Abstract
Objective
Energetic adaptations induced by bariatric surgery have not been studied in adolescents or for extended periods post-surgery. Energetic, metabolic and neuroendocrine responses to Roux-en-Y gastric bypass surgery (RYGB) were investigated in extremely obese adolescents.
Design and Methods
At baseline and at 1.5, 6 and 12 months post-baseline, 24-h room calorimetry, body composition and fasting blood biochemistries were measured in eleven obese adolescents relative to five matched controls.
Results
In RYGB group, mean weight loss was 44±19 kg at 12 months. Total energy expenditure (TEE), activity EE, basal metabolic rate (BMR), sleep EE and walking EE significantly declined by 1.5 months (p=0.001) and remained suppressed at 6 and 12 months. Adjusted for age, sex, FFM and FM, EE was still lower than baseline (p=0.001). Decreases in serum insulin, leptin, and T3, gut hormones, and urinary norepinephrine (NE) paralleled the decline in EE. Adjusted changes in TEE, BMR and/or sleep EE were associated with decreases in insulin, HOMA, leptin, TSH, total T3, PYY3–36, GLP2 and urinary NE and epinephrine (p=0.001–0.05).
Conclusions
Energetic adaptations in response to RYGB-induced weight loss are associated with changes in insulin, adipokines, thyroid hormones, gut hormones and sympathetic nervous system activity, and persist 12 months post-surgery.
Keywords: bariatric surgery, basal metabolic rate, total energy expenditure, calorimetry
Introduction
Bariatric surgery induces massive reductions in body weight that are associated with energetic adaptations that favor weight regain (1). These adaptations involve multiple signals including regulatory hormones from the gastrointestinal tract and pancreas impacting glucose homeostasis, adipokines affecting inflammation and insulin resistance, and the hypothalamic-pituitary axis (HPA) regulating energy balance in part through thyroid, autonomic nervous system, and adrenal mediators (2, 3).
The metabolic changes induced by bariatric surgery result in resolution or improvement in obesity-related comorbidities including type 2 diabetes, hyperlipidemia, liver disease, obstructive sleep apnea, pseudotumor cerebri, hypertension, and psychological disorders (3). Inevitably, bariatric surgery induces some loss of fat-free mass (FFM) which is undesirable since FFM is responsible for the majority of basal metabolism, regulation of core body temperature, cardiopulmonary function, skeletal integrity and mobility.
For extremely obese adolescents who have been unable to achieve a healthy weight with conventional treatment, Roux-en-Y gastric bypass surgery (RYGB) is an option (4). RYGB is a diversionary procedure which creates a very small gastric pouch considerably restricting meal size and promoting early satiety. A surgical anastomosis connects the gastric pouch to the mid-jejunum using a 125–150 cm Roux limb, diverting ingested macro- and micronutrients from the duodenum, decreasing the efficiency of micronutrient absorption (5).
Dietary energy restriction and weight loss elicit energetic adaptations or compensatory changes in energy expenditure that are greater than that accounted for by the residual active tissue mass (9). Decreased sympathetic nervous system (SNS) tone and circulating concentrations of leptin, and thyroid hormones act coordinately to favor weight regain. Energetic adaptations to bariatric surgery have been documented in adults mainly using portable respiration calorimeters (6), but also room respiration calorimetry (7) and doubly labeled water (8).
Persistence of energetic adaptations beyond the period of active weight loss by conventional means (9, 10) or bariatric surgery remains controversial (6). Studies demonstrate prolonged reduction in EE (9, 11, 12), while others show no persistence (13, 14). Whether energetic adaptations occur and persist in maturing adolescents is critical to understanding mechanisms of weight loss maintenance, and in particular, recidivism after RYGB.
The primary study objective was to investigate energetic, metabolic and neuroendocrine responses to RYGB in extremely obese adolescents at 1.5, 6 and 12 months after surgery. Specific aims were to: 1) monitor changes in weight and body composition using a multi-compartment model, 2) measure changes in neuroendocrine factors, 3) measure 24-h EE and substrate utilization using room respiration calorimetry, and 4) identify neuroendocrine factors associated with the changes in EE and substrate utilization.
Methods
Human subjects
A 12-month prospective study design was used to investigate energetic, metabolic and neuroendocrine responses to RYGB (n=11) in extremely obese adolescents. A control group (n=5) matched for initial weight, body mass index (BMI) and body composition was used to ascertain effects due to extreme obesity itself or protocol procedures. Anthropometry, body composition, 24-h room respiration calorimetry, and 12-h fasting blood and 24-h urine samples for neuroendocrine biochemistries were measured at baseline, and at 1.5, 6 and 12 months post-baseline to represent the following pre-surgical (baseline) and post-surgical phases: rapid weight loss (1.5 months after surgery), moderate weight loss (6 months after surgery), and minimal weight loss or weight maintenance (12 months after surgery). Controls were studied at baseline, and at 1.5, 6 and 12 months post-baseline.
Subjects were recruited from the Texas Children’s Hospital (TCH) Adolescent Bariatric Surgery Program. Adolescents electing surgery (RYGB group) or declining surgery and not enrolled in conventional weight loss programs (controls) were asked to participate. Inclusion criteria were Tanner stage IV or V and BMI≥50 kg/m2 or BMI≥40 kg/m2 with comorbidities. Exclusion criteria included a positive urine pregnancy test, and serious psychiatric or cognitive disorders.
Study participation did not interfere with the routine clinical care of the RYGB patients. Following bariatric surgery, regular and frequent follow-up visits assessed weight loss and monitored for postoperative complications, dietary progression, and adequacy of physical activity. Immediately postoperative until day 2, the patients ingested only clear liquids. Thereafter, patients were slowly advanced from a sugar-free full liquid diet to a soft diet, and finally to a regular diet. By six months post-surgery, the diet prescription was a regular diet consisting of 3 meals and 2 snacks per day, with an emphasis on high protein sources. A multivitamin mineral supplement with iron and supplemental calcium were prescribed.
Anthropometry
Body weight to the nearest 0.1 kg was measured with a digital scale (Tanita Corporation, model TBF-410, Arlington Heights, IL) and height to the nearest 1 mm was measured with a stadiometer (Seca, model 226l Chino, CA). Waist circumference was measured using a non-extensible metal tape measure.
Body Composition
Body composition was estimated using the Fuller three-compartment model based on total body water (TBW) and body volume (15). TBW was measured by the 2H isotope dilution following an oral dose (0.04 g/kg body weight) of deuterium oxide (2H2O). 2H abundances of baseline, 4- and 6-h post-dose urine samples were measured by gas-isotope-ratio mass spectrometry (16, 17). Body volume and body density were measured by air-displacement plethysmography (ADP) utilizing the BodPod (Life Measurements, Inc. Concord, CA).
Blood Chemistries
Serum glucose (Analox Instruments, Lundeburg, MA) and nonesterified fatty acids (NEFA) were measured by enzymatic-colorimetric techniques (Wako Diagnostics, Richmond, VA). Enzyme-linked-immunosorbent assays (ELISA) were used to measure serum insulin, resistin, adiponectin and glucagon-like peptide-2 (GLP2) (Millipore, Billerica, MD) and C-reactive protein (CRP) (Alpco Diagnostics, Salem, NH). Homeostatic model assessment (HOMA) was used to quantify insulin resistance (18). Radioimmunoassays (RIA) were used to measure serum leptin, peptide YY3–36 (PYY3–36), glucagon-like peptide-1(GLP1) (EMD Millipore, Billerica, MA), and thyroid stimulating hormone (TSH), total and free thyroxine (T4) and triiodothyronine (T3) and reverse T3 (Siemens, Deerfield, IL).
ELISAs were used to quantify urinary norepinephrine (NE) and epinephrine (E) (Rocky Mountain Diagnostics, Colorado Springs, CO). Urinary nitrogen concentrations were determined by Kjeldahl digestion (Kjeltec Auto Analyzer 1030; Tecator, Hoganas, Sweden) and a phenol-hypochlorite colorimetric reaction (19).
Room Respiration Calorimetry Protocol
Energy expenditure was measured for 24 hours in one of the two large 34-m3) calorimeters. The design, instrumentation and performance of the calorimeters have been published (20). During the 24-h calorimetry, subjects adhered to a schedule of physical activity (treadmill walking), feeding and sleeping. Heart rate and physical activity were recorded using Actiheart (CamNtech, Cambridge, UK). From the VO2, VCO2, and urinary nitrogen excretion, TEE, nonprotein energy expenditure (NPEE), respiratory quotient (RQ), and net substrate utilization (21).
