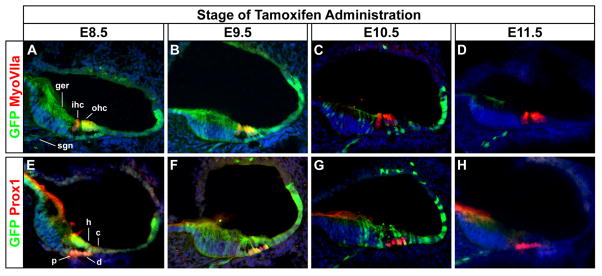
Transverse sections through the cochlear duct of TopCreER; RosaZsGreen/+ embryos (E18.5) exposed to tamoxifen at different developmental stages. (A–D) Co-labeling of the GFP+ (green) Wnt responsive lineage with MyoVIIa+ (red) hair cells. Inner and outer hair cells (ihc, ohc) showed co-labeling (yellow) when tamoxifen was administered between E8.5 and E9.5, but not thereafter. (E–H) Co-labeling of GFP (green) and Prox1 (red) a support cell marker that labels pillar (p) and Dieters’ (d) cells. Support cells showed co-labeling (yellow) when tamoxifen was administered between E8.5 and E10.5, but not thereafter. GFP staining was also detected in cell types adjacent to the organ of Corti, including the greater epithelial ridge (ger), Hensen’s cells (h), Claudius’ cells (c), and spiral ganglion neurons (sgn).
TopCreER labels hair cells along the length of the cochlear duct
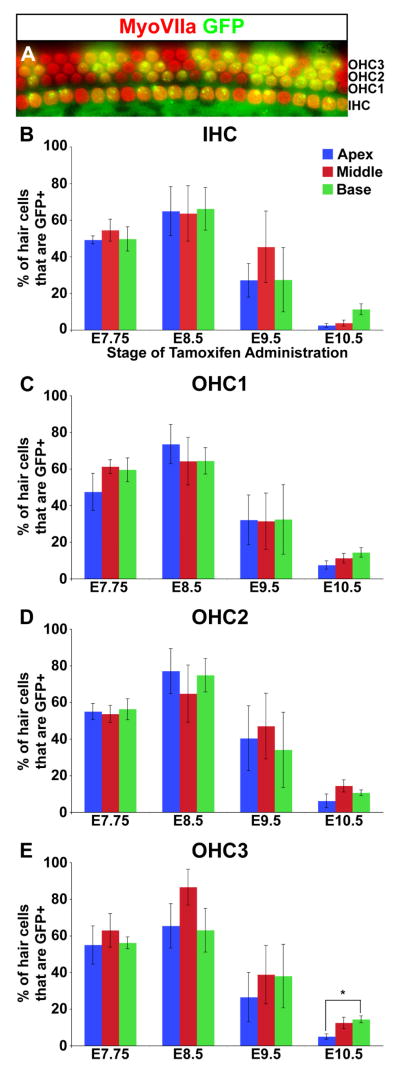
Hair cell differentiation initiates at mid-basal levels of the cochlear duct and then spreads in basal and apical orientations (Chen et al., 2002; Kelley 2006). Along the neural-abneural axis, inner hair cells are the first to mature, followed by outer hair cells (Montcouquiol and Kelley, 2003). To determine whether the distribution of Wnt responsive cells varied along the basal-apical or neural-abneural axes of the organ of Corti over time, we quantified the proportion of hair cells labeled with GFP at fixed positions along the cochlear duct at different tamoxifen administration time points.
The percentage of GFP labeled hair cells peaked at 64±21% when recombination was induced at E8.5 and then progressively declined to 35 ± 24% (E9.5) and 9 ± 6% (E10.5) at later induction times (Fig. 4A–E). Despite the temporal differences in labeling efficiency, the distribution of GFP+ hair cells remained uniform along the length of the cochlear duct. Moreover, GFP staining was equivalent between inner and outer hair cells, with the exception of a slight reduction in the third row of outer hair cells in apical compared to basal regions when recombination was initiated at E10.5 (Fig. 4E). Thus, the temporal kinetics of TopCreER activation of RosaZsGreen precedes most biases in hair cell differentiation that occur along the basal-apical and neural-abneural axes of the cochlear duct.
Figure 4. TopCreER marks hair cells along the entire length of the cochlear duct.
(A) An optical section through the cochlear sensory epithelium of an E18.5 TopCreER; RosaZsGreen/+ embryo exposed to tamoxifen at E8.5. The cochlea was immunostained with an antibody against MyoVIIa (red). GFP (green) was visualized by direct fluorescence. The majority of hair cells show co-labeling (yellow). (B–E) Bar graphs displaying the percentage of TopCreER labeled hair cells at distinct positions along the cochlear duct upon tamoxifen administration between E7.75 and E10.5. The maximum number of TopCreER labeled inner and outer hair cells was observed when tamoxifen was administered at E8.5. The third row of outer hair cells showed a significant reduction in labeling at the apex compared to mid and basal levels when exposed to tamoxifen at E10.5 (p<0.01). Error bars represent s.e.m.
