Abstract
Most researchers believe that neurogenesis in mature mammals is restricted only to the subgranular zone of the dentate gyrus and the subventricular zone of the lateral ventricle in the central nervous system. In the peripheral nervous system, neurogenesis is thought to be active only during prenatal development, with the exception of the olfactory neuroepithelium. However, sensory ganglia in the adult peripheral nervous system have been reported to contain precursor cells that can proliferate in vitro and be induced to differentiate into neurons. The occurrence of insult-induced neurogenesis, which has been reported by several investigators in the brain, is limited to a few recent reports for the peripheral nervous system. These reports suggest that damage to the adult nervous system induces mechanisms similar to those that control the generation of new neurons during prenatal development. Understanding conditions under which neurogenesis can be induced in physiologically non-neurogenic regions in adults is one of the major challenges for developing therapeutic strategies to repair neurological damage. However, the induced neurogenesis in the peripheral nervous system is still largely unexplored. This review presents the history of research on adult neurogenesis in the peripheral nervous system, which dates back more than 100 years and reveals the evidence on the under estimated potential for generation of new neurons in the adult peripheral nervous system.
Keywords: adult neurogenesis, peripheral nervous system, dorsal root ganglia, autonomic ganglia, stem cells, mammals
Abbreviations
PNS, peripheral nervous system; DRG, dorsal root ganglia; BAC, benzalkonium chloride; NG, nodose ganglion; NSC, neural stem cells
INTRODUCTION
In the healthy, intact peripheral nervous system (PNS), quality of sensation is dependent upon the density of sensory receptor organs, the number of innervating neurons, the size and degree of overlap of their receptive fields, and upon the cortical interpretation of the resulting topographically and qualitatively defined sensory input[1]. All these components are affected by damage of peripheral nerves or destruction of sensory neurons, and full function can be restored only by rapid and specific reinnervation by an adequate number of neurons. Therefore, it is likely that the single most important determinant of the poor sensory outcome after peripheral nerve damage is the extensive primary sensory neuronal death, resulting in failure of adequate reinnervation of target organs. It is now apparent that an end result of injury is that a considerable proportion of all neurons contributing to an injured nerve will die, with estimates ranging from 7 to 50%, depending on the exact nature of the experimental model[2,3,4,5,6,7,8,9,10]. Hence, in addition to axonal regeneration, the potential for functional recovery after injury depends on restoration of neuronal numbers and on development of appropriate neuronal phenotypes.
Previous studies have revealed that peripheral nerve injuries induce a cascade of events progressing throughout the plastic changes to restoration of damaged connections. In damaged neurons, axons begin to sprout after a delay of 3-14 days[11,12]. Nerve fibers grow by sprouting neurites that advance through the repair site only to be pruned down when the endoneurial tubes of the distal stump are reached[13]. Although neurite growth is facilitated by contact guidance from neurite outgrowth-promoting factors[14], it also is dependent upon the neurons’ inherent regenerative capacity. This is enhanced by adoption of the regenerative phenotype, partly in response to injury factors[5]. As a result, axons preferentially reinnervate the distal stump over neighbouring tissues, and display preferential reinnervation in the selection of endoneurial tubes[15,16,17]. Moreover, several studies show that damage to the adult nervous system induces factors and mechanisms that control neuronal proliferation, migration, differentiation, and connectivity during development[18,19,20]. Retrograde labelling studies suggest that some newly generated neurons may form appropriate long-distance connections[21]. Nevertheless, an increase in neurogenesis that results in addition to or replacement of a large proportion of existing neurons has not been reported in adult mammalian brain, although it does occur in some non-mammalian vertebrates[22].
Most studies indicate that generation of new neurons and replacement of damaged neurons in adult mammals is limited to the olfactory epithelium and hippocampus[23,24,25]. To the best of our knowledge, only our laboratory has reported large-scale neurogenesis in adult peripheral sensory ganglia[6,9]. However, low levels of spontaneous postnatal neurogenesis has been observed in adult rat dorsal root ganglia (DRG)[26]. In addition, a subpopulation of cells in adult rat DRG has been reported to express nestin and p75 neurotrophin receptor[27]. These cells formed clusters and spheres, and differentiated into neurons or glia, which formed secondary and tertiary neurospheres in cloning assays. Moreover, the cells expressing phenotypes of neural crest progenitors have been previously reported in DRG[27,28,29,30]. The literature presented above suggests that the limited nature of neurogenesis in adult sensory ganglia, and the nature of the differentiating process in these ganglia, depend upon the trophic environment. Understanding the conditions under which neurogenesis can be induced in physiologically non-neurogenic regions of the adult nervous system is one of the major challenges for developing therapeutic strategies to repair neurological damage.
Although the work cited above provides convincing evidence for injury-induced repair and neurogenesis in the adult nervous system, the precise mechanism by which this occurs is not understood. Moreover, there is no effective treatment for damage to the nervous system, and the lack of appropriate induction methods to stimulate large-scale plasticity and neurogenesis is an important problem. Without these methods, development of interventions to modify the course of injury and disease progression and improvement of functional outcomes in individuals following injury to the nervous system are highly unlikely.
We review the research dealing with adult neurogenesis in the PNS, which dates back more than 100 years, and reveal the current evidence on an underestimated potential for generation of new neurons outside the brain.