During 24-h calorimetry, the diet prescribed for the RYGB patients was served to both the RYGB and control groups. Food intake was provided as three meals and two snacks with a macronutrient composition consisting of 30% protein, 25% fat and 45% carbohydrate. Food intake was offered at 1.2 times BMR predicted for obese adolescents at baseline, and at 600, 1100 and 1400 kcal/d at 1.5, 6 and 12 months post-baseline, respectively.
BMR was measured after a 12-h fast upon awakening for 30 minutes. Sleeping EE was measured for the entire night sleep period, confirmed by heart rate and motion sensors. Activity energy expenditure (AEE) was computed as TEE-BMR-0.1TEE assuming diet-induced thermogenesis to be 10% of TEE. Physical activity level (PAL) was defined as TEE/BMR. Energy cost of walking was measured while walking at 2.5 mph for 15 minutes on a treadmill (Vision Fitness T9600)(22). The energy economy of walking (kcal·kg−1·km−1) was calculated as the ratio of the net EE standardized by weight per minute (kcal·kg−1·min−1) divided by speed (km/min).
Statistical Methods
Statistical analysis was performed using STATA (version 13.0, Statacorp, College Station, TX) and SAS (SAS Institute Inc., Cary, NC). Independent t tests for continuous variables and chi-square tests for categorical variables were used for descriptive analyses. A nonlinear regression with an exponential decay model was used to fit the weight data of the RYGB group (GraphPad Software, Inc., La Jolla, CA).
A linear mixed effects regression model for repeated measures was used where subjects were treated as random effects and group assignment (RYGB or control), measurement time from baseline, and potential interactions between group and time as fixed effects. As necessary, natural logarithms were used to transform data to better satisfy the linearity and distributional assumptions. Post-hoc comparisons using Tukey-Kramer for multiple comparisons with two-tailed statistical tests between time points were performed.
Results
A total of 11 adolescents (3M/8F) electing RYGB and 5 controls (3M/2F) participated. Mean age at enrollment was 16.5 ± 0.8 y in the RYGB and 14.8 ±1.2 y in controls (p=0.03). At baseline, weight, height, BMI, waist circumference, body volume, FFM, FM and percent FM did not differ between RYGB and controls.
Anthropometry and body composition of the RYGB and controls are summarized in Table 1. Adjusted for age and sex, significant group X time interactions were observed for all parameters (p=0.000–0.019). Highly significant (p=0.001) time effects for weight, BMI, waist circumference, and the body composition parameters were seen for the RYGB group only.
Table 1.
Anthropometric and body composition of the RYGB (n=11) and control (n=5) groups
| Baseline | Post-baseline | Time effect within group |
Post-hoc test1 | |||
|---|---|---|---|---|---|---|
| 1.5 mo | 6 mo | 12 mo | ||||
| Weight (kg) | (p-value) | |||||
| RYGB | 153.1 ± 28.7* | 136.9 ± 27.5 | 117.3 ± 30.6 | 106.6 ± 26.3 | <0.0001 | ab, ac, ad, bd |
| Control | 133.2 ± 24.9 | 133.0 ± 27.4 | 134.7 ± 31.6 | 131.4 ± 30.7 | 0.929 | |
| Height (m) | ||||||
| RYGB | 1.64 ± 0.07 | 1.64 ± 0.07 | 1.64 ± 0.05 | 1.64 ± 0.07 | 0.197 | NS |
| Control | 1.65 ± 0.06 | 1.66 ± 0.06 | 1.67 ± 0.06 | 1.68 ± 0.06 | 0.800 | |
| BMI (kg/m2) | ||||||
| RYGB | 57.0 ± 10.5 | 50.9 ± 10.4 | 45.3 ± 11.2 | 40.1 ± 10.7 | <0.0001 | ab, ac, ad, bc, bd |
| Control | 48.0 ± 8.7 | 48.3 ± 9.2 | 47.9 ± 10.3 | 46.0 ± 9.1 | 0.809 | |
| Waist circumference (cm) | ||||||
| RYGB | 133.7 ± 16.2 | 126.9 ± 21.3 | 115.9 ± 18.6 | 110.8 ± 18.0 | 0.016 | ac, ad, bc, bd |
| Control | 122.9 ± 16.0 | 127.2 ± 19.2 | 119.5 ± 19.4 | 119.2 ± 21.4 | 0.229 | |
| Body volume (L) | ||||||
| RYGB | 146.5 ± 24.6 | 139.3 ± 29.4 | 117.9 ± 32.6 | 105.3 ± 28.3 | <0.0001 | ab, ac, ad, bc, bd |
| Control | 131.4 ± 28.7 | 134.0 ± 29.3 | 127.0 ± 31.4 | 131.3 ± 31.1 | 0.889 | |
| TBW (kg) | ||||||
| RYGB | 54.1 ± 8.7 | 48.3 ± 7.8 | 46.2 ± 8.6 | 47.3 ± 10.3 | 0.001 | ab, ac, ad |
| Control | 49.4 ± 6.3 | 49.3 ± 5.8 | 50.2 ± 7.31 | 51.9 ± 8.70 | 0.441 | |
| FFM (kg) | ||||||
| RYGB | 72.9 ± 10.8 | 65.3 ± 9.6 | 62.8 ± 10.1 | 65.0 ± 12.6 | 0.001 | ab, ac, ad |
| Control | 66.0 ± 8.0 | 67.9 ± 8.1 | 68.3 ± 8.1 | 72.1 ± 13.5 | 0.352 | |
| FM (kg) | ||||||
| RYGB | 80.3 ± 20.9 | 71.6 ± 21.0 | 54.6 ± 21.9 | 41.5 ± 22.2 | <0.0001 | ab, ac, ad, bc, bd, cd |
| Control | 67.1 ± 17.0 | 65.1 ± 21.1 | 66.4 ± 23.7 | 59.3 ± 17.8 | 0.882 | |
| FM (%weight) | ||||||
| RYGB | 51.8 ± 5.2 | 51.6 ± 6.0 | 44.9 ± 8.6 | 37.0 ± 12.9 | 0.002 | ac, ad, bc, bd |
| Control | 49.8 ± 4.1 | 47.8 ± 7.8 | 47.8 ± 7.9 | 44.4 ± 4.70.738 | ||
Data are shown as mean ± SD.
Post-hoc estimation using Tukey-Kramer for multiple comparisons with p<0.05; letters refer to statistically significant differences between time points baseline (a),1.5 months (b), 6 months (c) and 12 months (d).
TBW, total body water; FFM, fat free mass; FM, fat mass.
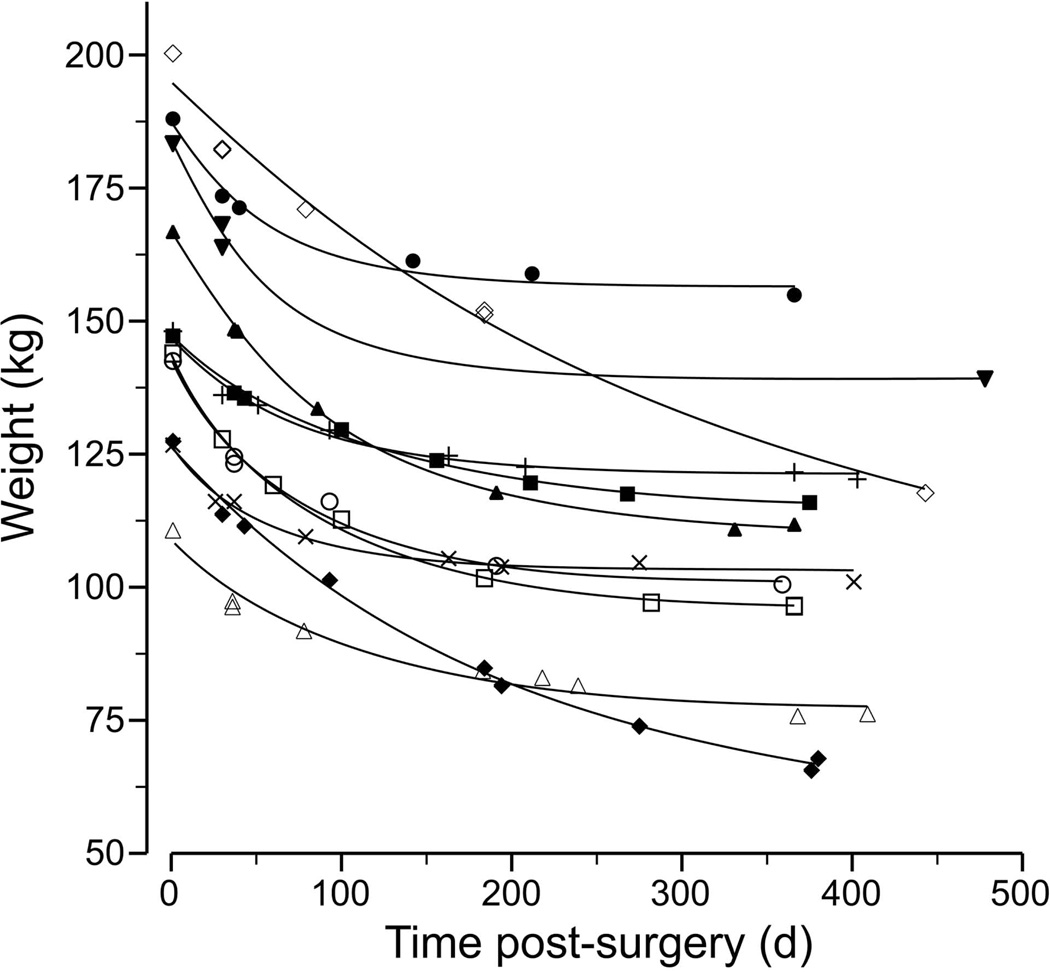
In the RYGB group, mean total weight lost was 44 ± 19 kg or 30 ± 11% of initial body weight at 12 months. Mean weight loss was −16, −18 and −10 kg from baseline to 1.5 months, 1.5 to 6 months and 6 to12 months, equivalent to 11, 14 and 9% of initial body weight, respectively. Substantial variation was seen in the rate of weight loss (299 ± 120 g/d during the first 1.5 months, 110 ± 62 g/d between 1.5 to 6 months, and 48 ± 45 g/d between 6 and 12 months). Based on multiple clinical weights, individual patterns of weight loss in the RYGB group were described by a negative exponential function (mean r2=0.98) (Figure 1). By 12 months, weight loss had reached a plateau in all (−5 ± 5 g/d) but two RYGB participants (−51 g/d and −77 g/d). No change in height was observed over the period of study in either group.