Contribution of Gbx2CreER fate mapped cells to the cochlear duct
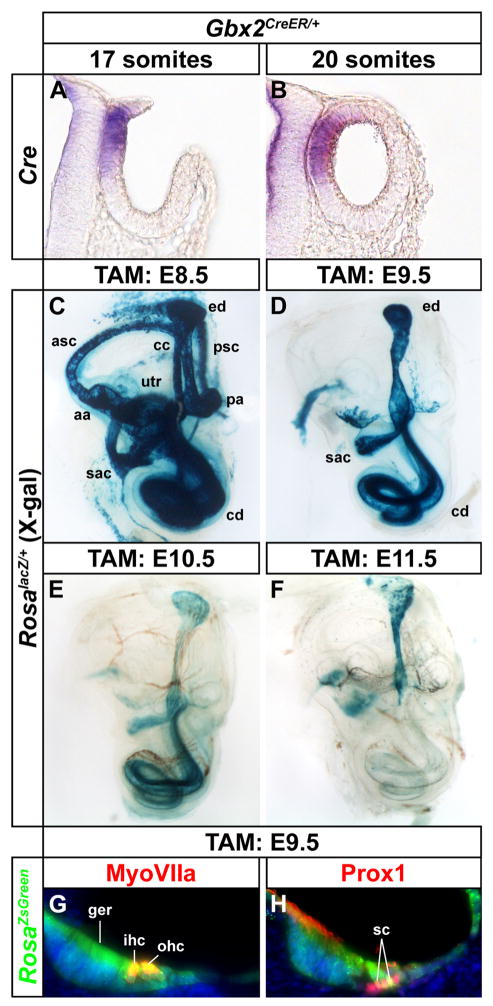
Given the unexpected nature of our findings that auditory hair cells and support cells derive from Wnt responsive progenitors in dorsomedial regions of the otic cup, we sought to confirm that this result was not an artifact of the TopCreER transgene. We therefore repeated the fate mapping experiments with Gbx2CreER/+, which also directs CreER expression to the dorsomedial wall of the otic cup and vesicle (Fig. 5A, B; Chen et al., 2009). Gbx2CreER/+; RosalacZ/+ embryos receiving a single dose of tamoxifen at E8.5 showed X-gal staining in the inner ear, including the anterior and posterior semicircular canals and associated cristae, endolymphatic duct, common crus, utricle, saccule and cochlear duct, which strongly resembled the pattern displayed by TopCreER; RosalacZ/+ embryos (compare Figs. 5C and 2B). The activation of lacZ expression in the cochlear duct of Gbx2CreER/+; RosalacZ/+ embryos was also evident when tamoxifen was administered at E9.5 and E10.5, but not thereafter (Fig. 5D–F). X-gal staining in the vestibular compartment of Gbx2CreER/+; RosalacZ/+ embryos was limited to the endolymphatic duct and portions of the utricle and saccule at tamoxifen induction times between E9.5 and E11.5 (Fig. 5D–F). The fate of dorsomedial cells labeled with Gbx2CreER/+; RosaZsGreen/+ at E9.5, included hair cells and support cells in the organ of Corti, as well as cells in the greater epithelial ridge, Hensen’s cells and Claudius’ cells (Fig. 5G, H). These results confirm with a second independent Cre line, that auditory hair and support cells derive from dorsomedial otic progenitors.
Figure 5. Gbx2CreER/+ labels cells contributing to both vestibular and auditory structures.
(A, B) Transverse sections of Gbx2CreER/+ embryos at otic cup (17 somite) and otic vesicle (20 somite) stages showing dorsomedial localization of Cre mRNA expression. (C–F) Whole mount views of X-gal stained inner ears (E18.5) exposed to tamoxifen at indicated developmental time points to induce Gbx2CreER dependent activation of RosalacZ. Tamoxifen administration between E8.5 and E10.5 labels vestibular and auditory structures, whereas after E11.5, labeling is restricted to the endolymphatic duct. (G, H) Transverse sections through the cochlear duct of Gbx2CreER/+; RosaZsGreen/+ embryos (E18.5) exposed to tamoxifen at E9.5 showing GFP co-labeling with MyoVIIa+ hair cells and Prox1+ support cells. Abbreviations: anterior semicircular canal, asc; posterior semicircular canal, psc; anterior ampulla, aa; posterior ampulla, pa; endolymphatic duct, ed; cochlear duct, cd; utricle, utr; saccule, sac; greater epithelial ridge, ger; inner hair cell, ihc; outer hair cells, ohc; support cells, sc.
