Abstract
Regular exercise has multi-system anti-aging effects. Here we summarize how exercise impacts the major hallmarks of aging. We propose that, besides searching for novel pharmaceutical targets of the aging process, more research efforts should be devoted to gaining insights into the molecular mediators of the benefits of exercise and to implement effective exercise interventions for elderly people.
Introduction
The number of people aged ≥60 years worldwide is expected to nearly triple by 2050, with the “oldest old” group (≥85 years) being the most rapidly expanding segment. A growing challenge is to maintain elderly people independently until end of life. In them, functional independence is directly dependent on physical fitness, i.e. “the ability to carry out daily tasks with vigor and alertness, without undue fatigue and with ample energy to enjoy [leisure] pursuits and to meet unforeseen emergencies.”1 In turn, physical fitness is determined by several measurable health-related phenotypes, including mainly cardiorespiratory fitness and muscle function.1 Among the physiological changes associated with aging, those affecting the cardiorespiratory and vascular system and skeletal muscles most affect physical fitness, whereas exercise can attenuate multi-system age declines, as explained below.
Summary of the Impact of Aging on the Main Physical Fitness—Related Phenotypes
Aging and cardiorespiratory fitness
Maximal oxygen uptake (VO2max; sometimes referred to as maximal aerobic capacity or simply aerobic capacity or aerobic endurance) is a main indicator of cardiorespiratory fitness. There is variability among studies,2–4 but the average rate of VO2max decline in old people is ≥4–5 mL·kg−1·min−1/decade.5 Mostly reduced maximal cardiac output6–10 but also a decline in maximal arteriovenous oxygen difference (a-vO2 diff) (minus ∼3%/decade)7,11 contribute to age reductions in VO2max.12,13 Aging skeletal muscles have a low capacity to use O2 due to several factors, such as decreased muscle mass (see below),14,15 increased peripheral resistance,16 reduced muscle capillary density,17 endothelial dysfunction,18 changes in skeletal muscle microcirculation,19 and reduced muscle oxidative capacity.20
Aging and muscle function
Muscle mass usually starts to decline after 25–30 years of age,21,22 such that on average 40% of muscle mass is lost by 80 years.22,23 In turn, a quantitative loss in muscle cross-sectional area is a major contributor to the decrease in muscle strength seen with advancing age, i.e., after 60–70 years of age.24 The term “sarcopenia” was originally created to refer to age-related loss of muscle mass with consequent loss of strength.25 There are now four international definitions of sarcopenia.26–29 In essence they all agree, requiring a measure of impaired walking capability (either low gait speed or a limited endurance [distance] in a 6-min walk), together with an appendicular lean mass of less than 2 standard deviations of a sex- and ethnically-corrected normal level for individuals 20–30 years old. Sarcopenia (see below for details on signaling pathways involved) occurs due to several age-related factors, such as gradual muscle denervation,23,30 diminished satellite cells,31 low muscle protein synthesis,32 low anabolic hormone levels,33 malnutrition,34 increased pro-inflammatory cytokines,35 oxidative stress,36 mitochondrial dysfunction,37–40 and physical inactivity.41 Although there are differences between studies, on average, 5%–13% and 11%–50% of people aged 60–70 years and ≥80 years, respectively, suffer sarcopenia,42–47 with a higher prevalence (68%) reported in nursing home residents ≥70 years.48 Sarcopenia needs to be differentiated from “cachexia,” a combination of both muscle and fat loss that is usually attributable to an excess of catabolic cytokines associated with a disease process, e.g., cancer.49–51 Sarcopenia is linked with increased disability, falls, hospitalization, nursing home admission, and mortality.48,52–54
Frailty and disability
A consequence of the aforementioned effects of aging on cardiorespiratory and muscle fitness (especially sarcopenia), alone or in combination with co-morbidities such as neurodegeneration, is the “frailty syndrome.”55 Although no single operational definition of the frailty syndrome has been agreed upon, two major definitions with proposed assessment tools have predominated over the past decade—the frailty phenotype, also known as Fried's definition,56 and the frailty index.57 The most widely cited is the frailty phenotype, which is operationalized as a syndrome meeting three or more of the following five phenotypic criteria: Weakness as measured by low grip strength, slowness (low walking speed), low level of physical activity, low energy or self-reported exhaustion, and unintentional weight loss.56 A prefrail stage, in which one or two criteria are present, identifies a subset at high risk of progressing to frailty. Older individuals with none of the above five criteria are classified as nonfrail. The frailty index was developed on the basis of a comprehensive geriatric assessment by counting the number of deficits accumulated, including diseases, physical and cognitive impairments, psychosocial risk factors, and common geriatric syndromes other than frailty.57,58 Whereas the frailty index may have clinical utility in risk assessment and stratification, it is less clear that it adds significant value to comprehensive geriatric assessment.59
Frailty is an important and growing problem in aging western populations, because it potentially affects 20%–30% of adults older than 75 years.60 For instance, the prevalence of prefrail and frail individuals is high in the Spanish population (41.8% and 8.4%, respectively) and increases with age, according to a recent a population-based study conducted on 2488 individuals aged 65 years and older in a central area of the country.61 On the other hand, frailty could eventually result in disability,62, i.e., “difficulty or dependency in carrying out activities necessary for independent living, including roles, tasks needed for self-care and household chores, and other activities important for a person's quality of life.”63
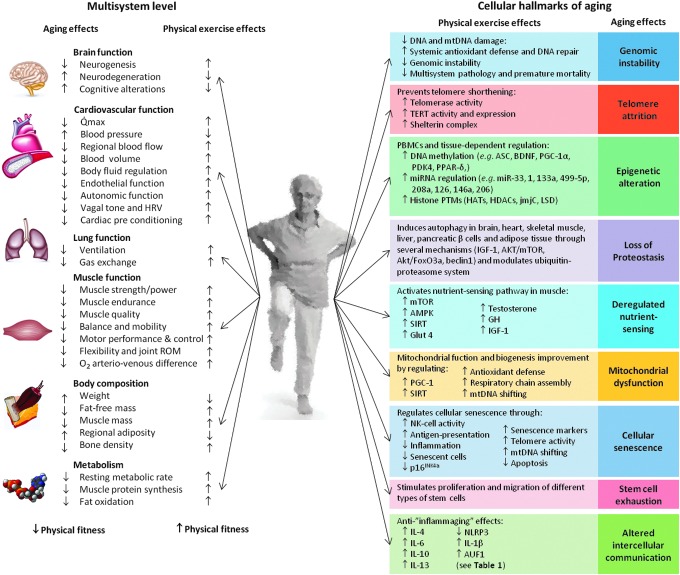
For the above-mentioned reasons, it is of paramount medical importance to attenuate age-related declines in physical fitness. Yet no single drug can reverse the age-related loss of physical fitness because none benefits all of the systems involved, whereas regular exercise has multi-system anti-aging effects (see below and Fig. 1, left column for a summary).
FIG. 1.
Summary of the main anti-aging effects of regular exercise vs. aging effects at the multi-systemic (left; see Chodzko-Zajko et al.64 for an in-depth review) and cellular level (right; see text). AKT, protein kinase B; AMPK, AMP-activated protein kinase; ASC, apoptosis-associated speck-like protein caspase; AUF1, AU-binding factor 1; BDNF, brain-derived neurotrophic factor; FoxO3a, human protein encoded by the FOXO3 gene; Glut 4, glucose transporter type 4; HATs, histone acetyltransferases; HDACs, histone deacetylases; HRV, heart rate variability; IGF-1, insulin-like growth factor 1; IL, interleukin; jmjC, jumonji C proteins; LSD, lysine-specific histone demethylase; miR, micro-RNA; mtDNA, mitochondrial DNA; mTOR, mammalian target of rapamycin; NK cell, natural killer cell; NLRP3, NOD-like receptor protein 3; PBMCs, peripheral blood mononucleated cells; PDK4, pyruvate dehydrogenase kinase isoenzyme 4; PGC-1, peroxisome proliferator-activated receptor gamma coactivator 1; PPAR-δ, peroxisome proliferator-activated receptor δ; PTMs, post-translational modifications; Qmax, maximal cardiac output; ROM, range of motion; SIRT, sirtuin; TERT, human telomerase reverse transcriptase. Color images available online at www.liebertpub.com/rej
Exercise Attenuates Fitness and Multisystem Age-Related Declines
Benefits of exercise (particularly aerobic exercise) in cardiorespiratory fitness/cardiovascular disease
Regular exercise, particularly dynamic exercise of moderate intensity (≤70% of VO2max or ≤80% of maximum heart rate) involving mostly the aerobic energy pathway and large muscle mass (e.g., brisk walking, bicycling) attenuates age declines in cardiorespiratory fitness (see Chodzko-Zajko et al.64 for an in-depth review and experts' recommendations). This type of exercise, commonly referred to as “aerobic exercise” (or “endurance exercise”), has a restoring effect on an important risk factor of cardiovascular disease (CVD), i.e., endothelial dysfunction.65–67 It also increases endothelial nitric oxide (NO•) production and thus vascular tone regulation. Regular bouts of exercise-increased laminar flow activate (through phosphorylation via protein kinase B, Akt) endothelial NO• synthase while attenuating NO• degradation into reactive oxygen species (ROS) and reactive nitrogen species.68 Together with increased angiogenesis (see further below), an additional benefit of aerobic exercise in endothelial health is stimulation of macrophage-mediated reverse cholesterol transport through activation of peroxisome proliferator-activated receptor gamma (PPARγ).69,70 Yet aging autonomic dysfunction has a synergistic effect together with endothelial dysfunction in increasing CVD risk71 and raises the risk of a leading cause of death in most industrialized countries, sudden death due to ventricular fibrillation.72
During aging, the sympathetic nervous system (SNS) outflow to several peripheral tissues increases to stimulate thermogenesis and thus to prevent increasing adiposity.73 Chronically activated SNS has deleterious effects on the cardiovascular system, i.e., reduced leg blood flow, increased arterial blood pressure, impaired baroreflex function and hypertrophy of large arteries; it can also increase insulin resistance, thereby raising the risk of metabolic syndrome.74,75 Importantly, aerobic exercise training (e.g., brisk walking) has a beneficial, dose–response effect in attenuating aging autonomic system dysfunction, with trained elderly individuals showing similar baroreflex function compared with their moderately active younger peers.76 Heart rate variability (HRV), a marker of autonomic function and a powerful predictor of CVD outcome (high HRV is associated with a better prognosis), increases with aerobic exercise training in old people.77 Reduced angiotensin II, increased NO•, and mainly improved vagal modulation and decreased sympathetic tone are implicated in the beneficial exercise effects on HRV.78
Benefits of exercise (particularly resistance exercise) in the aging muscle function
Training programs, especially if including resistance (strength) exercises (i.e., movements, such as weightlifting or exercises with resistance bands, performed against a specific external force that is regularly increased during training) are especially useful for improving muscle mass and/or strength in the elderly,79 including in the oldest old80 (see Table 1 for a summary of controlled exercise interventions [mostly based on resistance exercises] in the oldest old). In general, published resistance exercise interventions in old people had a duration ranging from 4 to 48 weeks and were in agreement with accepted or “traditional” exercise recommendations for older people,81 with the usual weekly schedule including two to three nonconsecutive sessions with one to three sets of 10–15 repetitions of classic weightlifting exercises, such as leg presses.
Table 1.
Summary of Controlled Exercise Interventions (Mostly Based on Resistance Exercises) in the Oldest Old
| Training | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Reference | Group | n (% women) | Mean age (SD or range in years) | Length (weeks) | Type | Frequency (sessions/week) | Sets | Exercises/session (repetitions per (×) exercise) | Intensity/duration | Effects |
| 356 | Exercise | 46 (72%) | 80 (7) | 24 | Low intensities+ | 3 | Flexibility, balance, coordination, movement speed and strength of all major muscle groups | 5–15 min (0–12 weeks) | ↑ Knee extension strength ↑ Fast walking speed ↑ Total free mass |
|
| Strength | 3 | 1–3 | 6–12× knee extension | 65–100% 1RM (12–24 weeks) |
||||||
| 1–3 | 6–12× knee flexion | 65–100% 1RM (12–24 weeks) |
||||||||
| 1–3 | 6–12× seated bench press | 65–100% 1RM (12–24 weeks) |
||||||||
| 1–3 | 6–12× seated row | 65–100% 1RM (12–24 weeks) |
||||||||
| 1–3 | 6–12× leg press | 65–100% 1RM (12–24 weeks) |
||||||||
| 1–3 | 6–12× biceps curl | 65–100% 1RM (12–24 weeks) |
||||||||
| Control | 44 (77%) | 81 (8) | 24 | 3 | Flexibility, balance, coordination, movement speed and strength of all major muscle groups | ↑ Knee extension strength ↑ Fast walking speed ↑ Total free mass (Significantly less pronounced than in the exercise group) |
||||
| 357 | Exercise | 11 (91%) | 88.6 (86–95) | 12 | Strength | 3 | 8 8 |
8x 90° knee flexion 8x 90° knee extension |
50–80% 1RM 50–80% 1RM |
↑ Extensor and flexor muscle strength |
| Control | 12 (92%) | 90.6 (86–95) | 12 | No PA | 2 | Social activities, including group gatherings | ↑ Extensor and flexor muscle strength (Significantly less pronounced than in the exercise group) | |||
| 358 | Exercise | 11 (NA) | 93.4 (3.2) | 12 | Strength+ | 2 | 8–10x upper body (1 exercise: seated bench press) | 40–60% 1RM High velocity |
↑ Quadriceps femoris CSA ↑ Hand grip ↑ Hip flexion strength ↑ Knee extension strength ↑ Upper-body 1RM ↑ Lower-body 1RM ↑ Maximal power at 30% 1RM ↑ Maximal power at 60% 1 RM ↓ Falls incidence |
|
| Balance + Gait + Stretching (cold down) |
8–10x lower body (bilateral leg extension and bilateral knee extension) | 40–60% 1RM High velocity 10 min 5 min |
||||||||
| Control | 13 (NA) | 90.1 (1.1) | 12 | Mobility | 4 | Active and passive movements | 30 min | = Quadriceps femoris CSA ↓ Hand grip strength ↓ Hip flexion strength ↓ Knee extension strength |
||
| 359 | Lifestyle integrated functional exercise | 107 (55%) | 82.8 (4.48) | 48 | Functional exercise | Several | Set of exercise | Ankle strength, knee strength and function effects were significantly better than in control group | ||
| Exercise | 105 (54%) | 84.0 (4.38) | 48 | Strength + Balance |
3 | 6× lower limb strength 7 exercise |
“Hard zone” | Ankle strength, knee strength and function effects were significantly better than in control group | ||
| Control | 105 (55%) | 83.5 (3.81) | 48 | Gentle + | 2 sessions, 1 booster session and 6 follow-up phone calls | Ankle strength, knee strength and function effects were significantly lower than in exercise or functional exercise group | ||||
| Flexibility | e.g., hip rotations | |||||||||
| 360 | Exercise | 15 (NA) | 86.7 (6.1) | 10 | Warm-up+ | 3 | 10 min | ↑ Muscle-strength ↑ BMI ↑ Mean lean body mass (no significant) |
||
| (77–98) | Strength+ | 1 | 10–15× muscle group | Elastic therabands and soft weights | ||||||
| Balance+ | Balls, balance disc, and blocks | 10 min | ||||||||
| Cool-down | 5 min | |||||||||
| Control | 15 (NA) | 86.9 (5.7) (77–94) |
10 | No effect ↓ Mean lean body mass (no significant) |
||||||
| 361 | Exercise | 25 (64%) | 86.2 (1.0) | 10 | Strength | 3 | 3 | 8× lhip extensor | 80% 1RM | ↑ Muscle-strength |
| (72–95) | 3 | 8× knee extensor | 80% 1RM | ↑ Thigh-muscle CSA | ||||||
| ↑ Stair-climbing power | ||||||||||
| Exercise+ | 25 (64%) | 87.2 (1.2) | 10 | Strength+ | 3 | 3 | 8× hip extensor | 80% 1RM | Idem exercise group | |
| Nutritional supplement | (76–98) | 240 mL liquid + CHO (60%) + fat (23%) + soy-based protein (17%) | 3 | 8× knee extensor | 80% 1RM | |||||
| Aerobic or flexibility activities | 3 activities (e.g., walking) |
|||||||||
| Nutritional supplement +Placebo activities |
24 (71%) | 85.7(1.2) (75–97) |
10 | 240 ml liquid + CHO (60%) + fat (23%) + soy-based protein (17%) Aerobic or flexibility activities |
3 | No effect | ||||
| 3 activities (e.g., walking) |
||||||||||
| Placebo activities +Placebo |
26 (54%) | 89.2 (0.8) (78–98) |
10 | Aerobic or flexibility activities | 3 | 3 activities (e.g., walking) |
No effect | |||
| supplement | Minimally nutritive (4 kcal) | |||||||||
| 362 | Exercise + Traditional balance training |
12 (50%) | 81.1 (6.0) (69–95) |
12 | Strength+ | 2 | Larger over extremity muscles (pulley stations) | 10–15 RM | ↑ Strength knee extension ↑ Sitting to standing test ↑ Static Balance |
|
| Large under extremity muscles (leg press and pulley stations) | 10–15 RM | |||||||||
| Step machine | 100 steps level 2 150 steps level 2 100 steps level 3 150 steps level 3 |
|||||||||
| Aerobic + Balance |
Static cycle Standing, one-legged balance and walking on different surfaces (open and closed eyes) |
> 15 min and > 3km | ||||||||
| Exercise + Visual computer feedback |
15 (80%) | 81.5 (7.7) (69–95) |
12 | Strength+ | Larger over extremity muscles (pulley stations) | 10–15 RM | ↑ Strength knee extension ↑ 6 minute walk test ↑ Training-specific performance |
|||
| Large under extremity muscles (leg press and pulley stations) | 10–15 RM | |||||||||
| Step machine | 100 steps level 2 150 steps level 2 100 steps level 3 150 steps level 3 |
|||||||||
| Aerobic+ | Static cycle | > 15 min and > 3km | ||||||||
| Balance | Visual computer feedback | |||||||||
| 363 | Exercise | 31 (NA) | 82.2 (4.1) (75–90) |
12 | Strength | 3 | 3 | 10x knee extensions | 70–90% of maximal | ↑ Leg extension strength ↑ Knee extension strength ↑ Knee flexion strength ↑ Ankle plantar flexion strength ↑ Handgrip strength ↑ Functional motor performance ↑ Balance Improvements persisted during 3-month follow-up with only moderate losses |
| 3 | 10× hip extension | 70–90% of maximal | ||||||||
| 2 | 10× left lifts | 70–90% of maximal | ||||||||
| 2 | 10× right lifts | 70–90% of maximal | ||||||||
| 2 | 15 lifts of bilateral plantar flexion (2–4 cm support) | |||||||||
| Functional-Balance training + Physiotherapy |
2 | Multifactorial activities e.g., massaging or stretching |
45 min per training session 25 min |
|||||||
| Control | 26 (NA) | 82.1 (4.8) (75–90) |
12 | Placebo activities + Physiotherapy |
3 | Motor activities (e.g., flexibility, calisthenics, ball games and memory tasks) | 1 hr | No effect during intervention and follow-up | ||
| 364 | Exercise | 62 (74%) | 82.3 (6.6) | 12 | Strength | 3 | Relevant muscle group | 70–80% 1RM | ↑ Knee extension strength ↑ Knee flexion strength ↑ Ankle flexion strength ↑ Handgrip strength ↑ Functional performance |
|
| Control | 60 (73%) | 82.9 (7.0) | 12 | Placebo activities | 2 | Flexibility exercise, calisthenics, low-intensity training with hand-held weights and ball games | No effect or ↓ | |||
| 365 | Exercise | 15 (80%) | 88.5 (1.0) (85–98) |
12 | Strength | 3 | 1 | Upper and lower extremities | 5–10 min | ↑ 1RM |
| 3 | 8x knee flexion+ | ↑ Isokinetic knee extension | ||||||||
| 8x knee extension | 50% 1RM (0–2 weeks) 80% 1RM (2–12 weeks) |
↑ Isometric knee extension | ||||||||
| Control | 15 (73%) | 90.9 (1.1) (85–98) |
12 | No PA | = 1RM = Isokinetic knee extension = Isometric knee extension |
|||||
| 366 | Exercise in long-term care-high mobility | 22 (NA) | 81.3 (5.3)* | 4 | Warm-up/stretching+ | 5 min | ↑ Mobility ↑ Balance ↑ Flexibility ↑ Knee strength ↑ Hip strength |
|||
| Aerobic + Strength |
Walking | 10–15 min | ||||||||
| Balance Cool-down/stretching |
1–2 1–2 |
2–10× lower body 2–10× upper body |
Soft-weights and Theraband | |||||||
| 10 min 5 min |
||||||||||
| Exercise in long-term care-low mobility | 14 (NA) | 81.3 (5.3)* | 4 | Warm-up/stretching + Aerobic+ |
5 min | ↑ Mobility ↑ Balance ↑ Flexibility ↑ Knee strength ↑ Hip strength |
||||
| Strength | Walking | < 1 min − 15 min | ||||||||
| Balance Cool-down/stretching |
1–2 1–2 |
2–10× lower body 2–10× upper body |
Soft-weights and Theraband 10 min 5 min |
|||||||
| Range of motion | 32 (94%) | 81.3 (5.3)* | 4 | Introductions | 10 min | ↑ Shoulder strength ↓ Hip Strength ↓ Mobility ↓ Functional ability |
||||
| Vocal exercises | 5 min | |||||||||
| Memory games | 5 min | |||||||||
| Range of motions | Fingers, hands, arms, knees, ankles | |||||||||
| Relaxation exercises | 5 min | |||||||||
| 367 | Exercise | 19 (95%) | 91.7 (6.3) | 12 | Aerobic | 5 | Walking | ↑ 10% weekly (maximum 30 min) (Baseline intensity defined by each participant) |
↑ Maximal walk endurance ↑ Maximal walk distance (significant) = Hand-grip strength = BMI |
|
| Control | 12 (100%) | 89.3 (4.4) | 12 | Social control | 1 | 30 min | = Maximal walk endurance ↑ Maximal walk distance (no significant) = Hand-grip strength = BMI |
|||
| 368 | Exercise | 36 (81%) | 83.7 (8) (67–98)* |
24 | Warm-up+ | 2 | 10 min | ↑ Quadriceps strength | ||
| Strength + Cool-down |
Isometric exercises major muscle groups | 25 min (Intensity was graduated by the n° of repetitions and the gravity-resistance) 10 min |
||||||||
| Control | 29 (90%) | 82 (9.6) (67–98)* |
24 | Reminiscence | 2 | Reminiscence therapy | 45 min | ↓ Quadriceps strength | ||
| 369 | Exercise | 21 (90%) | 81.4 (3.4) (75–96)* |
24 | Stretching + Range of movements exercises |
1 | 5–10× each exercise | Support of a chair or work surface |
No significant differences between the groups with regard to changes in any of the outcome variables (grip strength, ‘up & go’ test, ‘sit to stands' test, functional reach, spinal mobility, Barthel index, Philadelphia Geriatric Center Morale Scale) Trend toward improvement in comparison with control group (‘sit to stand ‘tests and ‘up & go’ tests) |
|
| Strength | 5–10× yellow-blue | Therabands | ||||||||
| Exercise | 20 (85%) | 82.9 (4.4) (75–96)* |
24 | Mobility | No significant differences between the groups with regard to changes in any of the outcome variables (grip strength, ‘up & go’ test, ‘sit to stand’ test, Functional reach, Spinal mobility, Barthel Index, Philadelphia Geriatric Center Morale Scale) Trend towards improvement in comparison with control group (‘sit to stand’ and ‘up & go’ tests) |
|||||
| Control | 28 (89%) | 81.9 (4.7) (75–96)* |
24 | Health education | 30 min | No significant differences between the groups with regard to changes in any of the outcome variables (grip strength, ‘up & go’ test, ‘sit to stand’ test, functional reach, spinal mobility, Barthel index, Philadelphia Geriatric Center Morale Scale) | ||||
| 370 | Exercise | 39 (67%) | 82.2 (1.3) | 8 | Strength + Balance + Functional mobility |
3 | Combined progressive exercises | 1 hour | ↑ 6-min walk test | |
| WBV | 38 (68%) | 80 (1.4) | 8 | Vibration + Strength + Balance + Functional mobility |
3 | 5 | Whole body vibration | 1 min 15–30 Hz 2–8 mm peak-to-peak | ↑ 6-min walk test (greater improvements than exercise group) |
|
| 371 | Exercise | 94 (81%) | 87 (8) | 32 | Strength | 5 | Upper body resistance training (arm curls or arm raises) | Implement every 2 hr for a possible total of 4 care episodes per day | ↑ Arm raise ↑ Arm curl ↑ Stands 30 sec |
|
| Aerobic | Walking or wheeling | 75–90% of the maximal distance | ||||||||
| Control | 96 (86%) | 88 (7) | 32 | UCG | ↓ Meter walked or wheeled | |||||
| 92 | Exercise | 20 (80%) | 92 (2) (90–96) |
8 | Mobility exercise + Stretching+ |
2 | 1 | Major muscle groups | 40.45 min | ↑ 1RM leg press ↑ FAC (in participants with FAC > 3) ↓ Falls (1.2 fewer per participant than in the control group) |
| Aerobic + Strength | Rest periods | |||||||||
| 3 3 |
1 2-3 1-8 |
Cycling (cycle ergometer) Leg press exercise 10× biceps curls, arm extension, arm side lifts, shoulder elevations, seated bench presses and calf raises |
10–15 min; RPE:12–13 30–70% 1RM ( ↑ 5%weekly) 1–3 kg or low-medium resistance bands |
|||||||
| Control | 20 (80%) | 92 (2) (90–97) |
8 | UCG | 5 | 1 | Mobility exercises in major muscle groups | 40–45 min | 14 of 18 participants showed ↑ RM leg press | |
| 372 | Exercise | 34 (100%) | 83.5 (4.1) | 10 | Warm-up+ | 2 | 15 min | = Abilities to carry out instrumental activities of daily living | ||
| Strength+ | 1-2 | 8–30× knee extension 8–30× knee flexion 8–30× hip abduction 8–30× hip adduction |
Gradually increased | |||||||
| Functional exercises+ | 2 | 15× exercise (e.g., rising from a chair or elbow flexion using light loads (1–2 kg)) |
30 min | |||||||
| Relaxation | 15 min | |||||||||
| Control | 34 (100%) | 82.6 (3.7) | 10 | Home exercise program | 2–3 | 2 | 15× functional exercises | = Abilities to carry out instrumental activities of daily living | ||
Of note, the mean age of the subjects in all the studies was ≥ 80 years.
