Abstract
Fragile X syndrome (FXS) is the most common cause of inherited intellectual disability and presents with markedly atypical speech-language, likely due to impaired vocal learning. Although current models have been useful for studies of some aspects of FXS, zebra finch is the only tractable lab model for vocal learning. The neural circuits for vocal learning in the zebra finch have clear relationships to the pathways in the human brain that may be affected in FXS. Further, finch vocal learning may be quantified using software designed specifically for this purpose. Knockdown of the zebra finch FMR1 gene may ultimately enable novel tests of therapies that are modality-specific, using drugs or even social strategies, to ameliorate deficits in vocal development and function. In this chapter, we describe the utility of the zebra finch model and present a hypothesis for the role of FMRP in the developing neural circuitry for vocalization.
9.2 Introduction to FXS and the vocal phenotype
Fragile X syndrome (FXS) is a genetic disease that results in a constellation of features, the most salient of which include intellectual disability and impaired speech and language. FXS is the most common cause of inherited intellectual disability, affecting 1:4000 males and 1:8000 females panethnically [reviewed in (Hagerman et al., 2009)], and 50%–90% of children with FXS have speech and language abnormalities such as perseveration and echolalia (Hagerman and Lampe, 1999; Kau et al., 2002; Roberts et al., 2007). FXS results from absent expression of the normal fragile X mental retardation protein (Pieretti et al., 1991; De Boulle et al., 1993) (FMRP; Fmrp in mice and rats; Fmrp in other species; for simplicity it will be written as FMRP hereafter), encoded by the gene FMR1. The FMR1 gene is expressed ubiquitously in the body, excluding the muscles, and -it is especially prominent in the testes and brain (Bachner et al., 1993; Devys et al., 1993; Hergersberg et al., 1995); in the brain the protein is primarily neuronal, expressed in glia only during development (Devys et al., 1993; Wang et al., 2004; Pacey and Doering, 2007). Within neurons it has been observed in both dendrites (Feng et al., 1997; Weiler et al., 1997; Greenough et al., 2001; Antar et al., 2004; Ling et al., 2004; Antar et al., 2005) and axons (Antar et al., 2006; Price et al., 2006; Tessier and Broadie, 2008).
Both speech and language are affected in FXS; our hypothesis is that these features are due to impaired speech and language learning. Before describing the speech and language impairments of FXS, collectively termed for our purposes as vocalization deficits, it is important to define speech and language. Speech is the learned sensorimotor control of vocal movements and sounds while language utilizes speech or signals to communicate a complex meaning, encompassing the cognitive processes required for this communication [reviewed in (Brainard and Doupe, 2002)]. Verbal language includes vocabulary and grammar while speech refers to the verbal production of language in terms of pronunciation and rhythm [reviewed in (Newbury and Monaco, 2010)].
One feature of FXS is delayed onset of vocalization. Before children use words to communicate, they use “prelinguistic” communication such as gestures and coordinated gaze, which are typically supplemented by spoken words by 12 months of age [reviewed in (Finestack et al., 2009)]. Children with FXS have delayed onset of this supplementation, with prolonged use of prelinguistic tools (Brady et al., 2006). In fact, vocal delays are found in 69% of children with FXS, based on parental reports (Ferrando-Lucas et al., 2003).
With regard to FXS speech, it has been shown to be perseverative (perseveration is the repetition of one’s own words or actions) (Ferrier et al., 1991) and less intelligible than typically developing (TD) peers (Barnes et al., 2009); intelligibility was even worse when the FXS boys presented with comorbid autism (Kover and Abbeduto, 2010). Interestingly, boys with FXS were shown to have phonological skills similar to TD boys who were of a younger age (Barnes et al., 2009). Additionally, children with FXS are perceived to speak more quickly than their TD peers; however, they actually do not (Zajac et al., 2006). This study was expanded recently to show that the articulation rate, as measured in syllables per second, is the same in FXS as in age-matched controls, but due to speech tone and rhythmicity characteristics (prosody) in children with FXS, their speech is perceived as faster (Zajac et al., 2009).
With regard to language, children with FXS have impaired receptive language capability, as measured by language comprehension (Price et al., 2007); comorbid autism presents with a further decreased receptive language capability (Lewis et al., 2006). Children with FXS also have impaired expressive language (Ke et al., 2005). This expressive language capability, as judged by expressive vocabulary, is not worsened by comorbid autism (Kover and Abbeduto, 2010). Syntax, which is the grammatical arrangement of words in a sentence, is a further measure of expressive language capability. Children with FXS have impaired syntactic skills, as measured by the complexity of their phrases, compared to TD peers and to age-matched children with non-specific intellectual disability. In contrast, children with FXS have stronger syntactic skills than children with Down syndrome (Price et al., 2008). Finally, syntactic ability does not depend on comorbidity with autism (Kover and Abbeduto, 2010).
An important additional feature of vocalization is pragmatics, which includes the “social norms” of communication, both nonverbal and verbal, such as eye contact and turn-taking [reviewed in (Noveck and Reboul, 2008)]. Pragmatic function in FXS is impaired in ways related to, but not identical to, autism (Abbeduto et al., 2008; Dalton et al., 2008).
The reduced intelligibility of speech in FXS individuals has been attributed to an impaired oral-motor system (Barnes et al., 2006). The generalized hypotonia, joint laxity and orofacial hypotonicity observed in FXS individuals may play a role in their unintelligible speech (Hodge, 1991; Hagerman et al., 1996; Hagerman and Lampe, 1999). In fact, FXS boys scored lower than typically developing boys on oral structure—particularly with regard to lip structure—and some oral function tasks (using lips, tongue and velopharynx), as patients performed less well on speech function tasks like repeating single and multiple syllable words (Barnes et al., 2006). In addition, other studies suggest that the FXS speech problems indicate higher-level motor encoding problems of linguistic information rather than peripheral articulatory deficits (Vilkman et al., 1988; Hodge, 1991; Hagerman et al., 1996). In addition, sensorimotor delays have been observed in FXS children as young as 9–12 months of age (Bailey et al., 2003; Grace et al., 2005), as well as both fine and gross motor skills (Ke et al., 2005; Zingerevich et al., 2009).
Importantly the vocal deficits are not due to cognitive impairment in general, but rather are unique to FXS, since they have been described in comparison to children with Down syndrome or idiopathic intellectual disability (Sudhalter et al., 1990; Ferrier et al., 1991; Belser and Sudhalter, 2001). While the speech-language deficits of FXS have been extensively established and characterized, as outlined above, in the realm of molecular biology, this phenotypic vocal quality of FXS is often overlooked. This paucity of studies is likely because to date there has not been a tractable model organism for the study of the atypical vocalizations.
Humans, songbirds, and certain cetaceans are vocal learners – so called because they have a sensitive period during postnatal (or posthatch) development in which they must hear the adult vocalizations as well as their own, in order to learn this vocalization (Doupe and Kuhl, 1999; Brainard and Doupe, 2002; Wilbrecht and Nottebohm, 2003). The atypical vocalization in FXS provides a unique opportunity to study the role of FMRP in a novel venue – vocal learning. Because FMRP is involved in learning and memory (Mercaldo et al., 2009), and humans are vocal learners, our hypothesis is that individuals with FXS have impaired vocal learning, leading to their impaired vocalization.
