Summary
Evolutionary modifications in nervous systems enabled organisms to adapt to their specific environments and underlie the remarkable diversity of behaviors expressed by animals. Resolving the pathways that shaped and modified neural circuits during evolution remains a significant challenge. Comparative studies have revealed a surprising conservation in the intrinsic signaling systems involved in early patterning of bilaterian nervous systems, but also raise the question of how neural circuit compositions and architectures evolved within specific animal lineages. In this Review we discuss the mechanisms that contributed to the emergence and diversity of animal nervous systems, focusing on the circuits governing vertebrate locomotion.
Introduction
The earliest nervous systems are thought to have consisted of distributed populations of sensory neurons and motor neurons that enabled animals to detect environmental changes and translate this information into specific motor actions (Holland, 2003). Execution of appropriate motor responses to stimuli is essential to the survival of an organism and one of the most fundamental aspects of nervous system function. Even the most complex regions of vertebrate nervous systems, such as the human cortex, can be considered as processing centers whose primary role is to interpret sensory information and transform it into specific motor commands.
In vertebrates much of the activity of the central nervous system is channeled into the brainstem and spinal cord with the sole purpose of coordinating the activation of muscles. The most well studied motor circuits in vertebrates are those that control walking and breathing, yet we know very little about the genetic modifications that facilitated the emergence of even these relatively simple animal behaviors. In the vertebrate lineage fundamental changes in the nervous system coincided with the transition from aquatic to terrestrial terrains, and necessitated the modulation and rewiring of existing locomotor and respiratory neuronal networks. A major goal has been to resolve how these essential motor circuits are constructed during development, and to determine how they evolved and diversified.
Comparisons of transcription factor profiles between diverse bilaterian species suggest deep conservation in the intrinsic signaling pathways controlling early nervous system patterning. Perhaps the most dramatic example is seen in the development of the visual system. Studies in mice and flies have demonstrated that key aspects of early eye development are controlled by a relatively small number of conserved fate determinants (Gehring, 2014). For example, the transcription factor Pax6/eyeless has a central role in the development of photodetection systems in both vertebrates and insects, and misexpression of mouse Pax6 can generate ectopic eyes in imaginal discs of Drosophila embryos (Halder et al., 1995). More recent studies indicate that a large number of transcription factors involved in early patterning along the dorsoventral and rostrocaudal axes are conserved in both vertebrates and invertebrates (Denes et al., 2007; Lowe et al., 2003), implying that the nervous system of the common ancestor to all bilaterians was already quite sophisticated (De Robertis and Sasai, 1996).
Given the remarkable conservation in the expression of key patterning genes, how did nervous systems evolve to generate new motor behaviors within various animal lineages? In this Review we discuss how alterations in developmental pathways enabled nervous systems to construct, and in some cases deconstruct, motor circuits that govern genetically predetermined locomotor behaviors. Because the link between neuronal identity and circuit connectivity has been closely examined in the spinal cord, we focus on the circuits governing the development of vertebrate motor systems, and describe how early intrinsic patterning systems impact circuit assembly and function. We discuss evidence that small changes in transcription factor activity can act as a major driving force for evolutionary modification of circuit architectures. Second, we argue that within the spinal cord a flexible system involving modulation of rostrocaudal positional information, acting in the context of a relatively uniform dorsoventral patterning system, can act to modify neuronal organization and connectivity within circuits governing a specific locomotor output.
Ancestral Origins of Neural Induction and Early Patterning
During the earliest phases of neural development regions of ectoderm are allocated to acquire neuronal characteristics. Naïve neural ectoderm subsequently acquires regional identities that prefigure the organization of motor circuits in the adult. On the surface, there appears to be fundamental differences in how nervous systems develop in distantly related species. Subsequent to neural induction the majority of neurons in Drosophila are specified in lineages that are governed through temporal specification codes, and a single progenitor can give rise to multiple neuronal classes (Kohwi and Doe, 2013). In contrast patterning in the vertebrate neural tube is driven by extrinsic morphogen-based signaling, and progenitors typically give rise to only a few classes of neurons (Jessell, 2000). Despite these significant differences, many species appear to use a common set of intrinsic determinants during early neural patterning. In this section we compare and contrast the mechanisms of neural induction and global patterning within the two major superphyla of bilaterians, protostomes (which includes arthropods and annelids) and deuterostomes (which includes chordates, hemichordates, and echinoderms) (Figure 1A).
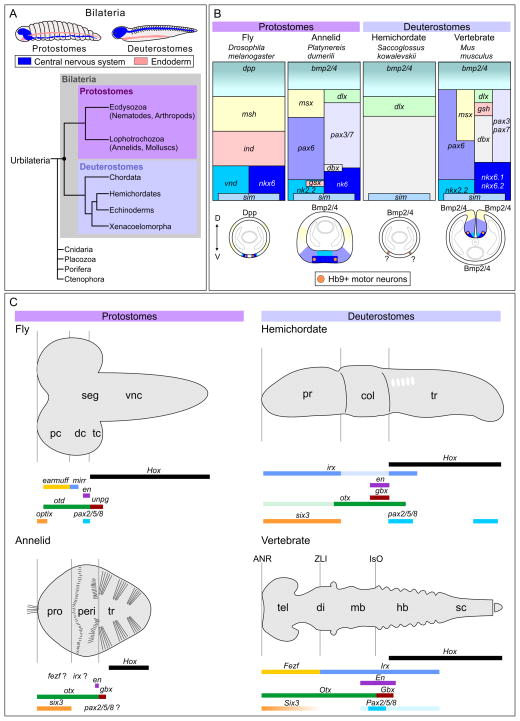
Figure 1. Neural Induction and Early Patterning in Bilateria.
(A) Traditional classification of bilateria. Bilaterians are a subgroup of eumetazoan animals characterized by a bilaterally symmetrical body plan and triploblastic development. Bilaterians are subdivided into protostomes (mouth-first) and deuterostomes (mouth-second). Top: The central nervous system (CNS) (in blue) forms ventrally in protostomes and dorsally in deuterostomes. Bottom: A simplified phylogenetic tree, showing the evolutionary relationships amongst bilaterians and other metazoan phyla.
(B) Conservation of gene expression patterns along the dorsoventral (DV) axis in protostomes (flies, annelids) and deuterostomes (hemichordates and vertebrates). In both protostomes and deuterostomes expression of neural identity genes is patterned by Bmps along the DV axis of the nerve cord (Esteves et al., 2014). Ventral patterning cues are not portrayed here as they are not homologous in different species (e.g., Dorsal in flies, Shh in vertebrates). As in vertebrates, cholinergic Hb9+ MNs derive from pax6+nk6+ progenitors and directly innervate muscles in annelids (Denes et al., 2007). In flies, there are additional MN populations (not depicted here) in addition to Hb9+ MNs. Although Bmp-Chordin signaling is present in hemichordates, many DV patterning genes are not expressed by the neuroectoderm (e.g., nk2.2 in endoderm). The Mnx gene which shares high homology with Hb9 homeodomain is expressed in the hemichordate ventral ectoderm implicating possible conservation in MN specification (Lowe et al., 2006). Homologous genes are color-coded. Schematics on the bottom represent cross-sections of the embryos.
(C) Conservation of anteroposterior patterning systems in bilaterians. Although protostomes do not have analogous neuroectodermal signaling centers present in developing vertebrate brains, key genes determining their boundaries are conserved along the anteroposterior axis. The en gene is also expressed at parasegmental boundaries in the epidermis of flies and annelids. In hemichordates, the expression of fezf (not shown here) is not adjacent to that of irx. Homologous genes are color-coded for comparison. pc, protocerebrum; dc, deutocerebrum; tc, tritocerebrum; seg, subesophageal ganglion; vnc, ventral nerve cord; pro, prostomium; peri, peristomium; tr, trunk (both in annelid and hemichordate); pr, proboscis; col, collar; tel, telencephalon; di, diencephalon; mb, midbrain; hb, hindbrain; sc, spinal cord. Comparisons between species represented in panel (A) and (B) do not take into account gene expression differences and therefore do not represent a true cladistics analysis. Furthermore, this model does not fully take into account the development of animals with unsegmented nervous systems such as in molluscs. Panel (A) is modified from (De Robertis, 2008; Philippe et al., 2011) and panel (B) is modified from (Denes et al., 2007; Mizutani and Bier, 2008).
Neural Induction and Dorsoventral Patterning in Bilaterians
The formation of bilaterian nervous systems is initiated through neural induction, a process where the neural plate is specified within a restricted region of ectoderm. In most species, neural induction involves bone morphogenetic protein (Bmp) signaling along the dorsoventral axis (De Robertis, 2008). Bmp signaling suppresses neural differentiation, the “default” fate of ectodermal cells, and promotes epidermal differentiation. In vertebrates Bmp antagonists (noggin, chordin, and follistatin) are secreted from the dorsal organizer, thereby differentiating ectodermal cells into neural tissue. Subsequently, gradients of dorsal Bmps, in conjunction with ventral sonic hedgehog (Shh) signaling, establish subdivisions of progenitor domains along the dorsoventral axis of the neural tube in chordates (Jessell, 2000).
Although there are significant morphological differences among bilaterian nervous systems, Bmp signaling plays a conserved role in both protostomes and deuterostomes (Figure 1A). For example, the vertebrate Bmp antagonist Chordin acts similarly to its Drosophila homolog sog (short gastrulation) in promoting neuronal fate (Holley et al., 1995). The Drosophila Bmp homolog dpp can phenocopy Bmp4 activity when expressed in Xenopus. Early Bmp expression is inversely correlated with the position where the CNS develops in both protostomes and deuterostomes, although the relative position of where the nervous system forms is distinct in both phyla. In protostomes the nerve cord forms ventrally, while in deuterostomes the nerve cord forms dorsally (Figure 1A). This relationship suggested a “dorsoventral inversion” hypothesis, where the central nervous systems of all bilaterians have a common origin, and an inversion of the dorsoventral axis occurred during deuterostome evolution (Arendt and Nubler-Jung, 1994; De Robertis and Sasai, 1996).
Further support for a common origin of bilaterian nervous systems have emerged from studies of neural development in protostome annelids. These studies revealed that the transcriptional regulatory networks required for early dorsoventral patterning in the vertebrate nerve cord are present in protostomes (Denes et al., 2007). Like other bilaterians Bmp signaling has a key role in annelid neural induction. Annelids also show a higher degree of similarity with vertebrates than Drosophila in the expression of neural patterning genes (Figure 1B). For example, the ventral determinants Nkx2.2 and Pax6 are expressed in mutually exclusive domains in both vertebrates and annelids, but this pattern is not conserved in fly (Kammermeier et al., 2001). In addition, like vertebrates annelid motor neurons (MNs) are generated from a ventral domain characterized by expression of the transcription factor Hb9, and these neurons are cholinergic. This contrasts with the embryonic CNS of Drosophila, where MNs are generated in multiple lineages and are typically glutamatergic.
