Abstract
Although air pollution has been suggested as a possible risk factor for type 2 diabetes mellitus (DM), results from existing epidemiologic studies have been inconsistent. We investigated the associations of prevalence and incidence of DM with long-term exposure to air pollution as estimated using annual average concentrations of particulate matter with an aerodynamic diameter of 2.5 μm or less (PM2.5) and nitrogen oxides at baseline (2000) in the Multi-Ethnic Study of Atherosclerosis. All participants were aged 45–84 years at baseline and were recruited from 6 US sites. There were 5,839 participants included in the study of prevalent DM and 5,135 participants without DM at baseline in whom we studied incident DM. After adjustment for potential confounders, we found significant associations of prevalent DM with PM2.5 (odds ratio (OR) = 1.09, 95% confidence interval (CI): 1.00, 1.17) and nitrogen oxides (OR = 1.18, 95% CI: 1.01, 1.38) per each interquartile-range increase (2.43 µg/m3 and 47.1 ppb, respectively). Larger but nonsignificant associations were observed after further adjustment for study site (for PM2.5, OR = 1.16, 95% CI: 0.94, 1.42; for nitrogen oxides, OR = 1.29, 95% CI: 0.94, 1.76). No air pollution measures were significantly associated with incident DM over the course of the 9-year follow-up period. Results were partly consistent with a link between long-term exposure to air pollution and the risk of type 2 DM. Additional studies with a longer follow-up time and a greater range of air pollution exposures, including high levels, are warranted to evaluate the hypothesized association.
Keywords: air pollution, diabetes, nitrogen oxides, particulate matter, prospective cohort study
Type 2 diabetes mellitus (DM) affects 11% of US adults, and the prevalence is double in people 65 years of age or older (1). It has been suggested that more than 90% of adult-onset DM is attributable to modifiable factors, such as lifestyle behaviors and diet (2). Increasing evidence suggests that environmental exposures also contribute to the development of diabetes (3, 4).
Recently, air pollution has been proposed as a risk factor for the development of type 2 DM (5). In a mouse model of diet-induced obesity, 6-month inhalation exposures to high concentrations of particulate matter with an aerodynamic diameter of 2.5 μm or less (PM2.5) induced insulin resistance and systemic inflammation and increased visceral adiposity (6). In the same mouse model, exposure to PM2.5 for 10 months induced insulin resistance, impaired glucose tolerance, lower circulating levels of adipokines (adiponectin and leptin), and mitochondrial alteration (7). Numerous studies have also shown associations of PM2.5 with systemic inflammatory responses and cardiac autonomic dysfunction (8–10), which might lead to diminished insulin action (11, 12).
However, human epidemiologic studies on the relationship between air pollution and DM have yielded inconsistent results (13), with some finding an association (14–16), others finding associations only in subpopulations (17–21), and still others finding no association (22). Such inconsistencies might be attributed to sociodemographic and geographic differences. More evidence in prospectively followed cohorts is required to determine whether air pollution exposures are causally related to the development of type 2 DM.
We investigated the associations of prevalent and incident DM with individual-level estimates of exposure to PM2.5 and nitrogen oxides and residential proximity to major roadways, a proxy of exposure to traffic-related pollution, in the Multi-Ethnic Study of Atherosclerosis (MESA). We also examined effect modification by sex, given that some studies reported a significant association between air pollution and DM only among women (19, 21, 22).
METHODS
Study population
MESA is a prospective cohort study of subclinical and clinical cardiovascular disease. We recruited a total of 6,814 participants comprising 4 racial/ethnic groups (black, white, Chinese, and Hispanic) aged 45–84 years who were free of clinically apparent cardiovascular disease at baseline between July 2000 and August 2002. Participants were enrolled from 6 US sites: Baltimore, Maryland; Chicago, Illinois; Forsyth County, North Carolina; Los Angeles County, California; New York, New York; and St. Paul, Minnesota. Details of the study design have been published previous (23). The present study included follow-up examinations during 4 different time periods: August 2002 to February 2004 (n = 6,233; 91.5%); March 2004 to September 2005 (n = 5,947; 87.3%); September 2005 to May 2007 (n = 5,818; 85.4%); and April 2010 to February 2012 (n = 4,716; 69.2%). For the analysis of prevalent DM at baseline, we excluded participants who were missing data on DM status (n = 10), exposure to air pollution (n = 258), or covariates (n = 750) (numbers in parentheses are not mutually exclusive), yielding a total of 5,839 participants. For the analysis of incident DM, we additionally excluded participants who had DM at baseline (n = 696) or who did not attend a follow-up visit (n = 8), leaving a total of 5,135 participants. Our study protocol was approved by each study site's institutional review board, and we obtained written informed consent from every participant.
Outcome ascertainment
We measured fasting serum glucose levels in a central laboratory with rate reflectance spectrophotometry using the thin-film adaption of the glucose oxidase method on the Vitros analyzer (Johnson & Johnson Clinical Diagnostics, Inc., Rochester, New York) on samples collected after a 12-hour overnight fast. DM was defined using the American Diabetes Association 2003 criteria (24): use of antidiabetes medication or a fasting glucose level of 126 mg/dL or greater at baseline for the study prevalence or at any follow-up examination for the study of incidence. Almost all of the DM cases in these middle-aged and older adults can be assumed to be type 2 DM. Person-years were computed from baseline to the date of examination at which incident DM was ascertained, loss to follow-up, or the date of the fifth examination, whichever came first.
