Abstract
Background & Aims
Weight loss following pharmacotherapy varies greatly. We aimed to examine associations of quantitative gastrointestinal and psychological traits with obesity, and to validate the ability of these traits to predict responses of obese individuals to pharmacotherapy.
Methods
In a prospective study, we measured gastric emptying (GE) of solids and liquids, fasting and postprandial gastric volume, satiation by nutrient drink test (volume to fullness and maximal tolerated volume), satiety following an ad-libitum buffet meal, gastrointestinal hormones, and psychological traits in 328 normal weight, overweight, or obese adults. We also analyzed data from 181 previously studied adults to assess associations between a subset of traits with body mass index and waist circumference. Latent dimensions associated with overweight or obesity were appraised by principal component analyses. We performed a proof-of-concept, placebo-controlled trial of extended-release phentermine and topiramate in 24 patients, to validate associations between quantitative traits and response to weight-loss therapy.
Results
In the prospective study, obesity was associated with fasting gastric volume (P=.03), accelerated GE (P<.001 for solids and P=.011 for liquids), lower postprandial levels of peptide tyrosine tyrosine (P=.003), and higher postprandial levels of glucagon-like peptide 1 (P<.001). In a combined analysis of data from all studies, obesity was associated with higher volume to fullness (n=509; P=.038) and satiety with abnormal waist circumference (n=271; P=.016). Principal component analysis identified latent dimensions that accounted for ∼81% of the variation among overweight and obese subjects, including satiety or satiation (21%), gastric motility (14%), psychological factors (13%), and gastric sensorimotor factors (11%). The combination of phentermine and topiramate caused significant weight loss, slowed GE, and decreased calorie intake; weight loss in response to phentermine and topiramate was significantly associated with calorie intake at the prior satiety test.
Conclusion
Quantitative traits are associated with high body mass index; they can distinguish obesity phenotypes and, in a proof-of-concept clinical trial, predicted response to pharmacotherapy for obesity.
Keywords: BMI, incretin, satietyphentermine, topiramate
Background
Obesity prevalence continues to increase worldwide1 and, in the United States, 69% of adults are overweight or obese2. Despite advances in understanding of some aspects of obesity pathophysiology, weight loss with current non-surgical treatments including diet, exercise and medications is highly variable3. For example, the high dose of extended release (ER) phentermine-topiramate, a recently approved medication for management of obesity, was associated with an average weight loss of 9.8%; however, only 48% of patients lost more than 10% of body weight, whereas 30% of patients lost less than 5% of body weight4.
Little is known about quantitative traits that predispose to weight gain and predict weight loss in response to non-surgical therapy5. Previous retrospective studies have identified specific individual demographic factors were independent predictors of successful weight loss. For example, older age, race, older age when first overweight, fewer self-implemented weight loss attempts, greater exercise self-efficacy, greater dietary restraint, fewer fat-related dietary behaviors, more sedentary activity level were independent predictors of successful weight loss in the Diabetes Prevention Program6, the sibutramine - STORM trial7 and the Weight loss maintenance trial8. Additionally, psychological and behavioral variables have been used to predict successful weight loss in women9, 10. However, the predictors of clinically-relevant weight loss with currently available obesity pharmacotherapy are unclear, after the withdrawal of sibutramine.
The gastrointestinal tract is essential in the regulation of food intake, a key component of the energy balance. The gastrointestinal system is the site of origin of satiation and satiety signals, which communicate and interact with the brain and other organs involved in energy homeostasis. The control of food intake, that is meal size and frequency of meals, is a major factor in the determination of the individual's weight status11. The most simplistic equation of obesity is based on the imbalance between caloric intake and insufficient energy expenditure. Despite the assumption that increased food intake results in higher body weight and/or BMI, this has not been demonstrated in controlled environment, such as the laboratory setting. This lack of correlation between food intake and body weight is attributed to behavioral inhibition of food intake by obese individuals in a testing environment12. Hence, it is still necessary to prove that individuals with higher BMI consume more calories and to appraise quantitative physiological and psychological factors that account for any discriminant factors.
Gastrointestinal functions such as gastric emptying and gastric volume influence food intake13 and they may therefore influence body weight. However, studies of gastric emptying in obesity have shown highly divergent or contradictory results. The vast majority of prior studies of gastrointestinal functions involved small cohorts14, 15 and used poorly validated methods16. In a prior study in 48 overweight or obese patients, we reported that obesity is associated with either normal17 or, paradoxically, lower postprandial gastric volume measured by noninvasive imaging (single photon emission computed tomography [SPECT]) and lower maximal tolerated volume in a satiation test18. On the other hand, bulimic patients have greater gastric volume19. Among psychological traits, higher anxiety and depression scores are associated with less weight loss after lifestyle modification programs20. Given these contradictory results on the associations of gastric functions with higher BMI in the published literature, it is essential to study a large and representative cohort of overweight and obese individuals, and also to appraise the potential association with psychological traits. In addition, since abdominal obesity, as defined by an abnormal waist circumference, is associated with metabolic syndrome and diabetes independently of body weight and BMI21, we also appraised the quantitative GI traits relative to waist circumference.
Our hypotheses were: first, there are definable phenotypes of obesity based on quantitative gastrointestinal physiological and psychological or behavioral traits; second, physiological traits predict short-term weight loss response to pharmacotherapy in obesity. Our three aims were: to examine the association of quantitative gastrointestinal and psychological traits with body mass index (BMI) and waist circumference; to identify latent dimensions in obesity; and to validate the use of quantitative traits in predicting response to a specific pharmacotherapeutic agent approved for treatment of obesity.
Materials and Methods
Study Design
Our study involved three cohorts comprising a total of 509 predominantly (91%) Caucasian adults of normal weight, overweight or obese [based on World Health Organization (WHO) classification]. We measured gastrointestinal and psychological traits in a prospectively studied cohort of 328 adults (cohort 1). In addition, we incorporated a databank of 181 normal weight, overweight or obese adults who had previously undergone the same measurements prior to any intervention using the same methods in our laboratory (cohort 2)18, 22-29. We assessed the association of the gastrointestinal and psychological traits with BMI and waist circumference. Latent dimensions were sought using principal component analysis in 231 participants in whom all the quantitative and psychological traits were prospectively collected. The third cohort consisted of 24 patients (randomly selected from the prospective cohort) who consented to participate in a randomized, placebo-controlled trial of the effects of phentermine-topiramate-ER on weight loss and quantitative traits. These data also served to appraise the ability of 5 preselected quantitative traits to predict weight loss in response to phentermine-topiramate-ER.
All protocols were approved by the Mayo Clinic Institutional Review Board, and research authorization to use data from the medical record was checked for all participants.
