Abstract
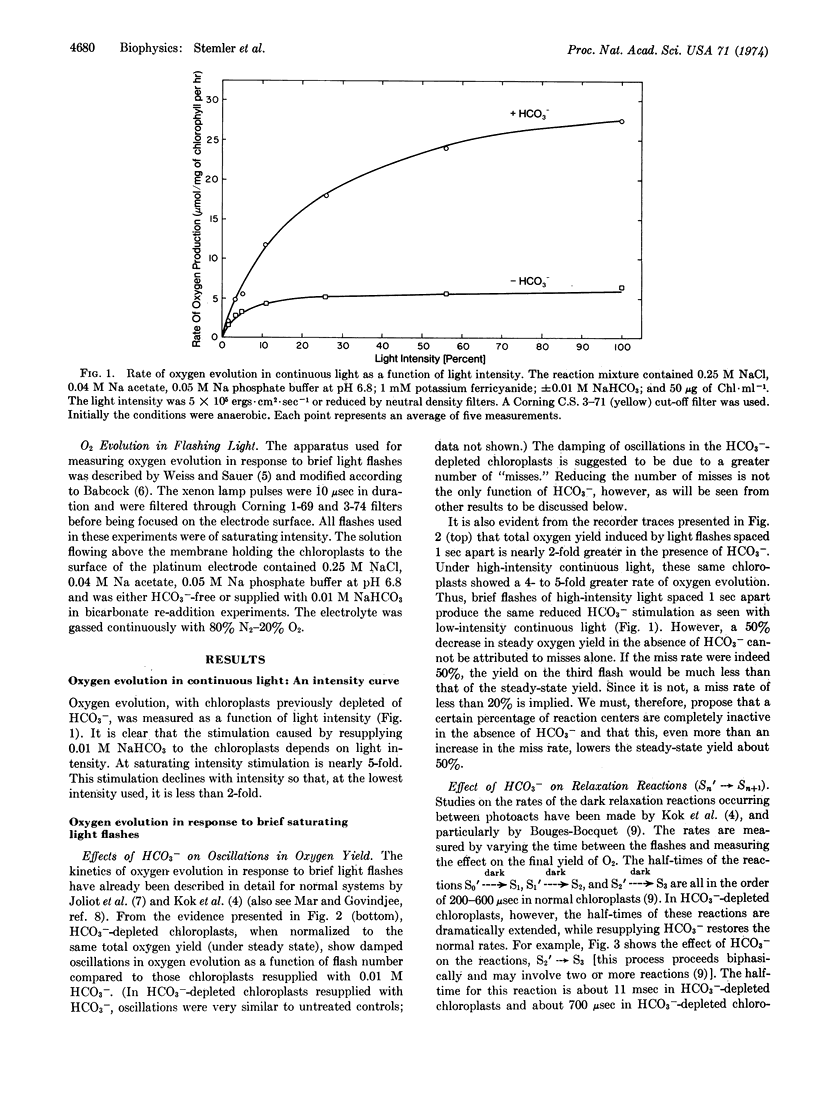
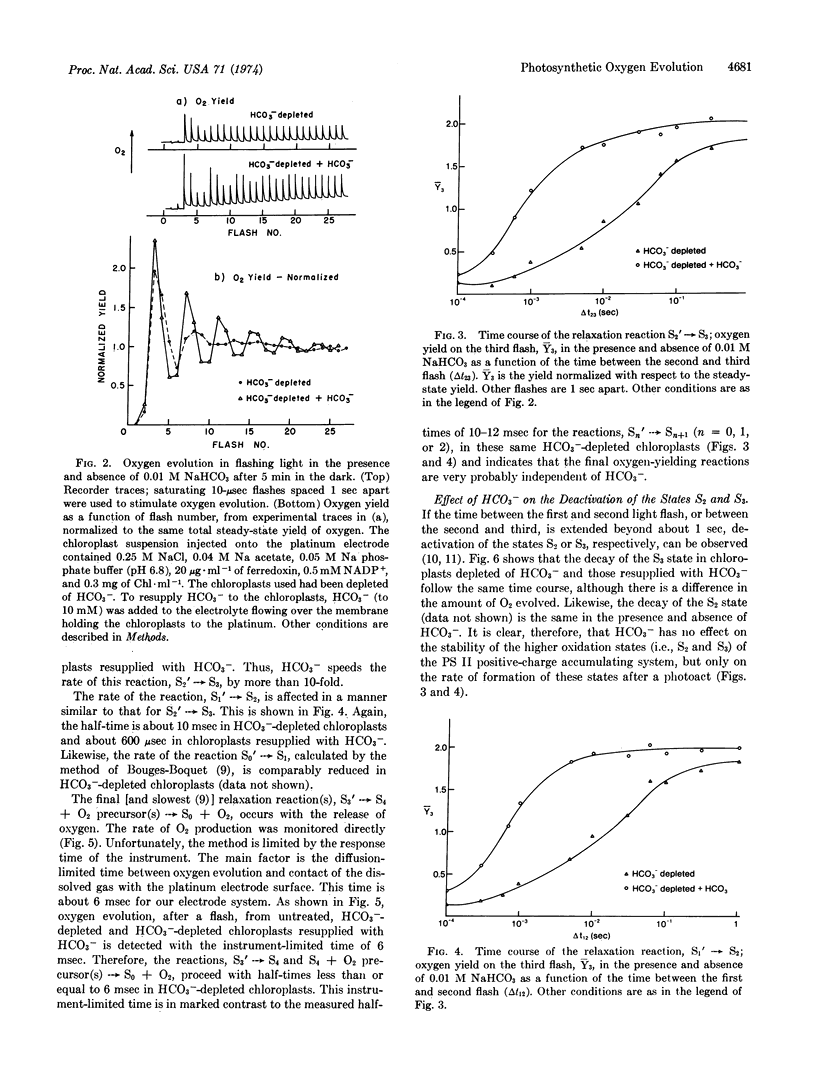
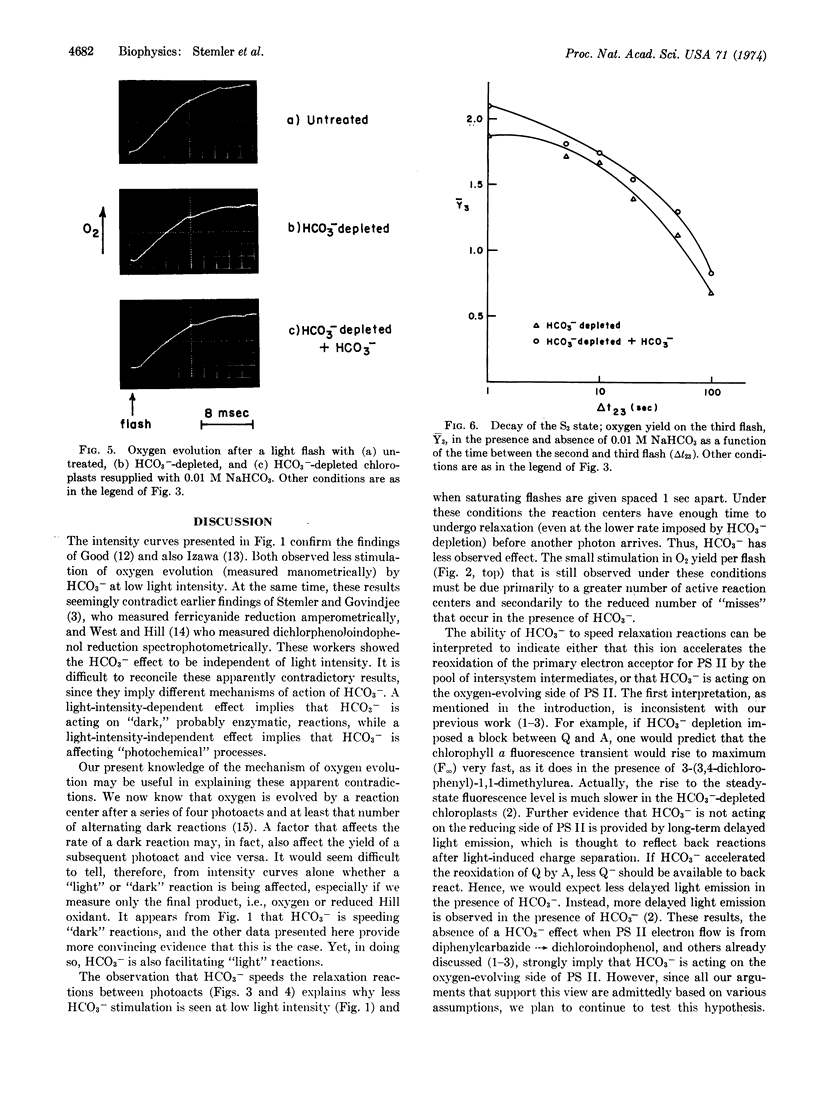
The ability of bicarbonate ion (HCO3-) to stimulate photosynthetic oxygen evolution in maize chloroplast fragments exposed to continuous light depends on light intensity. Stimulation by HCO3- is less at low intensities. In HCO3--depleted chloroplasts exposed to brief saturating light flashes, period 4 oscillations (in O2 yield per flash) are damped within three cycles. Readdition of HCO3- to these preparations restores the oscillatory pattern to higher flash numbers, indicating that HCO3- reduces the probability of “misses” in the photosystem II reaction center. The rate of the dark relaxation reaction Sn′ → Sn+1 (where S refers to the oxidation state of the oxygen-evolving mechanism and n = 0, 1, or 2), after a photoact in the photosystem II reaction center, is retarded in HCO3--depleted chloroplasts compared to the rate for this reaction in depleted chloroplasts to which HCO3- has