Abstract
Fetal Alcohol Spectrum Disorder (FASD) is caused by prenatal alcohol exposure, producing craniofacial, sensory, motor, and cognitive defects. FASD is highly prevalent in low socioeconomic populations, which are frequently accompanied by malnutrition. FASD-associated ocular pathologies include microphthalmia, optic nerve hypoplasia, and cataracts. The present study characterizes specific retinal tissue defects, identifies ethanol-sensitive stages during retinal development, and dissects the effect of nutrient supplements, such as retinoic acid (RA) and folic acid (FA) on ethanol-induced retinal defects. Exposure to pathophysiological concentrations of ethanol (during midblastula transition through somitogenesis; 2–24 hours post fertilization [hpf]) altered critical transcription factor expression involved in retinal cell differentiation, and produced severe retinal ganglion cell, photoreceptor, and Müller glial differentiation defects. Ethanol exposure did not alter retinal cell differentiation induction, but increased retinal cell death and proliferation. RA and FA nutrient co-supplementation rescued retinal photoreceptor and ganglion cell differentiation defects. Ethanol exposure during retinal morphogenesis stages (16–24 hpf) produced retinal defects like those seen with ethanol exposure between 2–24 hpf. Significantly, during an ethanol-sensitive time window (16–24 hpf), RA co-supplementation moderately rescued these defects, whereas FA co-supplementation showed significant rescue of optic nerve and photoreceptor differentiation defects. Interestingly, RA, but not FA, supplementation after ethanol exposure could reverse ethanol-induced optic nerve and photoreceptor differentiation defects. Our results indicate that various ethanol-sensitive events underlie FASD-associated retinal defects. Nutrient supplements like retinoids and folate were effective in alleviating ethanol-induced retinal defects.
Keywords: fetal alcohol spectrum disorder, retinal development, zebrafish, retinoic acid, folic acid
Introduction
Fetal alcohol spectrum disorder (FASD) caused by prenatal ethanol exposure is the most frequent preventable birth defect syndrome (May et al., 2009). FASD prevalence ranges from 0.3 to 5% within most populations, reaching as high as 8.9% in some low socioeconomic populations (May et al., 2009). Ethanol-induced defects include craniofacial, cardiac, central nervous system, learning, motor, sensory, and ocular defects. Ocular defects are frequently seen in children diagnosed with FASD, which include microphthalmia (reduced eye size), coloboma (incomplete optic fissure closure), optic nerve hypoplasia (ONH), cataracts, scotopic vision loss, low visual acuity, and abnormal electroretinograms (Hug, Fitzgerald, & Cibis, 2000; Strömland & Pinazo-Durán, 2002).
Ethanol exposure in vertebrate animal models recapitulates retinal defects seen in FASD patients. Experiments using mice showed that in utero ethanol exposure induced specific defects in rod photoreceptor sensitivity and dark adaptation (Katz & Fox, 1991). Studies on zebrafish embryos showed reduced electroretinogram responses, ONH, retinal lamination defects, inhibition of photoreceptor outer segment growth, and reduced visual acuity due to ethanol exposure during gastrulation through neurulation stages (2–24 hpf) (Arenzana et al., 2006; Bilotta, Saszik, Givin, Hardesty, & Sutherland, 2002; Matsui, Egana, Sponholtz, Adolph, & Dowling, 2006). Ethanol exposure during zebrafish retinal neurogenesis (24–48 hpf) also induced persistent microphthalmia (Kashyap, Frederickson, & Stenkamp, 2007). Shorter periods of ethanol exposure (12–24 hpf) were sufficient to induce microphthalmia, similar to that produced by longer treatments (Bilotta et al., 2002). However, cellular details of ethanol effects on retinal cell specification, differentiation, and potential protective measures remain unclear.
Proposed mechanisms underlying ethanol-induced ocular defects include increased cell death, developmental delay, and reduced cell differentiation (Kashyap et al., 2007). Developmental defects may be due to ethanol-induced RA signaling disruption, reactive oxygen species (ROS) generation, and epigenetic defects (Brocardo, Gil-Mohapel, & Christie, 2011; Kot-Leibovich & Fainsod, 2009; Marrs et al., 2010; Singh, Shiue, Schomberg, & Zhou, 2009; Zhou et al., 2011). In addition, low socioeconomic populations show increased FASD incidence, which correlates with nutritional deficiencies. Reduced absorption and increased excretion of essential vitamins in adults caused by ethanol consumption aggravates malnutrition (Lieber, 2003). Several studies showed nutritional compounds, including retinoids, folate, choline, and vitamin E partially rescued ethanol-induced developmental defects (Heaton, Paiva, & Siler-Marsiglio, 2011; Kot-Leibovich & Fainsod, 2009; Marrs et al., 2010; Mitchell, Paiva, & Heaton, 1999; Sarmah & Marrs, 2013; Thomas, Idrus, Monk, & Dominguez, 2010; Wang et al., 2009; Yelin et al., 2005). RA and FA were very effective in rescuing various developmental defects (Ballard, Sun, & Ko, 2012; Marrs et al., 2010; Sarmah & Marrs, 2013; Yelin et al., 2005).
Retinal structure and developmental mechanisms are highly conserved among vertebrates. Rapid and well-characterized developmental events in zebrafish offer opportunities to examine specific ethanol-induced retinal defects and design rescue experiments to study cellular details. Vertebrate retinal morphogenesis occurs through a series of tightly regulated processes involving retinal cell specification, lamination, and differentiation into various cell types which are tightly orchestrated by signaling pathways, including BMPs, Shh, FGFs, and retinoic acid (RA) (Lupo et al., 2005; Ohkubo, Chiang, & Rubenstein, 2002).
RA is a derivative of vitamin A (retinol), and RA signaling plays a crucial role during embryonic development. During retinal morphogenesis, RA performs distinct functions. RA is a morphogen for retinal dorsoventral patterning and RA induces terminal differentiation of unspecified photoreceptor progenitors and precursors into rod and cone photoreceptors in the outer nuclear layer (ONL) of the retina (Hyatt, Schmidt, Fadool, & Dowling, 1996; Prabhudesai, Cameron, & Stenkamp, 2005; Rhinn & Dollé, 2012). Several alcohol/aldehyde dehydrogenases (ADHs/ALDHs) tightly regulate RA biosynthesis, and RA-degrading enzymes control its catabolism during development. Retinaldehyde dehydrogenase enzymes are expressed in the dorsal (Raldh2) and ventral (Raldh3) regions of the zebrafish retina during retinal morphogenesis. Early in vitro studies showed competitive inhibition of ADHs by ethanol (Mezey & Holt, 1971) produces ethanol-induced RA signaling deficits during development, causing embryonic malformations (Duester, 1991), but it is unclear what other developmental signaling mechanisms are also disrupted by embryonic ethanol exposure.
FA is an essential vitamin that participates in nucleic acid synthesis and repair (Kamen, 1997). FA also plays a crucial role as a cofactor in 1-carbon metabolism as tetrahydrofolate, which is needed in DNA and histone methyl transfers. More recent studies identified ROS scavenging properties of FA (Ibrahim, Tousson, El-Masry, Arafa, & Akela, 2012; Joshi, Adhikari, Patro, Chattopadhyay, & Mukherjee, 2001). FA deficiency, consequently, produces a wide range of birth defects including severe ocular defects, such as microphthalmia, delayed lamination, and optic cup abnormalities (Maestro-de-las-Casas et al., 2013). Embryonic ethanol exposure affects FA metabolism, including reduced maternal-to-fetal folate transfer and reduced expression of folate metabolizing enzymes (Hutson, Stade, Lehotay, Collier, & Kapur, 2012). Ethanol-induced FA deficiency could alter histone and DNA methylation patterns as seen in ethanol-treated cell culture models (Mason & Choi, 2005; Singh et al., 2009; Zhou et al., 2011). Importantly, studies showed that FA supplementation rescued overall ethanol-induced morphological defects, particularly cardiac defects (Ballard et al., 2012; Sarmah & Marrs, 2013; Serrano, Han, Brinez, & Linask, 2010). Prenatal FA supplementation significantly reduces the risk of neural tube defects, congenital heart defects, and cleft lip/palate, and thus, FA is a recommended dietary supplement for pregnant mothers (Taruscio, Carbone, Granata, Baldi, & Mantovani, 2011).
Dose, duration, and timing of ethanol exposure greatly affect the severity of FASD, which is also influenced by intrinsic (genetic background) and extrinsic (nutrition and environment) factors. Here, experiments are presented that characterize effects of ethanol on cell differentiation pathways that produce retinal defects. Experiments were used to identify ethanol-sensitive developmental stages and examine effects of nutrient supplementation with RA and FA.
Materials and methods
Zebrafish husbandry and ethanol, RA, FA, inhibitor treatments
Zebrafish (Danio rerio; Hamilton; TL strain) were raised and housed under standard laboratory conditions (Westerfield, 2000) in accordance with Indiana University Policy on Animal Care and Use. The embryos were treated with 1-phenyl-2-thiourea (0.003%) from 6 hpf (shield) onward in order to prevent melanogenesis. Embryos were exposed to ethanol by incubation in the embryo medium containing ethanol (100 mM and 150 mM) for different periods between 2 to 24 hpf in Petri dishes wrapped with Parafilm® and maintained at 28.5 °C. Ethanol treatment dishes were placed in chambers with 2% ethanol to minimize ethanol volatilization. After treatment, embryos were rinsed with pre-warmed embryo medium and incubated in embryo medium until the desired stage was achieved.
RA (0.1 mM) and DEAB (1 mM) stock solutions (Sigma, St. Louis, MO, USA) were dissolved in DMSO. Citral (1 mM; T.C.I., Portland, OR, USA) stock solution was freshly prepared prior to treatment. The medium with citral was replaced with embryo medium containing freshly diluted citral (1 and 10 µM) every hour for 8 h (16–24 hpf) due to lability of citral in water. FA (1 mM, Sigma) stock solution was made fresh prior to treatment and diluted in embryo medium. RA and FA treatments were performed as previously described (Marrs et al., 2010; Sarmah & Marrs, 2013). Other concentrations of RA and FA have been tested previously (Marrs et al., 2010; Sarmah & Marrs, 2013), and 1 nM RA and 75 µM FA were chosen as optimal concentrations for treatments that minimized their toxic effects and displayed maximum rescue phenotypes.