Repression of neural induction by Bmps appears to have been lost in hemichordates, although Bmp-chordin signaling and orthologs of dorsoventral target genes are expressed (Lowe et al., 2006). This phenomenon may be due to its unique nervous system organization which consists of two nerve cords, one dorsal and one ventral, and a diffuse basiepidermal nerve net (Holland, 2003; Nomaksteinsky et al., 2009). A possible explanation provided by Arendt and colleagues is that hemichordates such as acorn worms might have modified their trunk neuroarchitecture due to the evolutionary changes in locomotor behaviors (Denes et al., 2007). Furthermore, a recent study provides additional evidence for conserved dorsoventral patterning cues in hemichordates. The hedgehog receptor patched is expressed ventrally in the collar nerve cord, while hedgehog is expressed in the endoderm of the buccal tube and the stomochord, similar to the relationship between ptc in the neural tube and Shh in the floor plate and notochord of vertebrates (Miyamoto and Wada, 2013).
Conservation of Rostrocaudal Patterning Cues in Bilaterians
Soon after neural induction in vertebrates, cells from neural plate acquire rostrocaudal positional identities and segregate into four major regions: the forebrain, midbrain, hindbrain and spinal cord. The anterior neural plate has three primary signaling centers that produce morphogens involved in rostrocaudal patterning: the anterior neural ridge (ANR), zona limitans intrathalamica (ZLI) and isthmic organizer (IsO). These neuroectodermal signaling centers were thought to have originated in the vertebrate central nervous system since they are either absent or divergent in other chordates (Bertrand et al., 2011; Holland et al., 2000; Imai et al., 2009; Irimia et al., 2010; Shimeld, 1999; Takatori et al., 2002). Recently, Lowe and colleagues provided evidence that inductive centers homologous to the ANR, ZLI and IsO are present in hemichordates, suggesting that they are ancient patterning systems that were present in early deuterostomes (Pani et al., 2012). Additionally, extensive analysis from Kirschner and colleagues revealed that the hemichordate nervous system shows remarkable conservation in rostrocaudal patterning (Lowe et al., 2003). While there are some differences in the rostrocaudal expression domains within the 22 orthologs of chordate neural patterning genes that were tested, the relative expression domains are very similar to vertebrates (Figure 1C).
Although the corresponding extrinsic signaling centers are absent from protostomes, early anteroposterior patterning has been reported in several species indicating that compartmental-like boundaries existed in the common bilaterian ancestor (Figure 1C). For example, recent studies reveal that the Drosophila brain has a tripartite ground plan similar to vertebrates and displays conserved expression of transcription factors that are key to the development of vertebrate nervous systems (otx2, gbx2, fezf, irx, pax2/5/8, Hox) (Hirth et al., 2003; Irimia et al., 2010). Similarly the segmental expression pattern of otx, gbx, and Hox genes in the protostome annelids parallels the pattern in Drosophila (Steinmetz et al., 2011). These results support the hypothesis that the nervous system of the common “urbilaterian” ancestor of all bilaterians had an organized CNS which was patterned by shared intrinsic signaling programs (De Robertis and Sasai, 1996).
Neuronal Class Specification, Guidance Systems, and Neuronal Organization
In vertebrates early patterning systems act on neuronal progenitors to prefigure cells to express a set of cell identity determinants at the time of cell cycle exit. The pattern of transcription factor expression in newly born neurons generates a remarkable diversity in cell types, a defining feature of most animal nervous systems. How neuronal cell types are specified is a first step towards elucidating how neurons are interconnected to establish a specific circuit. Here we outline the mechanisms through which large classes of neurons are specified, and the strategies through which neuronal subtypes essential within motor systems emerged in the vertebrate lineage. Recent evidence indicates that in some cases a transcription factor class present in multiple species can target the same genes that define the core physiological properties of a neuronal type.
Cell Fate Specification and Neurotransmitter Identity
Near the time of terminal differentiation transcription factors act to define the core physiological properties of neurons as well as features which allow them to establish their initial connectivity patterns. The nervous systems of many species contain thousands of molecularly and anatomically distinct cell types and it has historically been challenging to establish a unifying classification scheme (Masland, 2004). For simplicity we define the steps through which neurons acquire their identities as “class” and “subtype” specification programs. In vertebrates, neurons within a class typically derive from a single molecularly defined progenitor domain, use a common neurotransmission system, and form connections with similar types of neurons. Subtypes of neurons within a class are more loosely defined, but often express different sets of transcription factors, establish connections that are distinct from other subtypes, and can be morphologically distinct. In terms of evolutionary changes, neuronal classes are often present throughout animal species, whereas subtypes show the greatest evolutionary diversification.
A defining characteristic of neurons within a single class is the expression of genes encoding elements of neurotransmitter systems, including proteins involved in neurotransmitter synthesis and release. Expression of neurotransmitter genes appears to rely on the actions of transcription factors expressed in postmitotic cells, the identities of which have only been resolved in recent years. This question has been worked out in greatest clarity in C. elegans, where cohorts of genes involved in neurotransmission are controlled by a relatively small number of transcription factors acting on common cis-regulatory elements (Hobert, 2011). As these factors are capable of controlling a large number of genes that act in the same synthetic pathway, they have been called “terminal selectors”. Terminal selectors are typically expressed throughout the life of an organism, and their expression can be maintained through positive transcriptional autoregulation (Deneris and Hobert, 2014). Many of the regulatory proteins defined in C. elegans are functionally conserved in vertebrates. For example the C. elegans ETS family transcription factor ast-1 plays a critical role in regulating the battery of genes involved in dopamine synthesis (Flames and Hobert, 2009). In vertebrate olfactory neurons the ast-1 homolog Etv-1 directly controls the terminal synthetic enzyme required for dopamine synthesis, tyrosine hydroxylase. Similar conservation is observed in the regulation of glutamatergic fates by Lim homeodomain (HD) factors (Serrano-Saiz et al., 2013). The regulatory factors that control neurotransmitter synthesis in C. elegans are also tied to programs that regulate other features of a neuronal class, such as expression of ion channels, cell adhesion molecules, and determinants of axonal and dendritic morphology (Kratsios et al., 2012; Serrano-Saiz et al., 2013). These observations indicate that terminal selectors act on common cis-elements to establish and maintain the identity of a neuron throughout an animal’s lifespan.
Similar to C. elegans, regulation of neurotransmitter identity in vertebrates is linked to gene networks governing multiple aspects of neuronal identity and connectivity (Figure 2A). The motor neurons of vertebrates use acetylcholine (Ach) as the primary neurotransmitter to activate muscle and other neurons. Cholinergic gene batteries are directly regulated through complexes formed between the Lim HD proteins Isl1 and Lhx3 and their cofactor Lbd1 (Cho et al., 2014; Lee et al., 2012). This complex is also required to regulate the gene encoding the transcription factor Hb9 (Lee et al., 2008), a key determinant of multiple facets of MN subtype differentiation (Arber et al., 1999; Thaler et al., 1999). While vertebrates use Lim HD proteins to orchestrate Ach synthesis in MNs, C. elegans uses a distinct class of transcription factor, the COE family member unc-3 (Kratsios et al., 2012). Nematodes do, however, use Lim HD factors to regulate Ach synthesis in interneuron subtypes (Zhang et al., 2014). Another layer of complexity is apparent when one considers how MNs activate muscles in different model organisms. While vertebrates and C. elegans MNs use the cholinergic system, embryonic MNs of Drosophila activate muscles using glutamate, although both flies and mice require the same set of transcription factors (Hb9, Isl1, Lhx3) for diversifying MNs into subtypes. Protostome annelids also express class determinants similar to vertebrates, and their MNs are cholinergic (Denes et al., 2007). This observation supports the idea that the urbilaterian ancestor contained MNs that were similar to those of modern vertebrates. Flies and nematodes may have therefore evolved distinct mechanisms for controlling neurotransmitter systems in MNs.
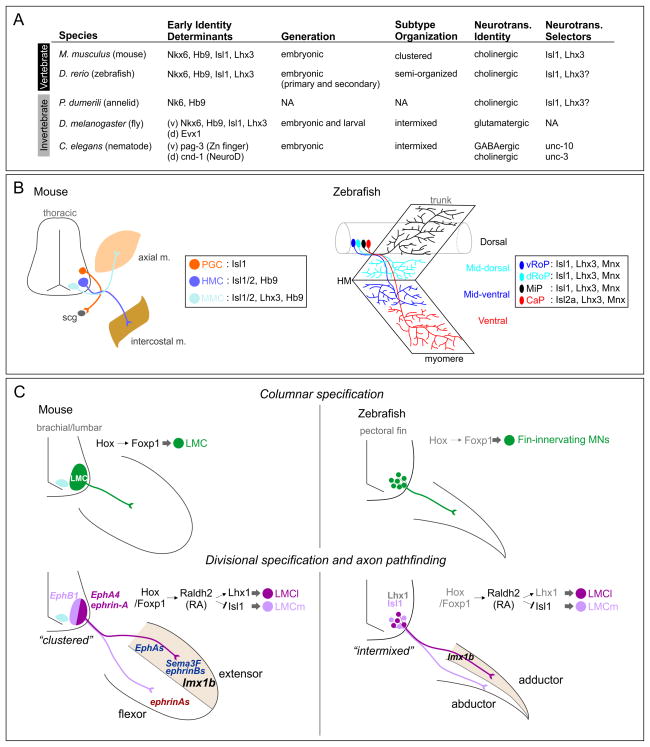
Figure 2. Motor Innervation Programs in Bilaterians.
(A) Table showing conservation and divergence of MN cell fate specification programs in invertebrates and vertebrates, emphasizing known conserved transcription factors. Several key transcription factors involved in MN specification are not indicated. NA, not assessed.
(B) Comparisons of MN organization and innervation patterns between mouse and zebrafish at trunk levels. Core MN determinants, Isl1/2, Hb9 and Lhx3, are expressed in different combinations in three distinct thoracic columns in mouse. scg, sympathetic chain ganglia. Zebrafish embryos contain four classes of primary MNs, vRoP (ventrally-projecting rostral primary), dRoP (dorsally-projecting RoP), MiP (medial primary) and CaP (caudal primary), and they do not organize into tightly clustered columns (Menelaou and McLean, 2012). They are classified by their specific innervation of axial muscles from dorsal to ventral. The stereotypic innervation patterns of each primary MN are depicted here. Although three Mnx proteins are detected within each primary MN subtype in zebrafish, Mnx proteints are only required in MiP MNs (Seredick et al., 2012).
