Abstract
Controlling amount of intrinsic S vacancies was achieved in ZnS spheres which were synthesized by a hydrothermal method using Zn and S powders in concentrated NaOH solution with NaBH4 added as reducing agent. These S vacancies efficiently extend absorption spectra of ZnS to visible region. Their photocatalytic activities for H2 production under visible light were evaluated by gas chromatograph, and the midgap states of ZnS introduced by S vacancies were examined by density functional calculations. Our study reveals that the concentration of S vacancies in the ZnS samples can be controlled by varying the amount of the reducing agent NaBH4 in the synthesis, and the prepared ZnS samples exhibit photocatalytic activity for H2 production under visible-light irradiation without loading noble metal. This photocatalytic activity of ZnS increases steadily with increasing the concentration of S vacancies until the latter reaches an optimum value. Our density functional calculations show that S vacancies generate midgap defect states in ZnS, which lead to visible-light absorption and responded.
In solving the global energy need and environmental pollution, hydrogen has attracted much attention for its potential to replace fossil fuels. Currently, however, pro-duction of hydrogen is mostly based on fossil fuels and on a process requiring high energy consumption1,2. Since the report of photocatalytic activity of TiO2 for hydrogen production, photocatalysis has become a desirable approach toward producing hydrogen with clean, environmentally friendly and economical process. In the past few decades, numerous photocatalysts have been found to exhibit high activities for water splitting3,4,5,6. These photocatalysts are mostly active only under UV light, which accounts for only 4% of the total sunlight. For practical applications, therefore, photocatalysts for hydrogen production need to operate under visible light.
ZnS is one of the most widely investigated photocatalysts because it rapidly generates electron-hole pairs under photoexcitation, and exhibits a relatively high activity for H2 production under UV light6,7. ZnS has a hexagonal structure and forms nanosheets or nanorods with large specific surface area7,8,9. Nevertheless, ZnS still has a substantially negative potential for excited electrons, and it does not respond to visible light due to its large band gap (~3.6 eV)10. Attempts have been made to extend the optical absorption of ZnS into the visible region by doping it with transition-metal ions (Au, Ni, Cu)10,11,12. However, these composite materials were differently to synthesize and hard to evaluate their intrinsic property of materials. Surface defects can also enhance light harvesting in photocatalytic materials13, and can also serve as adsorption sites where a charge transfer to the adsorbed species can prevent the recombination of photogenerated electrons and holes (namely, photogenerated charge carriers). However, when present in excessive amount, defects can act as traps for charge carriers leading to the recombination of photogenerated electrons and holes and hence decreasing the photocatalytic activity14,15,16,17. Therefore, controlling the amount of defects is great important to photocatalytic reaction. Vacancies are another kind of intrinsic defects in crystals, which are easily formed in quasi-two-dimensional materials because the exposed atoms on their surface can escape from the lattice hence affecting their physical and chemical properties18,19. Theoretical investigations have reported that S vacancies can decrease the band gap of ZnS, and the introduction of S vacancies is harder than Zn vacancies in ZnS crystals20,21. McCloy et al. introduced different vacancies into cubic ZnS crystals by chemical vapor deposition under various atmospheres and studied the change in their lattice parameters22. To our knowledge, there has been no study on S vacancies in ZnS with an aim for photocatalytic applications.
In the present work we first controlled amount of S vacancies in ZnS spheres, and characterized the ZnS samples with S vacancies by various experimental techniques and density functional calculations. The ZnS samples with controllable S vacancies were obtained via adding NaBH4 as a reducing agent in NaOH solution, and found to possess good photocatalytic activities for H2 production without loading noble metal under visible light irradiation. The probable cause for the visible-light photocatalytic activity of these ZnS materials was explored.
Results
XRD and SEM characterizations
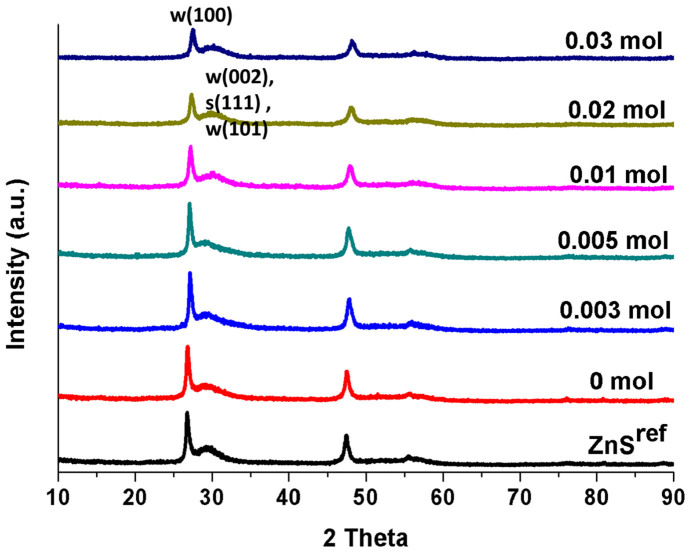
The XRD profiles for the ZnS samples synthesized using NaBH4 as reducing agent are given in Fig. 1, which is coordinated with that of ZnSref reported by Zhang et al10. These patterns have a strong and sharp peak at 2θ = 27.2°, which can be indexed as the (100) reflection of wurtzite ZnS, and the broader peak is the overlapping peaks of wurtzite (002), sphalerite (111), and wurtzite (101)10,23. These suggest that the {001} facet of wurtzite crystals is exposed, which is confirmed by SEM images (see below). The peaks of ZnS decrease with increasing the amount of NaBH4 (hence the amount of S vacancies), because S vacancies diminish the crystallinity of ZnS crystals.