Immunofluorescence
Whole-mount immunostaining was performed as previously described (Clendenon, Sarmah, Shah, Liu, & Marrs, 2012) using primary antibodies against HuC/D (Sigma, 1:1000), zpr-1 (ZIRC, Eugene, OR, USA; 1:1000), zpr-3 (ZIRC, 1:500), zrf-1 (ZIRC1:1000), zn-5 (ZIRC, 1:500), acetylated tubulin (Sigma, 1:500), and phospho-histone-3 (Millipore, Bellerica, MA, USA; 1:500). Alexa Fluor 488-conjugated anti-mouse and anti-rabbit secondary antibody (Molecular Probes, Grand Island, NY, USA) was used at 1:200 dilution. Alexa Fluor 488- conjugated phalloidin (Molecular Probes) was used at a 1:100 dilution. Nuclear staining was performed using TO-PRO-3 iodide at a 1:1000 dilution incubated for 1 h.
Apoptosis imaging
Acridine orange staining was performed to visualize apoptotic nuclei by incubating the dechorionated embryos with 5-µg/mL acridine orange for 3 min, followed by several washes in embryo medium. Live embryos were imaged immediately using a confocal microscope.
Microscopy
Laser scanning confocal images were acquired using a Zeiss Observer Z1 LSM 700 confocal microscope (40X 1.1 NA W objective; Carl Zeiss Microscopy, Thornwood, NY, USA). Several x-y focal plane images were captured to produce a z-stack or image volume. All embryos were deyolked and imaged from the ventral side, and z-sections were analyzed, always including the optic nerve for consistency. Differential interference contrast images of live embryos were obtained using Zeiss observer Z1 (20X 0.8 NA objective) with an Orca AG CCD camera (Hamamatsu Photonics, Bridgewater, NJ, USA). Brightfield dissecting microscope images were acquired using a color Leica DFC290 camera mounted on a Leica MZ12 stereomicroscope (Leica, Buffalo Grove, IL, USA).
Image analyses and cell counting
3D reconstruction of confocal slices was used to measure optic nerve widths using Image J software. The optic nerve widths were measured at the inner plexiform layer, which was determined using TO-PRO-3 staining. The measurement of zpr-1 intensity in the ONL was performed by highlighting the ONL using TO-PRO-3 nuclear stain channel in Carl Zeiss ZEN imaging software and exporting the highlighted images to the zpr-1 channel. The total intensity in the highlighted region was measured using Image J software.
Cell counting was performed for cell proliferation and cell apoptosis assays using ZEN software. ONL, inner nuclear layer (INL), and ciliary marginal zone (CMZ) regions in the cell proliferation cell counts were identified using TO-PRO-3 nuclear stain.
In situ hybridization
Whole mount in situ hybridization of zebrafish embryos was performed as previously described (Sarmah et al., 2010). Digoxigenin-labeled riboprobes for rx1 (retinal homeobox gene 1), shh (sonic hedgehog), crx (cone-rod homeobox), otx5 (orthodenticle homolog 5), neuroD (neurogenic differentiation), rho (rhodopsin), opn1sw1 (UV opsin), and opn1lw1 (red opsin) were synthesized using a Dig RNA labeling kit (Roche, Indianapolis, IN, USA).
Quantitative PCR analysis
RNA isolation and quantitative PCR analysis were performed as previously described (Sarmah et al., 2013) for 72 hpf treated and untreated embryos. The primers for rhodopsin and red opsin were used as previously described (Laranjeiro & Whitmore, 2014). Independent experiments in triplicates were performed using rsp15 endogenous control using the 7300 Real Time PCR system (Applied Biosystems, Grand Island, NY, USA).
Statistical analysis
One-way ANOVAs were used to test the group effect for each outcome, followed by pre-specified comparisons against the controls and comparisons against the rescue groups as appropriate for each analysis. A 5% significance level was used for each test (Biostatistics Services, IUSM).
Results
Zebrafish embryos treated with 100 mM and 150 mM ethanol (0.6 and 0.9% v/v, respectively) produced reproducible and consistent retinal defects. After ethanol treatment, embryos were returned to normal embryo medium until a specific developmental stage for analysis.
Chronic ethanol exposure affects retinal neurogenesis, gene expression, and tissue organization in the developing zebrafish retina
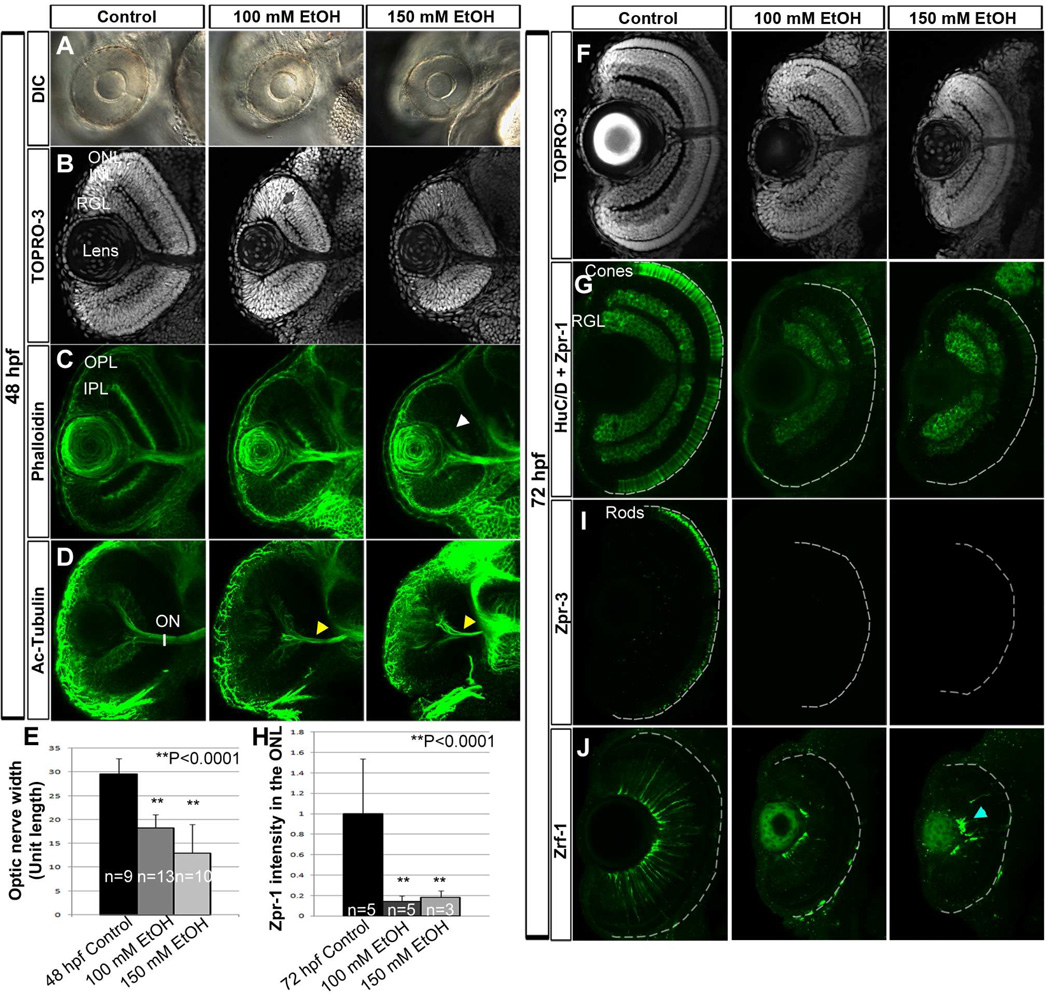
To examine the effect of chronic ethanol treatment during embryogenesis on retinal development, zebrafish embryos were exposed to ethanol from midblastula transition through somitogenesis stages (2–24 hpf). Examination of the retina at 48 hpf showed that ethanol-treated zebrafish embryos displayed microphthalmia and retinal lamination delay, as compared to untreated control embryos (Fig. 1A–C). Optic nerve width was measured by staining of the optic nerve using acetylated-tubulin antibody. Ethanol-treated embryos showed a significant decrease in optic nerve width relative to untreated controls (Fig. 1D and E). At 72 hpf, ethanol-treated embryos had formed retinal laminae (Fig. 1F), but failed to express normal levels of photoreceptor terminal differentiation markers, including markers for rods (zpr-3 antibody) and red-green double cones (zpr-1 antibody; Fig. 1G–I). These embryos also displayed highly aberrant Müller glial cell morphology as shown by zrf-1 antibody staining, which detects glial fibrillary acidic protein (GFAP; Fig. 1J). In untreated retinas, abundant Müller glial cells extended processes that spanned the entire retinal tissue, but in the ethanol-treated retinas the Müller glial cell processes collapsed onto the retinal ganglion cell layer.
Figure 1. Ethanol exposure disrupts tissue organization.
(A) DIC images demonstrating ethanol-induced microphthalmia at 48 hpf.
(B, C) TO-PRO-3 (B) and phalloidin (C) staining at 48 hpf showed formation of 3 distinct nuclear layers (RGL, INL, and ONL; B) and 2 plexiform layers (IPL and OPL; C) in control embryos, and severely disrupted lamination in ethanol-treated embryos (white arrowhead).
(D, E) 3D renderings of acetylated-tubulin-stained embryos showed severe ONH following ethanol treatment as compared to untreated controls (yellow arrowheads, D). (E) Optic nerve widths quantification of 100 mM and 150 mM ethanol-treated embryos showed significant reduction compared to untreated embryos.
(F–I) At 72 hpf, TO-PRO-3 staining showed delayed lamination in ethanol-treated embryos (F); zpr-1 and zpr-3 staining showed reduced differentiation of double cones (G) and rods (I). (H) Quantification of total zpr-1 intensity in ONL showed a significant ethanol-induced reduction in double cone terminal differentiation.
(J) Zrf-1 antibody staining in the retina revealed aberrant Müller glial cell morphology after ethanol treatment (blue arrowhead).
Anterior to the top; Ventral view; White dashed lines indicate retinal pigmented epithelium; RGL, retinal ganglion cell layer; ONL, outer nuclear layer; INL, inner nuclear layer; OPL, outer plexiform layer; IPL, inner plexiform layer; ON, optic nerve. Error bars indicate standard deviation.