POSTNATAL HISTOGENESIS IN THE PNS: THE HISTORICAL PERSPECTIVE
The occurrence of neurogenesis in the adult PNS has been postulated since the beginning of the 20th century when Hatai[31] reported data showing an age-related increase in the number of rat DRG neurons, and about ten years later when Miura[32] supported the possibility that adult neurogenesis may occur in the rat Auerbach plexus in case of hypertrophy of the intestinal wall. The changes occurring in the myenteric plexus in the sub-total intestinal stenosis model attracted the attention of several researchers in the 20th century. Ambrosi[33] reported that, after a short term intestinal occlusion, the number of myenteric neurons in rat increases not because of their proliferation (cell division), but due to differentiation of neuronal precursors induced by increased functional stimulation. These results were replicated by Bennighoff[34] in 1951 who showed that partial ligation of the adult rat small intestine induces not only smooth muscle hypertrophy in the loops upstream from the stenosis, but also increases the number of Auerbach's plexus neurons, thus confirming the possibility of postnatal neurogenesis in the mammal PNS. A few years later, in a dog experimental model of sub-total intestinal stenosis, Filogamo et al[35,36] showed that the adaptive process that occurs in the intestinal loops upstream from an obstruction is not only characterized by smooth muscle and myenteric neuron hypertrophy, but also by a five-fold increase in neuron numbers. Noteworthy, these authors, based on the absence of mitosis in nerve cells, postulated that postnatal neurogenesis in myenteric ganglia could be attributable to the persistence of a pool of poorly differentiated or undifferentiated cells capable of turning into neurons under the influence of exceptional stimuli, i.e. the presence, in the adult Auerbach's plexus, of a local stem-cell pool that anticipated for many years the recent definition of stem-cell niches[37,38,39,40].
Interestingly, it was also shown that when an intestinal loop upstream from the stenosis was excluded from the transit that connected it to the abdominal wall (Thiry-Vella method), thus inducing hypotrophy in its wall, the relevant decrease in the myenteric neuron size was not accompanied by a concurrent decrease in the number of neurons[41].
Starting from the middle of the 20th century and based on the above-mentioned evidence about PNS neurogenesis in the enteric nervous system, new interest developed about the sensory ganglia district, particularly with respect to the age-related increase already detectable in Hatai's work. Cavanaugh, in 1951[42], reported an age-related increase in DRG neurons in the rat, followed by Sosa et al[43] who obtained similar data in the rabbit. This evidence was further supported by Devor et al[44,45] in the rat and Tessler et al[46] in the cat. The evidence of an age-related increase in DRG neuron numbers was questioned based on the lively debate about cell counting methods that emerged in the 1980s[47]. The main critical issue was that the use of profile counts may lead to a false detection of neurogenesis in case of cell hypertrophy; Coggeshall and co-workers, using the newly devised design-based counting methods, denied the occurrence of age-related neurogenesis in adult rat DRGs[48,49]. The debate was further stimulated by Popken et al[50] who used both traditional model-based and new design-based stereological counting methods and detected a significant postnatal increase in DRG neuron numbers that “could be due either to neuron proliferation or to late differentiation of neurons that do not assume a typical appearance until adulthood”. While these results were further replicated by Farel et al[51,52], Mohammed et al[53] failed to detect a postnatal increase in rat DRG cell number, and Bergman et al[54] even reported a decrease in the number of DRG, although, unlike previous studies, these authors prolonged their observations through a very advanced age.
During the final decades of the 20th century, a similar debate also regarded neurogenesis in the enteric nervous system. In fact, the former works reporting myenteric neuron addition in the intestinal loops upstream from a partial occlusion were denied by Gabella[55], while supported by Giacobini-Robecchi et al[56,57,58,59] who showed, in the rat intestinal partial occlusion experimental model, the occurrence of unscheduled DNA synthesis (i.e., not followed by cell division) in some myenteric neurons. This evidence was supported by both autoradiography after 3H-thymidine administration and PCNA immunostaining, which revealed DNA neo-synthesis in myenteric neurons[56,57,59], and cytophotometry following Feulgen staining, which suggested that DNA neo-synthesis was not associated with cell division but led to a hyperdiploid DNA content[57,58]. Yet, electrophoretic analysis of the total genomic DNA suggested that the observed unscheduled DNA synthesis seen in myenteric neurons that form the loops upstream from a partial intestinal occlusion may be due to DNA amplification of selected genome sequences[60].
The subtotal intestinal stenosis was not the only experimental model where adult myenteric neurogenesis has been investigated. Cracco et al[61] applied locally benzalkonium chloride (BAC) to an intestinal tract and showed that it induced the selective destruction of about 90% of Auerbach's plexus neurons followed, after few weeks, by a relevant increase in the mean neuronal density in small ganglia located along the mesenteric nerves, i.e., the migration pathway of the neural crest cells that colonize the gut during development. The absence of mitoses suggested that the neurogenesis observed along mesenteric nerves of BAC-treated animals should be attributable to a late differentiation of poorly- or un-differentiated neural-crest-derived elements that remained, as a peripheral precursor cell niche, along their developmental migration pathway. Adult differentiation of the neural precursor niche might be triggered by diffusible factors released by the intestinal wall when a regenerative process takes place, such as after lumen stenosis or BAC-induced degeneration[62]. The action of local diffusible factors that stimulate neuronal differentiation in damaged intestine also can be postulated based on the evidence that transplantation of a neuronal precursor cell line (PC12) in the intestinal wall of BAC-treated animals indices their quick differentiation into mature neurons[63].