Figure 1. XRD patterns obtained for ZnSref and the ZnS samples prepared using different amounts of NaBH4 (0–0.03 mol), w(100) = wurtzite(100), w(002) = wurtzite (002), s(111) = sphalerite (111), and w(101) = wurtzite (101).
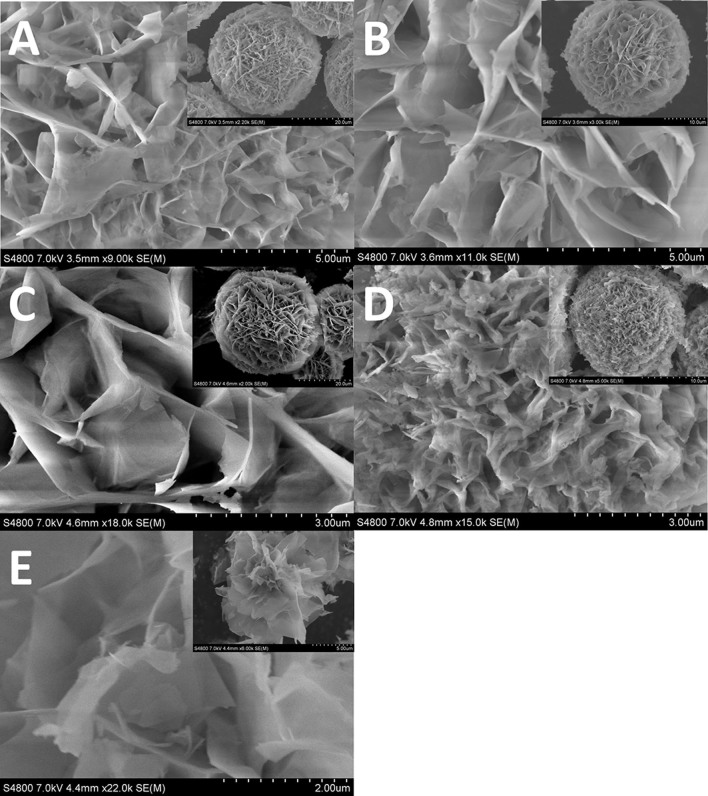
The morphologies of the prepared ZnS samples are shown in Fig. 2. The nanosheets of microspheres become crimped gradually with increasing the amount of NaBH4, because S vacancies enhance the stress in the nanosheets of ZnS. The morphology of the microsphere turns into a blossom as the amount of NaBH4 increases to 0.03 mol, in which nanosheets are already unfolded and broken. The morphologies of the microspheres are gradually ruptured, crisp and unfolded as the amount of NaBH4 is increased, which may decrease the specific surface areas of the samples reducing the photocatalytic activity.
Figure 2. SEM images of the ZnS samples synthesized using different amounts of NaBH4.
(A) 0.003, (B) 0.005, (C) 0.01, (D) 0.02, and (E) 0.03 mol NaBH4.
Optical absorption
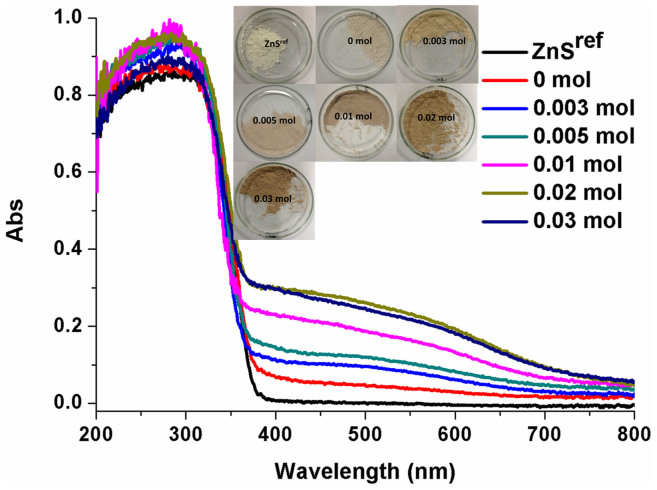
The UV-Vis diffuse reflectance spectra of the ZnS samples obtained under different conditions are presented in Fig. 3, with their colour changes presented in the inset. It is clearly seen that ZnSref is a typical direct gap semiconductor with no absorption in the visible light region. The ZnS samples, which obtained by the reaction between Zn and S powder without adding NaBH4 (0 mol), exhibited visible light absorption, but one cannot control their visible-light absorption and their photocatalytic activities are low. NaBH4 is a strongly reducing agent, and can reduce Zn2+ ions thereby creating S vacancies due to the charge balance requirement. Fig. 3 shows the absorption spectra measured for ZnSref and ZnS prepared without using NaBH4 as well as those prepared using NaBH4. The amount of NaBH4 used for synthesis clearly affects the visible-light absorption; the larger the amount of NaBH4, the stronger the absorption in the visible light.
Figure 3. UV-Vis diffuse reflectance spectra of ZnS synthesized using 0–0.03 mol of NaBH4.