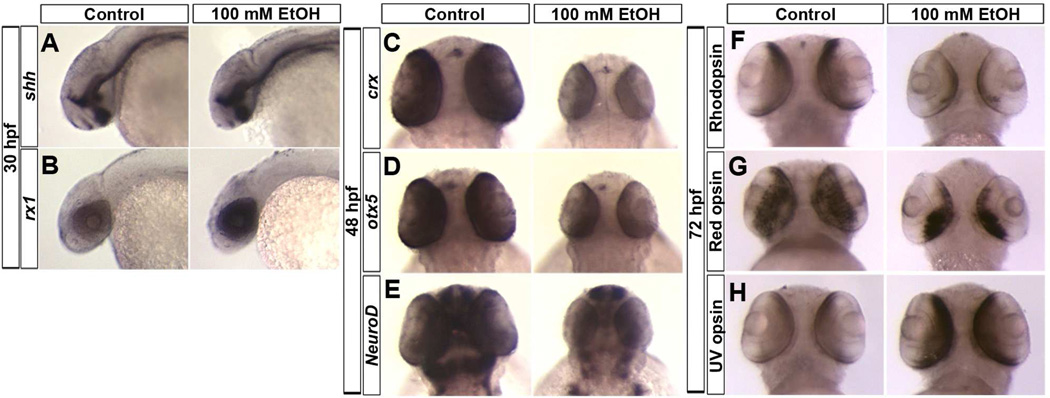
In situ hybridization (ISH) was used to examine ethanol effects on the expression patterns of genes involved in retinal neurogenesis induction, cell specification, and differentiation. The expression of retinal neurogenesis induction gene, shh, remained unchanged at 30 hpf (Fig. 2A). However, expression of retinal cell specification gene, rx1, increased after ethanol treatment (Fig. 2B). The expression of transcription factors involved in retinal cell differentiation, crx, otx5, and neuroD, was reduced in the retina at 48 hpf after ethanol exposure (Fig. 2C–E). Expression of terminal photoreceptor differentiation markers rhodopsin and red opsin showed altered pattern and downregulation. This was also reflected in quantitative PCR analysis for rhodopsin (100 and 150 mM ethanol-treated embryos, 0.29 and 0.097-fold change, respectively) and red opsin (100 and 150 mM ethanol-treated embryos, 0.52 and 0.215-fold change, respectively), which showed significant (p < 0.05) downregulation compared to control embryos. Conversely, UV opsin expression was increased after ethanol exposure (Fig. 2F–H).
Figure 2. Ethanol exposure alters expression of retinal transcription factors and opsin genes.
(A, B) In situ hybridization at 30 hpf showing expression pattern of shh and rx1. Ethanol exposure (100 mM) did not change shh expression pattern (A), but the embryos showed an increased expression of rx1 (B). Anterior to left.
(C–E) At 48 hpf, ethanol-treated embryos showed a reduced expression of transcription factors including crx (C), otx5 (D), and neuroD (E) in the retina compared to the control embryos.
(F–H) At 72 hpf, ethanol-treated embryos showed a reduced expression of rhodopsin (expressed in rods, F) and red opsin (red cones, G) and an increased expression of UV opsin (H). Anterior to the top; Dorsal view
Ethanol-induced retinal cell death accounts for microphthalmia
To determine the causes underlying ethanol-induced microphthalmia, three possibilities were examined: i) reduced number of cells initially induced for retinal differentiation, ii) increased retinal cell death, and (iii) reduced retinal cell proliferation.
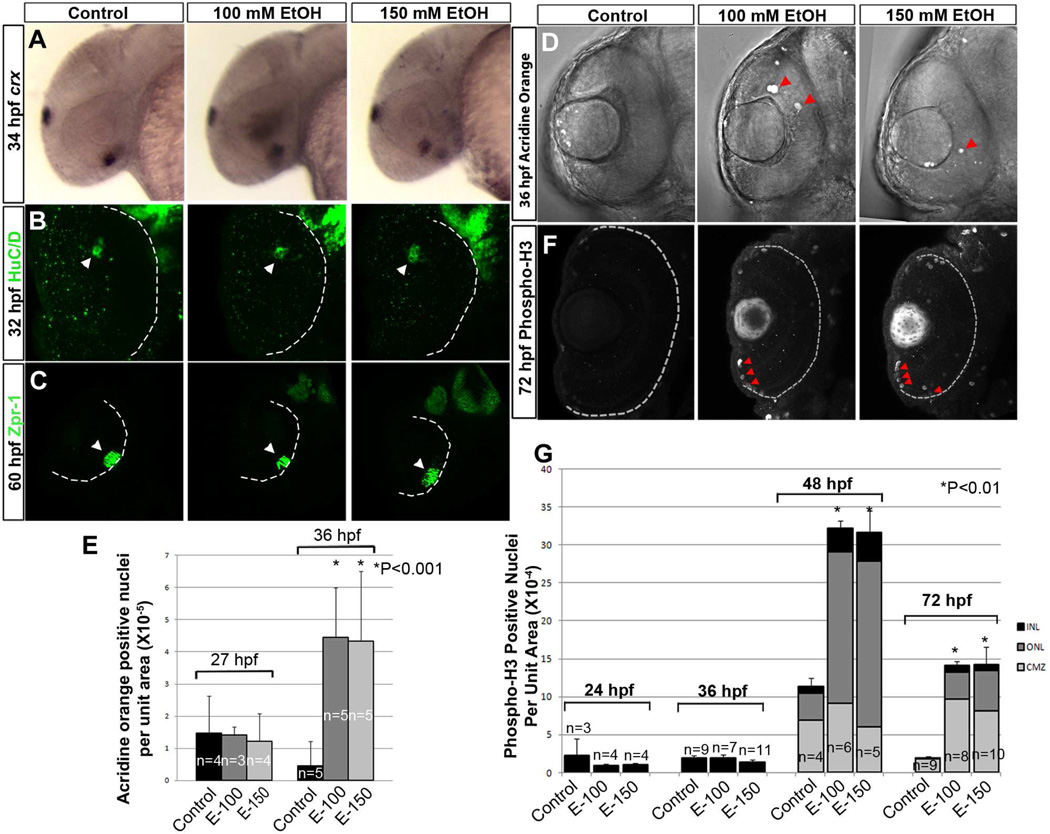
Expression domains of retinal cell specification marker, rx1, were comparable in control and ethanol-treated embryos, indicating no change in initial specification eye field (Fig. 2B). Induction of retinal differentiation was examined using crx ISH (retinal cell differentiation marker, at 34 hpf), HuC/D antibody staining (early neuronal marker) at early stages of ganglion cell differentiation (32 hpf), and zpr-1 antibody staining (red-green double cone photoreceptor marker) at early stages of photoreceptor differentiation (60 hpf). These experiments showed no difference in retinal cell specification and initial differentiation marker expression domains or timing after ethanol exposure (Fig. 2B; Fig. 3A–C).
Figure 3. Ethanol exposure did not change the cell differentiation induction in ventral retina.
(A–C) In situ hybridization using crx riboprobes (A, Anterior to left) and immunostaining detecting HuC/D protein (B) showed little or no difference in retinal ganglion cell differentiation after ethanol exposure. (C) zpr-1 antibody showed similar photoreceptor differentiation in the control and ethanol-treated embryos (white arrowheads).
(D) Acridine orange staining showed increased cell death after ethanol exposure (red arrowheads).
(E) Quantification of acridine orange-positive cells in the retina per unit area at 27 hpf and 40 hpf, showing little or no change in number of apoptotic cells at 27 hpf and a significant increase in cell death at 40 hpf in ethanol-treated embryos.
(F) Phospho-H3 staining showed an increase in the number of dividing cells after ethanol exposure (red arrowheads).
(G) Quantification of phospho-H3 positive nuclei per unit area of the retina showed no difference in the number of proliferating cells at 24 hpf between control and ethanol-treated retinas, but a significant increase in the number of cells undergoing division in the ONL and INL at 48 and 72 hpf. As 24 hpf retinal structure is not laminated, the cell counts at this stage represent the entire retinal area.
Anterior to the top; Ventral view; White dashed lines indicate future retinal pigmented epithelium. Error bars indicate standard deviation.
Acridine orange staining was used to measure apoptosis in embryonic retinas. Following completion of ethanol treatment, the number of acridine orange-positive cells per unit area did not differ from untreated control embryos at 27 hpf (Fig. 3E). However, at 36 hpf, there was a significant increase in the number of acridine orange-positive cells per unit area in the retina of ethanol-treated embryos (Fig. 3D and E).
To analyze cell proliferation in the retina, the mitosis marker phospho-histone-3 (H3) was examined using immunofluorescence. The number of phospho-H3-positive nuclei did not change following ethanol treatment at 24 and 36 hpf in the ethanol-treated retinas. However, there was a significant increase in the number of proliferating retinal cells per unit area after ethanol treatment at 48 and 72 hpf (Fig. 3F and G). Mitotic cells in the retinas of ethanol-treated embryos were mostly found in the outer nuclear layer (ONL) and inner nuclear layer (INL) at 48 hpf. These mitotic cells were predominantly found in the ciliary marginal zone (CMZ) in the retinas of the ethanol-treated embryos at 72 hpf (Fig. 3G).
Ethanol-induced retinal defects can be rescued by retinoic acid supplementation
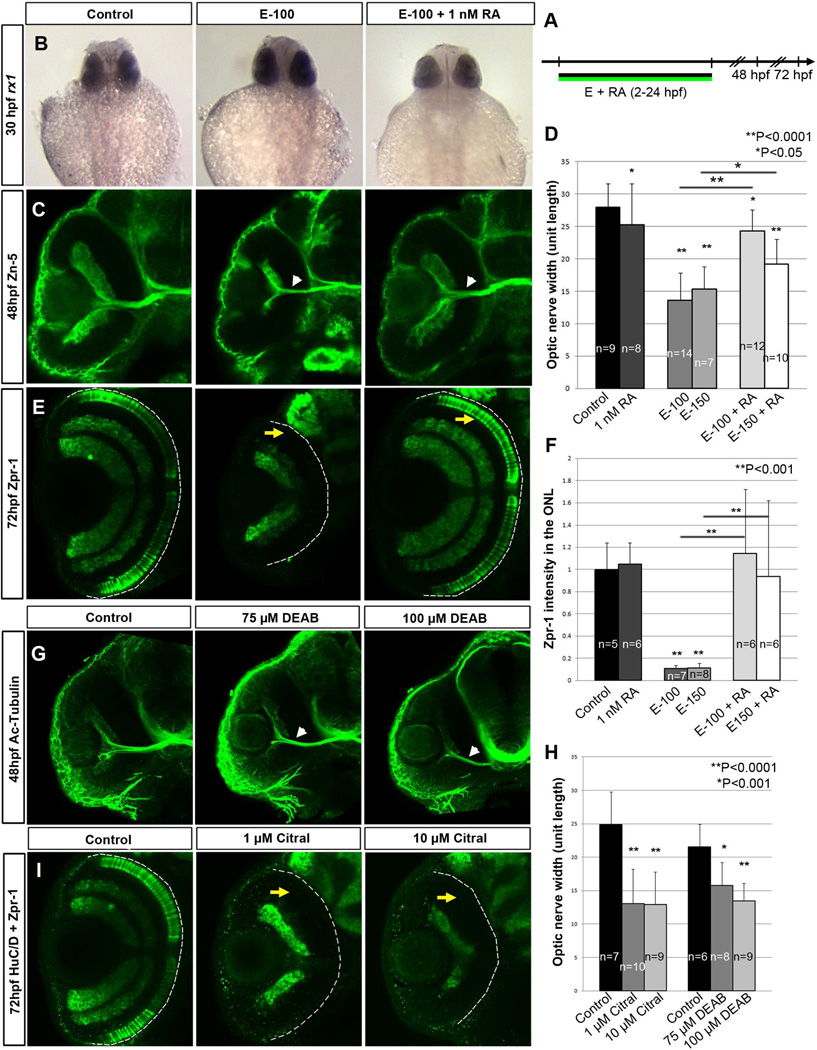
Previous studies showed that 1 nM RA supplementation rescued cardiac and other phenotypic defects in zebrafish embryos (Marrs et al., 2010; Sarmah & Marrs, 2013). To test RA rescue of ethanol-induced retinal defects, embryos were co-treated with ethanol and 1 nM RA from 2–24 hpf and compared with embryos treated with ethanol alone (Fig. 4A). rx1 expression was restored to near normal levels by RA supplementation (Fig. 4B). Co-treatment of RA with ethanol moderately rescued ONH (Fig. 4C and D) and completely rescued photoreceptor differentiation (Fig. 4E and F). These findings highlight that exogenous RA can restore retinal defects and indicate that ethanol exposure produced RA-signaling defects in the retina.