Participants
The entire study cohort consisted of 509 adults of normal weight (BMI 18-24.9kg/m2; n=85), overweight (BMI 25-29.9kg/m2; n=158), obesity class I (BMI 30-34.9kg/m2; n=135), or obesity class II or III (BMI ≥35kg/m2; n=131). The waist circumference was classified as normal (women <88cm and men <102cm) or abnormal (based on WHO classification). Anthropometric measurements were done during the screening visit in the morning, in a non-fasting state. Waist and hip circumferences at the end of normal expiration were obtained by trained physicians following the WHO guidelines.30 All participants were recruited by public advertisement as described elsewhere18. The main inclusion criteria were: men or women with body mass index >18kg/m2, age 18 years or older, and not on current treatment for other diseases other than hypothyroidism. Exclusion criteria were: a positive history of any systemic disease, concurrent treatment of gastrointestinal motility or psychological disorders (eating disorder, anxiety and depression) or weight loss medications. Permitted medications were stable doses (for at least 30 days prior to the studies) of birth control pills, estrogen, and L-thyroxine replacement. Women of childbearing potential had a negative pregnancy test within 48 hours of any test involving radioisotopes.
Quantitative Gastrointestinal, Behavioral and Psychological Traits
On different days, participants attended the Mayo Clinic Clinical Research Unit at 7:00 a.m. after an 8-hour fasting period, and the following validated quantitative traits were performed as in prior studies: Gastric emptying of solids and liquids by scintigraphy18; fasting and postprandial gastric volume by a validated SPECT31-33; satiation by nutrient drink test with Ensure® (Abbott Laboratories, Abbott Park, IL 60064)36; satiety by ad-libitum buffet meal to measure total caloric intake and macronutrient distribution in the chosen food18; and selected plasma gastrointestinal hormones18. These methods are described in detail in the Supplementary Appendix.
Self-administered questionnaires assessing affect, exercise performance, attitudes, satisfaction with body image, and eating behavior37-42 (details in Supplementary Appendix)
These psychological and behavioral traits were assessed by Hospital Anxiety and Depression Inventory37, AUDIT-C Alcoholism Screening Test41, Questionnaire on Eating and Weight Patterns-Revised40, the Multidimensional Body-Self Relations Questionnaire38, the Weight Efficacy Life-Style Questionnaire43, and the Physical Activity Stages of Change Questionnaire42. Each participant completed a series of validated questionnaires during their screening visit and after informed consent was signed; two participants had high anxiety levels at the screening visit, and their quantitative measurements were postponed until their anxiety levels on the Hospital Anxiety and Depression Scale had improved. Ten participants were excluded from the study based on responses to the questionnaires on “Eating and Weight Patterns-Revised” and “Eating Behaviors” that suggested possible eating disorders.
Randomized, Placebo-Controlled Trial of Phentermine-Topiramate-ER
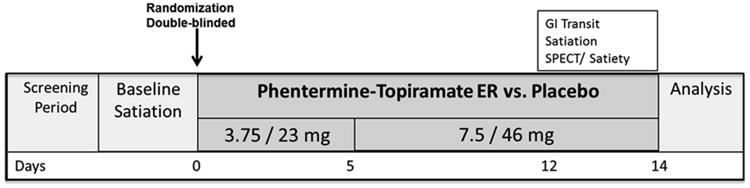
From the prospective cohort of ∼270 patients, the first 24 obese (BMI: 30-40kg/m2) adults who volunteered by responding to an invitation to participate in a therapeutic study were randomized in a parallel-group, double-blinded, 2-week treatment trial of placebo compared to phentermine-topiramate-ER at doses of 3.75 and 23mg respectively for the first 5 days, and 7.5 and 46mg for the next 10 days for a total of 15 days of combination treatment (Figure 1). Allocation was concealed from the clinical research team; randomization was conducted by an independent Mayo statistician who was otherwise not involved in the study, and communicated to and retained by the Study Research Pharmacist. Randomization was carried out within blocks of consecutive patients. Only the independent Mayo statistician and pharmacist knew the block size being used. The study was conducted in accordance with the Declaration of Helsinki and with Good Clinical Practice guidelines. Written informed consent was obtained from all patients before participation. The study was registered at ClinicalTrials.gov (#NCT01834404).
Figure 1.
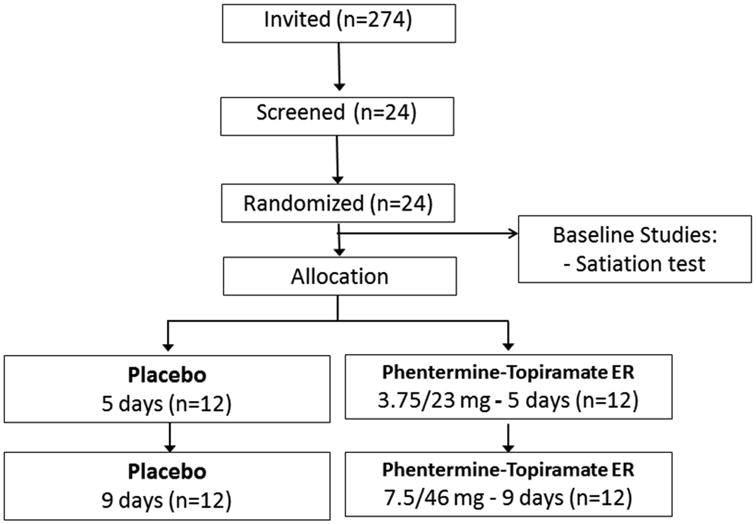
a) CONSORT flow chart for randomized controlled trial of effects of phentermine-topiramate-ER on weight loss and quantitative traits in obesity
b) Study protocol for randomized controlled trial of effects of phentermine-topiramate-ER on weight loss and quantitative traits measured on days 12 to 14 of treatment
After written informed consent and screening evaluation, participants completed questionnaires, underwent a physical exam, a baseline satiation (nutrient drink test) on Day 0 and a negative urine pregnancy test 48 hours prior doing tests involving radioactivity. They presented to the testing facility (Clinical Research Unit, Charlton Building, 7th floor, Mayo Clinic) after overnight fast for all quantitative measurements which were completed on separate days in this order in the last 3 days of the ∼12 days (+3) of medication administration: Scintigraphic gastric emptying, Satiation, and SPECT/satiety test.
Post-treatment weight was not available in one subject (who had been randomized to placebo), and thus an ITT analysis was performed imputing a value for “weight change” in this individual, based on the mean weight change in the remaining subjects with complete data (n=23).
Based on primary response measures and coefficient of variation29, 31, 44, 45, we estimated that with 12 patients per treatment arm, the mean effect size detectable (with 80% power based on a two sample t-test at a two-sided α level of 0.05) for GE T1/2 was 46 minutes, satiation (MTV) 439 ml, fasting GV92 ml and postprandial GV 93ml.