Figure 1.
Patterns of weight loss in adolescents undergoing RYGB surgery described by a negative exponential function
Body composition changed significantly in the RYGB group (p<0.001), but not in the controls. TBW and FFM loss occurred primarily in the first 1.5 months after surgery, with only minor (non-significant) changes thereafter. Hydration of FFM averaged 73.4% and did not differ by group or time. Total FFM loss averaged 8.3 ± 3.7 kg or 12 ± 5% of initial FFM. In contrast, FM decreased steadily over the 12 months post-surgery; total FM loss was 36 ± 20 kg or 47 ± 22% of initial FM.
Fasting blood chemistries and 24-h urinary catecholamines are presented in Table 2. Adjusted for age and sex, significant group X time interactions were seen for all parameters (p=0.0012–0.048). Further analysis revealed significant time effects for the RYGB group only. NEFA increased significantly in the RYGB group 1.5 months after surgery and then declined (p=0.001). Glucose was significantly lower than baseline at 1.5, 6 and 12 months post-surgery (p=0.001). Insulin and consequently HOMA were significantly lower after surgery (p=0.001). Adiponectin steadily increased and leptin decreased post-surgery (p=0.001), but resistin did not change. The inflammation marker CRP declined post-surgery (p=0.01). Thyroid status was altered by RYGB: fasting serum TSH (p=0.03) and total T3 (p=0.003) decreased post-surgery. Significant changes were not seen in total T4, reverse T3 or free T3 or T4. Fasting levels of PPY3–36 and GLP2 declined (p=0.001), but GLP1 did not change. Urinary excretion of norepinephrine, but not epinephrine, decreased after surgery (p=0.01).
Table 2.
Fasting blood chemistries and 24-h urinary catecholamines of the RYGB (n=11) and control (n=5) groups
| Baseline | Post-baseline | Time effect within group |
Post-hoc test1 |
|||
|---|---|---|---|---|---|---|
| 1.5 mo | 6 mo | 12 mo | ||||
| NEFA (mEq/L) | (p-value) | |||||
| RYGB | 0.72 ± 0.26* | 0.92 ± 0.23 | 0.61 ± 0.17 | 0.49 ± 0.17 | 0.014 | ab, bc |
| Control | 0.60 ± 0.11 | 0.70 ± 0.21 | 0.68 ± 0.17 | 0.70 ± 0.26 | 0.704 | |
| Glucose (mg/dL) | ||||||
| RYGB | 102.3 ± 21.4 | 91.6 ± 7.7 | 85.9 ± 7.1 | 86.8 ± 8.5 | 0.037 | ab, ac, ad |
| Control | 95.1 ± 6.0 | 98.2 ± 11.4 | 99.0 ± 7.3 | 98.9 ± 7.1 | 0.547 | |
| Insulin (µu/mL) | ||||||
| RYGB | 34.5 ± 24.0 | 13.1 ± 8.8 | 12.7 ± 7.1 | 9.1 ± 5.4 | 0.006 | ab, ac, ad |
| Control | 29.0 ± 10.4 | 23.1 ± 8.5 | 21.6 ± 11.6 | 22.8 ± 13.9 | 0.472 | |
| HOMA | ||||||
| RYGB | 8.1 ± 4.8 | 2.9 ± 1.7 | 2.6 ± 1.3 | 1.9 ± 1.1 | 0.001 | ab, ac, ad |
| Control | 6.7 ± 2.1 | 5.5 ± 1.7 | 5.2 ± 2.7 | 5.5 ± 3.3 | 0.600 | |
| Adiponectin (ng/mL) | ||||||
| RYGB | 6474 ± 2540 | 8422 ± 2688 | 8634 ± 3620 | 10900 ± 4820 | 0.002 | ab, ac, ad, bd, cd |
| Control | 6873 ± 4331 | 6686 ± 4229 | 7088 ± 4936 | 7690 ± 6502 | 0.909 | |
| Resistin (ng/mL) | ||||||
| RYGB | 11.79 ± 2.82 | 12.11 ± 4.46 | 12.47 ± 3.03 | 12.55 ± 3.88 | 0.931 | NS |
| Control | 9.44 ± 3.30 | 10.49 ± 2.76 | 10.90 ± 6.16 | 7.96 ± 2.45 | 0.714 | |
| Leptin (ng/mL) | ||||||
| RYGB | 71.0 ± 36.6 | 42.7 ± 24.5 | 35.8 ± 21.9 | 25.4 ± 24.1 | 0.001 | ab, ac, ad |
| Control | 66.2 ± 22.5 | 52.8 ± 19.1 | 60.0 ± 25.0 | 43.0 ± 14.9 | 0.238 | |
| CRP (ng/mL) | ||||||
| RYGB | 11360 ± 9801 | 4546 ± 3455 | 6945 ± 5986 | 3160 ± 4795 | 0.016 | |
| Control | 6433 ± 5537 | 6172 ± 3487 | 7252 ± 3557 | 5500 ± 4400 | 0.666 | |
| TSH (µIU/mL) | ||||||
| RYGB | 3.05 ± 1.36 | 2.10 ± 0.80 | 1.47 ± 0.51 | 1.65 ± 0.94 | 0.031 | ab, ac, ad |