9.3 The songbird as a model for vocal learning
The fragile X gene ortholog has been identified in a number of species and its knockdown has been induced in the mouse, fruit fly Drosophila melanogaster, zebra fish Danio rerio, and cricket Gryllus bimaculatus (Consortium, 1994; Zhang et al., 2001; Lin et al., 2006; Hamada et al., 2009); however, none of these species are vocal learners. Adult male mice and altricial mouse pups do emit ultrasonic vocalization, and a study has already been performed on murine FoxP2, an important speech gene mutated in a human familial speech disorder (Lai et al., 2001), showing a deleterious effect of a FoxP2 mutation on ultrasonic vocalization of mouse pups (Shu et al., 2005). Nonetheless, it is unclear if these murine vocalizations are learned (Sales, 1972; Branchi et al., 2001; Holy and Guo, 2005). Further, the mouse does not lend itself to our study because the particular brain regions involved in ultrasonic song have not been mapped. Additionally, the cricket model does not lend itself to our study because the deficit is likely purely motor and not due to a learning deficit in the realm of communication (Hamada et al., 2009. Currently, a model organism with a well-characterized neural circuit for vocalization is the songbird, specifically the zebra finch Taeniopygia guttata. In addition to their well-studied anatomy, songbirds have been proposed as invaluable animal models for studying the acquisition of a skilled motor sequence (Vu et al., 1994), as well as a behavioral sequence (Fee et al., 2004). For these reasons, and for its easy management in a laboratory setting, the zebra finch is an excellent animal model for studying the role of FMRP in vocal learning, thereby elucidating the biology of the vocal impairments observed in FXS.
Songbirds and humans are vocal learners. This type of vocalization is different from that of other avian species such as the chicken, for example, which when raised in isolation can still make the proper vocalizations as adults [reviewed in (Doupe and Kuhl, 1999)]. Researchers of birdsong have identified three stages in its learning and production – sensory, sensorimotor, and the final, crystallized song [reviewed in (Brainard and Doupe, 2002)].
In the sensory period, a ‘song template’ is formed as the young bird listens to his tutor and learns the tutor’s song. This sensory period of the zebra finch spans the first 60 days after hatching. During the sensorimotor period, from about posthatch day 23 (P23) through sexual maturity (approximately P90), the male bird begins to vocalize and to correct his song using auditory feedback, in order to match the tutor. At adulthood, the bird has an established, ‘crystallized’ song, which he will continue to sing for the remainder of his life. A feature of vocal learning is practice. The first vocalizations of a songbird (at about P35) are called subsong, akin to the babbling of a human infant. Human babbling begins at about month seven, followed by the first true word spoken at about one year of age, with continued vocal learning that diminishes markedly after sexual maturity (Doupe and Kuhl, 1999).
In addition to singing, the bird must be able to hear itself in order to crystallize its song properly, as must a human child hear him or herself in order to learn to speak properly [reviewed in (Doupe and Kuhl, 1999)]. A deaf songbird cannot learn proper song (Marler and Waser, 1977); similarly, hearing-impaired children cannot, without interventional training, learn proper adult vocalization (Ching et al., 2010).
Song learning and production utilizes many avian brain regions (called ‘song nuclei’); the five principle song nuclei comprise two merging pathways (Figure 1). The Anterior Forebrain Pathway (primarily for song learning) begins with nucleus HVC (letter-based name; formerly High Vocal Center), projecting to Area X then DLM, LMAN, and finally the RA. The Posterior Pathway (primarily for song production) also begins in the HVC, projecting directly to the RA. The RA then projects to the nucleus of the tracheosyringial region of the 12th cranial nerve (nXIIts) (songbirds have a dual-barreled voicebox, called a syrinx, comparable to the single-barreled human larynx). Each of these nuclei plays a role in song learning and/or production. For a summary of the zebra finch nuclei and the analogous mammalian brain regions, please see Table 1 and [(Reiner et al., 2004) and Reiner et al., (2004)].
Fig. 1. Map of the zebra finch ‘song circuit’.
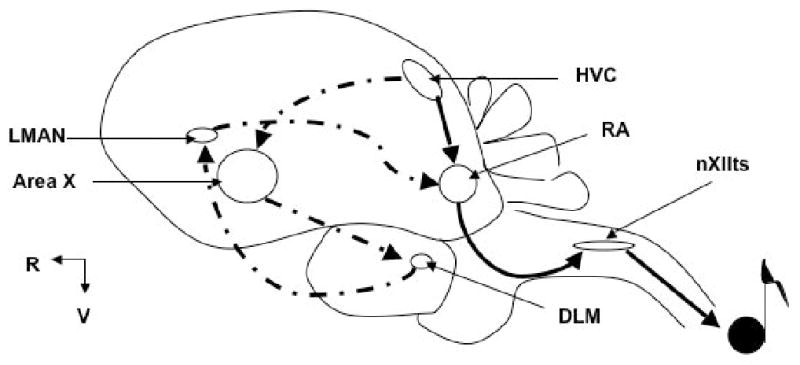
Schematic of sagital view of an adult male zebra finch. Anterior Forebrain Pathway for song learning shown in dashed lines; Posterior Pathway for song production shown in solid lines. (Rostral [R] left; Ventral [V] down. Not drawn to scale.) Song nuclei--Area X: letter-based name; DLM: DorsoLateral Medial nucleus of the thalamus; HVC: letter-based name; LMAN: Lateral Magnocellular nucleus of the Anterior Nidopallium; nXIIts: Nucleus TrachioSyringealis of cranial nerve XII; RA: Robust nucleus of the Arcopallium.
Table 1.
Analogous brain regions in zebra finch and mammals
| Zebra finch nuclei | Mammalian brain region | |
|---|---|---|
| abbreviation | Full name | |
| HVC (letter-based name) | Formerly High Vocal Center | Dorsal telencephalon* |
| Area X | Area X | Basal Ganglia |
| DLM | DorsoLateral Medial nucleus of the thalamus | Thalamus |
| LMAN | Lateral Magnocellular nucleus of Anterior Nidopallium | Dorsal telencephalon* |
| RA | Robust nucleus of the Arcopallium | Unique to avians but best described as vocal premotor cortex, similar to (but distinct from) the somatic telencephalon |
Derived from the telencephalic pallial sector of the developing brain (mammalian pallial derivatives are the neocortex, amygdala, and claustrum) [(Reiner et al., 2004) and Reiner et al., (2004)].
The Anterior Forebrain Pathway and the Posterior Pathway both begin with the HVC (letter-based name; formerly “High Vocal Center”). Behavioral learning (i.e., vocal learning) in the HVC requires rapid synaptic plasticity at this sensorimotor nucleus, in response to an instructive experience (Roberts et al., 2010). It is important to note that here, the term sensorimotor is used to denote the integration of sensory (such as visual, auditory, and proprioception) input and motor (such as song) output, not that the HVC is strictly involved in the sensorimotor (as opposed to sensory) phase of song learning.
Considering the Anterior Forebrain Pathway first, HVC projects to Area X, a nucleus considered to be the avian analog of the “direct” striatopallidothalamic pathway of the mammalian basal ganglia (Medina and Reiner, 1995; Farries and Perkel, 2002). Area X contains spiny neurons that respond to dopamine, thereby influencing song learning and maintenance (Ding et al., 2003). Furthermore, these spiny neurons express FOXP2 during song learning, and new neuron recruitment is increased during this period (Rochefort et al., 2007). Of note, lentivirally-driven knockdown of FoxP2 in Area X results in incomplete, inaccurate song learning (Haesler et al., 2007). Neurons in Area X project to the song nucleus DLM (DorsoLateral Medial nucleus of the thalamus) via the neurotransmitter GABA (Luo and Perkel, 1999).
DLM neurons are the avian equivalent of mammalian thalamocortical neurons. This alignment is due to both the strong inhibitory GABAergic input from Area X as well as its their intrinsic properties (Luo and Perkel, 2002). The Anterior Forebrain Pathway continues through DLM to LMAN (Lateral Magnocellular nucleus of the Anterior Nidopallium) via glutamatergic projections onto NMDA receptors as well as AMPA receptors on inhibitory interneurons that then use GABA to inhibit LMAN (Livingston and Mooney, 1997); plasticity related to these receptors has been shown to be involved in song learning (Bottjer, 2005). Interestingly, the NMDA receptors in LMAN decrease at the synapse between P32-40 (corresponding to the young finch’s maturation from fledgling to juvenile), suggesting that these synapses are important for sensory, not sensorimotor learning (Livingston and Mooney, 1997). LMAN itself remains important in residual plasticity and song maintenance during adulthood (Brainard and Doupe, 2001). Finally, neurons in LMAN project to the RA (Robust nucleus of the Arcopallium), a connection that has been shown to be critical for motor learning (Ölveczky et al., 2005).
