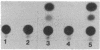
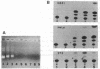
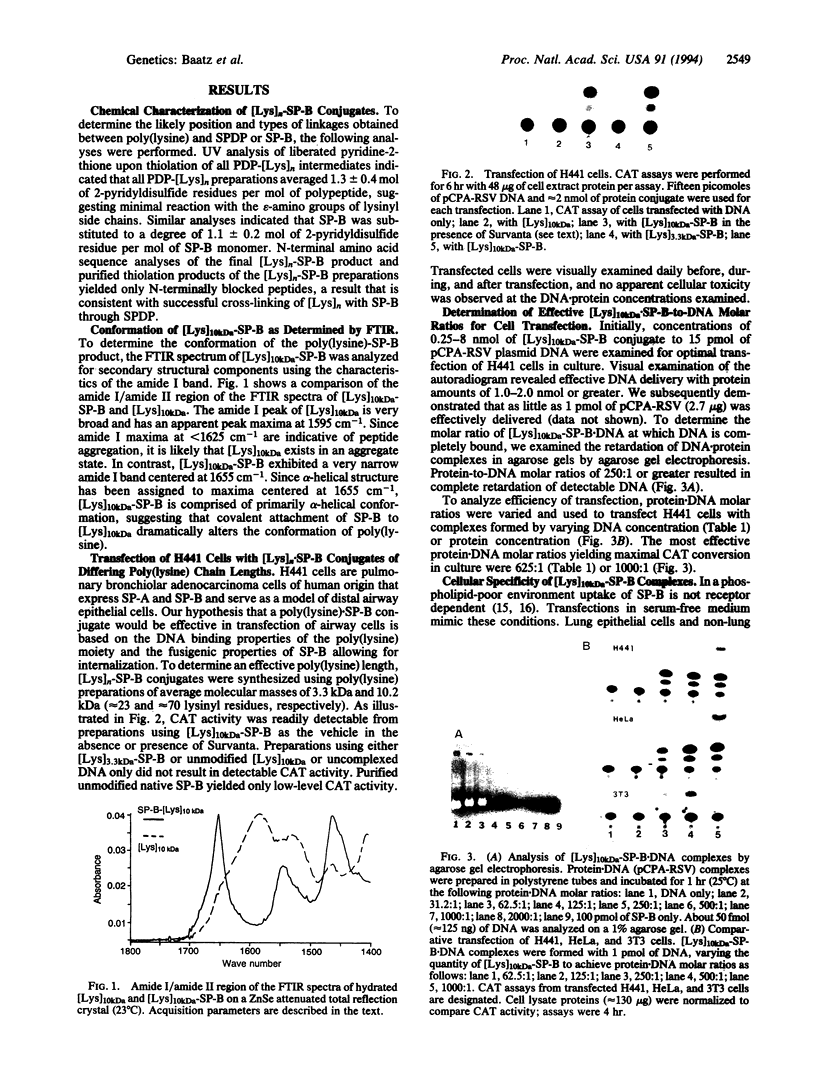
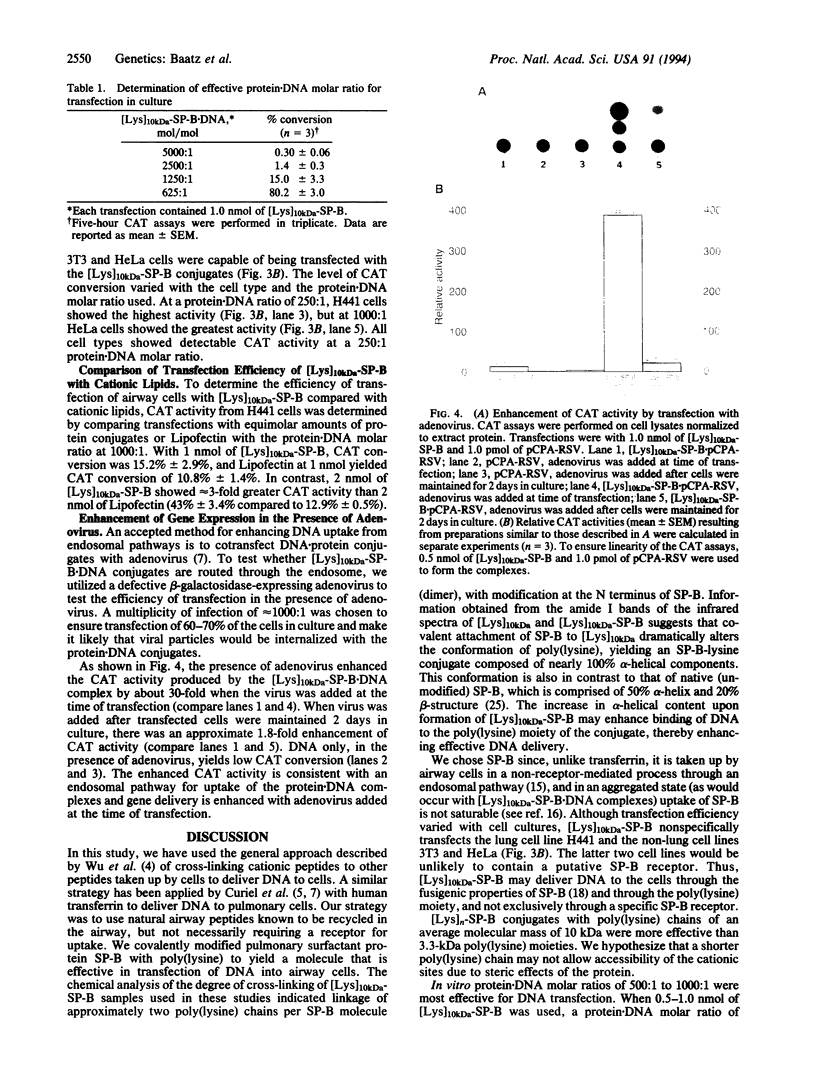
Abstract
Pulmonary surfactant lines the airway epithelium and creates a potential barrier to successful transfection of the epithelium in vivo. Based on the functional properties of pulmonary surfactant protein B (SP-B) and the fact that this protein is neither toxic nor immunogenic in the airway, we hypothesized that SP-B could be modified to deliver DNA to airway cells. We have modified native bovine SP-B by the covalent linkage of poly(lysine) (average molecular mass of 3.3 or 10 kDa) to the N terminus of SP-B and formed complexes between a test plasmid and the modified SP-B. Transfection efficiency was determined by transfection of pulmonary adenocarcinoma cells (H441) in culture with the test plasmid pCPA-RSV followed by measurement of activity of the reporter gene encoding chloramphenicol acetyltransferase (CAT). Transfections were performed with DNA.protein complexes using poly(lysine)10kDa-SP-B ([Lys]10kDa-SP-B) or poly(lysine)3.3kDa-SP-B ([Lys]3.3kDa-SP-B), and results were compared with transfections using unmodified poly(lysine).DNA, unmodified SP-B.DNA, or DNA only. For [Lys]10kDa-SP-B.pCPA-RSV preparations, CAT activity was readily detectable above the background of [Lys]3.3kDa-SP-B or unmodified SP-B. The SP-B-poly(lysine) conjugates were effective over a broad range of protein-to-DNA molar ratios, although they were optimal at approximately 500:1-1000:1. Transfection efficiency varied with the tested cell line but was not specific to airway cells. Addition of replication-defective adenovirus to the [Lys]10kDa-SP-B.pCPA-RSV complex enhanced CAT activity about 30-fold with respect to that produced by the [Lys]10kDa-SP-B.pCPA-RSV complex alone. This increase suggests routing of the adenoviral.[Lys]10kDa-SP-B.pCPA-RSV complex through an endosomal pathway. Effects of covalent modification on the secondary structure of SP-B were examined by Fourier transform infrared spectrometry (FTIR). Results of FTIR indicated that the conformation of [Lys]10kDa-SP-B was comprised primarily of alpha-helical structure compared with a predominantly aggregated structure of unmodified poly(lysine). We conclude that poly(lysine) conjugates of SP-B effectively deliver DNA in vitro and may have utility as DNA delivery vehicles to the airway in vivo.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baatz J. E., Smyth K. L., Whitsett J. A., Baxter C., Absolom D. R. Structure and functions of a dimeric form of surfactant protein SP-C: a Fourier transform infrared and surfactometry study. Chem Phys Lipids. 1992 Nov;63(1-2):91–104. doi: 10.1016/0009-3084(92)90026-l. [DOI] [PubMed] [Google Scholar]
- Bates S. R., Beers M. F., Fisher A. B. Binding and uptake of surfactant protein B by alveolar type II cells. Am J Physiol. 1992 Sep;263(3 Pt 1):L333–L341. doi: 10.1152/ajplung.1992.263.3.L333. [DOI] [PubMed] [Google Scholar]