Ventral movement of Wnt responsive cells occurs in an anteroposterior wave
Results from our tamoxifen time course experiments suggested that Wnt responsive cells in the dorsomedial otic cup move out of this territory at some point between E7.75 and E9.5 to occupy ventral positions along the medial wall of the elongating cochlear duct. To visualize the dynamic changes in the position of Wnt responsive cells during this temporal window, we induced recombination in TopCreER; RosaZsGreen/+ embryos at E7.75 and compared the location of Cre expressing cells with respect to their GFP+ descendants at the otic cup (E9, 17–19 somites) and early otic vesicle stages (E9.5, 20–25 somites). When collected at the 17 to 19-somite stage, GFP staining in the posterior third of the otic cup was restricted to a subpopulation of Cre expressing cells in the dorsomedial segment (Fig. 6A–A′). Not all Cre+ cells showed GFP activation at this stage, likely due to the short period of time between tamoxifen administration and embryo collection. At the mid point along the anterior-posterior otic axis, Cre and GFP staining were both confined to the same dorsomedial region (Fig. 6B–B′). Interestingly, in the anterior third of the otic cup, GFP staining extended beyond the ventral boundary of Cre expression, highlighting the first evidence of a ventral-ward displacement of Wnt responsive descendants (Fig. 6C–C′). At the 20–22 somite stage, GFP labeling in the posterior region of the newly closed otic vesicle remained aligned with Cre expression (Fig. 6D–D′). However, at this stage, GFP staining had now surpassed the ventral limit of Cre expression in both mid and anterior locations (Fig. 6E, E′-F, F′). The ventral ward displacement of GFP+ cells continued to advance in a posterior direction such that by the 23–25 somite stage, the ventral boundary of GFP staining had surpassed that of Cre at all positions along the anteroposterior axis of the otic vesicle (Fig. 6G–J, G′–I′). The discordance in Cre and GFP expression in the ear was only observed along the ventromedial wall, as these markers remained relatively fixed at the dorsolateral extent of their expression domains.
To exclude the possibility that the anterior to posterior wave of ventral-ward movement of Wnt responsive cells was due to the migration of anterior labeled cells to more posterior otic regions, we analyzed Ngn1Cre; RosatdTomato embryos to assess the fate of cells in anteroventromedial positions of the otic vesicle. At no point were these anteriorly labeled cells ever detected in posterior portions of the otic vesicle, ruling out an anterior to posterior migratory stream (Fig. S1). Taken together, our fate mapping experiments reveal that Wnt responsive cells in dorsomedial otic regions move ventrally according to a spatiotemporal sequence that initiates in the anterior otic cup at, or immediately prior to, the 17-somite stage and then spreads posteriorly over the course of 16 hours, reaching the posterior pole of the otic vesicle by the 25-somite stage.
Proliferative status of Wnt responsive cells
Given the frequent association of the Wnt/β̃catenin pathway with mitogenic activity, we wondered whether the ventral displacement of Wnt responsive cells correlated with enhanced proliferation. EdU incorporation experiments revealed that the mitotic index of GFP+ cells was equivalent between dorsomedial (Cre+) and ventromedial (Cre−) otic regions of 23–25 somite stage embryos and did not differ from that of all cells (GFP+ and GFP−) in this territory (Fig. 7A–K). Nonetheless, the mitotic index of dorsal otic progenitors was significantly increased on the dorsomedial (Wnt responsive) compared to dorsolateral (Wnt unresponsive) sides of the otic vesicle, particularly in medial and anterior regions (Fig. 7E, F, K). This finding may explain the preferential expansion of GFP+ cells along the medial otic wall. Moreover, the intercalation of proliferating cells (GFP−, EdU+) between GFP+ progenitors may also contribute to their ventral displacement (Fig. 7I, arrows). Finally, GFP+ cells were occasionally observed several cell diameters from their nearest GFP+ neighbors (Fig. 7G, arrow head), which is consistent with, but not proof of, migratory behavior. Thus, proliferative forces and possibly directed cell migration may explain the ventral movement of Wnt responsive cells along the medial side of the otic vesicle. Definitive confirmation of these cellular behaviors must await the results of live imaging experiments.
Discussion
Spatiotemporal dynamics of CreER mediated reporter activation
Our lineage tracing experiments determined that Wnt responsive progenitors in the dorsal otic cup contribute to not only a variety of dorsally derived vestibular structures but also to ventral inner ear fates, including auditory hair and support cells, during a brief temporal window between 17 and 25 somites (E9 – E9.5). Genetically inducible fate mapping is a powerful strategy to reveal the developmental lineage of distinct cell types over time (Jensen and Dymecki, 2014). However, without prior knowledge of the spatiotemporal dynamics of Cre activity, results from these experiments may be subject to misinterpretation. Three aspects of our analysis increase our confidence in the validity of our findings. Firstly, TopCreER expression is dorsally restricted throughout the early stages of otic development in a pattern highly reminiscent of Topgal – a sensitive reporter of Wnt signaling activity (Fig. 1 and Riccomagno et al., 2005). Secondly, the initial activation of RosaZsGreen closely matches TopCreER expression in dorsal otic cells and only becomes discordant over time, when GFP+ cells are displaced ventrally. This result suggests that the ventralward movement of labeled cells, rather than ectopic TopCreER transcription, accounts for the ventral GFP staining. Finally, the corroboration of our principal findings with a second independent Gbx2CreER strain increases the likelihood that they are not attributed to artifacts of either CreER mouse line.