Assessment of exposure to air pollution
We estimated ambient PM2.5 and nitrogen oxides concentrations for each participant over the follow-up period using the hierarchical spatiotemporal model from the Multi-Ethnic Study of Atherosclerosis and Air Pollution. This model, which has been described elsewhere (25, 26), leverages concentrations of PM2.5 and nitrogen oxides collected from the US Environmental Protection Agency's Air Quality System, supplemental measurements at the homes and within the communities of MESA participants, and a large suite of spatial covariates, including land use and traffic sources (27). Briefly, the hierarchical model decomposed the space-time field of concentrations into 3 facets: 1) spatially varying long-term averages; 2) spatially varying seasonal and long-term trends; and 3) spatially correlated but temporally independent residuals. Using 2-week average concentrations from each participant's home location at baseline, we computed the annual average concentrations in the calendar year 2000 and applied this data as a proxy measure of long-term exposure because there were high correlations between the estimated PM2.5 and nitrogen oxides concentrations in the year 2000 and those at the follow-up examinations. Pearson coefficients for the correlation between baseline and the follow-up PM2.5 concentrations ranged from 0.83 to 0.97 (except with examination 5; r = 0.55); for nitrogen oxides, they ranged from 0.88 to 0.99 (data not shown). We also computed 365-day average concentrations over the year preceding each examination date for each participant and applied this measure in a sensitivity analysis. We estimated residential proximity to major roadways at each visit, using ArcGIS 9.0 (ESRI, Redlands, California) and the Dynamap road network (TeleAtlas, Menlo Park, California). Participants were considered to live near a roadway if they resided within 100 m of an interstate or US highway (US Census Feature Class Codes A1 or A2) or within 50 m of a state or county highway (code A3) (28).
Other covariates
We obtained sociodemographic, behavioral, and medical data using questionnaires and calibrated devices. Educational attainment was categorized into less than a high school education, high school diploma, some college, and 4 or more years of college. Cigarette smoking and alcohol consumption were categorized into never, former, and current use. We defined family history of DM as positive if the participant had both a diabetic blood relative parent and a diabetic blood-relative sibling. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. Physical activity level was assessed as metabolic equivalent task-hours per day for walking, moderate- and vigorous-intensity sports, and conditioning activities reported on a physical activity questionnaire at baseline and at 2 follow-up examinations. Activity was categorized into tertiles (low, <2.8 hours/day; medium, 2.8–6.0 hours/day; high, >6.0 hours/day). Neighborhood socioeconomic status (NSES) index, a summary index of multiple NSES variables that was associated with air pollution exposure in MESA (29), was obtained from the MESA Neighborhood Study, an ancillary study to MESA (30). We collected data on 2 additional scales of neighborhood resources, walking environment and availability of healthy food, from the MESA Neighborhood Study and investigated them as potential confounders in a sensitivity analysis because of our previous findings that better neighborhood resources were associated with lower risks of obesity and DM (31, 32). We treated cigarette smoking, alcohol use, BMI, and physical activity level as time-varying covariates.
Statistical analysis
We used logistic regression models to estimate odds ratios and 95% confidence intervals for the prevalence of DM and Cox proportional hazards models to estimate hazard ratios and 95% confidence intervals for the incidence of DM associated with an interquartile-range (IQR) increase in residential concentrations of PM2.5 or nitrogen oxides and for the residential roadway proximity variable. To facilitate comparison with other studies, we also computed odds ratios and hazard ratios for a 10-µg/m3 increase in PM2.5. All models were adjusted for potential confounding factors that were considered in previous studies (17–20), such as age, sex, race/ethnicity, family history of DM, educational level, cigarette smoking status, alcohol consumption, physical activity level, NSES index, and BMI (model 1). We also evaluated the potential effect of site adjustment (model 2): Site was included as a covariate in logistic regression models but was stratified in Cox models. Residual and unmeasured confounding due to factors that vary across sites could be reduced with adjustment for site. Adjustment for site is essentially equivalent to estimating the association based only on within-site variability in the exposure. We evaluated independent associations of PM2.5 and nitrogen oxides with DM by including them in the models simultaneously (multipollutant models). To evaluate effect modification by sex, we applied separate logistic regression and Cox models stratified by sex. Significance (P value) of effect modification by sex was computed from the models that included cross-product terms between sex and all other covariates analogous to the stratified model. Effect modifications by race/ethnicity, educational level, and obesity (BMI ≥30) were evaluated in the same manner, but these analyses were considered exploratory because we had no a priori hypotheses.
We conducted several sensitivity analyses to verify whether our results were robust to several alternative model specifications. First, we examined whether the results differed after further adjustment for household income, pack-years of cigarette smoking, waist circumference, hypertension, or neighborhood resources (walking environment and availability of healthy food). Second, we used the 365-day average pollutant concentrations for the year preceding the baseline examination date for each participant as the exposure variables. We also examined PM2.5 concentrations estimated using data from the nearest monitoring center. Third, to address residential mobility, we restricted analyses to participants who had lived in baseline home for at least 10 years. Finally, we conducted site-specific logistic and Cox regressions and pooled the estimates using meta-analyses. Heterogeneity of associations was assessed using a χ2 test with 5 degrees of freedom (6 sites). All analyses were performed in R software, version 2.14 (R Foundation for Statistical Computing, Vienna, Austria; http://www.r-project.org).
RESULTS
At baseline, 696 of 5,839 participants (11.9%) had DM. During a median of 9 years of follow-up, 622 of 5,135 participants (12.1%) developed DM over the course of 39,102 person-years. Compared with persons without DM, persons with DM at baseline were older (mean age, 64.3 (standard deviation (SD), 9.4) years vs. 61.6 (SD, 10.2) years) and less likely to be female (47.0% vs. 53.3%), whereas age and sex distributions did not differ between the 2 groups (Table 1). Persons with DM at baseline or who developed DM during follow-up had higher BMIs and less favorable neighborhood resources, were more likely to have a family history of DM, and were more likely to be black or Hispanic, less educated, and less physically active. The mean concentrations of PM2.5 and nitrogen oxides in the year 2000 were 17.3 (SD, 3.1) µg/m3 and 56.5 (SD, 30.1) ppb, respectively, in persons with prevalent DM and 16.7 (SD, 2.8) µg/m3 and 49.7 (SD, 27.3) ppb, respectively, in persons without prevalent DM at baseline (P < 0.001 for the differences in concentrations between persons with and without prevalent DM at baseline). There was no difference in the mean concentrations of PM2.5 and nitrogen oxides between those who did and did not develop DM during follow-up. Approximately 27% of participants were living near major roadways at baseline. PM2.5 and nitrogen oxides concentrations were moderately correlated (r = 0.69, P < 0.0001).