- Bohinski R. J., Huffman J. A., Whitsett J. A., Lattier D. L. Cis-active elements controlling lung cell-specific expression of human pulmonary surfactant protein B gene. J Biol Chem. 1993 May 25;268(15):11160–11166. [PubMed] [Google Scholar]
- Breslin J. S., Weaver T. E. Binding, uptake, and localization of surfactant protein B in isolated rat alveolar type II cells. Am J Physiol. 1992 Jun;262(6 Pt 1):L699–L707. doi: 10.1152/ajplung.1992.262.6.L699. [DOI] [PubMed] [Google Scholar]
- Byler D. M., Susi H. Examination of the secondary structure of proteins by deconvolved FTIR spectra. Biopolymers. 1986 Mar;25(3):469–487. doi: 10.1002/bip.360250307. [DOI] [PubMed] [Google Scholar]
- Cotten M., Wagner E., Zatloukal K., Phillips S., Curiel D. T., Birnstiel M. L. High-efficiency receptor-mediated delivery of small and large (48 kilobase gene constructs using the endosome-disruption activity of defective or chemically inactivated adenovirus particles. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):6094–6098. doi: 10.1073/pnas.89.13.6094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curiel D. T., Agarwal S., Rømer M. U., Wagner E., Cotten M., Birnstiel M. L., Boucher R. C. Gene transfer to respiratory epithelial cells via the receptor-mediated endocytosis pathway. Am J Respir Cell Mol Biol. 1992 Mar;6(3):247–252. doi: 10.1165/ajrcmb/6.3.247. [DOI] [PubMed] [Google Scholar]
- Curiel D. T., Agarwal S., Wagner E., Cotten M. Adenovirus enhancement of transferrin-polylysine-mediated gene delivery. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8850–8854. doi: 10.1073/pnas.88.19.8850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curstedt T., Johansson J., Barros-Söderling J., Robertson B., Nilsson G., Westberg M., Jörnvall H. Low-molecular-mass surfactant protein type 1. The primary structure of a hydrophobic 8-kDa polypeptide with eight half-cystine residues. Eur J Biochem. 1988 Mar 15;172(3):521–525. doi: 10.1111/j.1432-1033.1988.tb13918.x. [DOI] [PubMed] [Google Scholar]
- Curstedt T., Jörnvall H., Robertson B., Bergman T., Berggren P. Two hydrophobic low-molecular-mass protein fractions of pulmonary surfactant. Characterization and biophysical activity. Eur J Biochem. 1987 Oct 15;168(2):255–262. doi: 10.1111/j.1432-1033.1987.tb13414.x. [DOI] [PubMed] [Google Scholar]
- Gao L., Wagner E., Cotten M., Agarwal S., Harris C., Rømer M., Miller L., Hu P. C., Curiel D. Direct in vivo gene transfer to airway epithelium employing adenovirus-polylysine-DNA complexes. Hum Gene Ther. 1993 Feb;4(1):17–24. doi: 10.1089/hum.1993.4.1-17. [DOI] [PubMed] [Google Scholar]
- Glasser S. W., Korfhagen T. R., Bruno M. D., Dey C., Whitsett J. A. Structure and expression of the pulmonary surfactant protein SP-C gene in the mouse. J Biol Chem. 1990 Dec 15;265(35):21986–21991. [PubMed] [Google Scholar]