The RA is the nucleus, which intersects the Anterior Forebrain Pathway and the Posterior Pathway (Figure 1). It is critical for song production and is analogous to mammalian premotor cortex (Nottebohm et al., 1976). The RA receives input from HVC and LMAN by approximately P30 (just preceding the onset of sensorimotor learning) (Konishi and Akutagawa, 1985; Mooney, 1992; Mooney and Rao, 1994), consolidates these inputs, and projects to the brainstem motor nucleus controlling song, nXIIts (tracheo-syringial nucleus of cranial nerve XII. Plasticity at RA has been proposed to play different roles temporally during song learning (Stark and Perkel, 1999). [For a model for the overall long term plasticity at the HVC-RA connections (via both pathways), see: (Fiete et al., 2007).] It is also interesting to note that feedback from the RA to the HVC has been observed, via intermediate song nuclei likely to be involved in the auditory pathway [reviewed in (Margoliash, 1997)]. In addition, ‘bottom-up’ feedback from the respiratory brainstem to RA and HVC during adult singing has also been observed (Ashmore et al., 2005). The complexities of the song circuit are fodder for elucidating studies into the role of FMRP in vocal learning.
9.4 FMRP expression in the song circuit
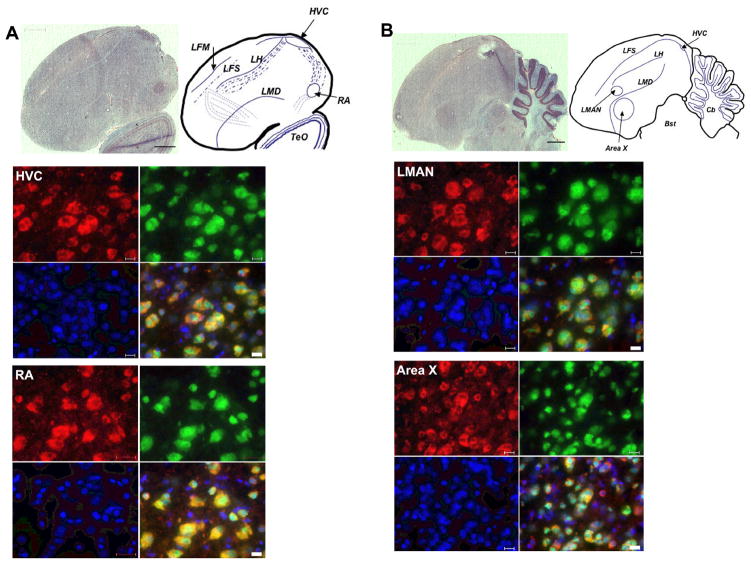
Synaptic plasticity, known to be abnormal in FXS [reviewed in (Bear et al., 2004)], plays a role at each connection between the nuclei in the song circuit; therefore, we hypothesized that FMRP plays a role in modulation of synaptic plasticity at one or more song nuclei, as part of a greater role in song learning. To test this hypothesis, we set out to examine the zebra finch song circuit for FMRP expression during development and thereby song learning. We found FMRP expressed in HVC, LMAN, Area X, and RA; expression was neuronal and primarily cytoplasmic just as in other species (Winograd et al., 2008) (Figure 2). [DLM is a heavily myelinated nucleus and we were unable to achieve adequate immunofluorescence in this region; therefore, we cannot make conclusions about the expression of FMRP in this thalamic brain region; FMRP expression in nXIIts was not examined.] Intriguingly, out of these four song nuclei, we observed elevated FMRP expression in the RA, as compared to surrounding neuropil, an effect that was consistent at P30 (Winograd et al., 2008) (Figure 3).
Fig. 2. FMRP is expressed in neurons in the male zebra finch brain.
Sagital brain sections containing A. HVC (letter-based name) and RA and B. LMAN (Lateral Magnocellular nucleus of the Anterior Nidopallium) and Area X were stained for anatomy with cresyl violet (bar = 1000μm) (upper left). Upper right is an accompanying sketch with the significant anatomical features indicated. Adjacent sections were co-stained with the zebra finch-specific Fmrp antibody 24 (red), the neuronal marker NeuN (green), and DAPI (blue). In the overlay, a yellow signal indicates co-fluorescence for red and green. Bar = 20μm. Bst: Brainstem; Cb: Cerebellum; LFM: Lamina frontalis suprema; LFS: Lamina frontalis superior; LH: Lamina hyperstriatica; LMD: Lamina medullaris dorsalis; TeO: Optic Tectum. Shown are images from a posthatch day (P) P30 brain; P60 and Adult males showed similar results (data not shown). [Reprinted with permission from (Winograd et al., 2008).]
Fig. 3. FMRP is consistently elevated in the RA nucleus of a P30 male zebra finch and variably expressed in P60 and Adult males.
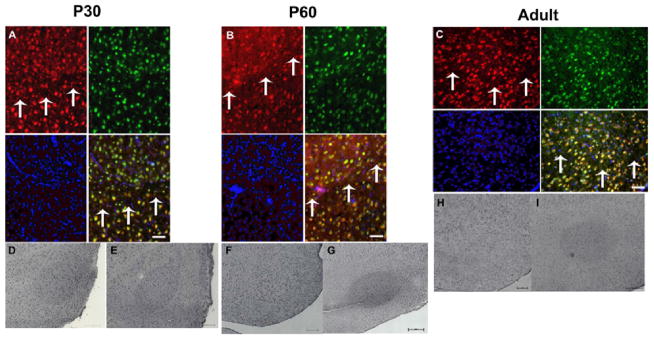
A–C. Representative fluorescent-immunohistochemistry using an antibody specific to zebra finch FMRP on a male P30 (A) P60 (B) and Adult (C) zebra finch RA. Shown are FMRP immunoreactivity (red), NeuN stain (green), and DAPI-labeled nuclei (blue), along with the overlay. Arrows denote ventral border of RA. Bar = 100μm. D–I. Representative DAB-IHC using anti-zebra finch FMRP antibody on a male P30 (D, E) P60 (F, G) and Adult (H, I) zebra finch RA. Bar = 200μm. [Reprinted with permission from (Winograd et al., 2008).]
RA receives inputs from both the nuclei HVC and LMAN as well as from intrinsic interneurons and therefore is not merely a relay nucleus from the HVC to the motor nucleus nXIIts [(Spiro et al., 1999) and see Fig. 1]. Synaptic plasticity in the RA has been proposed as a mechanism for song learning and production (Mooney, 1992; Stark and Perkel, 1999; Fee et al., 2004), and FMRP is necessary for normal synaptic plasticity [reviewed in (Bear et al., 2004)]. It is an exciting prospect that FMRP might be involved in the synaptic maturation of the RA that is associated with song learning.
A likely setting for the facilitation of this maturation is in the post-synaptic compartment of dendritic spines, where FMRP has been shown to play a role in synaptic plasticity in other species (Weiler and Greenough, 1999; Greenough et al., 2001; Michel et al., 2004). Specifically, it is possible that this role is via the NMDA receptors. Alterations in RA neurochemistry, such as NMDA receptor subunit expression, occur during song learning (Wang and Hessler, 2006). In fact, FMRP has recently been shown to modulate expression of NMDA receptors (Eadie et al., 2010; Edbauer et al., 2010).
