Abstract
Objective
This review will focus on the immunological aspects of adipose tissue and its potential role in development of chronic inflammation that instigates obesity-associated co-morbidities.
Design and Methods
The review utilized PubMed searches of current literature to examine adipose tissue leukocytosis.
Results
The adipose tissue of obese subjects becomes inflamed and contributes to the development of insulin resistance, type 2 diabetes and metabolic syndrome. Numerous immune cells including B cells, T cells, macrophages and neutrophils have been identified in adipose tissue, and obesity influences both the quantity and the nature of immune cell subtypes which emerges as an active immunological organ capable of modifying whole body metabolism through paracrine and endocrine mechanisms.
Conclusion
Adipose tissue is a large immunologically active organ during obesity that displays hallmarks of both and innate and adaptive immune response. Despite the presence of hematopoietic lineage cells in adipose tissue, it is presently unclear whether the adipose compartment has a direct role in immune-surveillance or host defense. Understanding the interactions between leukocytes and adipocytes may reveal the clinically relevant pathways that control adipose tissue inflammation and is likely to reveal mechanism by which obesity contributes to increased susceptibility to both metabolic and certain infectious disease.
Keywords: inflammation, cytokine, antigen, diabetes, metabolism, obesity
Introduction
Rising incidence of overweight and obesity has contributed to accelerating rates of type 2 diabetes and metabolic syndrome, which have large implications for global health. Epidemiological evidence indicates that obesity accounts for ~18% of all deaths for persons between 40 and 85 years of age in the United States (1). Thus, the development of safe and effective treatments for obesity and its co-morbidities, especially type 2 diabetes, is needed. The recognition that chronic low-grade inflammation contributes to obesity associated co-morbidities and that adipose tissue is a major immunologically active organ that contributes to this inflammation is growing.
It is established that adipose tissue is an active metabolic tissue that can secrete a variety of adipokines as well as pro- and anti-inflammatory proteins (e.g. tumor necrosis factor (TNF), interleukin-1β (IL-1β and adiponectin and IL-10 respectively) capable of modifying insulin sensitivity locally within adipose tissue and systemically. Enzymatic digestion of adipose tissue has been used to isolate an adipocyte fraction and a stromal vascular fraction, which consists of immune cells, pre-adipocytes, endothelial cells etc. Studies of different adipose depots have revealed that immune cells represent approximately two thirds of the stromal vascular fraction, which contains approximately 2 to 5 million cells/g of tissue (2). In morbidly obese subjects, adipose tissue can account for up to 50% of total body mass and represents a major compartment of the immune system capable of influencing systemic inflammation. Thus, the extensive expansion of adipose tissue during obesity increases its ability to act as an immunological tissue and control systemic inflammation and metabolism. However, some obese humans are not affected by obesity associated co-morbidities indicating that there is biological variation in response to excessive calories. This review article will focus on adipose tissue inflammation first by examining metabolically healthy and unhealthy obesity and then understanding adipose tissue leukocytosis by focusing on adipocyte damage and specific immune cell populations in order to understand the implications of adipose tissue inflammation on metabolic health and obesity associated co-morbidities.
Results
Inflammation and metabolically healthy and unhealthy obesity
Overwhelming evidence suggests that prolonged adipose tissue remodeling in response to caloric excess leads to chronic inflammation at the expense of reduced insulin-sensitivity. Clinically, chronic insulin resistance and pre-diabetes are linked to the development of type 2 diabetes, and lifestyle modifications and metformin treatment can reduce the risk of progression to diabetes (3). Interestingly, some obese subjects are metabolically healthy and relatively unaffected by obesity associated co-morbidities (4). Recent studies have begun addressing what separates metabolically healthy and unhealthy obese populations. Most definitions of metabolically healthy obesity include normal range measures of blood pressure, blood lipids (triglycerides, low-density lipoproteins, high-density lipoproteins, total cholesterol) and glucose homeostasis (fasting blood glucose, homeostatic model assessment). Some include inflammatory markers as criteria, and inflammation appears to separate both metabolically healthy lean and obese individuals from their metabolically unhealthy counter parts. The metabolically unhealthy populations have higher concentrations of complement C3, C-reactive protein and IL-6, and decreased adiponectin compared to matched metabolically healthy individuals (5). However, there is variation between studies, with some studies confirming differences in C-reactive protein (6) while others have not (7). Plasma concentrations of interferon-γ and TNF are also increased in metabolically unhealthy obese subjects (7). Interestingly, adipose tissue of the same subjects had increased expression of the macrophage marker cluster of differentiation 68 (CD68), and reduced tensile strength, which may be indicative of fibrosis (7). These studies add to other clinical data suggesting obesity associated co-morbidities are regulated by inflammation.
Although metabolically healthy obese individuals seem to be protected from obesity-associated disease, it stands to reason that the development of inflammation and insulin-resistance during obesity could be an adaptive mechanism to control organ dysfunction associated with the storage of excess energy. For example, over the last two decades there is strong evidence from studies in Pima Indians and other populations that lower rates of glucose:lipid oxidation or a low respiratory quotient is associated with a reduced rate of weight gain (8, 9, 10). From these early studies, it was hypothesized that a low rate of glucose oxidation leads to a concomitant reduction in glucose disposal, and insulin-resistance could be a biological adaptation to lower weight gain by reduced energy storage in adipose tissue.
Insulin resistance may reduce weight gain; however elevated glucose levels come at a price and contribute to inflammation. Increased glucose utilization by immune cells can control activation and polarization to more pro-inflammatory phenotypes. For example, T-helper 17 (Th17) cells are reliant on glycolysis, and inhibition of glycolysis switches T cell differentiation from a pro-inflammatory Th17 cells to the more anti-inflammatory regulatory T cells (11). Additionally, macrophage glucose metabolism is required for the secretion of IL-1β (12). Thus, a corollary would be that inflammation associated insulin resistance may be an early adaptive response to lower glycolytic rates in response to overnutrition. However, during chronic overnutrition the early inflammatory processes are not resolved and become maladaptive leading to pathological changes in organs and tissues that eventually lead to the development of diabetes and its complications. It is currently unclear whether inhibition of specific pro-inflammatory pathways will fully relieve obesity-associated tissue dysfunction, but come at the expense of continued energy storage in adipose as a result of increased insulin-sensitivity. Whether such adipocyte insulin sensitivity in response to energy excess leads to metabolically healthy obesity and continued weight gain remains to be fully established. Clearly, additional investigation linking inflammation to metabolic disease is warranted.