Figure 4. RA supplementation rescues ethanol-induced retinal defects.
(A) Timeline showing RA and ethanol co-supplementation from 2–24 hpf
(B–E) Treatment with low concentrations of RA (1 nM) rescued ethanol-induced retinal defects. (B) rx1 gene expression level was restored by RA and ethanol co-treatment. (C) ONH (white arrowheads) seen in ethanol-exposed embryos using zn-5 antibody staining ganglion cells, showed rescue after RA and ethanol co-treatment. (D) Quantification of optic nerve width showing a significant reduction after ethanol treatment, which was restored by RA co-treatment. (E) Restoration of terminally differentiated double cone photoreceptor (yellow arrows) after RA co-treatment as shown by zpr-1 staining. (F) Quantification of total zpr-1 intensity in the ONL showed a significant reduction after ethanol treatment, which was restored to control, levels following RA co-treatment.
(G–I) RA signaling inhibitors, DEAB and citral, induced retinal defects similar to ethanol. (G) ONH after treatment with DEAB (75 µM and 100 µM, white arrowheads). (H) Quantification of optic nerve size reduction after DEAB and citral treatment showed significant reduction compared to untreated embryos. (I) Terminal double cone photoreceptors differentiation using zpr-1 staining showed reduction/absence of differentiated photoreceptors (yellow arrows) after citral treatment (1 µM and 10 µM).
Error bars indicate standard deviation.
Effects of two known RA biosynthesis inhibitors, citral and 4-diethylaminobenzaldehyde (DEAB), were compared to ethanol to determine whether similar retinal defects are produced by directly disrupting RA synthesis (Fig. 4G–I). Embryos treated with these inhibitors displayed ONH at 48 hpf and red-green cone photoreceptor differentiation defects at 72 hpf. The similarity of retinal defects produced with RA biosynthesis inhibitors and ethanol supports the idea that ethanol affects RA levels in the retina.
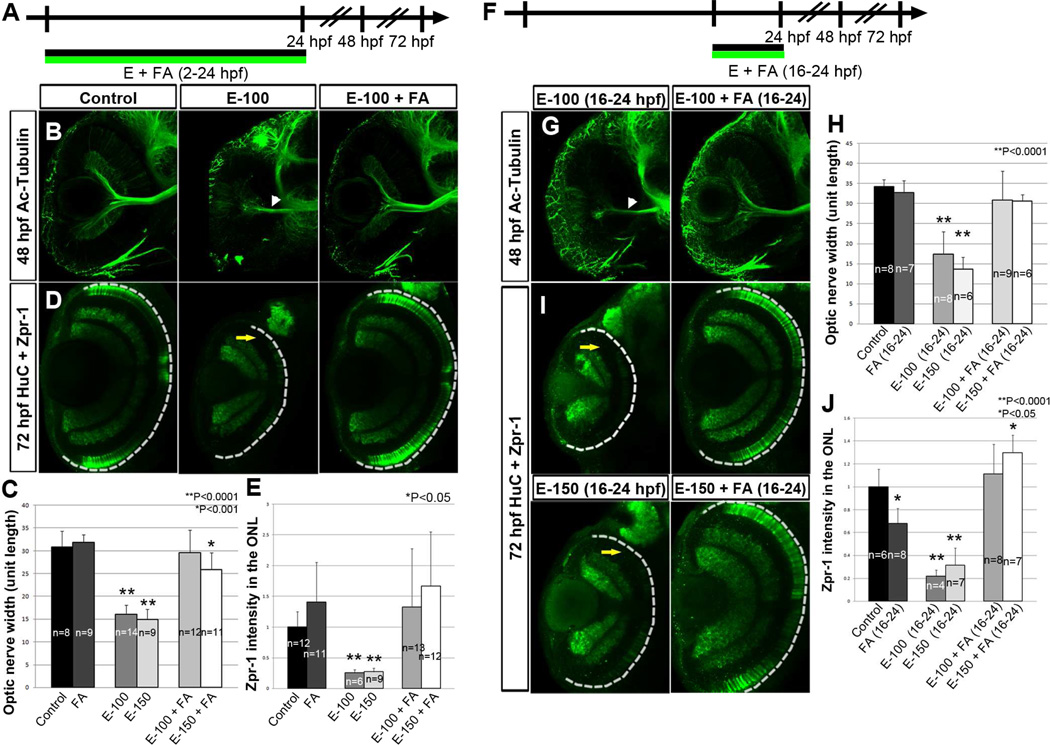
RA signaling in the zebrafish retina begins when raldh3 expression is activated in the ventral retina around 18 hpf (Ma, Chung, Liang, & Leung, 2010). To test whether ethanol-induced retinal defects coincide with this activation of RA signaling, embryos were treated with ethanol during different time windows, before and after the onset of raldh3 expression. The ethanol-treatment period in previous experiments (2–24 hpf) was subdivided into two windows, 2–16 hpf and 16–24 hpf, the latter encompassing raldh3 expression initiation of the previous treatment period (Fig. 5A). If ethanol-induced defects are due to reduced RA activity, then ethanol treatment only during RA biosynthesis should affect the cell differentiation processes, producing similar retinal defects as those seen in embryos treated with ethanol from 2–24 hpf, and not affecting retinal development as severely as by treating during an earlier period prior to RA biosynthesis (2–16 hpf). As expected, embryos treated with ethanol from 2–16 hpf showed only minimal retinal defects. However, embryos treated with ethanol during the 16–24 hpf time window showed thinner optic nerve widths and decreased red-green cone photoreceptor differentiation, as compared to control embryos (Fig. 5B–F). Similar to embryos treated with ethanol from 2–24 hpf, RA co-supplementation with ethanol during 16–24 hpf showed dramatic rescue of the optic nerve widths to near control levels, and were significantly different from embryos treated with only ethanol during this period (Fig. 5C). Embryos co-treated with ethanol and RA showed rescue of photoreceptor differentiation that was significantly different from ethanol-treated embryos (Fig. 5F). Less extensive rescue of ONH and photoreceptor differentiation in the 150 mM ethanol and RA co-treated embryos reflects more severe defects caused by higher ethanol concentrations.
Figure 5. Critical time window of ethanol exposure coincides with RA synthesis induction.
(A) Timeline showing windows of ethanol treatment from 2–16 hpf, 16–24 hpf, and RA treatment from 16–24 hpf.
(B–E) Ethanol treatment from 2–16 hpf showed near normal optic nerve, but exposure from 16–24 hpf showed ONH (white arrowhead, B). (C) Quantification of optic nerve width after ethanol treatment: ethanol alone from 2–16 and 16–24 hpf; ethanol + RA from 16–24 hpf. Embryos treated with ethanol alone from 16–24 hpf showed a significantly reduced optic nerve width, which was rescued by RA co-treatment. (D) TOPRO-3-iodide staining showing retinal lamination: ethanol alone from 2–16 hpf, near normal lamination; ethanol alone from 16–24 hpf, reduced retinal lamination; ethanol + RA from 16–24 hpf, normal lamination. (E) HuC/D and zpr-1 staining showed photoreceptor differentiation at 72 hpf: ethanol alone from 2–16 hpf, near normal photoreceptor; ethanol alone from 16–24 hpf, reduction of photoreceptor (yellow arrow); ethanol + RA from 16–24 hpf, near normal photoreceptor. (F) Quantification of total zpr-1 intensity in the ONL of the retina showed a significant decrease in photoreceptor marker expression after ethanol treatment (16–24 hpf) and subsequent rescue by RA co-treatment.
Error bars indicate standard deviation.
Ethanol-induced disruption of RA-dependent pathways during retinal morphogenesis
To further validate ethanol effects on RA-dependent developmental pathways during retinal morphogenesis, embryos were treated with ethanol during the gastrulation and somitogenesis periods, 2–24 hpf, and supplemented with RA only during the 2–16 or 16–24 hpf time windows (Fig. 6A). If the retinal defects are produced by RA inhibition only during the sensitive time window of 16–24 hpf, then RA supplementation during that time period alone should rescue the ethanol-induced defects. Embryos treated with ethanol from 2–24 hpf and RA from 2–16 hpf exhibited ONH, similar to embryos treated with ethanol alone (2–24 hpf) (Fig. 6B, C, I; E-100 2–24 vs. E-100 2–24 + RA 2–16, p = 0.3350). Embryos treated with ethanol from 2–24 hpf and RA from 16–24 hpf showed significant improvement of optic nerve defects, compared to ethanol-treated embryos (Fig. 6B and C). Presence of RA during the critical time window of 16–24 hpf was sufficient to completely rescue photoreceptor differentiation to normal levels (Fig. 6D). This indicates a strong disruption of RA-dependent developmental events during retinal morphogenesis due to ethanol exposure.
Figure 6. RA independent retinal defects and RA rescue after ethanol exposure.
(A) Timeline showing treatment with ethanol (2–24 hpf) and RA supplement (2–16 or 16–24 hpf).
(B) Embryos treated with ethanol (2–24 hpf) + RA (2–16 or 16–24 hpf) showed severe ONH.