Data provided by the total number of participants.
BMI, Body mass index; CHO, carbohydrate; CSA, cross-sectional area; FAC, functional ambulation classification; NA, not available; PA, physical activity; RPE, rating of perceived exertion (Borg's conventional scale, 6–20 points); 1RM, one repetition maximum; SD, standard deviation; SO, social activities; TG, training group; UCG, usual care group; WBV, whole-body vibration.
Other interventions feasible in old people include “high-velocity resistance training,” i.e., focusing on speed of movement,82,83 or even explosive-type heavy-resistance training, e.g., weightlifting exercises with a load equivalent to 75%–80% of one repetition maximum (1RM) and performed with maximal intentional acceleration of the training load during the concentric movement phase.84 In general, besides being feasible and well tolerated even by the oldest old,84 this alternative type of intervention would elicit similar85 or even higher improvements in functional performance and disability compared with more traditional, lower-velocity resistance training.86 Another approach is the use of a weighted vest, which has proved effective to improve perceived health in old people,87 as well as lateral stability, lower-body muscular strength, muscular power, and leg lean mass (and thus likely to reduce risk fall) in postmenopausal women aged 50–75 years.88 Preliminary findings also support the potential effectiveness of including a weighted stair climbing exercise in the training programs of old people.89 Others, however, found no significant benefits of a home-based intervention using a weighted vest.90
Benefits of exercise in the frail elderly
Although numerous studies have shown the benefits of resistance training in old people in general, less research has focused specifically on the frail elderly. This is an important issue because frailty status might influence the potential applicability and effectiveness of the different training programs. For instance, Faber et al. found that fall-preventive, moderate-intensity, group exercise programs (mostly based on walking and balance exercises) had positive effects on fall risk and physical performance in pre-frail but not in frail elderly (mean age 85 years).91
Fiatarone et al. reported that physically frail, oldest old men and women improved their leg muscle strength outcomes by 220% after a 10-week strength training program (three sets of eight repetitions at 80% of 1RM, three times a week).80 Serra-Serra-Rexach et al. found an increment of 17% in the leg press strength of nonagenarians after 8 weeks of progressive strength training with lower loads (two to three sets of eight to 10 repetitions at 30% of 1RM in the initial phase progressing to 70% of 1RM at the end of the intervention).92 Lustosa et al. found significant improvements in the knee extensor muscle power of pre-frail elderly women after 12 weeks of strength exercises of the lower extremities at 70% of 1RM, three times a week.93 Although more research is needed, higher-intensity exercises seem to elicit higher gains. Thus, Sullivan et al.94 found greater increases in the muscle strength of frail elderly after a 12-week resistance training program of progressive intensity (starting at 20% and ending at 80% of 1RM) compared with a continuous, lower intensity protocol (20% of 1RM during the entire 12-week period).
The American College of Sports Medicine recommends a multi-component (strength, endurance, flexibility, and balance) exercise program to maintain physical fitness in old adults.95 Villareal et al. studied the effect of such a multi-component exercise program on physical fitness in frail, obese older adults during 3 months, finding beneficial effects on muscle mass and physical function.96 Lord et al. found significant improvements in choice stepping reaction time test, 6-min walking distance, and simple reaction time requiring a hand press, after training (aerobic exercises, specific strengthening exercises, and activities for balance, hand-eye and foot-eye coordination, and flexibility) during 12 months in frail older people living in retirement villages.97 Eshani et al.98 assessed the effect of a 6-month aerobic training program on frail elderly (consisting of walking at 70%–75% of maximal heart rate during 20 min at the initial phase and progressing to 60 min at the end of the program) and found a 14% of increase in VO2max.
Evidence on the benefits of exercise interventions in elderly with frailty, co-morbidities, and subsequent physical disability comes from a recent meta-analysis by deVries et al.99 This study included data on 18 randomized controlled trials (2,580 participants in total) of physical training interventions in community-dwelling adults aged 60–85 years who were physically frail and/or had physical disability and/or multi-morbidity. Half of the included studies used multi-component training programs, and intervention duration ranged from 5 weeks to 18 months. There were statistically significant benefits with physical exercise therapy compared to no exercise in mobility and physical functioning. There were no differences in effectiveness with regard to the duration of the program (short vs. longer interventions), whereas high-intensity programs elicited greater gains compared to low-intensity ones. The interventions that showed the largest effect sizes were those using resistance training components.
Benefits of exercise (particularly, resistance exercise) in the aging muscle tissue—main signaling pathways involved in exercise adaptations
The molecular mechanisms involved in the activation of signal transduction cascades regulating the adaptations to exercise are dependent on specific signaling pathways activated or repressed by numerous stimuli such as alterations in metabolites concentrations, adenosine triphosphate (ATP)-to-adenosine diphosphate (ADP) ratio, calcium flux, intracellular pH, redox balance, ROS production, and intracellular oxygen pressure.100–103 Post-exercise changes in gene transcription involve immediate early genes, myogenic regulators, genes that regulate carbohydrate metabolism and lipid mobilization, transport and oxidation, mitochondrial metabolism, and oxidative phosphorylation, as well as transcriptional regulators of gene expression and mitochondrial biogenesis,104–107 or alterations in the DNA-binding activity of a variety of transcription factors, such as myocyte enhancer factor 2 (MEF2),107 histone deacetylases (HDACs),108 and nuclear respiratory factors (NRFs).109,110
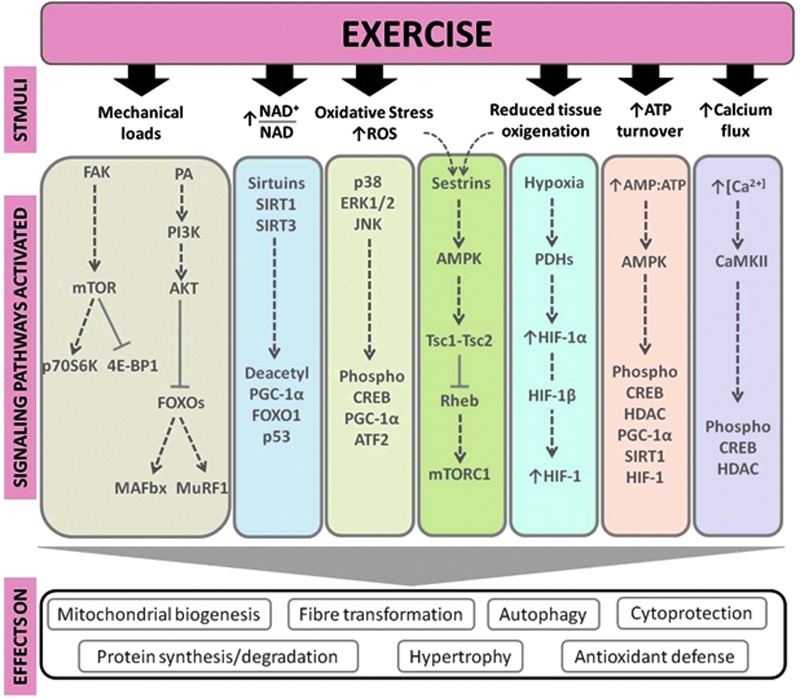
The most relevant signaling pathways modulated by exercise include calcium/calmodulin-dependent protein kinase (CaMKs) signaling, mitogen-activated protein kinases (MAPKs) signaling, ATP turnover and adenosine monophosphate (AMP)-activated protein kinase (AMPK) signaling, oxidized nicotinamide adenine dinucleotide (NAD+)/reduced nicotinamide adenine dinucleotide (NADH) ratio and sirtuins (SIRTs), activation of mammalian target of rapamycin (mTOR), oxygen sensing and the hypoxia-inducible factor 1 (HIF-1), mitochondrial biogenesis pathway, and the PPARγ co-activator-1α (PGC-1α) and −1β(PGC-1β).111 Accordingly, several important adaptations in skeletal muscle, such as mitochondrial biogenesis, anti-oxidant defense, hypertrophy, cytoprotection, and fiber transformation, are regulated primarily by these pathways. Below we summarize the main biological mechanisms and pathways through which exercise may attenuate sarcopenia (see also Fig. 2).
FIG. 2.
Main signaling pathways involved in the exercise effects in the skeletal muscle tissue. 4E-BP1, eukaryotic translation initiation factor 4E (eIF4E) binding protein; AKT, protein kinase B; AMP, adenosine monophosphate; AMPK, AMP activated protein kinase; ATF2, activating transcription factor 2; ATP, adenosine triphosphate; CaMKII, calmodulin-dependent protein kinase II; CREB, cAMP response-element-binding protein; ERK1/2, extracellular signal-regulated kinase 1 and 2; FAK, focal adhesion kinase; FoxO1, human protein encoded by the FOXO gene; FOXOs, Forkhead box-O transcription factors; HDACs, histone deacetylases; HIF, hypoxia-inducible factor; JNK, c-Jun NH2-terminal kinase; MAFbx, or Atrogin-1; MuRF-1, muscle RING-finger protein-1; mTOR, mammalian target of rapamycin; mTORC1, mTOR complex 1; NAD, nicotinamide adenine dinucleotide; PA, phosphatidic acid; p70S6K, ribosomal protein S6K; PDH, prolyl hydroxylase; PGC-1α, peroxisome proliferator-activated receptor-γ coactivator-1α; PI13K, phosphatidylinositol 3-kinase; Rheb, Ras homolog enriched in brain gene; ROS, reactive oxygen species; SIRT, sirtuin; Tsc1, tuberous sclerosis complex 1; Tsc2, tuberous sclerosis complex 2. Color images available online at www.liebertpub.com/rej
Calcium is implicated in the regulation of numerous intracellular proteins, such as protein kinase C, calcineurin, and CaMKs that mediate cellular signal transduction.112 Exercise increases CaMKII phosphorylation in an intensity-dependent manner. CaMKs and calcium signaling affect glucose transport,113 lipid uptake and oxidation,114 and skeletal muscle plasticity.115 In addition, the transcription factors cyclic AMP response element-binding protein (CREB), MEF2, and HDACs are CaMK targets involved in the regulation of skeletal muscle gene expression. Exercise also stimulates MAPK-related pathways, including extracellular signal-regulated kinase 1 and 2 (ERK1/2),116 p38,117 and c-Jun NH2-terminal kinase (JNK).116 For instance, p38 MAPK can stimulate upstream transcription factors of the PGC-1α gene through skeletal muscle contraction.118 Moreover, MAPKs regulate a wide range of physiological processes, such as differentiation, hypertrophy, inflammation, and gene expression.119
The role of ROS in the exercise-induced adaptations of skeletal muscles has been studied extensively, particularly with regard to aerobic exercise.120,121 Contracting skeletal muscles produce ROS, activating MAPK signaling and transcription factor nuclear factor-kappa B (NF-κB), thereby linking signal transduction to transcriptional processes.122 Acute exercise activates JNK signaling in a ROS-dependent manner, as evidenced by attenuated JNK signaling during exercise with infusion of the anti-oxidant N-acetylcysteine.123 Contraction-induced increases in interleukin-6 (IL-6) secretion, an exercise-associated cytokine with potent multi-organ metabolic effects124 (see further below), is JNK dependent,125 which attests to the likely importance of JNK signaling in mediating metabolic adaptations to exercise. AMPK is a serine/threonine kinase that modulates cellular metabolism acutely through phosphorylation of metabolic enzymes126 and, over time, via transcriptional regulation.127,128 Given the rate of ATP turnover during muscle contraction, AMPK acts as a signal transducer for metabolic adaptations by responding to an altered cellular energy status. Overall, AMPK activation preserves ATP by inhibiting both biosynthetic and anabolic pathways, while simultaneously stimulating catabolic pathways to re-establish cellular energy stores.129 Chronic AMPK activation modifies metabolic gene expression and stimulates mitochondrial biogenesis,127 partly via AMPK-induced modulation of the DNA-binding activity of transcription factors, including NRF-1, MEF2, and HDACs.127,130
Sestrins are a recently discovered hallmark of aging sarcopenia. Mammalian cells express sestrins in response to stress including DNA damage, oxidative stress, and hypoxia. Sestrins can inhibit the activity of the mTOR complex 1 (mTORC1) through activation of AMPK.131 Sestrins prevent sarcopenia, insulin resistance, diabetes, and obesity. They also extend life span and health span through activation of AMPK, suppression of mTORC1, and stimulation of autophagic signaling.131 Recently, we proposed a possible role of the AMPK-modulating functions of sestrins in the benefits produced by exercise in older subjects.132
The regulation of the SIRT family of protein deacetylases is NAD+ dependent.133 Both deacetylases SIRT1 and SIRT3 respond to elevations in [NAD+] and the NAD+/NADH ratio. Increased SIRT activity is associated with positive adaptations in skeletal muscle metabolism, including improved mitochondrial function and exercise performance.134,135 Likewise, the adaptive muscle growth consequent to mechanical loads induced by resistance exercise is largely determined by the enhanced skeletal muscle protein synthesis due to the activation of mTOR, ribosomal protein S6K (p70S6K), and downstream targets.136 p70S6K is a major regulator of muscle protein synthesis through pathways of protein translation and ribosome biogenesis involving eukaryotic translation initiation factor 4E (eIF4E), 4E binding protein 1 (4E-BP1), and elongation factor 2 (eEF2). Phosphorylation of 4E-BP1 by mTOR suppresses binding and inhibition of eIF4E by 4E-BP1. Phosphorylation of S6K leads to the phosphorylation of the 40S ribosomal protein S6 (rpS6) and eukaryotic translation initiation factor 4B (eIF4B). Collectively these events lead to the formation of the translation initiation complex and activate protein synthesis inducing cellular hypertrophy.137 Mechanosensory regulation of muscle protein synthesis also involves other signaling proteins, such as focal adhesion kinases (FAK), a class of transmembrane receptors that act as protein tyrosine kinases. FAK proteins are pivotal points for the transmission of contractile force throughout the skeletal muscle structure and a central component of integrin signaling. The grade of expression and activity of FAK in skeletal muscle is loading dependent,138,139 and contraction results in conformational modifications and activation of FAK phosphotransferase,138,140 which can trigger muscle protein synthesis through mTOR-dependent or -independent mechanisms.141
Oxygen sensing is also involved in the adaptations of skeletal muscle fibers to exercise, particularly aerobic exercise. Hypoxia-inducible factor (HIF), a heterodimeric transcription factor composed of two subunits (HIF-1α and HIF-1β), regulates the major signal transduction pathway sensitive to the intracellular partial pressure of oxygen. Activation of HIF-1 by many stimuli, including aerobic exercise, induces transcription of target genes involved in erythropoiesis, angiogenesis, glycolysis, and energy metabolism.105,142,143
Exercise benefits in neurodegeneration—main signaling pathways involved
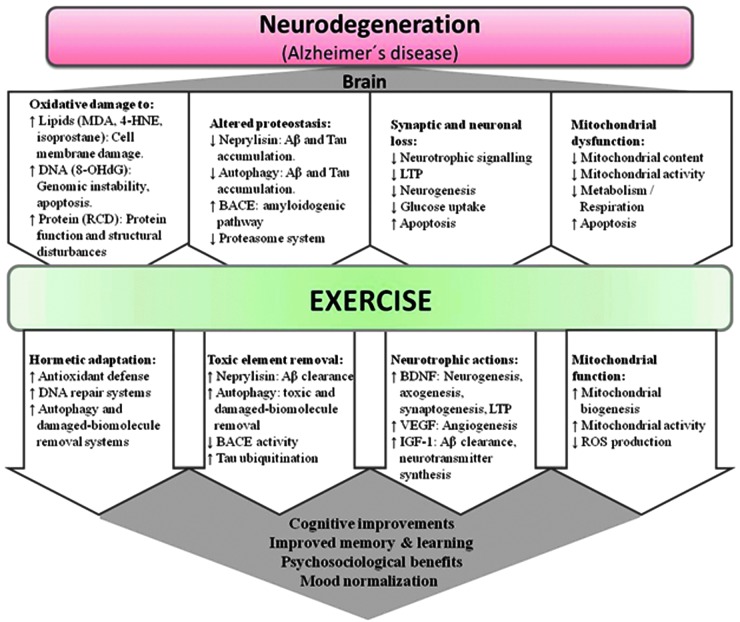
Physical exercise produces important benefits in several neurodegenerative diseases.144,145 Here we focus on the effects of exercise in Alzheimer's disease, which comprises per se 50%–56% of all causes of aging dementia (an additional 13%–17% is caused by Alzheimer's disease combined with vascular diseases).146 Exercise, especially aerobic exercise, is beneficial for patients with Alzheimer's disease,147 not only because it attenuates patients' physical and psychosocial dependence148 but it also because it improves several features of the pathophysiology of this disorder,149,150 including oxidative stress regulation, autophagy systems, neurotrophic signaling, mitochondrial biogenesis, angiogenesis, neurogenesis, and the modulation of specific amyloid-β (Aβ)-degrading enzymes (see Table 2 for a summary of intervention studies in humans and Fig. 3 for a summary of the putative molecular pathways involved).149,150
Table 2.