Adipose tissue leukocytosis
An understanding of adipose tissue leukocytosis began with the identification of macrophages in adipose tissue. Seminal findings by Weisberg et al. and Xu et al. demonstrated that macrophages are increased in adipose tissue during obesity and that macrophages are the primary source of TNF (13, 14). In addition to macrophages, other immune cells have been identified including: B cells (15), T cells (16), neutrophils (17, 18), eosinophils (19), and mast cells (20, 21). The cellular composition of adipose tissue is plastic and regulated by both acute and chronic stimuli including diet (13, 14, 18), body weight status (13, 14), cold exposure (22), feeding and fasting (23). The exact course of events leading to changes in the cellular composition of adipose tissue in response to high-fat diet is yet to be defined. However, it is known that neutrophils are recruited to adipose tissue within 3 days, macrophages within 2 weeks, and B cells and T cells are increased within 4 weeks of high-fat diet in mice (15, 18).
The total number of each cell type is important, however there are also changes in the quality of these cell populations. Obesity and insulin resistance have the capacity to skew immune cells from anti-inflammatory subtypes towards more pro-inflammatory subtypes. This includes a switching of macrophage polarization from M2-like cells to more pro-inflammatory M1-like cells (24) and the loss of regulatory T cells in adipose tissue (25). The local effects of these compositional changes during obesity drive adipose tissue inflammation and influence the capacity of adipocytes to store lipid, adipocyte insulin sensitivity, systemic glucose metabolism, and metabolic homeostasis. The underlying factors and cell types that drive adipose tissue inflammation is a very active field of research, yet there are still many unknown factors in the etiology of obesity-mediated disease.
Adipocyte stress and damage associate molecular patterns
In response to excess energy, adipocytes undergo hypertrophy and/or hyperplasia. Adipocyte size is correlated with insulin sensitivity in humans and those with smaller adipocytes have lower markers of inflammation (26, 27), however rodent models indicate that during the absence of certain proteins adipocytes can be large while the animal maintains insulin sensitivity (28). Histological analysis of adipose tissue reveals the presence of crown-like structures which consist of immune cells surrounding dead adipocytes that stain perilipin negative around their lipid droplets (29). The appearance of crown-like structures and adipocyte death follow a time course whereby there is increasing incidence from the onset of high fat-feeding to week 16. By week 20 crown-like structures are decreased compared to week 16, although compared to lean control animals the number is still elevated and the mice remain insulin resistant though to a lesser degree than at the peak of crown-like structure incidence (29). This indicates that remodeling of adipose tissue is an adaptive response, but cannot fully compensate for nutrient excess and eventually limits lipid storage leading to tissue dysfunction.
The precise way adipocytes die is unclear, however recent studies indicate that adipocyte death may proceed through pyroptosis (30). Pyroptosis is a specialized form of caspase-1 dependent cell death that involves the leaking of cytosolic constituents into the extracellular space. Leaking of the cytosolic constituents and exposure of the lipid droplet in the tissue causes the release of danger associated molecular patterns (DAMPs) that can be sensed by pattern recognition receptors (Figure 1). Metabolic DAMPs include free fatty acids (31), high glucose concentrations (32), ATP (33), ceramides (34) and other sphingolipids, and cholesterol (35, 36) and urate crystals. Recently, adipocytes have been shown to synthesize uric acid, and adipose tissue uric acid production increases during obesity (37). These metabolic DAMPs are capable of causing activation of macrophages and initiating adipose tissue inflammation.
Figure 1.
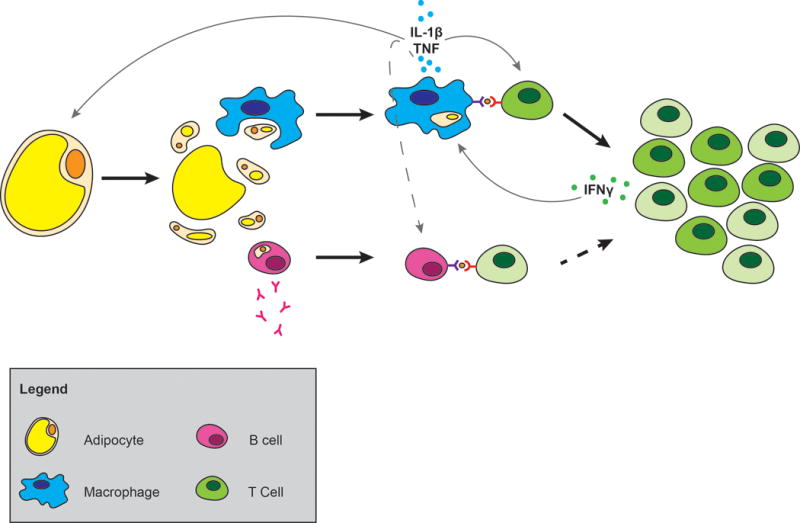
Generation of inflammation in adipose tissue. Adipocyte death releases cytosolic constituents and exposes the lipid droplet. Macrophages engulf or are activated by adipocyte constituents leading to macrophage production of cytokines. B cells are also activated leading to the production of antibodies and MHCII dependent T cell interaction which may contribute to insulin resistance. IL-1β secreted by macrophages and antigen presentation drives the clonal expansion of T cells and shifts the balance of T cells in adipose tissue from naïve to effector memory cells. Dashed lines indicate possible interactions between cells.