Finally, the potential for postnatal histogenesis, at least in case of stimulating conditions, also in the human enteric nervous system can be postulated based on the clinical evidence that intestinal inflammation induces an increase in the number of neurons in the myenteric ganglia[64,65]. Also in this case, it has been hypothesized that adult neuron addition in the human intestine might be attributable to the presence of a local niche of precursor cells capable of late proliferation and/or differentiation into mature neurons[66].
Twentieth-century studies of neuro differentiation, plasticity and regeneration led researchers to appreciate that neuronal damage can be compensated by reinnervation of target organs, remodeling of neuronal circuits and even generation of new neurons in adult PNS. They even reported some physiological levels of neural proliferation in the adult PNS. However, most reports were based on a less-rigorous approach involving neuronal counts and BrdU incorporation. The 21st century research introduced a palette of powerful tools to study adult neurogenesis. They will be presented and discussed in the reminder of this review.
RECENT ADVANCES IN POSTNATAL HISTOGENESIS IN THE PNS
The last few decades have seen a revival of debate about neurogenesis in the PNS. Ciaroni et al[26] showed that long-term BrdU administration (1 month) in vitamin E-deficient rats. This condition was expected to induce DRG neuron addition[67]; however, it also led to the presence of labelled nuclei in DRG neurons, suggesting that postnatal neurogenesis may be due to neuronal precursor proliferation in addition to the late differentiation of post mitotic cells. Another interesting element in favour of the possibility that histogenesis may occur in adult DRGs has been provided by the in vitro study of Namaka et al[68]. They showed that postnatal mouse DRG contains a neuronal precursor niche that proliferates in response to administration of different factors and can be induced to differentiate into mature sensory neurons.
Similar results have been obtained more recently by Li et al[27] who showed the presence in DRG explants of a subpopulation of cells expressing neural progenitors markers and able to differentiate into both glia and neurons under adequate culturing conditions. Noteworthy, these authors suggest, based on BrdU chasing, that these progenitors originate from satellite glial cells. The possibility that satellite glial cells represent the stem-cell niche of DRGs has also been supported by Muratori et al[69], based on electron microscopy observations. The intriguing possibility that a neural progenitor niche persists in sensory ganglia along adult life has also been supported by the study of Lagares et al[29] in the trigeminal ganglion. They showed that the number of sensory neurons in the rat trigeminal ganglion almost double between month 3 and month 8. This dramatic age-related neuron addition has been attributed to the differentiation due to the persistence of a neural crest-derived precursor cell niche.
Our recent studies[6,9] have revealed a > 50% reduction of nodose ganglion (NG) neuronal numbers by 30 days post capsaicin. By 60 days post-capsaicin, the total numbers of neurons in the ganglia from capsaicin-treated rats were not different from controls, suggesting that new neurons had been added to the NG following capsaicin-induced neuronal death (Figure 1). Moreover, the results revealed that a pool of neural progenitors that express nestin is present in NG in adult rats. Capsaicin treatment that induced cellular proliferation was revealed by significant populations of BrdU/Ki-67-labeled cells. Many of these newborn cells differentiated to become neurons; this process was revealed by significant BrdU incorporation in cells immunoreactive for the neuron-specific antigen PGP-9.5 and beta-III-tubulin found in NG collected 30 and 60 days post-capsaicin. The vast majority of newborn neurons matured and survived up to 300 days post treatment. Both the NG and the samples of cervical, thoracic and abdominal vagal trunks collected from capsaicin-treated rats revealed significantly more growth cones than samples collected from vehicle-treated or vagotomized rats. Furthermore, transmission electron micrographs of cervical vagus revealed the presence of very small axon profiles in the tissue from the capsaicin-treated rats, suggesting that newborn neurons extend their processes into vagal trunks following capsaicin-induced destruction. NG cultures from capsaicin-treated rats contained bipolar neurons, normally found only during development. To test the functional recovery of NG neurons, we injected the satiety molecule, CCK. The effect of CCK on food intake was restored by 300-day post-capsaicin. This restoration supported the possibility of a generation of functional neurons that replaced lost connections following capsaicin-induced destruction.
Figure 1.
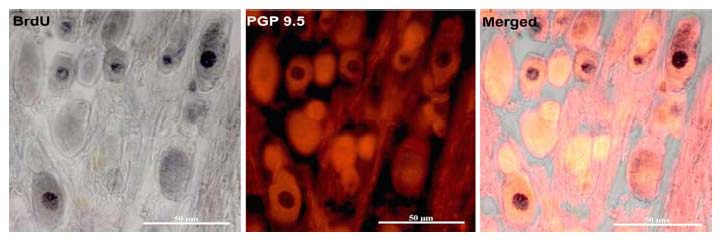
Sections of the nodose ganglion collected 60 days after intraperitoneal capsaicin treatment revealed significant numbers of bromodeoxyuridine-labeled (DNA synthesis marker) nuclei located in PGP 9.5-immunoreactive (neuronal marker) perikarya. This population of dual labelled cells indicates that after capsaicin-induced cell death, ganglionic progenitors entered the cell cycle, proceeded to divide and differentiated to become sensory neurons.