Summary of Controlled Exercise Interventions in Biomarkers of Neurodegeneration
| Reference | Group | n (Age, years) | Health status | Sex | Effects |
|---|---|---|---|---|---|
| 373 | High-intensity ET | Women: 10 (65.3 ± 9.4 years) Men: 9 (70.9 ± 6.7 years) |
Amnestic mild cognitive impairment | Both | BDNF increased in men but decreased in women. ET group improved cognitive function compared to controls. |
| Control (stretching) | Women: 5 (74.6 ± 11.1 years) Men: 5 (70.6 ± 6.1 years) |
||||
| 374 | High-intensity ET Control (stretching) |
29 (74.3 ± 2.8 years) | Glucose tolerance criteria for pre-diabetes or newly diagnosed | Both | Although BDNF did not change, ET group improved cognitive functions compared to controls. |
| 375 | ET Control |
20 (62 years) 19 (63 years) |
Coronary artery disease | Both | No changes were observed in VEGF concentrations for both groups. |
| 376 | High-intensity ST Moderate-intensity ST Control (stretch) |
62 (65–75 years) | Healthy | Men | IGF-1 increased in both ST groups. |
| 377 | ST (non-frail) ST (pre-frail) |
24 (70.5 ± 4.6 years) 24 (72.5 ± 4.3 years) |
Pre-frail and non- frail | Women | BDNF increased in both groups. No changes in GDNF or NGF. |
| 378 | ET Control (stretching) |
60 (67.6 ± 5.8 years) 60 (65.5 ± 5.4 years) |
Healthy | Both | ET increased the size of the hippocampus and BDNF. |
| 379 | ST | 21 (85.0 ± 4.5y) | Healthy | Women | No changes on VEGF after ST |
| 380 | Combined Control |
10 (66.1 ± 3.1 years) 10 (67.7 ± 5.2 years) |
Obese | Women | VEGF increased after 12 weeks of combined training. |
| 381 | ET ST |
181 (70.3 ± 4.5 years) 167 (71.0 ± 4.5 years) |
Healthy | Women | Only ST increased BDNF |
| 382 | Combined (aerobic and resistance) | 20 (92.3 ± 2.3 years) | Healthy | Both | No changes in BDNF, ACE, APP, EGF, and TNFα |
| Control | 20 (92.1 ± 2.3 years) | ||||
| 383 | Exercise + medical treatment Medical treatment |
20 (69 ± 8 years) 20 (70 ± 11 years) |
Peripheral artery disease | Both | Exercise increased circulating EPC counts and decreased ADMA levels. No changes in VEGF and SDF-1. |
| 384 | Multi-modal (aerobic, strength and motor fitness) | 25 (69.0 ± 3.1 years) | Healthy | Women | Exercise increased BDNF and cognitive performance. |
| Control | 24 (68.8 ± 3.5 years) | ||||
| 385 | ET Control (flexibility, toning and balance) |
30 (67.3 ± 5.8 years) 35 (65.4 ± 5.2 years) |
Healthy | Both | No changes in BDNF, IGF-1, VEGF |
| 386 | Acute exercise ET Control |
18 (62 ± 10 yeasrs) 7 (64 ± 6 years) 6 (56 ± 9) |
intermittent claudication | Both | No change was observed in VEGF concentrations in response to acute exercise and to the training |
ACE, angiotensin-converting enzyme; APP, amyloid precursor protein; DMA, asymmetric dimethylarginine; BDNF, brain-derived neurotrophic factor; EGF, epidermal growth factor; EPC, endothelial progenitor cells; ET, endurance training; GDNF, glial-derived neurotrophic factor; IGF-1, insulin like growth factor 1; NGF, nerve growth factor; SDF-1, stromal cell-derived factor-1; ST, strength training; TNFα, tumor necrosis factor-α; VEGF, vascular endothelial growth factor.
FIG. 3.
Main signaling pathways involved in the exercise effects in neurodegeneration, especially with regard to Alzheimer's disease. 4-HNE, 4-hydroxynonenal; 8-OHdG, 8-hydroxy-2′-deoxyguanosine; Aβ, amyloid-pβ; BACE, β-secretase; BDNF, brain-derived-neurotrophic factor; IGF-1, insulin-like growth factor 1; LTP, long-term potentiation; MDA, malondialdehyde; RCD, reactive carbonyl derivative; ROS, reactive oxygen species; VEGF, vascular endothelial growth factor. Color images available online at www.liebertpub.com/rej
Oxidative stress plays an important role in the etiology of Alzheimer's disease.151–154 The brain is especially sensitive to oxidative stress compared to other organs owing to its high metabolic rate (i.e., high O2 consumption) together with its relatively low anti-oxidant defense capacity and its high levels of polyunsaturated fatty acids and metals.151,155 Elevated levels of brain oxidative stress are found with normal aging,156,157 and this phenomenon is exacerbated in Alzheimer's disease due to additional sources of ROS such as Aβ accumulation and mitochondrial dysfunction.151,153,154,158 Although there is some controversy, aerobic exercise enhances anti-oxidant defense and mitigates oxidative damage in the brain of rodent models.150 Thus, exercise increases glutathione peroxidase (GPx) activity in whole brain,159 as well as superoxide dismutase (SOD) and GPx activity in the brainstem and corpus striatum.160 Using a triple transgenic mouse model of Alzheimer's disease, we recently showed that aerobic exercise training can increase hippocampal catalase mRNA levels.161
Exercise attenuates aging neurodegeneration partly by up-regulating neurotrophic factors, such as the brain-derived neurotrophic factor (BDNF).162,163 Circulating BDNF levels increase with aerobic exercise, especially when intensity is high.164–166 Exercise-produced BDNF can help maintain brain function and promote neuroplasticity167,168 as well as repairing motor neurons.169 Increased BDNF transcripts in exercised rodents' brains are well documented, which provides a biological explanation for the beneficial effect that exercise has in cognitive function, with tropomyosin receptor kinase (trkB), CREB, or synapsin I signaling been involved.170 These pathways are involved in synaptogenesis171 and long-term memory formation.172 Furthermore, the activation of the transcription factor CREB leads to induction of several genes that regulate neurotrophic effects, including those encoding PGC-1α,173 dynorphin, and BDNF.174 In addition, when the exercise induction of the BDNF pathway is blocked, aerobic exercise is unable to activate CREB and stimulate cognition.170,175 The hippocampal regulation of BDNF induced by exercise is mediated by neurotransmitters,167,176 neuroendocrine mechanisms,167 and insulin-like growth factor 1 (IGF-1) modulation.177,178 Thus, hippocampal IGF-1 levels increase with exercise training177 and have neurotrophic effects because IGF-1 activates BDNF signaling177 and increases trkB levels.179 Aerobic exercise induces other neurotrophic factors, such as vascular endothelial growth factor (VEGF),180 nerve growth factor (NGF),181 glial-derived neurotrophic factor (GDNF),182 neurotrophin (NT)-3,183 and NT-4/5,184 all of which act synergistically to induce neurogenesis and neuroplasticity.167,180
Although neurons are post-mitotic cells, neurogenesis can still occur in specific areas of the adult hippocampus185 with some stimuli such as ischemia/reperfusion, aging, metabolic pathology, or physical exercise being able to change the rate of neurogenesis.150 Van Praag and collaborators have extensively studied the effects of exercise, particularly of the aerobic type, on adult neurogenesis186 and have demonstrated that the newly formed neurons are associated with the cognitive and synaptic effects induced by exercise.187 This phenomenon is especially important in age-related neurodegeneration because attenuation of accelerated neuronal loss can prevent several age-related disorders, including Alzheimer's disease. Several signaling pathways induced by aerobic exercise have been suggested to mediate this process such as BDNF,188,189 VEGF,180 and IGF-1.190
Both age and disease-induced neurodegeneration are partially produced by a dysregulation of protein homeostasis (see further below on the exercise effects in aging “proteostasis”). Aerobic exercise increases proteolytic degradation by proteasomes and neprilysin, a specific Aβ-degrading enzyme.167,191 Activation of the brain proteasome is important in preventing Alzheimer's disease because proteasome inhibition produces Aβ accumulation, a hallmark of this disorder.192 The neurofibrillary tangles produced by an accumulation of hyperphosphorylated tau proteins is also an important hallmark of Alzheimer's disease. Lysine residues of tau are susceptible to ubiquitination, indicating interaction of tau aggregation by oligomerization and ubiquitination-mediated degradation through the proteasome system.193 The proteasome might also be involved in the learning process, because its inhibition in the hippocampus blocks long-term memory.194–195
Exercise and the Cellular Hallmarks of Aging
In a recent state-of-the-art review, López-Otín et al.196 nicely postulated nine hallmarks of aging that might be targeted in future pharmacological interventions—genomic instability, telomere attrition, epigenetic alterations, loss of protein homeostasis (proteostasis), deregulated nutrient sensing, mitochondrial dysfunction, cellular senescence, stem cell exhaustion, and altered intercellular communication. Although more research is needed, exercise, which is available at low cost and largely free of adverse effects,111 can influence, at least partly, most of these hallmarks (see Fig. 1, right column, for a summary).
Genomic instability
A 5-month aerobic exercise program prevented mitochondrial DNA (mtDNA) instability in multiple tissues of mtDNA mutator (progeroid) mice, thereby reducing multisystem pathology and preventing premature mortality.197 Oxidative damage to DNA occurs during the aging process.198 Resistance exercise decreases such damage in old people, as indicated by 8-hydroxy-2′-deoxyguanosine (8-OHdG) determination, through stimulation of endogenous anti-oxidant defense,199 whereas in rodent models aerobic exercise improves DNA repair mechanisms (e.g., proteasome complex),200 as well as NF-κB and PGC-1α signaling.121,201
Telomere attrition and telomerase activity
Accelerated telomere shortening is linked with numerous age-related chronic diseases and risk factors.202–210 On the other hand, there is increasing evidence supporting an association between habitual physical exercise, particularly aerobic exercise, and longer leukocyte telomere length.211–217 Leukocyte telomere length is also positively associated with cardiorespiratory fitness (expressed as VO2max),213,218,219 which, in turn, is associated with lower CVD and all-cause mortality.220 Long-term aerobic exercise can modulate leukocyte telomere length as well as the network of proteins that interact with telomeres, through activation and induction of telomerase enzyme activity (mediated by human telomerase reverse transcriptase [TERT]) and the shelterin complex (or telosome).217 In effect, TERT mRNA expression is up-regulated in leukocytes after exercise.221 Exercise also regulates the microRNAs (miRNAs) that control the downstream expression of genes involved in telomere homeostasis.221 The association between physical exercise and telomere length could also be due to lower oxidative stress and inflammation, exercise-induced regulation of telomeric genes, or a complex interplay between these processes.221,222 The effects of exercise on skeletal muscle tissue telomeres have been less studied compared with leukocytes, and the results are less conclusive.223,224
Epigenetic adaptations
The relationship between epigenetics regulation (e.g., DNA methylation) and aging is complex and controversial, i.e., hypomethylation or hypermethylation might be either beneficial or detrimental depending on the different cell types; and, whether manipulations of histone-modifying enzymes can influence aging through purely epigenetic mechanisms remains to be clarified.196 While keeping in mind the above-mentioned controversy, exercise seems to induce epigenetic modifications that can help attenuate age-deregulations,225 and several mechanisms, such as metabolic adaptations and transient hypoxia conditions, have been proposed recently.226 Regular aerobic exercise can modify genome-wide DNA methylation in humans.227 Animal216,228,229 and human research216 suggests that aerobic exercise induces, through epigenetic mechanisms, telomerase activity and the transcription of genes encoding telomere-stabilizing proteins. Both resistance and aerobic exercise can increase DNA methylation, cause histone modifications, and induce miRNAs in a wide range of tissues, including among others, muscle, brain, and cardiovascular system.225,226 Transient DNA hypomethylation of gene-specific promoter regions precedes increases in mRNA expression in response to acute exercise.230 In turn, these pulses of elevated mRNA during recovery from acute exercise facilitate protein synthesis and induce gradual structural remodeling and long-term functional adjustments.231 In general, these adaptations are intrinsic to the working skeletal muscle and collectively contribute to maximize substrate delivery, mitochondrial respiratory capacity, and contractile function during exercise. The net effect is promotion of optimal performance during a future exercise challenge, resulting in a robust defense of homeostasis in the face of metabolic perturbation and, consequently, enhanced resistance to fatigue.232,233 Several epigenetic mechanisms, including histone H4 deacetylation and loss of promoter methylation, have been implicated in the modified gene expression profile that occurs as an adaptation to aerobic exercise.234
Epigenetic mechanisms are not restricted to early stages of human development but are broad dynamic controllers of genomic plasticity in response to environmental factors such as exercise.235 For instance, in young adults, the class II HDACs 4 and 5 (transcriptional repressors) can translocate from the nucleus to the sarcoplasm of muscle fibers in response to aerobic exercise.108 Over-expression of HDAC5 in transgenic mice blocks the effects of exercise training, further suggesting a contribution of histone modifications in the transcriptomic response to muscle contraction.236 In human and mouse muscles, methylation of PGC-1α, mitochondrial transcription factor (TFAM), MEF2A, citrate synthase (CS), and pyruvate dehydrogenase kinase isozyme 4 (PDK4) gene promoters decreases after an acute bout of aerobic exercise.230 The degree of DNA methylation of a large number of genes changes in response to exercise in both skeletal muscles and adipose tissue.237,238 In addition, aerobic exercise-induced SIRT-1 regulates the tumor suppressor p53, PGC-1α, NF-κB as well as other transcription factors via its deacetylase activity.225,239
Chronic moderate aerobic exercise increases the methylation levels of the pro-inflammatory apoptosis-associated speck-like protein caspase (ASC) gene, which modulates IL-1β and IL-18 in the leukocytes of old people, thereby contributing to attenuation of age-related increases in pro-inflammatory cytokines.240 Aerobic exercise training also alters DNA methylation in a chronic manner.241 Thus, 48 hr after a bout of aerobic exercise, the DNA methylation profile of genes involved in diverse metabolic pathways, as well as in calcium and insulin signaling, was recently found to be differentially methylated in skeletal muscle.241 A majority of the detected genes in this study were chronically hypomethylated after exercise training. Both aerobic and resistance exercise also can help combating aging sarcopenia and frailty by modulating, through epigenetic mechanisms, several myogenic regulatory factors, e.g., myogenin, myoblast determination protein 1 (MyoD), or myogenic factors 5 (Myf5) and 6 (Myf6, also known as myogenic regulatory factor 4 [Mrf4] or herculin).242–244 Exercise also helps to attenuate the aging-related epigenetic deregulation of growth factors in neurodegenerative diseases, not only by up-regulating BDNF induction as mentioned above, but also by promoting remodeling of the chromatin containing the BDNF gene.245
Overall, the study of exercise-induced epigenetic modifications is just in its infancy. Yet the studies available have already provided new insights into the potential tissue-specific alterations in DNA methylation induced by exercise and into some of the mechanisms explaining the beneficial effects of regular exercise.
Loss of proteostasis
Proteostasis is defined as the protein homeostasis that is responsible for refolding or degrading altered proteins by several mechanisms, such as autophagy, proteasomal degradation, or chaperone-mediated folding. A loss of function in these processes leads to an aggregation of damaged proteins and thereby proteotoxic effects that have been associated with aging196,246 and age-related conditions such as Alzheimer's or Parkinson's disease.247
The autophagy–lysosomal and the ubiquitin–proteasome systems, two important proteostatic mechanisms, are impaired by aging.248,249 Exercise has a beneficial effect in autophagy.250 In aging mouse models, aerobic exercise induces autophagy in: (1) The brain, supporting its potential to promote elimination of damaging proteins causing neurodegeneration251; (2) heart252; or (3) muscle (besides preventing apoptosis), by modulating IGF-1, Akt/mTOR, and Akt/Forkhead box O3A (FoxO3a) signaling, thereby preventing loss of muscle mass/strength.253,254 Although data are still scarce in aging humans, autophagy muscle markers are up-regulated after combined exercise training (walking and moderate-intensity leg resistance exercises) in old women.255
Aerobic exercise induces autophagy in mice through activation of the BCL-2–beclin-1 complex,256 whereas beclin-1 disruption in transgenic mice reduces autophagy leading to neurodegeneration.257 Moreover, the aging human brain shows a down-regulation of beclin-1,258 whereas healthy centenarians have higher serum levels of beclin-1 compared with young controls, suggesting that elevated basal levels of autophagy may be related to healthy human exceptional longevity.259 MacKenzie et al. showed that acute high-resistance exercise evoked increased muscle protein synthesis and decreased protein degradation in rats, through activation of the class 3 phosphatidylinositol 3OH-kinase (PI3K) Vps34 mVps34,260 which is known to regulate autophagy by forming a complex with beclin-1.261 Using an atrogin-1 (also known as MAFbx) knockout mouse model, Zaglia et al. demonstrated that autophagy dysfunction promotes cardiomyopathy and premature death.262 Atrogin-1 is a muscle-specific ubiquitin ligase involved in muscle atrophy through FoxO signaling.263 Similar to skeletal muscle, atrogin-1 up-regulation in the heart leads to atrophy.264 Interestingly, aged atrogin-1 knockout mice have reduced tolerance to treadmill exercise and shortened life span compared with age-matched controls.262 In this animal model, muscle age-related increases in oxidative damage and apoptosis are attenuated by regular aerobic exercise, whereas both mechanisms are negatively correlated with autophagy.261
Deregulated nutrient sensing
Exercise exerts protective effects against age declines in the glucose-sensing somatotrophic axis,265 and also activates at the muscle level the three main interconnected nutrient-sensing systems, i.e., the amino acid–sensing mTOR pathway266,267 and the low energy–sensing AMPK and SIRT pathways,132,268 thereby promoting a beneficial muscle anabolic state. On the other hand, exercise improves insulin sensitivity through increased production of the glucose transporter type 4 (Glut 4).269
In addition, resistance exercise acutely increases the circulating levels of testosterone, growth hormone (GH), and IGF-1, with the magnitude of the effect usually increasing with higher exercise intensity270 or duration,271 shorter rest intervals,272 and higher exercising muscle mass.273,274 Thus, resistance exercise is a useful approach to prevent sarcopenia by virtue of its ability to increase protein synthesis275–277 and skeletal muscle mass.278,279 However, the rate of muscle protein synthesis in response to exercise training is lower in the elderly than in younger people,280,281 leading to a lower capacity to improve skeletal muscle strength and fiber size.282 On the other hand, supplementation with essential amino acids together with resistance training increases muscle protein synthesis both in young and old individuals, although such an effect is also attenuated in the latter, owing, at least partly, to lower ERK1/2 and mTOR signaling.280 (For a more extensive description about nutrient-sensing modulation by exercise, see above.)
Mitochondrial dysfunction
mtDNA mutations (typically deletions) accumulate with age in different tissues,283,284 including mainly the nervous and skeletal muscle tissue.37,285 Mutations in muscle mtDNA play a causal role in the physiological mechanisms implicated in sarcopenia,37–40 particularly in abnormalities of the electron transport system, muscle fiber atrophy, and breakage.37,39,40 Despite classic studies demonstrating exercise-induced mitochondrial biogenesis in young but not in aged mice,286 recent findings have shown that aerobic exercise training attenuates mitochondrial dysfunction and loss of mitochondrial content in the aging human skeletal muscle while increasing oxidative capacity and the activity of different electron transport chain protein complexes.287
The accumulated damage to the mitochondria due to the ROS generated from the electron transport chain is the base of the mitochondrial theory of aging first proposed by Harman.288 Oxidative damage to mtDNA increases with aging, affecting mtDNA replication and transcription, which, in turn, alters the functionality of mitochondrial proteins.289,290 Lanza et al. demonstrated that age-related decline in mitochondrial oxidative capacity was absent in endurance-trained humans, who showed elevated expression of mitochondrial proteins, mtDNA, and mitochondrial transcription factors.291 In mtDNA mutator mice, aerobic exercise promoted systemic mitochondrial biogenesis, prevented mtDNA depletion and mutations, increased mitochondrial oxidative capacity and respiratory chain assembly, restored mitochondrial morphology, and blunted pathological levels of apoptosis in multiple tissues. Thus, the authors concluded that a systemic “mitochondrial rejuvenation” occurred as a result of the training program.197
The lower mitochondrial enzyme activity commonly shown in older compared with younger adults17,292 is associated with a down-regulation of the mRNAs encoding mitochondrial proteins in skeletal muscle.293–295 Aged subjects have cytochrome c oxidase (COX)-deficient muscle fibers,296 especially in sarcopenic muscles or in those focal regions with higher content of mtDNA mutations.39,296–299 Yet resistance exercise training can reverse the muscle transcriptional signature of aging back to that of younger levels for most genes involved in mitochondrial function.299 Gomes et al.300 recently provided novel insights into the mechanisms responsible for the age decline of mitochondrial homeostasis by elegantly showing a regulatory pathway that is SIRT1-mediated and independent of PGC-1α and -β, with aging declining NAD+ levels and thereby reducing SIRT1 activity and leading to impaired oxidative phosphorylation (OXPHOS). Short treatment (1 week, or ∼8 months, when translated to the human life span) of 22-month-old mice with nicotinamide mononucleotide (NMN) (a precursor to NAD+ increasing NAD+ levels in vivo) reversed several biochemical indicators of muscle mitochondrial senescence, with increased OXPHOS transcripts in the gastrocnemius muscle. It was proposed that NMN or other compounds able to increase NAD+ are candidates to be included in the human anti-aging armamentarium. And yet NMN, as opposed to exercise, was unable to reverse other age-dependent whole-organism effects, such as loss of muscle strength.