Statistical Analysis
We analyzed the association of BMI class with quantitative traits using 1-way ANOVA models pairwise comparisons of quantitative traits in overweight, obesity class I and class II/III versus normal weight were examined using Dunnett's test. Associations with waist circumference were assessed using the rank sum test. The primary endpoints in Table 1 are parameters that reflect discrete physiological or psychological traits; therefore, no adjustment of the p-values was done for multiple tests among these endpoints, except for the two satiation endpoints (adjusted based on the Hochberg method46).
Table 1. Quantitative traits of satiation, gastric motor functions and gastrointestinal hormones in normal weight, overweight and obese patients (data show mean ± SEM).
| Group (BMI) | Normal weight (18-25 kg/m2) | Overweight (25-30kg/m2) | Obesity Class I (30-35kg/m2) | Obesity Class II/III (>35kg/m2) | P |
|---|---|---|---|---|---|
| Satiation test | |||||
| N (total=509) | 85 | 158 | 135 | 131 | |
| Gender (female %) | 71 | 61 | 67 | 75 | 0.032 |
| Mean BMI (kg/m2) | 22.8±0.2 | 27.6±0.1 | 32.4±0.1 | 38.9±0.3 | |
| Volume to fullness (mL) | 666.4±32.4 | 713.5±20.3 | 755.1±30.9 | 805.2±36.9* | 0.038# |
| MTV (mL) | 1248±42 | 1337±30 | 1283±34 | 1290±39 | 0.38# |
| Gastric motor functions and gastrointestinal hormones | |||||
| N (total=328) | 22 | 105 | 108 | 93 | |
| Age (y) | 38.8±3.1 | 37.3±1.2 | 38±1.2 | 37.5±1.2 | |
| Gender (female %) | 64 | 60 | 73 | 70 | 0.20 |
| Mean BMI (kg/m2) | 23±0.3 | 27.8±0.1 | 32.3±0.1 | 38.9±0.3 | |
| Solid GE T1/2 (min) | 124.2±4.1 | 100.2±2.5* | 99.4±2.5* | 106.6±3.3* | <0.001 |
| Liquid GE T1/2 (min) | 27.9±3.4 | 18.5±0.1* | 18.9±1* | 20±1.4* | 0.011§ |
| Fasting GV (mL) | 257.5±14.5 | 251.7±7 | 264.7±6.3 | 282.2±8.2 | 0.028 |
| Postprandial GV (mL) | 781.1±21.5 | 733.6±11.2 | 739.8±11.2 | 767±13.9 | 0.109 |
| Buffet meal intake (kcal) | NA | 948.1±31.9 | 972.7±30.7 | 987.5±32.1 | 0.66 |
| Ghrelin fasting (pg/ml) | NA | 85.2±7.2 | 69.5±3.6 | 70.14±6.7 | 0.063§ |
| CCK peak (pmol/L) | NA | 8.3±0.6 | 8.4±0.5 | 10.5±0.8 | 0.061§ |
| GLP-1 peak (pM) | 12.1±1.9 | 17.7 ±1.1* | 18.8±1.2* | 19.1±1.3* | <0.001§ |
| PYY peak (pg/ml) | 223.1±22.5 | 155.8±5.9* | 165.5±9.7* | 166.8±7.9 | 0.003§ |
| Psychological traits | |||||
| Anxiety (Scale 0-14) | NA | 2.7±0.2 | 4.0±0.3 | 3.7±0.3 | 0.006§ |
| Depression (Scale 0-14) | NA | 1.2±0.2 | 1.8±0.2 | 1.7±0.2 | 0.017§ |
| Body Image (Scale 9-45) | NA | 30.7±0.7 | 27.1±0.6 | 26.1±0.6 | <0.001 |
p value <0.05 when compared to normal weight (Dunnett's test)
based on a rank transformation;
adjusted for two endpoints (Hochberg method)
GE=gastric emptying; GV=gastric volume; MTV=maximum tolerated volume
A principal component analysis was examined to identify possible latent dimensions in 231 patients who had prospectively completed all quantitative and psychological traits measured. A rank transformation was used to compensate for several quantitative traits with non-normal distributions. Interpretations of the first 7 principal components (accounting for ∼81% of the variation among the quantitative traits) were assessed based on univariate associations using Spearman correlations. Significant associations were identified based on rs≥0.4 and p<0.0001. The principal component analyses were constructed to be uncorrelated with age and BMI.
Effects of phentermine-topiramate-ER on weight loss and quantitative traits were assessed using analysis of covariance adjusting for baseline satiation (volume to fullness and maximum tolerated volume), BMI, or gender as covariates depending on the specific quantitative trait.
In all statistical analyses, suitable transformations for skewness in the distributions of the measured responses or uneven variation were made as necessary.
Finally, we assessed potential differential treatment effects based on 5 pre-specified quantitative traits measured within two years of the treatment trial (satiety by buffet meal, satiation by volume to fullness, gastric emptying of solids, fasting gastric volume and peak plasma PYY). This was achieved by incorporating interaction terms for treatment by quantitative trait in separate ANCOVA models.
The authors had access to the study data and reviewed and approved the final manuscript.
Results
Association of Body Mass Index with Quantitative Traits
In the entire cohort of 509 participants [mean (±SD) age 37.5±12.2y; 67.2% females; 91% Caucasians of normal weight (85); overweight (158); obesity class I (135) or obesity class II (131)], obesity was associated with decreased satiation measured by a higher volume to reach fullness (p=0.038), but not maximal tolerated volume (p=0.38)(Table 1) on the nutrient drink test.
In the 328 prospectively studied participants [age 37.8±12y; 67.7% females; normal weight (22); overweight (105); obese class I (108) and obese class II (93)], obesity was associated with higher fasting GV (p=0.03), accelerated GE T1/2 for solids (p<0.001) and liquids (p=0.011).
In the overweight and obese groups, there were associations of BMI with lower peak postprandial PYY (p=0.003), higher peak postprandial GLP-1 (p<0.001), borderline lower fasting ghrelin (p=0.063), and borderline higher peak postprandial cholecystokinin (p=0.061) levels. There was no association of BMI with kcal intake at the buffet meal satiety test (Table 1).
Association of Abnormal BMI with Behavioral and Psychological Traits
In 274 participants [age 37.4±11.8y; 69.5% females; overweight (90); and obese (184)], 62% of obese individuals reported exercising regularly compared to 80% of overweight individuals (p=0.004, Supplementary Appendix Table 1A). In addition, obesity was associated with higher anxiety (p=0.006) and depression (p=0.017) scores, and with lower body image satisfaction (p<0.001) when compared to the overweight group (Table 1).