Singh et al[70] also suggested that DRG stem cells preserve their multipotency throughout adult life. They demonstrated the multipotency of neural stem cells (NSC) in adult DRG. Derived from DRG and after 4-5 years in culture without dissociating, the DRG NSC were found capable of proliferation. They expressed neuronal and glial markers: vesicular glutamate transporter 2 (VGluT2--glutamate terminals), transient receptor potential vanilloid 1 (TrpV1--capsaicin sensitive), phosphorylated 200 kDa neurofilament (pNF200--capsaicin insensitive, myelinated), and the serotonin transporter (5-HTT). NSC in adult DRGs also expressed nestin, Hu, and beta-III-tubulin (immature neuronal markers), green fibrillary acidic protein (astrocyte marker), as well as sensory neural marker TrpV1 (capsaicin sensitive) and pNF200 (mature, capsaicin insensitive, myelinated).
Our recent studies also documented the presence of BrdU-immunopositive neurons and neural progenitors labeled with Ki67, sox-2, nanog, nestin and neuroD, within DRGs after traumatic peripheral nerve crush (data not published). Time-lapse analysis of morphological changes following axonal damage, in addition to immunofluorescence characterization of cell phenotype in vitro and scan electron microscope analysis, suggested that the neuronal precursors are represented by satellite glial cells that actively proliferate after the lesion and are able to differentiate toward the neuronal lineage. Together these data led us to conclude that, within the DRG, a multipotent-progenitors-cell-niche exists throughout adulthood (data not published). The researchers (cited above) support the view that the adult sensory neurogenic niche may be located inside the ganglia (or in the nerve trunks nearby); however, it has been also shown that neural crest-derived boundary cap cells (i.e., those glial cells that mark the spinal cord/nerve boundary) can represent a late-migrating population of stem cells that can give rise to both new neurons and glia in DRGs[71,72]. This finding introduces the possibility that new neurons in the adult sensory ganglia may originate not only from endogenous precursors but also from migrating exogenous stem cells.
Similarly to the interest in peripheral sensory neuronal division, interest in the possibility of neurogenesis in the peripheral autonomic neuronal division has also been renewed over the last ten years. In particular, a couple of papers published in Neuron in 2002[73,74] from Morrison's laboratory at University of Michigan confirmed the presence of neuro-glial stem cells in the enteric nervous system. These neural crest-derived stem cells maintain the capability of self-renewing when cultured in vitro, although less than with foetal enteric stem cells. Yet, cell lineage assessment showed that these adult stem cells have a reduced neuronal sub-type potential[74]. The potential of adult neurogenesis in the adult intestine has been confirmed by Hanani et al[75] who observed the presence of differentiating new neurons in the myenteric plexus after belzalkonium chloride treatment.
Very recently, Joseph et al[76] carried out a comprehensive investigation on the stem-cell niche in the enteric nervous system and showed that these cells can be identified by CD49b expression and have the features of enteric glial cells. They also showed that while gliogenesis is very active, neurogenesis in the enteric nervous system appears to be very limited, although neurogenesis has the potential of forming neurons in culture. Interestingly, these authors assess enteric neurogenesis not only in normal condition but also in various pathological conditions, such as belzalkonium chloride treatment and partial gut stenosis. Their results again open the debate about partially contrasting evidence that injury can stimulate neurogenesis in the gut.
CONCLUSIONS AND FUTURE PERSPECTIVES
Review of the current literature regarding adult neurogenesis in the PNS shows that stem cells in the sensory ganglia may be induced to enter the cell cycle and differentiate to become mature and functional neurons. While the occurrence of an increase in peripheral neuron number, especially under particular stimuli, is widely acknowledged, the main question to be answered today appears to be if this phenomenon is the result of de novo neurogenesis (i.e., neural stem cell proliferation followed by neuronal differentiation) or if it is rather the late differentiation of post-mitotic neuronal precursors that have lost the capability to proliferate. Besides its biological importance, an answer to this question is also important in the clinical perspective, since the presence of a limited amount of neural precursors could have only a limited and temporarily effect in the neural repair mechanism after injury, compared to the presence of a true stem cell niche.
However, the answer to the previous question would become less relevant if more findings validate a third and alternative hypothesis, which gives the power or generation of new neuronal cells to the glia. Many promising studies are testing the possibility that satellite glia cells and Schwann cells can be neuronal precursors. Glia and neuronal progenitors may be stimulated to go back to a less-differentiated stage within their own lineage. Dedifferentiation may even go a step further and regress to a point where neural cells may switch lineage (transdifferentiation). These mechanisms and even re-entering the cell cycle by post mitotic, though immature, neurons may be responsible for the induced neurogenesis in the adult PNS. Interestingly, this alternative hypothesis combines and integrates the previous two wherein the glial cell is dispersed within the DRGs and, more in general, the PNS would represent a less-defined and more-expanded neurogenic niche.