Table 1.
Characteristics of Study Participants at Baseline Visit by Diabetes Mellitus Status, Multi-Ethnic Study of Atherosclerosis, 2000–2002
| Characteristic | DM at Baseline (n = 5,839) |
DM During Follow-up (n = 5,135)a |
||||||
|---|---|---|---|---|---|---|---|---|
| Yes (n = 696) |
No (n = 5,143) |
Yes (n = 622) |
No (n = 4,513) |
|||||
| Mean (SD) | % | Mean (SD) | % | Mean (SD) | % | Mean (SD) | % | |
| Age, years | 64.3 (9.4) | 61.6 (10.2)b | 61.0 (9.4) | 61.7 (10.3) | ||||
| BMIc | 30.5 (5.7) | 28.0 (5.3)b | 31.0 (5.9) | 27.6 (5.1)b | ||||
| Family history of DM | 64.2 | 34.8b | 48.9 | 32.9b | ||||
| Female sex | 47.0 | 53.3d | 52.6 | 53.4 | ||||
| Race/ethnicity | ||||||||
| White | 19.1 | 43.1b | 30.9 | 44.8b | ||||
| Black | 37.6 | 25.5 | 31.4 | 24.6 | ||||
| Hispanic | 31.0 | 20.4 | 26.2 | 19.6 | ||||
| Chinese | 12.2 | 11.0 | 11.6 | 11.0 | ||||
| Educational level | ||||||||
| <High school | 28.0 | 14.4b | 17.7 | 13.9d | ||||
| High school diploma | 19.7 | 18.0 | 19.6 | 17.8 | ||||
| Some college | 28.4 | 28.9 | 30.5 | 28.7 | ||||
| ≥4 Years of college | 23.9 | 38.7 | 32.2 | 39.6 | ||||
| Smoking status | ||||||||
| Never | 50.7 | 50.3 | 49.8 | 50.4 | ||||
| Former | 37.1 | 37.1 | 38.1 | 37.0 | ||||
| Current | 12.2 | 12.5 | 12.1 | 12.6 | ||||
| Alcohol use | ||||||||
| Never | 24.9 | 19.3b | 21.4 | 19.0 | ||||
| Former | 35.5 | 21.6 | 23.9 | 21.3 | ||||
| Current | 39.7 | 59.0 | 53.7 | 59.7 | ||||
| Physical activity levele | ||||||||
| Low (<2.8 hours/day) | 39.9 | 32.6f | 38.6 | 33.9d | ||||
| Medium (≤6.0 hours/day) | 31.3 | 33.7 | 34.7 | 32.7 | ||||
| High (>6.0 hours/day) | 28.7 | 33.7 | 26.7 | 33.4 | ||||
| Study site | ||||||||
| St. Paul, Minnesota | 13.9 | 15.9b | 13.7 | 16.2d | ||||
| Forsyth County, North Carolina | 14.4 | 15.9 | 18.0 | 15.6 | ||||
| Baltimore, Maryland | 14.9 | 15.0 | 16.2 | 14.8 | ||||
| New York, New York | 18.3 | 16.7 | 18.2 | 16.5 | ||||
| Chicago, Illinois | 12.6 | 18.7 | 14.6 | 19.2 | ||||
| Los Angeles, California | 25.9 | 17.9 | 19.3 | 17.7 | ||||
| Neighborhood factors | ||||||||
| NSES index | 0.12 (1.15) | −0.35 (1.39)b | −0.07 (1.23) | −0.39 (1.41)b | ||||
| Walking environment | 3.84 (0.26) | 3.93 (0.31)b | 3.86 (0.29) | 3.94 (0.31)b | ||||
| Availability of healthy food | 3.44 (0.43) | 3.50 (0.49)d | 3.42 (0.46) | 3.51 (0.49)b | ||||
| Air pollution in the year 2000 | ||||||||
| PM2.5, µg/m3 | 17.3 (3.1) | 16.7 (2.8)f | 16.8 (2.8) | 16.7 (2.8) | ||||
| Nitrogen oxides, ppb | 56.5 (30.1) | 49.7 (27.3)b | 51.3 (29.3) | 49.5 (27.0) | ||||
| Proximity to a major road | 26.7 | 25.1 | 24.8 | 25.2 | ||||
Abbreviations: BMI, body mass index; DM, diabetes mellitus; NSES, neighborhood socioeconomic status; PM2.5, particulate matter with an aerodynamic diameter of 2.5 µm or less; SD, standard deviation.
a We excluded 78 subjects with no follow-up data.
b P < 0.0001.
c Weight (kg)/height (m)2.
d P < 0.05.
e Tertile of physical activity which was assessed from metabolic equivalent task-hours per day.
f P < 0.001.
We found significant associations of prevalent DM with PM2.5 (OR = 1.09, 95% confidence interval (CI): 1.00, 1.17) and nitrogen oxides (OR = 1.18, 95% CI: 1.01, 1.38) per each IQR increase (2.43 µg/m3 and 47.1 ppb, respectively) in model 1, which was adjusted for age, sex, race/ethnicity, family history of DM, educational level, smoking status, alcohol consumption, physical activity level, NSES index, and BMI (Table 2). Larger but nonsignificant associations were observed in model 2, which was further adjusted for study site (for PM2.5, OR = 1.16, 95% CI: 0.94, 1.42; for nitrogen oxides, OR = 1.29, 95% CI: 0.94, 1.76). For a 10-µg/m3 increase in PM2.5, the adjusted odds ratios were 1.40 (95% CI: 1.02, 1.93) in model 1 and 1.82 (95% CI: 0.79, 4.19) in model 2. When both PM2.5 and nitrogen oxides were simultaneously included in the model, the odds ratios for both pollutants were reduced and were not statistically significant (Web Table 1, available at http://aje.oxfordjournals.org/). Proximity to major roadways was not associated with prevalent DM in any models (Web Table 2). No significant effect modification by sex was found in the associations between air pollution and prevalent DM (Table 2).
Table 2.