been resupplied. However, the final oxygen-evolving reaction after the accumulation of four positive charges appears to be independent of HCO3-. Bicarbonate has no effect on the dark deactivation of the higher oxidation states (S2 and S3) of the positive charge-accumulating system. We propose two alternate ways in which the kinetic model of oxygen evolution developed by Kok et al. [(1970) Photochem. Photobiol. 11, 457-475] can be extended to include the action of HCO3-.
Keywords: photosynthesis, Hill reaction, photochemical reactions of system II
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antoshechkin A. G. Chromosomal damage in Chinese hamster cells grown in U.V.-irradiated medium. Photochem Photobiol. 1970 Jan;11(1):49–52. doi: 10.1111/j.1751-1097.1970.tb05716.x. [DOI] [PubMed] [Google Scholar]
- Bouges-Bocquet B. Limiting steps in photosystem II and water decomposition in Chlorella and spinach chloroplasts. Biochim Biophys Acta. 1973 Apr 5;292(3):772–785. doi: 10.1016/0005-2728(73)90024-8. [DOI] [PubMed] [Google Scholar]
- Good N. E. Carbon Dioxide & the Hill Reaction. Plant Physiol. 1963 May;38(3):298–304. doi: 10.1104/pp.38.3.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kok B., Forbush B., McGloin M. Cooperation of charges in photosynthetic O2 evolution-I. A linear four step mechanism. Photochem Photobiol. 1970 Jun;11(6):457–475. doi: 10.1111/j.1751-1097.1970.tb06017.x. [DOI] [PubMed] [Google Scholar]
- Mar T., Govindjee Kinetic models of oxygen evolution in photosynthesis. J Theor Biol. 1972 Sep;36(3):427–446. doi: 10.1016/0022-5193(72)90001-x. [DOI] [PubMed] [Google Scholar]
- Stemler A., Govindjee Bicarbonate ion as a critical factor in photosynthetic oxygen evolution. Plant Physiol. 1973 Aug;52(2):119–123. doi: 10.1104/pp.52.2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West J., Hill R. Carbon dioxide and the reduction of indophenol and ferricyanide by chloroplasts. Plant Physiol. 1967 Jun;42(6):819–826. doi: 10.1104/pp.42.6.819. [DOI] [PMC free article] [PubMed] [Google Scholar]