(C) Quantification of optic nerve width showed significant reduction in ethanol-treated embryos and no rescue by RA supplementation during 2–16 hpf. A slight improvement was seen in RA-supplemented embryos from 16–24 hpf. (D, E) Zpr-1 staining intensity analysis showed reduction of photoreceptor differentiation in the embryos treated with ethanol alone (2–24 hpf) and ethanol (2–24 hpf) + RA (2–16 hpf) (yellow arrows); near normal differentiated photoreceptors in embryos treated with ethanol (2–24 hpf) + RA (16–24 hpf).
(F) Timeline showing treatment with ethanol (2–24 hpf) and RA (24–48 hpf and 48–72 hpf).
(G) Acetylated-tubulin staining showed ONH in ethanol-treated embryos, which was rescued by RA supplementation (24–48 hpf). (H) Quantification of optic nerve width showed significant rescue of ethanol-treated embryos with RA supplementation. (I–K) Terminal photoreceptor differentiation assay using zpr-1 staining showed reduced photoreceptor differentiation in embryos treated with ethanol (2–24 hpf; yellow arrow); near normal differentiated photoreceptors in embryos treated with ethanol (2–24 hpf) + RA (24–48 hpf); slight rescue of photoreceptor differentiation in ethanol-treated (2–24 hpf) + RA-treated (48–72 hpf) embryos.
Error bars indicate standard deviation.
Ethanol effects on retinal neurogenesis and photoreceptor development
If ethanol exposure produced a persistent alteration of RA biosynthesis and signaling, then RA supplementation at later stages (after ethanol exposure) should rescue retinal developmental defects caused by earlier ethanol exposure. To test this idea, embryos were ethanol-treated from 2–24 hpf, and RA was supplemented from 24–48 hpf (Fig. 6F). These embryos showed a significant rescue of ONH and photoreceptor differentiation (Fig. 6G–I, K). Interestingly, supplementation with RA during photoreceptor differentiation (48–72 hpf; Fig. 6F) improved expression of terminal red-green cone photoreceptor differentiation marker (Fig. 6J and K), suggesting a trend of photoreceptor differentiation rescue even at this later stage.
Prevention of ethanol-induced retinal defects by folic acid co-supplementation
To test the protective effects of FA on ethanol-induced retinal defects, embryos were co-supplemented with ethanol and 75 µM FA from 2–24 hpf (Fig. 7A). At 48 hpf, ethanol-treated embryos showed a significant reduction in optic nerve width, which was rescued by FA supplementation (Fig. 7B and C). Photoreceptor differentiation defects induced by ethanol exposure (2–24 hpf) were also rescued by FA co-supplementation, as revealed by zpr-1 staining at 72 hpf (Fig. 7D and E). Similarly, ethanol and FA co-treatment during the ethanol-sensitive time window from 16–24 hpf rescued ONH and photoreceptor differentiation effects at 48 and 72 hpf, respectively (Fig. 7G–J). These findings indicate that FA protects ocular morphogenetic events from ethanol toxicity. Comparison of FA and RA co-supplementation regimens during the ethanol-sensitive time window (16–24 hpf) show statistically normal optic nerve and photoreceptor differentiation after FA co-supplementation, as compared to partial rescue after RA co-supplementation (Fig. 5C and F; Fig. 7H and J). This indicated a surprisingly better rescue of ONH and photoreceptor differentiation by FA than RA during the ethanol-sensitive time period.
Figure 7. Folic acid co-supplementation prevented ethanol-induced retinal defects.
(A) Timeline demonstrating folic acid co-supplementation (2–24 hpf).
(B–E) Treatment with ethanol (2–24 hpf) produced ONH (white arrowhead; B), which was rescued by FA co-supplementation. (C) Optic nerve widths measured in acetylated-tubulin stained embryos showed rescue after FA co-supplementation. (D) Terminal photoreceptor differentiation marker expression, zpr-1, in the ONL showed rescue after FA co-treatment (yellow arrow). (E) Quantification of zpr-1 intensity in the ONL.
(F) Timeline showing FA co-treatment during the ethanol-sensitive time-window (16–24 hpf).
(G–J) FA supplementation (16–24 hpf) rescued ethanol-induced ONH and retinal lamination defects. (G, H) Acetylated-tubulin stained optic nerve width measurements showed significant ethanol-induced decrease, which was restored by FA treatment. (I, J) Zpr-1 staining showed photoreceptor differentiation defect was restored by FA co-treatment.
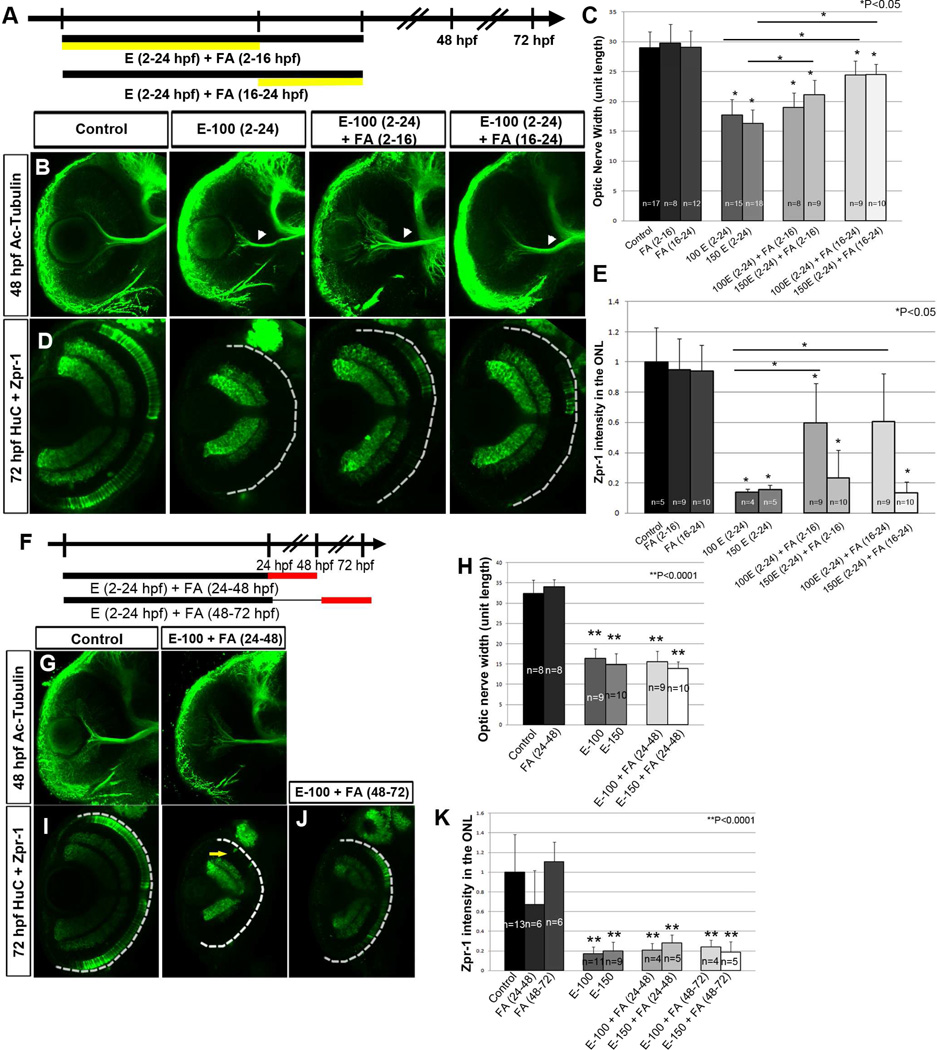
FA supplementation during both gastrulation (2–16 hpf) and retinal morphogenesis (16– 24 hpf) showed moderate rescue of ONH and photoreceptor differentiation defects in comparison to ethanol-treated embryos (2–24 hpf) (Fig. 8A–E). Similar experiments with RA pretreatment (Fig. 6A–E) showed that the 16–24 hpf time point was critical for RA rescue. However, in both RA and FA rescues, presence of ethanol during 2–24 hpf caused severe retinal defects, which were only partially rescued by supplementation during 2–16 or 16–24 hpf alone.
Figure 8. Folic acid rescues ethanol-induced retinal defects but does not restore.
(A) Timeline showing treatment with ethanol (2–24 hpf) and FA supplement (2–16 or 16–24 hpf).
(B) Embryos treated with ethanol (2–24 hpf) + FA (2–16 or 16–24 hpf) showed partial rescue of ONH (C) Quantification of optic nerve width showed slight rescue from FA supplementation during 2–16 hpf and 16–24 hpf. (D, E) Zpr-1 staining intensity analysis showed reduction of photoreceptor differentiation in the embryos treated with ethanol alone (2–24 hpf) and partial rescue in ethanol (2–24 hpf) + FA (2–16 hpf) and ethanol (2–24 hpf) + FA (16–24 hpf).
(F) Timeline showing FA treatment (24–48 and 48–72 hpf) after ethanol exposure (2–24 hpf)
(G–K) (G) Acetylated-tubulin staining showed ONH in ethanol (2–24 hpf) + FA (24–48 hpf) embryos. (H) Quantification of optic nerve width after FA supplementation (24–48 hpf). (I–K) Reduction/absence of photoreceptor differentiation was detected in zpr-1 stained embryos supplemented with FA from 24–48 hpf (I) and 48–72 hpf (J). (K) Quantification of total zpr-1 intensity in the ONL did not show rescue after FA supplementation (48–72 hpf).
Error bars indicate standard deviation.
To determine whether FA treatment following ethanol exposure could reverse ethanol-induced defects, ethanol-treated embryos were supplemented with FA during retinal neurogenesis (24–48 hpf) and photoreceptor differentiation stages (48–72 hpf; Fig. 8F). FA treatment following the ethanol-exposure period (2–24 hpf) during retinal neurogenesis (24– 48 hpf) or photoreceptor differentiation stages (48–72 hpf) did not rescue retinal cell differentiation defects (Fig. 8G–K). These findings reveal differences between RA- and FA-dependent pathways after retinal morphogenesis, as their supplementation during the different stages after ethanol treatment showed varying abilities to remedy ethanol-induced eye defects (Fig. 9).