Our tamoxifen time course experiments allowed us to label and trace the fates of TopCreER and Gbx2CreER expressing cells at distinct stages of inner ear development. When recombination was induced on or prior to E9.5, GFP expressing cells were observed in a steady ventralward stream along the medial wall of the otic vesicle. However, when tamoxifen was administered at slightly later stages, reporter activation in the cochlear duct was far less dynamic. The residual labeling of the auditory sensory epithelium in embryos receiving tamoxifen after E9.5 (TopCreER) and E10.5 (Gbx2CreER) is likely a consequence of slow Cre protein turnover in cells that have previously moved out of the Wnt responsive territory and are no longer actively transcribing Cre mRNA. This observation is consistent with previous work showing that Cre protein is stable for at least 40 hours in mammalian cells (Sauer and Henderson, 1988).
Wnt signaling, migratory behavior, and the evolutionary origin of the sensory epithelium
The description of cell movements during inner ear development is not without precedent. Streit (2002) observed extensive cell mixing and rearrangements between otic and non-otic cells prior to otic placode formation in the chick. Kil and Collazo (2001) also observed cell movements during the formation of sensory organs in frogs. Of particular significance was the recent report of an otic placode fate map using chick-quail chimeras (Sánchez-Guardado et al., 2014). In this study, a quail graft of the dorsal most ectoderm adjacent to future rhombomere 6 was shown to contribute to the sensory epithelium (basilar papilla) in the anterior portion of the cochlear duct of a chick host. Thus, the derivation of auditory sensory epithelial progenitors from dorsal otic regions may be a common feature of tetrapods as they adapted the cochlea as a hearing organ.
It is a widely held view that the inner ear of ancestral vertebrates functioned primarily as a vestibular device to detect angular and linear acceleration (Duncan and Fritzsch, 2012). During their adaptation to terrestrial life, vertebrates slowly acquired the ability to capture and transform air-borne sound vibrations into electrical signals through the evolution of middle ear bones, a dedicated auditory structure and appropriate neurotransmission pathways (Fritzsch et al., 2007; Sienknecht 2013). The inner ear components of the auditory apparatus, including the cochlear duct, sensory epithelium and associated nerve, emerged as ventral outgrowths of the otic vesicle, likely through the duplication and cooption of preexisting vestibular structures.
It is intriguing to speculate that the canonical Wnt signaling pathway, which is essential for vestibular morphogenesis and sensory cristae specification at early stages of otic development (Riccomagno et al., 2005; Rakowiecki and Epstein, 2013), was appropriated for additional use to facilitate the ventral displacement of prosensory progenitors along the medial wall of the cochlear duct. This model provides a compelling explanation for why Wnt1−/−; Wnt3a−/− double mutants exhibit cochlear outgrowth defects, despite the selective loss of Wnt signaling activity in dorsal otic regions (Riccomagno et al., 2005).
The Wnt pathway has been implicated in the regulation of cell movements in a variety of systems (Whangbo and Kenyon, 1999; Barker et al., 2007; Ahtiainen et al., 2014). For instance, the fate mapping of Wnt responsive cells in the intestinal epithelium demonstrates that cells generated at the base of the cript migrate in vertical coherent columns along the criptvillus axis to replenish older differentiated cells at the villus tips (Barker et al., 2007). Wnt signaling orchestrates the proliferative pressure that drives the upward movement of intestinal epithelial cells and also regulates their migratory properties by controlling the expression of Eph/ephrin signaling components (Batlle et al., 2002). Eph/ephrin signaling is associated with several aspects of inner ear development and hair cell innervation (Cowan et al., 2000; Coate et al., 2012; Defourny et al., 2013; Raft et al., 2014), but a specific role in auditory hair cell formation and/or cochlear morphogenesis has yet to be reported, possibly due to functional redundancy between the large number of family members expressed in the ear (Saeger et al., 2011). It will be interesting to determine whether similar mechanisms act to facilitate the migration of Wnt responsive progenitors in the gut and inner ear.
Conclusions
Our lineage tracing experiments reveal that the majority of hair and support cells within the organ of Corti derive from a population of dorsomedial otic progenitors that become ventrally displaced during a 16-hour window between 17 and 25 somites. Similar results were observed with both TopCreER and Gbx2CreER mouse lines. These findings demonstrate that Wnt responsive progenitors in the dorsomedial otic cup are the earliest known origins of the cochlear sensory epithelium. Furthermore, these two CreER mouse lines may be useful tools for the conditional inactivation of genes prior to their expression in auditory hair and support cells, or for the selective labeling of early otic progenitors in embryonic stem cell cultures with the potential to give rise to the sensory epithelium.