(C) MN organization and specification programs at limb/fin levels in mouse and zebrafish. In zebrafish, pectoral fin innervating MNs are considered to be secondary due to their late development and ventrolateral position relative to primary MNs (Myers, 1985). A GFP reporter under control of an Isl1 enhancer indicates that Isl1+ pectoral fin MNs selectively innervate abductor muscles (Uemura et al., 2005). Untested aspects of these models are shown in gray.
Convergence of cell fate determinants and neurotransmitter systems is also apparent when comparing different neuronal classes that share the same neurotransmitter identity. In addition to spinal MNs, cholinergic neurons are present in specific neurons of the vertebrate forebrain. Interestingly, the logic of the transcription factor network regulating cholinergic gene batteries are very similar in both regions. In MNs Lhx3 and Isl1 have key roles in regulation of cholinergic genes, while Lhx8 and Isl1 serve similar roles in the forebrain (Cho et al., 2014; Lopes et al., 2012). Thus in the context of neurotransmitter gene batteries, key targets can be regulated through highly conserved cis-regulatory elements. Evolutionary diversification of neurons using the same neurotransmitter system could in principle be achieved by utilization of multiple members of the same transcription factor family.
Ancestry and Evolution of Genetic Programs for Muscle Innervation
In addition to neurotransmitter systems a defining feature of neurons within a specific class are the types of cells with which they establish connections. Because of their central role in motor circuits, we will emphasize the connectivity patterns of motor neuron subtypes. The motor neurons of most species are characterized by the extension of axons outside the CNS, local connectivity with certain classes of interneurons and sensory neurons, as well as descending inputs from supraspinal areas. The basic program of peripheral connectivity with muscle is likely to be conserved across many bilaterian species, since determinants necessary for the selectivity of their peripheral projections are conserved in protostomes and deuterostomes. In mice and flies, ventrally projecting MNs can be defined by expression of Hb9, Nkx6, and Lim HD proteins, with each class member also acting at later stages to define the peripheral connectivity of MN subtypes.
A common feature of motor systems in many protostome and deuterostome species is the innervation of segmentally organized axial muscles by MNs. In tetrapods the selection of axial muscles is largely determined by the actions of Lim HD proteins and Hb9 (Figure 2B). Dorsal epaxial and ventral hypaxial muscles are innervated by motor columns that are defined by the expression of these factors. Hypaxial muscle, which includes intercostal and abdominal muscles are innervated by ventrally projecting MNs that express Isl1 and Hb9, while dorsal epaxial muscles are innervated by MNs expressing Lhx3 and Hb9 (Figure 2B). Lhx3 has a central role in differentiating dorsally from ventrally projecting MN subtypes, as misexpression of Lhx3 can suppress all other MN subtype specification programs and force motor axons to select a dorsal trajectory (Dasen et al., 2008; Sharma et al., 2000). In other species the logic of the Lim code with respect to the peripheral trajectories of motor axons is distinct. In zebrafish there is no clear correlation between the selection of dorsoventral trajectories of primary MNs and the expression of specific Lim HD proteins (Figure 2B), although MNs subtypes can be distinguished based on differential expression of these factors (Appel et al., 1995). Similarly in Drosophila the basic decision to project dorsally or ventrally involves a different class of transcription factors, where the Evx1 homolog even-skipped is required in dorsally projecting MNs, with Lim HD factors and Hb9 acting to define subtypes of ventrally projecting populations both in the embryonic and adult nervous system (Lacin et al., 2014; Landgraf and Thor, 2006).
A significant evolutionary advancement in the vertebrate lineage was the generation of MN subtypes that enabled the articulation of muscles in the limb. However, it is largely unknown at what stage in vertebrate evolution the program for limb innervation emerged. In vertebrates limb innervating MNs are organized into the lateral motor column (LMC), and are defined by the expression of the transcription factor Foxp1, and the retinoic acid synthetic enzyme Raldh2 (Figure 2C) (Dasen and Jessell, 2009). Amongst Foxp1+ limb-MNs, those projecting to the dorsal limb compartment express Lhx1, while those projecting ventrally express Isl1 (Dasen et al., 2008; Tsuchida et al., 1994). The establishment of this Lim HD code is essential for the peripheral connectivity of LMC axons. In the case of limb-innervating MNs, the effectors of these cell fate determinants have been well characterized, and include members of the Eph/ephrin signaling system, which are regulated by Lim HD proteins and determine the response of motor axons to ephrin signaling in the limb mesenchyme (Kao et al., 2012).
Analysis of limb-level MNs in other species suggests that some, but not all, aspects of appendage innervation programs are conserved amongst vertebrates (Figure 2C). Representatives of each of the four main classes of tetrapods (birds, reptiles, amphibians, and mammals) express similar profiles of transcription factors in LMC neurons (Jung et al., 2014). In zebrafish, the Lim HD code that defines the dorsoventral selection of motor axons appears to be conserved at the level of the pectoral fin (Uemura et al., 2005), and expression of Raldh2 has been reported in pectoral fin-level MNs (Begemann et al., 2001). However selective expression of Foxp1 by fin-level MNs has not been reported, nor is there any direct evidence that rostrocaudal positional identity determinants (e.g., Hox genes) have any role in MN subtype specification.
Many arthropod species also bear appendages involved in walking, although it appears that their leg innervation program arose independently. The common ancestor to protostomes and deuterostomes is thought to have lacked appendages, and this limbless state was preserved in early chordates, suggesting that the Foxp1/Lim HD code emerged in the vertebrate lineage. As a consequence of the independent origins of limb innervation programs, many basic features of MN organization and connectivity have diverged between vertebrates and invertebrates.
Evolution of Motor Neuron Somatotopic Organization
A highly varied feature of bilaterian motor systems is reflected in how motor neurons are organized. In tetrapods, MNs projecting to a common target zone or specific muscle are clustered in longitudinally arrayed columnar and pool groups. This organization creates a somatotopic map within the spinal cord that links cell body position to the peripheral trajectory of motor axons. The clustering of MNs is present in all tetrapods that have been examined, as well as some species of fish (Fetcho, 1987; Jung et al., 2014). In Drosophila and C. elegans, as well as aquatic vertebrates such as zebrafish, MNs targeting specific muscles do not cluster into coherent columnar groups (Thor and Thomas, 2002), although there is evidence that zebrafish MNs are dorsoventrally organized based on their activation at different locomotor speeds (Ampatzis et al., 2013). These observations raise the question of what is the significance of the clustering of MNs in tetrapods, and what evolutionary advantages it provides in terms of motor circuit connectivity and function. One possibility is that the complexity of vertebrate limb musculature necessitated a strategy to ensure that MNs receive selective inputs from other neuronal classes (e.g., interneurons and sensory neurons) on the basis of their position, rather than through specific molecular determinants.
In tetrapods the organization of MN cell bodies is controlled by signaling pathways that determine the migratory and adhesive properties of columnar and pool subtypes. A MN pool that targets a single muscle in the limb is clustered into groups of ~50–200 MNs that occupy a stereotypic intrasegmental and rostrocaudal position within the spinal cord. Members of the type II cadherin family have been implicated in pool clustering, as they display columnar- and pool-specific patterns of gene expression, and genetic manipulations that perturb cadherin expression or signaling randomizes of MN position (Demireva et al., 2011; Price et al., 2002). Expression of type II cadherins is regulated by intrinsic signaling systems including columnar-specific transcription factors such as Foxp1 and pool-restricted factors such as the Ets protein Pea3 (Dasen et al., 2008; Livet et al., 2002). In Foxp1 mutants cadherin expression is lost in LMC neurons and the position of MNs targeting a muscle is randomized within the spinal cord (Dasen et al., 2008; Surmeli et al., 2011).
The phenotype of Foxp1 mutants provides a means to test the hypothesis that settling position is a determining factor in the specificity of connections that MNs establishes centrally. Consistent with this idea, mutation in Foxp1, which scrambles MN cell body position, but otherwise preserves core features of MN identity, leads to formation of inappropriate connections between MNs and proprioceptive sensory neurons (Surmeli et al., 2011). This observation is consistent with the hypothesis that the organization of MN into clustered groups may have evolved to facilitate synaptic specificity within the context of an increased diversity of limb muscles in tetrapods (Fetcho, 1987).
Evolutionary Diversification of Effector Neurons in Motor Systems
The evolution of motor networks can be easily appreciated when one considers the diversity of locomotor behaviors exhibited by animals (e.g., swimming, walking, flying, and hopping). During vertebrate evolution fundamental changes in motor circuits accompanied the acquisition of paired-appendages and the transition of tetrapods from the sea to land (Figure 3A). The most primitive vertebrates are thought to have lacked paired appendages and are represented in modern species by agnathan (jawless) fish including lamprey and hagfish. Locomotion in agnathans is achieved through propagation of sinusoidal waves of muscle contraction along the body axis, and this locomotor strategy is observed in a range of species including nematodes, insect larvae, and snakes. While some modern fish can utilize the fins to generate a walking-like form of locomotion on the sea floor (Macesic and Kajiura, 2010), the predominant role of paired appendages in aquatic species is for steering, not propulsion. Therefore, the basic locomotor strategy in most fish is axial-based undulation, which has led to the proposal that motor circuits for walking evolved in species similar to modern amphibians (Murakami and Tanaka, 2011). Amphibians and reptiles appear to represent an intermediate step in the emergence of walking circuits, as some species display a combination of both undulatory and ambulatory locomotor behaviors (Figure 3A).
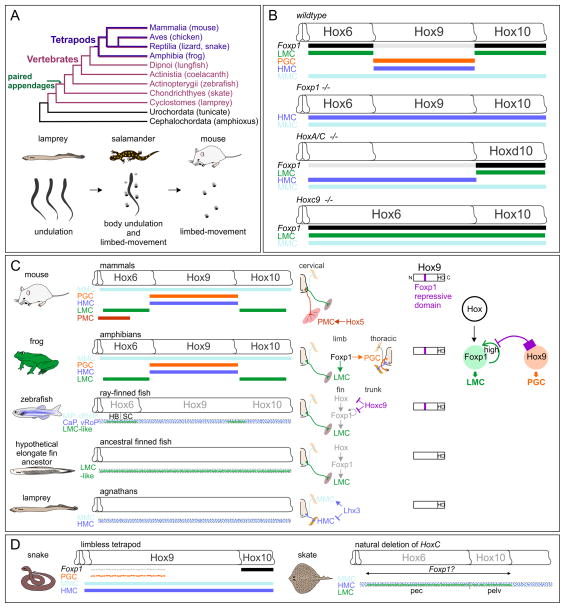
Figure 3. Evolutionary Diversity of Spinal Motor Neurons.