Advances in current research enable us to study the repertoire of genetic manipulations to uncover induction of neurogenesis outside the CNS. The transgenic or knock-in mouse lines expressing Cre recombinase, driven by promoters active in the specific ganglion cells will be a powerful tool to study adult neurogenesis in the PNS. Molecular mechanisms of induction and termination of neurogenesis in the PNS would be the next step to uncover in this exciting and novel research field. The existence of progenitor cells in the PNS would suggest that the PNS could serve as a source of committed autologous cells that may be stimulated to produce neurons in vivo. The discovery of progenitor cells in the PNS ultimately could also enable autologous grafting of new neurons into damaged areas of the CNS to replace neurons lost due to injury or disease, eliminating rejection as well as ethical issues related to the embryonic stem cell research.
We can thus conclude that the issue of postnatal histogenesis in the PNS still raises a number of interesting questions regarding both basic and clinical science, and hopefully the next few years will provide an answer to some of these as yet unanswered questions. Answering these questions will represent significant progress in the broader field of plasticity and regeneration in the adult nervous system.
Footnotes
Funding: Università degli Studi di Torino (ex-60% grant).
Conflicts of interest: None declared
(Edited by Dubory P/Marti-clua J/Zhao LJ/Song LP)
REFERENCES
- 1.Zhang M, Yannas IV. Peripheral nerve regeneration. Adv Biochem Eng Biotechnol. 2005;94:67–89. doi: 10.1007/b100000. [DOI] [PubMed] [Google Scholar]
- 2.Hiura A. Neuroanatomical effects of capsaicin on the primary afferent neurons. Arch Histol Cytol. 2000;63:199–215. doi: 10.1679/aohc.63.199. [DOI] [PubMed] [Google Scholar]
- 3.Tandrup T, Woolf CJ, Coggeshall RE. Delayed loss of small dorsal root ganglion cells after transection of the rat sciatic nerve. J Comp Neurol. 2000;422:172–180. doi: 10.1002/(sici)1096-9861(20000626)422:2<172::aid-cne2>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 4.Tandrup T, Jakobsen J. Long-term acrylamide intoxication induces atrophy of dorsal root ganglion A-cells and of myelinated sensory axons. J Neurocytol. 2002;31:79–87. doi: 10.1023/a:1022579817020. [DOI] [PubMed] [Google Scholar]
- 5.Navarro X, Vivo M, Valero-Cabre A. Neural plasticity after peripheral nerve injury and regeneration. Prog Neurobiol. 2007;82:163–201. doi: 10.1016/j.pneurobio.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 6.Czaja K, Burns GA, Ritter RC. Capsaicin-induced neuronal death and proliferation of the primary sensory neurons located in the nodose ganglia of adult rats. Neuroscience. 2008;154:621–630. doi: 10.1016/j.neuroscience.2008.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Welin D, Novikova LN, Wiberg M, et al. Survival and regeneration of cutaneous and muscular afferent neurons after peripheral nerve injury in adult rats. Exp Brain Res. 2008;186:315–323. doi: 10.1007/s00221-007-1232-5. [DOI] [PubMed] [Google Scholar]
- 8.Atlasi MA, Mehdizadeh M, Bahadori MH, et al. Morphological identification of cell death in dorsal root ganglion neurons following peripheral nerve injury and repair in adult rat. Iran Biomed J. 2009;13:65–72. [PubMed] [Google Scholar]
- 9.Gallaher ZR, Ryu V, Larios RM, et al. Neural proliferation and restoration of neurochemical phenotypes and compromised functions following capsaicin-induced neuronal damage in the nodose ganglion of the adult rat. Front Neurosci. 2011;5:12. doi: 10.3389/fnins.2011.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reid AJ, Mantovani C, Shawcross SG, et al. Phenotype of distinct primary sensory afferent subpopulations and caspase-3 expression following axotomy. Histochem Cell Biol. 2011;136:71–78. doi: 10.1007/s00418-011-0829-8. [DOI] [PubMed] [Google Scholar]
- 11.Du J, Fu C, Sretavan DW. Eph/ephrin signaling as a potential therapeutic target after central nervous system injury. Curr Pharm Des. 2007;13:2507–2518. doi: 10.2174/138161207781368594. [DOI] [PubMed] [Google Scholar]
- 12.Su HX, Cho EY. Sprouting of axon-like processes from axotomized retinal ganglion cells induced by normal and preinjured intravitreal optic nerve grafts. Brain Res. 2003;991:150–162. doi: 10.1016/j.brainres.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 13.Donnerer J. Regeneration of primary sensory neurons. Pharmacology. 2003;67:169–181. doi: 10.1159/000068405. [DOI] [PubMed] [Google Scholar]