| Control | 1.77 ± 0.97 | 1.66 ± 0.69 | 1.77 ± 1.01 | 1.58 ± 1.21 | 0.768 | |
| Total T3 (ng/dL) | ||||||
| RYGB | 115.0 ± 30.7 | 82.3 ± 23.8 | 91.5 ± 26.8 | 77.7 ± 17.9 | 0.003 | ab, ad |
| Control | 111.9 ± 37.2 | 104.1 ± 32.4 | 104.7 ± 32.6 | 97.1 ± 13.5 | 0.233 | |
| Total T4 (µg/dL) | ||||||
| RYGB | 7.77 ± 1.65 | 7.14 ± 1.64 | 7.82 ± 3.18 | 7.32 ± 1.23 | 0.480 | NS |
| Control | 6.18 ± 1.49 | 6.39 ± 0.97 | 6.20 ± 1.29 | 6.10 ± 2.47 | 0.027 | |
| Free T3 (pg/mL) | ||||||
| RYGB | 3.34 ± 0.49 | 2.82 ± 0.60 | 2.72 ± 0.57 | 2.59 ± 0.51 | 0.203 | |
| Control | 3.52 ± 0.70 | 3.27 ± 1.10 | 3.08 ± 0.93 | 3.14 ± 0.44 | 0.554 | |
| Free T4 (ng/dL) | ||||||
| RYGB | 1.31 ± 0.18 | 1.19 ± 0.22 | 1.29 ± 0.30 | 1.26 ± 0.25 | 0.525 | NS |
| Control | 1.10 ± 0.18 | 1.22 ± 0.16 | 1.15 ± 0.18 | 1.18 ± 0.43 | 0.541 | |
| Reverse T3 (ng/mL) | ||||||
| RYGB | 0.33 ± 0.08 | 0.34 ± 0.09 | 0.32 ± 0.08 | 0.27 ± 0.04 | 0.120 | bd |
| Control | 0.24 ± 0.08 | 0.27 ± 0.05 | 0.22 ± 0.06 | 0.20 ± 0.08 | 0.026 | |
| PYY3–36 (pg/mL) | ||||||
| RYGB | 105.2 ± 24.6 | 68.1 ± 20.8 | 89.5 ± 14.5 | 77.8 ± 28.4 | 0.001 | ab, ad, bc |
| Control | 77.0 ± 23.5 | 58.4 ± 22.4 | 72.1 ± 32.6 | 84.2 ± 17.0 | 0.240 | |
| GLP1 (pM) | ||||||
| RYGB | 16.1 ± 6.0 | 17.2 ± 10.3 | 15.6 ± 8.1 | 13.0 ± 6.6 | 0.903 | |
| Control | 13.1 ± 3.7 | 13.5 ± 7.2 | 8.6 ± 4.8 | 15.8 ± 10.1 | 0.365 | |
| GLP2 (ng/mL) | ||||||
| RYGB | 8.63 ± 1.83 | 5.38 ± 2.07 | 5.97 ± 0.65 | 6.23 ± 1.87 | 0.001 | ab, ac |
| Control | 5.58 ± 2.35 | 5.33 ± 2.03 | 6.64 ± 1.45 | 6.25 ± 2.55 | 0.341 | |
| Urinary NE (nmol/d) | ||||||
| RYGB | 326 ± 166 | 232 ± 168 | 250 ± 92 | 205 ± 91 | 0.014 | |
| Control | 296 ± 62 | 218 ± 86 | 300 ± 175 | 311 ± 105 | 0.632 | |
| Urinary E (nmol/d) | ||||||
| RYGB | 49.1 ± 14.8 | 42.5 ± 41.5 | 53.0 ± 19.1 | 43.3 ± 14.1 | 0.604 | |
| Control | 47.3 ± 9.1 | 48.2 ± 17.2 | 45.7 ± 32.1 | 57.8 ± 29.1 | 0.643 | |
Data are shown as mean ± SD. Models adjusted for age and sex; significant group X time interactions for all parameters (p=0.001–0.05)
Post-hoc estimation using Tukey-Kramer for multiple comparisons with p<0.05; letters refer to statistically significant differences between time points baseline (a),1.5 months (b), 6 months (c) and 12 months (d).
NEFA, nonesterified fatty acids; HOMA, homeostatic model assessment; CRP, C-reactive protein; TSH, thyroid stimulating hormone; T3, triiodothyronine; T4, thyroxine; PYY3–36, peptide YY3–36; GLP1, glucagon-like peptide-1; GLP2, glucagon-like peptide-2; NE, norepinephrine; E, epinephrine.
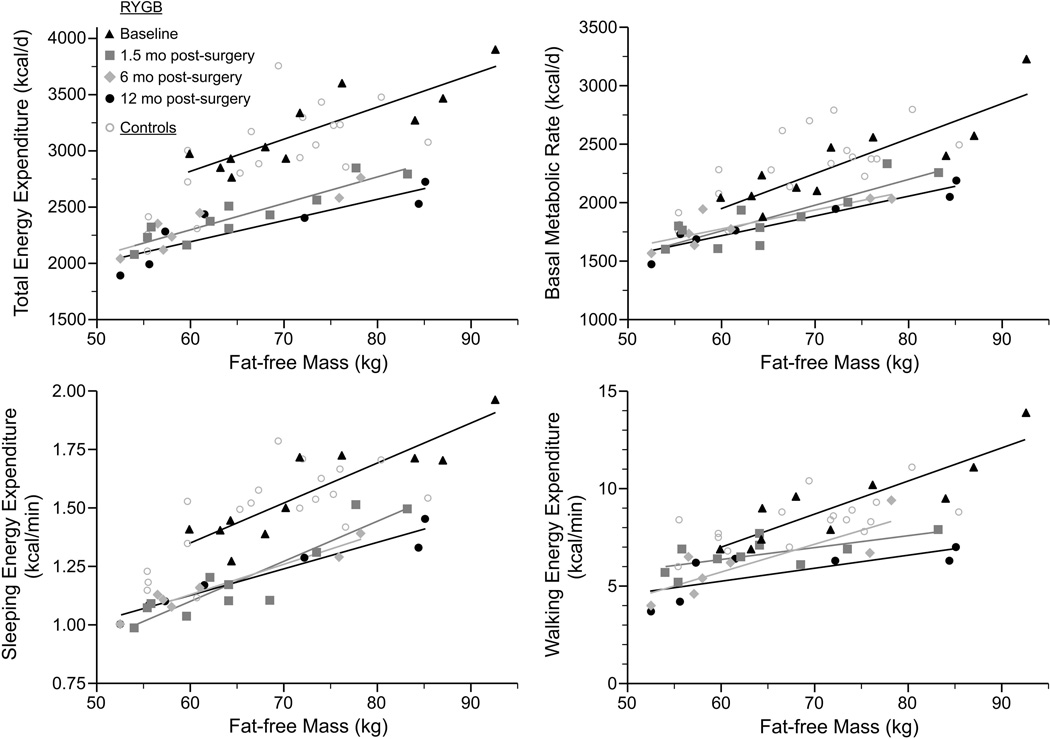
Total energy expenditure and its components measured by 24-h calorimetry are summarized in Table 3. Adjusted for age and sex, significant group X time interactions were seen for TEE and its components (p=0.001–0.01); significant time effects were observed in the RYGB group, but not controls. TEE, BMR, sleep EE declined by 24, 19, and 24% at 1.5 months, and then remained at the suppressed level at 6 and 12 months after surgery. Adjusted for age, sex, FFM and FM, post-surgical TEE (kcal/d), BMR (kcal/min) and sleep EE (kcal/min) were still significantly lower than baseline (p=0.001). TEE, BMR and sleep EE as a function of FFM are graphically displayed in Figure 2; the downward shift in TEE, BMR and sleep EE occurred in the initial 1.5 months post-surgery and then persisted. Similar to the pattern in EE, heart rate throughout the 24-h decreased significantly at 1.5 months after surgery (p=0.001), and remained at the lower level at 6 months (p=0.001) and 12 months (p=0.002).
Table 3.