To definitively test the role of FMRP in vocalization would entail knocking down FMRP in the zebra finch brain and observing any effects on song learning. In the zebra finch, gene knockout at the germline level, as accomplished with mouse and fly, is currently not feasible; therefore, epigenetic means such as silencing RNA must be employed. A future experiment would therefore be to use a viral vector to silence the gene encoding zebra finch FMRP in a young finch prior to the onset of song learning, and analyze the resultant song.
9.5 Feasibility of knockdown strategy
Researchers have used the zebra finch and its well-characterized song system to study the transcription factor FoxP2 (Haesler et al., 2004; Scharff and Haesler, 2005; Schulz et al., 2010). In a human family with a FOXP2 mutation, affected family members present with developmental verbal dyspraxia – difficulty with coordinated motor tasks, specifically in the lower face and jaw such that speech is impaired (Lai et al., 2001). There are also language processing impairments and significantly-below-average grammar skills (Vargha-Khadem et al., 1995). In the finch, FoxP2 is expressed ubiquitously in the brain, and principally in the striatum, and its knockdown in the zebra finch Area X via lentivirus-delivered short-hairpin silencing RNA results in imperfect imitation by a male zebra finch of his tutor’s song (Haesler et al., 2007). It is important to consider the contrast between the murine study where knockdown of FoxP2 did not affect ultrasonic vocalization, and this avian study, where it did. It is conceivable that certain proteins such as FoxP2 are required for vocal learning but not necessarily the production of innate vocalizations such as murine ultrasonic or non-learned avian calls. This specified role is also likely with FMRP, as people with FXS are capable of vocalization.
9.6 Strength of songbirds: ability to measure song learning
As described above, the charge of the young male zebra finch is to learn the song of the adult male tutor. Zebra finch song consists of a set of notes or syllables—the frequency of which can be measured in kilohertz over time (milliseconds). Thus, a song syllable is defined as a continuous, morphologically discrete trace on a song spectrogram (Sossinka and Bhoner, 1980). The adult song of the zebra finch male begins with several variations of the same introductory syllable, followed by a set of dissimilar syllables. The latter syllables are rendered in a stereotyped sequential order and constitute the “motif“. A motif lasts approximately 700 msec (Sossinka and Bhoner, 1980), with frequencies ranging from 0.5–0.8 kHz (Scharff and Nottebohm, 1991). Each adult male song is unique based on the nature of the syllables and their specific assembly into a motif. Thus, each zebra finch song is readily quantifiable. In order to do that, software (SA+) was designed to analyze how well a young male finch learns his tutor’s song (Tchernichovski et al., 2000). SA+ software is able to capture and analyze song at all stages of learning and compare it to the tutor; the program identifies individual syllables (notes) and analyzes their temporal structure, characterizing duration, mean pitch and mean frequency modulation. The software automatically generates and updates a syllable-table for each bird that summarizes every song syllable produced during vocal development. Thus, the song of the adult tutor can be specifically compared to the emerging song of the juvenile male.
9.7 What we could hope to learn from a knockdown zebra finch
Like FoxP2, FMRP is expressed throughout the brain; however, the role of this latter protein in a functional CNS circuit has not been investigated. The zebra finch provides a well-characterized functional CNS circuit for study in its song system.
As described in 1.2, individuals with FXS have developmental delays with sensorimotor deficits (Grace et al., 2005). In addition, a good animal model for the characterization of vocalization deficits is lacking (1.3). Numerous studies in rodent models expressing short hairpin RNAs from viruses have been successful in creating knockdown models (Xia et al., 2004; Bohn, 2005; Harper et al., 2005; Ralph et al., 2005; Raoul et al., 2005; Rodriguez-Lebron et al., 2005; Singer et al., 2005; Sapru et al., 2006). We expect that inducing loss of FMRP by viral-driven expression of short hairpin RNAs in the RA would have an effect on the ability to accurately learn the tutor song, since FMRP is elevated in the neuropil of RA (Fig. 3). We can imagine at least three possible outcomes of knocking down FMRP expression in RA: 1) the knockdown bird has the same phenotype as the RA-ablated bird where the song is lost (Nottebohm et al., 1976); 2) the knockdown bird is unable to produce normal song due to problems with motor production; 3) the knockdown bird is able to produce song but is unable to learn the tutor song.
If the first scenario is true and the knockdown bird is unable to sing, then we would suspect that FMRP is required for vocalization. This result would be surprising because although FXS patients have significant speech delays and deficits, vocalization is not absent. Thus, in zebra finch FMRP would appear to have a critical role in song production. This is a distinct possibility because in humans and rodents, loss of FMRP expression is sometimes compensated for by the other autosomal paralogs FXR1 and FXR2 (Siomi et al., 1995; Spencer et al., 2006). In fact, elimination of both FMRP and FXR2 was required to see a circadian defect in mice (Spencer et al., 2006). Although zebra finches express FXR1, they do not express FXR2 (unpublished results), thus, elimination of FMRP in the zebra finch may reveal defects not observed in other species.
The second possible outcome that we can envision is that knockdown of FMRP leads to problems with motor production. We would suspect that this is the case if the features of the song syllables are abnormal in the FMRP knockdown birds compared to those in the control birds. If we find that there are problems with motor production (versus motor learning), then adults should also be affected. We could test this prediction by introducing FMRP silencing viruses and control viruses into adult RA and then analyzing song production before and after virus introduction. If FMRP is required for producing song at any age, then we would expect altered song in adults injected with the silencing virus compared to control virus.
The third possible outcome, which is our hypothesized one, is that knockdown of FMRP expression affects motor learning and that in the absence of FMRP, the manipulated birds are unable to learn their tutor’s song. If FMRP is required for the introduction of variability by LMAN into the HVC-driven template song (Kao et al., 2005; Ölveczky et al., 2005), then we would expect early stereotypy, i.e., the song would crystallize before attaining similarity to the adult song. Thus, the mean similarity scores and accuracy scores of the analyzed bird song would be significantly different between the FMRP silenced birds and the control birds. This phenotype would be similar to that observed when LMAN was ablated (Scharff and Nottebohm, 1991). If the song stereotypes too soon, we would also expect to observe no change in song in the FMRP knockdown birds between P65 and adulthood. Further, the P65 song should not be normal – i.e., it should not be similar to the tutor song but should resemble immature song (Scharff and Nottebohm, 1991).
Alternatively, if the inability to learn song is due to a defect in motor learning because FMRP is required for reinforcing motor actions, then we would expect no normal stereotypy, i.e., the song would continue to change during adulthood. In this case, we would expect the song spectrograms of the knockdown pupils to change continually, where either syllables would be omitted or the duration of the syllable would be imprecisely copied between renditions. This would be reflected in differences in the mean similarity and accuracy scores between knockdown and control groups. Further, an individual’s own song would significantly vary from rendition to rendition, which would be reflected in the coefficient of variation.
9.8 Conclusion
In summary, FMRP is a protein involved in learning and memory, specifically in the realm of synaptic plasticity. People with FXS have impaired vocalization, which we propose is due to impaired vocal learning. The songbird zebra finch provides an exciting animal model for the study of the role of FMRP in vocal learning. We suggest a role for FMRP in the reorganization of the postsynaptic dendritic compartment in the song nucleus RA, possibly through effects on NMDA receptors. It is our hope that knowledge gained on the role of FMRP in song learning can be translated into therapeutics for FXS patients with impaired vocalization due to the absence of expression of FMRP. A knockdown zebra finch would be a useful model for the study of novel interventive approaches because song learning and production can be precisely quantified.
Acknowledgments
This work was supported in part by Public Health Service grant HD41591 from NICHD, the FRAXA foundation and the Spastic Paralysis Research Foundation of the Illinois-Eastern Iowa District of Kiwanis International to S. Ceman. C. Winograd was supported by the CMB training grant and a Neuroscience Program fellowship.