(A) Evolution of locomotor strategies. Top: A chordate phylogeny showing representative species of tetrapods (dark purple) and vertebrates (light purple). Chondrichthyans represent the most primitive species bearing paired appendages. Bottom: Comparisons of locomotor behaviors in lamprey, salamander and mouse.
(B) Altered MN columnar organization in Foxp1 and Hox mutants. In Foxp1 mutants Hox-dependent spinal MN columns (LMC and PGC) are transformed into an HMC-like “ground state”, which may represent a primitive condition. PMC neurons are present in Foxp1 mutants, but not depicted. Loss of LMC neurons at brachial levels is achieved only when HoxA and HoxC gene clusters are mutated. Lumbar LMC neurons are preserved in HoxA/C cluster mutant mice due to Hoxd10 activity. Deletion of the Hoxc9 gene causes global derepression of brachial Hox genes resulting in an extension of the brachial LMC throughout thoracic levels. MMC neurons are considered Hox-independent as their molecular profiles are preserved in each of these mutants.
(C) A model showing how MN organization has evolved with changes in body plans. A subset of MNs in agnathan vertebrates (represented by modern lampreys) may have lost Lhx3 activity, permitting the generation of HMC-like neurons. The acquisition of paired-appendages promoted the generation of LMC-like populations, which may have been initially present at most spinal levels. A repressive domain within Hox9 proteins necessary to suppress LMC specification appears to have emerged when the elongate fin split into pectoral and pelvic fins. Studies in zebrafish suggest the pectoral fin MNs were initially positioned in both the hindbrain (HB) and spinal cord (SC) (Ma et al., 2010). Pelvic fin innervating MNs do not align with Hox10 gene expression (Murata et al., 2010). In mammals, PMC neurons are specified by Hox5 proteins and are Foxp1-independent (Philippidou et al., 2012).
(D) In snake embryos expansion of Hoxc9 expression blocks LMC generation. The enlarged-finned fish skate, which naturally has lost the HoxC cluster, may have extended LMC population along the anteroposterior axis of the spinal cord.
Limb-based locomotion requires the precise coordination of individual muscles in the limb and hence, a more complex peripheral innervation program than is needed for undulatory locomotion. Insights into how motor circuits for walking emerged can be gleaned from understanding the mechanisms that fostered evolutionary changes in MN organization and connectivity.
Hox Networks in Spinal Motor Neuron Diversity and Organization
While all MNs share certain core properties, they are a highly diverse population which has evolved unique functions in different animal lineages. The spinal MNs of tetrapods are topographically organized into columns and pools, and express subtype identity determinants that allow them to make selective connections with their peripheral targets (Dasen and Jessell, 2009). In addition to the motor columns targeting epaxial (MMC), hypaxial (HMC), and limb muscles (LMC), several additional columnar subtypes appeared at different stages of vertebrate evolution. In tetrapods, the preganglionic column (PGC) is generated at thoracic levels and innervates the sympathetic chain ganglia (Figure 3B, C). Because sympathetic neurons are derived from neural crest cells, a migratory population that evolved in early chordates (Bronner and LeDouarin, 2012), PGC neurons likely emerged during neural crest diversification. A group of specialized MNs involved in respiratory function appeared later in vertebrate evolution. The phrenic motor column (PMC) neurons are generated at cervical levels of the spinal cord and innervate the diaphragm muscle. The PMC is unique to mammals, and is absent from birds, reptiles, and amphibians (Figure 3C). At limb levels, neurons within the LMC fractionate into ~50 motor pools and this diversity likely emerged concomitant with the increased complexity of tetrapod limb musculature.
How are MN columnar and pool subtypes specified during development? Motor neuron diversification relies on the large family of Hox genes, an evolutionarily conserved family of transcription factors essential in governing animal body plans along the rostrocaudal axis (McGinnis and Krumlauf, 1992). In tetrapods Hox genes are arrayed in 4 chromosomal clusters and their expression is governed through opposing FGF and rostral RA signaling gradients acting on neural progenitors (Dasen et al., 2003; Liu et al., 2001). Although Hox genes are restricted to specific rostrocaudal levels, they are widely expressed by most neuronal classes within the hindbrain and the spinal cord (Dasen et al., 2005). Efforts to elucidate their functions during neural patterning have focused largely on their roles in specifying MN subtype identities.
The role of Hox genes in MN specification has been investigated by genetic manipulation of their activities in mice and chick. The generation of segmentally-restricted MN subtypes (PMC, PGC, HMC, LMC neurons, and LMC pools) is governed by Hox genes expressed at specific levels, although the strategies involved vary significantly depending on the MN population. The columnar identity of limb-innervating MNs is controlled by multiple redundant Hox inputs, and only through combined deletion of the HoxA and HoxC cluster is LMC identity erased at forelimb levels (Jung et al., 2014; Lacombe et al., 2013). In contrast at thoracic levels MN subtypes rely on the single Hoxc9 gene, in the absence of Hoxc9 function HMC and PGC neurons acquire an LMC fate (Jung et al., 2010). This phenotype is due, in part, to the derepression of Hox genes expressed at forelimb levels (Figure 3B). At limb levels a network of ~20 Hox genes establishes the identity and connectivity of motor pools targeting limb muscles (Philippidou and Dasen, 2013). Given their central roles in MN subtype specification, modulation in Hox protein activities likely had a key role in the evolutionary diversification of motor circuits.
Origins of Motor Neuron Diversity
If Hox genes are involved in the diversification of motor neurons, at what stage in vertebrate evolution did this program first appear, and what are the ancestral MN populations that Hox genes acted upon? Insight into this question has emerged through analysis of a primary target of Hox proteins in MNs, the transcription factor Foxp1. In quadrupeds, a critical function of Hox proteins expressed by LMC neurons is to regulate expression of Foxp1. The majority of limb-level Hox proteins can induce high levels of Foxp1 when ectopically expressed in thoracic MNs, while the thoracic Hoxc9 protein represses Foxp1 levels when expressed at limb levels (Jung et al., 2014; Lacombe et al., 2013). In mice lacking the Foxp1 gene, MNs fail to express essential molecular determinants of Hox-dependent subtypes, and MNs that have lost Foxp1 retain expression of markers for thoracic HMC neurons (Dasen et al., 2008; Rousso et al., 2008). In contrast, dorsally projecting MMC neurons are unaffected by loss of Foxp1. As a consequence, mice with Foxp1 deletion consist largely of HMC and MMC subtypes extending throughout the spinal cord (Figure 3B).
The organization of MNs in Foxp1 mutants appears to resemble an ancestral state of the motor system present in primitive aquatic vertebrates lacking limbs, similar to modern agnathan species. Lamprey locomotion is driven by MMC-like neurons innervating segmentally iterated axial muscles that drive undulatory locomotion (Fetcho, 1992). While patterned Hox expression is present in lampreys (Takio et al., 2007), in this context Hox proteins presumably have no influence on spinal MN subtype diversification. The tetrapod motor system therefore likely coopted a preexisting Hox network to allow Foxp1 to be induced in HMC-like precursors. The emergence of LMC neurons in appendage-bearing vertebrates likely required evolutionary changes in functions of Hox proteins and/or modification of cis-regulatory elements within the Foxp1 gene.
Further evidence that HMC neurons serve as the evolutionary substrate for Hox-dependent MN diversification programs comes from analysis of the development of respiratory neurons in mammals. In mice PMC neurons require the actions of two Hox5 genes, Hoxa5 and Hoxc5. In Hox5 mutants molecular determinants for PMC neurons are lost, and the diaphragm fails to be properly innervated, leading to respiratory failure (Philippidou et al., 2012). In other tetrapod classes Hox5 proteins are expressed by cervical LMC neurons and were likely co-opted in mammals for regulating PMC-specific genes. This process may have been facilitated by partial duplication of cervical segments of the spinal cord (Hirasawa and Kuratani, 2013), which may have served to allow a new MN population to utilize Hox5 function in PMC specification, while preserving their function in LMC subtype specification. A similar strategy of co-option appears to have occurred during the development of insect nervous systems, as Hox genes have recently been shown to be instrumental in the development of peptidergic interneurons and leg-innervating motor neurons in Drosophila (Baek et al., 2013; Karlsson et al., 2010).
Hox Genes and the Evolutionary Diversification of Motor Effector Systems
Do changes in the profile of Hox gene activities contribute to the evolutionary diversification of motor circuits? Comparisons of Hox expression patterns amongst limb-bearing and limbless tetrapods have provided insights into this question. Snakes evolved from limb-bearing reptiles but presumably no longer require the MN subtypes necessary for limb motility. Analysis of Hox gene expression in snake embryos revealed that expression of the thoracic Hoxc9 gene is broadly extended along the rostrocaudal axis, and may account for the absence of LMC neurons (Figure 3D) (Jung et al., 2014). Furthermore, the ability of the Hoxc9 protein to repress limb-innervation programs relies on an N-terminal peptide motif present only in Hox9 proteins of vertebrates bearing paired appendages. This motif acts by blocking an autoregulatory circuit activated by limb-level Hox proteins which promotes high levels of Foxp1 expression in LMC neurons (Figure 3C). The repressive motif in Hoxc9 is present in both aquatic and terrestrial vertebrate species, including modern representatives of the most primitive fin-bearing vertebrates. These observations indicate that the repressive functions of Hoxc9 emerged at the time vertebrates acquired paired appendages.
Analysis of Hoxc9 activities suggests that Hox signaling contributed to the evolution of motor systems in early vertebrates. This interpretation is surprising, given that many Hox-dependent programs, such as clustering of MNs into columnar groups, and the alignment of Hox expression to specific MN subtypes are apparently not present in bony fish such as zebrafish (Appel et al., 1995; Murata et al., 2010). One possible explanation is that the utilization of Hox signaling in MNs may be more relevant in marine species that use fins as the primary mode of locomotion, such as in batoid chondrichthyans (rays and skates). In skates for example, the pectoral and pelvic fins develop adjacent to one another with no intervening “thoracic” level (Maxwell et al., 2008). Moreover, stingrays have a population of fin-innervating MNs extending over ~80 segments (Droge and Leonard, 1983). Interestingly, whole genome analysis of chondrichthyans revealed that elasmobranchs, which include skates and rays, lack the entire HoxC cluster (King et al., 2011). It is possible that removal of Hoxc9 gene in batoids allowed for the extension of fin-innervating MNs along the rostrocaudal axis of the spinal cord (Figure 3D).