Macrophage sensing of adipose tissue DAMPs and antigen presentation
Metabolic DAMPs and pathogen associated molecular patterns (PAMPs) are sensed by innate immune cells and lead to the activation of inflammatory pathways through pattern recognition receptors such as the toll-like and nucleotide-binding oligomerization domain containing protein-like receptor families (TLR and NLR). Analysis of the distribution of these receptors as well as macrophage markers has been difficult due to the enzymatic dispersion of adipose tissue. Enzymatic dispersion of adipose tissue separates adipose tissue into a buoyant adipocyte fraction and a stromal vascular pellet, however it is limited because of tight associations between adipocytes and macrophages and the presence of free floating macrophages in the adipocyte fraction (38).
All members of the TLR family (TLR 1–9) are expressed in adipose tissue to varied degrees of within the stromal vascular and the adipocyte fractions (39). In regards to adipose tissue inflammation, TLR4 has received the most attention. The interaction of TLR4 and fatty acids has been linked to the protein fetuin A (40). Fetuin A has been identified to be an endogenous ligand of TLR4 and is produced in large amounts by adipose tissue (40). In co-culture systems, fetuin A is released by adipocytes after exposure to free fatty acids and is able to polarize macrophages from an M2-like phenotype to an M1-like phenotype, similar to the change that occurs in adipose tissue during the development of obesity (40). In addition to its polarizing activities, fetuin A also serves as a chemoattractant for macrophages resulting in increased traffic to the tissue (40). Ablation of TLR4 reduces adipose tissue inflammation, yet its effects on whole body insulin sensitivity have not been consistent across studies with some showing increased insulin sensitivity (41, 42) while other show no change (42).
In addition to the toll-like receptors, NLRs are a family of cytosolic pattern recognition receptors that influence obesity. The NLR family, pyrin domain contain 3 (NLRP3) inflammasome in particular appears to promote adipose tissue inflammation and is responsive to a broad array of metabolic DAMPs. The NLRP3 inflammasome is a multi-protein scaffolding complex that oligomerizes upon exposure to DAMPs, through homotypic protein-protein interactions of NLRP3, apoptosis-associated speck-like protein containing a card, and pro-caspase-1 (43). Activation of the NLRP3 inflammasome results in the cleaving of the pro-caspase-1 zymogen to active caspase-1. Unlike the toll-like receptors whose primary effects on cytokine expression are mediated transcriptionally through nuclear factor kappa B, caspase-1 acts post-transcriptionally as a cysteine protease that cleaves pro-IL1β and pro-IL18 into their active forms, leading to their secretion. The NLRP3 inflammasome is able to sense free fatty acids (31), ATP (33), ceramides (34), glucotoxicity (32), cholesterol (35), amyloid-β (44, 45) and urate crystals (46). Interestingly, the active inflammasome complex is secreted or released during pyroptosis and functional in the extracellular space, producing IL-1β (47, 48). The secreted complex is also a danger signal, being engulfed by macrophages, transported to the lysosome and degraded slowly over time (47, 48). The exact mechanism of inflammasome activation is an active area of research, and it appears that in response to many DAMPs activation occurs in a K+ efflux dependent manner (49). Some of the DAMPs proceed through lysosome dependent processes, e.g. amyloid β, and require the fatty acid transport protein CD36 for import and inflammasome activation (50). Omega-3 fatty acids reduce inflammasome activation through G protein-coupled receptor 120 and 40 signaling and it will be interesting to see if other dietary constituents modulate inflammasome activation in the future (51). Most rodent studies indicate that ablating components of the inflammasome complex improve insulin sensitivity and glucose tolerance (31, 32, 34, 52), and human studies indicate that the inflammasome complex is active in type 2 diabetes and its activity is decreased by metformin (53). However, studies in rodents have also revealed that the NLRP3 inflammasome can cause dysbiosis, and in these animals this is linked to greater weight gain, inflammation and non-alcoholic fatty liver disease (54). These studies indicate that the NLRP3 inflammasome is a good target for drug development, but highlight the need to consider interactions with gut microbiota on clinical outcomes.
In lean animals, the balance of immune cells in adipose tissue is skewed to more anti-inflammatory phenotypes including M2-like macrophage and regulatory T-cells which are known to secrete the anti-inflammatory cytokines such as IL-10 (24). Over the course of obesity development, exposure of macrophages to DAMPs and PAMPs and recruitment of monocytes leads to increased polarization of macrophages to an M1-like phenotype and macrophage activation along with the expression of TNF and IL-1β. Interestingly, activation of nuclear factor kappa B in macrophages polarizes cells towards glycolysis, and this is required for transcription of pro-IL-1β (12). Alternatively, M2 macrophages rely on fatty acid oxidation and have a remodeling and reparative phenotype (55). Because diabetes is a state characterized by hyperglycemia and increased danger associated molecular patterns, this would appear to set the stage for uncontrolled inflammation. During the dynamic phase of weight loss in humans by very low calorie diet adipose tissue macrophages populations are not altered, however after 2 months of weight stabilization reductions in the total number of macrophages present in adipose tissue are apparent (56). In a more extreme case, 3 months after bariatric surgery markers of total macrophages and M1 like macrophages were decreased while M2-like macrophages markers were increased (57). Thus in both rodents and humans, macrophage phenotype varies with body composition and is modified by weight loss, but only when a steady state has been achieved indicating that macrophage populations are of functional importance during the active remodeling process during adipose tissue contraction and expansion.
Adipose tissue macrophages bridge innate and adaptive immunity through secretion of pro-inflammatory cytokines in response to DAMPs and PAMPs, but also by being the primary antigen presenting cells in adipose tissue and driving T cell proliferation. Adipose tissue macrophages are the primary cell type able to phagocytose antigens (OVA and Zymosan) in vitro and in vivo (58). Adipose tissue macrophages are capable of driving T cell proliferation in vitro, in an antigen dependent manner. Not only do they drive CD4+ T cell proliferation, but they also skew them towards a Th1 polarization, consistent with increased interferon-γ secretion from obese adipose tissue and increased plasma levels as well (Figure 1) (58). In addition to macrophages (58), B cell (15) and adipocytes (59) have also been demonstrated to present antigens. The relative contributions of these populations to antigen presentation during obesity needs further study. However, it appears that the macrophages and dendritic cells are the major cell type that participate in antigen processing and presentation. Thus, macrophages are necessary for the remodeling of adipose tissue, mediate adipose tissue inflammation through the production of pro-inflammatory cytokines and influence the adaptive immune response through antigen presentation. Future research is needed to determine the antigens that are presented.
