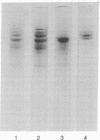
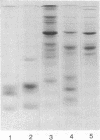
Abstract
Chromatin was reconstituted from the purified DNA and histones of chicken erythrocytes and the nonhistone proteins of either chicken reticulocytes or chicken liver. Reconstituted chromatins, native chicken reticulocyte chromatin, and free DNA were transcribed with Escherichia coli RNA polymerase and the concentrations of globin-specific sequences in the RNA products were measured by hybridization with [3H]DNA complementary to chicken globin messenger RNA. Reticulocyte, but not liver, nonhistone proteins were shown to activate the globin genes in reconstituted erythrocyte chromatin. The transcripts of native and reconstituted chromatins were indistinguishable in respect of both the total yield of the RNA and the fractional yield of globin-specific sequences.
Keywords: nonhistones, histones, chicken globin mRNA, complementary DNA, RNA polymerase
Full text
PDF

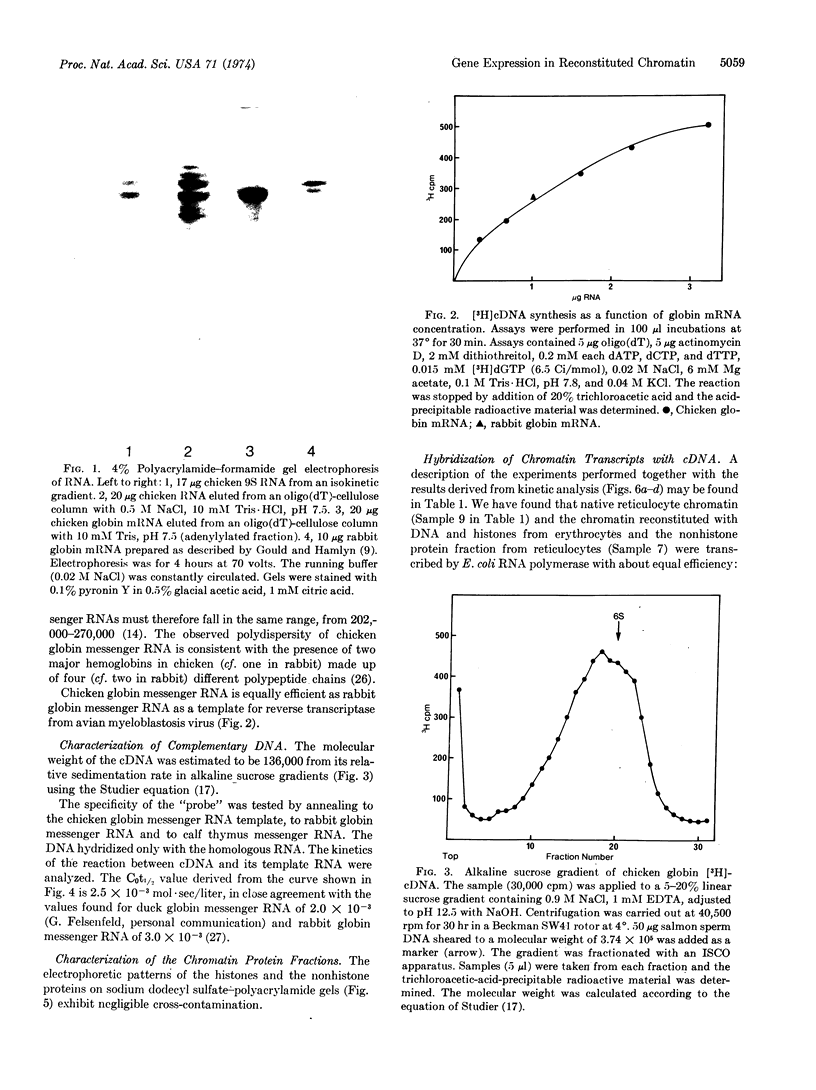
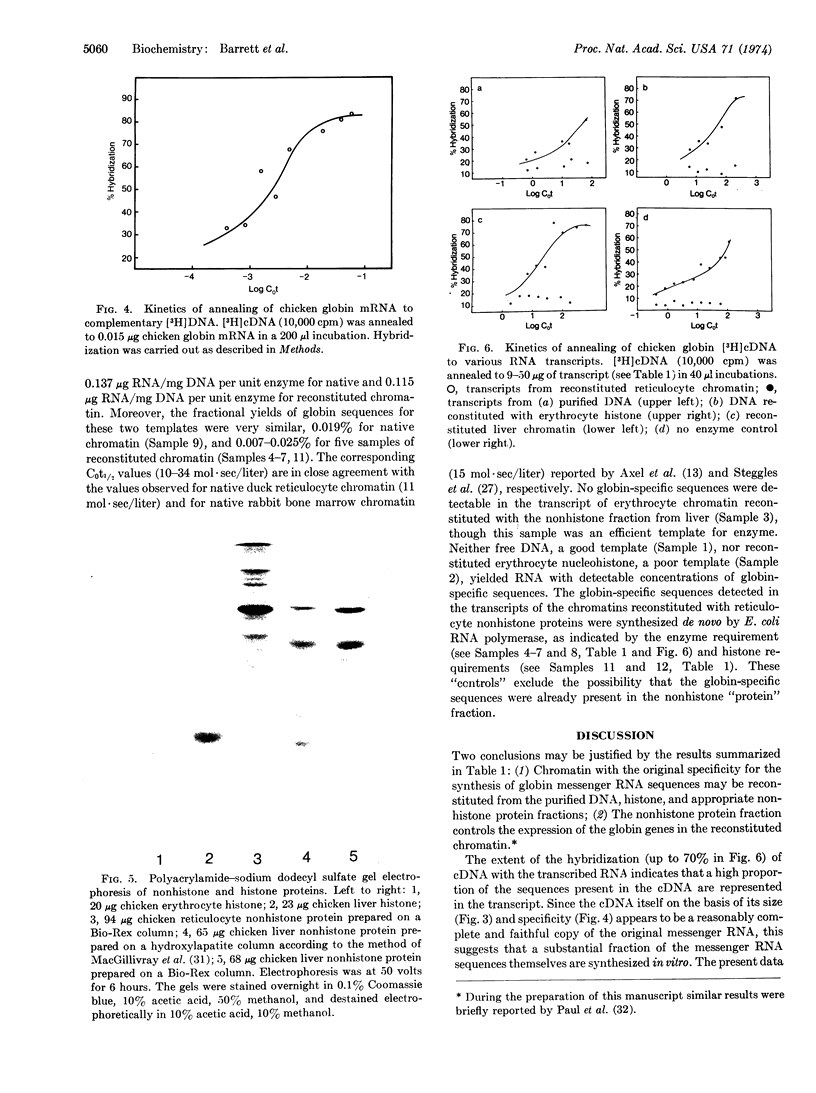
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axel R., Cedar H., Felsenfeld G. Synthesis of globin ribonucleic acid from duck-reticulocyte chromatin in vitro. Proc Natl Acad Sci U S A. 1973 Jul;70(7):2029–2032. doi: 10.1073/pnas.70.7.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bank A., Terada M., Metafora S., Dow L., Marks P. A. In vitro synthesis of DNA components of human genes for globins. Nat New Biol. 1972 Feb 9;235(58):167–169. doi: 10.1038/newbio235167a0. [DOI] [PubMed] [Google Scholar]
- Bishop J. O., Pemberton R., Baglioni C. Reiteration frequency of haemoglobin genes in the duck. Nat New Biol. 1972 Feb 23;235(60):231–234. doi: 10.1038/newbio235231a0. [DOI] [PubMed] [Google Scholar]
- Blobel G., Potter V. R. Nuclei from rat liver: isolation method that combines purity with high yield. Science. 1966 Dec 30;154(3757):1662–1665. doi: 10.1126/science.154.3757.1662. [DOI] [PubMed] [Google Scholar]
- DINGMAN C. W., SPORN M. B. STUDIES ON CHROMATIN. I. ISOLATION AND CHARACTERIZATION OF NUCLEAR COMPLEXES OF DEOXYRIBONUCLEIC ACID, RIBONUCLEIC ACID, AND PROTEIN FROM EMBRYONIC AND ADULT TISSUES OF THE CHICKEN. J Biol Chem. 1964 Oct;239:3483–3492. [PubMed] [Google Scholar]
- Dahmus M. E., Bonner J. Nucleoproteins in regulation of gene function. Fed Proc. 1970 May-Jun;29(3):1255–1260. [PubMed] [Google Scholar]