Being a source of H−, NaBH4 can reduce H2O to generate hydrogen. However, under the high-concentration NaOH solution used in our synthesis, the concentration of H+ in the solution is negligible so that reduction of H2O by H− is negligible. Thus, H− reduces Zn2+ ions of the ZnS crystal lattice thereby forming Zn0. To keep the charge balance, the amount of S atoms in the ZnS crystal lattice should decrease leading to S vacancies. With increasing the amount of NaBH4, more S vacancies are formed resulting in a darker colour of the samples (see the inset of Fig. 3). Therefore, visible-light absorption of ZnS crystals can be tuned by the concentration of S vacancies, which in turn can be controlled by varying the amount of NaBH4 in the synthesis process.
XPS
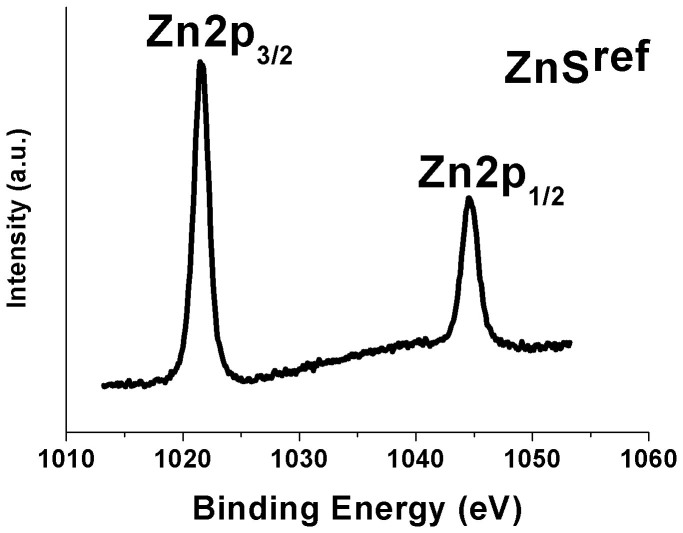
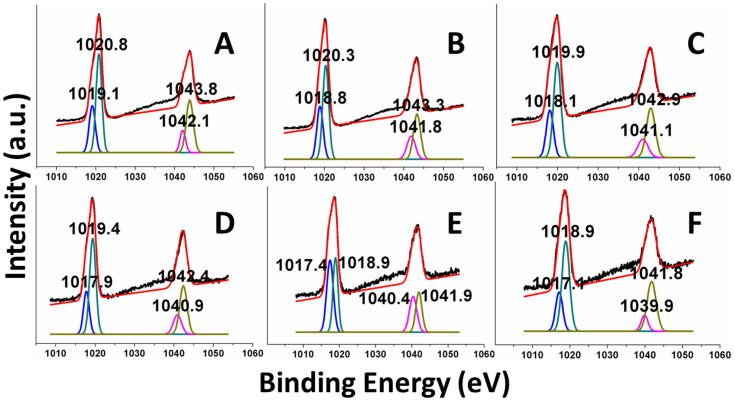
The chemical states of Zn2+ ions in ZnS samples were investigated by XPS (Full XPS survey scan display there was no impurity elements in the sample (Fig. S5, ESI†)) As shown in Fig. 4, the binding energies of the Zn 2p3/2 and Zn 2p1/2 peaks in ZnSref are about 1021.5 eV and 1044.5 eV, respectively24. When Zn and S powders were used for the synthesis without adding NaBH4, the resulting ZnS sample weakly absorbs visible light and a lower binding energy for both Zn 2p3/2 and Zn 2p1/2. This is attributed to the presence of some S vacancies20. With increasing the amount of NaBH4 during the synthesis, the binding energy for the Zn 2p3/2 and Zn 2p1/2 peaks of the ZnS samples shift gradually to a lower energy. This indicates that, to a certain degree, the concentration of S vacancies can be controlled by varying the amount of NaBH4 used. As depicted in Fig. 5, the Zn 2p3/2 peaks can be decomposed into two Gaussian peaks. With increasing the amount of NaBH4 from 0 to 0.03 mol, the major Zn 2p3/2 peaks change from 1020.8 eV to 1018.9 eV, and the minor Zn 2p3/2 peaks from 1019.1 eV to 1017.4 eV. At the same time, the major peaks of Zn 2p1/2 change from 1043.8 eV to 1041.8 eV, and the minor Zn 2p1/2 from 1042.1 eV to 1039.9 eV. All these peaks are lower in energy than that of ZnSref. It is reasonable to assign the major peaks to the Zn2+ ions away from the S vacancy sites, and the minor peaks to those Zn2+ ions adjacent to the S vacancy sites, and that the presence of Zn0 atoms around the S vacancy sites (see below) lowers the binding energies of the Zn2+ ions. EDS results also proved that absence of S element in ZnS samples (Fig S6, ESI†). The XPS results of S element in different samples were also investigated, and peaks of S 2p were all shifted to higher energy (Fig S7, ESI†).
Figure 4. XPS results of ZnSref sample.
Figure 5. Zn 2p3/2 spectra of the ZnS samples synthesized using different amounts of NaBH4.
(A) 0, (B) 0.003, (C) 0.005, (D) 0.01, (E) 0.02, and (F) 0.03 mol of NaBH4.