T cell subsets, expansion and contraction of populations
Obesity is associated with significant changes to local T cell populations in adipose tissue. Adipose tissue from lean animals is enriched with a unique population of CD4+CD25+Foxp3+ regulatory T cells, which are reduce by high-fat feeding (25). These regulatory T cells are unique, having high expression of the adipogenic transcription factor peroxisome proliferator-activated receptor γ (60). Regulatory T cells are known to express the anti-inflammatory cytokine IL-10 (25) and restrain immune responses by reducing the proliferation and activation of T cells. IL-10 reduces inflammatory signaling in adipocytes, so the function of these cells in adipose tissue is likely multi-factorial (61). Interestingly, the insulin sensitizing effects of the thiazolidinedione class of drugs that activate peroxisome proliferator-activated receptor γ may be due in large part to their effects on regulatory T cells (60). Although regulatory T cells are lost, during obesity there is an overall increase in total T cells in adipose tissue. These adipose tissue T cells are predominately memory T cells with a restricted T cell receptor repertoire (16, 62). Alterations of adipose tissue T cell populations during obesity are indicative of an active adaptive immune response and local proliferation of T cells. Ablation of T cells through genetic or antibody depletion leads to improvements in insulin signaling although the overall effects on glucose tolerance are variable (16, 62). T cell mediated inflammation is regulated by IL-6, and ablation of signal transducer and activator of transcription 3 signaling in T cells results in enhanced insulin sensitivity and increased markers of regulatory T cells in visceral adipose tissue during diet-induced obesity (63). In addition to cells of the innate immune system, the adaptive immune system appears to influence metabolic homeostasis, however the local adipose derived antigens driving this process have not been identified.
B cell contribution to adipose tissue inflammation and insulin resistance
B cells like macrophages express TLRs, however B cell expression of the NLRP3 inflammasome is very low (64). In addition, like macrophages B cells express major histocompatibility complex II (MHCII) and have the ability to present antigens to T cells. Adoptive transfer of MHCII null B cells to mice that lack B cells results in increased insulin sensitivity compared to transfer of wild type B cells, while MHCI null B cells were not different (Figure 1) (15). This indicates B cell antigen presentation to CD4+ T cells is a contributor to adipose tissue inflammation and systemic insulin resistance. Immunoglobulin G (IgG) produced by B cells has been shown to mediate insulin resistance during diet-induced obesity, but the exact mechanism involved here are unclear, and further research is needed to identify the targeted proteins and mechanisms by which this takes place (15). The contribution of adipose tissue and peripheral B cells to insulin resistance has received little attention. Animals that lack B cells have improved insulin sensitivity, and B cells appear to mediate their effects through interactions that promote Th17 polarization in T cells and a reduction in the regulatory T cell population (65). However, populations of IL-10 producing B cells have also been found in adipose tissue and appear to protect from insulin resistance (66). Although B cells mediate inflammation during obesity, the mechanisms of B cell infiltration and contribution to adipose tissue inflammation need to be substantiated including the relative contribution to adipose antigen presentation, production of pathogenic IgG and the production of cytokines.
Immunological structures of adipose tissues
Adipose tissue is not uniform in its distribution and has regionality based on immune structures including crown-like structures, fat associated lymphoid clusters, milky spots, but also on the occurrence of brite/beige adipocytes. However, the influence of adipose tissue regionality on adipose function is not well understood. Some adipose tissue depots, including the inguinal depot in rodents and many small depots in human and other mammals, contain lymph nodes (67). Initial studies on guinea pig adipose tissue depots demonstrated that adipose tissue explants located near lymph nodes compared to adipose tissue not adjacent to lymph nodes had enhanced capacity to suppress mitogen-stimulated proliferation of a mixed lymph node leukocyte preparation (67). Interestingly, the effects on proliferation were reversed by treatment with insulin. These findings indicate that the secreted products of adipose tissue near lymph nodes have immunosuppressive capacity, however the mechanism behind this is not known. These studies used intact adipose tissue explants, so it is unclear whether secreted products of adipocytes or immune cells were having the biological effects.
In addition to lymph nodes, adipose tissue can contain other structures including fat –associated lymphoid structures (FALCs) and milky spots. The function of these structures has not been determined, but they have characteristics of secondary lymphoid organs and are able to support both B and T-cell responses to antigens in the absence of conventional lymphoid organs (68). Interestingly, tertiary lymphoid organs have been identified with inflammatory disease including arthritis and atherosclerosis, and seem to arise during chronic inflammatory states (69). Whether these adipose tissue lymphoid structures are tertiary lymphoid structures has not been determined, but they do allow the coupling of innate and adaptive immune response (68). Interestingly, adipose tissue FALCs have been shown to harbor unique Lin−cKIT+SCA-1+ cells which have increased expression of the Th2 cytokines IL-5 and IL-13(70). Type 2 cytokines have been linked with cold-induced thermogenesis and the beiging of white adipose tissue, however it is unknown whether FALCs contributes to this process (71). Additionally, FALCs have also been shown to be capable of mediating secondary antibody responses and the Lin−cKIT+SCA-1+ cells promote B1 cell proliferation (70). These data imply that FALCs and milky spots provide adipose tissue niches capable of facilitating the adaptive immune response. However, it is not known what the physiological contribution of these adipose tissue structures is to local and/or systemic inflammation, whether they are able to respond to acute stimuli such as cold and if they are a main site for antigen presentation in the adipose tissue depot.
Obesity-associated inflammation and the response to pathogens
Obesity is associated with numerous diseases including: type 2 diabetes, gout, arthritis, atherosclerosis, liver disease, and increased susceptibility to certain pathogens. Both metabolic and inflammatory changes in adipose tissue drive obesity associated pathology. TNF, IL-1β and IL-6 have the ability to impair insulin action and glucose uptake in peripheral tissue. Although elevation in systemic IL-6 concentrations is commonly seen, changes in systemic IL-1β and TNF are harder to detect because of the low circulating amounts of these cytokines. Locally, these cytokines in adipose tissue have the ability to modulate adipocyte lipolysis and differentiation. Thus, these cytokines have the ability to limit fat storage in adipose tissue and redistribute this fat to other tissues producing ectopic fat.