Supplementary Material
Research Highlights.
Wnt responsive cells in the dorsomedial otic cup contribute to both vestibular and auditory progenitors.
TopCreER; RosaZsGreen labeled cells mark the earliest known origin of auditory hair cells
Ventral displacement of Wnt responsive progenitors proceeds in an anteroposterior direction
Acknowledgments
The authors thank Dr. Lisa Goodrich (Harvard Medical School) for kindly providing Ngn1Cre; RosatdTomato embryos. We also thank Dr. Yao Yao and Alex Rohacek for helpful comments on the manuscript. ASB was supported by Cell and Molecular Biology (T32-GM07229) and Genetics (T32-GM008216) training grants from the NIH/NIGMS. This work was supported by a grant from NIH/NIDCD (R01DC006254) to DJE.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahtiainen L, Lefebvre S, Lindfors PH, Renvoisé E, Shirokova V, Vartiainen MK, Thesleff I, Mikkola ML. Directional cell migration, but not proliferation, drives hair placode morphogenesis. Dev Cell. 2014;28:588–602. doi: 10.1016/j.devcel.2014.02.003. [DOI] [PubMed] [Google Scholar]
- Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, Clevers H. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–7. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- Batlle E, Henderson JT, Beghtel H, van den Born MM, Sancho E, Huls G, Meeldijk J, Robertson J, van de Wetering M, Pawson T, Clevers H. Beta-catenin and TCF mediate cell positioning in the intestinal epithelium by controlling the expression of EphB/ephrinB. Cell. 2002;111:251–63. doi: 10.1016/s0092-8674(02)01015-2. [DOI] [PubMed] [Google Scholar]
- Bermingham-McDonogh O, Oesterle EC, Stone JS, Hume CR, Huynh HM, Hayashi T. Expression of Prox1 during mouse cochlear development. J Comp Neurol. 2006;496:172–186. doi: 10.1002/cne.20944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bok J, Raft S, Kong KA, Koo SK, Dräger UC, Wu DK. Transient retinoic acid signaling confers anterior-posterior polarity to the inner ear. Proc Natl Acad Sci USA. 2011;108:161–6. doi: 10.1073/pnas.1010547108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooker R, Hozumi K, Lewis J. Notch ligands with contrasting functions: Jagged1 and Delta1 in the mouse inner ear. Development. 2006;133:1277–86. doi: 10.1242/dev.02284. [DOI] [PubMed] [Google Scholar]
- Brown AS, Epstein DJ. Otic ablation of smoothened reveals direct and indirect requirements for Hedgehog signaling in inner ear development. Development. 2011;138:3967–3976. doi: 10.1242/dev.066126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Streit A. Induction of the inner ear: stepwise specification of otic fate from multipotent progenitors. Hear Res. 2013;297:3–12. doi: 10.1016/j.heares.2012.11.018. [DOI] [PubMed] [Google Scholar]
- Chen L, Guo Q, Li JY. Transcription factor Gbx2 acts cell-nonautonomously to regulate the formation of lineage-restriction boundaries of the thalamus. Development. 2009;136:1317–1326. doi: 10.1242/dev.030510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P, Johnson JE, Zoghbi HY, Segil N. The role of Math1 in inner ear development: Uncoupling the establishment of the sensory primordium from hair cell fate determination. Development. 2002;129:2495–505. doi: 10.1242/dev.129.10.2495. [DOI] [PubMed] [Google Scholar]
- Coate TM, Raft S, Zhao X, Ryan AK, Crenshaw EB, 3rd, Kelley MW. Otic mesenchyme cells regulate spiral ganglion axon fasciculation through a Pou3f4/EphA4 signaling pathway. Neuron. 2012;73:49–63. doi: 10.1016/j.neuron.2011.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corwin JT, Warchol ME, Kelley MW. Hair cell development. Curr Opin Neurobiol. 1993;3:32–7. doi: 10.1016/0959-4388(93)90032-t. [DOI] [PubMed] [Google Scholar]
- Cowan CA, Yokoyama N, Bianchi LM, Henkemeyer M, Fritzsch B. EphB2 guides axons at the midline and is necessary for normal vestibular function. Neuron. 2000;26:417–30. doi: 10.1016/s0896-6273(00)81174-5. [DOI] [PubMed] [Google Scholar]
- Cullen KE. The vestibular system: multimodal integration and encoding of self-motion for motor control. Trends Neurosci. 2012;35:185–96. doi: 10.1016/j.tins.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DasGupta R, Fuchs E. Multiple roles for activated LEF/TCF transcription complexes during hair follicle development and differentiation. Development. 1999;126:4557–68. doi: 10.1242/dev.126.20.4557. [DOI] [PubMed] [Google Scholar]