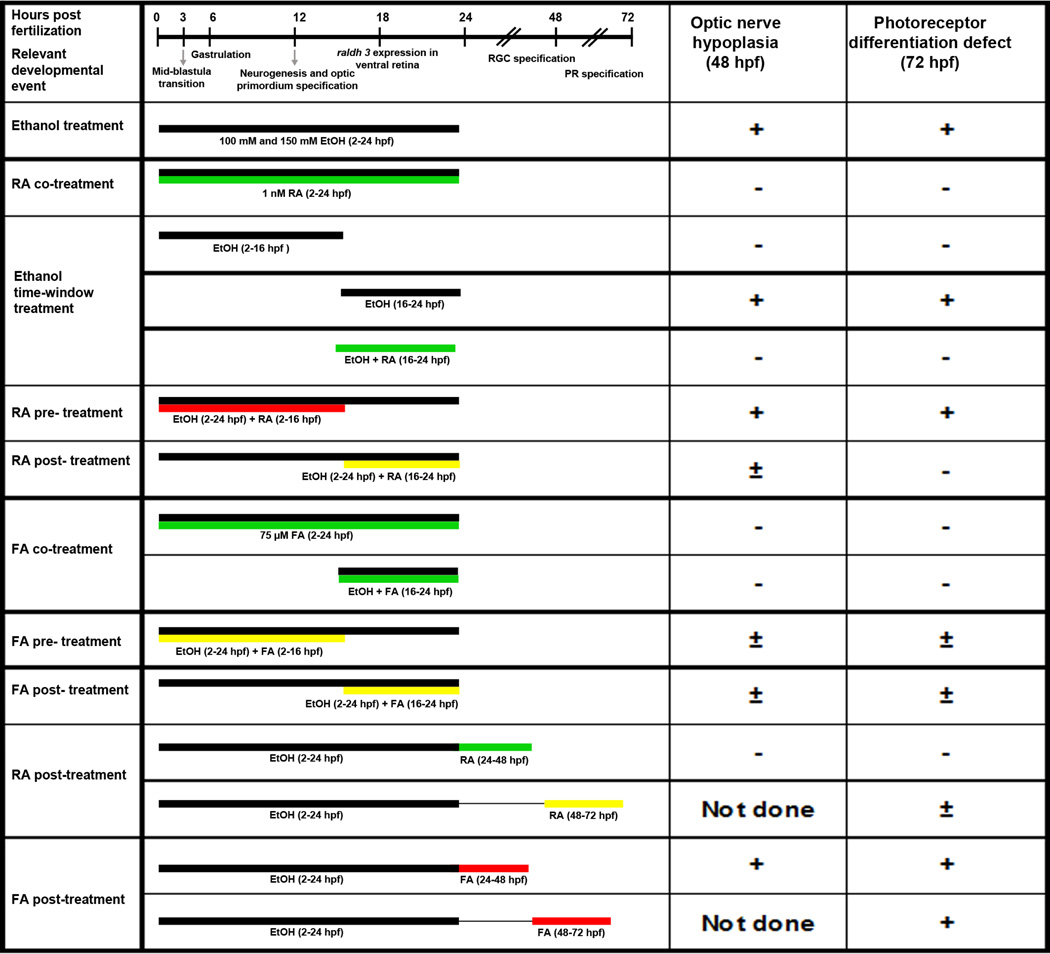
Figure 9. Schematic representation summarizing the various treatments.
The developmental timeline on the top shows numbers in hours post-fertilization (not to scale). Relevant developmental events occurring during that time are highlighted. Black bars represent ethanol exposure. Green bars represent significant rescue of retinal defects, including ONH and photoreceptor differentiation defects. Yellow bars represent partial rescue of retinal defects. Red bars represent little or no rescue. Observed defect is indicated by Positive sign (+); No observed defect is indicated by Negative sign (−); and partial defect is indicated by (±). Not done, experiment was not done because the treatment period was after the required time for the assay.
Discussion
Early development, including gastrulation and neurogenesis, is particularly sensitive to environmental teratogens, such as ethanol, which produce severe defects in the embryo (Gilbert-Barness, 2010; Lipinski et al., 2012). Ethanol is able to cross the placental barrier, allowing direct access to the developing embryo (Guerri & Sanchis, 1985). Ethanol-induced defects are greatly influenced by dose, duration, and genetic background (May & Gossage, 2011). Ethanol effects on development may be influenced by comorbid environmental and nutritional factors. The zebrafish is a useful model to examine ethanol effects on development, and this model allows control over many variables, including nutritional supplements (Ali, van Mil, & Richardson, 2011). Previous zebrafish-FASD model studies demonstrated loss of visual function at higher concentrations of ethanol (1–2% v/v) using electroretinography, due to reduced rod photoreceptor function, leading to scotopic vision loss (Bilotta et al., 2002; Matsui et al., 2006). Other studies showed that persistent microphthalmia resulted from ethanol exposure when ethanol was administered during retinal neurogenesis (24–48 hpf), showing slight rescue of photoreceptor differentiation by RA treatment (Kashyap et al., 2007; Kashyap, Frey, & Stenkamp, 2011). However, these studies did not differentiate particular retinal cell specification and differentiation events.
Our laboratory previously showed that treating zebrafish embryos with lower ethanol concentrations (0.6 and 0.9% v/v) produced reproducible defects in gastrulation cell movements, cell adhesion, and heart morphogenesis (Marrs et al., 2010; Sarmah & Marrs, 2013; Sarmah et al., 2013). Abnormal retinal cell differentiation events may be due to a combination of increased oxidative stress, increased cell death, disrupted cell signaling pathways, and altered epigenetic modifications induced by ethanol exposure (Muralidharan, Sarmah, Zhou, & Marrs, 2013). This study differentiates retinal developmental defects produced by ethanol exposure during midblastula transition, gastrulation, neurulation, and somitogenesis (2–24 hpf), and highlights an ethanol-sensitive critical time window during retinal morphogenesis (16–24 hpf). Rescue strategies designed in this study identified severe and persistent disruption of RA biosynthesis and signaling by ethanol exposure. Experiments tested the effect of nutritional supplements such as RA and FA during and after ethanol exposure. Significantly, this is the first study showing significant rescue of ethanol-induced retinal morphogenesis and differentiation defects using FA. Our experiments highlight the potential influence of nutritional status on teratogen exposure effects.
Ethanol severely perturbs retinal cell differentiation
Our experiments show that ethanol treatment during zebrafish early development affected expression patterns of critical genes involved in retinal development and morphogenesis, which may contribute to ocular defects. Measurement of these cellular defects allowed us to compare effects of different treatment regimens on specific cell differentiation events. Previous studies used eye diameter and optomotor response assays to compare several ethanol-exposure time window treatments, showing significant microphthalmia in embryos treated with ethanol during gastrulation and retinal morphogenesis (6–24 hpf), and similar defects when treated during retinal morphogenesis (12–24 hpf), highlighting this ethanol-sensitive developmental period (Bilotta et al., 2002). Increased susceptibility to ethanol during retinal morphogenetic stages is possibly due to disruption of multiple developmental pathways, including effects on epigenetic modifications and critical gene expression occurring at these times. Our studies showed that severe defects were produced by exposure during early retinal morphogenesis (16–24 hpf), resembling defects produced when ethanol was present during the entire gastrulation and retinal morphogenesis periods. Significantly, RA and FA co-supplementation could rescue retinal defects during this early retinal morphogenesis period. FA co-supplementation was more effective than RA, even during this critical time window (16–24 hpf) that coincides with RA biosynthesis when raldh3 expression is initiated. One-carbon metabolism and antioxidant functions of FA may protect the retina from developmental defects during the early retinal morphogenesis time window. Additional experiments will be needed to identify common developmental mechanisms protected by RA and FA supplements.
Microphthalmia could result from disrupting various processes, including initial retinal differentiation induction, cell death, and cell proliferation. Ethanol increased cell death and cell proliferation rates, but no difference was detected in the induction of ganglion or photoreceptor cell differentiation, indicating that increased cell death was a major factor contributing to the microphthalmia phenotype. Apoptosis rates were not significantly higher immediately following ethanol treatment (at 24 hpf), but were significantly increased at later stages of cell specification and neurogenesis. Cell proliferation rates, however, were higher at later stages (at 48 and 72 hpf). The lag between cell death and cell proliferation suggests a cause and effect relationship indicating that cell proliferation is compensatory. This suggests that ethanol effects on gene expression mechanisms during retinal specification and differentiation lead to increased apoptosis, rather than inducing acute cellular toxicity. Perhaps, the effects of ethanol prevent progression of normal terminal differentiation leading to cell death, accompanied by compensatory cell proliferation.
Effect of ethanol on RA signaling during retinal development
Several investigators proposed inhibitory effects of ethanol on RA biosynthesis. Experiments using Xenopus embryos showed phenotypic similarities between ethanol treatments and RA signaling inhibitors or RALDH gene knockdown (Yelin et al., 2005). Ethanol exposure also produced downregulation of RA-responsive hox gene expression and early limb genes (Johnson et al., 2007; Kot-Leibovich & Fainsod, 2009). Rescue of many craniofacial features and cardiac defects by RA supplementation in zebrafish strongly suggests that RA deficiency is induced by ethanol exposure in developing embryos (Marrs et al., 2010; Sarmah & Marrs, 2013). The present study demonstrates rescue of ethanol-induced retinal defects by co-supplementing RA at low concentrations. If RA synthesis inhibition was partially responsible for the FASD phenotype, then ethanol-sensitive developmental stages should coincide with the onset of RA signaling in the retina at 18 hpf. Indeed, a critical time window for ethanol-sensitive retinal defects coincides with RA signaling (16–24 hpf). RA rescue of photoreceptor differentiation and optic nerve defects during the 16–24 hpf critical time window showed that ethanol-induced defects were masked by RA supplementation. However, the retinal defects induced by ethanol were not limited to this developmental period. Treating embryos with ethanol from 2–24 hpf and co-supplementing with RA during 16–24 hpf did not rescue all ethanol-induced defects, indicating that retinal developmental pathways other than RA signaling were also affected by ethanol. Persistent RA deficiency and signaling was indicated by rescue of optic nerve defects by RA treatment (24–48 hpf) after ethanol treatment from 2–24 hpf, and rescue of photoreceptor differentiation defects by RA treatment (24–48 and 48–72 hpf) after ethanol treatment from 2– 24 hpf.
RA signaling influences photoreceptor differentiation and patterning in the retina. Studies showed that exogenous RA promoted rods and red cones and decreased blue and UV cones without significantly affecting green cones, and vice versa when RA signaling was inhibited using specific chemical inhibitors (Hyatt et al., 1996; Prabhudesai et al., 2005). Thus, opsin expression patterns after ethanol treatment, showing downregulation of rhodopsin and red opsin and upregulation of UV opsin expression, indicate that ethanol treatment produced decreased RA levels during photoreceptor differentiation. Restoration of red-green double cones by RA supplementation at later stages (24–48 and 48–72 hpf) suggests persistent reduction in RA signaling due to ethanol exposure during early stages (2–24 hpf). This reveals a possibility of reducing the severity of retinal defects caused by ethanol exposure by improving nutrition during later stages of development.