Energy expenditure, heart rate and physical activity during 24-h calorimetry of the RYGB (n=11) and control (n=5) groups
| Baseline | Post-baseline | Time effect within group |
Post-hoc test1 |
|||
|---|---|---|---|---|---|---|
| 1.5 mo | 6 mo | 12 mo | ||||
| TEE (kcal/d) | (p-value) | |||||
| RYGB | 3189 ± 358* | 2421 ± 243 | 2363 ± 256 | 2323 ± 294 | 0.001 | ab, ac, ad |
| Control | 3110 ± 512 | 2859 ± 374 | 2927 ± 421 | 2887 ± 575 | 0.072 | |
| PAL | ||||||
| RYGB | 1.39 ± 0.07 | 1.30 ± 0.12 | 1.32 ± 0.08 | 1.24 ± 0.12 | 0.001 | ab, ad, cd |
| Control | 1.36 ± 0.07 | 1.29 ± 0.07 | 1.27 ± 0.11 | 1.27 ± 0.07 | 0.212 | |
| AEE (kcal/d) | ||||||
| RYGB | 535 ± 111 | 306 ± 115 | 309 ± 130 | 257 ± 118 | 0.001 | ab, ac, ad |
| Control | 495 ± 183 | 332 ± 133 | 304 ± 221 | 303 ± 132 | 0.160 | |
| HR (bpm) | ||||||
| RYGB | 85.8 ± 6.1 | 71.6 ± 9.5 | 72.6 ± 3.6 | 70.5 ± 7.6 | 0.002 | ab, ac, ad |
| Control | 84.0 ± 11.6 | 79.3 ± 12.7 | 80.1 ± 9.4 | 77.5 ± 13.1 | 0.484 | |
| Actiheart (counts/d) | ||||||
| RYGB | 24202 ± 4082 | 20619 ± 8099 | 22716 ± 7356 | 19839 ± 5419 | 0.326 | NS |
| Control | 29088 ± 8200 | 27650 ± 5211 | 27187 ± 13405 | 22836 ± 13178 | 0.977 | |
| BMR (kcal/d) | ||||||
| RYGB | 2335 ± 374 | 1909 ± 333 | 1818 ± 189 | 1877 ± 305 | 0.001 | ab, ac, ad |
| Control | 2304 ± 319 | 2241 ± 329 | 2331 ± 321 | 2296 ± 450 | 0.150 | |
| BMR HR (bpm) | ||||||
| RYGB | 76.6 ± 7.7 | 64.4 ± 10.3 | 62.5 ± 5.0 | 64.9 ± 6.7 | 0.001 | ab, ac, ad |
| Control | 73.9 ± 8.1 | 75.6 ± 8.5 | 72.3 ± 10.5 | 71.7 ± 13.0 | 0.874 | |
| Sleep EE (kcal/min) | ||||||
| RYGB | 1.57 ± 0.21 | 1.19 ± 0.18 | 1.17 ± 0.13 | 1.20 ± 0.16 | <0.0001 | ab, ac, ad |
| Control | 1.52 ± 0.22 | 1.41 ± 0.20 | 1.51 ± 0.17 | 1.49 ± 0.24 | 0.085 | |
| Sleep HR (bpm) | ||||||
| RYGB | 75.7 ± 7.3 | 62.0 ± 10.2 | 61.0 ± 4.6 | 63.8 ± 7.0 | 0.002 | ab, ac |
| Control | 72.2 ± 10.8 | 71.0 ± 13.4 | 72.5 ± 9.6 | 69.8 ± 12.6 | 0.816 | |
| Walking EE at 2.5 mph (kcal/min) | ||||||
| RYGB | 9.2 ± 2.2 | 6.6 ± 0.8 | 6.1 ± 1.8 | 5.7 ± 1.3 | <0.0001 | ab, ac, ad |
| Control | 8.7 ± 1.1 | 8.2 ± 1.0 | 7.7 ± 1.2 | 9.0 ± 2.0 | 0.597 | |
| Walking HR at 2.5 mph (bpm) | ||||||
| RYGB | 143 ± 14.0 | 128 ± 12.3 | 121 ± 9.7 | 112 ± 11.1 | 0.003 | ab, ac, ad |
| Control | 146 ± 7.0 | 135 ± 14.3 | 124 ± 8.6 | 141 ± 7.7 | 0.019 | ac |
| Energy economy of walking (kcal·kg−1·km−1) | ||||||
| RYGB | 0.013 ± 0.002 | 0.010 ± 0.001 | 0.010 ± 0.001 | 0.010 ± 0.001 | 0.001 | ab, ad |
| Control | 0.013 ± 0.003 | 0.013 ± 0.002 | 0.012 ± 0.002 | 0.012 ± 0.002 | 0.847 | |
Data are shown as mean ± SD. Models adjusted for age and sex; significant group X time interactions for all parameters (p=0.001–0.01)
Post-hoc estimation using Tukey-Kramer for multiple comparisons with p<0.05; letters refer to statistically significant differences between time points baseline (a),1.5 months (b), 6 months (c) and 12 months (d).
TEE, total energy expenditure; PAL, physical activity level; AEE, activity energy expenditure; HR, heart rate; BMR, basal metabolic rate.
Figure 2.
Relationship between total, basal, sleeping and walking energy expenditure and fat-free mass in RYGB and control groups at baseline and 1.5, 6 and 12 months post-baseline
In addition to changes in basal energy requirements, the energy expended in physical activity also fell. AEE declined by 41% and the energy cost of walking dropped by 28% at 1.5 months after surgery (p=0.001). Adjusted for age, sex, FFM and FM, post-surgical AEE (kcal/d) and walking EE (kcal/min) were still significantly lower than baseline (p=0.001–0.0001) (Figure 2). The energy economy of walking (kcal·kg−1·km−1) also decreased at 1.5 months post-surgery (p=0.001) and persisted at the lower level at 6 and 12 months post-surgery.
Substrate utilization was significantly altered in the RYGB, but not controls (Table 4). At 1.5 months post-surgery, 24-h RQ and NPRQ declined sharply, reflective of increased fat utilization and decreased carbohydrate utilization (p=0.001). Thereafter, the changes in fat and carbohydrate utilization reversed, approaching baseline values. At 1.5 months post-surgery, protein utilization dropped significantly (p=0.001), but was restored at 6 and 12 months. Adjusted for age, sex, FFM, FM and energy balance, the time effects for substrate utilization were still significant (p=0.001–0.05).
Table 4.
Substrate Utilization during 24-h calorimetry of the RYGB (n=11) and control (n=5) groups
| Baseline | Post-baseline | Time effect within group |
Post-hoc test1 |
|||
|---|---|---|---|---|---|---|
| 1.5 mo | 6 mo | 12 mo | ||||
| RQ | (p-value) | |||||
| RYGB | 0.85 ± 0.03* | 0.76 ± 0.02 | 0.81 ± 0.03 | 0.82 ± 0.03 | <0.0001 | ab, bc, bd |
| Control | 0.83 ± 0.02 | 0.82 ± 0.03 | 0.81 ± 0.05 | 0.84 ± 0.04 | 0.385 | |
| Protein utilization (g/d) | ||||||
| RYGB | 97 ± 43 | 34 ± 12 | 70 ± 20 | 77 ± 12 | <0.0001 | ab, bc, bd |
| Control | 101 ± 21 | 80 ± 23 | 70 ± 31 | 95 ± 28 | 0.208 | |
| Carbohydrate utilization (g/d) | ||||||
| RYGB | 331 ± 72 | 94 ± 46 | 169 ± 48 | 172 ± 54 | <0.0001 | ab, ac, ad |
| Control | 293 ± 94 | 222 ± 76 | 227 ± 144 | 282 ± 130 | 0.567 | |
| Fat utilization (g/d) | ||||||
| RYGB | 142 ± 40 | 197 ± 30 | 140 ± 35 | 131 ± 38 | 0.001 | ab, bc, bd, cd |
| Control | 149 ± 19 | 164 ± 44 | 174 ± 39 | 133 ± 33 | 0.352 | |
| Protein utilization (%EE) | ||||||
| RYGB | 14.2 ± 5.3 | 6.5 ± 2.2 | 14.2 ± 4.6 | 16.0 ± 3.6 | <0.0001 | ab, bd |
| Control | 15.5 ± 3.2 | 13.2 ± 4.0 | 11.4 ± 5.0 | 15.5 ± 3.5 | 0.424 | |
| Carbohydrate utilization (%EE) | ||||||
| RYGB | 43.9 ± 9.9 | 16.3 ± 8.2 | 30.0 ± 8.7 | 31.3 ± 10.2 | <.0001 | ab, bc, bd |
| Control | 38.6 ± 6.5 | 32.5 ± 10.4 | 31.2 ± 17.1 | 39.8 ± 12.3 | 0.333 | |
| Fat utilization (%EE) | ||||||
| RYGB | 41.7 ± 9.0 | 76.8 ± 8.6 | 55.6 ± 10.9 | 52.5 ± 11.0 | <0.0001 | ab, bc, bd |
| Control | 45.7 ± 6.5 | 54.1 ± 12.2 | 57.1 ± 14.6 | 44.5 ± 11.8 | 0.375 | |
| NPRQ | ||||||
| RYGB | 0.85 ± 0.03 | 0.76 ± 0.02 | 0.81 ± 0.03 | 0.81 ± 0.03 | <.0001 | ab, bc, bd |
| Control | 0.84 ± 0.02 | 0.81 ± 0.04 | 0.81 ± 0.05 | 0.84 ± 0.04 | 0.375 | |
| Carbohydrate utilization (%NPEE) | ||||||
| RYGB | 51.2 ± 10.9 | 17.5 ± 8.8 | 35.3 ± 11.4 | 37.4 ± 12.2 | <0.0001 | ab, bc, bd |
| Control | 45.7 ± 7.3 | 37.8 ± 13.1 | 34.9 ± 17.3 | 47.2 ± 14.1 | 0.466 | |
| Fat utilization (%NPEE) | ||||||
| RYGB | 48.8 ± 10.9 | 82.5 ± 8.8 | 64.7 ± 11.4 | 62.6 ± 12.2 | <0.0001 | ab, bc, bd |
| Control | 54.3 ± 7.3 | 62.2 ± 13.1 | 65.1 ± 17.3 | 52.8 ± 14.1 | 0.456 | |
Data are shown as mean ± SD. Models adjusted for age and sex; significant program by time interactions for all parameters (p=0.000–0.04)
Post-hoc estimation using Tukey-Kramer for multiple comparisons with p<0.05; letters refer to statistically significant differences between time points baseline (a),1.5 months (b), 6 months (c) and 12 months (d).
