Abstract
Accumulation of 45Ca++ was found to occur in membrane vesicles of E. coli prepared by lysis with a French pressure cell. The uptake occurs by active transport, requiring an energy source. Substrates of the electron transport chain, including D-lactate, reduced phenazine methosulfate, and NADH, stimulated accumulation, but this effect was blocked by the addition of cyanide. ATP could also stimulate accumulation, and this effect was blocked by dicyclohexylcarbodiimide. Uncouplers of oxidative phosphorylation inhibited the accumulation driven by either type of energy source.
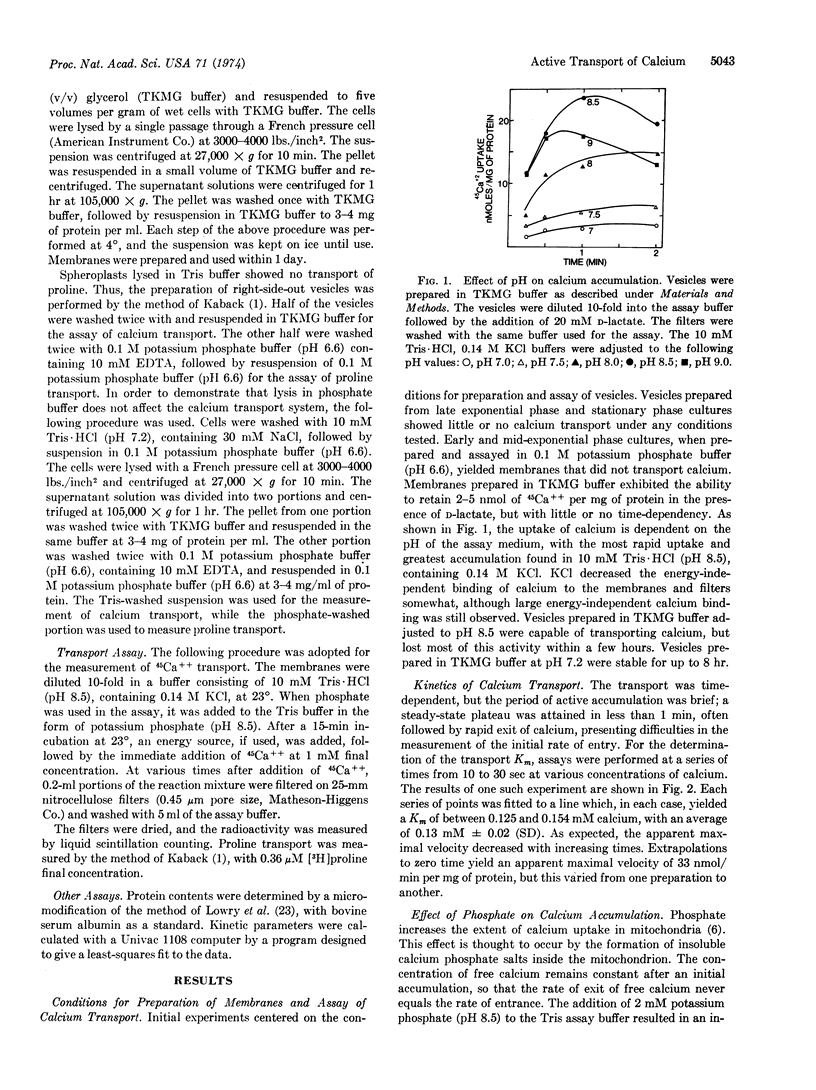
Accumulation of calcium is rapid, reaching the steady-state plateau within 1 min. Addition of phosphate to the assay buffer results in a prolongation of the reaction, allowing for the time-dependent accumulation of calcium for as long as 30 min.
Vesicles prepared by lysis with a French pressure cell exhibit almost no ability to accumulate proline, while vesicles prepared by the method of Kaback transport proline but exhibit little energy-dependent transport of calcium. It is suggested that the accumulation of calcium in these vesicles, which are believed to be inverted, reflects a system that in vivo is responsible for the active extrusion of calcium from the cells.