Effect of ethanol on retinal photoreceptor progenitor and precursor cells
In an uninjured teleost retina, retinal stem cells that generate new retinal tissue reside in the CMZ (Wehman, Staub, Meyers, Raymond, & Baier, 2005). Slowly dividing progenitors in the INL generate rapidly dividing precursors in the ONL (Julian, Ennis, & Korenbrot, 1998; Otteson, D'Costa, & Hitchcock, 2001; Raymond & Rivlin, 1987). Müller glial cells are also stem cells in the central retina since they proliferate in response to injury, producing rod precursors (Yurco & Cameron, 2005). Upregulation of rx1 gene expression and increased phospho-H3-stained cells in specific laminae of the retina following ethanol treatment suggest that damage due to ethanol exposure triggers expansion of retinal progenitor populations. Initial ethanol responses at 48 hpf showed an increased cell proliferation in the ONL and INL, indicating an activation of progenitor and precursor cells. At later stages (72 hpf), an ethanol-induced increase in cell proliferation was seen in the CMZ, which may represent ongoing proliferation of retinal stem cells that may be needed to replenish the progenitor and precursor populations. Analysis of these cell populations will provide valuable insights into ethanol teratogenesis mechanisms.
A recent study on zebrafish embryos showed that ethanol exposure prevented cell cycle exit in the retina (Chung et al., 2013). Photoreceptor differentiation after exogenous RA application indicates that there are persistent precursor and progenitor cells in the ethanol-treated embryo retina that can differentiate. The differentiation status of these precursors still needs to be defined. However, presence of RA is sufficient to induce their terminal differentiation. Together, these data indicate an increased number of precursor cells in the ONL and INL lacking appropriate signals for terminal differentiation. Abnormal terminal differentiation may lead to the increased apoptosis observed at 40 hpf. Our results provide evidence that ethanol induces persistent defects in retinal stem and progenitor cell populations, which prevent complete regeneration of normal retinal tissue. RA may be an important missing signal, supplementation of which leads to restoration of photoreceptor differentiation. Interestingly, RA supplementation during the period when increased apoptosis was observed (24–48 hpf) could restore retinal defects to near normal levels in comparison to later supplementation (48–72 hpf).
Preventive role of FA in FASD
FA functions as a cofactor for methyl group transfers in 1-carbon metabolism, and FA has antioxidant properties, affecting a wide range of developmental processes. In the present study, FA co-supplementation with ethanol during 2–24 hpf and 16–24 hpf rescued retinal defects. FA supplementation (2–16 and 16–24 hpf) during ethanol exposure (2–24 hpf) could partially rescue ethanol-induced defects. A similar experiment with RA showed no rescue during 2–16 hpf and partial rescue from 16–24 hpf, potentially due to activation of RA biosynthesis starting at 18 hpf. Unlike RA supplementation, FA rescue was effective during 2–16 hpf and 16– 24 hpf time windows. This indicates that mechanisms underlying RA and FA rescue are different. FA supplementation (24–48 hpf or 48–72 hpf) following ethanol treatments (2–24 hpf) did not rescue retinal defects, showing that, in contrast to RA supplementation, FA supplementation could protect retinal morphogenesis only during ethanol treatment. Although the molecular details underlying FA rescue are unclear, exogenous FA may prevent abnormal epigenetic processes, and may prevent disruption by reactive oxygen species generated by ethanol exposure (Brocardo et al., 2011; Zhou et al., 2011). Additional studies will be required to dissect the differences between RA and FA supplementation and explore the potential protective and therapeutic benefits.
A previous study from our laboratory showed prevention of cardiac defects induced by ethanol exposure by FA co-supplementation (Sarmah & Marrs, 2013). FA co-supplementation rescued defects in both myocardium and endocardium, whereas RA co-supplementation with ethanol did not rescue endocardial cushion formation defects. During eye development, where RA plays a critical role in the differentiation and maturation of photoreceptors, RA was able to remedy retinal developmental defects even after ethanol exposure ended. These findings highlight organ-to-organ differences in ethanol-sensitive signaling and timing, illustrating complexity of the underlying molecular basis of FASD. Despite this complexity, nutritional status heavily influences birth defect type and severity.
In summary, this study illustrates novel cellular mechanisms of ethanol teratogenicity during ocular development. Our experiments also reveal therapeutic potential of specific molecules that stimulate developmental signaling pathways. Additional research examining epigenetic changes, apoptosis, and signaling pathway disruptions will allow us to identify specific targets of ethanol during development, and facilitate design of therapeutic and preventive measures for this frequent and devastating disorder.
Highlights.
A zebrafish FASD model showed retinal defects similar to those seen in FASD patients.
Ethanol exposure severely disrupted retinal morphogenesis.
Retinoic acid and folic acid co-supplementation rescued ethanol-induced retinal cell differentiation defects.
Retinoic acid supplementation but not folic acid rescued retinal cell differentiation defects after ethanol exposure.
Acknowledgments
National Institutes of Health P50 AA07611 project (PI, David Crabb) and R21 AA0022396 to J.A.M. supported these studies. Plasmid probes to detect rx1, shh, crx, otx5, neuroD, rhodopsin, UV opsin, and red opsin were generously provided by Drs. Pamela Raymond (University of Michigan), Stephen Ekker (Mayo Clinic Cancer Center), Qin Liu (University of Akron), and Yuk Fai Leung (Purdue University). We thank M. Farrell and members of the Marrs laboratory for helpful discussion.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing interests
The authors declare no conflict of interest.
References
- Ali S, van Mil HG, Richardson MK. Large-scale assessment of the zebrafish embryo as a possible predictive model in toxicity testing. PLoS One. 2011;6:e21076. doi: 10.1371/journal.pone.0021076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arenzana FJ, Carvan MJ, 3rd, Aijón J, Sánchez-González R, Arévalo R, Porteros A. Teratogenic effects of ethanol exposure on zebrafish visual system development. Neurotoxicology and Teratology. 2006;28:342–348. doi: 10.1016/j.ntt.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Ballard MS, Sun M, Ko J. Vitamin A, folate, and choline as a possible preventive intervention to fetal alcohol syndrome. Medical Hypotheses. 2012;78:489–493. doi: 10.1016/j.mehy.2012.01.014. [DOI] [PubMed] [Google Scholar]
- Bilotta J, Saszik S, Givin CM, Hardesty HR, Sutherland SE. Effects of embryonic exposure to ethanol on zebrafish visual function. Neurotoxicology and Teratology. 2002;24:759–766. doi: 10.1016/s0892-0362(02)00319-7. [DOI] [PubMed] [Google Scholar]
- Brocardo PS, Gil-Mohapel J, Christie BR. The role of oxidative stress in fetal alcohol spectrum disorders. Brain Research Reviews. 2011;67:209–225. doi: 10.1016/j.brainresrev.2011.02.001. [DOI] [PubMed] [Google Scholar]
- Chung HY, Chang CT, Young HW, Hu SP, Tzou WS, Hu CH. Ethanol inhibits retinal and CNS differentiation due to failure of cell cycle exit via an apoptosis-independent pathway. Neurotoxicology and Teratology. 2013;38:92–103. doi: 10.1016/j.ntt.2013.05.006. [DOI] [PubMed] [Google Scholar]
- Clendenon SG, Sarmah S, Shah B, Liu Q, Marrs JA. Zebrafish cadherin-11 participates in retinal differentiation and retinotectal axon projection during visual system development. Developmental Dynamics. 2012;241:442–454. doi: 10.1002/dvdy.23729. [DOI] [PubMed] [Google Scholar]
- Duester G. A hypothetical mechanism for fetal alcohol syndrome involving ethanol inhibition of retinoic acid synthesis at the alcohol dehydrogenase step. Alcoholism: Clinical and Experimental Research. 1991;15:568–572. doi: 10.1111/j.1530-0277.1991.tb00562.x. [DOI] [PubMed] [Google Scholar]
- Gilbert-Barness E. Teratogenic causes of malformations. Annals of Clinical and Laboratory Science. 2010;40:99–114. [PubMed] [Google Scholar]
- Guerri C, Sanchis R. Acetaldehyde and alcohol levels in pregnant rats and their fetuses. Alcohol. 1985;2:267–270. doi: 10.1016/0741-8329(85)90057-6. [DOI] [PubMed] [Google Scholar]
- Heaton MB, Paiva M, Siler-Marsiglio K. Ethanol influences on Bax translocation, mitochondrial membrane potential, and reactive oxygen species generation are modulated by vitamin E and brain-derived neurotrophic factor. Alcoholism: Clinical and Experimental Research. 2011;35:1122–1133. doi: 10.1111/j.1530-0277.2011.01445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hug TE, Fitzgerald KM, Cibis GW. Clinical and electroretinographic findings in fetal alcohol syndrome. Journal of AAPOS. 2000;4:200–204. doi: 10.1067/mpa.2000.105278. [DOI] [PubMed] [Google Scholar]
- Hutson JR, Stade B, Lehotay DC, Collier CP, Kapur BM. Folic acid transport to the human fetus is decreased in pregnancies with chronic alcohol exposure. PLoS One. 2012;7:e38057. doi: 10.1371/journal.pone.0038057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyatt GA, Schmitt EA, Fadool JM, Dowling JE. Retinoic acid alters photoreceptor development in vivo. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:13298–13303. doi: 10.1073/pnas.93.23.13298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim W, Tousson E, El-Masry T, Arafa N, Akela M. The effect of folic acid as an antioxidant on the hypothalamic monoamines in experimentally induced hypothyroid rat. Toxicology and Industrial Health. 2012;28:253–261. doi: 10.1177/0748233711410913. [DOI] [PubMed] [Google Scholar]
- Johnson CS, Zucker RM, Hunter ES, 3rd, Sulik KK. Perturbation of retinoic acid (RA)-mediated limb development suggests a role for diminished RA signaling in the teratogenesis of ethanol. Birth Defects Research. Part A, Clinical and Molecular Teratology. 2007;79:631–641. doi: 10.1002/bdra.20385. [DOI] [PubMed] [Google Scholar]