Evolution of the Neural Circuits Governing Locomotion
While analyses of MN specification programs have revealed important insights into how peripheral innervation patterns have evolved, locomotor behaviors in vertebrates driven through assemblies of rhythmically active neural circuits residing in the brainstem and spinal cord. These networks, termed central pattern generators (CPGs), are composed of several classes of locally connected interneuron subtypes that provide the primary drive to MNs during basic motor actions. Both axial-based undulatory and limb-based ambulatory locomotion rely on CPG activities (Grillner and Jessell, 2009), and there is emerging evidence that limb CPGs evolved from cooption of preexisting undulatory motor circuits (Bagnall and McLean, 2014; Sillar et al., 2008).
The most thoroughly investigated CPG circuits in tetrapods are the locomotor networks residing within the spinal cord and the respiratory rhythm generator in the brainstem. Recent studies indicate that locomotor CPG circuits are constructed in a modular fashion, and alteration in their neuronal components can have a dramatic effect on gait characteristics. Changes in the composition and connectivity of neurons within CPGs likely contributed to evolutionary adaptations in motor behaviors.
Commissural Interneurons and Locomotor CPG Output
In tetrapods spinal CPGs can facilitate two types of locomotor output, one which ensures coordination between left and right halves of the spinal cord in animals that walk (L-R CPGs), and a second which facilitates reciprocal activation of extensor and flexor muscles within an appendage (E-F CPGs) (Goulding and Pfaff, 2005). Amongst vertebrate species there is considerable diversity in how CPG circuits are organized. While bipedal and quadrupedal animals typically alternate left and right limbs during walking, most avian species and several terrestrial species (e.g., rabbits, kangaroos, bats, and desert jerboas) utilize CPGs that activate muscles in both limbs synchronously. The mode of L-R CPG operation appears to be a consequence of the activities of excitatory and inhibitory commissural interneurons (CINs) that project their axons across the midline of the spinal cord (Vallstedt and Kullander, 2013). CINs control L-R CPGs via the connections that they establish with MNs and interneurons. Hemisection of the spinal cord leads to discoordination in L-R CPGs, while preserving E-F CPG function, indicating the circuits governing limb alternation rely on CINs (Kjaerulff and Kiehn, 1996; Lanuza et al., 2004).
The activities of L-R CPGs are coordinated through two distinct CIN-driven microcircuits (Bernhardt et al., 2013). One circuit ensures L-R alternation through reciprocal inhibition of the contralateral half of the spinal cord when the ipsilateral side is active. A parallel CPG circuit facilitates synchronous activity and predominates in the absence of inhibitory CIN connections. Evidence supporting this model comes from genetic manipulations that shift the balance in excitation-inhibition ratios across the midline (Figure 4A). Eph/ephrin signaling plays an essential role in CIN guidance, where expression of ephrinB3 in midline spinal populations prevents the crossing of excitatory EphA4-positive ipsilaterally projecting interneurons (IINs). Mutation in EphA4 or its ligand ephrinB3 in mice leads to inappropriate excitatory projections to the contralateral side of the spinal cord (Kullander et al., 2003). Mice lacking EphA4 or ephrinB3 display a hopping-like motor behavior, likely due to a shift in the balance from inhibitory towards excitatory connections with MNs. The spinal autonomy of this L-R CPG defect was confirmed using fictive locomotor assays that measure the activities of MN through ventral root recordings in isolated spinal cords (Figure 4B). Complete L-R synchrony is also observed in mice mutant for the midline attractant Netrin1, which disables the ability of inhibitory CINs to cross the midline (Rabe et al., 2009). Netrin signaling also controls commissural axon guidance in Drosophila, suggesting deep conservation in the signaling pathways ensuring communication between both halves of the nerve cord (Zarin et al., 2014).
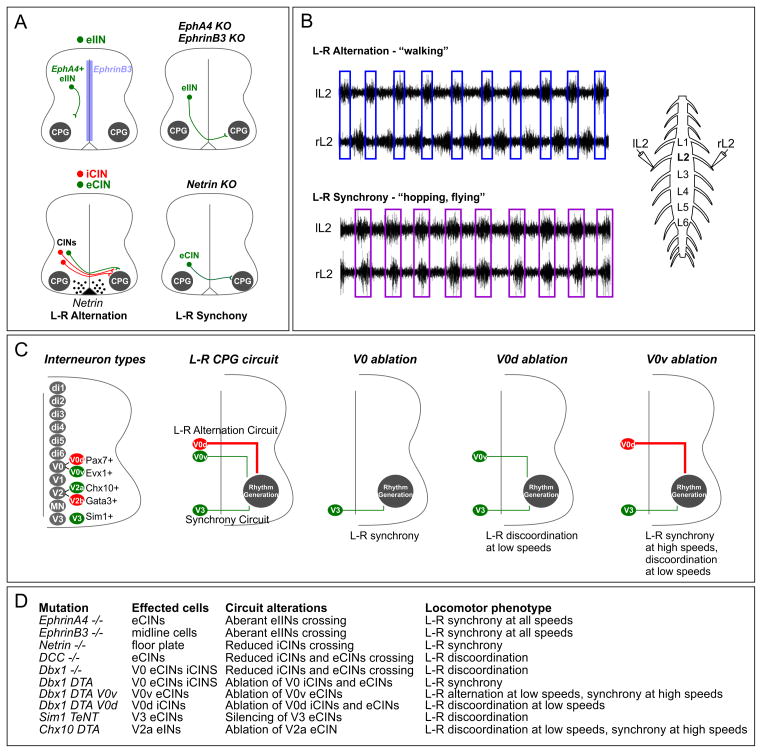
Figure 4. Central Pattern Generators and Locomotor Behaviors.
(A) Genetic mutations in guidance systems that lead to synchronous bilateral activation of limb-level MNs (hopping) in mice. Mutations in EphA4 or ephrinB3 cause multiple classes of excitatory ipsilaterally projecting interneurons (eIINs) to aberrantly cross the ventral midline. Mutation in netrin causes fewer inhibitory commissural interneurons (iCINs) to cross, but preserves some eCINs projections.
(B) Examples of fictive locomotor assays in mice. Ventral root recordings from lumbar level L2 showing bursts of MN activation at regular intervals. In control mice bursts recorded from left L2 (lL2) and right L2 (rL2) alternate. In netrin mutants both sides of the spinal cord burst in phase. Images are modified from (Rabe et al., 2009).
(C) Intrinsic factors involved in CIN specification. Excitatory and inhibitory CINs are derived from multiple progenitor domains that are defined by transcription factor expression. Factors expressed by postmitotic neurons are indicated. Both V0d and V0v interneurons are derived from progenitors expressing Dbx1. Genetic silencing of V0 populations causes changes in the connections between CINs and target cells on the contralateral side of the spinal cord.
(D) Partial list of genetic manipulations that affect left right alternation. Locomotor phenotypes described represent analysis using either fictive locomotor or behavior assays, or the combinations of both.
The role of specific classes of spinal interneurons in locomotor CPGs has been closely examined in mice, and many of the cell types required in L-R CPG circuits have been genetically characterized (Arber, 2012). Interneurons originating from the p0 progenitor domain generate two types of postmitotic commissural interneuron subtypes, V0v and V0d, characterized by specific transcription factor profiles and neurotransmitter systems (Figure 4C). All V0 neurons derive from progenitors expressing the transcription factor Dbx1. This population further segregates into an excitatory population expressing Evx1 (V0v) and an inhibitory population expressing Pax7 (V0d). Mutation in Dbx1 leads to discoordination in left-right alternation characterized by episodes of synchronous activity (Lanuza et al., 2004). Genetic silencing of both V0 populations leads to synchronous activation of both sides of the spinal cord (Talpalar et al., 2013). These genetic manipulations indicate that V0 neurons have a key role in the establishing L-R CPG spinal circuitry. Given the variety of vertebrate species capable of synchronous muscle activation it seems likely that modification in V0 interneuron subtype distributions, and/or changes in the levels of Eph/ephrin or netrin signaling could account for evolutionary adaptations of L-R CPG output.
Diversification of Locomotor Gaits
While genetic manipulation in L-R CPGs can transform locomotor output from walking to hopping, most animals are capable of displaying a variety of gaits that are intermediates of these extremes. Within a species, gait changes are often associated with the speed of locomotion, as well as how movements are coordinated between the forelimbs and hindlimbs. In quadrupeds the relative phase of locomotor gaits between left and right limbs tends to shift from purely L-R alternation at slow speeds, such as walking, to more synchronized at higher speeds. Switch in gaits at different speeds appears to involve several populations of spinal interneurons. Consistent with this idea, mutations that affect the relative distribution of interneuron subtypes display phenotypes at specific locomotor velocities (Talpalar et al., 2013). For example ablation of the inhibitory V0d populations leads to locomotor discoordination at low speeds but normal alternation at high speeds, while ablation of excitatory V0v interneurons leads to hopping only at medium and high locomotor speeds. Additional interneuron populations, including excitatory V3 domain-derived CINs and V2a-ipsilateral excitatory interneurons play critical roles in establishing L-R alternation (Crone et al., 2009; Zhang et al., 2008). These studies indicate multiple interneuron classes are involved in the maintenance and fine tuning of CPG function (Figure 4D).
A more direct test of the role of cell fate determinants in the evolution of locomotor behaviors has emerged from analyses of the genetic determinants controlling gait patterns in horses. Certain horse breeds are capable of a special gait called pacing, where two legs on one side of the body are moved in a synchronized manner. Genome-wide association analysis identified a single transcription factor, Dmrt3, which when mutated allows for the ability to perform pacing (Andersson et al., 2012). Heterozygote mutant horses also display an alternate gait, suggesting this mutation acts as a dominant negative. In mice Dmrt3 is expressed by a single class of dorsal (di6) spinal interneurons, and mutation of this gene causes locomotor discoordination at high locomotor speeds.
Rostrocaudal Positional Information and Locomotor Circuits
The organization of CPG circuits within the vertebrate nervous system also appears to rely on rostrocaudal positional information. Lesion studies in rats indicate that locomotor CPG circuits reside at specific rostrocaudal levels of the spinal cord. For example, the CPGs controlling hindlimb muscles extend from lower thoracic to upper lumbar levels (Kjaerulff and Kiehn, 1996). A classic set of experiments underscoring the role of rostrocaudal positional identities involved neural tube transposition experiments in chick embryos (Narayanan and Hamburger, 1971; Straznicky, 1963). When brachial (wing) levels of chick neural tube is grafted to lumbar levels, hatched chicks loose left-right alternation and instead exhibit synchronous activation of leg muscles, resulting in hopping motor behaviors. Conversely, when brachial neural tube is replaced by lumbar tissue, chicks alternate wing movements. These studies suggest that positional specification along the rostrocaudal axis is essential for the development of CPG circuits associated with level-specific locomotor output. It is tempting to speculate that the same Hox-dependent pathways that confer MN subtype identities along the rostrocaudal axis are similarly used in local CPG circuits to establish species specific locomotor behaviors.