Besides the above-mentioned benefits of resistance exercise on muscle strength until end of life (which were summarized in Table 1), regular exercise has a profound beneficial effect on human mitochondrial function/biogenesis,301 with this effect being both PGC-1 and SIRT mediated.111 An active lifestyle attenuates aging mitochondrial dysfunction, promoting longevity through pathways common to the effects of caloric restriction.291 In addition, some “myokines” (see further below for the definition of myokine) have a mitochondrial rejuvenating effect, e.g., visfatin, a NAD+ biosynthetic enzyme that stimulates the SIRT-1 pathway.252
Cellular senescence
Cellular senescence is defined as a stable arrest of the cell cycle coupled with stereotyped phenotypic changes, and its regulation during aging is a complex process. Indeed, the same phenomenon, i.e., elimination of senescent cells, that is beneficial to delay age-related pathologies and thus to promote longevity, could also stimulate cancer development.196 Besides inducing secretion of anti-tumorigenic myokines such as secreted protein acidic and rich in cysteine (SPARC, also known as basement membrane protein [BM]-40), calprotectin, or leukemia inhibitory factor,302 exercise, mainly aerobic exercise, may decrease cancer incidence and help improve cancer prognosis through several mechanisms, including greater natural killer (NK) cell activity, enhanced antigen presentation, reduced inflammation, and prevention of functional senescent cells' accumulation.303 Telomere-associated proteins regulate cellular senescence and, as described above, are up-regulated by exercise. Moreover, aerobic exercise increases the aortic expression of telomere repeat-binding factor 2 and Ku70 and reduces the expression of apoptosis regulators, such as cell-cycle-checkpoint kinase 2, p16INK4a, and p53 or survival regulators.216
Telomere-associated proteins, as well as p16INK4a/Rb and p19ARF/p53 signaling, are considered main pathways in the control of human aging and age-associated pathologies.196 p16INK4a and p21 are cell cycle inhibitors that are up-regulated in senescent cells.304,305 p21 is a downstream target of p53 and telomere dysfunction, whereas p16INK4a appears to be up-regulated in a p53- and telomere-independent manner.306 Sousa-Victor et al. recently highlighted the importance of p16INK4a in the modulation of cellular senescence. In a geriatric mouse model, muscle satellite cells lose their quiescent state owing to deregulation of p16INK4a, whereas repressing p16INK4a restores muscle regenerative capacity.307 Thus, we suggested the importance of p16INK4a modulation as a new target for combating aging-related chronic diseases.308 Lifestyle factors, including smoking and physical aerobic exercise practice, have been associated with p16INK4a mRNA levels in peripheral blood T ymphocytes,309 a biomarker of human aging.310 Thus, physical exercise is inversely correlated with p16INK4a mRNA levels, i.e., higher amounts of physical exercise leads to down-regulation of p16INK4a in blood cells, which might promote protective effects against age-dependent alterations.309
As exposed above, cellular senescence plays a key role not only in cancer development311,312 but also in aging.313 In fact, cell senescence is one of the major paradigms of aging research through the acquisition of the senescence-associated secretory phenotype (SASP) or senescence-messaging secretome.314 SASP is a DNA damage response, which, through production of inflammatory, growth-promoting, and remodeling factors can potentially explain how senescent cells alter tissue microenvironments.315 Different animal model investigations have shown that exercise modulates senescence associated to aging. Thus, 12 weeks of aerobic (swimming) exercise training suppressed liver senescence markers and down-regulated inflammatory mediators by reducing gamma glutamyltranspeptidase activity and levels of p53, p21, and IL-6 in a d-galactose–induced senescence rat model.316 Werner et al.228 studied the effect of aerobic exercise on telomere-regulating and cellular senescence mechanisms at the cardiac level in wild-type, endothelial NO• synthase (eNOS)-deficient and TERT-deficient mice models. Their results showed that exercise up-regulated cardiac telomere-stabilizing proteins, promoted anti-senescent effects, and provided protection against doxorubicin-induced cardiomyopathy. Werner et al.216 also studied the effects of aerobic exercise on vascular telomere biology and endothelial apoptosis in mice and the effects of long-term aerobic training on telomere biology in circulating leukocytes in humans. Besides improving telomere biology in the thoracic aorta and in mononuclear cells, exercise reduced the vascular expression of apoptosis regulators. Moreover, endurance athletes had increased telomerase activity and down-regulated cell cycle inhibitors compared with sedentary subjects. These findings are supported by Song et al.,309 who found that in humans aerobic exercise reduced the expression of DNA damage biomarkers and correlated positively with p16INK4a expression and negatively with telomere length in peripheral blood T lymphocytes.
Stem cell exhaustion
The decline in the regenerative potential of tissues is a main characteristic of aging, whereas exercise is one of the most potent stimuli for the proliferation/migration of the different adult stem cell subsets from their home tissue (e.g., bone marrow) to target damaged tissues for subsequent engraftment and regeneration.252 Thus, regular exercise attenuates age-associated reduction in the endothelium-reparative capacity of endothelial progenitor cells.317 Exercise activates mesenchymal stem cells, which are pluripotent progenitors with a wide variety of therapeutic potential (e.g., as vehicles of anti-cancer genes) and promotes proliferation of neural stem cells, thereby contributing to improve brain regenerative capacity and cognitive ability.252
Arguably the most affected stem cell type during aging is the myogenic one, known as satellite cells.318 Although the human skeletal muscle tissue maintains myofiber replacement and repair potential throughout most of life, the efficiency of this process declines with aging, owing to satellite cell alterations. Age-reduced number or functionality of these myogenic cells prevents proper maintenance of muscle mass.318–321 Specifically, aging atrophy of type II muscle fibers is accompanied by a specific decline in the content of type II muscle fiber satellite cells.318 Thus, since sarcopenia is associated with atrophy of type II muscle fibers, its pathophysiological mechanisms are closely related with the decline in satellite cell content with aging.31 Both aging reductions in muscle mass and strength are positively correlated with muscle fiber type specific cross-sectional area, myonuclear content, and satellite cell content.322
Animal studies have demonstrated that aerobic exercise increases myofibers that contain higher numbers of satellite cells in both young and old rats,323 and also promotes expansion of the satellite cell pool in young and old mice.324 The contribution of these stem cells to skeletal muscle regeneration has been well documented.325,326 As stated by Hawke and Garry,326 because adult myofibers are post-mitotic cells, the regulation of skeletal muscle is dependent on a small population of resident cells that are the satellite cells. The regulation of satellite cells involves several mechanisms, including immune response, neurotransmitters, neurotrophic and vascular factors (among other growth factors such as IGF-1327), cytokines such as IL-6,328 testosterone, or NO•, most of which are modulated by exercise.111
Not only in young adults329 but also during aging, resistance training is able to induce skeletal muscle satellite cell proliferation and differentiation, thereby resulting in hypertrophy of type II fibers.330 The latter phenomenon, in turn, attenuates the pro-sarcopenic physiological events related to type II fiber atrophy associated with aging,31,318, 322 On the other hand, although resistance training in the elderly of both sexes can counteract the loss of muscle mass and strength,331 a recent study reported that satellite cell induction in response to a single bout of resistance exercise is delayed in old men.332 McKay et al.333 also showed that, compared to young adults, muscle levels of myostatin, a protein that inhibits muscle differentiation and growth in the myogenesis process, were two-fold higher in older individuals, who also had 35% fewer basal stem cells and a type II fiber-specific impairment in stem cell content. The authors concluded that the co-localization of myostatin with satellite cells explains the worsened myogenic capacity of the aged skeletal muscle.333 In fact, an aging-blunted activation of type II muscle fiber satellite cells in response to an acute bout of resistance exercise was recently shown by Snijders et al.332 In addition, the satellite cell response to resistance exercise is related to the extent of muscular hypertrophy induced by training.334
Altered intercellular communication
Aging is associated with altered intercellular communication leading to inflammation or “inflammaging.”196 Several mechanisms are responsible for this process, including accumulation of pro-inflammatory tissue damage, immune dysfunction, release of pro-inflammatory cytokines by senescent cells, higher activation of NF-κB, or impaired autophagy regulation.196,335 These events activate the NOD-like receptor protein 3 (NLRP3) “inflammasome,” characterized by elevations in IL-1β, tumor necrosis factor-α (TNF-α), and interferons.335,336 Interestingly, calorie restriction and exercise-mediated weight loss in obese individuals with type 2 diabetes lead to a reduction in adipose tissue expression of the NLRP3 inflammasome and IL-1β, and thus to reduced inflammation.337
The decay factor AUF1 (AU-binding factor 1, also known as heterogeneous nuclear ribonucleoprotein D or hnRNP D) is implicated in the cessation of the inflammatory response (by mediating cytokine mRNA degradation) and also in the maintenance of telomere length by modulating TERT.338 Down-regulation of AUF1 leads to accelerated cellular senescence and premature aging in mice, which is rescued by normalizing the expression of this factor.196 Lai et al. found that chronic muscle contractile activity increased different AUF1 isoforms (p37, p40, and p45) in the muscle of healthy rats, resulting in improved muscle plasticity in response to subsequent contractile activity.339 Senescent cells transmit their condition to other cells through multiple mechanisms, including ROS, growth factors, and interleukins.340 As mentioned above, chronic physical exercise (mostly of the aerobic type) decreases ROS damage, and it does so by decreasing ROS production at the mitochondrial level while up-regulating endogenous anti-oxidant defense.121
Importantly, skeletal muscle fibers produce hundreds of secreted factors or “myokines” (including the above-mentioned neurotrophins) with a potential drug-like effect at the local and systemic levels, i.e., proteins, growth factors, cytokines, or metallopeptidases, and this secretory capacity increases during and after exercise training (see Fiuza-Luces252 for an in-depth review and Table 3 for some illustrative examples). Systemic low-level inflammation and related conditions such as CVD or cancer can be attenuated by the cumulative effect of regular exercise bouts, during which the muscle can release IL-6, arguably the myokine prototype.341 This, in turn, creates a healthy milieu by inducing the production of the anti-inflammatory cytokines IL-1 receptor antagonist (IL-1Ra), IL-10, or TNF soluble receptors (sTNF-R) while inhibiting the pro-inflammatory cytokine TNF-α.341 The release of IL-6 from working muscles increases with exercise intensity342 and duration,343 and endogenous NO• and the interaction between Ca2+/nuclear factor of activated T cell (NFAT) and glycogen/p38 MAPK are the proposed upstream signals leading to its secretion.344 Other anti-inflammatory myokines include IL-4, IL-10, or IL-13.345,346 Thus, life-long aerobic exercise training is associated with lower inflammation levels.347–349 Higher levels of aerobic exercise have also been associated with lower levels of C-reactive protein (CRP), IL-6, and TNF-α in people aged 70–79 years.350 However, although exercise training is known to have beneficial anti-inflammatory effects across a broad spectrum of organs and systems, more research is needed in the elderly, particularly to determine if the molecular mechanisms and pathways involved are similar in old people compared with younger population segments.351
Table 3.
Examples of Myokines Released During Exercise with a Potential Anti-Aging Effect
| Name of molecule | Main tissue(s) of origin | Main type of exercise inducing its release/secretion | Main target tissue(s) | Main biological effect(s) associated with exercise-induced release/secretion | Main aging hallmark targeted | Potential future anti-aging application | Illustrative references |
|---|---|---|---|---|---|---|---|
| Brain-derived neurotrophic factor (BDNF) | Central nervous system Vascular endothelial cells, platelets, lymphocytes, eosinophils, monocytes, pituitary gland, working skeletal muscle |
Moderate-intense “aerobic” exercise (e.g., brisk walking) | Brain Motor neurons |
↑ Neuroplasticity ↑ Motor unit regeneration |
Cellular senescence in the brain | Protection against neurodegeneration (including possibly dementia) | 167–169 |
| Interleukin-4 (IL-4) and IL-13 | Lymphocytes, mast cells and neutrophils Various origins (brain, cancer cells, liver, fibroblasts, and muscle cells) Working muscles |
Resistance exercise (e.g., weightlifting) | Skeletal muscle | ↑ Muscle growth | Loss of muscle proteostasis | Aging muscle atrophy/sarcopenia | 387–389 |
| IL-6 (also termed interferon, beta 2) | Working muscles Immune cells Adipocytes |
Intense ‘aerobic’ exercise (e.g., brisk/very brisk walking) | Skeletal muscle Adipose tissue Pituitary gland-liver Immune cells |
↑ Muscle lipolysis ↑ Muscle growth ↑ Adipocyte lipolysis ↑ Liver-glucose release to blood ↓Inflammation ↑Immunomodulation |
Altered inter-cellular communication (‘inflammaging’) | Age-related cardio-metabolic diseases | 129, 390–393 |
| IL-15 | Working muscles Various origins (lymphoid tissues, kidney, brain, cardiac muscle, lung, pancreas, testis, liver, placenta, epithelial cells, and activated macrophages, and maybe adipocytes) |
Mainly resistance exercise | Skeletal muscle Adipose tissue |
Promotes muscle anabolism/inhibits catabolism Anti-obesogenic (↓mainly visceral fat) effect Insulin-sensitizing effect |
Loss of muscle proteostasis | Aging muscle wasting/sarcopenia | 394–397 |
| Leukemia inhibitory factor (LIF) | Working muscles Central nervous system |
Mainly resistance exercise | Skeletal muscle | Mainly local (autocrine/paracrine effect): ↑ Muscle growth (satellite cell proliferation) ↑ Muscle regeneration |
Loss of muscle proteostasis | Aging muscle wasting/sarcopenia | 398–401 |
| Myostatin (also termed, growth differentiation factor 8 [GDF8]) | Skeletal muscle | Both “aerobic” and resistance exercise | Skeletal muscle Adipose tissue |
Main effects associated to myostatin inhibition which can be partly achieved by exercise are: ↑Muscle growth ↓Adiposity ↑Insulin sensitivity |
Attenuation of disease/age muscle wasting Obesity/diabetes prevention |
Use of exercise as a coadjuvant of myostatin-inhibition therapies for muscle wasting | 402–406 |
| Visfatin (also known as (nicotinamide phosphoribosyltransferase [NAMPT] or pre-B cell enhancing factor [PBEF]) | Ubiquitous expression in human tissues, including adipose and skeletal muscle tissue (i.e., it is both an adypokine and a myokine), liver, bone marrow, lymphocytes, β-cells and human islets, heart | “Aerobic” exercise | Skeletal muscle and adipose tissue | AMPK activation→↑ sirtuin1 (SIRT1)→peroxisome proliferator-activated receptor γ co-activator α (PGC-1α) It provides NAD+ |
Mitochondrial dysfunction | Exercise as a major component of anti-aging medicine | 407–410 |
The information provided in the table is based (and adapted from) a previous review paper by the authors.111
AMPK AMP-activated protein kinase; NAD+, oxidized nicotinamide adenine dinucleotide.
Perspective
The benefits of regular exercise are such that a dose–response is usually observed in humans. Higher levels of moderate-to-vigorous exercise (≥450 min/week, clearly above the minimum international recommendations of 150 min/week) are associated with longer life expectancy.352 Furthermore, elite athletes—those humans sustaining the highest possible exercise levels (e.g., former participants in the Tour de France cycling race or former Olympic marathoners)—usually live considerably longer than the general population.353
Physical exercise has a profound effect on the expression of a substantial proportion of our genome, which has evolved to optimize aerobic metabolism in an environment of food scarcity. Thus, physical inactivity is becoming a major public health problem worldwide.354 Exercise certainly cannot reverse the aging process, but it does attenuate many of its deleterious systemic and cellular effects. Most common age-associated chronic conditions are diseases of physiology and thus physiological interventions, of which physical exercise is arguably the best example, are largely the answer.355 We propose that more research efforts should be devoted to gain insights into the molecular mediators of the exercise benefits. Besides putting more anti-aging drugs on the market, it would be wise to determine which are the most effective combinations, types, and dosages (frequency, duration, intensity) of exercise for older people and to implement efficient exercise interventions for this and younger population segments. Note: For the purposes of simplicity in this review, we have mostly used the common term “exercise” instead of “physical activity.”
Acknowledgments
Research in the field of exercise and health by A. Lucia is funded by Fondo de Investigaciones Sanitarias (FIS, grant no. PI12/00914).
Author Disclosure Statement
No competing financial interests exist.
All co-authors fully reviewed the entire manuscript and participated in at least some of its parts, including literature search (all authors), figure design (F.S.-G., N.G., H.P.-G.), tables edition (A.S.-L., C.F.-L., N.G.), or writing and editing of a significant part of the manuscript (all authors).