Obesity is also associated with impairments in response to pathogens. This is of vital importance because of the concern about pandemic outbreaks of pathogens such as influenza virus. Indeed, during the 2009 worldwide H1N1 influenza pandemic, the obese individuals were at increased risk for morbidity and mortality. Similarly, rodent models indicate that diet-induced obesity increases mortality in response to challenge with influenza (72). Contributing mechanisms appear to be defects in CD8+ T cells including an impaired memory T cell response (73, 74), impaired lung wound healing (75) and increased leptin concentrations (76). Furthermore, new evidence suggests that obese mice infected with influenza develop greater lung inflammation and damage due to dysfunctional regulatory T cell response and alterations in cytotoxic CD8+ T cells (73, 77). Obesity has been shown to cause central defects in T cell development that are associated with reduced production of naïve T cells by the thymus, which leads to reduced T cell receptor diversity, a mechanism associated with reduced immune surveillance (62). In addition, the response to vaccines is also impaired by obesity, as is heterologous immunity (78), thus limiting the protection provided by vaccines. With a growing obese population worldwide, developing therapeutic strategies to limit obesity-related influenza morbidity and mortality is needed.
Conclusions
There is a growing literature that demonstrates adipose tissue has several features of an immunological organ. The leukocyte subsets in adipose tissue have been shown to play important role in restoration of homeostasis as well as in pathogenesis of several obesity-associated chronic diseases that stem from inflammation. Whether adipose tissue participates in classical immunological function such as host defense against pathogens is not known. Furthermore, if obesity associated expansion of immune cell populations in adipose tissue contribute to systemic disturbances in immune-surveillance that are linked to risk of certain infections and cancers in obese individuals is also unclear. Yet, initial studies in the Pima Indians led to the hypothesis that insulin-resistance is an adaptive mechanism to control weight gain (8). Emerging studies also suggest that certain macrophage subsets can directly control lipid homeostasis and energy expenditure through production of catecholamines (22). Thus, a deeper understanding of how adipose tissue immune cells contribute to maintenance of homeostasis and development of chronic inflammation will be important in understanding mechanisms of obesity associated complications. It is interesting that distinct metabolically healthy and unhealthy individuals occur in both lean and obese populations. For the metabolically unhealthy individuals, it will be important to determine if inflammation influences the development of metabolic disease and also affects their response to pathogens irrespective of body mass. Though there are many drugs currently targeted to improve glucose utilization, many of which have additional anti-inflammatory effects, it is still unclear if drugs specifically targeting inflammatory signaling pathways will reverse existing pathology or provide additional benefits in combination with drugs that regulate classical metabolic pathways. Future research into how energy substrates modify immune cell function, the physiological function of FALCs, and the mechanisms of adipose-immune crosstalk may aid in development of approaches to combat obesity-associated chronic diseases.
Acknowledgments
Research in Dixit Lab is supported in part by the National Institutes of Health (AG043608, AG31797, DK090556 and AI105097). Ryan Grant is supported by Purdue University. We thank Ann Liu for assistance with figure design and creation.
Footnotes
Conflicts of interest: The authors have no conflicts of interest to declare.
Author Contributions: Both authors contributed to the conception, writing and editing of the manuscript.
References
- 1.Masters RK, Reither EN, Powers DA, Yang YC, Burger AE, Link BG. The Impact of Obesity on US Mortality Levels: The Importance of Age and Cohort Factors in Population Estimates. AM J Public Health. 2013;103:1895–1901. doi: 10.2105/AJPH.2013.301379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grant R, Youm YH, Ravussin A, Dixit VD. Quantification of adipose tissue leukocytosis in obesity. Methods Mol Biol. 2013;1040:195–209. doi: 10.1007/978-1-62703-523-1_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sims EA. Are there persons who are obese, but metabolically healthy? Metabolism. 2001;50:1499–1504. doi: 10.1053/meta.2001.27213. [DOI] [PubMed] [Google Scholar]
- 5.Phillips CM, Perry IJ. Does Inflammation Determine Metabolic Health Status in Obese and Nonobese Adults? J Clin Endocrinol Metab. 2013;98:E1610–1619. doi: 10.1210/jc.2013-2038. [DOI] [PubMed] [Google Scholar]
- 6.Perez-Martinez P, Alcala-Diaz J, Delgado-Lista J, Garcia-Rios A, Gomez-Delgado F, Marin-Hinojosa C, et al. Metabolical phenotypes of obesity influence triglyceride and inflammation homeostasis. Eur J Clin Invest. 2014;44:1053–1064. doi: 10.1111/eci.12339. [DOI] [PubMed] [Google Scholar]
- 7.Lackey DE, Burk DH, Ali MR, Mostaedi R, Smith WH, Park J, et al. Contributions of adipose tissue architectural and tensile properties toward defining healthy and unhealthy obesity. Am J Physiol Endocrinol Metab. 2014;306:E233–246. doi: 10.1152/ajpendo.00476.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Swinburn BA, Nyomba BL, Saad MF, Zurlo F, Raz I, Knowler WC, et al. Insulin resistance associated with lower rates of weight gain in Pima Indians. J Clin Invest. 1991;88:168–173. doi: 10.1172/JCI115274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zurlo F, Lillioja S, Esposito-Del Puente A, Nyomba BL, Raz I, Saad MF, et al. Low ratio of fat to carbohydrate oxidation as predictor of weight gain: study of 24-h RQ. Am J Physiol. 1990;259:E650–657. doi: 10.1152/ajpendo.1990.259.5.E650. [DOI] [PubMed] [Google Scholar]
