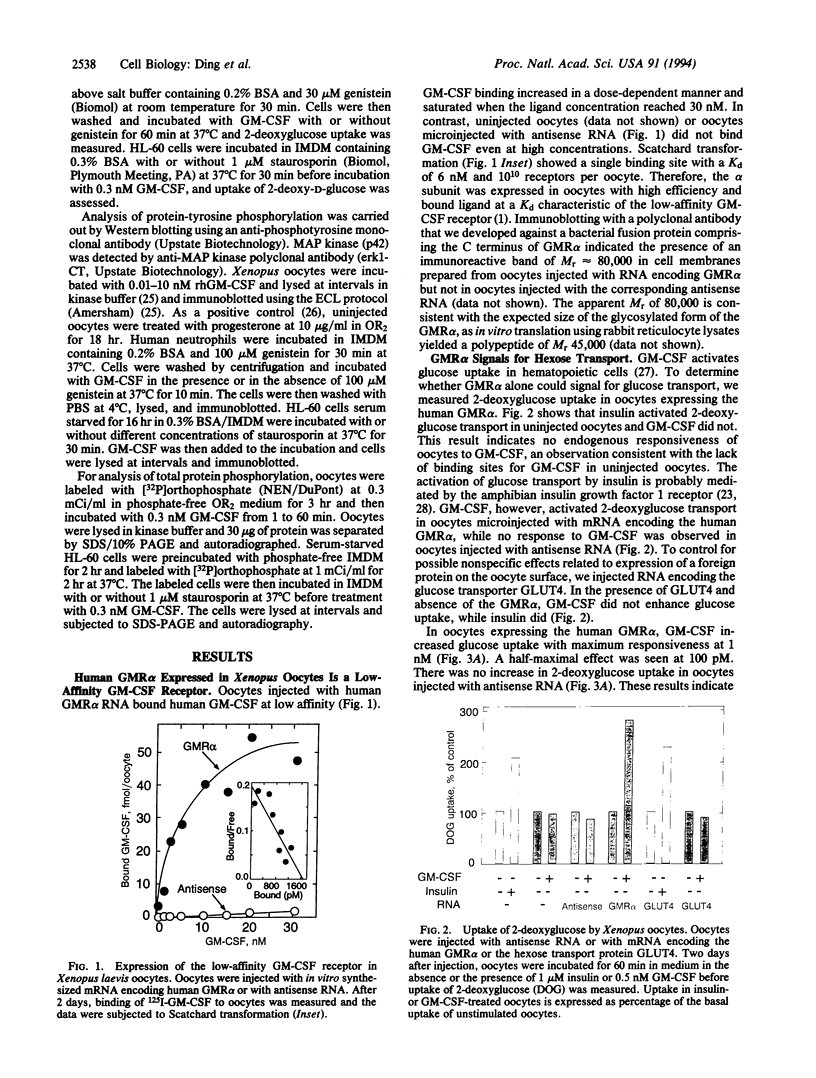
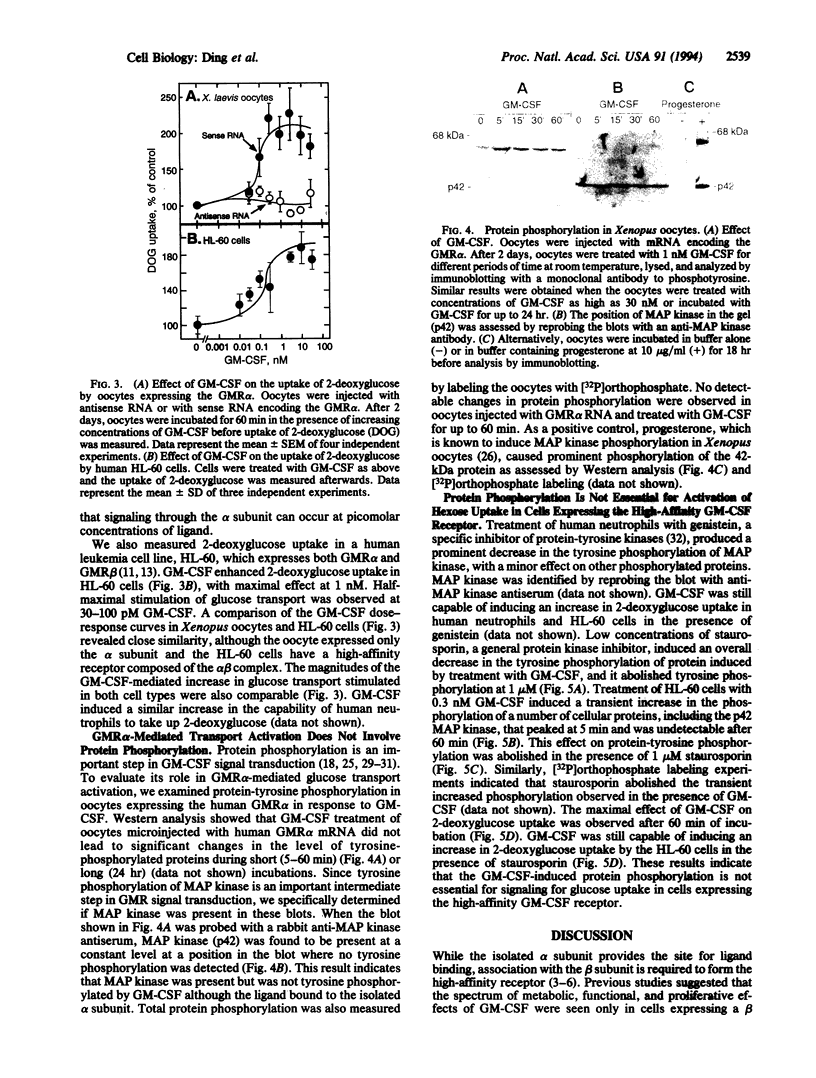
Abstract
The receptor for granulocyte-macrophage colony-stimulating factor (GM-CSF) is composed of an alpha and beta subunit, which together form the high-affinity receptor. The alpha subunit by itself binds ligand at low affinity, whereas the isolated beta subunit does not bind GM-CSF. It is generally believed that the high-affinity receptor is responsible for the multiple functions of GM-CSF and that the isolated alpha subunit (GMR alpha) does not transduce a signal. Xenopus laevis oocytes injected with RNA encoding human GMR alpha expressed up to 10(10) low-affinity sites for GM-CSF (Kd = 6 nM). GM-CSF binding to the alpha subunit expressed in Xenopus oocytes caused activation of 2-deoxyglucose transport through endogenous glucose transporters. 2-Deoxyglucose transport was stimulated by similar low concentrations of GM-CSF in HL-60 leukemia cells as well as normal human neutrophils and Xenopus oocytes expressing GMR alpha. Engagement of the isolated alpha subunit in oocytes did not lead to protein phosphorylation or tyrosine phosphorylation of mitogen-activated protein kinase (MAP kinase). Staurosporin and genistein inhibited GM-CSF-induced tyrosine phosphorylation of MAP kinase in human neutrophils and HL-60 cells without affecting GM-CSF-stimulated uptake of 2-deoxyglucose. These results provide direct evidence that the isolated alpha subunit signals for hexose transport and can do so without engagement of the kinase cascade. Our data also indicate that signaling for hexose uptake may occur in a phosphorylation-independent manner in cells expressing the high-affinity GM-CSF receptor.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiyama T., Ishida J., Nakagawa S., Ogawara H., Watanabe S., Itoh N., Shibuya M., Fukami Y. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J Biol Chem. 1987 Apr 25;262(12):5592–5595. [PubMed] [Google Scholar]
- Baldwin G. C., Gasson J. C., Kaufman S. E., Quan S. G., Williams R. E., Avalos B. R., Gazdar A. F., Golde D. W., DiPersio J. F. Nonhematopoietic tumor cells express functional GM-CSF receptors. Blood. 1989 Mar;73(4):1033–1037. [PubMed] [Google Scholar]
- Baldwin G. C., Golde D. W., Widhopf G. F., Economou J., Gasson J. C. Identification and characterization of a low-affinity granulocyte-macrophage colony-stimulating factor receptor on primary and cultured human melanoma cells. Blood. 1991 Aug 1;78(3):609–615. [PubMed] [Google Scholar]
- Birnbaum M. J. Identification of a novel gene encoding an insulin-responsive glucose transporter protein. Cell. 1989 Apr 21;57(2):305–315. doi: 10.1016/0092-8674(89)90968-9. [DOI] [PubMed] [Google Scholar]