Further evidence suggesting a role for Hox function in locomotor circuits has emerged from studies of the embryonic nervous system in Drosophila. Fly larvae display segmental-level specific patterns of peristaltic locomotion that are used in exploratory behaviors. Recent genetic experiments have shown that these motor patterns can be displayed in the absence of brain function, indicating they reflect the activities of CPG circuits residing within the nerve cord (Berni et al., 2012). Larvae with combined mutations in the Hox genes Ubx and abdA show no peristaltic movement of abdominal segments, while ubiquitous misexpression of either of these genes extends the number of segments displaying abdominal-specific muscle contractions (Dixit et al., 2008). Although the neurons responsible for the altered behaviors have not been fully resolved, these observations implicate key roles for Hox genes in larval locomotion. Collectively, work in vertebrates and flies suggest changes in rostrocaudal positional information provided by Hox genes can impact locomotor behaviors through modifying CPG organization.
Perspectives
Studies on the evolution and development of locomotor circuits have provided key insights into the mechanisms through which nervous systems have diversified to establish new motor behaviors. Although comparative studies in vertebrates and invertebrates provide evidence for conservation in transcription factor profiles during early neural patterning, in the future it will be informative to determine whether these ancestral relationships extend to the target genes they regulate. A recent study on the gene networks involved in segmentation of the hindbrain indicates that many of the cis-regulatory elements controlling the expression of Hox genes and their targets in mice are functionally conserved in lamprey (Parker et al., 2014). Because aspects of class features are often shared between vertebrate and invertebrate neurons, such as the neurotransmitter systems of mammalian and annelid motor neurons, it seems likely that in many cases conservation will extend to target genes.
Modulation in the rostrocaudal expression profiles of Hox genes appears to be capable of eliciting global changes in the organization of effector neurons within locomotor circuits. These observations suggest that modulation in the expression of a small number of key regulatory factors can reorganize the structure of preexisting circuits, independent of changes in the downstream genes they regulate, or creation of new neuronal classes. However in many cases the development of new circuits relies on the generation of completely novel cell types. The appearance of a new cis-regulatory element in the Fezf2 gene fostered the generation of corticospinal motor neurons in mammals (Shim et al., 2012), a population of projection neurons essential for communication between the brain and spinal cord. Changes in cis-elements also were likely essential in the establishment of the gene regulatory network controlling neural crest lineages in vertebrates, and the developmental of the peripheral nervous systems (Bronner and LeDouarin, 2012).
While this review has focused on spinal circuits controlling relatively simple aspects of locomotor behaviors, in the future it will be important to resolve how the circuits governing more refined motor tasks evolved in the vertebrate lineages. Perhaps the most relevant for understanding mammalian evolution is the development of circuits controlling articulation of muscles in the hand. Recent studies have defined many of the anatomical features and functional properties of the neurons responsible for skilled forelimb movement in mice (Azim et al., 2014; Esposito et al., 2014). Given the vast number of novel motor behaviors that were enabled through the development of circuits for hand control it will be revealing to determine how spinal and supraspinal networks evolved to establish these sophisticated motor functions.
Acknowledgments
We thank Catarina Catela and Olivia Hanley for feedback on the manuscript, and Oliver Hobert, Klas Kullander, and David Mclean for valuable insights. J.S.D. is supported by funding from HHMI and the NIH (R01 NS062822).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ampatzis K, Song J, Ausborn J, El Manira A. Pattern of innervation and recruitment of different classes of motoneurons in adult zebrafish. J Neurosci. 2013;33:10875–10886. doi: 10.1523/JNEUROSCI.0896-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson LS, Larhammar M, Memic F, Wootz H, Schwochow D, Rubin CJ, Patra K, Arnason T, Wellbring L, Hjalm G, et al. Mutations in DMRT3 affect locomotion in horses and spinal circuit function in mice. Nature. 2012;488:642–646. doi: 10.1038/nature11399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appel B, Korzh V, Glasgow E, Thor S, Edlund T, Dawid IB, Eisen JS. Motoneuron fate specification revealed by patterned LIM homeobox gene expression in embryonic zebrafish. Development. 1995;121:4117–4125. doi: 10.1242/dev.121.12.4117. [DOI] [PubMed] [Google Scholar]
- Arber S. Motor circuits in action: specification, connectivity, and function. Neuron. 2012;74:975–989. doi: 10.1016/j.neuron.2012.05.011. [DOI] [PubMed] [Google Scholar]
- Arber S, Han B, Mendelsohn M, Smith M, Jessell TM, Sockanathan S. Requirement for the homeobox gene Hb9 in the consolidation of motor neuron identity. Neuron. 1999;23:659–674. doi: 10.1016/s0896-6273(01)80026-x. [DOI] [PubMed] [Google Scholar]
- Arendt D, Nubler-Jung K. Inversion of dorsoventral axis? Nature. 1994;371:26. doi: 10.1038/371026a0. [DOI] [PubMed] [Google Scholar]
- Azim E, Jiang J, Alstermark B, Jessell TM. Skilled reaching relies on a V2a propriospinal internal copy circuit. Nature. 2014;508:357–363. doi: 10.1038/nature13021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baek M, Enriquez J, Mann RS. Dual role for Hox genes and Hox co-factors in conferring leg motoneuron survival and identity in Drosophila. Development. 2013;140:2027–2038. doi: 10.1242/dev.090902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagnall MW, McLean DL. Modular organization of axial microcircuits in zebrafish. Science. 2014;343:197–200. doi: 10.1126/science.1245629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begemann G, Schilling TF, Rauch GJ, Geisler R, Ingham PW. The zebrafish neckless mutation reveals a requirement for raldh2 in mesodermal signals that pattern the hindbrain. Development. 2001;128:3081–3094. doi: 10.1242/dev.128.16.3081. [DOI] [PubMed] [Google Scholar]
- Bernhardt NR, Memic F, Kullander K. Genetic analysis of left-right coordination of locomotion. Front Biosci (Landmark Ed) 2013;18:21–35. doi: 10.2741/4085. [DOI] [PubMed] [Google Scholar]
- Berni J, Pulver SR, Griffith LC, Bate M. Autonomous circuitry for substrate exploration in freely moving Drosophila larvae. Curr Biol. 2012;22:1861–1870. doi: 10.1016/j.cub.2012.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand S, Camasses A, Somorjai I, Belgacem MR, Chabrol O, Escande ML, Pontarotti P, Escriva H. Amphioxus FGF signaling predicts the acquisition of vertebrate morphological traits. Proc Natl Acad Sci U S A. 2011;108:9160–9165. doi: 10.1073/pnas.1014235108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronner ME, LeDouarin NM. Development and evolution of the neural crest: an overview. Dev Biol. 2012;366:2–9. doi: 10.1016/j.ydbio.2011.12.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho HH, Cargnin F, Kim Y, Lee B, Kwon RJ, Nam H, Shen R, Barnes AP, Lee JW, Lee S, et al. Isl1 directly controls a cholinergic neuronal identity in the developing forebrain and spinal cord by forming cell type-specific complexes. PLoS Genet. 2014;10:e1004280. doi: 10.1371/journal.pgen.1004280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone SA, Zhong G, Harris-Warrick R, Sharma K. In mice lacking V2a interneurons, gait depends on speed of locomotion. J Neurosci. 2009;29:7098–7109. doi: 10.1523/JNEUROSCI.1206-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasen JS, De Camilli A, Wang B, Tucker PW, Jessell TM. Hox repertoires for motor neuron diversity and connectivity gated by a single accessory factor, FoxP1. Cell. 2008;134:304–316. doi: 10.1016/j.cell.2008.06.019. [DOI] [PubMed] [Google Scholar]
- Dasen JS, Jessell TM. Hox networks and the origins of motor neuron diversity. Curr Top Dev Biol. 2009;88:169–200. doi: 10.1016/S0070-2153(09)88006-X. [DOI] [PubMed] [Google Scholar]
- Dasen JS, Liu JP, Jessell TM. Motor neuron columnar fate imposed by sequential phases of Hox-c activity. Nature. 2003;425:926–933. doi: 10.1038/nature02051. [DOI] [PubMed] [Google Scholar]
- Dasen JS, Tice BC, Brenner-Morton S, Jessell TM. A Hox regulatory network establishes motor neuron pool identity and target-muscle connectivity. Cell. 2005;123:477–491. doi: 10.1016/j.cell.2005.09.009. [DOI] [PubMed] [Google Scholar]
- De Robertis EM. Evo-devo: variations on ancestral themes. Cell. 2008;132:185–195. doi: 10.1016/j.cell.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Robertis EM, Sasai Y. A common plan for dorsoventral patterning in Bilateria. Nature. 1996;380:37–40. doi: 10.1038/380037a0. [DOI] [PubMed] [Google Scholar]
- Demireva EY, Shapiro LS, Jessell TM, Zampieri N. Motor neuron position and topographic order imposed by beta- and gamma-catenin activities. Cell. 2011;147:641–652. doi: 10.1016/j.cell.2011.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deneris ES, Hobert O. Maintenance of postmitotic neuronal cell identity. Nat Neurosci. 2014;17:899–907. doi: 10.1038/nn.3731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denes AS, Jekely G, Steinmetz PR, Raible F, Snyman H, Prud’homme B, Ferrier DE, Balavoine G, Arendt D. Molecular architecture of annelid nerve cord supports common origin of nervous system centralization in bilateria. Cell. 2007;129:277–288. doi: 10.1016/j.cell.2007.02.040. [DOI] [PubMed] [Google Scholar]