- Glasser S. W., Korfhagen T. R., Weaver T., Pilot-Matias T., Fox J. L., Whitsett J. A. cDNA and deduced amino acid sequence of human pulmonary surfactant-associated proteolipid SPL(Phe). Proc Natl Acad Sci U S A. 1987 Jun;84(12):4007–4011. doi: 10.1073/pnas.84.12.4007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawgood S., Benson B. J., Schilling J., Damm D., Clements J. A., White R. T. Nucleotide and amino acid sequences of pulmonary surfactant protein SP 18 and evidence for cooperation between SP 18 and SP 28-36 in surfactant lipid adsorption. Proc Natl Acad Sci U S A. 1987 Jan;84(1):66–70. doi: 10.1073/pnas.84.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazinski T. A., Ladd P. A., DeMatteo C. A. Localization and induced expression of fusion genes in the rat lung. Am J Respir Cell Mol Biol. 1991 Mar;4(3):206–209. doi: 10.1165/ajrcmb/4.3.206. [DOI] [PubMed] [Google Scholar]
- Jung G., Köhnlein W., Lüders G. Biological activity of the antitumor protein neocarzinostatin coupled to a monoclonal antibody by N-succinimidyl 3-(2-pyridyldithio)-propionate. Biochem Biophys Res Commun. 1981 Jul 30;101(2):599–606. doi: 10.1016/0006-291x(81)91301-2. [DOI] [PubMed] [Google Scholar]
- Korfhagen T. R., Bruno M. D., Glasser S. W., Ciraolo P. J., Whitsett J. A., Lattier D. L., Wikenheiser K. A., Clark J. C. Murine pulmonary surfactant SP-A gene: cloning, sequence, and transcriptional activity. Am J Physiol. 1992 Nov;263(5 Pt 1):L546–L554. doi: 10.1152/ajplung.1992.263.5.L546. [DOI] [PubMed] [Google Scholar]
- Rice W. R., Sarin V. K., Fox J. L., Baatz J., Wert S., Whitsett J. A. Surfactant peptides stimulate uptake of phosphatidylcholine by isolated cells. Biochim Biophys Acta. 1989 Nov 28;1006(2):237–245. doi: 10.1016/0005-2760(89)90202-6. [DOI] [PubMed] [Google Scholar]
- Rosenfeld M. A., Yoshimura K., Trapnell B. C., Yoneyama K., Rosenthal E. R., Dalemans W., Fukayama M., Bargon J., Stier L. E., Stratford-Perricaudet L. In vivo transfer of the human cystic fibrosis transmembrane conductance regulator gene to the airway epithelium. Cell. 1992 Jan 10;68(1):143–155. doi: 10.1016/0092-8674(92)90213-v. [DOI] [PubMed] [Google Scholar]
- SELA M., FUCHS S., ARNON R. Studies on the chemical basis of the antigenicity of proteins. 5. Synthesis, characterization and immunogenicity of some multichain and linear polypeptides containing tyrosine. Biochem J. 1962 Oct;85:223–235. doi: 10.1042/bj0850223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seth P., Fitzgerald D. J., Willingham M. C., Pastan I. Role of a low-pH environment in adenovirus enhancement of the toxicity of a Pseudomonas exotoxin-epidermal growth factor conjugate. J Virol. 1984 Sep;51(3):650–655. doi: 10.1128/jvi.51.3.650-655.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffer K., Hawgood S., Düzgünes N., Goerke J. Interactions of the low molecular weight group of surfactant-associated proteins (SP 5-18) with pulmonary surfactant lipids. Biochemistry. 1988 Apr 19;27(8):2689–2695. doi: 10.1021/bi00408a008. [DOI] [PubMed] [Google Scholar]