- Budel L. M., Elbaz O., Hoogerbrugge H., Delwel R., Mahmoud L. A., Löwenberg B., Touw I. P. Common binding structure for granulocyte macrophage colony-stimulating factor and interleukin-3 on human acute myeloid leukemia cells and monocytes. Blood. 1990 Apr 1;75(7):1439–1445. [PubMed] [Google Scholar]
- Cannistra S. A., Koenigsmann M., DiCarlo J., Groshek P., Griffin J. D. Differentiation-associated expression of two functionally distinct classes of granulocyte-macrophage colony-stimulating factor receptors by human myeloid cells. J Biol Chem. 1990 Jul 25;265(21):12656–12663. [PubMed] [Google Scholar]
- Chiba S., Shibuya K., Miyazono K., Tojo A., Oka Y., Miyagawa K., Takaku F. Affinity purification of human granulocyte macrophage colony-stimulating factor receptor alpha-chain. Demonstration of binding by photoaffinity labeling. J Biol Chem. 1990 Nov 15;265(32):19777–19781. [PubMed] [Google Scholar]
- Chiba S., Shibuya K., Piao Y. F., Tojo A., Sasaki N., Matsuki S., Miyagawa K., Miyazono K., Takaku F. Identification and cellular distribution of distinct proteins forming human GM-CSF receptor. Cell Regul. 1990 Mar;1(4):327–335. doi: 10.1091/mbc.1.4.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba S., Tojo A., Kitamura T., Urabe A., Miyazono K., Takaku F. Characterization and molecular features of the cell surface receptor for human granulocyte-macrophage colony-stimulating factor. Leukemia. 1990 Jan;4(1):29–36. [PubMed] [Google Scholar]
- Desbois C., Capeau J., Hainault I., Wicek D., Reynet C., Veissière D., Caron M., Picard J., Guerre-Millo M., Cherqui G. Differential role of insulin receptor autophosphorylation sites 1162 and 1163 in the long-term insulin stimulation of glucose transport, glycogenesis, and protein synthesis. J Biol Chem. 1992 Jul 5;267(19):13488–13497. [PubMed] [Google Scholar]
- DiPersio J., Billing P., Kaufman S., Eghtesady P., Williams R. E., Gasson J. C. Characterization of the human granulocyte-macrophage colony-stimulating factor receptor. J Biol Chem. 1988 Feb 5;263(4):1834–1841. [PubMed] [Google Scholar]
- Dumont J. N. Oogenesis in Xenopus laevis (Daudin). I. Stages of oocyte development in laboratory maintained animals. J Morphol. 1972 Feb;136(2):153–179. doi: 10.1002/jmor.1051360203. [DOI] [PubMed] [Google Scholar]
- Duronio V., Clark-Lewis I., Federsppiel B., Wieler J. S., Schrader J. W. Tyrosine phosphorylation of receptor beta subunits and common substrates in response to interleukin-3 and granulocyte-macrophage colony-stimulating factor. J Biol Chem. 1992 Oct 25;267(30):21856–21863. [PubMed] [Google Scholar]