Contributor Information
C Winograd, Email: cwinogr2@illinois.edu.
S Ceman, Email: sceman@life.illinois.edu.
9.9 References
- Abbeduto L, Murphy MM, Kover ST, Giles ND, Karadottir S, Amman A, Bruno L, Kim JS, Schroeder S, Anderson JA, Nollin KA. Signaling noncomprehension of language: a comparison of fragile X syndrome and Down syndrome. Am J Ment Retard. 2008;113:214–230. doi: 10.1352/0895-8017(2008)113[214:SNOLAC]2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antar LN, Afroz R, Dictenberg JB, Carroll RC, Bassell GJ. Metabotropic glutamate receptor activation regulates fragile x mental retardation protein and FMR1 mRNA localization differentially in dendrites and at synapses. J Neurosci. 2004;24:2648–2655. doi: 10.1523/JNEUROSCI.0099-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antar LN, Dictenberg JB, Plociniak M, Afroz R, Bassell GJ. Localization of FMRP-associated mRNA granules and requirement of microtubules for activity-dependent trafficking in hippocampal neurons. Genes Brain Behav. 2005;4:350–359. doi: 10.1111/j.1601-183X.2005.00128.x. [DOI] [PubMed] [Google Scholar]
- Antar LN, Li C, Zhang H, Carroll RC, Bassell GJ. Local functions for FMRP in axon growth cone motility and activity-dependent regulation of filopodia and spine synapses. Mol Cell Neurosci. 2006;32:37–48. doi: 10.1016/j.mcn.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Ashmore RC, Wild JM, Schmidt MF. Brainstem and forebrain contributions to the generation of learned motor behaviors for song. J Neurosci. 2005;25:8543–8554. doi: 10.1523/JNEUROSCI.1668-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachner D, Steinbach P, Worhle D, Just W, Vogel M, Hameister H. Enhanced FMR1 expression in testes. Nat Genet. 1993;4:155–116. doi: 10.1038/ng0693-115. [DOI] [PubMed] [Google Scholar]
- Bailey DBJ, Skinner D, Sparkman KL. Discovering fragile X syndrome: family experiences and perceptions. Pediatrics. 2003;111:407–416. doi: 10.1542/peds.111.2.407. [DOI] [PubMed] [Google Scholar]
- Barnes E, Roberts J, Long SH, Martin GE, Berni MC, Mandulak KC, Sideris J. Phonological accuracy and intelligibility in connected speech of boys with fragile X syndrome or Down syndrome. J Speech Lang Hear Res. 2009;52:1048–1061. doi: 10.1044/1092-4388(2009/08-0001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes EF, Roberts J, Mirrett P, Sideris J, Misenheimer J. A comparison of oral structure and oral-motor function in young males with fragile X syndrome and Down syndrome. J Speech Lang Hear Res. 2006;49:903–917. doi: 10.1044/1092-4388(2006/065). [DOI] [PubMed] [Google Scholar]
- Bear MF, Huber KM, Warren ST. The mGluR theory of fragile X mental retardation. Trends Neurosci. 2004;27:370–377. doi: 10.1016/j.tins.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Belser RC, Sudhalter V. Conversational characteristics of children with fragile X syndrome: repetitive speech. Am J Ment Retard. 2001;106:28–38. doi: 10.1352/0895-8017(2001)106<0028:CCOCWF>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Bohn MC. Society for Neuroscience (Short Course) Washington, D. C: 2005. Overview of gene therapy and viral vectors for CNS applications. [Google Scholar]
- Bottjer SW. Silent synapses in a thalamo-cortical circuit necessary for song learning in zebra finches. J Neurophysiol. 2005;94:3698–3707. doi: 10.1152/jn.00282.2005. [DOI] [PubMed] [Google Scholar]
- Brady N, Skinner D, Roberts J, Hennon E. Communication in young children with fragile X syndrome: a qualitative study of mothers’ perspectives. Am J Speech Lang Pathol. 2006;15:353–364. doi: 10.1044/1058-0360(2006/033). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brainard MS, Doupe AJ. Postlearning consolidation of birdson: stabilizing effects of age and anterior forebrain lesions. J Neurosci. 2001;21:2501–2517. doi: 10.1523/JNEUROSCI.21-07-02501.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brainard MS, Doupe AJ. What songbirds teach us about learning. Nature. 2002;417:351–358. doi: 10.1038/417351a. [DOI] [PubMed] [Google Scholar]
- Branchi I, Santucci D, Alleva E. Ultrasonic vocalisation emitted by infant rodents: a tool for assessment of neurobehavioural development. Behav Brain Res. 2001;125:49–56. doi: 10.1016/s0166-4328(01)00277-7. [DOI] [PubMed] [Google Scholar]
- Ching TY, Crowe K, Martin V, Day J, Mahler N, Youn S, Street L, Cook C, Orsini J. Language development and everyday functioning of children with hearing loss assessed at 3 years of age. Int J Speech Lang Pathol. 2010;12:124–131. doi: 10.3109/17549500903577022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consortium D-BFX. FMR1 knockout mice: a model to study fragile X mental retardation. Cell. 1994;78:23–33. [PubMed] [Google Scholar]
- Dalton KM, Holsen L, Abbeduto L, Davidson RJ. Brain function and gaze fixation during facial-emotion processing in fragile X and autism. Autism Res. 2008;1:231–239. doi: 10.1002/aur.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Boulle K, Verkerk AJMH, Reyniers E, Vits L, Hendrickx J, Van Roy B, Van den Bos F, de Graaff E, Oostra BA, Willems PJ. A point mutation in the FMR-1 gene associated with fragile X mental retardation. Nat Genet. 1993;3:31–35. doi: 10.1038/ng0193-31. [DOI] [PubMed] [Google Scholar]
- Devys D, Lutz Y, Rouyer N, Bellocq J-P, Mandel J-L. The FMR-1 protein is cytoplasmic, most abundant in neurons, and appears normal in carriers of the fragile X premutation. Nat Genet. 1993;4:335–340. doi: 10.1038/ng0893-335. [DOI] [PubMed] [Google Scholar]
- Ding L, Perkel DJ, Farries MA. Presynaptic depression of glutamatergic synaptic transmission by D1-like dopamine receptor activation in the avian basal ganglia. J Neurosci. 2003;23:6086–6095. doi: 10.1523/JNEUROSCI.23-14-06086.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doupe AJ, Kuhl PK. Birdsong and human speech: common themes and mechanisms. Annu Rev Neurosci. 1999;22:567–631. doi: 10.1146/annurev.neuro.22.1.567. [DOI] [PubMed] [Google Scholar]
- Eadie BD, Cushman J, Kannangara TS, Fanselow MS, Christie BR. NMDA receptor hypofunction in the dentate gyrus and impaired context discrimination in adult Fmr1 knockout mice. Hippocampus. 2010 doi: 10.1002/hipo.20890. [DOI] [PubMed] [Google Scholar]
- Edbauer D, Neilson JR, Foster KA, Wang CF, Seeburg DP, Batterton MN, Tada T, Dolan BM, Sharp PA, Sheng M. Regulation of synaptic structure and function by FMRP-associated microRNAs miR-125b and miR-132. Neuron. 2010;65:373–384. doi: 10.1016/j.neuron.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farries MA, Perkel DJ. A telencephalic nucleus essential for song learning contains neurons with physiological characteristics of both striatum and globus pallidus. J Neurosci. 2002;22:3776–3787. doi: 10.1523/JNEUROSCI.22-09-03776.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fee MS, Kozhevnikov A, Hahnloser R. Neural mechanisms of vocal sequence generation in the songbird. Ann N Y Acad Sci. 2004;1016:153–170. doi: 10.1196/annals.1298.022. [DOI] [PubMed] [Google Scholar]