- Gilmour R. S., Paul J. RNA transcribed from reconstituted nucleoprotein is similar to natural RNA. J Mol Biol. 1969 Feb 28;40(1):137–139. doi: 10.1016/0022-2836(69)90301-5. [DOI] [PubMed] [Google Scholar]
- Gilmour R. S., Paul J. Role of non-histone components in determining organ specificity of rabbit chromatins. FEBS Lett. 1970 Aug 17;9(4):242–244. doi: 10.1016/0014-5793(70)80366-0. [DOI] [PubMed] [Google Scholar]
- Gilmour R. S., Paul J. Tissue-specific transcription of the globin gene in isolated chromatin. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3440–3442. doi: 10.1073/pnas.70.12.3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould H. J., Hamlyn P. H. The molecular weight of rabbit globin messenger RNA's. FEBS Lett. 1973 Mar 15;30(3):301–304. doi: 10.1016/0014-5793(73)80674-x. [DOI] [PubMed] [Google Scholar]
- Gross-Bellard M., Oudet P., Chambon P. Isolation of high-molecular-weight DNA from mammalian cells. Eur J Biochem. 1973 Jul 2;36(1):32–38. doi: 10.1111/j.1432-1033.1973.tb02881.x. [DOI] [PubMed] [Google Scholar]
- Imaizumi T., Diggelmann H., Scherrer K. Demonstration of globin messenger sequences in giant nuclear precursors of messenger RNA of avian erythroblasts. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1122–1126. doi: 10.1073/pnas.70.4.1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy S., Simpson R. T., Sober H. A. Fractionation of chromatin components. Biochemistry. 1972 Apr 25;11(9):1547–1554. [PubMed] [Google Scholar]
- MacGillivray A. J., Carroll Dana, Paul J. The heterogeneity of the non-histone chromatin proteins from mouse tissues. FEBS Lett. 1971 Mar 16;13(4):204–208. doi: 10.1016/0014-5793(71)80536-7. [DOI] [PubMed] [Google Scholar]
- Panyim S., Bilek D., Chalkley R. An electrophoretic comparison of vertebrate histones. J Biol Chem. 1971 Jul 10;246(13):4206–4215. [PubMed] [Google Scholar]
- Paul J., Gilmour R. S., Affara N., Birnie G., Harrison P., Hell A., Humphries S., Windass J., Young B. The globin gene: structure and expression. Cold Spring Harb Symp Quant Biol. 1974;38:885–890. doi: 10.1101/sqb.1974.038.01.090. [DOI] [PubMed] [Google Scholar]
- Paul J., Gilmour R. S. Organ-specific restriction of transcription in mammalian chromatin. J Mol Biol. 1968 Jul 14;34(2):305–316. doi: 10.1016/0022-2836(68)90255-6. [DOI] [PubMed] [Google Scholar]
- Penman S. RNA metabolism in the HeLa cell nucleus. J Mol Biol. 1966 May;17(1):117–130. doi: 10.1016/s0022-2836(66)80098-0. [DOI] [PubMed] [Google Scholar]