- Eder M., Griffin J. D., Ernst T. J. The human granulocyte-macrophage colony-stimulating factor receptor is capable of initiating signal transduction in NIH3T3 cells. EMBO J. 1993 Apr;12(4):1647–1656. doi: 10.1002/j.1460-2075.1993.tb05810.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott M. J., Vadas M. A., Eglinton J. M., Park L. S., To L. B., Cleland L. G., Clark S. C., Lopez A. F. Recombinant human interleukin-3 and granulocyte-macrophage colony-stimulating factor show common biological effects and binding characteristics on human monocytes. Blood. 1989 Nov 15;74(7):2349–2359. [PubMed] [Google Scholar]
- Forsayeth J. R., Caro J. F., Sinha M. K., Maddux B. A., Goldfine I. D. Monoclonal antibodies to the human insulin receptor that activate glucose transport but not insulin receptor kinase activity. Proc Natl Acad Sci U S A. 1987 May;84(10):3448–3451. doi: 10.1073/pnas.84.10.3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasson J. C., Kaufman S. E., Weisbart R. H., Tomonaga M., Golde D. W. High-affinity binding of granulocyte-macrophage colony-stimulating factor to normal and leukemic human myeloid cells. Proc Natl Acad Sci U S A. 1986 Feb;83(3):669–673. doi: 10.1073/pnas.83.3.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gearing D. P., King J. A., Gough N. M., Nicola N. A. Expression cloning of a receptor for human granulocyte-macrophage colony-stimulating factor. EMBO J. 1989 Dec 1;8(12):3667–3676. doi: 10.1002/j.1460-2075.1989.tb08541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Cambronero J., Huang C. K., Gomez-Cambronero T. M., Waterman W. H., Becker E. L., Sha'afi R. I. Granulocyte-macrophage colony-stimulating factor-induced protein tyrosine phosphorylation of microtubule-associated protein kinase in human neutrophils. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7551–7555. doi: 10.1073/pnas.89.16.7551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton J. A., Vairo G., Lingelbach S. R. Activation and proliferation signals in murine macrophages: stimulation of glucose uptake by hemopoietic growth factors and other agents. J Cell Physiol. 1988 Mar;134(3):405–412. doi: 10.1002/jcp.1041340311. [DOI] [PubMed] [Google Scholar]
- Hayashida K., Kitamura T., Gorman D. M., Arai K., Yokota T., Miyajima A. Molecular cloning of a second subunit of the receptor for human granulocyte-macrophage colony-stimulating factor (GM-CSF): reconstitution of a high-affinity GM-CSF receptor. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9655–9659. doi: 10.1073/pnas.87.24.9655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janicot M., Lane M. D. Activation of glucose uptake by insulin and insulin-like growth factor I in Xenopus oocytes. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2642–2646. doi: 10.1073/pnas.86.8.2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanakura Y., Druker B., Cannistra S. A., Furukawa Y., Torimoto Y., Griffin J. D. Signal transduction of the human granulocyte-macrophage colony-stimulating factor and interleukin-3 receptors involves tyrosine phosphorylation of a common set of cytoplasmic proteins. Blood. 1990 Aug 15;76(4):706–715. [PubMed] [Google Scholar]