References
- 1.Garber CE, Blissmer B, Deschenes MR, Franklin BA, Lamonte MJ, Lee IM, Nieman DC, Swain DP, American College of Sports M. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: Guidance for prescribing exercise. Med Sci Sports Exerc 2011;43:1334–1359 [DOI] [PubMed] [Google Scholar]
- 2.Hollenberg M, Yang J, Haight TJ, Tager IB. Longitudinal changes in aerobic capacity: Implications for concepts of aging. J Gerontol A Biol Sci Med Sci 2006;61:851–858 [DOI] [PubMed] [Google Scholar]
- 3.Stathokostas L, Jacob-Johnson S, Petrella RJ, Paterson DH. Longitudinal changes in aerobic power in older men and women. J Appl Physiol 2004;97:781–789 [DOI] [PubMed] [Google Scholar]
- 4.Fleg JL, Morrell CH, Bos AG, Brant LJ, Talbot LA, Wright JG, Lakatta EG. Accelerated longitudinal decline of aerobic capacity in healthy older adults. Circulation 2005;112:674–682 [DOI] [PubMed] [Google Scholar]
- 5.Shephard RJ. Maximal oxygen intake and independence in old age. Br J Sports Med 2009;43:342–346 [DOI] [PubMed] [Google Scholar]
- 6.Hagberg JM, Allen WK, Seals DR, Hurley BF, Ehsani AA, Holloszy JO. A hemodynamic comparison of young and older endurance athletes during exercise. J Appl Physiol 1985;58:2041–2046 [DOI] [PubMed] [Google Scholar]
- 7.Ogawa T, Spina RJ, Martin WH, 3rd, Kohrt WM, Schechtman KB, Holloszy JO, Ehsani AA. Effects of aging, sex, and physical training on cardiovascular responses to exercise. Circulation 1992;86:494–503 [DOI] [PubMed] [Google Scholar]
- 8.Stratton JR, Levy WC, Cerqueira MD, Schwartz RS, Abrass IB. Cardiovascular responses to exercise. Effects of aging and exercise training in healthy men. Circulation 1994;89:1648–1655 [DOI] [PubMed] [Google Scholar]
- 9.Hawkins SA, Marcell TJ, Victoria Jaque S, Wiswell RA. A longitudinal assessment of change in VO2max and maximal heart rate in master athletes. Med Sci Sports Exerc 2001;33:1744–1750 [DOI] [PubMed] [Google Scholar]
- 10.Eskurza I, Donato AJ, Moreau KL, Seals DR, Tanaka H. Changes in maximal aerobic capacity with age in endurance-trained women: 7-yr follow-up. J Appl Physiol 2002;92:2303–2308 [DOI] [PubMed] [Google Scholar]
- 11.Hossack KF, Bruce RA. Maximal cardiac function in sedentary normal men and women: Comparison of age-related changes. J Appl Physiol 1982;53:799–804 [DOI] [PubMed] [Google Scholar]
- 12.Rivera AM, Pels AE, 3rd, Sady SP, Sady MA, Cullinane EM, Thompson PD. Physiological factors associated with the lower maximal oxygen consumption of master runners. J Appl Physiol 1989;66:949–954 [DOI] [PubMed] [Google Scholar]
- 13.Wiebe CG, Gledhill N, Jamnik VK, Ferguson S. Exercise cardiac function in young through elderly endurance trained women. Med Sci Sports Exerc 1999;31:684–691 [DOI] [PubMed] [Google Scholar]
- 14.Proctor DN, Joyner MJ. Skeletal muscle mass and the reduction of VO2max in trained older subjects. J Appl Physiol 1997;82:1411–1415 [DOI] [PubMed] [Google Scholar]
- 15.Toth MJ, Gardner AW, Ades PA, Poehlman ET. Contribution of body composition and physical activity to age-related decline in peak VO2 in men and women. J Appl Physiol 1994;77:647–652 [DOI] [PubMed] [Google Scholar]
- 16.Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part I: Aging arteries: a “set up” for vascular disease. Circulation 2003;107:139–146 [DOI] [PubMed] [Google Scholar]
- 17.Coggan AR, Spina RJ, King DS, Rogers MA, Brown M, Nemeth PM, Holloszy JO. Histochemical and enzymatic comparison of the gastrocnemius muscle of young and elderly men and women. J Gerontol 1992;47:B71–B76 [DOI] [PubMed] [Google Scholar]
- 18.Schrage WG, Eisenach JH, Joyner MJ. Ageing reduces nitric-oxide- and prostaglandin-mediated vasodilatation in exercising humans. J Physiol 2007;579:227–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Degens H. Age-related changes in the microcirculation of skeletal muscle. Adv Exp Med Biol 1998;454:343–348 [DOI] [PubMed] [Google Scholar]
- 20.Conley KE, Jubrias SA, Esselman PC. Oxidative capacity and ageing in human muscle. J Physiol 2000;526(Pt 1):203–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Janssen I, Heymsfield SB, Wang ZM, Ross R. Skeletal muscle mass and distribution in 468 men and women aged 18–88 yr. J Appl Physiol. 2000;89:81–88 [DOI] [PubMed] [Google Scholar]
- 22.Lexell J, Taylor CC, Sjostrom M. What is the cause of the ageing atrophy? Total number, size and proportion of different fiber types studied in whole vastus lateralis muscle from 15- to 83-year-old men. J Neurol Sci 1988;84:275–294 [DOI] [PubMed] [Google Scholar]
- 23.Saini A, Faulkner S, Al-Shanti N, Stewart C. Powerful signals for weak muscles. Ageing Res Rev 2009;8:251–267 [DOI] [PubMed] [Google Scholar]
- 24.Frontera WR, Hughes VA, Fielding RA, Fiatarone MA, Evans WJ, Roubenoff R. Aging of skeletal muscle: A 12-yr longitudinal study. J Appl Physiol 2000;88:1321–1326 [DOI] [PubMed] [Google Scholar]
- 25.Morley JE, Baumgartner RN, Roubenoff R, Mayer J, Nair KS. Sarcopenia. J Lab Clin Med 2001;137:231–243 [DOI] [PubMed] [Google Scholar]
- 26.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel JP, Rolland Y, Schneider SM, Topinkova E, Vandewoude M, Zamboni M. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010;39:412–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muscaritoli M, Anker SD, Argiles J, Aversa Z, Bauer JM, Biolo G, Boirie Y, Bosaeus I, Cederholm T, Costelli P, Fearon KC, Laviano A, Maggio M, Rossi Fanelli F, Schneider SM, Schols A, Sieber CC. Consensus definition of sarcopenia, cachexia and pre-cachexia: Joint document elaborated by Special Interest Groups (SIG) “cachexia-anorexia in chronic wasting diseases” and “nutrition in geriatrics.” Clin Nutr 2010;29:154–159 [DOI] [PubMed] [Google Scholar]
- 28.Fielding RA, Vellas B, Evans WJ, Bhasin S, Morley JE, Newman AB, Abellan van Kan G, Andrieu S, Bauer J, Breuille D, Cederholm T, Chandler J, De Meynard C, Donini L, Harris T, Kannt A, Keime Guibert F, Onder G, Papanicolaou D, Rolland Y, Rooks D, Sieber C, Souhami E, Verlaan S, Zamboni M. Sarcopenia: An undiagnosed condition in older adults. Current consensus definition: Prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Dir Assoc 2011;12:249–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morley JE, Abbatecola AM, Argiles JM, Baracos V, Bauer J, Bhasin S, Cederholm T, Coats AJ, Cummings SR, Evans WJ, Fearon K, Ferrucci L, Fielding RA, Guralnik JM, Harris TB, Inui A, Kalantar-Zadeh K, Kirwan BA, Mantovani G, Muscaritoli M, Newman AB, Rossi-Fanelli F, Rosano GM, Roubenoff R, Schambelan M, Sokol GH, Storer TW, Vellas B, von Haehling S, Yeh SS, Anker SD. Sarcopenia with limited mobility: An international consensus. J Am Med Dir Assoc 2011;12:403–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deschenes MR. Effects of aging on muscle fibre type and size. Sports Med 2004;34:809–824 [DOI] [PubMed] [Google Scholar]
- 31.Verdijk LB, Koopman R, Schaart G, Meijer K, Savelberg HH, van Loon LJ. Satellite cell content is specifically reduced in type II skeletal muscle fibers in the elderly. Am J Physiol Endocrinol Metab 2007;292:E151–E157 [DOI] [PubMed] [Google Scholar]
- 32.Kumar V, Selby A, Rankin D, Patel R, Atherton P, Hildebrandt W, Williams J, Smith K, Seynnes O, Hiscock N, Rennie MJ. Age-related differences in the dose-response relationship of muscle protein synthesis to resistance exercise in young and old men. J Physiol 2009;587:211–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Volpi E, Nazemi R, Fujita S. Muscle tissue changes with aging. Curr Opin Clin Nutr Metab Care 2004;7:405–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Doherty TJ. Invited review: Aging and sarcopenia. J Appl Physiol 2003;95:1717–1727 [DOI] [PubMed] [Google Scholar]
- 35.Kamel HK. Sarcopenia and aging. Nutr Rev 2003;61:157–167 [DOI] [PubMed] [Google Scholar]
- 36.Howard C, Ferrucci L, Sun K, Fried LP, Walston J, Varadhan R, Guralnik JM, Semba RD. Oxidative protein damage is associated with poor grip strength among older women living in the community. J Appl Physiol 2007;103:17–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McKenzie D, Bua E, McKiernan S, Cao Z, Aiken JM. Mitochondrial DNA deletion mutations: A causal role in sarcopenia. Eur J Biochem 2002;269:2010–2015 [DOI] [PubMed] [Google Scholar]
- 38.Pak JW, Herbst A, Bua E, Gokey N, McKenzie D, Aiken JM. Mitochondrial DNA mutations as a fundamental mechanism in physiological declines associated with aging. Aging Cell 2003;2:1–7 [DOI] [PubMed] [Google Scholar]
- 39.Wanagat J, Cao Z, Pathare P, Aiken JM. Mitochondrial DNA deletion mutations colocalize with segmental electron transport system abnormalities, muscle fiber atrophy, fiber splitting, and oxidative damage in sarcopenia. FASEB J 2001;15:322–332 [DOI] [PubMed] [Google Scholar]
- 40.Aiken J, Bua E, Cao Z, Lopez M, Wanagat J, McKenzie D, McKiernan S. Mitochondrial DNA deletion mutations and sarcopenia. Ann NY Acad Sci 2002;959:412–423 [DOI] [PubMed] [Google Scholar]
- 41.Cesari M, Pahor M. Target population for clinical trials on sarcopenia. J Nutr Health Aging 2008;12:470–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR, Garry PJ, Lindeman RD. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol 1998;147:755–763 [DOI] [PubMed] [Google Scholar]
- 43.Frisoli A, Jr., Chaves PH, Ingham SJ, Fried LP. Severe osteopenia and osteoporosis, sarcopenia, and frailty status in community-dwelling older women: Results from the Women's Health and Aging Study (WHAS) II. Bone 2011;48:952–957 [DOI] [PubMed] [Google Scholar]
- 44.Janssen I. Influence of sarcopenia on the development of physical disability: The Cardiovascular Health Study. J Am Geriatr Soc 2006;54:56–62 [DOI] [PubMed] [Google Scholar]
- 45.Janssen I, Heymsfield SB, Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc 2002;50:889–896 [DOI] [PubMed] [Google Scholar]
- 46.Lauretani F, Russo CR, Bandinelli S, Bartali B, Cavazzini C, Di Iorio A, Corsi AM, Rantanen T, Guralnik JM, Ferrucci L. Age-associated changes in skeletal muscles and their effect on mobility: An operational diagnosis of sarcopenia. J Appl Physiol 2003;95:1851–1860 [DOI] [PubMed] [Google Scholar]
- 47.Rolland Y, Lauwers-Cances V, Cournot M, Nourhashemi F, Reynish W, Riviere D, Vellas B, Grandjean H. Sarcopenia, calf circumference, and physical function of elderly women: A cross-sectional study. J Am Geriatr Soc 2003;51:1120–1124 [DOI] [PubMed] [Google Scholar]
- 48.Landi F, Liperoti R, Fusco D, Mastropaolo S, Quattrociocchi D, Proia A, Tosato M, Bernabei R, Onder G. Sarcopenia and mortality among older nursing home residents. J Am Med Dir Assoc 2012;13:121–126 [DOI] [PubMed] [Google Scholar]
- 49.Yeh SS, Lovitt S, Schuster MW. Pharmacological treatment of geriatric cachexia: Evidence and safety in perspective. J Am Med Dir Assoc 2007;8:363–377 [DOI] [PubMed] [Google Scholar]
- 50.Evans WJ, Morley JE, Argiles J, Bales C, Baracos V, Guttridge D, Jatoi A, Kalantar-Zadeh K, Lochs H, Mantovani G, Marks D, Mitch WE, Muscaritoli M, Najand A, Ponikowski P, Rossi Fanelli F, Schambelan M, Schols A, Schuster M, Thomas D, Wolfe R, Anker SD. Cachexia: A new definition. Clin Nutr 2008;27:793–799 [DOI] [PubMed] [Google Scholar]
- 51.Argiles JM, Anker SD, Evans WJ, Morley JE, Fearon KC, Strasser F, Muscaritoli M, Baracos VE. Consensus on cachexia definitions. J Am Med Dir Assoc 2010;11:229–230 [DOI] [PubMed] [Google Scholar]
- 52.Rolland Y, Czerwinski S, Abellan Van Kan G, Morley JE, Cesari M, Onder G, Woo J, Baumgartner R, Pillard F, Boirie Y, Chumlea WM, Vellas B. Sarcopenia: Its assessment, etiology, pathogenesis, consequences and future perspectives. J Nutr Health Aging 2008;12:433–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cesari M, Vellas B. Sarcopenia: A novel clinical condition or still a matter for research? J Am Med Dir Assoc 2012;13:766–767 [DOI] [PubMed] [Google Scholar]
- 54.von Haehling S, Morley JE, Anker SD. An overview of sarcopenia: Facts and numbers on prevalence and clinical impact. J Cachexia Sarcopenia Muscle 2010;1:129–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Heuberger RA. The frailty syndrome: A comprehensive review. J Nutr Gerontol Geriatr 2011;30:315–368 [DOI] [PubMed] [Google Scholar]
- 56.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, McBurnie MA. Frailty in older adults: Evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001;56:M146–M156 [DOI] [PubMed] [Google Scholar]
- 57.Jones DM, Song X, Rockwood K. Operationalizing a frailty index from a standardized comprehensive geriatric assessment. J Am Geriatr Soc 2004;52:1929–1933 [DOI] [PubMed] [Google Scholar]
- 58.Searle SD, Mitnitski A, Gahbauer EA, Gill TM, Rockwood K. A standard procedure for creating a frailty index. BMC Geriatr 2008;8:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen X, Mao G, Leng SX. Frailty syndrome: An overview. Clin Interv Aging 2014;9:433–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mohler MJ, Fain MJ, Wertheimer AM, Najafi B, Nikolich-Zugich J. The Frailty syndrome: Clinical measurements and basic underpinnings in humans and animals. Exp Gerontol 2014;54:6–13 [DOI] [PubMed] [Google Scholar]
- 61.Garcia-Garcia FJ, Gutierrez Avila G, Alfaro-Acha A, Amor Andres MS, De Los Angeles De La Torre Lanza M, Escribano Aparicio MV, Humanes Aparicio S, Larrion Zugasti JL, Gomez-Serranillo Reus M, Rodriguez-Artalejo F, Rodriguez-Manas L. The prevalence of frailty syndrome in an older population from Spain. The Toledo Study for Healthy Aging. J Nutr Health Aging 2011;15:852–856 [DOI] [PubMed] [Google Scholar]
- 62.Sternberg SA, Wershof Schwartz A, Karunananthan S, Bergman H, Mark Clarfield A. The identification of frailty: A systematic literature review. J Am Geriatr Soc 2011;59:2129–2138 [DOI] [PubMed] [Google Scholar]
- 63.Fried LP, Ferrucci L, Darer J, Williamson JD, Anderson G. Untangling the concepts of disability, frailty, and comorbidity: Implications for improved targeting and care. J Gerontol A Biol Sci Med Sci 2004;59:255–263 [DOI] [PubMed] [Google Scholar]
- 64.Chodzko-Zajko WJ, Proctor DN, Fiatarone Singh MA, Minson CT, Nigg CR, Salem GJ, Skinner JS. American College of Sports Medicine position stand. Exercise and physical activity for older adults. Med Sci Sports Exerc 2009;41:1510–530 [DOI] [PubMed] [Google Scholar]
- 65.DeSouza CA, Shapiro LF, Clevenger CM, Dinenno FA, Monahan KD, Tanaka H, Seals DR. Regular aerobic exercise prevents and restores age-related declines in endothelium-dependent vasodilation in healthy men. Circulation 2000;102:1351–1357 [DOI] [PubMed] [Google Scholar]
- 66.Green DJ, Maiorana A, O'Driscoll G, Taylor R. Effect of exercise training on endothelium-derived nitric oxide function in humans. J Physiol 2004;561:1–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Walsh JH, Yong G, Cheetham C, Watts GF, O'Driscoll GJ, Taylor RR, Green DJ. Effects of exercise training on conduit and resistance vessel function in treated and untreated hypercholesterolaemic subjects. Eur Heart J 2003;24:1681–1689 [DOI] [PubMed] [Google Scholar]
- 68.Gielen S, Sandri M, Erbs S, Adams V. Exercise-induced modulation of endothelial nitric oxide production. Curr Pharmaceut Biotech 2011;12:1375–1384 [DOI] [PubMed] [Google Scholar]
- 69.Butcher LR, Thomas A, Backx K, Roberts A, Webb R, Morris K. Low-intensity exercise exerts beneficial effects on plasma lipids via PPARgamma. Med Sci Sports Exerc 2008;40:1263–1270 [DOI] [PubMed] [Google Scholar]
- 70.Thomas AW, Davies NA, Moir H, Watkeys L, Ruffino JS, Isa SA, Butcher LR, Hughes MG, Morris K, Webb R. Exercise-associated generation of PPARgamma ligands activates PPARgamma signaling events and upregulates genes related to lipid metabolism. J Appl Physiol 2012;112:806–815 [DOI] [PubMed] [Google Scholar]
- 71.Charkoudian N, Joyner MJ, Barnes SA, Johnson CP, Eisenach JH, Dietz NM, Wallin BG. Relationship between muscle sympathetic nerve activity and systemic hemodynamics during nitric oxide synthase inhibition in humans. Am J Physiol Heart Circ Physiol 2006;291:H1378–H1383 [DOI] [PubMed] [Google Scholar]
- 72.Billman GE. Cardiac autonomic neural remodeling and susceptibility to sudden cardiac death: Effect of endurance exercise training. Am J Physiol Heart Circ Physiol 2009;297:H1171–H1193 [DOI] [PubMed] [Google Scholar]
- 73.Seals DR, Dinenno FA. Collateral damage: Cardiovascular consequences of chronic sympathetic activation with human aging. Am J Physiol Heart Circ Physiol 2004;287:H1895–H1905 [DOI] [PubMed] [Google Scholar]
- 74.Baron AD, Laakso M, Brechtel G, Hoit B, Watt C, Edelman SV. Reduced postprandial skeletal muscle blood flow contributes to glucose intolerance in human obesity. J Clin Endocrinol Metab 1990;70:1525–1533 [DOI] [PubMed] [Google Scholar]
- 75.Lind L, Lithell H. Decreased peripheral blood flow in the pathogenesis of the metabolic syndrome comprising hypertension, hyperlipidemia, and hyperinsulinemia. Am Heart J 1993;125:1494–1497 [DOI] [PubMed] [Google Scholar]
- 76.Monahan KD, Dinenno FA, Tanaka H, Clevenger CM, DeSouza CA, Seals DR. Regular aerobic exercise modulates age-associated declines in cardiovagal baroreflex sensitivity in healthy men. J Physiol 2000;529(Pt 1):263–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nolan RP, Jong P, Barry-Bianchi SM, Tanaka TH, Floras JS. Effects of drug, biobehavioral and exercise therapies on heart rate variability in coronary artery disease: A systematic review. Eur J Cardiovasc Prev Rehabil 2008;15:386–396 [DOI] [PubMed] [Google Scholar]
- 78.Routledge FS, Campbell TS, McFetridge-Durdle JA, Bacon SL. Improvements in heart rate variability with exercise therapy. Can J Cardiol 2010;26:303–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liu CJ, Latham NK. Progressive resistance strength training for improving physical function in older adults. Cochrane Database Syst Rev 2009CD002759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fiatarone MA, Marks EC, Ryan ND, Meredith CN, Lipsitz LA, Evans WJ. High-intensity strength training in nonagenarians. Effects on skeletal muscle. JAMA 1990;263:3029–3034 [PubMed] [Google Scholar]
- 81.Nelson ME, Rejeski WJ, Blair SN, Duncan PW, Judge JO, King AC, Macera CA, Castaneda-Sceppa C. Physical activity and public health in older adults: Recommendation from the American College of Sports Medicine and the American Heart Association. Circulation 2007;116:1094–1105 [DOI] [PubMed] [Google Scholar]
- 82.Earles DR, Judge JO, Gunnarsson OT. Velocity training induces power-specific adaptations in highly functioning older adults. Arch Phys Med Rehabil 2001;82:872–878 [DOI] [PubMed] [Google Scholar]
- 83.Evans WJ. High-velocity resistance training for increasing peak muscle power in elderly women. Clin J Sport Med 2003;13:66. [DOI] [PubMed] [Google Scholar]
- 84.Caserotti P, Aagaard P, Larsen JB, Puggaard L. Explosive heavy-resistance training in old and very old adults: Changes in rapid muscle force, strength and power. Scand J Med Sci Sports 2008;18:773–782 [DOI] [PubMed] [Google Scholar]
- 85.Sayers SP, Bean J, Cuoco A, LeBrasseur NK, Jette A, Fielding RA. Changes in function and disability after resistance training: Does velocity matter?: A pilot study. Am J Phys Med Rehabil 2003;82:605–613 [DOI] [PubMed] [Google Scholar]
- 86.Fielding RA, LeBrasseur NK, Cuoco A, Bean J, Mizer K, Fiatarone Singh MA. High-velocity resistance training increases skeletal muscle peak power in older women. J Am Geriatr Soc 2002;50:655–662 [DOI] [PubMed] [Google Scholar]
- 87.Greendale GA, Hirsch SH, Hahn TJ. The effect of a weighted vest on perceived health status and bone density in older persons. Qual Life Res 1993;2:141–152 [DOI] [PubMed] [Google Scholar]
- 88.Shaw JM, Snow CM. Weighted vest exercise improves indices of fall risk in older women. J Gerontol A Biol Sci Med Sci 1998;53:M53–M58 [DOI] [PubMed] [Google Scholar]
- 89.Bean J, Herman S, Kiely DK, Callahan D, Mizer K, Frontera WR, Fielding RA. Weighted stair climbing in mobility-limited older people: A pilot study. J Am Geriatr Soc 2002;50:663–670 [DOI] [PubMed] [Google Scholar]
- 90.Greendale GA, Salem GJ, Young JT, Damesyn M, Marion M, Wang MY, Reuben DB. A randomized trial of weighted vest use in ambulatory older adults: Strength, performance, and quality of life outcomes. J Am Geriatr Soc 2000;48:305–311 [DOI] [PubMed] [Google Scholar]
- 91.Faber MJ, Bosscher RJ, Chin APMJ, van Wieringen PC. Effects of exercise programs on falls and mobility in frail and pre-frail older adults: A multicenter randomized controlled trial. Arch Phys Med Rehabil 2006;87:885–896 [DOI] [PubMed] [Google Scholar]
- 92.Serra-Rexach JA, Bustamante-Ara N, Hierro Villaran M, Gonzalez Gil P, Sanz Ibanez MJ, Blanco Sanz N, Ortega Santamaria V, Gutierrez Sanz N, Marin Prada AB, Gallardo C, Rodriguez Romo G, Ruiz JR, Lucia A. Short-term, light- to moderate-intensity exercise training improves leg muscle strength in the oldest old: A randomized controlled trial. J Am Geriatr Soc 2011;59:594–602 [DOI] [PubMed] [Google Scholar]