- Daudet N, Lewis J. Two contrasting roles for Notch activity in chick inner ear development: specification of prosensory patches and lateral inhibition of hair-cell differentiation. Development. 2005;132:541–51. doi: 10.1242/dev.01589. [DOI] [PubMed] [Google Scholar]
- Daudet N, Ariza-McNaughton L, Lewis J. Notch signalling is needed to maintain, but not to initiate, the formation of prosensory patches in the chick inner ear. Development. 2007;134:2369–78. doi: 10.1242/dev.001842. [DOI] [PubMed] [Google Scholar]
- Defourny J, Poirrier AL, Lallemend F, Mateo Sánchez S, Neef J, Vanderhaeghen P, Soriano E, Peuckert C, Kullander K, Fritzsch B, Nguyen L, Moonen G, Moser T, Malgrange B. Ephrin-A5/EphA4 signalling controls specific afferent targeting to cochlear hair cells. Nat Commun. 2013;4:1438. doi: 10.1038/ncomms2445. [DOI] [PubMed] [Google Scholar]
- Duncan JS, Fritzsch B. Transforming the vestibular system one molecule at a time: the molecular and developmental basis of vertebrate auditory evolution. Adv Exp Med Biol. 2012;739:173–86. doi: 10.1007/978-1-4614-1704-0_11. [DOI] [PubMed] [Google Scholar]
- Epstein DJ, Martinu L, Michaud JL, Losos KM, Fan C, Joyner AL. Members of the bHLH-PAS family regulate Shh transcription in forebrain regions of the mouse CNS. Development. 2000;127:4701–4709. doi: 10.1242/dev.127.21.4701. [DOI] [PubMed] [Google Scholar]
- Fekete DM, Muthukumar S, Karagogeos D. Hair cells and supporting cells share a common progenitor in the avian inner ear. J Neurosci. 1998;18:7811–21. doi: 10.1523/JNEUROSCI.18-19-07811.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forristall CA, Stellabotte F, Castillo A, Collazo A. Embryological manipulations in the developing Xenopus inner ear reveal an intrinsic role for Wnt signaling in dorsal-ventral patterning. Dev Dyn. 2014 Feb 5; doi: 10.1002/dvdy.24116. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Freyer L, Aggarwal V, Morrow BE. Dual embryonic origin of the mammalian otic vesicle forming the inner ear. Development. 2011;138:5403–14. doi: 10.1242/dev.069849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch B, Beisel KW, Pauley S, Soukup G. Molecular evolution of the vertebrate mechanosensory cell and ear. Int J Dev Biol. 2007;51:663–78. doi: 10.1387/ijdb.072367bf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Géléoc GS, Holt JR. Sound strategies for hearing restoration. Science. 2014;344:1241062. doi: 10.1126/science.1241062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groves AK, Fekete DM. Shaping sound in space: the regulation of inner ear patterning. Development. 2012;139:245–57. doi: 10.1242/dev.067074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasson T, Gillespie PG, Garcia JA, MacDonald RB, Zhao Y, Yee AG, Mooseker MS, Corey DP. Unconventional myosins in inner-ear sensory epithelia. J Cell Biol. 1997;137:1287–1307. doi: 10.1083/jcb.137.6.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indra AK, Warot X, Brocard J, Bornert JM, Xiao JH, Chambon P, Metzger D. Temporally-controlled site-specific mutagenesis in the basal layer of the epidermis: comparison of the recombinase activity of the tamoxifen-inducible Cre-ER(T) and Cre-ER(T2) recombinases. Nucleic Acids Res. 1999;27:4324–4327. doi: 10.1093/nar/27.22.4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacques BE, Puligilla C, Weichert RM, Ferrer-Vaquer A, Hadjantonakis AK, Kelley MW, Dabdoub A. A dual function for canonical Wnt/β-catenin signaling in the developing mammalian cochlea. Development. 2012;139:4395–404. doi: 10.1242/dev.080358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayasena CS, Ohyama T, Segil N, Groves AK. Notch signaling augments the canonical Wnt pathway to specify the size of the otic placode. Development. 2008;135:2251–61. doi: 10.1242/dev.017905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen P, Dymecki SM. Essentials of recombinase-based genetic fate mapping in mice. Methods Mol Biol. 2014;1092:437–54. doi: 10.1007/978-1-60327-292-6_26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Wang L, Beier KT, Cepko CL, Fekete DM, Brigande JV. Lineage analysis of the late otocyst stage mouse inner ear by transuterine microinjection of a retroviral vector encoding alkaline phosphatase and an oligonucleotide library. PLoS ONE. 2013;8:e69314. doi: 10.1371/journal.pone.0069314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley MW. Regulation of cell fate in the sensory epithelia of the inner ear. Nat Rev Neurosci. 2006;7:837–49. doi: 10.1038/nrn1987. [DOI] [PubMed] [Google Scholar]