RQ, respiratory quotient; EE, energy expenditure; NPRQ, nonprotein RQ; NPEE, nonprotein EE.
Mixed-effects linear regression models adjusted for age, sex, FFM and FM were used to explore neuroendocrine mechanisms associated with suppressed EE following RYGB (Table 5; Figure 3). Changes in TEE, BMR and/or sleep EE were associated with changes in insulin, HOMA, adiponectin, leptin, TSH, total T3, PYY3–36, GLP2, and urinary NE and E. Substrate utilization was not associated with neuroendocrine alterations; however, fat utilization was positively associated with fasting serum NEFA.
Table 5.
Associations between energy expenditure and fasting serum hormones and urinary catecholamine excretion in RYGB group (n=11)
| TEE | BMR | Sleep EE | HR | |||||
|---|---|---|---|---|---|---|---|---|
| β (se) | p-value | β (se) | p-value | β (se) | p-value | β (se) | p-value | |
| Independent variables | ||||||||
| Insulin (µu/mL)* | 328 (71) | 0.001 | 204 (68) | 0.008 | 0.14 (0.04) | 0.001 | 7.68 (2.58) | 0.008 |
| HOMA* | 338 (57) | 0.0001 | 210 (59 | 0.002 | 0.17 (0.03) | 0.001 | 7.88 (2.27) | 0.003 |
| Adiponectin (ng/mL)* | 372 (149) | 0.023 | 219 (129) | 0.108 | −0.18 (0.08) | 0.042 | −8.2 (5.0) | 0.117 |
| Resistin (ng/mL) | 18.4 (15.6) | 0.253 | 7.91 (11.4) | 0.495 | 0.01 (0.01) | 0.333 | 0.27 (0.53) | 0.615 |
| Leptin (ng/mL) | 367 (94) | 0.001 | 127 (80.6) | 0.132 | 0.12 (0.05) | 0.043 | 2.93 (3.17) | 0.366 |
| CRP (ng/mL)* | 63.5 (39.8) | 0.128 | 28.8 (29.3) | 0.338 | 0.02 (0.02) | 0.265 | 3.61 (0.99) | 0.002 |
| TSH (µIU/mL)* | 266 (110) | 0.027 | 166 (83) | 0.062 | 0.12 (0.06) | 0.056 | 5.07 (3.80) | 0.200 |
| Total T3 (ng/dL) | 624 (143) | 0.001 | 497 (119) | 0.001 | 0.33 (0.08) | 0.001 | 13.28 (4.94) | 0.015 |
| Total T4 (ng/dL) | 189 (248) | 0.455 | 11.1 (179) | 0.951 | 0.08 (0.12) | 0.542 | 11.13 (6.77) | 0.118 |
| Free T3 (pg/mL) | 188 (99) | 0.075 | 120 (74) | 0.124 | 0.10 (0.05) | 0.053 | 5.5 (2.26) | 0.026 |
| Free T4 (ng/dL) | 184 (228) | 0.431 | 60.5 (170) | 0.726 | 0.09 (0.12) | 0.443 | 8.92 (4.81) | 0.081 |
| Reverse T3 (ng/mL) | −1298 (719) | 0.091 | 772 (530) | 0.165 | −0.61 (0.36) | 0.109 | −8.48 (23.54) | 0.724 |
| PYY3–36 (pg/mL) | 5.68 (1.33) | 0.001 | 2.94 (1.38) | 0.049 | 0.002 (0.0007) | 0.004 | 0.16 (0.05) | 0.007 |
| GLP1 (pM) | −1.79 (2.37) | 0.460 | 2.04 (1.72) | 0.252 | −0.0008 (0.001) | 0.307 | 0.12 (0.05) | 0.023 |
| GLP2 (ng/mL) | 68.5 (16.5) | 0.001 | 60.2 (15.5) | 0.001 | 0.04 (0.01) | 0.003 | 2.24 (0.51) | 0.001 |
| Urinary NE (nmol/d) | 181.4 (56.5) | 0.005 | 135 (60) | 0.037 | 0.10 (0.03) | 0.004 | 5.74 (1.94) | 0.009 |
| Urinary E (nmol/d) | 167.4 (54.1) | 0.006 | 116 (70) | 0.114 | 0.07 (0.05) | 0.151 | 2.83 (2.14) | 0.201 |
Natural logarithm transformed. Models adjusted for age, sex, FFM and FM.
NEFA, nonesterified fatty acids; HOMA, homeostatic model assessment; CRP, C-reactive protein; TSH, thyroid stimulating hormone; T3, triiodothyronine; T4, thyroxine; PYY3–36, peptide YY3–36; GLP1, glucagon-like peptide-1; GLP2, glucagon-like peptide-2; NE, norepinephrine; E, epinephrine.
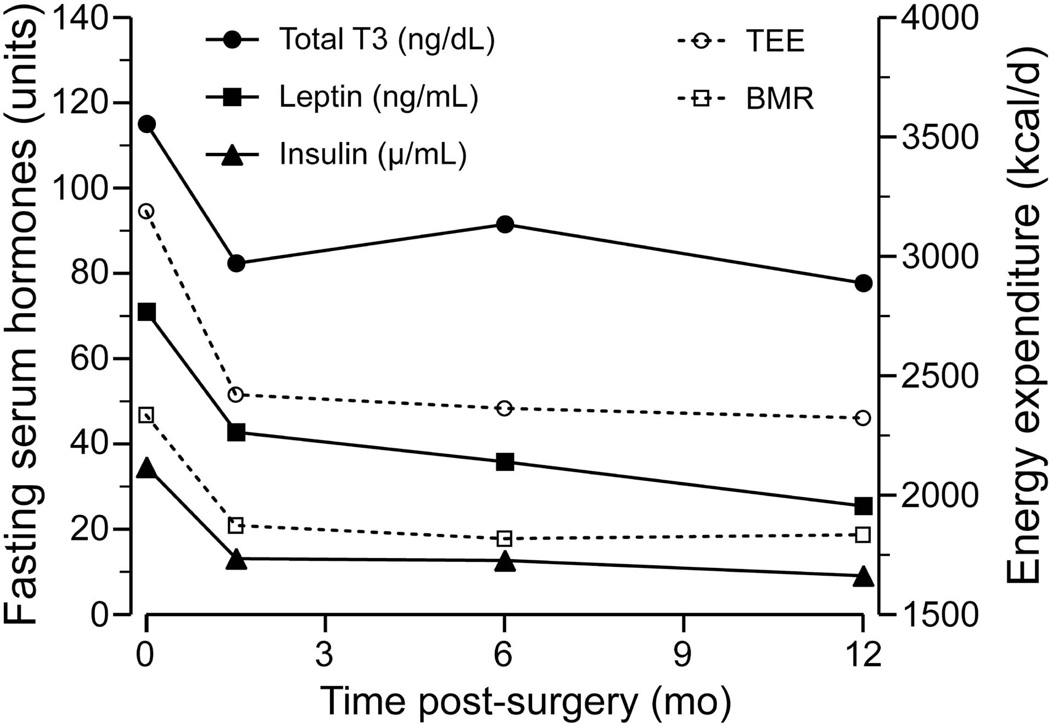
Figure 3.
Changes in total energy expenditure (TEE), basal metabolic rate (BMR), and fasting serum hormones in adolescents at baseline and 1.5, 6 and 12 months post-surgery
Discussion
Here within, we demonstrate that energetic adaptations in response to RYGB in severely obese adolescents 1) are not totally explained by weight, FFM or FM loss; 2) are associated with changes in insulin, leptin, adiponectin, T3, gut hormones, and SNS activity; and 3) persist 12 months after surgery despite a diminishing rate of weight loss.
RYGB-induced energetic adaptations were observed in TEE and its components after surgery. The majority of the adaptive thermogenesis occurred by 1.5 months after surgery, coincident with the decline in FFM and biochemical changes. TEE, AEE, BMR, sleep EE and walking EE declined by 24, 41, 19, 24, and 28% at 1.5 months, and then remained at the suppressed level at 6 and 12 months after surgery. Heart rate paralleled the decline in EE, probably driven by the autonomic nervous system (23). Despite the significant slowing of weight loss by 12 months post-surgery, the pre-surgical relationship between EE and weight or FFM was not restored. Lower energy economy of walking post-surgically also indicated energy conservation. Within the confines of the calorimeter, AEE and PAL declined in both groups, possibly due to habituation to the room calorimeter at follow-up.