- 93.Lustosa LP, Silva JP, Coelho FM, Pereira DS, Parentoni AN, Pereira LS. Impact of resistance exercise program on functional capacity and muscular strength of knee extensor in pre-frail community-dwelling older women: A randomized crossover trial. Rev Bras Fisioter 2011;15:318–324 [PubMed] [Google Scholar]
- 94.Sullivan DH, Roberson PK, Smith ES, Price JA, Bopp MM. Effects of muscle strength training and megestrol acetate on strength, muscle mass, and function in frail older people. J Am Geriatr Soc 2007;55:20–28 [DOI] [PubMed] [Google Scholar]
- 95.Haskell WL, Lee IM, Pate RR, Powell KE, Blair SN, Franklin BA, Macera CA, Heath GW, Thompson PD, Bauman A. Physical activity and public health: Updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Circulation 2007;116:1081–1093 [DOI] [PubMed] [Google Scholar]
- 96.Villareal DT, Smith GI, Sinacore DR, Shah K, Mittendorfer B. Regular multicomponent exercise increases physical fitness and muscle protein anabolism in frail, obese, older adults. Obesity (Silver Spring) 2011;19:312–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lord SR, Castell S, Corcoran J, Dayhew J, Matters B, Shan A, Williams P. The effect of group exercise on physical functioning and falls in frail older people living in retirement villages: A randomized, controlled trial. J Am Geriatr Soc 2003;51:1685–1692 [DOI] [PubMed] [Google Scholar]
- 98.Ehsani AA, Spina RJ, Peterson LR, Rinder MR, Glover KL, Villareal DT, Binder EF, Holloszy JO. Attenuation of cardiovascular adaptations to exercise in frail octogenarians. J Appl Physiol 2003;95:1781–1788 [DOI] [PubMed] [Google Scholar]
- 99.de Vries NM, van Ravensberg CD, Hobbelen JS, Olde Rikkert MG, Staal JB, Nijhuis-van der Sanden MW. Effects of physical exercise therapy on mobility, physical functioning, physical activity and quality of life in community-dwelling older adults with impaired mobility, physical disability and/or multi-morbidity: A meta-analysis. Ageing Res Rev 2012;11:136–149 [DOI] [PubMed] [Google Scholar]
- 100.Sakamoto K, Goodyear LJ. Invited review: Intracellular signaling in contracting skeletal muscle. J Appl Physiol 2002;93:369–383 [DOI] [PubMed] [Google Scholar]
- 101.Ji LL, Gomez-Cabrera MC, Steinhafel N, Vina J. Acute exercise activates nuclear factor (NF)-kappaB signaling pathway in rat skeletal muscle. FASEB J 2004;18:1499–1506 [DOI] [PubMed] [Google Scholar]
- 102.Vina J, Sanchis-Gomar F, Martinez-Bello V, Gomez-Cabrera MC. Exercise acts as a drug; the pharmacological benefits of exercise. Br J Pharmacol 2012;167:1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Egan B, Zierath JR. Exercise metabolism and the molecular regulation of skeletal muscle adaptation. Cell Metab 2013;17:162–184 [DOI] [PubMed] [Google Scholar]
- 104.Pilegaard H, Saltin B, Neufer PD. Exercise induces transient transcriptional activation of the PGC-1alpha gene in human skeletal muscle. J Physiol 2003;546:851–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mahoney DJ, Parise G, Melov S, Safdar A, Tarnopolsky MA. Analysis of global mRNA expression in human skeletal muscle during recovery from endurance exercise. FASEB J 2005;19:1498–1500 [DOI] [PubMed] [Google Scholar]
- 106.Coffey VG, Shield A, Canny BJ, Carey KA, Cameron-Smith D, Hawley JA. Interaction of contractile activity and training history on mRNA abundance in skeletal muscle from trained athletes. Am J Physiol Endocrinol Metab 2006;290:E849–E855 [DOI] [PubMed] [Google Scholar]
- 107.Louis E, Raue U, Yang Y, Jemiolo B, Trappe S. Time course of proteolytic, cytokine, and myostatin gene expression after acute exercise in human skeletal muscle. J Appl Physiol 2007;103:1744–1751 [DOI] [PubMed] [Google Scholar]
- 108.McGee SL, Fairlie E, Garnham AP, Hargreaves M. Exercise-induced histone modifications in human skeletal muscle. J Physiol 2009;587:5951–5958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Baar K, Wende AR, Jones TE, Marison M, Nolte LA, Chen M, Kelly DP, Holloszy JO. Adaptations of skeletal muscle to exercise: Rapid increase in the transcriptional coactivator PGC-1. FASEB J 2002;16:1879–1886 [DOI] [PubMed] [Google Scholar]
- 110.Wright DC, Han DH, Garcia-Roves PM, Geiger PC, Jones TE, Holloszy JO. Exercise-induced mitochondrial biogenesis begins before the increase in muscle PGC-1alpha expression. J Biol Chem 2007;282:194–199 [DOI] [PubMed] [Google Scholar]
- 111.Fiuza-Luces C, Garatachea N, Berger NA, Lucia A. Exercise is the real polypill. Physiology (Bethesda) 2013;28:330–358 [DOI] [PubMed] [Google Scholar]
- 112.Berchtold MW, Brinkmeier H, Muntener M. Calcium ion in skeletal muscle: Its crucial role for muscle function, plasticity, and disease. Physiol Rev 2000;80:1215–1265 [DOI] [PubMed] [Google Scholar]
- 113.Wright DC, Hucker KA, Holloszy JO, Han DH. Ca2+ and AMPK both mediate stimulation of glucose transport by muscle contractions. Diabetes 2004;53:330–335 [DOI] [PubMed] [Google Scholar]
- 114.Raney MA, Turcotte LP. Evidence for the involvement of CaMKII and AMPK in Ca2+-dependent signaling pathways regulating FA uptake and oxidation in contracting rodent muscle. J Appl Physiol 2008;104:1366–1373 [DOI] [PubMed] [Google Scholar]
- 115.Wu H, Kanatous SB, Thurmond FA, Gallardo T, Isotani E, Bassel-Duby R, Williams RS. Regulation of mitochondrial biogenesis in skeletal muscle by CaMK. Science 2002;296:349–352 [DOI] [PubMed] [Google Scholar]
- 116.Goodyear LJ, Chang PY, Sherwood DJ, Dufresne SD, Moller DE. Effects of exercise and insulin on mitogen-activated protein kinase signaling pathways in rat skeletal muscle. Am J Physiol 1996;271:E403–E408 [DOI] [PubMed] [Google Scholar]
- 117.Gomez-Cabrera MC, Borras C, Pallardo FV, Sastre J, Ji LL, Vina J. Decreasing xanthine oxidase mediated oxidative stress prevents useful cellular adaptations to exercise in rats. J Physiol 2005;567(Pt 1):113–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Akimoto T, Pohnert SC, Li P, Zhang M, Gumbs C, Rosenberg PB, Williams RS, Yan Z. Exercise stimulates Pgc-1alpha transcription in skeletal muscle through activation of the p38 MAPK pathway. J Biol Chem 2005;280:19587–19593 [DOI] [PubMed] [Google Scholar]
- 119.Long YC, Widegren U, Zierath JR. Exercise-induced mitogen-activated protein kinase signalling in skeletal muscle. Proc Nutr Soc 2004;63:227–232 [DOI] [PubMed] [Google Scholar]
- 120.Salminen A, Vihko V. Endurance training reduces the susceptibility of mouse skeletal muscle to lipid peroxidation in vitro. Acta Physiol Scand 1983;117:109–113 [DOI] [PubMed] [Google Scholar]
- 121.Gomez-Cabrera MC, Domenech E, Vina J. Moderate exercise is an antioxidant: Upregulation of antioxidant genes by training. Free Radic Biol Med 2008;44:126–131 [DOI] [PubMed] [Google Scholar]
- 122.Powers SK, Duarte J, Kavazis AN, Talbert EE. Reactive oxygen species are signalling molecules for skeletal muscle adaptation. Exp Physiol 2010;95:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Petersen AC, McKenna MJ, Medved I, Murphy KT, Brown MJ, Della Gatta P, Cameron-Smith D. Infusion with the antioxidant N-acetylcysteine attenuates early adaptive responses to exercise in human skeletal muscle. Acta Physiol (Oxf) 2012;204:382–392 [DOI] [PubMed] [Google Scholar]
- 124.Pedersen BK, Febbraio MA. Muscles, exercise and obesity: Skeletal muscle as a secretory organ. Nat Rev Endocrinol 2012;8:457–465 [DOI] [PubMed] [Google Scholar]
- 125.Whitham M, Chan MH, Pal M, Matthews VB, Prelovsek O, Lunke S, El-Osta A, Broenneke H, Alber J, Bruning JC, Wunderlich FT, Lancaster GI, Febbraio MA. Contraction-induced interleukin-6 gene transcription in skeletal muscle is regulated by c-Jun terminal kinase/activator protein-1. J Biol Chem 2012;287:10771–10779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Carling D, Hardie DG. The substrate and sequence specificity of the AMP-activated protein kinase. Phosphorylation of glycogen synthase and phosphorylase kinase. Biochim Biophys Acta 1989;1012:81–86 [DOI] [PubMed] [Google Scholar]
- 127.Bergeron R, Ren JM, Cadman KS, Moore IK, Perret P, Pypaert M, Young LH, Semenkovich CF, Shulman GI. Chronic activation of AMP kinase results in NRF-1 activation and mitochondrial biogenesis. Am J Physiol Endocrinol Metab 2001;281:E1340–E1346 [DOI] [PubMed] [Google Scholar]
- 128.Jager S, Handschin C, St-Pierre J, Spiegelman BM. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proc Natl Acad Sci USA 2007;104:12017–12022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kahn BB, Alquier T, Carling D, Hardie DG. AMP-activated protein kinase: Ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab 2005;1:15–25 [DOI] [PubMed] [Google Scholar]
- 130.McGee SL, van Denderen BJ, Howlett KF, Mollica J, Schertzer JD, Kemp BE, Hargreaves M. AMP-activated protein kinase regulates GLUT4 transcription by phosphorylating histone deacetylase 5. Diabetes 2008;57:860–867 [DOI] [PubMed] [Google Scholar]
- 131.Lee JH, Budanov AV, Karin M. Sestrins orchestrate cellular metabolism to attenuate aging. Cell Metab 2013;18:792–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sanchis-Gomar F. Sestrins: Novel antioxidant and AMPK-modulating functions regulated by exercise? J Cell Physiol 2013;228:1647–1650 [DOI] [PubMed] [Google Scholar]
- 133.Schwer B, Verdin E. Conserved metabolic regulatory functions of sirtuins. Cell Metab 2008;7:104–112 [DOI] [PubMed] [Google Scholar]
- 134.Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P, Elliott P, Geny B, Laakso M, Puigserver P, Auwerx J. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell 2006;127:1109–1122 [DOI] [PubMed] [Google Scholar]
- 135.Gerhart-Hines Z, Rodgers JT, Bare O, Lerin C, Kim SH, Mostoslavsky R, Alt FW, Wu Z, Puigserver P. Metabolic control of muscle mitochondrial function and fatty acid oxidation through SIRT1/PGC-1alpha. EMBO J 2007;26:1913–1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Bodine SC, Stitt TN, Gonzalez M, Kline WO, Stover GL, Bauerlein R, Zlotchenko E, Scrimgeour A, Lawrence JC, Glass DJ, Yancopoulos GD. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol 2001;3:1014–1019 [DOI] [PubMed] [Google Scholar]
- 137.Sandri M. Signaling in muscle atrophy and hypertrophy. Physiology (Bethesda). 2008;23:160–170 [DOI] [PubMed] [Google Scholar]
- 138.Klossner S, Durieux AC, Freyssenet D, Flueck M. Mechano-transduction to muscle protein synthesis is modulated by FAK. Eur J Appl Physiol 2009;106:389–398 [DOI] [PubMed] [Google Scholar]
- 139.Durieux AC, D'Antona G, Desplanches D, Freyssenet D, Klossner S, Bottinelli R, Fluck M. Focal adhesion kinase is a load-dependent governor of the slow contractile and oxidative muscle phenotype. J Physiol 2009;587:3703–3717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wilkinson SB, Phillips SM, Atherton PJ, Patel R, Yarasheski KE, Tarnopolsky MA, Rennie MJ. Differential effects of resistance and endurance exercise in the fed state on signalling molecule phosphorylation and protein synthesis in human muscle. J Physiol 2008;586:3701–3717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Philp A, Hamilton DL, Baar K. Signals mediating skeletal muscle remodeling by resistance exercise: PI3-kinase independent activation of mTORC1. J Appl Physiol 2011;110:561–568 [DOI] [PubMed] [Google Scholar]
- 142.Schmutz S, Dapp C, Wittwer M, Vogt M, Hoppeler H, Fluck M. Endurance training modulates the muscular transcriptome response to acute exercise. Pflugers Arch 2006;451:678–687 [DOI] [PubMed] [Google Scholar]
- 143.Taylor CT. Mitochondria and cellular oxygen sensing in the HIF pathway. Biochem J 2008;409:19–26 [DOI] [PubMed] [Google Scholar]
- 144.Meeusen R. Exercise, nutrition and the brain. Sports Med 2014;44(Suppl 1):S47–S56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ellis T, Motl RW. Physical activity behavior change in persons with neurologic disorders: Overview and examples from Parkinson disease and multiple sclerosis. J Neurol Phys Ther 2013;37:85–90 [DOI] [PubMed] [Google Scholar]
- 146.Querfurth HW, LaFerla FM. Alzheimer's disease. N Engl J Med 2010;362:329–344 [DOI] [PubMed] [Google Scholar]
- 147.Lucia A, Ruiz JR. Exercise is beneficial for patients with Alzheimer's disease: A call for action. Br J Sports Med 2011;45:468–469 [DOI] [PubMed] [Google Scholar]
- 148.Santana-Sosa E, Barriopedro MI, Lopez-Mojares LM, Perez M, Lucia A. Exercise training is beneficial for Alzheimer's patients. Int J Sports Med 2008;29:845–850 [DOI] [PubMed] [Google Scholar]
- 149.Radak Z, Hart N, Sarga L, Koltai E, Atalay M, Ohno H, Boldogh I. Exercise plays a preventive role against Alzheimer's disease. J Alzheimers Dis. 2010;20:777–783 [DOI] [PubMed] [Google Scholar]
- 150.Radak Z, Ihasz F, Koltai E, Goto S, Taylor AW, Boldogh I. The redox-associated adaptive response of brain to physical exercise. Free Radic Res 2014;48:84–92 [DOI] [PubMed] [Google Scholar]
- 151.Pratico D. Oxidative stress hypothesis in Alzheimer's disease:A reappraisal. Trends Pharmacol Sci 2008;29:609–615 [DOI] [PubMed] [Google Scholar]
- 152.Markesbery WR. Oxidative stress hypothesis in Alzheimer's disease. Free Radic Biol Med 1997;23:134–147 [DOI] [PubMed] [Google Scholar]
- 153.Smith MA, Perry G, Richey PL, Sayre LM, Anderson VE, Beal MF, Kowall N. Oxidative damage in Alzheimer's. Nature 1996;382:120–121 [DOI] [PubMed] [Google Scholar]
- 154.Balazs L, Leon M. Evidence of an oxidative challenge in the Alzheimer's brain. Neurochem Res 1994;19:1131–1137 [DOI] [PubMed] [Google Scholar]
- 155.Coyle JT, Puttfarcken P. Oxidative stress, glutamate, and neurodegenerative disorders. Science 1993;262:689–695 [DOI] [PubMed] [Google Scholar]
- 156.Bondy SC. Reactive oxygen species: Relation to aging and neurotoxic damage. Neurotoxicology 1992;13:87–100 [PubMed] [Google Scholar]
- 157.Pacifici RE, Davies KJ. Protein, lipid and DNA repair systems in oxidative stress: The free-radical theory of aging revisited. Gerontology 1991;37:166–180 [DOI] [PubMed] [Google Scholar]
- 158.Markesbery WR, Carney JM. Oxidative alterations in Alzheimer's disease. Brain Pathol 1999;9:133–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Hara M, Iigo M, Ohtani-Kaneko R, Nakamura N, Suzuki T, Reiter RJ, Hirata K. Administration of melatonin and related indoles prevents exercise-induced cellular oxidative changes in rats. Biol Signals 1997;6:90–100 [DOI] [PubMed] [Google Scholar]
- 160.Somani SM, Ravi R, Rybak LP. Effect of exercise training on antioxidant system in brain regions of rat. Pharmacol Biochem Behav 1995;50:635–639 [DOI] [PubMed] [Google Scholar]
- 161.Garcia-Mesa Y, Pareja-Galeano H, Bonet-Costa V, Revilla S, Gomez-Cabrera MC, Gambini J, Gimenez-Llort L, Cristòfol R, Viña J, Sanfeliu C. Physical exercise neuroprotects ovariectomized 3xTg-AD mice through BDNF mechanisms. Psychoneuroendocrinology 2014;45:154–166 [DOI] [PubMed] [Google Scholar]
- 162.Knaepen K, Goekint M, Heyman EM, Meeusen R. Neuroplasticity - exercise-induced response of peripheral brain-derived neurotrophic factor: A systematic review of experimental studies in human subjects. Sports Med 2010;40:765–801 [DOI] [PubMed] [Google Scholar]
- 163.Nagahara AH, Merrill DA, Coppola G, Tsukada S, Schroeder BE, Shaked GM, Wang L, Blesch A, Kim A, Conner JM, Rockenstein E, Chao MV, Koo EH, Geschwind D, Masliah E, Chiba AA, Tuszynski MH. Neuroprotective effects of brain-derived neurotrophic factor in rodent and primate models of Alzheimer's disease. Nat Med 2009;15:331–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Ferris LT, Williams JS, Shen CL. The effect of acute exercise on serum brain-derived neurotrophic factor levels and cognitive function. Med Sci Sports Exerc 2007;39:728–734 [DOI] [PubMed] [Google Scholar]
- 165.Schmidt-Kassow M, Schadle S, Otterbein S, Thiel C, Doehring A, Lotsch J, Kaiser J. Kinetics of serum brain-derived neurotrophic factor following low-intensity versus high-intensity exercise in men and women. Neuroreport 2012;23:889–893 [DOI] [PubMed] [Google Scholar]
- 166.Winter B, Breitenstein C, Mooren FC, Voelker K, Fobker M, Lechtermann A, Krueger K, Fromme A, Korsukewitz C, Floel A, Knecht S. High impact running improves learning. Neurobiol Learning Memory 2007;87:597–609 [DOI] [PubMed] [Google Scholar]
- 167.Cotman CW, Berchtold NC. Exercise: A behavioral intervention to enhance brain health and plasticity. Trends Neurosci 2002;25:295–301 [DOI] [PubMed] [Google Scholar]
- 168.Gomez-Pinilla F, Ying Z, Roy RR, Molteni R, Edgerton VR. Voluntary exercise induces a BDNF-mediated mechanism that promotes neuroplasticity. J Neurophysiol 2002;88:2187–2195 [DOI] [PubMed] [Google Scholar]
- 169.Mousavi K, Parry DJ, Jasmin BJ. BDNF rescues myosin heavy chain IIB muscle fibers after neonatal nerve injury. Am J Physiol Cell Physiol 2004;287:C22–C29 [DOI] [PubMed] [Google Scholar]
- 170.Vaynman S, Ying Z, Gomez-Pinilla F. Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. Eur J Neurosci 2004;20:2580–2590 [DOI] [PubMed] [Google Scholar]
- 171.Murphy DD, Segal M. Morphological plasticity of dendritic spines in central neurons is mediated by activation of cAMP response element binding protein. Proc Natl Acad Sci USA 1997;94:1482–1487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Miyamoto E. Molecular mechanism of neuronal plasticity: Induction and maintenance of long-term potentiation in the hippocampus. J Pharmacol Sci. 2006;100:433–442 [DOI] [PubMed] [Google Scholar]
- 173.Cheng A, Wan R, Yang JL, Kamimura N, Son TG, Ouyang X, Luo Y, Okun E, Mattson MP. Involvement of PGC-1alpha in the formation and maintenance of neuronal dendritic spines. Nat Commun 2012;3:1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Carlezon WA, Jr., Duman RS, Nestler EJ. The many faces of CREB. Trends Neurosci 2005;28:436–445 [DOI] [PubMed] [Google Scholar]
- 175.Vaynman S, Ying Z, Gomez-Pinilla F. Interplay between brain-derived neurotrophic factor and signal transduction modulators in the regulation of the effects of exercise on synaptic-plasticity. Neuroscience 2003;122:647–657 [DOI] [PubMed] [Google Scholar]
- 176.Russo-Neustadt AA, Chen MJ. Brain-derived neurotrophic factor and antidepressant activity. Curr Pharm Des 2005;11:1495–1510 [DOI] [PubMed] [Google Scholar]
- 177.Ding Q, Vaynman S, Akhavan M, Ying Z, Gomez-Pinilla F. Insulin-like growth factor I interfaces with brain-derived neurotrophic factor-mediated synaptic plasticity to modulate aspects of exercise-induced cognitive function. Neuroscience 2006;140:823–833 [DOI] [PubMed] [Google Scholar]
- 178.Llorens-Martin M, Torres-Aleman I, Trejo JL. Growth factors as mediators of exercise actions on the brain. Neuromolecular Med 2008;10:99–107 [DOI] [PubMed] [Google Scholar]
- 179.McCusker RH, McCrea K, Zunich S, Dantzer R, Broussard SR, Johnson RW, Kelley KW. Insulin-like growth factor-I enhances the biological activity of brain-derived neurotrophic factor on cerebrocortical neurons. J Neuroimmunol 2006;179:186–190 [DOI] [PubMed] [Google Scholar]
- 180.Fabel K, Fabel K, Tam B, Kaufer D, Baiker A, Simmons N, Kuo CJ, Palmer TD. VEGF is necessary for exercise-induced adult hippocampal neurogenesis. Eur J Neurosci 2003;18:2803–2812 [DOI] [PubMed] [Google Scholar]
- 181.Neeper SA, Gomez-Pinilla F, Choi J, Cotman CW. Physical activity increases mRNA for brain-derived neurotrophic factor and nerve growth factor in rat brain. Brain Res 1996;726:49–56 [PubMed] [Google Scholar]
- 182.Revilla S, Sunol C, Garcia-Mesa Y, Gimenez-Llort L, Sanfeliu C, Cristofol R. Physical exercise improves synaptic dysfunction and recovers the loss of survival factors in 3xTg-AD mouse brain. Neuropharmacology 2014;81:55–63 [DOI] [PubMed] [Google Scholar]
- 183.Koo HM, Lee SM, Kim MH. Spontaneous Wheel running exercise induces brain recovery via neurotrophin-3 expression following experimental traumatic brain injury in rats. J Phys Ther Sci 2013;25:1103–1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Chung JY, Kim MW, Bang MS, Kim M. Increased expression of neurotrophin 4 following focal cerebral ischemia in adult rat brain with treadmill exercise. PLoS One 2013;8:e52461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nat Med 1998;4:1313–1317 [DOI] [PubMed] [Google Scholar]
- 186.Vivar C, Potter MC, van Praag H. All about running: Synaptic plasticity, growth factors and adult hippocampal neurogenesis. Curr Top Behav Neurosci 2013;15:189–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci USA 1999;96:13427–13431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Rossi C, Angelucci A, Costantin L, Braschi C, Mazzantini M, Babbini F, Fabbri ME, Tessarollo L, Maffei L, Berardi N, Caleo M. Brain-derived neurotrophic factor (BDNF) is required for the enhancement of hippocampal neurogenesis following environmental enrichment. Eur J Neurosci Oct 2006;24:1850–1856 [DOI] [PubMed] [Google Scholar]