- Dixit R, Vijayraghavan K, Bate M. Hox genes and the regulation of movement in Drosophila. Dev Neurobiol. 2008;68:309–316. doi: 10.1002/dneu.20589. [DOI] [PubMed] [Google Scholar]
- Droge MH, Leonard RB. Organization of spinal motor nuclei in the stingray, Dasyatis sabina. Brain Res. 1983;276:201–211. doi: 10.1016/0006-8993(83)90727-8. [DOI] [PubMed] [Google Scholar]
- Esposito MS, Capelli P, Arber S. Brainstem nucleus MdV mediates skilled forelimb motor tasks. Nature. 2014;508:351–356. doi: 10.1038/nature13023. [DOI] [PubMed] [Google Scholar]
- Esteves FF, Springhorn A, Kague E, Taylor E, Pyrowolakis G, Fisher S, Bier E. BMPs regulate msx gene expression in the dorsal neuroectoderm of Drosophila and vertebrates by distinct mechanisms. PLoS Genet. 2014;10:e1004625. doi: 10.1371/journal.pgen.1004625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fetcho JR. A review of the organization and evolution of motoneurons innervating the axial musculature of vertebrates. Brain Res. 1987;434:243–280. doi: 10.1016/0165-0173(87)90001-4. [DOI] [PubMed] [Google Scholar]
- Fetcho JR. The spinal motor system in early vertebrates and some of its evolutionary changes. Brain Behav Evol. 1992;40:82–97. doi: 10.1159/000113905. [DOI] [PubMed] [Google Scholar]
- Flames N, Hobert O. Gene regulatory logic of dopamine neuron differentiation. Nature. 2009;458:885–889. doi: 10.1038/nature07929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring WJ. The evolution of vision. Wiley Interdiscip Rev Dev Biol. 2014;3:1–40. doi: 10.1002/wdev.96. [DOI] [PubMed] [Google Scholar]
- Goulding M, Pfaff SL. Development of circuits that generate simple rhythmic behaviors in vertebrates. Curr Opin Neurobiol. 2005;15:14–20. doi: 10.1016/j.conb.2005.01.017. [DOI] [PubMed] [Google Scholar]
- Grillner S, Jessell TM. Measured motion: searching for simplicity in spinal locomotor networks. Curr Opin Neurobiol. 2009;19:572–586. doi: 10.1016/j.conb.2009.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halder G, Callaerts P, Gehring WJ. Induction of ectopic eyes by targeted expression of the eyeless gene in Drosophila. Science. 1995;267:1788–1792. doi: 10.1126/science.7892602. [DOI] [PubMed] [Google Scholar]
- Hirasawa T, Kuratani S. A new scenario of the evolutionary derivation of the mammalian diaphragm from shoulder muscles. J Anat. 2013;222:504–517. doi: 10.1111/joa.12037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirth F, Kammermeier L, Frei E, Walldorf U, Noll M, Reichert H. An urbilaterian origin of the tripartite brain: developmental genetic insights from Drosophila. Development. 2003;130:2365–2373. doi: 10.1242/dev.00438. [DOI] [PubMed] [Google Scholar]
- Hobert O. Regulation of terminal differentiation programs in the nervous system. Annu Rev Cell Dev Biol. 2011;27:681–696. doi: 10.1146/annurev-cellbio-092910-154226. [DOI] [PubMed] [Google Scholar]
- Holland LZ, Holland NN, Schubert M. Developmental expression of AmphiWnt1, an amphioxus gene in the Wnt1/wingless subfamily. Dev Genes Evol. 2000;210:522–524. doi: 10.1007/s004270000089. [DOI] [PubMed] [Google Scholar]
- Holland ND. Early central nervous system evolution: an era of skin brains? Nat Rev Neurosci. 2003;4:617–627. doi: 10.1038/nrn1175. [DOI] [PubMed] [Google Scholar]
- Holley SA, Jackson PD, Sasai Y, Lu B, De Robertis EM, Hoffmann FM, Ferguson EL. A conserved system for dorsal-ventral patterning in insects and vertebrates involving sog and chordin. Nature. 1995;376:249–253. doi: 10.1038/376249a0. [DOI] [PubMed] [Google Scholar]
- Imai KS, Stolfi A, Levine M, Satou Y. Gene regulatory networks underlying the compartmentalization of the Ciona central nervous system. Development. 2009;136:285–293. doi: 10.1242/dev.026419. [DOI] [PubMed] [Google Scholar]
- Irimia M, Pineiro C, Maeso I, Gomez-Skarmeta JL, Casares F, Garcia-Fernandez J. Conserved developmental expression of Fezf in chordates and Drosophila and the origin of the Zona Limitans Intrathalamica (ZLI) brain organizer. Evodevo. 2010;1:7. doi: 10.1186/2041-9139-1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessell TM. Neuronal specification in the spinal cord: inductive signals and transcriptional codes. Nat Rev Genet. 2000;1:20–29. doi: 10.1038/35049541. [DOI] [PubMed] [Google Scholar]
- Jung H, Lacombe J, Mazzoni EO, Liem KF, Jr, Grinstein J, Mahony S, Mukhopadhyay D, Gifford DK, Young RA, Anderson KV, et al. Global control of motor neuron topography mediated by the repressive actions of a single hox gene. Neuron. 2010;67:781–796. doi: 10.1016/j.neuron.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung H, Mazzoni EO, Soshnikova N, Hanley O, Venkatesh B, Duboule D, Dasen JS. Evolving hox activity profiles govern diversity in locomotor systems. Dev Cell. 2014;29:171–187. doi: 10.1016/j.devcel.2014.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kammermeier L, Leemans R, Hirth F, Flister S, Wenger U, Walldorf U, Gehring WJ, Reichert H. Differential expression and function of the Drosophila Pax6 genes eyeless and twin of eyeless in embryonic central nervous system development. Mech Dev. 2001;103:71–78. doi: 10.1016/s0925-4773(01)00328-8. [DOI] [PubMed] [Google Scholar]
- Kao TJ, Law C, Kania A. Eph and ephrin signaling: lessons learned from spinal motor neurons. Semin Cell Dev Biol. 2012;23:83–91. doi: 10.1016/j.semcdb.2011.10.016. [DOI] [PubMed] [Google Scholar]
- Karlsson D, Baumgardt M, Thor S. Segment-specific neuronal subtype specification by the integration of anteroposterior and temporal cues. PLoS Biol. 2010;8:e1000368. doi: 10.1371/journal.pbio.1000368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King BL, Gillis JA, Carlisle HR, Dahn RD. A natural deletion of the HoxC cluster in elasmobranch fishes. Science. 2011;334:1517. doi: 10.1126/science.1210912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjaerulff O, Kiehn O. Distribution of networks generating and coordinating locomotor activity in the neonatal rat spinal cord in vitro: a lesion study. J Neurosci. 1996;16:5777–5794. doi: 10.1523/JNEUROSCI.16-18-05777.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohwi M, Doe CQ. Temporal fate specification and neural progenitor competence during development. Nat Rev Neurosci. 2013;14:823–838. doi: 10.1038/nrn3618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kratsios P, Stolfi A, Levine M, Hobert O. Coordinated regulation of cholinergic motor neuron traits through a conserved terminal selector gene. Nat Neurosci. 2012;15:205–214. doi: 10.1038/nn.2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullander K, Butt SJ, Lebret JM, Lundfald L, Restrepo CE, Rydstrom A, Klein R, Kiehn O. Role of EphA4 and EphrinB3 in local neuronal circuits that control walking. Science. 2003;299:1889–1892. doi: 10.1126/science.1079641. [DOI] [PubMed] [Google Scholar]
- Lacin H, Zhu Y, Wilson BA, Skeath JB. Transcription factor expression uniquely identifies most postembryonic neuronal lineages in the Drosophila thoracic central nervous system. Development. 2014;141:1011–1021. doi: 10.1242/dev.102178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacombe J, Hanley O, Jung H, Philippidou P, Surmeli G, Grinstein J, Dasen JS. Genetic and functional modularity of Hox activities in the specification of limb-innervating motor neurons. PLoS Genet. 2013;9:e1003184. doi: 10.1371/journal.pgen.1003184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landgraf M, Thor S. Development of Drosophila motoneurons: specification and morphology. Semin Cell Dev Biol. 2006;17:3–11. doi: 10.1016/j.semcdb.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Lanuza GM, Gosgnach S, Pierani A, Jessell TM, Goulding M. Genetic identification of spinal interneurons that coordinate left-right locomotor activity necessary for walking movements. Neuron. 2004;42:375–386. doi: 10.1016/s0896-6273(04)00249-1. [DOI] [PubMed] [Google Scholar]
- Lee S, Cuvillier JM, Lee B, Shen R, Lee JW, Lee SK. Fusion protein Isl1-Lhx3 specifies motor neuron fate by inducing motor neuron genes and concomitantly suppressing the interneuron programs. Proc Natl Acad Sci U S A. 2012;109:3383–3388. doi: 10.1073/pnas.1114515109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Lee B, Joshi K, Pfaff SL, Lee JW, Lee SK. A regulatory network to segregate the identity of neuronal subtypes. Dev Cell. 2008;14:877–889. doi: 10.1016/j.devcel.2008.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JP, Laufer E, Jessell TM. Assigning the positional identity of spinal motor neurons: rostrocaudal patterning of Hox-c expression by FGFs, Gdf11, and retinoids. Neuron. 2001;32:997–1012. doi: 10.1016/s0896-6273(01)00544-x. [DOI] [PubMed] [Google Scholar]
- Livet J, Sigrist M, Stroebel S, De Paola V, Price SR, Henderson CE, Jessell TM, Arber S. ETS gene Pea3 controls the central position and terminal arborization of specific motor neuron pools. Neuron. 2002;35:877–892. doi: 10.1016/s0896-6273(02)00863-2. [DOI] [PubMed] [Google Scholar]
- Lopes R, Verhey van Wijk N, Neves G, Pachnis V. Transcription factor LIM homeobox 7 (Lhx7) maintains subtype identity of cholinergic interneurons in the mammalian striatum. Proc Natl Acad Sci U S A. 2012;109:3119–3124. doi: 10.1073/pnas.1109251109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe CJ, Terasaki M, Wu M, Freeman RM, Jr, Runft L, Kwan K, Haigo S, Aronowicz J, Lander E, Gruber C, et al. Dorsoventral patterning in hemichordates: insights into early chordate evolution. PLoS Biol. 2006;4:e291. doi: 10.1371/journal.pbio.0040291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe CJ, Wu M, Salic A, Evans L, Lander E, Stange-Thomann N, Gruber CE, Gerhart J, Kirschner M. Anteroposterior patterning in hemichordates and the origins of the chordate nervous system. Cell. 2003;113:853–865. doi: 10.1016/s0092-8674(03)00469-0. [DOI] [PubMed] [Google Scholar]