As expected on very low calorie diets, negative energy balance caused a shift towards increased fat oxidation. At 1.5 months after surgery, mean 24-h RQ was 0.76, and fat utilization had increased markedly to 77% of EE, a finding likely explained best by a shift from use of exogenous (dietary) energy intake to use of endogenous fat stores for fuel during the rapid weight loss phase associated with hypocaloric dietary intake early postoperatively. The measurements at 6 and 12 months demonstrated a decrease in fat utilization and an increase in carbohydrate utilization, despite continued fat mass loss (utilization).
As first observed in the Minnesota Experiment by Keys et al. (24), caloric restriction resulted in a reduction in basal metabolic rate (BMR) that was greater than that accounted for by the loss in weight and FFM. In these adolescents, RYGB resulted in substantial reductions in weight (30% of initial weight), as in other studies (25), and FM (47% of initial FM) at 12 months post-surgery, but FFM (12% of initial FFM) appeared to be relatively conserved. FFM loss occurred primarily in the first 1.5 months after surgery, and plateaued thereafter. In these adolescents, the proportion of total weight loss was 22% as FFM, and 78% as FM at 12 months post-surgery. In adults, the proportion of weight loss was 31% as FFM with RYGB (26).
The neuroendocrine mechanisms underlying the energetic responses to weight loss induced by RYGB are not well elucidated but may involve insulin, adipokines, thyroid hormones, gut hormones, and SNS activity. Our data demonstrates that decreases in fasting serum insulin, leptin, and T3, and gut hormones, and 24-h urinary excretion of NE parallel the fall in TEE, BMR and sleep EE, and that the effects on EE are statistically independent of FFM and FM losses.
After RYGB, there was a rapid improvement in insulin sensitivity associated with changes in total T3, leptin and adiponectin. The fall in insulin (27) and leptin with weight loss (29) acts to decrease SNS activity (30), thereby lowering BMR independently of changes in weight. Changes in NE likely contribute to the suppressed EE through direct effects on skeletal muscle and indirect effects on thyroid hormones (28). Adipokines (leptin, adiponectin, resistin) interact both centrally and peripherally to regulate energy intake and EE (29). Leptin can reduce FM centrally through inhibition of appetite, stimulation of thermogenesis and fat oxidation. In these adolescents, leptin correlated closely with FM loss. Leptin was a strong predictor of the adaptations in TEE, BMR and sleep EE. We did not observe a significant effect of leptin on substrate utilization.
Thyroid hormones, specifically TSH and T3, decreased after RYGB-induced weight loss in these adolescents. Elevated TSH and T3 levels in obese individuals have been shown to normalize with substantial weight loss (30). The changes in T3 were associated with adaptations in TEE, BMR and sleep EE, confirming the role of thyroid hormones in the regulation of energy metabolism. A reduction in thyroid activity acts to decrease oxygen consumption, slow cellular metabolism and conserve energy stores (31). In conventional weight loss, plasma T3 fell in conjunction with 24-h TEE (32).
Changes in gut hormones after RYGB have been hypothesized to mediate enhanced satiety, while effects on EE are uncertain. In humans, the effects of GLP1 and PYY on EE are emerging but inconsistent (6). PYY was positively correlated with resting EE (33) and negatively correlated in another study (34). Infusion of PYY3–36 tended to reduce EE in lean and obese adults (35). In our study, fasting PYY3–36 and GLP2 declined after RYGB, and were positively associated with TEE, BMR and sleep EE.
A series of studies explored whether energetic adaptations were a result of caloric restriction during active weight loss or maintenance of a reduced weight after conventional weight loss (9, 23,28, 36, 37). Maintenance of reduced weight was accompanied by increased skeletal muscle work efficiency, decreased serum T3 and urinary norepinephrine excretion (28). Our results corroborate these findings in that energetic adaptations were not observed in controls subjected to acute caloric restriction during 24-h calorimetry, and TEE and its components remained suppressed after RYGB-induced weight loss plateaued at 12 months.
Controversy exists over the persistence of energetic adaptations after weight loss (6, 10, 36, 38). The suppression of TEE, BMR, sleep EE, AEE and walking EE observed in these adolescents after RYGB clearly persisted, despite the fact that weight loss had plateaued in most cases. In 12 adults undergoing RYGB, TEE and sleep EE were reduced at 6 months and persisted at 12 months (7). In another study, patients who regained weight two years after RYGB had lower resting EE (39). After conventional weight loss, a disproportionate reduction in EE persisted in individuals who maintained a body weight reduction of ≥10% for greater than one year (9).
RYGB, when used for appropriate patients, clearly can afford substantial health benefits for extremely obese adolescents. This study demonstrated that RYGB improved insulin sensitivity, decreased heart rate and reduced inflammation, but also induced persistent energetic, metabolic and endocrine adaptations that favor weight regain. Elucidating the adaptations induced by weight loss after RYGB will be instrumental in guiding the clinical management of these patients to prevent recidivism and future research into alternate surgical and non-surgical treatments for morbid obesity.
What is already known about this subject?
Energetic adaptations occur after conventional weight loss and Roux-en-Y gastric bypass surgery (RYGB) in adults
Energy adaptations following conventional weight loss and semi-starvation involve changes in autonomic nervous system, thyroid hormones, insulin, adiposity-derived hormones and gut hormones
Weight loss and regain after RYGB are highly variable in adolescents, yet the underlying mechanisms are unclear
What does this study add?
Energetic adaptations involving all components of energy expenditure (basal, activity, sleep and walking) occur in adolescents following RYGB
Energy adaptations are possibly mediated by insulin, leptin, T3, gut hormones and norepinephrine in weight-reduced adolescents.
Energy adaptations persist up to 12 months in adolescents after RYGB
Acknowledgements
NFB, MLB and WWW designed the study; NRM, TAW, ALA, MRP, FAV, RJS carried out the experiments; YL and IFZ analyzed the data. All authors participated in manuscript preparation and provided approval of the final version. This ancillary study to Teen-LABS and was funded by USDA/ARS (Cooperative Agreement No.58-6250-0-008) and National Institute of Diabetes and Digestive and Kidney Diseases with a grant to Cincinnati Children's Hospital Medical Center (U01DK072493/ UM1 DK072493) PI:Thomas Inge, MD, PhD and the University of Cincinnati (UM1DK095710) PI:C. Ralph Buncher, ScD and Todd Jenkins, PhD, MPH. We gratefully acknowledge significant contributions made by Teen-LABS Consortium as well as our parent study LABS Consortium (U01DK066557). The contents of this publication do not necessarily reflect views or policies of USDA, nor does mention of trade names, commercial products, or organizations imply endorsement by U.S. Government.
Footnotes
Competing interests: The authors have no competing interests.