Association of Waist Circumference with Quantitative Traits
In 264 participants [age 37.4±11.8y; 69.5% females; normal waist circumference 59; and abnormal waist circumference 205], there were associations with age (p=0.08) and, as expected, with BMI (p<0.001). Abnormal waist circumference was associated with increased caloric intake at the ad libitum buffet meal test (p=0.016) (Table 2), manifested as increased intake for all macronutrients (carbohydrates [p=0.06], protein [p=0.008], and fat [p=0.004]) compared to those with normal waist circumference. Abnormal waist circumference was not associated with gastric emptying, gastric volume, satiation, or gastrointestinal hormones.
Table 2. Quantitative traits of satiation, gastric motor functions and gastrointestinal hormones based on waist circumference (WC) in both genders; normal weight circumference is based on less than 102cm for men and 88cm for women (data mean±SEM).
| Data show mean ± SEM | Normal WC | Abnormal WC | P# |
|---|---|---|---|
| Demographics | |||
| N (total=264) | 59 | 205 | |
| Age (y) | 35.3±1.7 | 37.9±0.8 | 0.08 |
| Gender (female %) | 49 | 75 | <0.001 |
| Mean BMI (kg/m2) | 28.02±0.2 | 34.5±0.3 | <0.001 |
| Waist circumference (cm) | 87.9±1 | 104.9±0.7 | |
| Satiation / Satiety | |||
| Volume to fulness (mL) | 664.7±32.8 | 723.3±24.1 | 0.22 |
| MTV (mL) | 1240±47.9 | 1303±30.7 | 0.45 |
| Buffet meal intake (kcal) | 893.5±32.9 | 992.7±21.6 | 0.016 |
| CHO intake at buffet meal (kcal) | 476 ± 16.8 | 525.2± 12.0 | 0.06 |
| Protein intake at buffet meal (kcal) | 200.8 ± 7.6 | 225.6 ±4.8 | 0.008 |
| Fat intake at buffet meal (kcal) | 204.3 ± 9 | 236.7 ± 5.4 | 0.004 |
| Gastric motor functions and gastrointestinal hormones | |||
| Solid GE T1/2 (min) | 99.7±3.5 | 99.7±1.9 | 0.76 |
| Liquid GE T1/2 (min) | 18.2±1.4 | 19±0.7 | 0.39 |
| Fasting GV (mL) | 273.3±9.9 | 274.6±5.3 | 0.84 |
| Postprandial GV (mL) | 753.4±16.4 | 754.1±8.7 | 0.97 |
| Ghrelin fasting (pg/ml) | 70.7±5.6 | 73.18±3.6 | 0.72 |
| CCK peak (pmol/L) | 8.2±0.7 | 9.3±0.4 | 0.13 |
| GLP-1 peak (pM) | 16.2±0.9 | 19.1±0.9 | 0.22 |
| PYY peak (pg/ml) | 148.9±6.5 | 167.4±5.8 | 0.25 |
| Psychological traits | |||
| Anxiety (Scale 0-14) | 2.9±0.3 | 3.6±0.2 | 0.12 |
| Depression (Scale 0-14) | 1.2±0.2 | 1.7±0.1 | 0.038 |
| Body Image Satisfaction (Scale 9-45) | 30.8±0.8 | 27.2±0.4 | <0.001 |
CHO=carbohydrates; GE=gastric emptying; GV=gastric volume; MTV=maximum tolerated volume;
based on Rank sum test
Association of Waist Circumference with Behavioral and Psychological Traits
Among the 264 overweight or obese participants, 64% of obese individuals reported exercising regularly compared to 83% of overweight individuals (p=0.005) (Supplementary Appendix Table 1B). Abnormal waist circumference was associated with increased depression score (p=0.038) and lower body image satisfaction (p<0.001), but not with anxiety score (Table 2) when compared to those with normal waist.
Latent Dimensions of Obesity Based on Principal Components Analysis
The principal component analysis identified four main latent dimensions (each with rs>0.4, p<0.0001) accounting together for ∼81% of the variation in the rank scales of the traits among overweight and obese subjects: satiety [kcal at buffet meal, satiation volume to fullness and maximal tolerated volume, peak postprandial GLP-1 and PYY (21%)]; gastric capacity [fasting and postprandial gastric volumes (14%)]; psychological [anxiety, depression, body image satisfaction (13%)]; gastric motor and sensory functions [postprandial symptoms after liquid nutrient drink test, gastric emptying, peak postprandial PYY levels (11%)] (Table 3). In addition, separate contributions to the overall variation were identified for principal component analyses reflecting peak postprandial GLP-1 levels (9%), symptoms 30 minutes post-satiation (6%), and body image satisfaction (6%).
Table 3. Latent dimensions of quantitative traits identified in association with obesity based on r≥0.4 and p<0.0001, using principal components (PC) analysis.
| Quantitative Trait | pc1 | pc2 | pc3 | pc4 |
|---|---|---|---|---|
| Latent Dimension (LD) | Satiety/ Satiation | Gastric capacity | Psychological | Gastric motor/sensory |
| Satiety (kcal intake) | 0.67 | |||
| Satiation (volume to fullness) | 0.74 | |||
| Satiation (MTV) | 0.80 | |||
| Satiation Symptoms | 0.61 | |||
| Solid GE T1/2 | -0.46 | 0.53 | ||
| Fasting gastric volume | 0.83 | |||
| Postprandial Δ gastric volume | -0.81 | |||
| Postprandial peak GLP-1 | 0.40 | |||
| Postprandial peak PYY | 0.51 | 0.42 | ||
| HADS Anxiety | 0.77 | |||
| HADS Depression | 0.74 | |||
| Body Image Satisfaction | -0.48 | |||
| Attributable proportion (%) of quantitative trait variance based on LD | 21 | 14 | 13 | 11 |
GE=gastric emptying; GV=gastric volume; HADS=Hospital Anxiety and Depression scale; LD=latent dimension; MTV=maximum tolerated volume; VAS=visual analog score
Effects of Phentermine-Topiramate-ER on Weight Loss and Quantitative Traits
The two treatment groups were balanced for age, gender, and BMI (Table 4). After two weeks of treatment, patients on phentermine-topiramate-ER lost 1.42±0.4kg when compared to patients on placebo who, on average, lost 0.23±0.4kg (p=0.03, based on least square mean analysis, adjusted for gender). There were no adverse effects reported in either treatment group.