- Ma LH, Gilland E, Bass AH, Baker R. Ancestry of motor innervation to pectoral fin and forelimb. Nat Commun. 2010;1:49. doi: 10.1038/ncomms1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macesic LJ, Kajiura SM. Comparative punting kinematics and pelvic fin musculature of benthic batoids. J Morphol. 2010;271:1219–1228. doi: 10.1002/jmor.10865. [DOI] [PubMed] [Google Scholar]
- Masland RH. Neuronal cell types. Curr Biol. 2004;14:R497–500. doi: 10.1016/j.cub.2004.06.035. [DOI] [PubMed] [Google Scholar]
- Maxwell EE, Frobisch NB, Heppleston AC. Variability and conservation in late chondrichthyan development: ontogeny of the winter skate (Leucoraja ocellata) Anat Rec (Hoboken) 2008;291:1079–1087. doi: 10.1002/ar.20719. [DOI] [PubMed] [Google Scholar]
- McGinnis W, Krumlauf R. Homeobox genes and axial patterning. Cell. 1992;68:283–302. doi: 10.1016/0092-8674(92)90471-n. [DOI] [PubMed] [Google Scholar]
- Menelaou E, McLean DL. A gradient in endogenous rhythmicity and oscillatory drive matches recruitment order in an axial motor pool. J Neurosci. 2012;32:10925–10939. doi: 10.1523/JNEUROSCI.1809-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto N, Wada H. Hemichordate neurulation and the origin of the neural tube. Nat Commun. 2013;4:2713. doi: 10.1038/ncomms3713. [DOI] [PubMed] [Google Scholar]
- Mizutani CM, Bier E. EvoD/Vo: the origins of BMP signalling in the neuroectoderm. Nat Rev Genet. 2008;9:663–677. doi: 10.1038/nrg2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami Y, Tanaka M. Evolution of motor innervation to vertebrate fins and limbs. Dev Biol. 2011;355:164–172. doi: 10.1016/j.ydbio.2011.04.009. [DOI] [PubMed] [Google Scholar]
- Murata Y, Tamura M, Aita Y, Fujimura K, Murakami Y, Okabe M, Okada N, Tanaka M. Allometric growth of the trunk leads to the rostral shift of the pelvic fin in teleost fishes. Dev Biol. 2010;347:236–245. doi: 10.1016/j.ydbio.2010.07.034. [DOI] [PubMed] [Google Scholar]
- Myers PZ. Spinal motoneurons of the larval zebrafish. J Comp Neurol. 1985;236:555–561. doi: 10.1002/cne.902360411. [DOI] [PubMed] [Google Scholar]
- Narayanan CH, Hamburger V. Motility in chick embryos with substitution of lumbosacral by brachial and brachial by lumbosacral spinal cord segments. J Exp Zool. 1971;178:415–431. doi: 10.1002/jez.1401780402. [DOI] [PubMed] [Google Scholar]
- Nomaksteinsky M, Rottinger E, Dufour HD, Chettouh Z, Lowe CJ, Martindale MQ, Brunet JF. Centralization of the deuterostome nervous system predates chordates. Curr Biol. 2009;19:1264–1269. doi: 10.1016/j.cub.2009.05.063. [DOI] [PubMed] [Google Scholar]
- Pani AM, Mullarkey EE, Aronowicz J, Assimacopoulos S, Grove EA, Lowe CJ. Ancient deuterostome origins of vertebrate brain signalling centres. Nature. 2012;483:289–294. doi: 10.1038/nature10838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker HJ, Bronner ME, Krumlauf R. A Hox regulatory network of hindbrain segmentation is conserved to the base of vertebrates. Nature. 2014;514:490–493. doi: 10.1038/nature13723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippe H, Brinkmann H, Copley RR, Moroz LL, Nakano H, Poustka AJ, Wallberg A, Peterson KJ, Telford MJ. Acoelomorph flatworms are deuterostomes related to Xenoturbella. Nature. 2011;470:255–258. doi: 10.1038/nature09676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippidou P, Dasen JS. Hox genes: choreographers in neural development, architects of circuit organization. Neuron. 2013;80:12–34. doi: 10.1016/j.neuron.2013.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippidou P, Walsh CM, Aubin J, Jeannotte L, Dasen JS. Sustained Hox5 gene activity is required for respiratory motor neuron development. Nat Neurosci. 2012;15:1636–1644. doi: 10.1038/nn.3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price SR, De Marco Garcia NV, Ranscht B, Jessell TM. Regulation of motor neuron pool sorting by differential expression of type II cadherins. Cell. 2002;109:205–216. doi: 10.1016/s0092-8674(02)00695-5. [DOI] [PubMed] [Google Scholar]
- Rabe N, Gezelius H, Vallstedt A, Memic F, Kullander K. Netrin-1-dependent spinal interneuron subtypes are required for the formation of left-right alternating locomotor circuitry. J Neurosci. 2009;29:15642–15649. doi: 10.1523/JNEUROSCI.5096-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousso DL, Gaber ZB, Wellik D, Morrisey EE, Novitch BG. Coordinated actions of the forkhead protein Foxp1 and Hox proteins in the columnar organization of spinal motor neurons. Neuron. 2008;59:226–240. doi: 10.1016/j.neuron.2008.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seredick SD, Van Ryswyk L, Hutchinson SA, Eisen JS. Zebrafish Mnx proteins specify one motoneuron subtype and suppress acquisition of interneuron characteristics. Neural Dev. 2012;7:35. doi: 10.1186/1749-8104-7-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano-Saiz E, Poole RJ, Felton T, Zhang F, De La Cruz ED, Hobert O. Modular control of glutamatergic neuronal identity in C. elegans by distinct homeodomain proteins. Cell. 2013;155:659–673. doi: 10.1016/j.cell.2013.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma K, Leonard AE, Lettieri K, Pfaff SL. Genetic and epigenetic mechanisms contribute to motor neuron pathfinding. Nature. 2000;406:515–519. doi: 10.1038/35020078. [DOI] [PubMed] [Google Scholar]
- Shim S, Kwan KY, Li M, Lefebvre V, Sestan N. Cis-regulatory control of corticospinal system development and evolution. Nature. 2012;486:74–79. doi: 10.1038/nature11094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimeld SM. The evolution of the hedgehog gene family in chordates: insights from amphioxus hedgehog. Dev Genes Evol. 1999;209:40–47. doi: 10.1007/s004270050225. [DOI] [PubMed] [Google Scholar]
- Sillar KT, Combes D, Ramanathan S, Molinari M, Simmers J. Neuromodulation and developmental plasticity in the locomotor system of anuran amphibians during metamorphosis. Brain Res Rev. 2008;57:94–102. doi: 10.1016/j.brainresrev.2007.07.018. [DOI] [PubMed] [Google Scholar]
- Steinmetz PR, Kostyuchenko RP, Fischer A, Arendt D. The segmental pattern of otx, gbx, and Hox genes in the annelid Platynereis dumerilii. Evol Dev. 2011;13:72–79. doi: 10.1111/j.1525-142X.2010.00457.x. [DOI] [PubMed] [Google Scholar]
- Straznicky K. Function of Heterotopic Spinal Cord Segments Investigation in the Chick. Acta Biol Acad Sci Hung. 1963;14:143–153. [PubMed] [Google Scholar]
- Surmeli G, Akay T, Ippolito GC, Tucker PW, Jessell TM. Patterns of spinal sensory-motor connectivity prescribed by a dorsoventral positional template. Cell. 2011;147:653–665. doi: 10.1016/j.cell.2011.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takatori N, Satou Y, Satoh N. Expression of hedgehog genes in Ciona intestinalis embryos. Mech Dev. 2002;116:235–238. doi: 10.1016/s0925-4773(02)00150-8. [DOI] [PubMed] [Google Scholar]
- Takio Y, Kuraku S, Murakami Y, Pasqualetti M, Rijli FM, Narita Y, Kuratani S, Kusakabe R. Hox gene expression patterns in Lethenteron japonicum embryos--insights into the evolution of the vertebrate Hox code. Dev Biol. 2007;308:606–620. doi: 10.1016/j.ydbio.2007.05.009. [DOI] [PubMed] [Google Scholar]
- Talpalar AE, Bouvier J, Borgius L, Fortin G, Pierani A, Kiehn O. Dual-mode operation of neuronal networks involved in left-right alternation. Nature. 2013;500:85–88. doi: 10.1038/nature12286. [DOI] [PubMed] [Google Scholar]
- Thaler J, Harrison K, Sharma K, Lettieri K, Kehrl J, Pfaff SL. Active suppression of interneuron programs within developing motor neurons revealed by analysis of homeodomain factor HB9. Neuron. 1999;23:675–687. doi: 10.1016/s0896-6273(01)80027-1. [DOI] [PubMed] [Google Scholar]
- Thor S, Thomas JB. Motor neuron specification in worms, flies and mice: conserved and ‘lost’ mechanisms. Curr Opin Genet Dev. 2002;12:558–564. doi: 10.1016/s0959-437x(02)00340-4. [DOI] [PubMed] [Google Scholar]
- Tsuchida T, Ensini M, Morton SB, Baldassare M, Edlund T, Jessell TM, Pfaff SL. Topographic organization of embryonic motor neurons defined by expression of LIM homeobox genes. Cell. 1994;79:957–970. doi: 10.1016/0092-8674(94)90027-2. [DOI] [PubMed] [Google Scholar]
- Uemura O, Okada Y, Ando H, Guedj M, Higashijima S, Shimazaki T, Chino N, Okano H, Okamoto H. Comparative functional genomics revealed conservation and diversification of three enhancers of the isl1 gene for motor and sensory neuron-specific expression. Dev Biol. 2005;278:587–606. doi: 10.1016/j.ydbio.2004.11.031. [DOI] [PubMed] [Google Scholar]
- Vallstedt A, Kullander K. Dorsally derived spinal interneurons in locomotor circuits. Ann N Y Acad Sci. 2013;1279:32–42. doi: 10.1111/j.1749-6632.2012.06801.x. [DOI] [PubMed] [Google Scholar]
- Zarin AA, Asadzadeh J, Labrador JP. Transcriptional regulation of guidance at the midline and in motor circuits. Cell Mol Life Sci. 2014;71:419–432. doi: 10.1007/s00018-013-1434-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Bhattacharya A, Nelson JC, Abe N, Gordon P, Lloret-Fernandez C, Maicas M, Flames N, Mann RS, Colon-Ramos DA, et al. The LIM and POU homeobox genes ttx-3 and unc-86 act as terminal selectors in distinct cholinergic and serotonergic neuron types. Development. 2014;141:422–435. doi: 10.1242/dev.099721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Narayan S, Geiman E, Lanuza GM, Velasquez T, Shanks B, Akay T, Dyck J, Pearson K, Gosgnach S, et al. V3 spinal neurons establish a robust and balanced locomotor rhythm during walking. Neuron. 2008;60:84–96. doi: 10.1016/j.neuron.2008.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]