- Feng Y, Gutekunst CA, Eberhart DE, Yi H, Warren ST, Hersch SM. Fragile X mental retardation protein: nucleocytoplasmic shuttling and association with somatodendritic ribosomes. J Neurosci. 1997;17:1539–1547. doi: 10.1523/JNEUROSCI.17-05-01539.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrando-Lucas MT, Banús-Gómez P, López-Pérez G. Aspects of cognition and language in children with fragile X syndrome. Rev Neurol. 2003;36(Suppl 1):S137–142. [PubMed] [Google Scholar]
- Ferrier LJ, Bashir AS, Meryash DL, Johnston J, Wolff P. Conversational skills of individuals with fragile-X syndrome: a comparison with autism and Down syndrome. Dev Med Child Neurol. 1991;33:776–788. doi: 10.1111/j.1469-8749.1991.tb14961.x. [DOI] [PubMed] [Google Scholar]
- Fiete IR, Fee MS, Seung HS. Model of birdsong learning based on gradient estimation by dynamic perturbation of neural conductances. J Neurophysiol. 2007;98:2038–2057. doi: 10.1152/jn.01311.2006. [DOI] [PubMed] [Google Scholar]
- Finestack LH, Richmond EK, Abbeduto L. Language Development in Individuals with Fragile X Syndrome. Top Lang Disord. 2009;29:133–148. doi: 10.1097/tld.0b013e3181a72016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace TB, Cassandra DD, Martie LS, Donald B, DDH, Jane ER, Penny LM. Video Analysis of Sensory-Motor Features in Infants with Fragile X Syndrome at 9–12 Months of Age. Journal of Autism and Developmental Disorders. 2005;V35:645. doi: 10.1007/s10803-005-0008-7. [DOI] [PubMed] [Google Scholar]
- Greenough WT, Klintsova AY, Irwin SA, Galvez R, Bates KE, Weiler IJ. Synaptic regulation of protein synthesis and the fragile X protein. Proc Natl Acad Sci U S A. 2001;98:7101–7106. doi: 10.1073/pnas.141145998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigsby J, Kemper MB, Hagerman RJ. Verbal learning and memory among heterozygous fragile X females. Am J Med Genet. 1992;43:111–115. doi: 10.1002/ajmg.1320430116. [DOI] [PubMed] [Google Scholar]
- Haesler S, Rochefort C, Georgi B, Licznerski P, Osten P, Scharff C. Incomplete and inaccurate vocal imitation after knockdown of FoxP2 in songbird basal ganglia nucleus Area X. PLoS Biology. 2007;5:2885–2897. doi: 10.1371/journal.pbio.0050321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haesler S, Wada K, Nshdejan A, Morrisey EE, Lints T, Jarvis ED, Scharff C. FoxP2 Expression in Avian Vocal Learners and Non-Learners. J Neurosci. 2004;24:3164–3175. doi: 10.1523/JNEUROSCI.4369-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagerman RJ, Lampe ME. Handbook of Neurodevelopmental and Genetic disorders in children. Guilford Press; 1999. [Google Scholar]
- Hagerman RJ, Staley LW, O’Conner R, Lugenbeel K, Nelson D, McLean SD, Taylor A. Learning-disabled males with a fragile X CGG expansion in the upper premutation size range. Pediatrics. 1996;97:122–126. [PubMed] [Google Scholar]
- Hagerman RJ, Berry-Kravis E, Kaufmann WE, Ono MY, Tartaglia N, Lachiewicz A, Kronk R, Delahunty C, Hessl D, Visootsak J, Picker J, Gane L, Tranfaglia M. Advances in the Treatment of Fragile X Syndrome. Pediatrics. 2009;123:378–390. doi: 10.1542/peds.2008-0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada A, Miyawaki K, Honda-sumi E, Tomioka K, Mito T, Ohuchi H, Noji S. Loss-of-function analyses of the Fragile X-related and dopamine receptor genes by RNA interference in the cricket Gryllus bimaculatus. Dev Dynamics. 2009;238:2025–2033. doi: 10.1002/dvdy.22029. [DOI] [PubMed] [Google Scholar]
- Harper SQ, Staber PD, He X, Eliason SL, Martins IH, Mao Q, Yang L, Kotin RM, Paulson HL, Davidson BL. RNA interference improves motor and neuropathological abnormalities in a Huntington’s disease mouse model. Proc Natl Acad Sci U S A. 2005;102:5820–5825. doi: 10.1073/pnas.0501507102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hergersberg M, Matsuo K, Gassman M, Schaffner W, Lüscher B, Rülicke T, Aguzzi A. Tissue-specific expression of a FMR1/beta-galactosidase fusion gene in transgenic mice. Hum Mol Genet. 1995;4:359–366. doi: 10.1093/hmg/4.3.359. [DOI] [PubMed] [Google Scholar]
- Hodge MM. Assessing early speech motor function. Clin Commun Disord. 1991;1:69–85. [PubMed] [Google Scholar]
- Holy TE, Guo Z. Ultrasonic Songs of Male Mice. PLoS Biology. 2005:3. doi: 10.1371/journal.pbio.0030386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao MH, Doupe AJ, Brainard MS. Contributions of an avian basal ganglia-forebrain circuit to real-time modulation of song. Nature. 2005;433:638–643. doi: 10.1038/nature03127. [DOI] [PubMed] [Google Scholar]
- Kau AS, Meyer WA, Kaufmann WE. Early development in males with Fragile X syndrome: a review of the literature. Microsc Res Tech. 2002;57:174–178. doi: 10.1002/jemt.10069. [DOI] [PubMed] [Google Scholar]
- Ke J-Y, Chen C-L, Chen Y-J, Chen C-H, Lee L-F, Chiang T-M. Features of developmental functions and autistic profiles in children with fragile X syndrome. Chang Gung Med J. 2005;28:551–558. [PubMed] [Google Scholar]
- Konishi M, Akutagawa E. Neuronal growth, atrophy and death in a sexually dimorphic song nucleus in the zebra finch. Nature. 1985;315:145–147. doi: 10.1038/315145a0. [DOI] [PubMed] [Google Scholar]
- Kover ST, Abbeduto L. Expressive language in male adolescents with fragile X sydrome with and without comorbid autism. J Intellect Disabil Res. 2010;54:246–265. doi: 10.1111/j.1365-2788.2010.01255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai CS, Fisher SE, Hurst JA, Vargha-Khadem F, Monaco AP. A forkhead-domain gene is mutated in a severe speech and language disorder. Nature. 2001;413:519–523. doi: 10.1038/35097076. [DOI] [PubMed] [Google Scholar]
- Lewis P, Abbeduto L, Murphy M, Richmond E, Giles N, Bruno L, Schroeder S. Cognitive, language and social-cognitive skills of individuals with fragile X syndrome with and without autism. J Intellect Disabil Res. 2006;50:532–545. doi: 10.1111/j.1365-2788.2006.00803.x. [DOI] [PubMed] [Google Scholar]
- Lin SL, Chang SJ, Ying SY. First in vivo evidence of microRNA-induced fragile X mental retardation syndrome. Mol Psychiatry. 2006;11:616–617. doi: 10.1038/sj.mp.4001828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling SC, Fahrner PS, Greenough WT, Gelfand VI. Transport of Drosophila fragile X mental retardation protein-containing ribonucleoprotein granules by kinesin-1 and cytoplasmic dynein. Proc Natl Acad Sci U S A. 2004;101:17428–17433. doi: 10.1073/pnas.0408114101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingston FS, Mooney R. Development of intrinsic and synaptic properties in a forebrain nucleus essential to avian song learning. J Neurosci. 1997;17:8997–9009. doi: 10.1523/JNEUROSCI.17-23-08997.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo M, Perkel DJ. A GABAergic, strongly inhibitory projection to a thalamic nucleus in the zebra finch song system. J Neurosci. 1999;19:6700–6711. doi: 10.1523/JNEUROSCI.19-15-06700.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo M, Perkel DJ. Intrinsic and synaptic properties of neurons in an avian thalamic nucleus during song learning. J Neurophysiol. 2002;88:1903–1914. doi: 10.1152/jn.2002.88.4.1903. [DOI] [PubMed] [Google Scholar]