Odds Ratios for the Prevalence of Diabetes Mellitus at Baseline per Each Interquartile-Range Increasea in Concentrations of Air Pollutants Among Study Participants (n = 5,839), Multi-Ethnic Study of Atherosclerosis, 2000–2002
| Pollutant and Model | All |
Men |
Women |
P for Interactionb | |||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | OR | 95% CI | ||
| PM2.5 | |||||||
| Model 1c | 1.09 | 1.00, 1.17d | 1.04 | 0.94, 1.16 | 1.14 | 1.02, 1.28d | 0.27 |
| Model 2e | 1.16 | 0.94, 1.42 | 1.31 | 0.98, 1.74 | 1.04 | 0.78, 1.39 | 0.27 |
| Nitrogen oxides | |||||||
| Model 1c | 1.18 | 1.01, 1.38d | 1.07 | 0.85, 1.33 | 1.33 | 1.06, 1.66d | 0.17 |
| Model 2e | 1.29 | 0.94, 1.76 | 1.40 | 0.89, 2.20 | 1.21 | 0.79, 1.87 | 0.65 |
Abbreviations: CI, confidence interval; OR, odds ratio; PM2.5, particulate matter with an aerodynamic diameter of 2.5 µm or less.
a The interquartile range was 2.43 µg/m3 for PM2.5 and 47.1 ppb for nitrogen oxides.
b P value for the interaction term between sex and the air pollutant.
c Model 1 was adjusted for sex (except in the stratified analyses by sex), age, race/ethnicity, family history of diabetes mellitus, educational level, smoking status, alcohol consumption, physical activity level, neighborhood socioeconomic status index, and body mass index.
d P < 0.05.
e Model 2 was adjusted for all of the variables in model 1 and study site.
Neither PM2.5 concentration nor nitrogen oxides concentration was associated with the incidence of DM over the 9-year follow-up in the entire population (Table 3). We found significant effect modification of the association between nitrogen oxides and incident DM by sex in model 1 (P for interaction = 0.03). In stratified analyses, there was a nonsignificant positive association in women (hazard ratio (HR) = 1.17, 95% CI: 0.95, 1.43) in model 1, whereas a nonsignificant inverse association was seen in men (HR = 0.84, 95% CI: 0.67, 1.06). However, this significant effect modification did not remain in model 2 after adjustment for study site (P for interaction = 0.40). The association between PM2.5 and incident DM was not modified by sex. Race/ethnicity, educational level, and obesity did not modify the associations of PM2.5 and nitrogen oxides with either DM incidence or prevalence (data not shown).
Table 3.
Hazard Ratios for the Incidence of Diabetes Mellitus per Each Interquartile-Range Increasea in Concentrations of Air Pollutants Among Study Participants (n = 5,135), Multi-Ethnic Study of Atherosclerosis, 2000–2012
| Pollutant and Model | All |
Men |
Women |
P for Interactionb | |||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | OR | 95% CI | ||
| PM2.5 | |||||||
| Model 1c | 1.02 | 0.95, 1.10 | 1.00 | 0.90, 1.11 | 1.04 | 0.94, 1.16 | 0.56 |
| Model 2d | 1.05 | 0.87, 1.26 | 1.00 | 0.75, 1.32 | 1.10 | 0.85, 1.41 | 0.71 |
| Nitrogen oxides | |||||||
| Model 1c | 1.00 | 0.86, 1.16 | 0.84 | 0.67, 1.06 | 1.17 | 0.95, 1.43 | 0.03 |
| Model 2d | 1.04 | 0.77, 1.40 | 0.91 | 0.59, 1.42 | 1.20 | 0.80, 1.80e | 0.40 |
Abbreviations: CI, confidence interval; OR, odds ratio; PM2.5, particulate matter with an aerodynamic diameter of 2.5 µm or less.
a The interquartile range was 2.43 µg/m3 for PM2.5 and 47.1 ppb for nitrogen oxides.
b P value for the interaction term between sex and the air pollutant.
c Model 1 was adjusted for sex (except in the stratified analyses by sex), age, race/ethnicity, family history of diabetes mellitus, educational level, smoking status, alcohol consumption, physical activity level, neighborhood socioeconomic status index, and body mass index.
d Model 2 was adjusted for all of the variables in model 1 and study site.
e P < 0.1.
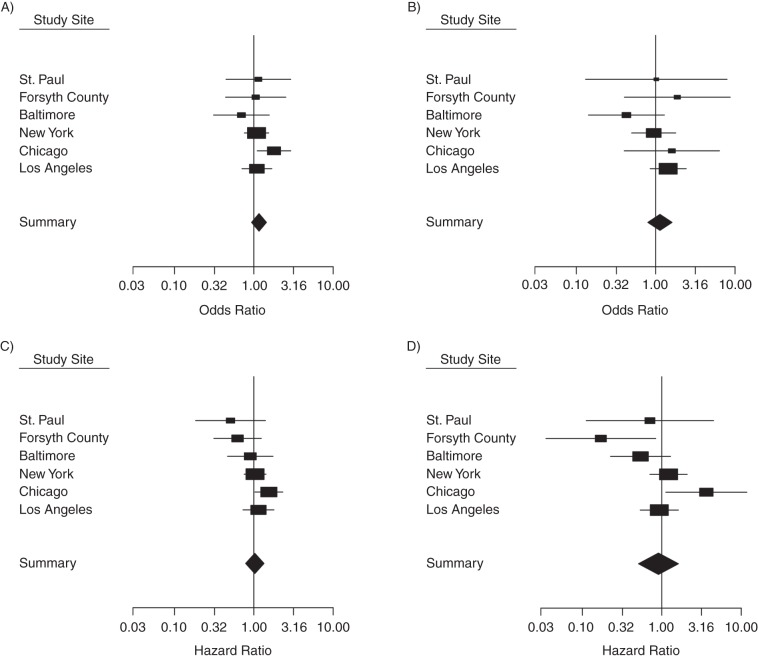
Figure 1 shows site-specific analyses. For prevalent DM, Chicago and Los Angeles showed positive associations with both PM2.5 and nitrogen oxides, whereas Baltimore showed inverse associations. For incident DM, Chicago, Los Angeles, and New York showed positive associations, whereas St. Paul, Forsyth County, and Baltimore had inverse associations, which resulted in an overall null association. Chicago was the only site where significant positive associations were found both cross-sectionally and prospectively (for prevalent DM and PM2.5, OR =1.80, 95% CI: 1.10, 2.92; for incident DM and PM2.5, HR = 1.55, 95% CI: 1.03, 2.32; and for incident DM and nitrogen oxides, HR = 3.64, 95% CI: 1.12, 11.8). There was statistical evidence of significant heterogeneity in the hazard ratio estimates in relation to nitrogen oxides across the sites (P = 0.035).