- 189.Bath KG, Akins MR, Lee FS. BDNF control of adult SVZ neurogenesis. Dev Psychobiol 2012;54:578–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Llorens-Martin M, Torres-Aleman I, Trejo JL. Mechanisms mediating brain plasticity: IGF1 and adult hippocampal neurogenesis. Neuroscientist 2009;15:134–148 [DOI] [PubMed] [Google Scholar]
- 191.Lazarov O, Robinson J, Tang YP, Hairston IS, Korade-Mirnics Z, Lee VM, Hersh LB, Sapolsky RM, Mirnics K, Sisodia SS. Environmental enrichment reduces Abeta levels and amyloid deposition in transgenic mice. Cell 2005;120:701–713 [DOI] [PubMed] [Google Scholar]
- 192.Dehvari N, Mahmud T, Persson J, Bengtsson T, Graff C, Winblad B, Ronnback A, Behbahani H. Amyloid precursor protein accumulates in aggresomes in response to proteasome inhibitor. Neurochem Int 2012;60:533–542 [DOI] [PubMed] [Google Scholar]
- 193.Lee MJ, Lee JH, Rubinsztein DC. Tau degradation: The ubiquitin-proteasome system versus the autophagy-lysosome system. Prog Neurobiol 2013;105:49–59 [DOI] [PubMed] [Google Scholar]
- 194.Lopez-Salon M, Alonso M, Vianna MR, Viola H, Mello e Souza T, Izquierdo I, Pasquini JM, Medina JH. The ubiquitin-proteasome cascade is required for mammalian long-term memory formation. Eur J Neurosci 2001;14:1820–1826 [DOI] [PubMed] [Google Scholar]
- 195.Karpova A, Mikhaylova M, Thomas U, Knopfel T, Behnisch T. Involvement of protein synthesis and degradation in long-term potentiation of Schaffer collateral CA1 synapses. J Neurosci 2006;26:4949–4955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell 2013;153:1194–1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Safdar A, Bourgeois JM, Ogborn DI, Little JP, Hettinga BP, Akhtar M, Thompson JE, Melov S, Mocellin NJ, Kujoth GC, Prolla TA, Tarnopolsky MA. Endurance exercise rescues progeroid aging and induces systemic mitochondrial rejuvenation in mtDNA mutator mice. Proc Natl Acad Sci USA 2011;108:4135–4140 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 198.Gomez-Cabrera MC, Sanchis-Gomar F, Garcia-Valles R, Pareja-Galeano H, Gambini J, Borras C, Vina J. Mitochondria as sources and targets of damage in cellular aging. Clin Chem Lab Med 2012;50:1287–1295 [DOI] [PubMed] [Google Scholar]
- 199.Parise G, Brose AN, Tarnopolsky MA. Resistance exercise training decreases oxidative damage to DNA and increases cytochrome oxidase activity in older adults. Exp Gerontol 2005;40:173–180 [DOI] [PubMed] [Google Scholar]
- 200.Radak Z, Naito H, Kaneko T, Tahara S, Nakamoto H, Takahashi R, Cardozo-Pelaez F, Goto S. Exercise training decreases DNA damage and increases DNA repair and resistance against oxidative stress of proteins in aged rat skeletal muscle. Pflugers Archiv 2002;445:273–278 [DOI] [PubMed] [Google Scholar]
- 201.Leick L, Lyngby SS, Wojtaszewski JF, Pilegaard H. PGC-1alpha is required for training-induced prevention of age-associated decline in mitochondrial enzymes in mouse skeletal muscle. Exp Gerontol 2010;45:336–342 [DOI] [PubMed] [Google Scholar]
- 202.Aviv A, Valdes A, Gardner JP, Swaminathan R, Kimura M, Spector TD. Menopause modifies the association of leukocyte telomere length with insulin resistance and inflammation. J Clin Endocrinol Metab 2006;91:635–640 [DOI] [PubMed] [Google Scholar]
- 203.Benetos A, Okuda K, Lajemi M, Kimura M, Thomas F, Skurnick J, Labat C, Bean K, Aviv A. Telomere length as an indicator of biological aging: The gender effect and relation with pulse pressure and pulse wave velocity. Hypertension 2001;37:381–385 [DOI] [PubMed] [Google Scholar]
- 204.Brouilette S, Singh RK, Thompson JR, Goodall AH, Samani NJ. White cell telomere length and risk of premature myocardial infarction. Arterioscler Thromb Vasc Biol 2003;23:842–846 [DOI] [PubMed] [Google Scholar]
- 205.Gardner JP, Li S, Srinivasan SR, Chen W, Kimura M, Lu X, Berenson GS, Aviv A. Rise in insulin resistance is associated with escalated telomere attrition. Circulation 2005;111:2171–2177 [DOI] [PubMed] [Google Scholar]
- 206.Jeanclos E, Schork NJ, Kyvik KO, Kimura M, Skurnick JH, Aviv A. Telomere length inversely correlates with pulse pressure and is highly familial. Hypertension 2000;36:195–200 [DOI] [PubMed] [Google Scholar]
- 207.Nawrot TS, Staessen JA, Gardner JP, Aviv A. Telomere length and possible link to X chromosome. Lancet 2004;363:507–510 [DOI] [PubMed] [Google Scholar]
- 208.Samani NJ, Boultby R, Butler R, Thompson JR, Goodall AH. Telomere shortening in atherosclerosis. Lancet 2001;358:472–473 [DOI] [PubMed] [Google Scholar]
- 209.Sampson MJ, Winterbone MS, Hughes JC, Dozio N, Hughes DA. Monocyte telomere shortening and oxidative DNA damage in type 2 diabetes. Diabetes Care 2006;29:283–289 [DOI] [PubMed] [Google Scholar]
- 210.Valdes AM, Andrew T, Gardner JP, Kimura M, Oelsner E, Cherkas LF, Aviv A, Spector TD. Obesity, cigarette smoking, and telomere length in women. Lancet 2005;366:662–664 [DOI] [PubMed] [Google Scholar]
- 211.Cherkas LF, Hunkin JL, Kato BS, Richards JB, Gardner JP, Surdulescu GL, Kimura M, Lu X, Spector TD, Aviv A. The association between physical activity in leisure time and leukocyte telomere length. Arch Intern Med 2008;168:154–158 [DOI] [PubMed] [Google Scholar]
- 212.Denham J, Nelson CP, O'Brien BJ, Nankervis SA, Denniff M, Harvey JT, Marques FZ, Codd V, Zukowska-Szczechowska E, Samani NJ, Tomaszewski M, Charchar FJ. Longer leukocyte telomeres are associated with ultra-endurance exercise independent of cardiovascular risk factors. PLoS One 2013;8:e69377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.LaRocca TJ, Seals DR, Pierce GL. Leukocyte telomere length is preserved with aging in endurance exercise-trained adults and related to maximal aerobic capacity. Mech Ageing Dev 2010;131:165–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Ludlow AT, Zimmerman JB, Witkowski S, Hearn JW, Hatfield BD, Roth SM. Relationship between physical activity level, telomere length, and telomerase activity. Med Sci Sports Exerc 2008;40:1764–1771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Puterman E, Lin J, Blackburn E, O'Donovan A, Adler N, Epel E. The power of exercise: Buffering the effect of chronic stress on telomere length. PLoS One 2010;5:e10837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Werner C, Furster T, Widmann T, Poss J, Roggia C, Hanhoun M, Scharhag J, Buchner N, Meyer T, Kindermann W, Haendeler J, Bohm M, Laufs U. Physical exercise prevents cellular senescence in circulating leukocytes and in the vessel wall. Circulation 2009;120:2438–2447 [DOI] [PubMed] [Google Scholar]
- 217.Laye MJ, Solomon TP, Karstoft K, Pedersen KK, Nielsen SD, Pedersen BK. Increased shelterin mRNA expression in peripheral blood mononuclear cells and skeletal muscle following an ultra-long-distance running event. J Appl Physiol 2012;112:773–781 [DOI] [PubMed] [Google Scholar]
- 218.Osthus IB, Sgura A, Berardinelli F, Alsnes IV, Bronstad E, Rehn T, Stobakk PK, Hatle H, Wisloff U, Nauman J. Telomere length and long-term endurance exercise: Does exercise training affect biological age? A pilot study. PLoS One 2012;7:e52769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Savela S, Saijonmaa O, Strandberg TE, Koistinen P, Strandberg AY, Tilvis RS, Pitkala KH, Miettinen TA, Fyhrquist F. Physical activity in midlife and telomere length measured in old age. Exp Gerontol 2013;48:81–84 [DOI] [PubMed] [Google Scholar]
- 220.Kodama S, Saito K, Tanaka S, Maki M, Yachi Y, Asumi M, Sugawara A, Totsuka K, Shimano H, Ohashi Y, Yamada N, Sone H. Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: A meta-analysis. JAMA 2009;301:2024–2035 [DOI] [PubMed] [Google Scholar]
- 221.Chilton WL, Marques FZ, West J, Kannourakis G, Berzins SP, O'Brien BJ, Charchar FJ. Acute exercise leads to regulation of telomere-associated genes and microRNA expression in immune cells. PLoS One 2014;9:e92088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Tosato M, Zamboni V, Ferrini A, Cesari M. The aging process and potential interventions to extend life expectancy. Clin Interv Aging 2007;2:401–412 [PMC free article] [PubMed] [Google Scholar]
- 223.Ludlow AT, Roth SM. Physical activity and telomere biology: Exploring the link with aging-related disease prevention. J Aging Res 2011;2011:790378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Rae DE, Vignaud A, Butler-Browne GS, Thornell LE, Sinclair-Smith C, Derman EW, Lambert MI, Collins M. Skeletal muscle telomere length in healthy, experienced, endurance runners. Eur J Appl Physiol 2010;109:323–330 [DOI] [PubMed] [Google Scholar]
- 225.Ntanasis-Stathopoulos J, Tzanninis JG, Philippou A, Koutsilieris M. Epigenetic regulation on gene expression induced by physical exercise. J Musculoskelet Neuronal Interact 2013;13:133–146 [PubMed] [Google Scholar]
- 226.Pareja-Galeano H, Sanchis-Gomar F, Garcia-Gimenez JL. Physical exercise and epigenetic modulation: Elucidating intricate mechanisms. Sports Med 2014;44:429—436 [DOI] [PubMed] [Google Scholar]
- 227.Ling C, Ronn T. Epigenetic adaptation to regular exercise in humans. Drug Discov Today 2014; doi: 10.1016/j.drudis.2014.03.006 [DOI] [PubMed] [Google Scholar]
- 228.Werner C, Hanhoun M, Widmann T, Kazakov A, Semenov A, Poss J, Bauersachs J, Thum T, Pfreundschuh M, Muller P, Haendeler J, Bohm M, Laufs U. Effects of physical exercise on myocardial telomere-regulating proteins, survival pathways, and apoptosis. J Am Coll Cardiol 2008;52:470–482 [DOI] [PubMed] [Google Scholar]
- 229.Wolf SA, Melnik A, Kempermann G. Physical exercise increases adult neurogenesis and telomerase activity, and improves behavioral deficits in a mouse model of schizophrenia. Brain Behav Immun 2011;25:971–980 [DOI] [PubMed] [Google Scholar]
- 230.Barres R, Yan J, Egan B, Treebak JT, Rasmussen M, Fritz T, Caidahl K, Krook A, O'Gorman DJ, Zierath JR. Acute exercise remodels promoter methylation in human skeletal muscle. Cell Metab 2012;15:405–411 [DOI] [PubMed] [Google Scholar]
- 231.Perry CG, Lally J, Holloway GP, Heigenhauser GJ, Bonen A, Spriet LL. Repeated transient mRNA bursts precede increases in transcriptional and mitochondrial proteins during training in human skeletal muscle. J Physiol 2010;588:4795–4810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Holloszy JO, Coyle EF. Adaptations of skeletal muscle to endurance exercise and their metabolic consequences. J Appl Physiol 1984;56:831–838 [DOI] [PubMed] [Google Scholar]
- 233.Booth FW, Thomason DB. Molecular and cellular adaptation of muscle in response to exercise: Perspectives of various models. Physiol Rev 1991;71:541–585 [DOI] [PubMed] [Google Scholar]
- 234.Rasmussen M, Zierath JR, Barres R. Dynamic epigenetic responses to muscle contraction. Drug Discov Today 2014;19:1010–1014 [DOI] [PubMed] [Google Scholar]
- 235.Rasmussen M, Zierath JR, Barres R. Dynamic epigenetic responses to muscle contraction. Drug Discov Today 2014 [DOI] [PubMed] [Google Scholar]
- 236.Potthoff MJ, Wu H, Arnold MA, Shelton JM, Backs J, McAnally J, Richardson JA, Bassel-Duby R, Olson EN. Histone deacetylase degradation and MEF2 activation promote the formation of slow-twitch myofibers. J Clin Invest 2007;117:2459–2467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Dayeh TA, Olsson AH, Volkov P, Almgren P, Ronn T, Ling C. Identification of CpG-SNPs associated with type 2 diabetes and differential DNA methylation in human pancreatic islets. Diabetologia 2013;56:1036–1046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Nitert MD, Dayeh T, Volkov P, Elgzyri T, Hall E, Nilsson E, Yang BT, Lang S, Parikh H, Wessman Y, Weishaupt H, Attema J, Abels M, Wierup N, Almgren P, Jansson PA, Ronn T, Hansson O, Eriksson KF, Groop L, Ling C. Impact of an exercise intervention on DNA methylation in skeletal muscle from first-degree relatives of patients with type 2 diabetes. Diabetes 2012;61:3322–3332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Kaliman P, Parrizas M, Lalanza JF, Camins A, Escorihuela RM, Pallas M. Neurophysiological and epigenetic effects of physical exercise on the aging process. Ageing Res Rev 2011;10:475–486 [DOI] [PubMed] [Google Scholar]
- 240.Nakajima K, Takeoka M, Mori M, Hashimoto S, Sakurai A, Nose H, Higuchi K, Itano N, Shiohara M, Oh T, Taniguchi S. Exercise effects on methylation of ASC gene. Int J Sports Med 2010;31:671–675 [DOI] [PubMed] [Google Scholar]
- 241.Ronn T, Volkov P, Davegardh C, Dayeh T, Hall E, Olsson AH, Nilsson E, Tornberg A, Dekker Nitert M, Eriksson KF, Jones HA, Groop L, Ling C. A six months exercise intervention influences the genome-wide DNA methylation pattern in human adipose tissue. PLoS Genet 2013;9:e1003572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Sanchis-Gomar F, Garcia-Gimenez JL, Perez-Quilis C, Gomez-Cabrera MC, Pallardo FV, Lippi G. Physical exercise as an epigenetic modulator: Eustress, the “positive stress” as an effector of gene expression. J Strength Cond Res 2012;26:3469–3472 [DOI] [PubMed] [Google Scholar]
- 243.Perdiguero E, Sousa-Victor P, Ballestar E, Munoz-Canoves P. Epigenetic regulation of myogenesis. Epigenetics 2009;4:541–550 [DOI] [PubMed] [Google Scholar]
- 244.Raue U, Slivka D, Jemiolo B, Hollon C, Trappe S. Myogenic gene expression at rest and after a bout of resistance exercise in young (18–30 yr) and old (80–89 yr) women. J Appl Physiol 2006;101:53–59 [DOI] [PubMed] [Google Scholar]
- 245.Gomez-Pinilla F, Zhuang Y, Feng J, Ying Z, Fan G. Exercise impacts brain-derived neurotrophic factor plasticity by engaging mechanisms of epigenetic regulation. Eur J Neurosci 2011;33:383–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Koga H, Kaushik S, Cuervo AM. Protein homeostasis and aging: The importance of exquisite quality control. Ageing Res Rev 2010;10:205–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Powers ET, Morimoto RI, Dillin A, Kelly JW, Balch WE. Biological and chemical approaches to diseases of proteostasis deficiency. Annu Rev Biochem 2009;78:959–991 [DOI] [PubMed] [Google Scholar]
- 248.Rubinsztein DC, Marino G, Kroemer G. Autophagy and aging. Cell 2011;146:682–695 [DOI] [PubMed] [Google Scholar]
- 249.Tomaru U, Takahashi S, Ishizu A, Miyatake Y, Gohda A, Suzuki S, Ono A, Ohara J, Baba T, Murata S, Tanaka K, Kasahara M. Decreased proteasomal activity causes age-related phenotypes and promotes the development of metabolic abnormalities. Am J Pathol 2012;180:963–972 [DOI] [PubMed] [Google Scholar]
- 250.Tanida I. Autophagosome formation and molecular mechanism of autophagy. Antioxid Redox Signal 2010;14:2201–2214 [DOI] [PubMed] [Google Scholar]
- 251.He C, Sumpter R, Jr., Levine B. Exercise induces autophagy in peripheral tissues and in the brain. Autophagy 2012;8:1548–1551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Fiuza-Luces C, Delmiro A, Soares-Miranda L, Gonzalez-Murillo A, Martinez-Palacios J, Ramirez M, Lucia A, Moran M. Exercise training can induce cardiac autophagy at end-stage chronic conditions: Insights from a graft-versus-host-disease mouse model. Brain Behav Immun. 2014;39:56–60 [DOI] [PubMed] [Google Scholar]
- 253.Kim YA, Kim YS, Oh SL, Kim HJ, Song W. Autophagic response to exercise training in skeletal muscle with age. J Physiol Biochem 2013;69:697–705 [DOI] [PubMed] [Google Scholar]
- 254.Luo L, Lu AM, Wang Y, Hong A, Chen Y, Hu J, Li X, Qin ZH. Chronic resistance training activates autophagy and reduces apoptosis of muscle cells by modulating IGF-1 and its receptors, Akt/mTOR and Akt/FOXO3a signaling in aged rats. Exp Gerontol 2013;48:427–436 [DOI] [PubMed] [Google Scholar]
- 255.Wohlgemuth SE, Lees HA, Marzetti E, Manini TM, Aranda JM, Daniels MJ, Pahor M, Perri MG, Leeuwenburgh C, Anton SD. An exploratory analysis of the effects of a weight loss plus exercise program on cellular quality control mechanisms in older overweight women. Rejuvenation Res 2011;14:315–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.He C, Bassik MC, Moresi V, Sun K, Wei Y, Zou Z, An Z, Loh J, Fisher J, Sun Q, Korsmeyer S, Packer M, May HI, Hill JA, Virgin HW, Gilpin C, Xiao G, Bassel-Duby R, Scherer PE, Levine B. Exercise-induced BCL2-regulated autophagy is required for muscle glucose homeostasis. Nature 2012;481:511–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Pickford F, Masliah E, Britschgi M, Lucin K, Narasimhan R, Jaeger PA, Small S, Spencer B, Rockenstein E, Levine B, Wyss-Coray T. The autophagy-related protein beclin 1 shows reduced expression in early Alzheimer disease and regulates amyloid beta accumulation in mice. J Clin Invest 2008;118:2190–2199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Lipinski MM, Zheng B, Lu T, Yan Z, Py BF, Ng A, Xavier RJ, Li C, Yankner BA, Scherzer CR, Yuan J. Genome-wide analysis reveals mechanisms modulating autophagy in normal brain aging and in Alzheimer's disease. Proc Natl Acad Sci USA 2010;107:14164–14169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Emanuele E, Minoretti P, Sanchis-Gomar F, Pareja-Galeano H, Yilmaz Y, Garatachea N, Lucia A. Can enhanced autophagy be associated with human longevity? Serum levels of the autophagy biomarker beclin-1 are increased in healthy centenarians. Rejuvenation Res 2014;17:518–524 [DOI] [PubMed] [Google Scholar]
- 260.MacKenzie MG, Hamilton DL, Murray JT, Taylor PM, Baar K. mVps34 is activated following high-resistance contractions. J Physiol 2009;587:253–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Wohlgemuth SE, Seo AY, Marzetti E, Lees HA, Leeuwenburgh C. Skeletal muscle autophagy and apoptosis during aging: Effects of calorie restriction and life-long exercise. Exp Gerontol 2010;45:138–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Zaglia T, Milan G, Ruhs A, Franzoso M, Bertaggia E, Pianca N, Carpi A, Carullo P, Pesce P, Sacerdoti D, Sarais C, Catalucci D, Kruger M, Mongillo M, Sandri M. Atrogin-1 deficiency promotes cardiomyopathy and premature death via impaired autophagy. J Clin Invest 2014;124:2410–2424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Sandri M, Sandri C, Gilbert A, Skurk C, Calabria E, Picard A, Walsh K, Schiaffino S, Lecker SH, Goldberg AL. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell 2004;117:399–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Zaglia T, Milan G, Franzoso M, Bertaggia E, Pianca N, Piasentini E, Voltarelli VA, Chiavegato D, Brum PC, Glass DJ, Schiaffino S, Sandri M, Mongillo M. Cardiac sympathetic neurons provide trophic signal to the heart via beta2-adrenoceptor-dependent regulation of proteolysis. Cardiovasc Res 2013;97:240–250 [DOI] [PubMed] [Google Scholar]
- 265.Coiro V, Volpi R, Gramellini D, Maffei ML, Volta E, Melani A, Chiodera P. Effect of physical training on age-related reduction of GH secretion during exercise in normally cycling women. Maturitas 2010;65:392–395 [DOI] [PubMed] [Google Scholar]
- 266.Fujita S, Rasmussen BB, Cadenas JG, Drummond MJ, Glynn EL, Sattler FR, Volpi E. Aerobic exercise overcomes the age-related insulin resistance of muscle protein metabolism by improving endothelial function and Akt/mammalian target of rapamycin signaling. Diabetes 2007;56:1615–1622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Mayhew DL, Kim JS, Cross JM, Ferrando AA, Bamman MM. Translational signaling responses preceding resistance training-mediated myofiber hypertrophy in young and old humans. J Appl Physiol 2009;107:1655–1662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Pasiakos SM. Exercise and amino acid anabolic cell signaling and the regulation of skeletal muscle mass. Nutrients 2012;4:740–758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Mann S, Beedie C, Balducci S, Zanuso S, Allgrove J, Bertiato F, Jimenez A. Changes in insulin sensitivity in response to different modalities of exercise: A review of the evidence. Diabetes Metab Res Rev 2014;30:237–268 [DOI] [PubMed] [Google Scholar]
- 270.Kraemer WJ, Marchitelli L, Gordon SE, Harman E, Dziados JE, Mello R, Frykman P, McCurry D, Fleck SJ. Hormonal and growth factor responses to heavy resistance exercise protocols. J Appl Physiol 1990;69:1442–1450 [DOI] [PubMed] [Google Scholar]
- 271.Gotshalk LA, Loebel CC, Nindl BC, Putukian M, Sebastianelli WJ, Newton RU, Hakkinen K, Kraemer WJ. Hormonal responses of multiset versus single-set heavy-resistance exercise protocols. Can J Appl Physiol 1997;22:244–255 [DOI] [PubMed] [Google Scholar]
- 272.Kraemer WJ, Noble BJ, Clark MJ, Culver BW. Physiologic responses to heavy-resistance exercise with very short rest periods. Int J Sports Med 1987;8:247–252 [DOI] [PubMed] [Google Scholar]
- 273.Kraemer WJ, Ratamess NA. Hormonal responses and adaptations to resistance exercise and training. Sports Med 2005;35:339–361 [DOI] [PubMed] [Google Scholar]
- 274.West DW, Kujbida GW, Moore DR, Atherton P, Burd NA, Padzik JP, De Lisio M, Tang JE, Parise G, Rennie MJ, Baker SK, Phillips SM. Resistance exercise-induced increases in putative anabolic hormones do not enhance muscle protein synthesis or intracellular signalling in young men. J Physiol 2009;587:5239–5247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Hartman JW, Moore DR, Phillips SM. Resistance training reduces whole-body protein turnover and improves net protein retention in untrained young males. Appl Physiol Nutr Metab 2006;31:557–564 [DOI] [PubMed] [Google Scholar]