- Margoliash D. Functional organization of forebrain pathways for song production and perception. J Neurobiol. 1997;33:671–693. doi: 10.1002/(sici)1097-4695(19971105)33:5<671::aid-neu12>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Marler P, Waser MS. Role of auditory feedback in canary song development. J Comp Physiol Psychol. 1977;91:8–16. doi: 10.1037/h0077303. [DOI] [PubMed] [Google Scholar]
- Medina L, Reiner A. Neurotransmitter organization and connectivity of the basal ganglia in vertebrates: implicatios for the evolution of basal ganglia. Brain Behav Evol. 1995;46:235–258. doi: 10.1159/000113277. [DOI] [PubMed] [Google Scholar]
- Mercaldo V, Descalzi G, Zhuo M. Fragile X mental retardation protein in learning-related synaptic plasticity. Mol Cells. 2009;28:501–507. doi: 10.1007/s10059-009-0193-x. [DOI] [PubMed] [Google Scholar]
- Michel CI, Kraft R, Restifo LL. Defective neuronal development in the mushroom bodies of Drosophila fragile X mental retardation 1 mutants. J Neurosci. 2004;24:5798–5809. doi: 10.1523/JNEUROSCI.1102-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney R. Synaptic basis for developmental plasticity in a birdsong nucleus. J Neurosci. 1992;12:2464–2477. doi: 10.1523/JNEUROSCI.12-07-02464.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney R, Rao M. Waiting periods versus early innervation: the development of axonal connections in the zebra finch song system. J Neurosci. 1994;14:6532–6543. doi: 10.1523/JNEUROSCI.14-11-06532.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newbury DF, Monaco AP. Genetic advances in the study of speech and language disorders. Neuron. 2010;68:309–320. doi: 10.1016/j.neuron.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nottebohm F, Stokes TM, Leonard CM. Central control of song in the canary, Serinus canarius. J Comp Neurol. 1976;165:457–486. doi: 10.1002/cne.901650405. [DOI] [PubMed] [Google Scholar]
- Noveck IA, Reboul A. Experimental pragmatics: a Gricean turn in the study of language. Trends Cogn Sci. 2008;12:425–431. doi: 10.1016/j.tics.2008.07.009. [DOI] [PubMed] [Google Scholar]
- Ölveczky BP, Andalman AS, Fee MS. Vocal experimentation in the juvenile songbird requires a basal ganglia circuit. PLoS Biol. 2005;3:e153. doi: 10.1371/journal.pbio.0030153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacey LK, Doering LC. Developmental expression of FMRP in the astrocyte lineage: implications for fragile X syndrome. Glia. 2007;55:1601–1609. doi: 10.1002/glia.20573. [DOI] [PubMed] [Google Scholar]
- Pieretti M, Zhang F, Fu YH, Warren ST, Oostra BA, Caskey CT, Nelson DL. Absence of expression of the FMR-1 gene in fragile X syndrome. Cell. 1991;66:817–822. doi: 10.1016/0092-8674(91)90125-i. [DOI] [PubMed] [Google Scholar]
- Price J, Roberts J, Vandergrift N, Martin G. Language comprehension in boys with fragile X syndrome and boys with Down syndrome. J Intellect Disabil Res. 2007;51:318–326. doi: 10.1111/j.1365-2788.2006.00881.x. [DOI] [PubMed] [Google Scholar]
- Price JR, Roberts JE, Hennon EA, Berni MC, Anderson KL, Sideris J. Syntactic complexity during conversation of boys with fragile X syndrome and Down syndrome. J Speech Lang Hear Res. 2008;51:3–15. doi: 10.1044/1092-4388(2008/001). [DOI] [PubMed] [Google Scholar]
- Price TJ, Flores CM, Cervero F, Hargreaves KM. The RNA binding and transport proteins staufen and fragile X mental retardation protein are expressed by rat primary afferent neurons and localize to peripheral and central axons. Neuroscience. 2006;141:2107–2116. doi: 10.1016/j.neuroscience.2006.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralph GS, Radcliffe PA, Day DM, Carthy JM, Leroux MA, Lee DC, Wong LF, Bilsland LG, Greensmith L, Kingsman SM, Mitrophanous KA, Mazarakis ND, Azzouz M. Silencing mutant SOD1 using RNAi protects against neurodegeneration and extends survival in an ALS model. Nat Med. 2005;11:429–433. doi: 10.1038/nm1205. [DOI] [PubMed] [Google Scholar]
- Raoul C, Abbas-Terki T, Bensadoun JC, Guillot S, Haase G, Szulc J, Henderson CE, Aebischer P. Lentiviral-mediated silencing of SOD1 through RNA interference retards disease onset and progression in a mouse model of ALS. Nat Med. 2005;11:423–428. doi: 10.1038/nm1207. [DOI] [PubMed] [Google Scholar]
- Reiner A, Perkel DJ, Bruce LL, Butler AB, Csillag A, Kuenzel W, Medina L, Paxinos G, Shimizu T, Striedter G, Wild M, Ball GF, Surand S, Guturkun O, Lee DW, Mello CV, Powers A, White SA, Hough G, Kubikova L, Smulders TV, Wada K, Dugas-Ford J, Husband S, Yamamoto K, Yu J, Siang C, Jarvis E. Revised Nomenclature for Avian Telencephalon and some related brainstem nuclei. J Comp Neurol. 2004;473:377–414. doi: 10.1002/cne.20118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner A, Perkel DJ, Mello CV, Jarvis ED. Songbirds and the revised avian brain nomenclature. Ann NY Acad Sci. 2004;1016:77–108. doi: 10.1196/annals.1298.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts J, Price J, Barnes E, Nelson L, Burchinal M, Hennon EA, Moskowitz L, Edwards A, Malkin C, Anderson K, Misenheimer J, Hooper SR. Receptive vocabulary, expressive vocabulary, and speech production of boys with fragile X syndrome in comparison to boys with down syndrome. Am J Ment Retard. 2007;112:177–193. doi: 10.1352/0895-8017(2007)112[177:RVEVAS]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Roberts TF, Tschida KA, Klein ME, Mooney R. Rapid spine stabilization and synaptic enhancement at the onset of behavioral learning. Nature. 2010;463:948–952. doi: 10.1038/nature08759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochefort C, He X, Scotto-Lomassese S, Scharff C. Recruitment of FoxP2-expressing neurons to Area X varies during song development. Dev Neurobiol. 2007;67:809–817. doi: 10.1002/dneu.20393. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Lebron E, Denovan-Wright EM, Nash K, Lewin AS, Mandel RJ. Intrastriatal rAAV-mediated delivery of anti-huntingtin shRNAs induces partial reversal of disease progression in R6/1 Huntington’s disease transgenic mice. Molecular Therapy. 2005;12:618. doi: 10.1016/j.ymthe.2005.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sales G. Ultrasound and mating behavior in rodents with some observations on other behavioural situations. J Zool Lond. 1972;168:149–164. [Google Scholar]
- Sapru MK, Yates JW, Hogan S, Jiang L, Halter J, Bohn MC. Silencing of human [alpha]-synuclein in vitro and in rat brain using lentiviral-mediated RNAi. Experimental Neurology. 2006;198:382. doi: 10.1016/j.expneurol.2005.12.024. [DOI] [PubMed] [Google Scholar]