Figure 1.
Site-specific and pooled summary estimates (odds ratios for prevalent diabetes mellitus and hazard ratios for incident diabetes mellitus), Multi-Ethnic Study of Atherosclerosis, 2000–2012. The study sites were Baltimore, Maryland; Chicago, Illinois; Forsyth County, North Carolina; Los Angeles County, California; New York, New York; and St. Paul, Minnesota. A) Prevalence of diabetes mellitus in relation to concentrations of particulate matter with an aerodynamic diameter of 2.5 µm or less. B) Prevalence of diabetes mellitus in relation to nitrogen oxides concentrations. C) Incidence of diabetes mellitus in relation to concentrations of particulate matter with an aerodynamic diameter of 2.5 µm or less. D) Incidence of diabetes mellitus in relation to nitrogen oxides. All analyses were adjusted for sex, age, race/ethnicity, family history of diabetes mellitus, educational level, smoking status, alcohol consumption, physical activity level, neighborhood socioeconomic status index, and body mass index.
In sensitivity analyses, additional adjustment for household income, waist circumference, pack-years of smoking, hypertension, or walking environment did not change the results (Web Table 3). The strength of the association between nitrogen oxides and incident DM increased after adjustment for availability of healthy food, although the association remained not statistically significant (HR = 1.12, 95% CI: 0.94, 1.34) (Web Table 3). The overall results were similar when we examined the annual average concentrations preceding the baseline examination date and baseline PM2.5 concentrations estimated from the nearest monitoring site (Web Table 4). When the analyses were restricted to participants who had lived in their current home for more than 10 years, the results seemed to be robust (Web Table 5).
DISCUSSION
In the present large, community-based, multiethnic prospective study, higher long-term exposures to PM2.5 and nitrogen oxides estimated as the annual averages in the year 2000 were significantly associated with prevalent DM at baseline. We found larger but less significant associations after adjustment for study site, which suggests that air pollution might have stronger within-site associations with prevalent DM. In contrast, long-term exposure to air pollution was not associated with the development of DM over a 9-year follow-up period. Significant effect modification by sex of the association between nitrogen oxides and incident DM was observed, with an inverse association in men and a positive association in women. No significant association was found between proximity to roadways and either prevalent or incident DM. Our sensitivity analyses suggest that the observed associations are robust to or might be stronger with adjustment for neighborhood resources, such as walking environment and availability of healthy food.
Associations of air pollution exposures with prevalent and incident DM were generally larger after adjustment for study site. Also, the standard errors became wider, as expected, because power was lost because of a reduction in the overall variability in our exposure estimates after adjustment for site. Certain previous studies in this population (33) and other multisite studies (34) have also indicated that the associations were strengthened after accounting for site. Because adjustment for site results in estimates that rely exclusively on within-site variability, stronger associations can be found after adjustment for site if the within-site associations are bigger than the between-site associations. This appeared true in our study, especially in the case prevalent DM, because larger associations were found after adjustment for study site. Although PM2.5 concentrations are typically relatively homogeneous over large geographic areas (35, 36), PM2.5 concentrations in our data showed large within-site variations that were comparable to between-site variations, especially in Chicago (range, 12.6–27.9 µg/m3) and Los Angeles (17.2–26.0 µg/m3), the sites where odds ratios were positive and higher (for Chicago, OR = 1.80, 95% CI: 1.10, 2.92; for Los Angeles, OR =1.09, 95% CI: 0.71, 1.69). Relatively large within-site variations in nitrogen oxides concentrations were also found in Chicago (range, 22.6–69.8 ppb) and Los Angeles (15.9–136 ppb), where odds ratios were positive and higher (for Chicago, OR = 1.60, 95% CI: 0.40, 6.36; for Los Angeles, OR = 1.44, 95% CI: 0.85, 2.42). Interestingly, adjustment for study site differentially influenced the associations with prevalent DM by sex: The magnitudes of the odds ratio for the associations of both PM2.5 and nitrogen oxides with prevalent DM increased in men after adjustment for study site but decreased in women (Table 2). This suggests that confounding factors associated with site might be differentially distributed by sex, although a similar pattern was not observed with incident DM. In summary, the models without adjustment for site provide estimates of associations based on both within-site and between-site variability, whereas the models with adjustment for site rely exclusively on within-site variability. Therefore, measures of associations derived from models adjusted for site cannot be confounded by site-specific factors.
Animal studies have suggested that there are biological mechanisms that connect air pollution exposure with insulin resistance and type 2 DM (5, 37). Long-term exposure to fine particulate matter might induce impaired glucose tolerance (7), increase macrophage levels and inflammation in visceral adipose tissue (6), induce endoplasmic reticulum stress in the liver and lungs (38), and alter mitochondrial functions and brown adipose tissue functions (39). Our cross-sectional findings support this hypothesis. However, our prospective findings do not support the hypothesis that long-term exposure to air pollution is associated with incident DM.