- 14.Yoshii S, Shima M, Oka M, et al. Nerve regeneration along collagen filament and the presence of distal nerve stump. Neurol Res. 2004;26:145–150. doi: 10.1179/016164104225013770. [DOI] [PubMed] [Google Scholar]
- 15.Rajan B, Polydefkis M, Hauer P, et al. Epidermal reinnervation after intracutaneous axotomy in man. J Comp Neurol. 2003;457:24–36. doi: 10.1002/cne.10460. [DOI] [PubMed] [Google Scholar]
- 16.Redett R, Jari R, Crawford T, et al. Peripheral pathways regulate motoneuron collateral dynamics. J Neurosci. 2005;25:9406–9412. doi: 10.1523/JNEUROSCI.3105-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kovacic U, Tomsic M, Sketelj J, et al. Collateral sprouting of sensory axons after end-to-side nerve coaptation--a longitudinal study in the rat. Exp Neurol. 2007;203:358–369. doi: 10.1016/j.expneurol.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 18.Taupin P. Adult neurogenesis and neuroplasticity. Restor Neurol Neurosci. 2006;24:9–15. [PubMed] [Google Scholar]
- 19.Ghashghaei HT, Lai C, Anton ES. Neuronal migration in the adult brain: are we there yet? Nat Rev Neurosci. 2007;8:141–151. doi: 10.1038/nrn2074. [DOI] [PubMed] [Google Scholar]
- 20.Navarro X. Neural plasticity after nerve injury and regeneration. Int Rev Neurobiol. 2009;87:483–505. doi: 10.1016/S0074-7742(09)87027-X. [DOI] [PubMed] [Google Scholar]
- 21.Suarez V, Guntinas-Lichius O, Streppel M, et al. The axotomy-induced neuropeptides galanin and pituitary adenylate cyclase-activating peptide promote axonal sprouting of primary afferent and cranial motor neurones. Eur J Neurosci. 2006;24:1555–1564. doi: 10.1111/j.1460-9568.2006.05029.x. [DOI] [PubMed] [Google Scholar]
- 22.Chapouton P, Jagasia R, Bally-Cuif L. Adult neurogenesis in non-mammalian vertebrates. Bioessays. 2007;29:745–757. doi: 10.1002/bies.20615. [DOI] [PubMed] [Google Scholar]
- 23.Suh H, Deng W, Gage FH. Signaling in adult neurogenesis. Ann Rev Cell Dev Biol. 2009;25:253–275. doi: 10.1146/annurev.cellbio.042308.113256. [DOI] [PubMed] [Google Scholar]
- 24.Sun J, Sun J, Ming GL, et al. Epigenetic regulation of neurogenesis in the adult mammalian brain. Eur J Neurosci. 2011;33:1087–1093. doi: 10.1111/j.1460-9568.2011.07607.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Winner B, Kohl Z, Gage FH. Neurodegenerative disease and adult neurogenesis. Eur J Neurosci. 2011;33:1139–1151. doi: 10.1111/j.1460-9568.2011.07613.x. [DOI] [PubMed] [Google Scholar]
- 26.Ciaroni S, Cecchini T, Cuppini R, et al. Are there proliferating neuronal precursors in adult rat dorsal root ganglia? Neurosci Lett. 2000;281:69–71. doi: 10.1016/s0304-3940(00)00785-0. [DOI] [PubMed] [Google Scholar]
- 27.Li HY, Say EH, Zhou XF. Isolation and characterization of neural crest progenitors from adult dorsal root ganglia. Stem Cells. 2007;25:2053–2065. doi: 10.1634/stemcells.2007-0080. [DOI] [PubMed] [Google Scholar]
- 28.Arora DK, Cosgrave AS, Howard MR, et al. Evidence of postnatal neurogenesis in dorsal root ganglion: role of nitric oxide and neuronal restrictive silencer transcription factor. J Mol Neurosci. 2007;32:97–107. doi: 10.1007/s12031-007-0014-7. [DOI] [PubMed] [Google Scholar]
- 29.Lagares A, Li HY, Zhou XF, et al. Primary sensory neuron addition in the adult rat trigeminal ganglion: evidence for neural crest glio-neuronal precursor maturation. J Neurosci. 2007;27:7939–7953. doi: 10.1523/JNEUROSCI.1203-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gu Y, Wang J, Ding F, et al. Neurotrophic actions of bone marrow stromal cells on primary culture of dorsal root ganglion tissues and neurons. J Mol Neurosci. 2010;40:332–341. doi: 10.1007/s12031-009-9304-6. [DOI] [PubMed] [Google Scholar]
- 31.Hatai S. Number and size of the spinal ganglion cells and dorsal root fibers in the white rat at different ages. J Comp Neurol. 1902;12:107–124. [Google Scholar]
- 32.Miura M. Muscularis mucosae et Muscularis propria gastrointestinalis. Phisiol Patol Forschung. 1913;26:193–234. [Google Scholar]
- 33.Ambrosi F. Sulle alterazioni istologiche dei gangli e dei nervi del tubo digerente nell’occlusione intestinale. Arch De Vecchi. 1942:215–240. [Google Scholar]
- 34.Bennighoff A. Vemerhung und vergrosserung von nervzellen bei hypertrophie des innervationgebietes. Z Naturforsch. 1951;6:38–44. [Google Scholar]
- 35.Filogamo G, Vigliani F. Le cellules du plexus myentérique (d’Auerbach) dans la sténose expérimentale de l’intestin. C R Assoc Anat. 1953;78:683–692. [Google Scholar]