Reference List
- 1.Karra E, Yousseif A, Batterham RL. Mechanisms facilitating weight loss and resolution of type 2 diabetes following bariatric surgery. Trends Endocrinol Metab. 2010;21(6):337–344. doi: 10.1016/j.tem.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 2.Beckman LM, Beckman TR, Earthman CP. Changes in gastrointestinal hormones and leptin after Roux-en-Y gastric bypass procedure: a review. J Am Diet Assoc. 2010;110(4):571–584. doi: 10.1016/j.jada.2009.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Inge TH, Miyano G, Bean J, et al. Reversal of type 2 diabetes mellitus and improvements in cardiovascular risk factors after surgical weight loss in adolescents. Pediatrics. 2009;123(1):214–222. doi: 10.1542/peds.2008-0522. [DOI] [PubMed] [Google Scholar]
- 4.Inge TH. Bariatric surgery for morbidly obese adolescents: is there a rationale for each intervention? Growth Hormone & IGF Research. 2006;16:S15–S19. doi: 10.1016/j.ghir.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 5.Leslie D, Kellogg TA, Ikramuddin S. Bariatric surgery primer for the internist: keys to the surgical consultation. Med Clin North Am. 2007;91:353–381. doi: 10.1016/j.mcna.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 6.Thivel D, Brakonieki K, Duche P, Morio B, Boirie Y, Laferrere B. Surgical weight loss: impact on energy expenditure. Obes Surg. 2013;23(2):255–266. doi: 10.1007/s11695-012-0839-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tamboli RA, Hossain HA, Marks PA, et al. Body composition and energy metabolism following Roux-en-Y gastric bypass surgery. Obesity (Silver Spring) 2010;18(9):1718–1724. doi: 10.1038/oby.2010.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Gemert WG, Westerterp KR, van Acker BA, et al. Energy, substrate and protein metabolism in morbid obesity before, during and after massive weight loss. Int J Obes Relat Metab Disord. 2000;24(6):711–718. doi: 10.1038/sj.ijo.0801230. [DOI] [PubMed] [Google Scholar]
- 9.Rosenbaum M, Hirsch J, Gallagher DA, Leibel RL. Long-term persistence of adaptive thermogenesis in subjects who have maintained a reduced body weight. Am J Clin Nutr. 2008;88(4):906–912. doi: 10.1093/ajcn/88.4.906. [DOI] [PubMed] [Google Scholar]
- 10.Schwartz A, Kuk JL, Lamothe G, Doucet E. Greater than predicted decrease in resting energy expenditure and weight loss: results from a systematic review. Obesity (Silver Spring) 2012;20(11):2307–2310. doi: 10.1038/oby.2012.34. [DOI] [PubMed] [Google Scholar]
- 11.Weyer C, Walford RL, Harper IT, et al. Energy metabolism after 2 y of energy restriction: the biosphere 2 experiment. Am J Clin Nutr. 2000;72(4):946–953. doi: 10.1093/ajcn/72.4.946. [DOI] [PubMed] [Google Scholar]
- 12.Weyer C, Pratley RE, Salbe AD, Bogardus C, Ravussin E, Tataranni PA. Energy expenditure, fat oxidation, and body weight regulation: a study of metabolic adaptation to long-term weight change. J Clin Endocrinol Metab. 2000;85(3):1087–1094. doi: 10.1210/jcem.85.3.6447. [DOI] [PubMed] [Google Scholar]
- 13.Astrup A, Buemann B, Christensen NJ, Madsen J. 24-hour energy expenditure and sympathetic activity in postobese women consuming a high-carbohydrate diet. Am J Physiol. 1992;262(3 Pt 1):E282–E288. doi: 10.1152/ajpendo.1992.262.3.E282. [DOI] [PubMed] [Google Scholar]
- 14.Weinsier RL, Hunter GR, Zuckerman PA, et al. Energy expenditure and free-living physical activity in black and white women: comparison before and after weight loss. Am J Clin Nutr. 2000;71(5):1138–1146. doi: 10.1093/ajcn/71.5.1138. [DOI] [PubMed] [Google Scholar]
- 15.Fuller NJ, Jebb SA, Laskey MA, Coward WA, Elia M. Four-component model for the assessment of body composition in humans: comparison with alternative methods, and evaluation of the density and hydration of fat-free mass. Clin Sci. 1992;82:687–693. doi: 10.1042/cs0820687. [DOI] [PubMed] [Google Scholar]
- 16.Wong WW, Lee LS, Klein PD. Deuterium and oxygen-18 measurements on microliter samples of urine, plasma, saliva, and human milk. Am J Clin Nutr. 1987;45:905–913. doi: 10.1093/ajcn/45.5.905. [DOI] [PubMed] [Google Scholar]
- 17.Wong WW, Clark LL, Llaurador M, Klein PD. A new zinc product for the reduction of water in physiological fluids to hydrogen gas for 2H/1H isotope ratio measurements. Eur J Clin Nutr. 1992;46:69–71. [PubMed] [Google Scholar]
- 18.Matthews DR, Hosker JP, Rudensky AS, Naylor A, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta cell function from fasting glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 19.Wetherburn MW. Phenol-hypochlorite reaction for determination of ammonia. Analytical Chem. 1967;39:971. [Google Scholar]
- 20.Moon JK, Vohra FA, Valerio Jimenez OS, Puyau MR, Butte NF. Closed-loop control of carbon dioxide concentration and pressure improves response of room respiration calorimeters. J Nutr. 1995;125:220–228. doi: 10.1093/jn/125.2.220. [DOI] [PubMed] [Google Scholar]
- 21.Livesey G, Elia M. Estimation of energy expenditure, net carbohydrate utilization, and net fat oxidation and synthesis by indirect calorimetry: evaluation of errors with special reference to the detailed composition of fuels. Am J Clin Nutr. 1988;47:608–628. doi: 10.1093/ajcn/47.4.608. [DOI] [PubMed] [Google Scholar]
- 22.Schmidt-Nielsen K. Locomotion: energy cost of swimming, flying, and running. Science. 1972;177(45):222–228. doi: 10.1126/science.177.4045.222. [DOI] [PubMed] [Google Scholar]
- 23.Rosenbaum M, Goldsmith R, Bloomfield D, et al. Low-dose leptin reverses skeletal muscle, autonomic, and neuroendocrine adaptations to maintenance of reduced weight. J Clin Invest. 2005;115:3579–3586. doi: 10.1172/JCI25977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Keys A, Brozek J, Henschel A, Mickelson O, Taylor HL. The Biology of Human Starvation. Minneapolis: University of Minneapolis Press; 1950. [Google Scholar]
- 25.Inge TH, Zeller MH, Lawson ML, Daniels SR. A critical appraisal of evidence supporting a bariatric surgical approach to weight management for adolescents. J Pediatr. 2006 doi: 10.1016/j.jpeds.2005.03.021. [DOI] [PubMed] [Google Scholar]
- 26.Chaston TB, Dixon JB, O'Brien PE. Changes in fat-free mass during significant weight loss: a systematic review. Int J Obes. 2007;31:743–750. doi: 10.1038/sj.ijo.0803483. [DOI] [PubMed] [Google Scholar]
- 27.Emdin M, Gastaldelli A, Muscelli E, et al. Hyperinsulinemia and autonomic nervous system dysfunction in obesity: effects of weight loss. Circulation. 2001;103(4):513–519. doi: 10.1161/01.cir.103.4.513. [DOI] [PubMed] [Google Scholar]
- 28.Rosenbaum M, Hirsch J, Murphy E, Leibel RL. Effects of changes in body weight on carbohydrate metabolism, catecholamine excretion, and thyroid function. Am J Clin Nutr. 2000;71:1421–1432. doi: 10.1093/ajcn/71.6.1421. [DOI] [PubMed] [Google Scholar]
- 29.Harwood HJ., Jr The adipocyte as an endocrine organ in the regulation of metabolic homeostasis. Neuropharmacology. 2011 doi: 10.1016/j.neuropharm.2011.12.010. [DOI] [PubMed] [Google Scholar]
- 30.Reinehr T. Obesity and thyroid function. Molecular and cellular endocrin. 2010;316:165–171. doi: 10.1016/j.mce.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 31.McAninch EA, Bianco AC. Thyroid hormone signaling in energy homeostasis and energy metabolism. Ann N Y Acad Sci. 2014;1311:77–87. doi: 10.1111/nyas.12374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heilbronn LK, de JL, Frisard MI, et al. Effect of 6-month calorie restriction on biomarkers of longevity, metabolic adaptation, and oxidative stress in overweight individuals: a randomized controlled trial. JAMA. 2006;295(13):1539–1548. doi: 10.1001/jama.295.13.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hill BR, De Souza MJ, Williams NI. Characterization of the diurnal rhythm of peptide YY and its association with energy balance parameters in normal-weight premenopausal women. Am J Physiol Endocrinol Metab. 2011;301(2):E409–E415. doi: 10.1152/ajpendo.00171.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guo Y, Ma L, Enriori PJ, et al. Physiological evidence for the involvement of peptide YY in the regulation of energy homeostasis in humans. Obesity (Silver Spring) 2006;14(9):1562–1570. doi: 10.1038/oby.2006.180. [DOI] [PubMed] [Google Scholar]
- 35.Sloth B, Holst JJ, Flint A, Gregersen NT, Astrup A. Effects of PYY1–36 and PYY3–36 on appetite, energy intake, energy expenditure, glucose and fat metabolism in obese and lean subjects. Am J Physiol Endocrinol Metab. 2007;292(4):E1062–E1068. doi: 10.1152/ajpendo.00450.2006. [DOI] [PubMed] [Google Scholar]
- 36.Leibel RL, Rosenbaum M, Hirsch J. Changes in energy expenditure resulting from altered body weight. N Engl J Med. 1995;332(10):621–628. doi: 10.1056/NEJM199503093321001. [DOI] [PubMed] [Google Scholar]
- 37.Rosenbaum M, Vandenborne K, Goldsmith R, et al. Effects of experimental weight perturbation on skeletal muscle work efficiency in human subjects. Am J Physiol Regul Integr Comp Physiol. 2003;285(1):R183–R192. doi: 10.1152/ajpregu.00474.2002. [DOI] [PubMed] [Google Scholar]
- 38.Rosenbaum M, Leibel RL. Adaptive thermogenesis in humans. Int J Obes (Lond) 2010;34(Suppl 1):S47–S55. doi: 10.1038/ijo.2010.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Faria SL, Kelly E, Faria OP. Energy expenditure and weight regain in patients submitted to Roux-en-Y gastric bypass. Obes Surg. 2009;19(7):856–859. doi: 10.1007/s11695-009-9842-6. [DOI] [PubMed] [Google Scholar]