Previous epidemiologic studies on the association between long-term exposure to air pollution and incident DM have had mixed findings (13). A study conducted in 1,775 women from West Germany found significant positive associations between the annual average traffic-related air pollution concentration in 1990 and the incidence of type 2 DM between 1990 and 2006 (HRs ranged from 1.15 for particulate matter or nitrogen dioxide concentrations estimated from a traffic emission inventory to 1.42 for nitrogen dioxide concentration estimated using land-use regression) (20). In the Black Women's Health Study conducted in Los Angeles (n = 3,992), every IQR increase in the annual average concentration of nitrogen oxides (12.4 ppb) was associated with a 25% higher risk of incident type 2 DM over a mean follow-up of 10 years (95% CI: 1.07, 1.46) (17). In a study of 62,012 residents in Ontario, Canada, a 10-µg/m3 increase in PM2.5 concentration was associated with an 11% higher risk of incident DM (14). In the Danish Diet, Cancer, and Health Cohort Study (n =51,818 subjects with 9.7 years of follow-up), Andersen et al. (18) found no associations of various measures of traffic-related air pollution with DM (defined as hospital admission for diabetes or use of medication or blood glucose tests); however, they did find a significant association when the analysis was restricted to confirmed cases of DM, although the magnitude of the association was small (per each 4.9-µg/m3 increase in nitrogen dioxide, HR = 1.04, 95% CI: 1.00, 1.08). Another large study (n = 88,460) conducted in 2 prospective cohorts from the Nurses’ Health Study and the Health Professional Follow-Up Study found no significant associations of the 1989 annual average concentrations of particulate matter with an aerodynamic diameter of 10 μm or less and PM2.5 with incident DM between 1989 and 2002 (19).
It is unclear why exposure to air pollution was associated with prevalent but not incident DM in our study. One possible explanation is that persons who were more susceptible to DM had already developed DM before baseline. Individuals who were free of diabetes at baseline could be relatively healthier and less susceptible to the potential effects air pollution. Interestingly, higher age, which is a well-established risk factor for type 2 DM, was also associated with prevalent but not incident DM (Table 1). At baseline, the prevalence of DM was higher in men than in women (13.3% vs. 10.7%), whereas the incidence of DM was comparable between men and women (12.3% vs. 12.0%) (data not shown). On the other hand, the 9-year follow-up time might not have been long enough to capture the long-term, effects of air pollution on the development of DM. Another possible reason is that air pollution concentrations during the study period were not high enough to increase the risk of developing DM. Over the past several decades, air quality in the United States has improved: The annual average PM2.5 concentrations dropped 33% from 2000 to 2012, and concentrations of particulate matter with an aerodynamic diameter of 10 µm or less dropped 39% from 1990 to 2012 (40). A similar improvement was observed in our study sites between 2000 and 2012 (data not shown). Therefore, we might have low power to detect associations of incident DM with those low levels of air pollution. To the extent that exposures at baseline are correlated with similarly patterned but higher exposures in the past, our cross-sectional results could reflect the effects of prior exposures when air pollution concentrations were much higher.
Our finding of a stronger association between nitrogen oxides and incident DM in women is in agreement with previous findings (18, 19, 21), although it is difficult to interpret the inverse association in men. It is unclear why women might be more susceptible to air pollution in relation to DM. Brook et al. (21) conducted a cross-sectional study in Hamilton and Toronto, Canada (n = 7,634) and reported a significant association between nitrogen dioxide concentration and prevalent DM in women (for each ppb increase in nitrogen dioxide, which is equivalent to an OR of 1.17 for an IQR increase of 4 ppb, OR = 1.04, 95% CI: 1.00, 1.08) but not in men. Puett et al. (19) did not find a significant association when they examined data from the Nurses’ Health Study (women) and the Health Professional Follow-Up Study (men) together, but they did observe a significant association between roadway proximity and incident DM in the Nurses’ Health Study alone. The Danish Diet, Cancer, and Health Cohort Study also found a statistically significant association between nitrogen dioxide and confirmed DM only among women (18). Both sex-related biological differences (e.g., hormone-dependent physiological response) and socially determined sex differences (e.g., differential activity pattern and related exposure measurement accuracy) (41) might account for the observed difference between the sexes.
Our study has numerous strengths, including a large, prospective cohort with multicity and multiethnic groups in whom we examined both prevalent and incident DM that was objectively identified via medication use and fasting glucose levels; the quality of the fine-scale intra-urban modeling of air pollution exposure assessment; and high-quality covariate assessments. Nonetheless, this study has several limitations that need to be considered. Our exposure measures were based on annual averages from the year 2000, and we assumed that the exposures were time constant. An alternative approach could involve using time-varying PM2.5 and nitrogen oxides concentrations estimated at each visit, which might capture subsequent exposures (after baseline) that could be more relevant to disease risk. Given the high correlations between baseline and follow-up visit concentrations, the present study was able to contrast the rank order of pollution exposures but could not properly capture the impact of improved air quality (reduced ambient concentrations) on the risk of DM.
In conclusion, our study provides evidence that supports the association between long-term exposure to air pollution and the prevalence of DM. However, our results do not support the hypothesis that long-term exposure to air pollution is associated with incidence of DM, although short follow-up times and low exposure concentrations during the follow-up period might have limited our ability to detect an association. Much higher concentrations of air pollution have been reported in recently urbanized cities in Asia and Latin America (42, 43), where type 2 DM is an increasing public health concern. Given that the prevalence of type 2 DM is increasing worldwide and that people are exposed to a wide range of fine particulate concentrations, additional large studies with longer follow-up and a greater range in air pollution concentrations, including high levels, are warranted to evaluate the hypothesized association.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Department of Epidemiology, School of Public Health, University of Michigan, Ann Arbor, Michigan (Sung Kyun Park, Sara D. Adar, Marie S. O'Neill, Ana V. Diez-Roux); Department of Environmental Health Sciences, School of Public Health, University of Michigan, Ann Arbor, Michigan (Sung Kyun Park, Marie S. O'Neill); Department of Epidemiology and Biostatistics, School of Public Health, Drexel University, Philadelphia, Pennsylvania (Amy H. Auchincloss); Department of Biostatistics, School of Public Health, University of Washington, Seattle, Washington (Adam Szpiro); Department of Epidemiology and Prevention, Wake Forest University, Winston-Salem, North Carolina (Alain G. Bertoni); Department of Environmental Health Sciences, Bloomberg School of Public Health, Johns Hopkins University, Baltimore, Maryland (Ana Navas-Acien); Department of Epidemiology, Bloomberg School of Public Health, Johns Hopkins University, Baltimore, Maryland (Ana Navas-Acien); Welch Center for Prevention, Epidemiology and Clinical Research, Bloomberg School of Public Health, Johns Hopkins University, Baltimore, Maryland (Ana Navas-Acien); Department of Environmental and Occupational Health Sciences, University of Washington, Seattle, Washington (Joel D. Kaufman); Department of Medicine, University of Washington, Seattle, Washington (Joel D. Kaufman); and Department of Epidemiology, University of Washington, Seattle, Washington (Joel D. Kaufman).