- Kiernan AE, Pelling AL, Leung KK, Tang AS, Bell DM, Tease C, Lovell-Badge R, Steel KP, Cheah KS. Sox2 is required for sensory organ development in the mammalian inner ear. Nature. 2005;434:1031–5. doi: 10.1038/nature03487. [DOI] [PubMed] [Google Scholar]
- Kiernan AE, Xu J, Gridley T. The Notch ligand JAG1 is required for sensory progenitor development in the mammalian inner ear. PLoS Genet. 2006;2:e4. doi: 10.1371/journal.pgen.0020004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kil SH, Collazo A. Origins of inner ear sensory organs revealed by fate map and time-lapse analyses. Dev Biol. 2001;233:365–379. doi: 10.1006/dbio.2001.0211. [DOI] [PubMed] [Google Scholar]
- Ladher RK, Anakwe KU, Gurney AL, Schoenwolf GC, Francis-West PH. Identification of synergistic signals initiating inner ear development. Science. 2000;290:1965–1967. doi: 10.1126/science.290.5498.1965. [DOI] [PubMed] [Google Scholar]
- Ladher RK, Wright TJ, Moon AM, Mansour SL, Schoenwolf GC. FGF8 initiates inner ear induction in chick and mouse. Genes Dev. 2005;19:603–13. doi: 10.1101/gad.1273605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CW, Van De Water TR, Ruben RJ. The fate mapping of the eleventh and twelfth day mouse otocyst: an in vitro study of the sites of origin of the embryonic inner ear sensory structures. J Morphol. 1978;157:249–67. doi: 10.1002/jmor.1051570302. [DOI] [PubMed] [Google Scholar]
- Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, Ng LL, Palmiter RD, Hawrylycz MJ, Jones AR, Lein ES, Zeng H. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci. 2010;13:133–140. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann ZF, Kelley MW. Development of tonotopy in the auditory periphery. Hear Res. 2011;276:2–15. doi: 10.1016/j.heares.2011.01.011. [DOI] [PubMed] [Google Scholar]
- Matz MV, Fradkov AF, Labas YA, Savitsky AP, Zaraisky AG, Markelov ML, Lukyanov SA. Fluorescent proteins from nonbioluminescent Anthozoa species. Nat Biotech. 1999;17:969–73. doi: 10.1038/13657. [DOI] [PubMed] [Google Scholar]
- Mayordomo R, Rodríguez-Gallardo L, Alvarez IS. Morphological and quantitative studies in the otic region of the neural tube in chick embryos suggest a neuroectodermal origin for the otic placode. J Anat. 1998;193 (Pt 1):35–48. doi: 10.1046/j.1469-7580.1998.19310035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montcouquiol M, Kelley MW. Planar and vertical signals control cellular differentiation and patterning in the mammalian cochlea. J Neurosci. 2003;23:9469–78. doi: 10.1523/JNEUROSCI.23-28-09469.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morsli H, Choo D, Ryan A, Johnson R, Wu DK. Development of the mouse inner ear and origin of its sensory organs. J Neurosci. 1998;18:3327–35. doi: 10.1523/JNEUROSCI.18-09-03327.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morsli H, Tuorto F, Choo D, Postiglione MP, Simeone A, Wu DK. Otx1 and Otx2 activities are required for the normal development of the mouse inner ear. Development. 1999;126:2335–43. doi: 10.1242/dev.126.11.2335. [DOI] [PubMed] [Google Scholar]
- Munnamalai V, Fekete DM. Wnt signaling during cochlear development. Semin Cell Dev Biol. 2013;24:480–9. doi: 10.1016/j.semcdb.2013.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohyama T, Mohamed OA, Taketo MM, Dufort D, Groves AK. Wnt signals mediate a fate decision between otic placode and epidermis. Development. 2006;133:865–75. doi: 10.1242/dev.02271. [DOI] [PubMed] [Google Scholar]
- Ohyama T, Basch ML, Mishina Y, Lyons KM, Segil N, Groves AK. BMP signaling is necessary for patterning the sensory and nonsensory regions of the developing mammalian cochlea. J Neurosci. 2010;30:15044–51. doi: 10.1523/JNEUROSCI.3547-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono K, Kita T, Sato S, O’Neill P, Mak SS, Paschaki M, Ito M, Gotoh N, Kawakami K, Sasai Y, Ladher RK. FGFR1-Frs2/3 signalling maintains sensory progenitors during inner ear hair cell formation. PLoS Genet. 2014;10:e1004118. doi: 10.1371/journal.pgen.1004118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan W, Jin Y, Stanger B, Kiernan AE. Notch signaling is required for the generation of hair cells and supporting cells in the mammalian inner ear. Proc Natl Acad Sci USA. 2010;107:15798–803. doi: 10.1073/pnas.1003089107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan W, Jin Y, Chen J, Rottier RJ, Steel KP, Kiernan AE. Ectopic expression of activated notch or SOX2 reveals similar and unique roles in the development of the sensory cell progenitors in the mammalian inner ear. J Neurosci. 2013;33:16146–57. doi: 10.1523/JNEUROSCI.3150-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips BT, Bolding K, Riley BB. Zebrafish fgf3 and fgf8 encode redundant functions required for otic placode induction. Dev Biol. 2001;235:351–65. doi: 10.1006/dbio.2001.0297. [DOI] [PubMed] [Google Scholar]