Table 4. Effects of phentermine-topiramate-ER and placebo on obesity and quantitative traits in a proof-of-concept, randomized, double-blind trial.
| Placebo | Phentermine-Topiramate-ER | p | |
|---|---|---|---|
| Baseline measurements | |||
| N | 12 | 12 | |
| Age (y) | 38.2±2.4 | 31.8±1.8 | |
| BMI (kg/m2) | 33.9±1.9 | 35.8±0.9 | |
| Waist (cm) | 111.2±2.2 | 108.5±2.2 | |
| Fasting plasma glucose (mg/dL) | 96.5±3.6 | 94.2±2.7 | |
| Volume to fullness (ml) | 710±95 | 712±86 | |
| Maximum tolerated volume (ml) | 1227±111 | 1368±111 | |
| Effects of Treatment | |||
| - Weight change | |||
| Baseline weight (kg) | 105.1±3.0 | 99.8±3.1 | |
| Post treatment weight (kg) | 105.3 ± 3.5 | 98.4 ±3.0 | |
| Weight Change (kg)† | -0.23 ± 0.4 | -1.42 ± 0.4 | 0.03 |
| - Primary Endpoints† | |||
| Solid GE T ½ min | 88 ± 7 | 109 ± 7 | 0.057 |
| Fasting gastric volume (mL) | 261 ± 25 | 227 ± 25 | 0.36 |
| Postprandial gastric volume (mL) | 681 ± 37 | 680 ± 37 | 0.99 |
| Volume to fullness (mL) | 630±61.1 | 570±63.2 | 0.45 |
| Maximum tolerated volume (mL) | 1108 ± 79 | 966 ± 79 | 0.22 |
| Buffet meal intake (Kcal) | 988 ± 79 | 728 ± 79 | 0.032 |
| - Secondary Endpoints | |||
| Solid Ge: proportion emptied @ 2hr† | 0.66 ± 0.03 | 0.56 ± 0.03 | 0.052 |
| Solid GE: proportion remaining @ 4hr† | 0.16 ± 0.02 | 0.09 ± 0.02 | 0.030§ |
| Δ Postprandial GV (mL)† | 420 ± 24 | 453 ± 24 | 0.35 |
| Fasting Ghrelin | 82.6 ± 10.8 | 78.1±5.6 | 0.72 |
| Peak CCK (pg/mL) | 8.3 ± 1 | 8.1 ± 0.9 | 0.90 |
| Peak GLP-1 (pg/mL) | 11.9 ± 1.6 | 13.0 ± 1.8 | 0.54# |
| Peak PYY (pg/mL) | 166±15.7 | 195.3±21.2 | 0.26# |
Data show least-square means±SE
GE=gastric emptying; GV=gastric volume
based on a Rank transformation
Rank sum test
Phentermine-topiramate-ER resulted in a decrease in caloric intake [mean difference (Δ) 206 kcal, p=0.032] when compared to the placebo group in the satiety test. Active treatment group also had borderline significant delay in GE T1/2 (Δ 19min, p=0.057), and percent of the meal emptied at 2 hours (mean Δ relative to placebo 10%, p=0.052) and 4 hours (mean Δ relative to placebo 6%, p=0.030). There were no effects of treatment on fasting and postprandial gastric volume, satiation, liquid gastric emptying or gastrointestinal hormones (ghrelin, cholecystokinin, GLP-1 and PYY).
Quantitative Traits as Predictors of Response to Phentermine-Topiramate-ER
Among the 5 pre-specified traits, we noted that satiety by ad-libitum buffet meal conducted in the same participants prior to the proof-of-concept treatment trial was significantly correlated with the post-treatment satiety test result for all subjects (r=0.76, p<0.001) and in each intervention group: phentermine-topiramate ER (r=0.60, p=0.04) and placebo (r=0.92, p<0.001) groups (Figure 2, upper panel). A differential effect of phentermine-topiramate-ER on weight loss was identified (p=0.029) with higher kcal intake at the prior satiety test, predisposing to greater weight loss in the active treatment group (Figure 2, lower panel).
Figure 2.
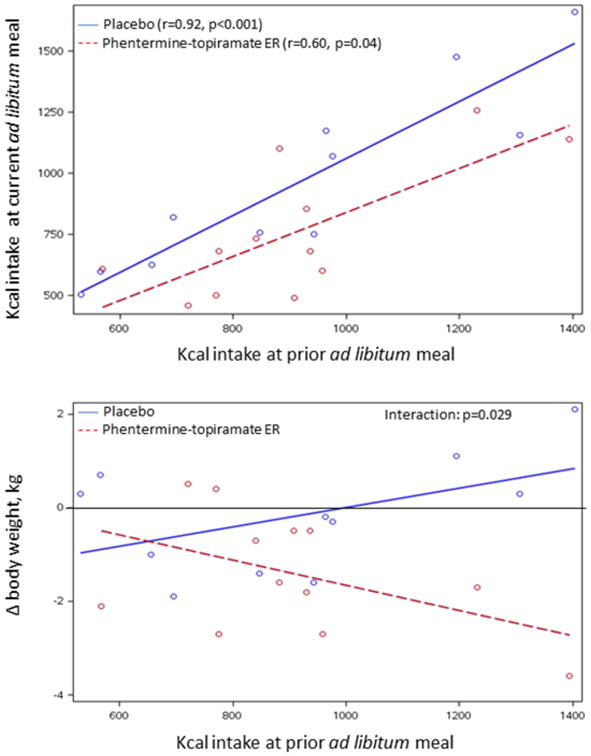
Upper panel: Relationship of kcal intake at prior ad-libitum meal and kcal intake in response to randomized treatment; note that there are significant associations in both treatment groups. Lower panel: Association of change in body weight (in response to randomized treatment with placebo or phentermine-topiramate-ER) and kcal intake at prior ad-libitum meal. Note the kcal intake at baseline prior to treatment is associated with the degree of weight loss on treatment with phentermine-topiramate-ER with no observed effect with placebo treatment. This is illustrated by p=0.029 for the drug treatment interaction.
There were no differential treatment effects on weight change associated with solid GE T1/2, fasting gastric volume, volume to fullness (satiation) and peak postprandial PYY.
Discussion
Quantitative traits of gastric function and satiation, as well as behavioral traits are associated with higher BMI compared to normal BMI. These traits identify distinct obesity phenotypes, and a simple measurement of satiety predicts short-term response to obesity pharmacotherapy.