- 10.Marra M, Scalfi L, Covino A, Esposito-Del Puente A, Contaldo F. Fasting respiratory quotient as a predictor of weight changes in non-obese women. Int J Obes Relat Metab Disord. 1998;22:601–603. doi: 10.1038/sj.ijo.0800612. [DOI] [PubMed] [Google Scholar]
- 11.Shi LZ, Wang R, Huang G, Vogel P, Neale G, Green DR, et al. HIF1alpha-dependent glycolytic pathway orchestrates a metabolic checkpoint for the differentiation of TH17 and Treg cells. J Exp Med. 2011;208:1367–1376. doi: 10.1084/jem.20110278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tannahill GM, Curtis AM, Adamik J, Palsson-McDermott EM, McGettrick AF, Goel G, et al. Succinate is an inflammatory signal that induces IL-1beta through HIF-1alpha. Nature. 2013;496:238–242. doi: 10.1038/nature11986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Winer DA, Winer S, Shen L, Wadia PP, Yantha J, Paltser G, et al. B cells promote insulin resistance through modulation of T cells and production of pathogenic IgG antibodies. Nat Med. 2011;17:610–617. doi: 10.1038/nm.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nishimura S, Manabe I, Nagasaki M, Eto K, Yamashita H, Ohsugi M, et al. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med. 2009;15:914–920. doi: 10.1038/nm.1964. [DOI] [PubMed] [Google Scholar]
- 17.Elgazar-Carmon V, Rudich A, Hadad N, Levy R. Neutrophils transiently infiltrate intra-abdominal fat early in the course of high-fat feeding. J Lipid Res. 2008;49:1894–1903. doi: 10.1194/jlr.M800132-JLR200. [DOI] [PubMed] [Google Scholar]
- 18.Talukdar S, Oh da Y, Bandyopadhyay G, Li D, Xu J, McNelis J, et al. Neutrophils mediate insulin resistance in mice fed a high-fat diet through secreted elastase. Nat Med. 2012;18:1407–1412. doi: 10.1038/nm.2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu D, Molofsky AB, Liang HE, Ricardo-Gonzalez RR, Jouihan HA, Bando JK, et al. Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science. 2011;332:243–247. doi: 10.1126/science.1201475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hellman B, Larsson S, Westman S. Mast cell content and fatty acid metabolism in the epididymal fat pad of obese mice. Acta Physiol Scand. 1963;58:255–262. doi: 10.1111/j.1748-1716.1963.tb02647.x. [DOI] [PubMed] [Google Scholar]
- 21.Liu J, Divoux A, Sun J, Zhang J, Clement K, Glickman JN, et al. Genetic deficiency and pharmacological stabilization of mast cells reduce diet-induced obesity and diabetes in mice. Nat Med. 2009;15:940–945. doi: 10.1038/nm.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nguyen KD, Qiu Y, Cui X, Goh YP, Mwangi J, David T, et al. Alternatively activated macrophages produce catecholamines to sustain adaptive thermogenesis. Nature. 2011;480:104–108. doi: 10.1038/nature10653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kosteli A, Sugaru E, Haemmerle G, Martin JF, Lei J, Zechner R, et al. Weight loss and lipolysis promote a dynamic immune response in murine adipose tissue. J Clin Invest. 2010;120:3466–3479. doi: 10.1172/JCI42845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117:175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feuerer M, Herrero L, Cipolletta D, Naaz A, Wong J, Nayer A, et al. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat Med. 2009;15:930–939. doi: 10.1038/nm.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arner E, Westermark PO, Spalding KL, Britton T, Ryden M, Frisen J, et al. Adipocyte turnover: relevance to human adipose tissue morphology. Diabetes. 2010;59:105–109. doi: 10.2337/db09-0942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hardy OT, Perugini RA, Nicoloro SM, Gallagher-Dorval K, Puri V, Straubhaar J, et al. Body mass index-independent inflammation in omental adipose tissue associated with insulin resistance in morbid obesity. Surg Obes Relat Dis. 2011;7:60–67. doi: 10.1016/j.soard.2010.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khan T, Muise ES, Iyengar P, Wang ZV, Chandalia M, Abate N, et al. Metabolic dysregulation and adipose tissue fibrosis: role of collagen VI. Mol Cell Biol. 2009;29:1575–1591. doi: 10.1128/MCB.01300-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Strissel KJ, Stancheva Z, Miyoshi H, Perfield JW, 2nd, DeFuria J, Jick Z, et al. Adipocyte death, adipose tissue remodeling, and obesity complications. Diabetes. 2007;56:2910–2918. doi: 10.2337/db07-0767. [DOI] [PubMed] [Google Scholar]
- 30.Giordano A, Murano I, Mondini E, Perugini J, Smorlesi A, Severi I, et al. Obese adipocytes show ultrastructural features of stressed cells and die of pyroptosis. J Lipid Res. 2013;54:2423–2436. doi: 10.1194/jlr.M038638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wen H, Gris D, Lei Y, Jha S, Zhang L, Huang MT, et al. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat Immunol. 2011;12:408–415. doi: 10.1038/ni.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou R, Tardivel A, Thorens B, Choi I, Tschopp J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat Immunol. 2010;11:136–140. doi: 10.1038/ni.1831. [DOI] [PubMed] [Google Scholar]
- 33.Mariathasan S, Weiss DS, Newton K, McBride J, O’Rourke K, Roose-Girma M, et al. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440:228–232. doi: 10.1038/nature04515. [DOI] [PubMed] [Google Scholar]