- Pirvola U, Ylikoski J, Trokovic R, Hébert JM, McConnell SK, Partanen J. FGFR1 is required for the development of the auditory sensory epithelium. Neuron. 2002;35:671–80. doi: 10.1016/s0896-6273(02)00824-3. [DOI] [PubMed] [Google Scholar]
- Quiñones HI, Savage TK, Battiste J, Johnson JE. Neurogenin 1 (Neurog1) expression in the ventral neural tube is mediated by a distinct enhancer and preferentially marks ventral interneuron lineages. Dev Biol. 2010;340:283–92. doi: 10.1016/j.ydbio.2010.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raft S, Koundakjian EJ, Quiñones H, Jayasena CS, Goodrich LV, Johnson JE, Segil N, Groves AK. Cross-regulation of Ngn1 and Math1 coordinates the production of neurons and sensory hair cells during inner ear development. Development. 2007;134:4405–15. doi: 10.1242/dev.009118. [DOI] [PubMed] [Google Scholar]
- Raft S, Andrade LR, Shao D, Akiyama H, Henkemeyer M, Wu DK. Ephrin-B2 governs morphogenesis of endolymphatic sac and duct epithelia in the mouse inner ear. Dev Biol. 2014;390:51–67. doi: 10.1016/j.ydbio.2014.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakowiecki S, Epstein DJ. Divergent roles for Wnt/β-catenin signaling in epithelial maintenance and breakdown during semicircular canal formation. Development. 2013;140:1730–9. doi: 10.1242/dev.092882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riccomagno MM, Takada S, Epstein DJ. Wnt-dependent regulation of inner ear morphogenesis is balanced by the opposing and supporting roles of Shh. Genes Dev. 2005;19:1612–23. doi: 10.1101/gad.1303905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeger BM, Suhm M, Neubüser A. Ephrin/ephrin receptor expression during early stages of mouse inner ear development. Dev Dyn. 2011;240:1578–85. doi: 10.1002/dvdy.22632. [DOI] [PubMed] [Google Scholar]
- Sánchez-Guardado LÓ, Puelles L, Hidalgo-Sánchez M. Fate map of the chicken otic placode. Development. 2014;141:2302–12. doi: 10.1242/dev.101667. [DOI] [PubMed] [Google Scholar]
- Sauer B, Henderson N. Site-specific DNA recombination in mammalian cells by the Cre recombinase of bacteriophage P1. Proc Natl Acad Sci U S A. 1988;85:5166–70. doi: 10.1073/pnas.85.14.5166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwander M, Kachar B, Müller U. The Cell Biology of Hearing. J Cell Bio. 2010;190:9–20. doi: 10.1083/jcb.201001138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi F, Hu L, Jacques BE, Mulvaney JF, Dabdoub A, Edge AS. β-Catenin Is Required for Hair-Cell Differentiation in the Cochlea. J Neurosci. 2014;34:6470–9. doi: 10.1523/JNEUROSCI.4305-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sienknecht UJ. Developmental origin and fate of middle ear structures. Hear Res. 2013;301:19–26. doi: 10.1016/j.heares.2013.01.019. [DOI] [PubMed] [Google Scholar]
- Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- Stevens CB, Davies AL, Battista S, Lewis JH, Fekete DM. Forced activation of Wnt signaling alters morphogenesis and sensory organ identity in the chicken inner ear. Dev Biol. 2003;261:149–64. doi: 10.1016/s0012-1606(03)00297-5. [DOI] [PubMed] [Google Scholar]
- Streit A. Extensive cell movements accompany formation of the otic placode. Dev Biol. 2002;249:237–54. doi: 10.1006/dbio.2002.0739. [DOI] [PubMed] [Google Scholar]
- Whangbo J, Kenyon C. A Wnt signaling system that specifies two patterns of cell migration in C. elegans. Mol Cell. 1999;4:851–8. doi: 10.1016/s1097-2765(00)80394-9. [DOI] [PubMed] [Google Scholar]
- Yamamoto N, Chang W, Kelley MW. Rbpj regulates development of prosensory cells in the mammalian inner ear. Dev Biol. 2011;353:367–79. doi: 10.1016/j.ydbio.2011.03.016. [DOI] [PubMed] [Google Scholar]
- Yu WM, Goodrich LV. Morphological and physiological development of auditory synapses. Hear Res. 2014 Feb 5; doi: 10.1016/j.heares.2014.01.007. S0378–5955(14)00015-X. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.