Our findings suggest that, compared to normal weight controls, those who are overweight or obese have significant differences in gastrointestinal quantitative traits: lower satiation manifested as higher Ensure® volume intake to feel fullness; accelerated gastric emptying of liquids and solids; increased fasting gastric volume; and decreased peak postprandial serum PYY. In addition, we noted higher caloric intake to record satiety in individuals with abnormal waist circumference, and there was an expected increase of peak GLP-1 in response to accelerated gastric emptying. It is interesting to note that there were also numerical trends in increasing volume to fullness, accelerating GE T1/2 and increasing fasting gastric volume between overweight and obesity class I and II groups. Our current data represent the largest sample of overweight or obese patients who have undergone the validated quantitative trait measurements to date. In contrast, a comprehensive review showed equivocal effects (slow, fast or normal gastric emptying) in obesity based on multiple small studies14. We have previously published47 normal value data for gastric emptying T1/2 for 319 healthy controls for the same solid meal: 5-95%ile was 78.4 to 174 minutes. Using these data, we documented that 1/105 overweight, 1/108 class I obesity, and 2/93 class II/III obesity had evidence of delayed gastric emptying at baseline. In contrast, the absolute percentages of accelerated gastric emptying of solids in these groups were 10, 24 and 20% respectively.
Here we also report the first clear evidence that obesity is associated with higher caloric intake in a controlled-laboratory setting. This is associated with approximately 50kcal higher intake per 5kg/m2 of BMI. This numerical trend is seen also in individuals with abnormal weight circumference; thus, individuals with abnormal weight circumference consumed 100kcal more than the normal weight circumference overweight or obese controls. With the exception of the ad-libitum total calorie intake at the satiety test, abnormal waist circumference is not associated with changes in quantitative gastrointestinal traits.
The observed gastrointestinal quantitative traits in the larger patient cohort in the present study provide greater confidence in the observations of gastrointestinal dysfunction in overweight and obesity, and may explain, in part, the pathophysiology of weight gain and obesity. Thus, individuals with higher BMI tolerated a higher caloric volume to, and patients with abnormal waist circumference ingested more calories at ad-libitum meal. Increased calorie intake may be facilitated by the larger fasting gastric volume, faster gastric emptying of solids, or a decrease in the satiety hormone, PYY. Lower PYY level in obesity was previously reported48. Reduction of the increased fasting gastric volume by bariatric procedures reverses the larger fasting gastric volume and is one of the mechanisms for weight loss3. Whereas, our current study does not discriminate between cause or consequence of obesity, it suggests phenotypic subgroups may be identified based on pathophysiological mechanisms.
In a proof-of-concept, randomized clinical trial, we compared effects of phentermine-topiramate-ER and placebo on weight and quantitative traits, and explored the ability to predict weight loss based on a priori selected quantitative traits. Phentermine-topiramate-ER resulted in the expected weight loss even in a short-term (2-week) trial, slowed gastric emptying, and decreased caloric intake in a standardized satiety test. These findings suggest that phentermine-topiramate-ER has specific effects, reversing the acceleration of gastric emptying of solids, as well as reducing the calories ingested in an ad-libitum meal, both traits associated with BMI. These data enhance understanding of the mechanisms of action of this drug, although the current study cannot identify which of the two medications in the combination is responsible for the pharmacodynamic effects. We have used the conventional description of phentermine-topiramate-ER as a centrally acting agent; however, the effects on stomach emptying may suggest either a peripheral action or an effect mediated through the vagal nuclei. In addition, the effect of phentermine-topiramate-ER on weight loss (which is known to be highly variable in clinical trials) is significantly associated with increased calorie intake during an ad-libitum buffet meal, but not with the other gastric functions or PYY. Our findings suggest that in the short-term, obese individuals, who consume over a 1000 calories during ad-libitum buffet meal may lose 1kg or more per week on phentermine-topiramate ER while the individuals on placebo had no response or actually gained weight (Figure 2, lower panel). This suggests that measuring satiety may facilitate prediction of efficacy over the short term. Future studies will need to appraise the prediction of the response to therapy over longer periods (12 weeks to 12 months).
In a principal component analysis conducted in 231 overweight or obese individuals and excluding the normal weight controls since they do not have the phenotype to be predicted (that is, BMI >25kg.m2), we identified latent dimensions accounting together for 81% of overweight and obesity variance, including 4 main latent dimensions: satiety (21%), gastric capacity (14%), psychological (13%), and gastric motor-sensory functions (11%). These latent dimensions in obesity may serve as biomarkers to enrich selection of patients for treatment, based on the pharmacological effects of the medication. While this principle was illustrated for the centrally-acting phentermine-topiramate-ER (satiety predicted weight loss response), it is conceivable that other biomarkers such as rapid gastric emptying of solids in obesity may potentially predict the response to amylin agonists such as pramlintide, or GLP-1 agonists such as exenatide or liraglutide that are being tested as weight loss remedies49.
Higher scores of anxiety and depression have been previously reported to be correlated with weight loss in obese patients20. In our cohort, obese individuals had higher anxiety and depression scores without meeting severity criteria for major depression or clinical anxiety.
In conclusion, obesity is associated with decreased satiation, accelerated gastric emptying, increased fasting gastric volume, and decreased peak postprandial PYY, all of which may individually be associated with increased calorie intake and predispose to, or perpetuate, obesity. Whereas, our current study does not discriminate between cause or consequence of obesity, it suggests phenotypic subgroups may be identified based on pathophysiological mechanisms. Identifying a prominent phenotype such as abnormal satiety, abnormal gastric motor function, or affect provides the opportunity to select patients for pharmacotherapeutic approaches based on the mechanisms of action of the medications. This observation has public health relevance as it would usher in a new era of matching patients based on quantitative traits to pharmacotherapy, potentially enhancing drug efficacy in treatment of obesity, and reducing expenditures for both validating the efficacy of such medications and prescribing them to obese individuals in clinical practice.
Supplementary Material
Supplementary Appendix eTable 1A. Exercise behaviors and attitudes in overweight or obese (BMI >30kg/m2) participants
Supplementary Appendix eTable 1B. Exercise behaviors and attitudes in overweight or obese participants with normal or abnormal weight circumference
Supplementary Appendix eTable 2. Demographics of participants randomized to placebo or phentermine-topiramate-ER
Acknowledgments
Funding: This research and Dr. Camilleri were funded by NIH RO1-DK67071; medication used for this study was provided by VIVUS; no other support was provided. This study was supported by CCaTS grant #UL1-TR000135 from NIH (Endoscopy, Physiology and Imaging Core; and Nursing Core in the Mayo Clinical Research Unit).