Surface areas of ZnS
An important factor affecting the photocatalytic activity of a ZnS sample is its surface area (Table 1). Fig S2 (ESI†) shows the typical isotherms of N2 adsorption onto the ZnS sample obtained using 0.01 mol of NaBH4, with the pore size distributions in the inset. These isotherms exhibit type IV isotherms with hysteresis loops according to the IUPAC classification25, which implies the presence of mesopores. The pore size distribution curves (the inset in Fig S2) indicate that the sample possesses mesopores and macropores in a wide size range of 10 to over 90 nm. Use of NaBH4 can increase the BET of the sample to a certain degree. The ZnS sample obtained with 0.01 mol of NaBH4 possesses a relatively large specific surface area, while use of a greater amount of NaBH4 decreases by destroying the microspheres.
Table 1. BET surface areas and pore structures of the ZnS samples obtained using different amounts of NaBH4.
| NaBH4 (mol) | Surface area (m2/g) | Pore volume (cc/g) | Pore size (Ǻ) |
|---|---|---|---|
| 0 | 113.1 | 0.55 | 97.7 |
| 0.003 | 87.4 | 0.65 | 148.3 |
| 0.005 | 93.9 | 0.36 | 94.2 |
| 0.01 | 129.3 | 0.61 | 87 |
| 0.02 | 112.9 | 0.5 | 89.1 |
| 0.03 | 90.1 | 0.53 | 117.6 |
Photocatalytic production of H2
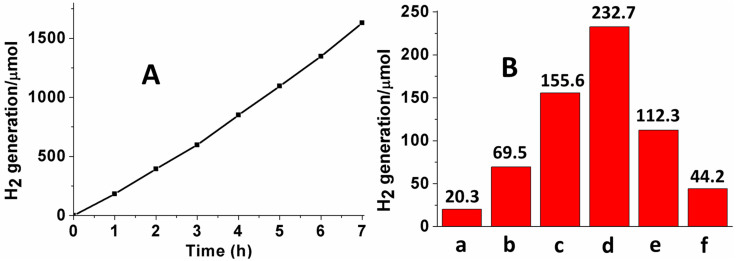
The ability of the ZnS samples (0.5 g) for photocatalytic hydrogen generations under visible light (λ > 420 nm) irradiation was evaluated by using 100 mL aqueous solution containing Na2S (0.25 M) and Na2SO3 (0.5 M) as sacrificial agents. The H2 production of the ZnS sample (obtained with 0.01 mol of NaBH4) as a function of the irradiation time is presented in Fig. 6A, which shows that the activity of the sample has no decrease after 7 h irradiation (The XPS results of this sample, before and after photocatalytic reaction, were also show in Fig S4, ESI†). The hydrogen production rates of the ZnS samples synthesized using different amounts of NaBH4 (0–0.03 mol), displayed in Fig. 6B, show that the production rate increases until the amount of NaBH4 reaches 0.01 mol but drops abruptly when it is beyond 0.01 mol. This means that the S vacancies enhance the visible light absorption resulting in an enhanced high photocatalytic activity. However, when present in excess amount, vacancies act as recombination centers for photogenerated carriers decreasing the photocatalytic activity. With increasing the amount of NaBH4 to 0.02 and 0.03 mol, lots of hierarchical microspheres are broken, which can also contribute to the precipitous decline of the H2 production rate. Cycling photocatalytic experiments of ZnS were also done for evaluating the stability of the ZnS samples (Fig S8, ESI†), which exhibited good stability after 3 times photocatalytic reactions.
Figure 6. Photocatalytic reactions results.
(A) Photocatalytic production of H2 by the ZnS sample (0.5 g) obtained using 0.01 mol NaBH4 as a function of the visible-light irradiation time. (B) Hydrogen production rate by ZnS samples obtained using different amounts of NaBH4 (a = 0 mol, b = 0.003 mol, c = 0.005 mol, d = 0.01 mol, e = 0.02 mol, and f = 0.03 mol).
PL spectra
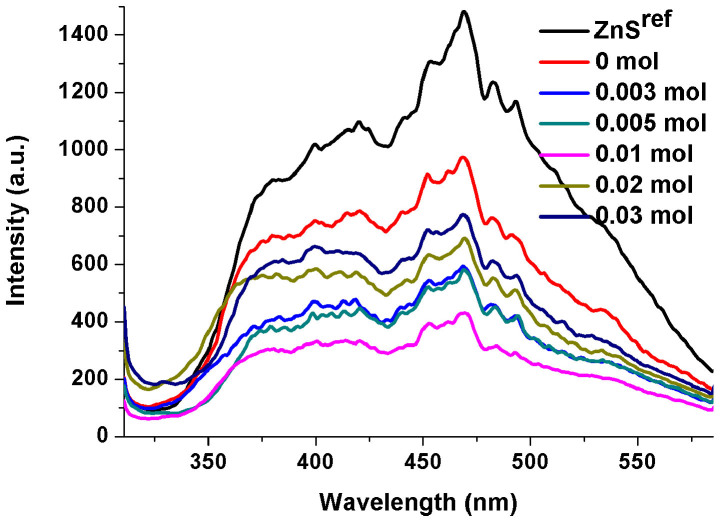
The photocatalytic activity is enhanced when photogenerated electron-hole pairs are efficiently separated. PL emission is an effective way of estimating the ability of a sample to separate these photogenerated carriers. The PL spectra of different ZnS samples are presented in Fig. 7, which were carried out on a Hitachi F-4500 fluorescence spectrophotometer at room temperature and obtained with excitation wavelength at 300 nm. It is observed that ZnSref has the highest intensity of photoluminescence emission, indicating that the photogenerated carriers are quickly recombined. The PL intensity of the ZnS samples decreases with increasing the amount of NaBH4 reaches 0.01 mol, but decreases when it is beyond 0.01 mol. It clearly indicates that the S vacancies can become centers for the recombination of photogenerated electrons and holes when present in excessive amount. This finding is consistent with the results of the photocatalytic activities of these ZnS samples.