- 34.Vandanmagsar B, Youm YH, Ravussin A, Galgani JE, Stadler K, Mynatt RL, et al. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med. 2011;17:179–188. doi: 10.1038/nm.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464:1357–1361. doi: 10.1038/nature08938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Youm YH, Kanneganti TD, Vandanmagsar B, Zhu X, Ravussin A, Adijiang A, et al. The Nlrp3 inflammasome promotes age-related thymic demise and immunosenescence. Cell Rep. 2012;1:56–68. doi: 10.1016/j.celrep.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tsushima Y, Nishizawa H, Tochino Y, Nakatsuji H, Sekimoto R, Nagao H, et al. Uric acid secretion from adipose tissue and its increase in obesity. J Biol Chem. 2013;288:27138–27149. doi: 10.1074/jbc.M113.485094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ebke LA, Nestor-Kalinoski AL, Slotterbeck BD, Al-Dieri AG, Ghosh-Lester S, Russo L, et al. Tight association between macrophages and adipocytes in obesity: implications for adipocyte preparation. Obesity (Silver Spring) 2014;22:1246–1255. doi: 10.1002/oby.20634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kopp A, Buechler C, Neumeier M, Weigert J, Aslanidis C, Scholmerich J, et al. Innate immunity and adipocyte function: ligand-specific activation of multiple Toll-like receptors modulates cytokine, adipokine, and chemokine secretion in adipocytes. Obesity (Silver Spring) 2009;17:648–656. doi: 10.1038/oby.2008.607. [DOI] [PubMed] [Google Scholar]
- 40.Pal D, Dasgupta S, Kundu R, Maitra S, Das G, Mukhopadhyay S, et al. Fetuin-A acts as an endogenous ligand of TLR4 to promote lipid-induced insulin resistance. Nat Med. 2012;18:1279–1285. doi: 10.1038/nm.2851. [DOI] [PubMed] [Google Scholar]
- 41.Nguyen MT, Favelyukis S, Nguyen AK, Reichart D, Scott PA, Jenn A, et al. A subpopulation of macrophages infiltrates hypertrophic adipose tissue and is activated by free fatty acids via Toll-like receptors 2 and 4 and JNK-dependent pathways. J Biol Chem. 2007;282:35279–35292. doi: 10.1074/jbc.M706762200. [DOI] [PubMed] [Google Scholar]
- 42.Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006;116:3015–3025. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140:821–832. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 44.Heneka MT, Kummer MP, Stutz A, Delekate A, Schwartz S, Vieira-Saecker A, et al. NLRP3 is activated in Alzheimer’s disease and contributes to pathology in APP/PS1 mice. Nature. 2013;493:674–678. doi: 10.1038/nature11729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Halle A, Hornung V, Petzold GC, Stewart CR, Monks BG, Reinheckel T, et al. The NALP3 inflammasome is involved in the innate immune response to amyloid-beta. Nat Immunol. 2008;9:857–865. doi: 10.1038/ni.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Busso N, So A. Mechanisms of inflammation in gout. Arthritis Res Ther. 2010;12:206. doi: 10.1186/ar2952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Franklin BS, Bossaller L, De Nardo D, Ratter JM, Stutz A, Engels G, et al. The adaptor ASC has extracellular and ‘prionoid’ activities that propagate inflammation. Nat Immunol. 2014;15:727–737. doi: 10.1038/ni.2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Baroja-Mazo A, Martin-Sanchez F, Gomez AI, Martinez CM, Amores-Iniesta J, Compan V, et al. The NLRP3 inflammasome is released as a particulate danger signal that amplifies the inflammatory response. Nat Immunol. 2014;15:738–748. doi: 10.1038/ni.2919. [DOI] [PubMed] [Google Scholar]
- 49.Munoz-Planillo R, Kuffa P, Martinez-Colon G, Smith BL, Rajendiran TM, Nunez G. K(+) efflux is the common trigger of NLRP3 inflammasome activation by bacterial toxins and particulate matter. Immunity. 2013;38:1142–1153. doi: 10.1016/j.immuni.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sheedy FJ, Grebe A, Rayner KJ, Kalantari P, Ramkhelawon B, Carpenter SB, et al. CD36 coordinates NLRP3 inflammasome activation by facilitating intracellular nucleation of soluble ligands into particulate ligands in sterile inflammation. Nat Immunol. 2013;14:812–820. doi: 10.1038/ni.2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yan Y, Jiang W, Spinetti T, Tardivel A, Castillo R, Bourquin C, et al. Omega-3 fatty acids prevent inflammation and metabolic disorder through inhibition of NLRP3 inflammasome activation. Immunity. 2013;38:1154–1163. doi: 10.1016/j.immuni.2013.05.015. [DOI] [PubMed] [Google Scholar]
- 52.Stienstra R, van Diepen JA, Tack CJ, Zaki MH, van de Veerdonk FL, Perera D, et al. Inflammasome is a central player in the induction of obesity and insulin resistance. Proc Natl Acad Sci U S A. 2011;108:15324–15329. doi: 10.1073/pnas.1100255108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee HM, Kim JJ, Kim HJ, Shong M, Ku BJ, Jo EK. Upregulated NLRP3 inflammasome activation in patients with type 2 diabetes. Diabetes. 2013;62:194–204. doi: 10.2337/db12-0420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Henao-Mejia J, Elinav E, Jin C, Hao L, Mehal WZ, Strowig T, et al. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature. 2012;482:179–185. doi: 10.1038/nature10809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vats D, Mukundan L, Odegaard JI, Zhang L, Smith KL, Morel CR, et al. Oxidative metabolism and PGC-1beta attenuate macrophage-mediated inflammation. Cell Metab. 2006;4:13–24. doi: 10.1016/j.cmet.2006.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kovacikova M, Sengenes C, Kovacova Z, Siklova-Vitkova M, Klimcakova E, Polak J, et al. Dietary intervention-induced weight loss decreases macrophage content in adipose tissue of obese women. Int J Obes (Lond) 2011;35:91–98. doi: 10.1038/ijo.2010.112. [DOI] [PubMed] [Google Scholar]
- 57.Cancello R, Henegar C, Viguerie N, Taleb S, Poitou C, Rouault C, et al. Reduction of macrophage infiltration and chemoattractant gene expression changes in white adipose tissue of morbidly obese subjects after surgery-induced weight loss. Diabetes. 2005;54:2277–2286. doi: 10.2337/diabetes.54.8.2277. [DOI] [PubMed] [Google Scholar]