- Kanakura Y., Druker B., Wood K. W., Mamon H. J., Okuda K., Roberts T. M., Griffin J. D. Granulocyte-macrophage colony-stimulating factor and interleukin-3 induce rapid phosphorylation and activation of the proto-oncogene Raf-1 in a human factor-dependent myeloid cell line. Blood. 1991 Jan 15;77(2):243–248. [PubMed] [Google Scholar]
- Kitamura T., Sato N., Arai K., Miyajima A. Expression cloning of the human IL-3 receptor cDNA reveals a shared beta subunit for the human IL-3 and GM-CSF receptors. Cell. 1991 Sep 20;66(6):1165–1174. doi: 10.1016/0092-8674(91)90039-2. [DOI] [PubMed] [Google Scholar]
- Linnekin D., Farrar W. L. Signal transduction of human interleukin 3 and granulocyte-macrophage colony-stimulating factor through serine and tyrosine phosphorylation. Biochem J. 1990 Oct 15;271(2):317–324. doi: 10.1042/bj2710317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez A. F., Eglinton J. M., Lyons A. B., Tapley P. M., To L. B., Park L. S., Clark S. C., Vadas M. A. Human interleukin-3 inhibits the binding of granulocyte-macrophage colony-stimulating factor and interleukin-5 to basophils and strongly enhances their functional activity. J Cell Physiol. 1990 Oct;145(1):69–77. doi: 10.1002/jcp.1041450111. [DOI] [PubMed] [Google Scholar]
- Metcalf D., Nicola N. A., Gearing D. P., Gough N. M. Low-affinity placenta-derived receptors for human granulocyte-macrophage colony-stimulating factor can deliver a proliferative signal to murine hemopoietic cells. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4670–4674. doi: 10.1073/pnas.87.12.4670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazawa K., Hendrie P. C., Mantel C., Wood K., Ashman L. K., Broxmeyer H. E. Comparative analysis of signaling pathways between mast cell growth factor (c-kit ligand) and granulocyte-macrophage colony-stimulating factor in a human factor-dependent myeloid cell line involves phosphorylation of Raf-1, GTPase-activating protein and mitogen-activated protein kinase. Exp Hematol. 1991 Dec;19(11):1110–1123. [PubMed] [Google Scholar]
- Moller D. E., Benecke H., Flier J. S. Biologic activities of naturally occurring human insulin receptor mutations. Evidence that metabolic effects of insulin can be mediated by a kinase-deficient insulin receptor mutant. J Biol Chem. 1991 Jun 15;266(17):10995–11001. [PubMed] [Google Scholar]
- Okuda K., Sanghera J. S., Pelech S. L., Kanakura Y., Hallek M., Griffin J. D., Druker B. J. Granulocyte-macrophage colony-stimulating factor, interleukin-3, and steel factor induce rapid tyrosine phosphorylation of p42 and p44 MAP kinase. Blood. 1992 Jun 1;79(11):2880–2887. [PubMed] [Google Scholar]