Figure 7. PL spectra of different ZnS samples (λexc = 300 nm).
Density functional characterization of the defect states
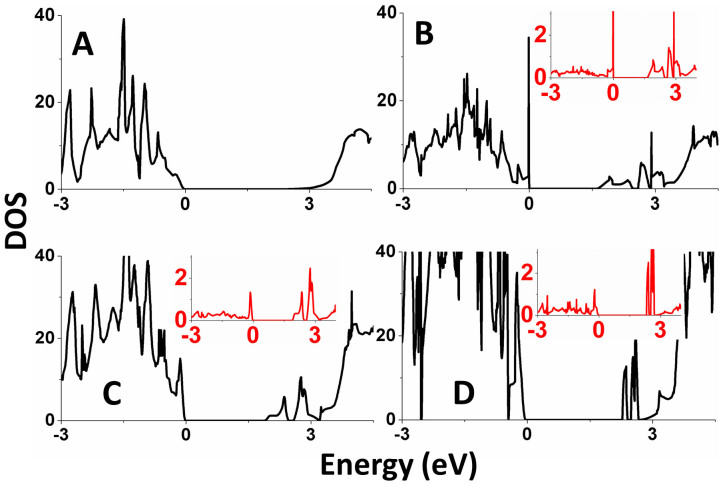
The density of states (DOS) plot calculated for ZnS with no S vacancy is presented in Fig. 8A, which shows a band gap of ~3.0 eV. The DOS plots calculated for the model ZnS systems with one S vacancy in the 2 × 2 × 2, 4 × 2 × 2 and 4 × 4 × 2 supercells are presented in Figs. 9B–D, respectively, where the insets show the projected DOS (PDOS) plots calculated for the four Zn atoms surrounding the S vacancy. These plots reveal that the S vacancy generates new states within the band gap of defect-free ZnS. The presence of these midgap defect states causes a visible-light absorption for the ZnS samples with S vacancies. It is of interest to discuss the formation of these defect states from the viewpoint of the local electronic structure around an S vacancy.
Figure 8. Total DOS and PDOS plots calculated for various ZnS with and without S vacancy.
The PDOS plots, shown in the inset, are calculated for the four Zn atoms surrounding the S vacancy. (A) Hexagonal ZnS without S vacancy in which there are 32 atoms per 2 × 2 × 2 supercell. (B) ZnS in which there is one S vacancy per 2 × 2 × 2 supercell. (C) ZnS in which there is one S vacancy per 4 × 2 × 2 supercell. (D) ZnS in which there is one S vacancy per 4 × 4 × 2 supercell.
Figure 9. The examination of the relaxed structures.
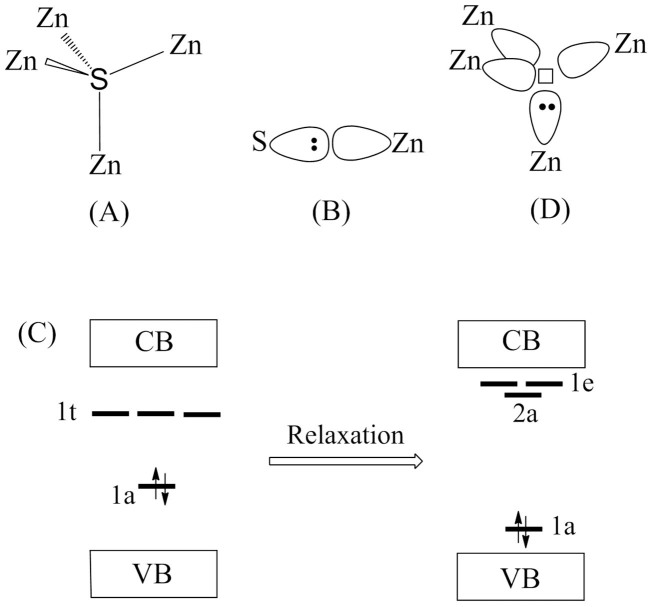
(A) SZn4 tetrahedron of ZnS. (B) Zn-S bonding as a combination of a filled sp3 hybrid orbital of S2− with an empty sp3 orbital of Zn2+. (C) Schematic diagram showing the midgap defect states of ZnS created by an S vacancy before (left) and after (right) relaxing the structures around the vacancy site. (D) Overall effect of an S vacancy showing the formation of one of the four ZnS3 pyramids surrounding the S vacancy getting two electrons. The VB and CB refer to valence and conduction bands, respectively. Here, for simplicity, it was assumed that the unrelaxed Zn4 tetrahedron has a Td symmetry, and the relaxed Zn4 tetrahedron a C3 symmetry.