- Joshi R, Adhikari S, Patro BS, Chattopadhyay S, Mukherjee T. Free radical scavenging behavior of folic acid: evidence for possible antioxidant activity. Free Radical Biology & Medicine. 2001;30:1390–1399. doi: 10.1016/s0891-5849(01)00543-3. [DOI] [PubMed] [Google Scholar]
- Julian D, Ennis K, Korenbrot JI. Birth and fate of proliferative cells in the inner nuclear layer of the mature fish retina. The Journal of Comparative Neurology. 1998;394:271–282. [PubMed] [Google Scholar]
- Kamen B. Folate and antifolate pharmacology. Seminars in Oncology. 1997;24:S18-S30–S18-S39. [PubMed] [Google Scholar]
- Kashyap B, Frederickson LC, Stenkamp DL. Mechanisms for persistent microphthalmia following ethanol exposure during retinal neurogenesis in zebrafish embryos. Visual Neurosciences. 2007;24:409–421. doi: 10.1017/S0952523807070423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashyap B, Frey RA, Stenkamp DL. Ethanol-induced microphthalmia is not mediated by changes in retinoic acid or sonic hedgehog signaling during retinal neurogenesis. Alcoholism: Clinical and Experimental Research. 2011;35:1644–1661. doi: 10.1111/j.1530-0277.2011.01511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz LM, Fox DA. Prenatal ethanol exposure alters scotopic and photopic components of adult rat electroretinograms. Investigative Ophthalmology & Visual Science. 1991;32:2861–2872. [PubMed] [Google Scholar]
- Kot-Leibovich H, Fainsod A. Ethanol induces embryonic malformations by competing for retinaldehyde dehydrogenase activity during vertebrate gastrulation. Disease Models & Mechanisms. 2009;2:295–305. doi: 10.1242/dmm.001420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laranjeiro R, Whitmore D. Transcription factors involved in retinogenesis are coopted by the circadian clock following photoreceptor differentiation. Development. 2014;141:2644–2656. doi: 10.1242/dev.104380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieber CS. Relationships between nutrition, alcohol use, and liver disease. Alcohol Research & Health. 2003;27:220–231. [PMC free article] [PubMed] [Google Scholar]
- Lipinski RJ, Hammond P, O'Leary-Moore SK, Ament JJ, Pecevich SJ, Jiang Y, et al. Ethanol-induced face-brain dysmorphology patterns are correlative and exposure-stage dependent. PLoS One. 2012;7:e43067. doi: 10.1371/journal.pone.0043067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupo G, Liu Y, Qiu R, Chandraratna RA, Barsacchi G, He RQ, et al. Dorsoventral patterning of the Xenopus eye: a collaboration of Retinoid, Hedgehog and FGF receptor signaling. Development. 2005;132:1737–1748. doi: 10.1242/dev.01726. [DOI] [PubMed] [Google Scholar]
- Ma AC, Chung MI, Liang R, Leung AY. A DEAB-sensitive aldehyde dehydrogenase regulates hematopoietic stem and progenitor cells development during primitive hematopoiesis in zebrafish embryos. Leukemia. 2010;24:2090–2099. doi: 10.1038/leu.2010.206. [DOI] [PubMed] [Google Scholar]
- Maestro-de-las-Casas C, Pérez-Miguelsanz J, López-Gordillo Y, Maldonado E, Partearroyo T, Varela-Moreiras G, et al. Maternal folic acid-deficient diet causes congenital malformations in the mouse eye. Birth Defects Research. Part A, Clinical and Molecular Teratology. 2013;97:587–596. doi: 10.1002/bdra.23176. [DOI] [PubMed] [Google Scholar]
- Marrs JA, Clendenon SG, Ratcliffe DR, Fielding SM, Liu Q, Bosron WF. Zebrafish fetal alcohol syndrome model: effects of ethanol are rescued by retinoic acid supplement. Alcohol. 2010;44:707–715. doi: 10.1016/j.alcohol.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason JB, Choi SW. Effects of alcohol on folate metabolism: implications for carcinogenesis. Alcohol. 2005;35:235–241. doi: 10.1016/j.alcohol.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Matsui JI, Egana AL, Sponholtz TR, Adolph AR, Dowling JE. Effects of ethanol on photoreceptors and visual function in developing zebrafish. Investigative Ophthalmology & Visual Science. 2006;47:4589–4597. doi: 10.1167/iovs.05-0971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Gossage JP. Maternal risk factors for fetal alcohol spectrum disorders: not as simple as it might seem. Alcohol Research & Health. 2011;34:15–26. [PMC free article] [PubMed] [Google Scholar]
- May PA, Gossage JP, Kalberg WO, Robinson LK, Buckley D, Manning M, et al. Prevalence and epidemiologic characteristics of FASD from various research methods with an emphasis on recent in-school studies. Developmental Disabilities Research Reviews. 2009;15:176–192. doi: 10.1002/ddrr.68. [DOI] [PubMed] [Google Scholar]
- Mezey E, Holt PR. The inhibitory effect of ethanol on retinol oxidation by human liver and cattle retina. Experimental and Molecular Pathology. 1971;15:148–156. doi: 10.1016/0014-4800(71)90095-5. [DOI] [PubMed] [Google Scholar]
- Mitchell JJ, Paiva M, Heaton MB. Vitamin E and beta-carotene protect against ethanol combined with ischemia in an embryonic rat hippocampal culture model of fetal alcohol syndrome. Neuroscience Letters. 1999;263:189–192. doi: 10.1016/s0304-3940(99)00144-5. [DOI] [PubMed] [Google Scholar]
- Muralidharan P, Sarmah S, Zhou FC, Marrs JA. Fetal alcohol spectrum disorder (FASD) associated neural defects: complex mechanisms and potential therapeutic targets. Brain Sciences. 2013;3:964–991. doi: 10.3390/brainsci3020964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkubo Y, Chiang C, Rubenstein JL. Coordinate regulation and synergistic actions of BMP4, SHH and FGF8 in the rostral prosencephalon regulate morphogenesis of the telencephalic and optic vesicles. Neuroscience. 2002;111:1–17. doi: 10.1016/s0306-4522(01)00616-9. [DOI] [PubMed] [Google Scholar]
- Otteson DC, D'Costa AR, Hitchcock PF. Putative stem cells and the lineage of rod photoreceptors in the mature retina of the goldfish. Developmental Biology. 2001;232:62–76. doi: 10.1006/dbio.2001.0163. [DOI] [PubMed] [Google Scholar]
- Prabhudesai SN, Cameron DA, Stenkamp DL. Targeted effects of retinoic acid signaling upon photoreceptor development in zebrafish. Developmental Biology. 2005;287:157–167. doi: 10.1016/j.ydbio.2005.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond PA, Rivlin PK. Germinal cells in the goldfish retina that produce rod photoreceptors. Developmental Biology. 1987;122:120–138. doi: 10.1016/0012-1606(87)90338-1. [DOI] [PubMed] [Google Scholar]
- Rhinn M, Dollé P. Retinoic acid signalling during development. Development. 2012;139:843–858. doi: 10.1242/dev.065938. [DOI] [PubMed] [Google Scholar]
- Sarmah S, Barrallo-Gimeno A, Melville DB, Topczewski J, Solnica-Krezel L, Knapik EW. Sec24D-dependent transport of extracellular matrix proteins is required for zebrafish skeletal morphogenesis. PLoS One. 2010;5:e10367. doi: 10.1371/journal.pone.0010367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarmah S, Marrs JA. Complex cardiac defects after ethanol exposure during discrete cardiogenic events in zebrafish: prevention with folic acid. Developmental Dynamics. 2013;242:1184–1201. doi: 10.1002/dvdy.24015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarmah S, Muralidharan P, Curtis CL, McClintick JN, Buente BB, Holdgrafer DJ, et al. Ethanol exposure disrupts extraembryonic microtubule cytoskeleton and embryonic blastomere cell adhesion, producing epiboly and gastrulation defects. Biology Open. 2013;2:1013–1021. doi: 10.1242/bio.20135546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano M, Han M, Brinez P, Linask KK. Fetal alcohol syndrome: cardiac birth defects in mice and prevention with folate. American Journal of Obstetrics and Gynecology. 2010;203:75.e7–75.e15. doi: 10.1016/j.ajog.2010.03.017. [DOI] [PubMed] [Google Scholar]
- Singh RP, Shiue K, Schomberg D, Zhou FC. Cellular epigenetic modifications of neural stem cell differentiation. Cell Transplantation. 2009;18:1197–1211. doi: 10.3727/096368909X12483162197204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strömland K, Pinazo-Durán MD. Ophthalmic involvement in the fetal alcohol syndrome: clinical and animal model studies. Alcohol and Alcoholism. 2002;37:2–8. doi: 10.1093/alcalc/37.1.2. [DOI] [PubMed] [Google Scholar]
- Taruscio D, Carbone P, Granata O, Baldi F, Mantovani A. Folic acid and primary prevention of birth defects. Biofactors. 2011;37:280–284. doi: 10.1002/biof.175. [DOI] [PubMed] [Google Scholar]
- Thomas JD, Idrus NM, Monk BR, Dominguez HD. Prenatal choline supplementation mitigates behavioral alterations associated with prenatal alcohol exposure in rats. Birth Defects Research. Part A, Clinical and Molecular Teratology. 2010;88:827–837. doi: 10.1002/bdra.20713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LL, Zhang Z, Li Q, Yang R, Pei X, Xu Y, et al. Ethanol exposure induces differential microRNA and target gene expression and teratogenic effects which can be suppressed by folic acid supplementation. Human Reproduction. 2009;24:562–579. doi: 10.1093/humrep/den439. [DOI] [PubMed] [Google Scholar]
- Wehman AM, Staub W, Meyers JR, Raymond PA, Baier H. Genetic dissection of the zebrafish retinal stem-cell compartment. Developmental Biology. 2005;281:53–65. doi: 10.1016/j.ydbio.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Westerfield M. The Zebrafish Book. Eugene, OR: The University of Oregon Press; 2000. [Google Scholar]
- Yelin R, Schyr RB, Kot H, Zins S, Frumkin A, Pillemer G, et al. Ethanol exposure affects gene expression in the embryonic organizer and reduces retinoic acid levels. Developmental Biology. 2005;279:193–204. doi: 10.1016/j.ydbio.2004.12.014. [DOI] [PubMed] [Google Scholar]
- Yurco P, Cameron DA. Responses of Müller glia to retinal injury in adult zebrafish. Vision Research. 2005;45:991–1002. doi: 10.1016/j.visres.2004.10.022. [DOI] [PubMed] [Google Scholar]
- Zhou FC, Balaraman Y, Teng M, Liu Y, Singh RP, Nephew KP. Alcohol alters DNA methylation patterns and inhibits neural stem cell differentiation. Alcoholism: Clinical and Experimental Research. 2011;35:735–746. doi: 10.1111/j.1530-0277.2010.01391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]