Footnotes
Clinical trial registration: ClinicalTrials.gov #NCT01834404
Authors' contributions: A. Acosta: fellow co-investigator; conduct of the study; writing and critical revision of the manuscript
M. Camilleri: study concept and design; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript; obtained funding
A. Shin: fellow co-investigator; conduct of the study; critical revision of the manuscript
M. Vazquez-Roque: fellow co-investigator; conduct of the study; critical revision of the manuscript
J. Iturrino: fellow co-investigator; conduct of the study; critical revision of the manuscript
D. Burton: technical support; study supervision
J. O'Neill: study coordinator
D. Eckert: study coordinator
A.R. Zinsmeister: staff statistician; study design; analysis and interpretation of data; critical revision of the manuscript
Conflicts of Interest for All Authors: None
Authors' names in bold designate shared co-first authorship.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ng M, Fleming T, Robinson M, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014 doi: 10.1016/S0140-6736(14)60460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Flegal KM, Carroll MD, Kit BK, et al. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999-2010. JAMA : the journal of the American Medical Association. 2012;307:491–497. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 3.Acosta A, Abu Dayyeh BK, Port JD, et al. Recent advances in clinical practice challenges and opportunities in the management of obesity. Gut. 2014 doi: 10.1136/gutjnl-2013-306235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gadde KM, Allison DB, Ryan DH, et al. Effects of low-dose, controlled-release, phentermine plus topiramate combination on weight and associated comorbidities in overweight and obese adults (CONQUER): a randomised, placebo-controlled, phase 3 trial. Lancet. 2011;377:1341–1352. doi: 10.1016/S0140-6736(11)60205-5. [DOI] [PubMed] [Google Scholar]
- 5.Stubbs J, Whybrow S, Teixeira P, et al. Problems in identifying predictors and correlates of weight loss and maintenance: implications for weight control therapies based on behaviour change. Obesity reviews : an official journal of the International Association for the Study of Obesity. 2011;12:688–708. doi: 10.1111/j.1467-789X.2011.00883.x. [DOI] [PubMed] [Google Scholar]
- 6.Delahanty LM, Peyrot M, Shrader PJ, et al. Pretreatment, psychological, and behavioral predictors of weight outcomes among lifestyle intervention participants in the Diabetes Prevention Program (DPP) Diabetes care. 2013;36:34–40. doi: 10.2337/dc12-0733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hansen D, Astrup A, Toubro S, et al. Predictors of weight loss and maintenance during 2 years of treatment by sibutramine in obesity. Results from the European multi-centre STORM trial. Sibutramine Trial of Obesity Reduction and Maintenance International journal of obesity and related metabolic disorders : journal of the International Association for the Study of Obesity. 2001;25:496–501. doi: 10.1038/sj.ijo.0801481. [DOI] [PubMed] [Google Scholar]
- 8.Svetkey LP, Ard JD, Stevens VJ, et al. Predictors of long-term weight loss in adults with modest initial weight loss, by sex and race. Obesity. 2012;20:1820–8. doi: 10.1038/oby.2011.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Teixeira PJ, Going SB, Sardinha LB, et al. A review of psychosocial pre-treatment predictors of weight control. Obesity reviews : an official journal of the International Association for the Study of Obesity. 2005;6:43–65. doi: 10.1111/j.1467-789X.2005.00166.x. [DOI] [PubMed] [Google Scholar]
- 10.Teixeira PJ, Going SB, Houtkooper LB, et al. Pretreatment predictors of attrition and successful weight management in women. International journal of obesity and related metabolic disorders : journal of the International Association for the Study of Obesity. 2004;28:1124–33. doi: 10.1038/sj.ijo.0802727. [DOI] [PubMed] [Google Scholar]
- 11.Blundell J, Gillett A. Control of food intake in the obese. Obesity research. 2001;9(Suppl 4) doi: 10.1038/oby.2001.129. [DOI] [PubMed] [Google Scholar]
- 12.French SA, Epstein LH, Jeffery RW, et al. Eating behavior dimensions. Associations with energy intake and body weight. A review Appetite. 2012;59:541–9. doi: 10.1016/j.appet.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Delgado-Aros S, Cremonini F, Castillo JE, et al. Independent influences of body mass and gastric volumes on satiation in humans. Gastroenterology. 2004;126:432–40. doi: 10.1053/j.gastro.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 14.Park MI, Camilleri M. Gastric motor and sensory functions in obesity. Obesity research. 2005;13:491–500. doi: 10.1038/oby.2005.51. [DOI] [PubMed] [Google Scholar]
- 15.Seimon RV, Brennan IM, Russo A, et al. Gastric emptying, mouth-to-cecum transit, and glycemic, insulin, incretin, and energy intake responses to a mixed-nutrient liquid in lean, overweight, and obese males. American journal of physiology Endocrinology and metabolism. 2013;304:E294–300. doi: 10.1152/ajpendo.00533.2012. [DOI] [PubMed] [Google Scholar]
- 16.Szarka LA, Camilleri M. Methods for measurement of gastric motility. American journal of physiology Gastrointestinal and liver physiology. 2009;296:G461–75. doi: 10.1152/ajpgi.90467.2008. [DOI] [PubMed] [Google Scholar]
- 17.Kim DY, Camilleri M, Murray JA, et al. Is there a role for gastric accommodation and satiety in asymptomatic obese people? Obesity research. 2001;9:655–61. doi: 10.1038/oby.2001.89. [DOI] [PubMed] [Google Scholar]
- 18.Vazquez Roque M, Camilleri M, Stephens D, et al. Gastric sensorimotor functions and hormone profile in normal weight, overweight, and obese people. Gastroenterology. 2006;131:1717–1724. doi: 10.1053/j.gastro.2006.10.025. [DOI] [PubMed] [Google Scholar]
- 19.Geliebter A, Hashim SA. Gastric capacity in normal, obese, and bulimic women. Physiology & behavior. 2001;74:743–6. doi: 10.1016/s0031-9384(01)00619-9. [DOI] [PubMed] [Google Scholar]
- 20.Buscemi S, Castellini G, Batsis JA, et al. Psychological and behavioural factors associated with long-term weight maintenance after a multidisciplinary treatment of uncomplicated obesity. Eating and weight disorders : EWD. 2013;18:351–8. doi: 10.1007/s40519-013-0059-2. [DOI] [PubMed] [Google Scholar]
- 21.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–421. [PubMed] [Google Scholar]
- 22.Delgado-Aros S, Camilleri M, Cremonini F, et al. Contributions of gastric volumes and gastric emptying to meal size and postmeal symptoms in functional dyspepsia. Gastroenterology. 2004;127:1685–94. doi: 10.1053/j.gastro.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 23.Delgado-Aros S, Camilleri M, Castillo E, et al. Effect of gastric volume or emptying on meal-related symptoms after liquid nutrients in obesity: a pharmacologic study. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2005;3:997–1006. doi: 10.1016/s1542-3565(05)00285-5. [DOI] [PubMed] [Google Scholar]