- Opresko L. K., Wiley H. S. Functional reconstitutional of the human epidermal growth factor receptor system in Xenopus oocytes. J Cell Biol. 1990 Oct;111(4):1661–1671. doi: 10.1083/jcb.111.4.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park L. S., Friend D., Gillis S., Urdal D. L. Characterization of the cell surface receptor for human granulocyte/macrophage colony-stimulating factor. J Exp Med. 1986 Jul 1;164(1):251–262. doi: 10.1084/jem.164.1.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park L. S., Friend D., Price V., Anderson D., Singer J., Prickett K. S., Urdal D. L. Heterogeneity in human interleukin-3 receptors. A subclass that binds human granulocyte/macrophage colony stimulating factor. J Biol Chem. 1989 Apr 5;264(10):5420–5427. [PubMed] [Google Scholar]
- Park L. S., Martin U., Sorensen R., Luhr S., Morrissey P. J., Cosman D., Larsen A. Cloning of the low-affinity murine granulocyte-macrophage colony-stimulating factor receptor and reconstitution of a high-affinity receptor complex. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4295–4299. doi: 10.1073/pnas.89.10.4295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessin J. E., Bell G. I. Mammalian facilitative glucose transporter family: structure and molecular regulation. Annu Rev Physiol. 1992;54:911–930. doi: 10.1146/annurev.ph.54.030192.004403. [DOI] [PubMed] [Google Scholar]
- Polotskaya A., Zhao Y., Lilly M. L., Kraft A. S. A critical role for the cytoplasmic domain of the granulocyte-macrophage colony-stimulating factor alpha receptor in mediating cell growth. Cell Growth Differ. 1993 Jun;4(6):523–531. [PubMed] [Google Scholar]
- Posada J., Cooper J. A. Requirements for phosphorylation of MAP kinase during meiosis in Xenopus oocytes. Science. 1992 Jan 10;255(5041):212–215. doi: 10.1126/science.1313186. [DOI] [PubMed] [Google Scholar]
- Quelle F. W., Quelle D. E., Wojchowski D. M. Interleukin 3, granulocyte-macrophage colony-stimulating factor, and transfected erythropoietin receptors mediate tyrosine phosphorylation of a common cytosolic protein (pp100) in FDC-ER cells. J Biol Chem. 1992 Aug 25;267(24):17055–17060. [PubMed] [Google Scholar]
- Raines M. A., Golde D. W., Daeipour M., Nel A. E. Granulocyte-macrophage colony-stimulating factor activates microtubule-associated protein 2 kinase in neutrophils via a tyrosine kinase-dependent pathway. Blood. 1992 Jun 15;79(12):3350–3354. [PubMed] [Google Scholar]