- 36.Filogamo G, Vigliani F. Ricerche sperimentali sulla correlazione tra estensione del territorio di innervazione e grandezza e numero delle cellule gangliari del plesso mienterico (di Auerbach) nel cane. Riv Patol Nerv Ment. 1954;75:1–32. [PubMed] [Google Scholar]
- 37.Geuna S, Borrione P, Fornaro M, et al. Adult stem cells and neurogenesis: historical roots and state of the art. Anat Rec. 2001;265:132–141. doi: 10.1002/ar.1135. [DOI] [PubMed] [Google Scholar]
- 38.Geuna S, Borrione P, Filogamo G. Postnatal histogenesis in the peripheral nervous system. Int J Dev Neurosci. 2002;20:475–479. doi: 10.1016/s0736-5748(02)00059-x. [DOI] [PubMed] [Google Scholar]
- 39.Bonfanti L, Peretto P. Adult neurogenesis in mammals--a theme with many variations. Eur J Neurosci. 2011;34:930–950. doi: 10.1111/j.1460-9568.2011.07832.x. [DOI] [PubMed] [Google Scholar]
- 40.Martino G, Pluchino S, Bonfanti L, et al. Brain regeneration in physiology and pathology: the immune signature driving therapeutic plasticity of neural stem cells. Physiol Rev. 2011;91:1281–1304. doi: 10.1152/physrev.00032.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Filogamo G, Lievre C. Comportamento delle cellule nervose del plesso mienterico dell’intestino normale ed ipertrofico, nell’ansa alla Thiry-Vella. Boll Soc Piem Chir. 1955;25:1–3. [Google Scholar]
- 42.Cavanaugh MW. Quantitative effects of peripheral innervation on nerves and spinal ganglion cells. J Comp Neurol. 1951;94:181–219. doi: 10.1002/cne.900940203. [DOI] [PubMed] [Google Scholar]
- 43.Sosa JM, de Zorrilla NB. Morphological variations of the Golgi apparatus in spinal ganglion nerve cells related to ageing. Acta Anat. 1966;64:475–497. doi: 10.1159/000142848. [DOI] [PubMed] [Google Scholar]
- 44.Devor M, Govrin-Lippman R. Neurogenesis in adult rat dorsal root ganglia. Neurosci Lett. 1985;61:189–194. doi: 10.1016/0304-3940(85)90423-9. [DOI] [PubMed] [Google Scholar]
- 45.Devor M, Govrin-Lippman R, Frank I, et al. Neurogenesis in adult rat dorsal root ganglia: On counting and the count. Somatos Mot Res. 1991;3:139–167. doi: 10.3109/08990229109144724. [DOI] [PubMed] [Google Scholar]
- 46.Tessler A, Himes BT, Krieger NR, et al. Sciatic nerve transection produces death of dorsal root ganglion cells and reversible loss of substance P in spinal cord. Brain Res. 1985;332:209–218. doi: 10.1016/0006-8993(85)90590-6. [DOI] [PubMed] [Google Scholar]
- 47.Geuna S. The revolution of counting “tops”: two decades of the disector principle in morphological research. Microsc Res Tech. 2005;66:270–274. doi: 10.1002/jemt.20167. [DOI] [PubMed] [Google Scholar]
- 48.La Forte RA, Melville S, Chung K, et al. Absence of neurogenesis in adult rat dorsal root ganglion cells. Somatosens Mot Res. 1991;8:3–7. doi: 10.3109/08990229109144723. [DOI] [PubMed] [Google Scholar]
- 49.Pover C, Barnes MC, Coggeshall RE. Do primary afferent cell numbers change in relation to increasing weight and surface area in adult rats? Somatos Mot Res. 1994;11:163–167. doi: 10.3109/08990229409028869. [DOI] [PubMed] [Google Scholar]
- 50.Popken GJ, Farel PB. Sensory neuronnumber in neonatal and adult rats estimated by means of stereologic and profile-based methods. J Comp Neurol. 1997;386:8–15. [PubMed] [Google Scholar]
- 51.Farel PB. Sensory neuron addition in juvenile rat: time course and specificity. J Comp Neurol. 2002;449:158–165. doi: 10.1002/cne.10274. [DOI] [PubMed] [Google Scholar]
- 52.Farel PB. Latedifferentiationcontributes to the apparent increase in sensoryneuronnumber in juvenile rat. Brain Res Dev Brain Res. 2003;144:91–98. doi: 10.1016/s0165-3806(03)00163-9. [DOI] [PubMed] [Google Scholar]
- 53.Mohammed HA, Santer RM. Total neuronal numbers of rat lumbosacral primary afferent neurons do not change with age. Neurosci Lett. 2001;304:149–152. doi: 10.1016/s0304-3940(01)01781-5. [DOI] [PubMed] [Google Scholar]
- 54.Bergman E, Ulfhake B. Loss of primary sensory neurons in the very old rat: Neuron number estimates using the dissector method and confocal optical sectioning. J Comp Neurol. 1998;396:211–222. [PubMed] [Google Scholar]
- 55.Gabella G. Size of neurons and glial cells in the intramural ganglia of the hypertrophic intestine of the guinea pig. J Neurocytol. 1984;13:73–84. doi: 10.1007/BF01148319. [DOI] [PubMed] [Google Scholar]
- 56.Giacobini-Robecchi MG, Cannas M, Filogamo G. Increase in the number and volume of myenteric neurons in the adult rat. Int J Dev Neurosci. 1985;3:673–675. doi: 10.1016/0736-5748(85)90058-9. [DOI] [PubMed] [Google Scholar]