- Stribling R., Brunette E., Liggitt D., Gaensler K., Debs R. Aerosol gene delivery in vivo. Proc Natl Acad Sci U S A. 1992 Dec 1;89(23):11277–11281. doi: 10.1073/pnas.89.23.11277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stripp B. R., Sawaya P. L., Luse D. S., Wikenheiser K. A., Wert S. E., Huffman J. A., Lattier D. L., Singh G., Katyal S. L., Whitsett J. A. cis-acting elements that confer lung epithelial cell expression of the CC10 gene. J Biol Chem. 1992 Jul 25;267(21):14703–14712. [PubMed] [Google Scholar]
- Vandenbussche G., Clercx A., Clercx M., Curstedt T., Johansson J., Jörnvall H., Ruysschaert J. M. Secondary structure and orientation of the surfactant protein SP-B in a lipid environment. A Fourier transform infrared spectroscopy study. Biochemistry. 1992 Sep 29;31(38):9169–9176. doi: 10.1021/bi00153a008. [DOI] [PubMed] [Google Scholar]
- Wagner E., Zatloukal K., Cotten M., Kirlappos H., Mechtler K., Curiel D. T., Birnstiel M. L. Coupling of adenovirus to transferrin-polylysine/DNA complexes greatly enhances receptor-mediated gene delivery and expression of transfected genes. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):6099–6103. doi: 10.1073/pnas.89.13.6099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitsett J. A., Hull W. M., Luse S. Failure to detect surfactant protein-specific antibodies in sera of premature infants treated with survanta, a modified bovine surfactant. Pediatrics. 1991 Apr;87(4):505–510. [PubMed] [Google Scholar]
- Whitsett J. A., Hull W. M., Ohning B., Ross G., Weaver T. E. Immunologic identification of a pulmonary surfactant-associated protein of molecular weight = 6000 daltons. Pediatr Res. 1986 Aug;20(8):744–749. doi: 10.1203/00006450-198608000-00009. [DOI] [PubMed] [Google Scholar]
- Wilkinson D. G., Bailes J. A., McMahon A. P. Expression of the proto-oncogene int-1 is restricted to specific neural cells in the developing mouse embryo. Cell. 1987 Jul 3;50(1):79–88. doi: 10.1016/0092-8674(87)90664-7. [DOI] [PubMed] [Google Scholar]
- Wu C. H., Wilson J. M., Wu G. Y. Targeting genes: delivery and persistent expression of a foreign gene driven by mammalian regulatory elements in vivo. J Biol Chem. 1989 Oct 15;264(29):16985–16987. [PubMed] [Google Scholar]
- Xu J. J., Richardson C., Ford C., Spencer T., Yao L. J., Mackie G., Hammond G., Possmayer F. Isolation and characterization of the cDNA for pulmonary surfactant-associated protein-B (SP-B) in the rabbit. Biochem Biophys Res Commun. 1989 Apr 14;160(1):325–332. doi: 10.1016/0006-291x(89)91659-8. [DOI] [PubMed] [Google Scholar]
- Zhu N., Liggitt D., Liu Y., Debs R. Systemic gene expression after intravenous DNA delivery into adult mice. Science. 1993 Jul 9;261(5118):209–211. doi: 10.1126/science.7687073. [DOI] [PubMed] [Google Scholar]