The examination of the relaxed structures around the S vacancies show that one of the four ZnS3 pyramids surrounding the S vacancy becomes strongly pyramidal (with 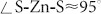 ) while the remaining three ZnS3 pyramids do not undergo a strong change in shape (with
) while the remaining three ZnS3 pyramids do not undergo a strong change in shape (with 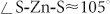 ). In ZnS each S2− ion is surrounded by four Zn2+ ions to form a SZn4 tetrahedron (Fig. 9A), and each Zn2+ ion surrounded by four S2− ions to form a ZnS4 tetrahedron. To a first approximation, the bonding in ZnS can be described in terms of the sp3 hybridization for the Zn and S atoms so that each Zn-S bond results from a bonding combination of a filled sp3 orbital of S2− (i.e., a sp3 lone pair) with an empty sp3 orbital of Zn2+ (Fig. 9B). When an S atom is removed from a Zn4 tetrahedron, each of the four Zn atoms surrounding the S vacancy becomes a ZnS3 pyramid with one sp3 dangling bond. If the structure around the vacancy site is not relaxed, then the interactions between the four dangling bonds lead to the midgap states 1a and 1t (1a < 1t) of the Zn4 tetrahedron lying approximately in the middle of the gap between the valence band maximum (VBM) and conduction band minimum (CBM) (left of Fig. 9C) (see Fig S3, ESI† for the DOS plot in the case of no geometry relaxation around the S vacancy). When the structure around the vacancy site is relaxed, the energy gap between the 1a and 1t levels becomes large with the 1t level splitting (right of Fig. 9C) due to the symmetry lowering. As a consequence, the 1a level comes close to the VBM and becomes doubly filled, while the three empty levels come close to the CBM. In the relaxed structure around an S vacancy, one ZnS3 pyramid that becomes strongly pyramidal (
). In ZnS each S2− ion is surrounded by four Zn2+ ions to form a SZn4 tetrahedron (Fig. 9A), and each Zn2+ ion surrounded by four S2− ions to form a ZnS4 tetrahedron. To a first approximation, the bonding in ZnS can be described in terms of the sp3 hybridization for the Zn and S atoms so that each Zn-S bond results from a bonding combination of a filled sp3 orbital of S2− (i.e., a sp3 lone pair) with an empty sp3 orbital of Zn2+ (Fig. 9B). When an S atom is removed from a Zn4 tetrahedron, each of the four Zn atoms surrounding the S vacancy becomes a ZnS3 pyramid with one sp3 dangling bond. If the structure around the vacancy site is not relaxed, then the interactions between the four dangling bonds lead to the midgap states 1a and 1t (1a < 1t) of the Zn4 tetrahedron lying approximately in the middle of the gap between the valence band maximum (VBM) and conduction band minimum (CBM) (left of Fig. 9C) (see Fig S3, ESI† for the DOS plot in the case of no geometry relaxation around the S vacancy). When the structure around the vacancy site is relaxed, the energy gap between the 1a and 1t levels becomes large with the 1t level splitting (right of Fig. 9C) due to the symmetry lowering. As a consequence, the 1a level comes close to the VBM and becomes doubly filled, while the three empty levels come close to the CBM. In the relaxed structure around an S vacancy, one ZnS3 pyramid that becomes strongly pyramidal (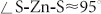 ) has its dangling bond doubly filled becoming a lone pair (Fig. 9D). Then the optical excitations associated with the filled defect level close the VBM and the empty defect levels below the CBM are responsible for the visible-light absorption of the ZnS samples with S vacancies. Furthermore, trapping of photogenerated electrons and holes by these defect states helps slow down their recombination, thereby enhancing the photocatalytic activities of ZnS samples with S vacancies.
) has its dangling bond doubly filled becoming a lone pair (Fig. 9D). Then the optical excitations associated with the filled defect level close the VBM and the empty defect levels below the CBM are responsible for the visible-light absorption of the ZnS samples with S vacancies. Furthermore, trapping of photogenerated electrons and holes by these defect states helps slow down their recombination, thereby enhancing the photocatalytic activities of ZnS samples with S vacancies.
In summary, aimed at finding photocatalytic ZnS systems for H2 production operating under visible-light irradiation, we synthesized a new ZnS samples with controlled amount of S vacancies by the hydrothermal reaction of zinc and sulfur powders with NaBH4 added as a reducing agent. The concentration of S vacancies in the ZnS samples can be easily controlled by varying the amount of NaBH4. The as-prepared ZnS samples exhibit enhanced visible-light absorption with increasing the concentration of S vacancies. The photocatalytic activities of these samples for H2 production, evaluated under visible light irradiation, show that the photocatalytic activity increases with increasing the concentration of S vacancies until it reaches the optimum value, but decreases sharply when it goes beyond the optimum value because, when present in excessive amount, S vacancies destroy both crystal structure and morphologies of the samples and also play as recombination sites of photogenerated electrons and holes. Our density functional analysis reveals that S vacancies generate midgap defect states in ZnS, which are responsible for visible-light absorption and also act as trap centers for electrons and holes improving the separation efficiency of photogenerated charge carries. This work provides a novel method to induce and control S vacancies in crystals for enhancing their photocatalytic H2 production under visible light.
Methods
Matrials
All chemicals in this work (i.e., Zn powder, sublimed sulfur, NaOH, NaBH4, Na2S·9H2O, Na2SO3) were AR reagents, and were used without any further purification.
Preparation of ZnS
Samples of ZnS with S vacancies were synthesized by mixing Zn powder and sublimed sulfur in 50 ml of 21 M NaOH solution. After the suspension was cooled to room temperature, a various amount of NaBH4 (0–0.03 mol) was added to control the amount of S vacancies in ZnS crystals. After constant stirring for 2 h, the mixed solution was transferred into a 120 ml sealed Teflon-lined autoclave and was heated at 230°C for at least 12 h. The autoclave was slowly cooled down to room temperature, and the obtained sample was washed with distilled water, and was finally dried at 40°C overnight. For the purpose of comparison, we prepared a reference ZnS sample following the method proposed by Zhang et al.10 with slight modification10, which will be hereafter referred to as ZnSref; a certain amount of ZnO was dissolved in 50 ml of 21 M NaOH solution, and Na2S·9H2O was added after the solution cooled down. The suspension was transferred into a 120 mL sealed Teflon-lined autoclave and followed by hydrothermal treatment at 230°C for at least 12 h. The sample was also collected after washing and drying.