- 57.Giacobini-Robecchi MG, Poncino A, Geuna S, et al. DNA content in neurons of Auerbach's plexus under experimental conditions in adult rats. Int J Dev Neurosci. 1988;6:109–115. doi: 10.1016/0736-5748(88)90034-2. [DOI] [PubMed] [Google Scholar]
- 58.Poncino A, Geuna S, Scherini E, et al. DNA synthesis in adult neurons: tetraploidy of hyperdiploidy? Int J Dev Neurosci. 1991;8:621–623. doi: 10.1016/0736-5748(90)90054-6. [DOI] [PubMed] [Google Scholar]
- 59.Corvetti G, Fornaro M, Geuna S, et al. Unscheduled DNA synthesis in rat adult myenteric neurons: an immunohistochemical study. Neuroreport. 2001;12:2165–2168. doi: 10.1097/00001756-200107200-00024. [DOI] [PubMed] [Google Scholar]
- 60.Giacobini-Robecch MG, Borrione P, Canavese M, et al. Electrophoretic analysis of Auerbach plexus neurons DNA isolated from the rat hypertrophic gut. Int J Dev Neurosci. 1995;13:635–637. doi: 10.1016/0736-5748(95)00035-f. [DOI] [PubMed] [Google Scholar]
- 61.Cracco C, Filogamo G. Mesenteric neurons in the adult rat are responsive to ileal treatment with benzalkonium chloride. Int J Dev Neurosci. 1993;11:49–61. doi: 10.1016/0736-5748(93)90034-b. [DOI] [PubMed] [Google Scholar]
- 62.Williams TH, Jew JY. Neuronal responses to gut hypertrophy. J Autonom Nerv Syst. 1991;33:204–206. [Google Scholar]
- 63.Filogamo G, Cracco C. Models of neuronal plasticity and repair in the enteric nervous system: a review. Ital J Anat Embryol. 1995;100:185–195. [PubMed] [Google Scholar]
- 64.Storsteen KA, Kemohan JW, Bargen JA. The myenteric plexus in chronic ulcerative colitis. Surg Gynecol Obstet. 1953;97:335–343. [PubMed] [Google Scholar]
- 65.Davis DR, Dockerry MB, Mayo CW. The mesenteric plexus in regional enteritis: a study of the number of ganglion cells in the ileum in 24 cases. Surg Gynecol Obstet. 1955;101:208–216. [PubMed] [Google Scholar]
- 66.Sharkey KA, Parr EJ. The enteric nervous system in intestinal inflammation. Can J Gastroenterol. 1996;10:335–341. [Google Scholar]
- 67.Cecchini T, Cuppini R, Ciaroni S, et al. Changes in the number of primary sensory neurons in normal and vitamin E-deficient rats during aging. Somatos Mot Res. 1995;12:317–327. doi: 10.3109/08990229509093665. [DOI] [PubMed] [Google Scholar]
- 68.Namaka MP, Sawchuk M, MacDonald SC, et al. Neurogenesis in postnatal mouse dorsal root ganglia. Exp Neurol. 2001;172:60–69. doi: 10.1006/exnr.2001.7761. [DOI] [PubMed] [Google Scholar]
- 69.Muratori L, Ronchi G, Cunotto A, et al. Amsterdam: 7th Meeting of the Federation of European Socieites for Neuroscience; 2010. The number of neurons drammatically increases as a consequence of peripheral nerve crush injury in adult rat. [Google Scholar]
- 70.Singh RP, Cheng YH, Nelson P, et al. Retentive multipotency of adult dorsal root ganglia stem cells. Cell Transplant. 2009;18:55–68. doi: 10.3727/096368909788237177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Maro GS, Vermeren M, Voiculescu O, et al. Neural crest boundary cap cells constitute a source of neuronal and glial cells of the PNS. Nat Neurosci. 2004;7:930–938. doi: 10.1038/nn1299. [DOI] [PubMed] [Google Scholar]
- 72.Coulpier F, Le Crom S, Maro GS, et al. Novel features of boundary cap cells revealed by the analysis of newly identified molecular markers. Glia. 2009;57:1450–1457. doi: 10.1002/glia.20862. [DOI] [PubMed] [Google Scholar]
- 73.Bixby S, Kruger GM, Mosher JT, et al. Cell-intrinsic differences between stem cells from different regions of the peripheral nervous system regulate the generation of neural diversity. Neuron. 2002;35:643–656. doi: 10.1016/s0896-6273(02)00825-5. [DOI] [PubMed] [Google Scholar]
- 74.Kruger GM, Mosher JT, Bixby S, et al. Neural crest stem cells persist in the adult gut but undergo changes in self-renewal, neuronal subtype potential, and factor responsiveness. Neuron. 2002;35:657–669. doi: 10.1016/s0896-6273(02)00827-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hanani M, Ledder O, Yutkin V, et al. Regeneration of myenteric plexus in the mouse colon after experimental denervation with benzalkonium chloride. J Comp Neurol. 2003;462:315–327. doi: 10.1002/cne.10721. [DOI] [PubMed] [Google Scholar]
- 76.Joseph NM, He S, Quintana E, et al. Enteric glia are multipotent in culture but primarily form glia in the adult rodent gut. J Clin Invest. 2011;121:3398–3411. doi: 10.1172/JCI58186. [DOI] [PMC free article] [PubMed] [Google Scholar]