- Raines M. A., Liu L., Quan S. G., Joe V., DiPersio J. F., Golde D. W. Identification and molecular cloning of a soluble human granulocyte-macrophage colony-stimulating factor receptor. Proc Natl Acad Sci U S A. 1991 Sep 15;88(18):8203–8207. doi: 10.1073/pnas.88.18.8203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth R. A., Zhang B., Chin J. E., Kovacina K. Substrates and signalling complexes: the tortured path to insulin action. J Cell Biochem. 1992 Jan;48(1):12–18. doi: 10.1002/jcb.240480104. [DOI] [PubMed] [Google Scholar]
- Sakamaki K., Miyajima I., Kitamura T., Miyajima A. Critical cytoplasmic domains of the common beta subunit of the human GM-CSF, IL-3 and IL-5 receptors for growth signal transduction and tyrosine phosphorylation. EMBO J. 1992 Oct;11(10):3541–3549. doi: 10.1002/j.1460-2075.1992.tb05437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh T., Nakafuku M., Miyajima A., Kaziro Y. Involvement of ras p21 protein in signal-transduction pathways from interleukin 2, interleukin 3, and granulocyte/macrophage colony-stimulating factor, but not from interleukin 4. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3314–3318. doi: 10.1073/pnas.88.8.3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung C. K. Insulin receptor signaling through non-tyrosine kinase pathways: evidence from anti-receptor antibodies and insulin receptor mutants. J Cell Biochem. 1992 Jan;48(1):26–32. doi: 10.1002/jcb.240480106. [DOI] [PubMed] [Google Scholar]
- Taketazu F., Chiba S., Shibuya K., Kuwaki T., Tsumura H., Miyazono K., Miyagawa K., Takaku F. IL-3 specifically inhibits GM-CSF binding to the higher affinity receptor. J Cell Physiol. 1991 Feb;146(2):251–257. doi: 10.1002/jcp.1041460209. [DOI] [PubMed] [Google Scholar]
- Tavernier J., Devos R., Cornelis S., Tuypens T., Van der Heyden J., Fiers W., Plaetinck G. A human high affinity interleukin-5 receptor (IL5R) is composed of an IL5-specific alpha chain and a beta chain shared with the receptor for GM-CSF. Cell. 1991 Sep 20;66(6):1175–1184. doi: 10.1016/0092-8674(91)90040-6. [DOI] [PubMed] [Google Scholar]
- Vera J. C., Rosen O. M. Reconstitution of an insulin signaling pathway in Xenopus laevis oocytes: coexpression of a mammalian insulin receptor and three different mammalian hexose transporters. Mol Cell Biol. 1990 Feb;10(2):743–751. doi: 10.1128/mcb.10.2.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whetton A. D., Bazill G. W., Dexter T. M. Haemopoietic cell growth factor mediates cell survival via its action on glucose transport. EMBO J. 1984 Feb;3(2):409–413. doi: 10.1002/j.1460-2075.1984.tb01821.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams G. T., Smith C. A., Spooncer E., Dexter T. M., Taylor D. R. Haemopoietic colony stimulating factors promote cell survival by suppressing apoptosis. Nature. 1990 Jan 4;343(6253):76–79. doi: 10.1038/343076a0. [DOI] [PubMed] [Google Scholar]