Sample characterizations
XRD patterns were recorded on a Bruker AXS D8 Advance powder diffractometer (Cu Kα X-ray tube, λ = 0.154056 nm). The morphologies of the samples were obtained using SEM (Hitachi S-4800). The chemical energy-dispersive X-ray spectrum (EDS) were examined by an energy dispersive X-ray spectrometer equipped in the SEM machine. The surface areas of the as-prepared samples were measured by the BET method using nitrogen adsorption-desorption isotherms on a Micromeritics ASAP 2020 apparatus at liquid nitrogen temperature. UV-Vis diffuse reflectance spectra were obtained for dry-pressed disk samples by using a Shimadzu UV 2550 recording spectrophotometer equipped with an integrating sphere, and BaSO4 was used as a reference. PL spectra were carried out on a Hitachi F-4500 fluorescence spectrophotometer at room temperature and the excitation wavelength was 300 nm.
Details of calculations
Our density functional calculations employed the projector augmented wave method as implemented in the Vienna ab initio simulation package26,27,28,29, the generalized gradient approximation of PBE for exchange and correlation corrections30,31 with plane wave cutoff energies of 400 eV, and a threshold of self-consistent-field energy convergence of 10−4 eV. The atomic positions were fully optimized until all the residual forces are smaller than 0.01 eV/Å. The structure of hexagonal ZnS was constructed on the basis of 2 × 2 × 2, 4 × 2 × 2 and 4 × 4 × 2 supercells containing 32, 64 and 128 atoms, respectively. Various model structures of sulfur-deficient ZnS were constructed by removing one sulfur atom from the 32-atom 2 × 2 × 2 supercell (see Fig. S1 ESI†), 64-atom 4 × 2 × 2 supercell, and 128-atom 4 × 4 × 2 supercell.
Photocatalytic activity for H2 prodution
A top irradiation vessel connected to a glass-enclosed gas-circulation system was used to evaluate the photocatalytic hydrogen evolution of the ZnS samples. In a typical photocatalytic experiment, 0.5 g sample was suspended in 100 mL aqueous solution containing Na2S·9H2O (0.35 M) and Na2SO3 (0.25 M) as sacrificial agents. The temperature was maintained at 5°C. The visible light source was obtained from a 300 W Xe arc lamp (PLS-SXE 300, Beijing Trusttech Co. Ltd.) equipped with an ultraviolet cutoff filter (λ > 420 nm). The amount of H2 produced was determined with a gas chromatograph (Varian GC-3800) equipped with thermal conductivity detector.
Author Contributions
G.W., B.B.H., Z.Z.L. and Z.Y.W. conceived the project, carried out the experiments and analysed the experimental data. Z.J.L., Y.D. and M.-H.W. performed the density functional theory calculations. All authors contributed to writing the manuscript.
Supplementary Material
SI
Acknowledgments
This work was financially supported by research grants from the National Basic Research Program of China (the 973 Program; 2013CB632401), the National Natural Science Foundation of China (21333006, 11374190, and 51231091).
References
- Cortright R. D., Davda R. R. & Dumesic J. A. Hydrogen from catalytic reforming of biomass-derived hydrocarbons in liquid water. Nature 418, 964–967 (2002). [DOI] [PubMed] [Google Scholar]
- Turner J. A. Sustainable Hydrogen Production. Science 305, 972–974 (2004). [DOI] [PubMed] [Google Scholar]
- Kudo A. & Miseki Y. Heterogeneous photocatalyst materials for water splitting. Chem. Soc. Rev 38, 253–278 (2009). [DOI] [PubMed] [Google Scholar]
- Abe R., Higashi M., Sayama K., Abe Y. & Sugihara H. Photocatalytic activity of R3MO7 and R2Ti2O7 (R = Y, Gd, La; M = Nb, Ta) for water splitting into H2 and O2. J. Phys. Chem. B 110, 2219–2226 (2006). [DOI] [PubMed] [Google Scholar]
- Li Q. et al. Highly efficient visible-light-driven photocatalytic hydrogen production of CdS-cluster-decorated graphene nanosheets. J. Am. Chem. Soc 133, 10878–10884 (2011). [DOI] [PubMed] [Google Scholar]
- Hu J. S. et al. Mass production and high photocatalytic activity of ZnS nanoporous nanoparticles. Angew. Chem. Int. Ed 44, 1269–1273 (2005). [DOI] [PubMed] [Google Scholar]
- Zhang J. et al. Visible light photocatalytic H2-production activity of CuS/ZnS porous nanosheets based on photoinduced interfacial charge transfer. Nano. Lett 11, 4774–4779 (2011). [DOI] [PubMed] [Google Scholar]
- Chen X. J. et al. Kinetically controlled synthesis of wurtzite ZnS nanorods through mild thermolysis of a covalent organic-inorganic network. Inorg. Chem 42, 3100–3106 (2003). [DOI] [PubMed] [Google Scholar]