- Ross J., Aviv H., Scolnick E., Leder P. In vitro synthesis of DNA complementary to purified rabbit globin mRNA (RNA-dependent DNA polymerase-reticulocyte-hemoglobin-density gradient centrifugation-oligo(dT) primer). Proc Natl Acad Sci U S A. 1972 Jan;69(1):264–268. doi: 10.1073/pnas.69.1.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Carrilo A., Beato M., Schutz G., Feigelson P., Allfrey V. G. Cell-free translation of the globin message within polydisperse high-molecular-weight ribonucleic acid of avian erythrocytes. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3641–3645. doi: 10.1073/pnas.70.12.3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STUDIER F. W. SEDIMENTATION STUDIES OF THE SIZE AND SHAPE OF DNA. J Mol Biol. 1965 Feb;11:373–390. doi: 10.1016/s0022-2836(65)80064-x. [DOI] [PubMed] [Google Scholar]
- Staynov D. Z., Pinder J. C., Gratzer W. B. Molecular weight determination of nucleic acids by gel electrophoresis in non-aqueous solution. Nat New Biol. 1972 Jan 26;235(56):108–110. doi: 10.1038/newbio235108a0. [DOI] [PubMed] [Google Scholar]
- Steggles A. W., Wilson G. N., Kantor J. A., Picciano D. J., Falvey A. K., Anderson W. F. Cell-free transcription of mammalian chromatin: transcription of globin messenger RNA sequences from bone-marrow chromatin with mammalian RNA polymerase. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1219–1223. doi: 10.1073/pnas.71.4.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma I. M., Temple G. F., Fan H., Baltimore D. In vitro synthesis of DNA complementary to rabbit reticulocyte 10S RNA. Nat New Biol. 1972 Feb 9;235(58):163–167. doi: 10.1038/newbio235163a0. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Williamson R., Drewienkiewicz C. E., Paul J. Globin messenger sequences in high molecular weight RNA from embryonic mouse liver. Nat New Biol. 1973 Jan 17;241(107):66–68. doi: 10.1038/newbio241066a0. [DOI] [PubMed] [Google Scholar]
- Zentgraf H., Deumling B., Franke W. W. Isolation and characterization of nuclei from bird erythrocytes. Exp Cell Res. 1969 Aug;56(2):333–337. doi: 10.1016/0014-4827(69)90022-6. [DOI] [PubMed] [Google Scholar]
- Zirkin B. R. A cytochemical study of the nonhistone protein content of condensed and extended chromatin. Exp Cell Res. 1973 Apr;78(2):394–398. doi: 10.1016/0014-4827(73)90084-0. [DOI] [PubMed] [Google Scholar]