This research was supported by contracts N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168 and N01-HC-95169 from the National Heart, Lung, and Blood Institute and by grants UL1-TR-000040 and UL1-RR-025005 from the National Center for Research Resources. S.K.P. was supported by the National Institute of Environmental Health Sciences grant K01-ES016587. This publication was developed under a STAR research assistance agreement, no. RD831697 (to the Multi-Ethnic Study of Atherosclerosis and Air Pollution), awarded by the US Environmental Protection Agency (EPA).
We thank the other investigators and staff the Multi-Ethnic Study of Atherosclerosis for their valuable contributions. A full list of participating investigators and institutions can be found at http://www.mesa-nhlbi.org.
This article has not been formally reviewed by the EPA. The views expressed in this document are solely those of the authors, and the EPA does not endorse any products or commercial services mentioned in this publication.
Conflict of interest: none declared.
REFERENCES
- 1.Centers for Disease Control and Prevention. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2011. National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States, 2011. http://www.cdc.gov/diabetes/pubs/pdf/ndfs_2011.pdf. Accessed October 22, 2012. [Google Scholar]
- 2.Willett WC. Balancing life-style and genomics research for disease prevention. Science. 2002;296(5568):695–698. doi: 10.1126/science.1071055. [DOI] [PubMed] [Google Scholar]
- 3.Thayer KA, Heindel JJ, Bucher JR, et al. Role of environmental chemicals in diabetes and obesity: a National Toxicology Program workshop review. Environ Health Perspect. 2012;120(6):779–789. doi: 10.1289/ehp.1104597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kuo CC, Moon K, Thayer KA, et al. Environmental chemicals and type 2 diabetes: an updated systematic review of the epidemiologic evidence. Curr Diab Rep. 2013;13(6):831–849. doi: 10.1007/s11892-013-0432-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rajagopalan S, Brook RD. Air pollution and type 2 diabetes: mechanistic insights. Diabetes. 2012;61(12):3037–3045. doi: 10.2337/db12-0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sun Q, Yue P, Deiuliis JA, et al. Ambient air pollution exaggerates adipose inflammation and insulin resistance in a mouse model of diet-induced obesity. Circulation. 2009;119(4):538–546. doi: 10.1161/CIRCULATIONAHA.108.799015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu X, Liu C, Xu Z, et al. Long-term exposure to ambient fine particulate pollution induces insulin resistance and mitochondrial alteration in adipose tissue. Toxicol Sci. 2011;124(1):88–98. doi: 10.1093/toxsci/kfr211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Delfino RJ, Staimer N, Tjoa T, et al. Circulating biomarkers of inflammation, antioxidant activity, and platelet activation are associated with primary combustion aerosols in subjects with coronary artery disease. Environ Health Perspect. 2008;116(7):898–906. doi: 10.1289/ehp.11189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dubowsky SD, Suh H, Schwartz J, et al. Diabetes, obesity, and hypertension may enhance associations between air pollution and markers of systemic inflammation. Environ Health Perspect. 2006;114(7):992–998. doi: 10.1289/ehp.8469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park SK, O'Neill MS, Vokonas PS, et al. Effects of air pollution on heart rate variability: the VA Normative Aging Study. Environ Health Perspect. 2005;113(3):304–309. doi: 10.1289/ehp.7447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carnethon MR, Golden SH, Folsom AR, et al. Prospective investigation of autonomic nervous system function and the development of type 2 diabetes: the Atherosclerosis Risk in Communities Study, 1987–1998. Circulation. 2003;107(17):2190–2195. doi: 10.1161/01.CIR.0000066324.74807.95. [DOI] [PubMed] [Google Scholar]
- 12.Meigs JB, Hu FB, Rifai N, et al. Biomarkers of endothelial dysfunction and risk of type 2 diabetes mellitus. JAMA. 2004;291(16):1978–1986. doi: 10.1001/jama.291.16.1978. [DOI] [PubMed] [Google Scholar]
- 13.Park SK, Wang W. Ambient air pollution and type 2 diabetes mellitus: a systematic review of epidemiologic research. Curr Environ Health Rep. 2014;1(3):275–286. doi: 10.1007/s40572-014-0017-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen H, Burnett RT, Kwong JC, et al. Risk of incident diabetes in relation to long-term exposure to fine particulate matter in Ontario, Canada. Environ Health Perspect. 2013;121(7):804–810. doi: 10.1289/ehp.1205958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brook RD, Cakmak S, Turner MC, et al. Long-term fine particulate matter exposure and mortality from diabetes in Canada. Diabetes Care. 2013;36(10):3313–3320. doi: 10.2337/dc12-2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raaschou-Nielsen O, Sørensen M, Ketzel M, et al. Long-term exposure to traffic-related air pollution and diabetes-associated mortality: a cohort study. Diabetologia. 2013;56(1):36–46. doi: 10.1007/s00125-012-2698-7. [DOI] [PubMed] [Google Scholar]
- 17.Coogan PF, White LF, Jerrett M, et al. Air pollution and incidence of hypertension and diabetes mellitus in black women living in Los Angeles. Circulation. 2012;125(6):767–772. doi: 10.1161/CIRCULATIONAHA.111.052753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andersen ZJ, Raaschou-Nielsen O, Ketzel M, et al. Diabetes incidence and long-term exposure to air pollution: a cohort study. Diabetes Care. 2012;35(1):92–98. doi: 10.2337/dc11-1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Puett RC, Hart JE, Schwartz J, et al. Are particulate matter exposures associated with risk of type 2 diabetes? Environ Health Perspect. 2011;119(3):384–389. doi: 10.1289/ehp.1002344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krämer U, Herder C, Sugiri D, et al. Traffic-related air pollution and incident type 2 diabetes: results from the SALIA cohort study. Environ Health Perspect. 2010;118(9):1273–1279. doi: 10.1289/ehp.0901689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brook RD, Jerrett M, Brook JR, et al. The relationship between diabetes mellitus and traffic-related air pollution. J Occup Environ Med. 2008;50(1):32–38. doi: 10.1097/JOM.0b013e31815dba70. [DOI] [PubMed] [Google Scholar]