- Wang Y. H. et al. NaOH concentration effect on the oriented attachment growth kinetics of ZnS. J. Phys. Chem. B 111, 5290–5294 (2007). [DOI] [PubMed] [Google Scholar]
- Zhang J. Y. et al. Enhanced photocatalytic hydrogen production activities of Au-loaded ZnS flowers. ACS Appl. Mater. Interfaces 5, 1031–1037 (2013). [DOI] [PubMed] [Google Scholar]
- Kudo A. & Sekizawa M. Photocatalytic H2 evolution under visible light irradiation on Ni-doped ZnS photocatalyst. Chem. Commun 1371–1372 (2000) 10.1039/b003297m. [DOI] [Google Scholar]
- Arai T. et al. Cu-Doped ZnS Hollow Particle with High Activity for Hydrogen Generation from Alkaline Sulfide Solution under Visible Light. Chem. Mater 20, 1997–2000 (2008). [Google Scholar]
- Zhang W., Zhong Z. Y., Wang Y. S. & Xu R. Doped Solid Solution: (Zn0.95Cu0.05)1−xCdxS Nanocrystals with High Activity for H2 Evolution from Aqueous Solutions under Visible Light. J. Phys. Chem. C 112, 17635–17642 (2008). [Google Scholar]
- Wang J. P. et al. Oxygen Vacancy Induced Band-Gap Narrowing and Enhanced Visible Light Photocatalytic Activity of ZnO. ACS. Appl. Mater. Interfaces 4, 4024–4030 (2012). [DOI] [PubMed] [Google Scholar]
- Kong M. et al. Tuning the relative concentration ratio of bulk defects to surface defects in TiO2 nanocrystals leads to high photocatalytic efficiency. J. Am. Chem. Soc 133, 16414–16417 (2011). [DOI] [PubMed] [Google Scholar]
- Linsebigler A. L., Lu G. Q. & Yates J. T. Jr. Photocatalysis on TiOn Surfaces: Principles, Mechanisms, and Selected Results. Chem. Rev 95, 735–758 (1995). [Google Scholar]
- Lv Y. H. et al. The surface oxygen vacancy induced visible activity and enhanced UV activity of a ZnO1−x photocatalyst. Catal. Sci. Technol. 3, 3136–3146 (2013). [Google Scholar]
- Niu P., Liu G. & Cheng H. M. Nitrogen vacancy-promoted photocatalytic activity of graphitic carbon nitride. J. Phys. Chem. C 116, 11013–11018 (2012). [Google Scholar]
- Liu G. et al. Enhanced Photoactivity of Oxygen-Deficient Anatase TiO2 Sheets with Dominant {001} Facets. J. Phys. Chem. C 113, 21784–21788 (2009). [Google Scholar]
- Guang M. L. et al. Vacancy Associates Promoting Solar-Driven Photocatalytic Activity of Ultrathin Bismuth Oxychloride Nanosheets. J. Am. Chem. Soc 135, 10411 (2013). [DOI] [PubMed] [Google Scholar]
- Chen J. H., Zeng X. Q., Chen Y. & Zhang H. P. First-principle theory calculations of electronic structure of sphalerite with vacancy and impurity. Chin. J. Nonferrous. Met 20, 765–771 (2010). [Google Scholar]
- McCloy J. S., Wolf W., Wimmer E. & Zelinski B. J. Impact of hydrogen and oxygen defects on the lattice parameter of chemical vapor deposited zinc sulfide. J. Appl. Phys. 113, 023706(1–7) (2013). [Google Scholar]
- Hong Y. P., Lin Z., Huang J., Wang Y. J. & Huang F. Study on the influence of lattice integrity and phase composition to the photocatalytic efficiency of ZnS material. Nanoscale 3, 1512–1515 (2011). [DOI] [PubMed] [Google Scholar]
- Biswal N., Das D. P., Martha S. & Parida K. M. Efficient hydrogen production by composite photocatalyst CdS-ZnS/Zirconium-titanium phosphate (ZTP) under visible light illumination. Int. J. Hydrogen Energy 36, 13452–13460 (2011). [Google Scholar]
- Li D. S. et al. The Mass Production of ZnS Nanoarchitecture via Thermodynamic Design. Cryst. Growth. Des 8, 2324–2328 (2008). [Google Scholar]
- Kresse G. & Furthmüller J. Efficiency of ab-initio total energy calculations for metals and semiconductors. Comput. Mater. Sci. 6, 15–50 (1996). [Google Scholar]
- Kresse G. & Furthmüller J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996). [DOI] [PubMed] [Google Scholar]
- Wang Z. Y. et al. Enhanced Ferromagnetism and Tunable Saturation Magnetization of Mn/C-Codoped GaN Nanostructures Synthesized by Carbothermal Nitridation. J. Am. Chem. Soc 130, 16366–16373 (2008). [DOI] [PubMed] [Google Scholar]
- Wang G. et al. Cu2(OH)PO4, a Near-Infrared-Activated Photocatalyst. Angew. Chem. Int. Ed 52, 4810–4813 (2013). [DOI] [PubMed] [Google Scholar]
- Perdew J. P. et al. Atoms, molecules, solids, and surfaces: Applications of the generalized gradient approximation for exchange and correlation. Phys. Rev. B 1992, 46, 6671–6687. [DOI] [PubMed] [Google Scholar]
- Perdew J. P., Burke K. & Ernzerhof M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett 77, 3865–3868 (1996). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SI