- 24.Delgado-Aros S, Kim DY, Burton D, et al. Effect of GLP-1 on gastric volume, emptying, maximum volume ingested, and postprandial symptoms in humans. American journal of physiology Gastrointestinal and liver physiology. 2002;282:31. doi: 10.1152/ajpgi.2002.282.3.G424. [DOI] [PubMed] [Google Scholar]
- 25.Vazquez-Roque M, Camilleri M, Vella A, et al. Association of genetic variation in cannabinoid mechanisms and gastric motor functions and satiation in overweight and obesity. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2011;23:637. doi: 10.1111/j.1365-2982.2011.01711.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Odunsi ST, Vazquez-Roque MI, Camilleri M, et al. Effect of alginate on satiation, appetite, gastric function, and selected gut satiety hormones in overweight and obesity. Obesity. 2010;18:1579–84. doi: 10.1038/oby.2009.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Castillo EJ, Delgado-Aros S, Camilleri M, et al. Effect of oral CCK-1 agonist GI181771X on fasting and postprandial gastric functions in healthy volunteers. American journal of physiology Gastrointestinal and liver physiology. 2004;287:G363–9. doi: 10.1152/ajpgi.00074.2004. [DOI] [PubMed] [Google Scholar]
- 28.Andrews CN, Bharucha AE, Camilleri M, et al. Nitrergic contribution to gastric relaxation induced by glucagon-like peptide-1 (GLP-1) in healthy adults. American journal of physiology Gastrointestinal and liver physiology. 2007;292:G1359–65. doi: 10.1152/ajpgi.00403.2006. [DOI] [PubMed] [Google Scholar]
- 29.Grudell AB, Sweetser S, Camilleri M, et al. A controlled pharmacogenetic trial of sibutramine on weight loss and body composition in obese or overweight adults. Gastroenterology. 2008;135:1142–54. doi: 10.1053/j.gastro.2008.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.World Health Organization. Waist circumference and waist–hip ratio: report of a WHO expert consultation. Geneva, Switzerland: 2008. [Google Scholar]
- 31.Bouras EP, Delgado-Aros S, Camilleri M, et al. SPECT imaging of the stomach: comparison with barostat, and effects of sex, age, body mass index, and fundoplication. Single photon emission computed tomography. Gut. 2002;51:781–6. doi: 10.1136/gut.51.6.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Delgado-Aros S, Camilleri M, Castillo EJ, et al. Effect of gastric volume or emptying on meal-related symptoms after liquid nutrients in obesity: a pharmacologic study. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2005;3:997–1006. doi: 10.1016/s1542-3565(05)00285-5. [DOI] [PubMed] [Google Scholar]
- 33.Breen M, Camilleri M, Burton D, et al. Performance characteristics of the measurement of gastric volume using single photon emission computed tomography. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2011;23:308–315. doi: 10.1111/j.1365-2982.2010.01660.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bouras E, Delgado-Aros S, Camilleri M, et al. SPECT imaging of the stomach: comparison with barostat, and effects of sex, age, body mass index, and fundoplication. Single photon emission computed tomography. Gut. 2002;51:781–786. doi: 10.1136/gut.51.6.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.De Schepper H, Camilleri M, Cremonini F, et al. Comparison of gastric volumes in response to isocaloric liquid and mixed meals in humans. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2004;16:567–573. doi: 10.1111/j.1365-2982.2004.00533.x. [DOI] [PubMed] [Google Scholar]
- 36.Chial H, Camilleri C, Delgado-Aros S, et al. A nutrient drink test to assess maximum tolerated volume and postprandial symptoms: effects of gender, body mass index and age in health. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2002;14:249–253. doi: 10.1046/j.1365-2982.2002.00326.x. [DOI] [PubMed] [Google Scholar]
- 37.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta psychiatrica Scandinavica. 1983;67:361–70. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 38.Lloyd-Richardson EE, King TK, Forsyth LH, et al. Body image evaluations in obese females with binge eating disorder. Eating behaviors. 2000;1:161–71. doi: 10.1016/s1471-0153(00)00016-7. [DOI] [PubMed] [Google Scholar]
- 39.Yanovski SZ. Binge eating disorder: current knowledge and future directions. Obes Res. 1993;1:306–24. doi: 10.1002/j.1550-8528.1993.tb00626.x. [DOI] [PubMed] [Google Scholar]
- 40.Clark MM, King TK. Eating self-efficacy and weight cycling: a prospective clinical study. Eating behaviors. 2000;1:47–52. doi: 10.1016/s1471-0153(00)00009-x. [DOI] [PubMed] [Google Scholar]
- 41.Bush K, Kivlahan DR, McDonell MB, et al. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP) Alcohol Use Disorders Identification Test Archives of internal medicine. 1998;158:1789–95. doi: 10.1001/archinte.158.16.1789. [DOI] [PubMed] [Google Scholar]
- 42.Clark MM, Novotny PJ, Patten CA, et al. Motivational readiness for physical activity and quality of life in long-term lung cancer survivors. Lung Cancer. 2008;61:117–22. doi: 10.1016/j.lungcan.2007.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cargill BR, Clark MM, Pera V, et al. Binge eating, body image, depression, and self-efficacy in an obese clinical population. Obesity research. 1999;7:379–86. doi: 10.1002/j.1550-8528.1999.tb00421.x. [DOI] [PubMed] [Google Scholar]
- 44.Chial HJ, Camilleri C, Delgado-Aros S, et al. A nutrient drink test to assess maximum tolerated volume and postprandial symptoms: effects of gender, body mass index and age in health. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2002;14:249–53. doi: 10.1046/j.1365-2982.2002.00326.x. [DOI] [PubMed] [Google Scholar]
- 45.Gurevich-Panigrahi T, Panigrahi S, Wiechec E, et al. Obesity: pathophysiology and clinical management. Current medicinal chemistry. 2009;16:506–21. doi: 10.2174/092986709787315568. [DOI] [PubMed] [Google Scholar]
- 46.Hochberg Y. A Sharper Bonferroni Procedure for Multiple Tests of Significance. Biometrika. 1988;75:800–802. [Google Scholar]
- 47.Camilleri M, Iturrino J, Bharucha A, et al. Performance characteristics of scintigraphic measurement of gastric emptying of solids in healthy participants. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2012;24:1076. doi: 10.1111/j.1365-2982.2012.01972.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Batterham RL, Cohen MA, Ellis SM, et al. Inhibition of food intake in obese subjects by peptide YY3-36. The New England journal of medicine. 2003;349:941–8. doi: 10.1056/NEJMoa030204. [DOI] [PubMed] [Google Scholar]
- 49.Bray G, Ryan D. Update on obesity pharmacotherapy. Annals of the New York Academy of Sciences. 2014 doi: 10.1111/nyas.12328. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Appendix eTable 1A. Exercise behaviors and attitudes in overweight or obese (BMI >30kg/m2) participants
Supplementary Appendix eTable 1B. Exercise behaviors and attitudes in overweight or obese participants with normal or abnormal weight circumference
Supplementary Appendix eTable 2. Demographics of participants randomized to placebo or phentermine-topiramate-ER