- 22.Dijkema MB, Mallant SF, Gehring U, et al. Long-term exposure to traffic-related air pollution and type 2 diabetes prevalence in a cross-sectional screening-study in the Netherlands. Environ Health. 2011;10:76. doi: 10.1186/1476-069X-10-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bild DE, Bluemke DA, Burke GL, et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156(9):871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 24.Genuth S, Alberti KG, Bennett P, et al. Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care. 2003;26(11):3160–3167. doi: 10.2337/diacare.26.11.3160. [DOI] [PubMed] [Google Scholar]
- 25.Szpiro AA, Sampson PD, Sheppard L, et al. Predicting intra-urban variation in air pollution concentrations with complex spatio-temporal dependencies. Environmetrics. 2010;21(6):606–631. doi: 10.1002/env.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sampson PD, Szpiro AA, Sheppard L, et al. Pragmatic estimation of a spatio-temporal air quality model with irregular monitoring data. Atmos Environ. 2011;45(36):6593–6606. [Google Scholar]
- 27.Cohen MA, Adar SD, Allen RW, et al. Approach to estimating participant pollutant exposures in the Multi-Ethnic Study of Atherosclerosis and Air Pollution (MESA Air) Environ Sci Technol. 2009;43(13):4687–4693. doi: 10.1021/es8030837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.U.S. Census Bureau. Washington, DC: 2000. Redistricting Census 2000 TIGER/Line Files Technical Documentation. https://www.census.gov/geo/maps-data/data/pdfs/tiger/rd_2ktiger/tgrrd2k.pdf . Accessed December 2, 2013. [Google Scholar]
- 29.Hajat A, Diez-Roux AV, Adar SD, et al. Air pollution and individual and neighborhood socioeconomic status: evidence from the Multi-Ethnic Study of Atherosclerosis (MESA) Environ Health Perspect. 2013;121(11-12):1325–1333. doi: 10.1289/ehp.1206337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mujahid MS, Diez Roux AV, Morenoff JD, et al. Assessing the measurement properties of neighborhood scales: from psychometrics to ecometrics. Am J Epidemiol. 2007;165(8):858–867. doi: 10.1093/aje/kwm040. [DOI] [PubMed] [Google Scholar]
- 31.Auchincloss AH, Diez Roux AV, Mujahid MS, et al. Neighborhood resources for physical activity and healthy foods and incidence of type 2 diabetes mellitus: the Multi-Ethnic Study of Atherosclerosis. Arch Intern Med. 2009;169(18):1698–1704. doi: 10.1001/archinternmed.2009.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mujahid MS, Diez Roux AV, Shen M, et al. Relation between neighborhood environments and obesity in the Multi-Ethnic Study of Atherosclerosis. Am J Epidemiol. 2008;167(11):1349–1357. doi: 10.1093/aje/kwn047. [DOI] [PubMed] [Google Scholar]
- 33.Van Hee VC, Szpiro AA, Prineas R, et al. Association of long-term air pollution with ventricular conduction and repolarization abnormalities. Epidemiology. 2011;22(6):773–780. doi: 10.1097/EDE.0b013e31823061a9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miller KA, Siscovick DS, Sheppard L, et al. Long-term exposure to air pollution and incidence of cardiovascular events in women. N Engl J Med. 2007;356(5):447–458. doi: 10.1056/NEJMoa054409. [DOI] [PubMed] [Google Scholar]
- 35.Burton RM, Suh HH, Koutrakis P. Spatial variation in particulate concentrations within metropolitan Philadelphia. Environ Sci Technol. 1996;30(2):400–407. [Google Scholar]
- 36.Gomišček B, Hauck H, Stopper S, et al. Spatial and temporal variations of PM1, PM2.5, PM10 and particle number concentration during the AUPHEP—project. Atmos Environ. 2004;38(24):3917–3934. [Google Scholar]
- 37.Liu C, Ying Z, Harkema J, et al. Epidemiological and experimental links between air pollution and type 2 diabetes. Toxicol Pathol. 2013;41(2):361–373. doi: 10.1177/0192623312464531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Laing S, Wang G, Briazova T, et al. Airborne particulate matter selectively activates endoplasmic reticulum stress response in the lung and liver tissues. Am J Physiol Cell Physiol. 2010;299(4):C736–C749. doi: 10.1152/ajpcell.00529.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu Z, Xu X, Zhong M, et al. Ambient particulate air pollution induces oxidative stress and alterations of mitochondria and gene expression in brown and white adipose tissues. Part Fibre Toxicol. 2011;8:20. doi: 10.1186/1743-8977-8-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.EPA. U.S. Environmental Protection Agency; 2013. Air trends: particulate matter. http://www.epa.gov/airtrends/pm.html. Accessed November 21, 2013. [Google Scholar]
- 41.Clougherty JE. A growing role for gender analysis in air pollution epidemiology. Environ Health Perspect. 2010;118(2):167–176. doi: 10.1289/ehp.0900994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Green J, Sanchez S. Air quality in Latin American: an overview. http://www.cleanairinstitute.org/calidaddelaireamericalatina/cai-report-english.pdf . Accessed November 22, 2013.
- 43.Silva RA, West JJ, Zhang Y, et al. Global premature mortality due to anthropogenic outdoor air pollution and the contribution of past climate change. Environ Res Lett. 2013;8(3):034005. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.