- 58.Morris DL, Cho KW, Delproposto JL, Oatmen KE, Geletka LM, Martinez-Santibanez G, et al. Adipose tissue macrophages function as antigen-presenting cells and regulate adipose tissue CD4+ T cells in mice. Diabetes. 2013;62:2762–2772. doi: 10.2337/db12-1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Deng T, Lyon CJ, Minze LJ, Lin J, Zou J, Liu JZ, et al. Class II major histocompatibility complex plays an essential role in obesity-induced adipose inflammation. Cell Metab. 2013;17:411–422. doi: 10.1016/j.cmet.2013.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cipolletta D, Feuerer M, Li A, Kamei N, Lee J, Shoelson SE, et al. PPAR-gamma is a major driver of the accumulation and phenotype of adipose tissue Treg cells. Nature. 2012;486:549–553. doi: 10.1038/nature11132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lira FS, Rosa JC, Pimentel GD, Seelaender M, Damaso AR, Oyama LM, et al. Both adiponectin and interleukin-10 inhibit LPS-induced activation of the NF-kappaB pathway in 3T3-L1 adipocytes. Cytokine. 2012;57:98–106. doi: 10.1016/j.cyto.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 62.Yang H, Youm YH, Vandanmagsar B, Ravussin A, Gimble JM, Greenway F, et al. Obesity increases the production of proinflammatory mediators from adipose tissue T cells and compromises TCR repertoire diversity: implications for systemic inflammation and insulin resistance. J Immunol. 2010;185:1836–1845. doi: 10.4049/jimmunol.1000021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Priceman SJ, Kujawski M, Shen S, Cherryholmes GA, Lee H, Zhang C, et al. Regulation of adipose tissue T cell subsets by Stat3 is crucial for diet-induced obesity and insulin resistance. Proc Natl Acad Sci U S A. 2013;110:13079–13084. doi: 10.1073/pnas.1311557110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Guarda G, Zenger M, Yazdi AS, Schroder K, Ferrero I, Menu P, et al. Differential expression of NLRP3 among hematopoietic cells. J Immunol. 2011;186:2529–2534. doi: 10.4049/jimmunol.1002720. [DOI] [PubMed] [Google Scholar]
- 65.Defuria J, Belkina AC, Jagannathan-Bogdan M, Snyder-Cappione J, Carr JD, Nersesova YR, et al. B cells promote inflammation in obesity and type 2 diabetes through regulation of T-cell function and an inflammatory cytokine profile. Proc Natl Acad Sci U S A. 2013;110:5133–5138. doi: 10.1073/pnas.1215840110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nishimura S, Manabe I, Takaki S, Nagasaki M, Otsu M, Yamashita H, et al. Adipose Natural Regulatory B Cells Negatively Control Adipose Tissue Inflammation. Cell Metab. 2013;18:759–766. doi: 10.1016/j.cmet.2013.09.017. [DOI] [PubMed] [Google Scholar]
- 67.Pond CM, Mattacks CA. Interactions between adipose tissue around lymph nodes and lymphoid cells in vitro. J Lipid Res. 1995;36:2219–2231. [PubMed] [Google Scholar]
- 68.Rangel-Moreno J, Moyron-Quiroz JE, Carragher DM, Kusser K, Hartson L, Moquin A, et al. Omental milky spots develop in the absence of lymphoid tissue-inducer cells and support B and T cell responses to peritoneal antigens. Immunity. 2009;30:731–743. doi: 10.1016/j.immuni.2009.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mohanta SK, Yin C, Peng L, Srikakulapu P, Bontha V, Hu D, et al. Artery tertiary lymphoid organs contribute to innate and adaptive immune responses in advanced mouse atherosclerosis. Circ Res. 2014;114:1772–1787. doi: 10.1161/CIRCRESAHA.114.301137. [DOI] [PubMed] [Google Scholar]
- 70.Moro K, Yamada T, Tanabe M, Takeuchi T, Ikawa T, Kawamoto H, et al. Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature. 2010;463:540–544. doi: 10.1038/nature08636. [DOI] [PubMed] [Google Scholar]
- 71.Qiu YF, Nguyen KD, Odegaard JI, Cui XJ, Tian XY, Locksley RM, et al. Eosinophils and Type 2 Cytokine Signaling in Macrophages Orchestrate Development of Functional Beige Fat. Cell. 2014;157:1292–1308. doi: 10.1016/j.cell.2014.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Smith AG, Sheridan PA, Harp JB, Beck MA. Diet-induced obese mice have increased mortality and altered immune responses when infected with influenza virus. J Nutr. 2007;137:1236–1243. doi: 10.1093/jn/137.5.1236. [DOI] [PubMed] [Google Scholar]
- 73.Karlsson EA, Sheridan PA, Beck MA. Diet-induced obesity in mice reduces the maintenance of influenza-specific CD8+ memory T cells. J Nutr. 2010;140:1691–1697. doi: 10.3945/jn.110.123653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Karlsson EA, Sheridan PA, Beck MA. Diet-induced obesity impairs the T cell memory response to influenza virus infection. J Immunol. 2010;184:3127–3133. doi: 10.4049/jimmunol.0903220. [DOI] [PubMed] [Google Scholar]
- 75.O’Brien KB, Vogel P, Duan S, Govorkova EA, Webby RJ, McCullers JA, et al. Impaired wound healing predisposes obese mice to severe influenza virus infection. J Infect Dis. 2012;205:252–261. doi: 10.1093/infdis/jir729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang AJ, To KK, Li C, Lau CC, Poon VK, Chan CC, et al. Leptin mediates the pathogenesis of severe 2009 pandemic influenza A(H1N1) infection associated with cytokine dysregulation in mice with diet-induced obesity. J Infect Dis. 2013;207:1270–1280. doi: 10.1093/infdis/jit031. [DOI] [PubMed] [Google Scholar]
- 77.Kanneganti TD, Dixit VD. Immunological complications of obesity. Nat Immunol. 2012;13:707–712. doi: 10.1038/ni.2343. [DOI] [PubMed] [Google Scholar]
- 78.Milner JJ, Sheridan PA, Karlsson EA, Schultz-Cherry S, Shi Q, Beck MA. Diet-Induced Obese Mice Exhibit Altered Heterologous Immunity during a Secondary 2009 Pandemic H1N1 Infection. J Immunol. 2013;191:2474–2485. doi: 10.4049/jimmunol.1202429. [DOI] [PMC free article] [PubMed] [Google Scholar]