Keywords: membrane orientation, energy coupling, calcium uptake
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altendorf K. H., Staehelin L. A. Orientation of membrane vesicles from Escherichia coli as detected by freeze-cleave electron microscopy. J Bacteriol. 1974 Feb;117(2):888–899. doi: 10.1128/jb.117.2.888-899.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astle L., Cooper C. Relationship of sidedness of mitochondrial inner membrane vesicles to their enzymic properties. Biochemistry. 1974 Jan 1;13(1):154–160. doi: 10.1021/bi00698a024. [DOI] [PubMed] [Google Scholar]
- Berger E. A. Different mechanisms of energy coupling for the active transport of proline and glutamine in Escherichia coli. Proc Natl Acad Sci U S A. 1973 May;70(5):1514–1518. doi: 10.1073/pnas.70.5.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan T. L., Greenawalt J. W., Pedersen P. L. Biochemical and ultrastructural properties of a mitochondrial inner membrane fraction deficient in outer membrane and matrix activities. J Cell Biol. 1970 May;45(2):291–305. doi: 10.1083/jcb.45.2.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futai M. Orientation of membrane vesicles from Escherichia coli prepared by different procedures. J Membr Biol. 1974;15(1):15–28. doi: 10.1007/BF01870079. [DOI] [PubMed] [Google Scholar]
- HAYASHI S., KOCH J. P., LIN E. C. ACTIVE TRANSPORT OF L-ALPHA-GLYCEROPHOSPHATE IN ESCHERICHIA COLI. J Biol Chem. 1964 Sep;239:3098–3105. [PubMed] [Google Scholar]
- Hertzberg E. L., Hinkle P. C. Oxidative phosphorylation and proton translocation in membrane vesicles prepared from Escherichia coli. Biochem Biophys Res Commun. 1974 May 7;58(1):178–184. doi: 10.1016/0006-291x(74)90908-5. [DOI] [PubMed] [Google Scholar]
- Inesi G. Active transport of calcium ion in sarcoplasmic membranes. Annu Rev Biophys Bioeng. 1972;1:191–210. doi: 10.1146/annurev.bb.01.060172.001203. [DOI] [PubMed] [Google Scholar]
- Kaback H. R., Milner L. S. Relationship of a membrane-bound D-(-)-lactic dehydrogenase to amino acid transport in isolated bacterial membrane preparations. Proc Natl Acad Sci U S A. 1970 Jul;66(3):1008–1015. doi: 10.1073/pnas.66.3.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Larsen S. H., Adler J., Gargus J. J., Hogg R. W. Chemomechanical coupling without ATP: the source of energy for motility and chemotaxis in bacteria. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1239–1243. doi: 10.1073/pnas.71.4.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawford H. G., Haddock B. A. Respiration-driven proton translocation in Escherichia coli. Biochem J. 1973 Sep;136(1):217–220. doi: 10.1042/bj1360217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardi F. J., Reeves J. P., Kaback H. R. Mechanisms of active transport in isolated bacterial membrane vesicles. 8. Valinomycin-induced rubidium transport. J Biol Chem. 1973 May 25;248(10):3551–3565. [PubMed] [Google Scholar]
- Loyter A., Christiansen R. O., Steensland H., Saltzgaber J., Racker E. Energy-linked ion translocation in submitochondrial particles. I. Ca++ accumulation in submitochondrial particles. J Biol Chem. 1969 Aug 25;244(16):4422–4427. [PubMed] [Google Scholar]
- Mitchell P. Chemiosmotic coupling in oxidative and photosynthetic phosphorylation. Biol Rev Camb Philos Soc. 1966 Aug;41(3):445–502. doi: 10.1111/j.1469-185x.1966.tb01501.x. [DOI] [PubMed] [Google Scholar]
- Pedersen P. L., Coty W. A. Energy-dependent accumulation of calcium and phosphate by purified inner membrane vesicles of rat liver mitochondria. J Biol Chem. 1972 May 25;247(10):3107–3113. [PubMed] [Google Scholar]
- Reeves J. P. Transient pH changes during D-lactate oxidation by membrane vesicles. Biochem Biophys Res Commun. 1971 Nov;45(4):931–936. doi: 10.1016/0006-291x(71)90427-x. [DOI] [PubMed] [Google Scholar]
- Schatzmann H. J. ATP-dependent Ca++-extrusion from human red cells. Experientia. 1966 Jun 15;22(6):364–365. doi: 10.1007/BF01901136. [DOI] [PubMed] [Google Scholar]
- Silver S., Kralovic M. L. Manganese accumulation by Escherichia coli: evidence for a specific transport system. Biochem Biophys Res Commun. 1969 Mar 10;34(5):640–645. doi: 10.1016/0006-291x(69)90786-4. [DOI] [PubMed] [Google Scholar]
- Tanaka S., Lerner S. A., Lin E. C. Replacement of a phosphoenolpyruvate-dependent phosphotransferase by a nicotinamide adenine dinucleotide-linked dehydrogenase for the utilization of mannitol. J Bacteriol. 1967 Feb;93(2):642–648. doi: 10.1128/jb.93.2.642-648.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VASINGTON F. D. Calcium ion uptake by fragments of rat liver mitochondria and its dependence on electron transport. J Biol Chem. 1963 May;238:1841–1847. [PubMed] [Google Scholar]
- VASINGTON F. D., MURPHY J. V. Ca ion uptake by rat kidney mitochondria and its dependence on respiration and phosphorylation. J Biol Chem. 1962 Aug;237:2670–2677. [PubMed] [Google Scholar]
- van Thienen G., Postma P. W. Coupling between energy conservation and active transport of serine in Escherichia coli. Biochim Biophys Acta. 1973 Oct 25;323(3):429–440. doi: 10.1016/0005-2736(73)90188-0. [DOI] [PubMed] [Google Scholar]