- Scharff C, Nottebohm F. A comparative study of the behavioral deficits following lesions of various parts of the zebra finch song system: implications for vocal learning. J Neurosci. 1991;11:2896–2913. doi: 10.1523/JNEUROSCI.11-09-02896.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharff C, Haesler S. An evolutionary perspective on FoxP2: strictly for the birds? Current Opinion in Neurobiology. 2005;15:694. doi: 10.1016/j.conb.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Schulz SB, Haesler S, Scharff C, Rochefort C. Knockdown of FoxP2 alters spine density in Area X of the zebra finch. Genes Brain Behav. 2010 doi: 10.1111/j.1601-183X.2010.00607.x. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Shu W, Cho JY, Jiang Y, Zhang M, Weisz D, Elder GA, Schmeidler J, De Gasperi R, Sosa MAG, Rabidou D, Santucci AC, Perl D, Morrisey E, Buxbaum JD. Altered ultrasonic vocalization in mice with a disruption in the Foxp2 gene. PNAS. 2005;102:9643–9648. doi: 10.1073/pnas.0503739102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer O, Marr RA, Rockenstein E, Crews L, Coufal NG, Gage FH, Verma IM, Masliah E. Targeting BACE1 with siRNAs ameliorates Alzheimer disease neuropathology in a transgenic model. Nat Neurosci. 2005;8:1343–1349. doi: 10.1038/nn1531. [DOI] [PubMed] [Google Scholar]
- Siomi MC, Siomi H, Sauer WH, Srinivasan S, Nussbaum RL, Dreyfuss G. FXR1, an autosomal homolog of the fragile X mental retardation protein. EMBO J. 1995;14:2401–2408. doi: 10.1002/j.1460-2075.1995.tb07237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sossinka R, Bhoner J. Song types in the zebra finch Poephila guttata castanotis. Z Tierpsychol. 1980;53:123–132. [Google Scholar]
- Spencer CM, Serysheva E, Yuva-Paylor LA, Oostra BA, Nelson DL, Paylor R. Exaggerated behavioral phenotypes in Fmr1/Fxr2 double knockout mice reveal a functional genetic interaction between Fragile X-related proteins. Hum Mol Genet. 2006;15:1984–1994. doi: 10.1093/hmg/ddl121. [DOI] [PubMed] [Google Scholar]
- Spiro JE, Dalva MB, Mooney R. Long-rage inhibition within the zebra finch song nucleus RA can coordinate the firing of multiple projection neurons. J Neurophysiol. 1999;81:3007–3020. doi: 10.1152/jn.1999.81.6.3007. [DOI] [PubMed] [Google Scholar]
- Stark LL, Perkel DJ. Two-stage, input-specific synaptic maturation in a nucleus essential for vocal production in the zebra finch. J Neurosci. 1999;19:9107–9116. doi: 10.1523/JNEUROSCI.19-20-09107.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudhalter V, Cohen IL, Silverman W, Wolf-Schein EG. Conversational analyses of males with fragile X, Down syndrome, and autism: comparison of the emergence of deviant language. Am J Ment Retard. 1990;94:431–441. [PubMed] [Google Scholar]
- Tchernichovski O, Nottebohm F, Ho CE, Pesaran B, Mitra PP. A procedure for an automated measurement of song similarity. Anim Behav. 2000;59:1167–1176. doi: 10.1006/anbe.1999.1416. [DOI] [PubMed] [Google Scholar]
- Tessier CR, Broadie K. Drosophila fragile X mental retardation protein developmentally regulates activity-dependent axon pruning. Development. 2008;135:1547–1557. doi: 10.1242/dev.015867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargha-Khadem F, Watkins K, Alcock K, Fletcher P, Passingham R. Praxic and nonverbal cognitive deficits in a large family with a genetically transmitted speech and language disorder. Proc Natl Acad Sci U S A. 1995;92:930–933. doi: 10.1073/pnas.92.3.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilkman E, Niemi J, Ikonen U. Fragile X speech pathology in Finnish. Brain Lang. 1988;34:203–221. doi: 10.1016/0093-934x(88)90133-2. [DOI] [PubMed] [Google Scholar]
- Vu ET, Mazurek ME, Kuo YC. Identification of a forebrain motor programming network for the learned song of zebra finches. J Neurosci. 1994;14:6924–6934. doi: 10.1523/JNEUROSCI.14-11-06924.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Ku L, Osterhout DJ, Li W, Ahmadian A, Liang Z, Feng Y. Developmentally-programmed FMRP expression in oligodendrocytes: a potential role of FMRP in regulating translation in oligodendroglia progenitors. Hum Mol Genet. 2004;13:79–89. doi: 10.1093/hmg/ddh009. [DOI] [PubMed] [Google Scholar]
- Wang J, Hessler NA. Coordination of presynaptic and postsynaptic maturation in a zebra finch forebrain motor control nucleus during song learning. Eur J Neurosci. 2006;24:2859–2869. doi: 10.1111/j.1460-9568.2006.05173.x. [DOI] [PubMed] [Google Scholar]
- Weiler IJ, Greenough WT. Synaptic synthesis of the Fragile X protein: possible involvement in synapse maturation and elimination. Am J Med Genet. 1999;83:248–252. doi: 10.1002/(sici)1096-8628(19990402)83:4<248::aid-ajmg3>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Weiler IJ, Irwin SA, Klintsova AY, Spencer CM, Brazelton AD, Miyashiro K, Comery TA, Patel B, Eberwine J, Greenough WT. Fragile X mental retardation protein is translated near synapses in response to neurotransmitter activation. Proc Natl Acad Sci U S A. 1997;94:5395–5400. doi: 10.1073/pnas.94.10.5395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilbrecht L, Nottebohm F. Vocal learning in birds and humans. Ment Retard Dev Disabil Res Rev. 2003;9:135–148. doi: 10.1002/mrdd.10073. [DOI] [PubMed] [Google Scholar]
- Winograd C, Clayton D, Ceman S. Expression of fragile X mental retardation protein within the vocal control system of developing and adult male zebra finches. Neuroscience. 2008;157:132–142. doi: 10.1016/j.neuroscience.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia H, Mao Q, Eliason SL, Harper SQ, Martins IH, Orr HT, Paulson HL, Yang L, Kotin RM, Davidson BL. RNAi suppresses polyglutamine-induced neurodegeneration in a model of spinocerebellar ataxia. Nat Med. 2004;10:816. doi: 10.1038/nm1076. [DOI] [PubMed] [Google Scholar]
- Zajac DJ, Harris AA, Roberts JE, Martin GE. Direct magnitude estimation of articulation rate in boys with fragile X syndrome. J Speech Lang Hear Res. 2009;52:1370–1379. doi: 10.1044/1092-4388(2009/07-0208). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zajac DJ, Roberts JE, Hennon EA, Harris AA, Barnes EF, Misenheimer J. Articulation rate and vowel space characteristics of young males with fragile X syndrome: preliminary acoustic findings. J Speech Lang Hear Res. 2006;49:1147–1155. doi: 10.1044/1092-4388(2006/082). [DOI] [PubMed] [Google Scholar]
- Zhang YQ, Bailey AM, Matthies HJ, Renden RB, Smith MA, Speese SD, Rubin GM, Broadie K. Drosophila fragile X-related gene regulates the MAP1B homolog Futsch to control synaptic structure and function. Cell. 2001;107:591–603. doi: 10.1016/s0092-8674(01)00589-x. [DOI] [PubMed] [Google Scholar]
- Zingerevich C, Greiss-Hess L, Lemons-Chitwood K, Harris SW, Hessl D, Cook K, Hagerman RJ. Motor abilities of children diagnosed with fragile X syndrome with and without autism. J Intellect Disabil Res. 2009;53:11–18. doi: 10.1111/j.1365-2788.2008.01107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]