- 276.Yarasheski KE, Zachwieja JJ, Bier DM. Acute effects of resistance exercise on muscle protein synthesis rate in young and elderly men and women. Am J Physiol 1993;265:E210–E214 [DOI] [PubMed] [Google Scholar]
- 277.Yarasheski KE, Pak-Loduca J, Hasten DL, Obert KA, Brown MB, Sinacore DR. Resistance exercise training increases mixed muscle protein synthesis rate in frail women and men >/=76 yr old. Am J Physiol 1999;277:E118–E125 [DOI] [PubMed] [Google Scholar]
- 278.Esmarck B, Andersen JL, Olsen S, Richter EA, Mizuno M, Kjaer M. Timing of postexercise protein intake is important for muscle hypertrophy with resistance training in elderly humans. J Physiol 2001;535:301–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Kosek DJ, Kim JS, Petrella JK, Cross JM, Bamman MM. Efficacy of 3 days/wk resistance training on myofiber hypertrophy and myogenic mechanisms in young vs. older adults. J Appl Physiol 2006;101:531–544 [DOI] [PubMed] [Google Scholar]
- 280.Drummond MJ, Dreyer HC, Pennings B, Fry CS, Dhanani S, Dillon EL, Sheffield-Moore M, Volpi E, Rasmussen BB. Skeletal muscle protein anabolic response to resistance exercise and essential amino acids is delayed with aging. J Appl Physiol 2008;104:1452–1461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Welle S, Thornton C, Statt M. Myofibrillar protein synthesis in young and old human subjects after three months of resistance training. Am J Physiol 1995;268:E422–E427 [DOI] [PubMed] [Google Scholar]
- 282.Aniansson A, Grimby G, Rundgren A. Isometric and isokinetic quadriceps muscle strength in 70-year-old men and women. Scand J Rehabil Med 1980;12:161–168 [PubMed] [Google Scholar]
- 283.Linnane AW, Baumer A, Maxwell RJ, Preston H, Zhang CF, Marzuki S. Mitochondrial gene mutation: The ageing process and degenerative diseases. Biochem Int 1990;22:1067–1076 [PubMed] [Google Scholar]
- 284.Cortopassi GA, Arnheim N. Detection of a specific mitochondrial DNA deletion in tissues of older humans. Nucleic Acids Res 1990;18:6927–6933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Cortopassi GA, Shibata D, Soong NW, Arnheim N. A pattern of accumulation of a somatic deletion of mitochondrial DNA in aging human tissues. Proc Natl Acad Sci USA 1992;89:7370–7374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Derbre F, Gomez-Cabrera MC, Nascimento AL, Sanchis-Gomar F, Martinez-Bello VE, Tresguerres JA, Fuentes T, Gratas-Delamarche A, Monsalve M, Vina J. Age associated low mitochondrial biogenesis may be explained by lack of response of PGC-1alpha to exercise training. Age (Dordr) 2012;34:669–679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Broskey NT, Greggio C, Boss A, Boutant M, Dwyer A, Schlueter L, Hans D, Gremion G, Kreis R, Boesch C, Canto C, Amati F. Skeletal muscle mitochondria in the elderly: Effects of physical fitness and exercise training. J Clin Endocrinol Metab 2014;99:1852–1861 [DOI] [PubMed] [Google Scholar]
- 288.Harman D. Aging: A theory based on free radical and radiation chemistry. J Gerontol 1956;11:298–300 [DOI] [PubMed] [Google Scholar]
- 289.Hebert SL, Lanza IR, Nair KS. Mitochondrial DNA alterations and reduced mitochondrial function in aging. Mech Ageing Dev 2010;131:451–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Short KR, Bigelow ML, Kahl J, Singh R, Coenen-Schimke J, Raghavakaimal S, Nair KS. Decline in skeletal muscle mitochondrial function with aging in humans. Proc Natl Acad Sci USA 2005;102:5618–5623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Lanza IR, Short DK, Short KR, Raghavakaimal S, Basu R, Joyner MJ, McConnell JP, Nair KS. Endurance exercise as a countermeasure for aging. Diabetes 2008;57:2933–2942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Rooyackers OE, Adey DB, Ades PA, Nair KS. Effect of age on in vivo rates of mitochondrial protein synthesis in human skeletal muscle. Proc Natl Acad Sci USA 1996;93:15364–15369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Welle S, Bhatt K, Thornton CA. High-abundance mRNAs in human muscle: Comparison between young and old. J Appl Physiol 2000;89:297–304 [DOI] [PubMed] [Google Scholar]
- 294.Zahn JM, Sonu R, Vogel H, Crane E, Mazan-Mamczarz K, Rabkin R, Davis RW, Becker KG, Owen AB, Kim SK. Transcriptional profiling of aging in human muscle reveals a common aging signature. PLoS Genet Jul 2006;2:e115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Melov S, Tarnopolsky MA, Beckman K, Felkey K, Hubbard A. Resistance exercise reverses aging in human skeletal muscle. PLoS One 2007;2:e465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Brierley EJ, Johnson MA, Lightowlers RN, James OF, Turnbull DM. Role of mitochondrial DNA mutations in human aging: Implications for the central nervous system and muscle. Ann Neurol 1998;43:217–223 [DOI] [PubMed] [Google Scholar]
- 297.Bua E, Johnson J, Herbst A, Delong B, McKenzie D, Salamat S, Aiken JM. Mitochondrial DNA-deletion mutations accumulate intracellularly to detrimental levels in aged human skeletal muscle fibers. Am J Hum Genet 2006;79:469–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Bua EA, McKiernan SH, Wanagat J, McKenzie D, Aiken JM. Mitochondrial abnormalities are more frequent in muscles undergoing sarcopenia. J Appl Physiol 2002;92:2617–2624 [DOI] [PubMed] [Google Scholar]
- 299.Tarnopolsky MA. Mitochondrial DNA shifting in older adults following resistance exercise training. Appl Physiol Nutr Metabolism 2009;34:348–354 [DOI] [PubMed] [Google Scholar]
- 300.Gomes AP, Price NL, Ling AJ, Moslehi JJ, Montgomery MK, Rajman L, White JP, Teodoro JS, Wrann CD, Hubbard BP, Mercken EM, Palmeira CM, de Cabo R, Rolo AP, Turner N, Bell EL, Sinclair DA. Declining NAD(+) Induces a pseudohypoxic state disrupting nuclear-mitochondrial communication during aging. Cell 2013;155:1624–1638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Howald H, Hoppeler H, Claassen H, Mathieu O, Straub R. Influences of endurance training on the ultrastructural composition of the different muscle fiber types in humans. Pflugers Arch 1985;403:369–376 [DOI] [PubMed] [Google Scholar]
- 302.Broholm C, Pedersen BK. Leukaemia inhibitory factor–an exercise-induced myokine. Exercise Immunol Rev 2010;16:77–85 [PubMed] [Google Scholar]
- 303.Bigley AB, Spielmann G, LaVoy EC, Simpson RJ. Can exercise-related improvements in immunity influence cancer prevention and prognosis in the elderly? Maturitas 2013;76:51–56 [DOI] [PubMed] [Google Scholar]
- 304.Alcorta DA, Xiong Y, Phelps D, Hannon G, Beach D, Barrett JC. Involvement of the cyclin-dependent kinase inhibitor p16 (INK4a) in replicative senescence of normal human fibroblasts. Proc Natl Acad Sci USA 1996;93:13742–13747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Stein GH, Drullinger LF, Soulard A, Dulic V. Differential roles for cyclin-dependent kinase inhibitors p21 and p16 in the mechanisms of senescence and differentiation in human fibroblasts. Mol Cell Biol 1999;19:2109–2117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Herbig U, Jobling WA, Chen BP, Chen DJ, Sedivy JM. Telomere shortening triggers senescence of human cells through a pathway involving ATM, p53, and p21(CIP1), but not p16(INK4a). Mol Cell 2004;14:501–513 [DOI] [PubMed] [Google Scholar]
- 307.Sousa-Victor P, Gutarra S, Garcia-Prat L, Rodriguez-Ubreva J, Ortet L, Ruiz-Bonilla V, Jardi M, Ballestar E, Gonzalez S, Serrano AL, Perdiguero E, Munoz-Canoves P. Geriatric muscle stem cells switch reversible quiescence into senescence. Nature 2014;506:316–321 [DOI] [PubMed] [Google Scholar]
- 308.Pareja-Galeano H, Sanchis-Gomar F, Lucia A. p16INK4a, NAD, and sestrins: New targets for combating aging-related chronic illness? J Cell Physiol 2014;229:1575–1576 [DOI] [PubMed] [Google Scholar]
- 309.Song Z, von Figura G, Liu Y, Kraus JM, Torrice C, Dillon P, Rudolph-Watabe M, Ju Z, Kestler HA, Sanoff H, Lenhard Rudolph K. Lifestyle impacts on the aging-associated expression of biomarkers of DNA damage and telomere dysfunction in human blood. Aging Cell 2010;9:607–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Liu Y, Sanoff HK, Cho H, Burd CE, Torrice C, Ibrahim JG, Thomas NE, Sharpless NE. Expression of p16(INK4a) in peripheral blood T-cells is a biomarker of human aging. Aging Cell 2009;8:439–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Perez-Mancera PA, Young AR, Narita M. Inside and out: The activities of senescence in cancer. Nat Rev Cancer 2014;14:547–558 [DOI] [PubMed] [Google Scholar]
- 312.Coppe JP, Desprez PY, Krtolica A, Campisi J. The senescence-associated secretory phenotype: The dark side of tumor suppression. Annu Rev Pathol 2010;5:99–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.van Deursen JM. The role of senescent cells in ageing. Nature 2014;509:439–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Kuilman T, Peeper DS. Senescence-messaging secretome: SMS-ing cellular stress. Nat Rev Cancer 2009;9:81–94 [DOI] [PubMed] [Google Scholar]
- 315.Tchkonia T, Zhu Y, van Deursen J, Campisi J, Kirkland JL. Cellular senescence and the senescent secretory phenotype: Therapeutic opportunities. J Clin Invest 2013;123:966–972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Huang CC, Chiang WD, Huang WC, Huang CY, Hsu MC, Lin WT. Hepatoprotective effects of swimming exercise against D-galactose-induced senescence rat model. Evid Based Complement Alternat Med 2013;2013:275431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Xia WH, Li J, Su C, Yang Z, Chen L, Wu F, Zhang YY, Yu BB, Qiu YX, Wang SM, Tao J. Physical exercise attenuates age-associated reduction in endothelium-reparative capacity of endothelial progenitor cells by increasing CXCR4/JAK-2 signaling in healthy men. Aging Cell 2012;11:111–119 [DOI] [PubMed] [Google Scholar]
- 318.Verdijk LB, Snijders T, Drost M, Delhaas T, Kadi F, van Loon LJ. Satellite cells in human skeletal muscle; from birth to old age. Age (Dordr) 2014;36:545–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Jang YC, Sinha M, Cerletti M, Dall'Osso C, Wagers AJ. Skeletal muscle stem cells: Effects of aging and metabolism on muscle regenerative function. Cold Spring Harb Symp Quant Biol 2011;76:101–111 [DOI] [PubMed] [Google Scholar]
- 320.Renault V, Thornell LE, Eriksson PO, Butler-Browne G, Mouly V. Regenerative potential of human skeletal muscle during aging. Aging Cell 2002;1:132–139 [DOI] [PubMed] [Google Scholar]
- 321.Gopinath SD, Rando TA. Stem cell review series: Aging of the skeletal muscle stem cell niche. Aging Cell 2008;7:590–598 [DOI] [PubMed] [Google Scholar]
- 322.Verdijk LB, Snijders T, Beelen M, Savelberg HH, Meijer K, Kuipers H, Van Loon LJ. Characteristics of muscle fiber type are predictive of skeletal muscle mass and strength in elderly men. J Am Geriatr Soc 2010;58:2069–2075 [DOI] [PubMed] [Google Scholar]
- 323.Shefer G, Rauner G, Yablonka-Reuveni Z, Benayahu D. Reduced satellite cell numbers and myogenic capacity in aging can be alleviated by endurance exercise. PLoS One 2010;5:e13307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Shefer G, Rauner G, Stuelsatz P, Benayahu D, Yablonka-Reuveni Z. Moderate-intensity treadmill running promotes expansion of the satellite cell pool in young and old mice. FEBS J 2013;280:4063–4073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Kawiak J, Brzoska E, Grabowska I, Hoser G, Streminska W, Wasilewska D, Machaj EK, Pojda Z, Moraczewski J. Contribution of stem cells to skeletal muscle regeneration. Folia Histochem Cytobiol 2006;44:75–79 [PubMed] [Google Scholar]
- 326.Hawke TJ, Garry DJ. Myogenic satellite cells: Physiology to molecular biology. J Appl Physiol 2001;91:534–551 [DOI] [PubMed] [Google Scholar]
- 327.Grubb A, Joanisse S, Moore DR, Bellamy LM, Mitchell CJ, Phillips SM, Parise G. IGF-1 colocalizes with muscle satellite cells following acute exercise in humans. Appl Physiol Nutr Metab 2014;39:514–518 [DOI] [PubMed] [Google Scholar]
- 328.McKay BR, Ogborn DI, Baker JM, Toth KG, Tarnopolsky MA, Parise G. Elevated SOCS3 and altered IL-6 signaling is associated with age-related human muscle stem cell dysfunction. Am J Physiol Cell Physiol 2013;304:C717–C728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Cermak NM, Snijders T, McKay BR, Parise G, Verdijk LB, Tarnopolsky MA, Gibala MJ, Van Loon LJ. Eccentric exercise increases satellite cell content in type II muscle fibers. Med Sci Sports Exerc 2013;45:230–237 [DOI] [PubMed] [Google Scholar]
- 330.Verdijk LB, Gleeson BG, Jonkers RA, Meijer K, Savelberg HH, Dendale P, van Loon LJ. Skeletal muscle hypertrophy following resistance training is accompanied by a fiber type-specific increase in satellite cell content in elderly men. J Gerontol A Biol Sci Med Sci 2009;64:332–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Leenders M, Verdijk LB, van der Hoeven L, van Kranenburg J, Nilwik R, van Loon LJ. Elderly men and women benefit equally from prolonged resistance-type exercise training. J Gerontol A Biol Sci Med Sci 2013;68:769–779 [DOI] [PubMed] [Google Scholar]
- 332.Snijders T, Verdijk LB, Smeets JS, McKay BR, Senden JM, Hartgens F, Parise G, Greenhaff P, van Loon LJ. The skeletal muscle satellite cell response to a single bout of resistance-type exercise is delayed with aging in men. Age (Dordr) 2014;36:9699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.McKay BR, Ogborn DI, Bellamy LM, Tarnopolsky MA, Parise G. Myostatin is associated with age-related human muscle stem cell dysfunction. FASEB J 2012;26:2509–2521 [DOI] [PubMed] [Google Scholar]
- 334.Bellamy LM, Joanisse S, Grubb A, Mitchell CJ, McKay BR, Phillips SM, Baker S, Parise G. The acute satellite cell response and skeletal muscle hypertrophy following resistance training. PLoS One 2014;9:e109739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Salminen A, Kaarniranta K, Kauppinen A. Inflammaging: Disturbed interplay between autophagy and inflammasomes. Aging (Albany NY) 2012;4:166–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Green DR, Galluzzi L, Kroemer G. Mitochondria and the autophagy-inflammation-cell death axis in organismal aging. Science 2011;333:1109–1112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Vandanmagsar B, Youm YH, Ravussin A, Galgani JE, Stadler K, Mynatt RL, Ravussin E, Stephens JM, Dixit VD. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med 2011;17:179–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Pont AR, Sadri N, Hsiao SJ, Smith S, Schneider RJ. mRNA decay factor AUF1 maintains normal aging, telomere maintenance, and suppression of senescence by activation of telomerase transcription. Mol Cell 2012;47:5–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Lai RY, Ljubicic V, D'Souza D, Hood DA. Effect of chronic contractile activity on mRNA stability in skeletal muscle. Am J Physiol Cell Physiol 2010;299:C155–C163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Nelson G, Wordsworth J, Wang C, Jurk D, Lawless C, Martin-Ruiz C, von Zglinicki T. A senescent cell bystander effect: Senescence-induced senescence. Aging Cell 2012;11:345–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Pedersen BK. Muscular interleukin-6 and its role as an energy sensor. Med Sci Sports Exerc 2012;44:392–396 [DOI] [PubMed] [Google Scholar]
- 342.Ostrowski K, Schjerling P, Pedersen BK. Physical activity and plasma interleukin-6 in humans—effect of intensity of exercise. Eur J Appl Physiol 2000;83:512–515 [DOI] [PubMed] [Google Scholar]
- 343.Fischer CP. Interleukin-6 in acute exercise and training: What is the biological relevance? Exerc Immunol Rev 2006;12:6–33 [PubMed] [Google Scholar]
- 344.Pedersen BK, Febbraio MA. Muscle as an endocrine organ: Focus on muscle-derived interleukin-6. Physiol Rev 2008;88:1379–1406 [DOI] [PubMed] [Google Scholar]
- 345.Pedersen BK. Muscle as a secretory organ. Compr Physiol 2013;3:1337–1362 [DOI] [PubMed] [Google Scholar]
- 346.Della Gatta PA, Garnham AP, Peake JM, Cameron-Smith D. Effect of exercise training on skeletal muscle cytokine expression in the elderly. Brain Behav Immun 2014;39:80–86 [DOI] [PubMed] [Google Scholar]
- 347.Mikkelsen UR, Couppe C, Karlsen A, Grosset JF, Schjerling P, Mackey AL, Klausen HH, Magnusson SP, Kjaer M. Life-long endurance exercise in humans: Circulating levels of inflammatory markers and leg muscle size. Mech Ageing Dev 2013;134:531–540 [DOI] [PubMed] [Google Scholar]
- 348.Nicklas BJ, Brinkley TE. Exercise training as a treatment for chronic inflammation in the elderly. Exerc Sport Sci Rev 2009;37:165–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Beavers KM, Brinkley TE, Nicklas BJ. Effect of exercise training on chronic inflammation. Clin Chim Acta 2010;411:785–793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Colbert LH, Visser M, Simonsick EM, Tracy RP, Newman AB, Kritchevsky SB, Pahor M, Taaffe DR, Brach J, Rubin S, Harris TB. Physical activity, exercise, and inflammatory markers in older adults: Findings from the Health, Aging and Body Composition Study. J Am Geriatr Soc 2004;52:1098–1104 [DOI] [PubMed] [Google Scholar]
- 351.Woods JA, Wilund KR, Martin SA, Kistler BM. Exercise, inflammation and aging. Aging Dis 2012;3:130–140 [PMC free article] [PubMed] [Google Scholar]
- 352.Moore SC, Patel AV, Matthews CE, Berrington de Gonzalez A, Park Y, Katki HA, Linet MS, Weiderpass E, Visvanathan K, Helzlsouer KJ, Thun M, Gapstur SM, Hartge P, Lee IM. Leisure time physical activity of moderate to vigorous intensity and mortality: A large pooled cohort analysis. PLoS Med 2012;9:e1001335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Garatachea N, Santos-Lozano A, Sanchis-Gomar F, Fiuza-Luces C, Pareja-Galeano H, Emanuele E, Lucía A. Elite athletes live longer than the general population: A meta-analysis. Mayo Clin Proc 2014;89:1195–1200 [DOI] [PubMed] [Google Scholar]
- 354.Lee IM, Shiroma EJ, Lobelo F, Puska P, Blair SN, Katzmarzyk PT. Effect of physical inactivity on major non-communicable diseases worldwide: An analysis of burden of disease and life expectancy. Lancet 2012;380:219–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Joyner MJ. Why physiology matters in medicine. Physiology (Bethesda) 2011;26:72–75 [DOI] [PubMed] [Google Scholar]
- 356.Binder EF, Brown M, Sinacore DR, Steger-May K, Yarasheski KE, Schechtman KB. Effects of extended outpatient rehabilitation after hip fracture: A randomized controlled trial. JAMA 2004;292:837–846 [DOI] [PubMed] [Google Scholar]
- 357.Bruunsgaard H, Bjerregaard E, Schroll M, Pedersen BK. Muscle strength after resistance training is inversely correlated with baseline levels of soluble tumor necrosis factor receptors in the oldest old. J Am Geriatr Soc 2004;52:237–241 [DOI] [PubMed] [Google Scholar]
- 358.Cadore EL, Casas-Herrero A, Zambom-Ferraresi F, Idoate F, Millor N, Gomez M, Rodriguez-Manas L, Izquierdo M. Multicomponent exercises including muscle power training enhance muscle mass, power output, and functional outcomes in institutionalized frail nonagenarians. Age 2014;36:773–785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Clemson L, Fiatarone Singh MA, Bundy A, Cumming RG, Manollaras K, O'Loughlin P, Black D. Integration of balance and strength training into daily life activity to reduce rate of falls in older people (the LiFE study): Randomised parallel trial. BMJ 2012;345:e4547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Dorner T, Kranz A, Zettl-Wiedner K, Ludwig C, Rieder A, Gisinger C. The effect of structured strength and balance training on cognitive function in frail, cognitive impaired elderly long-term care residents. Aging Clin Exp Res 2007;19:400–405 [DOI] [PubMed] [Google Scholar]
- 361.Fiatarone MA, O'Neill EF, Ryan ND, Clements KM, Solares GR, Nelson ME, Roberts SB, Kehayias JJ, Lipsitz LA, Evans WJ. Exercise training and nutritional supplementation for physical frailty in very elderly people. N Engl J Med 1994;330:1769–1775 [DOI] [PubMed] [Google Scholar]
- 362.Hagedorn DK, Holm E. Effects of traditional physical training and visual computer feedback training in frail elderly patients. A randomized intervention study. Eur J Phys Rehab Medi 2010;46:159–168 [PubMed] [Google Scholar]
- 363.Hauer K, Rost B, Rutschle K, Opitz H, Specht N, Bartsch P, Oster P, Schlierf G. Exercise training for rehabilitation and secondary prevention of falls in geriatric patients with a history of injurious falls. J Am Geriatr Soc 2001;49:10–20 [DOI] [PubMed] [Google Scholar]
- 364.Hauer K, Schwenk M, Zieschang T, Essig M, Becker C, Oster P. Physical training improves motor performance in people with dementia: A randomized controlled trial. J Am Geriatr Soc 2012;60:8–15 [DOI] [PubMed] [Google Scholar]
- 365.Kryger AI, Andersen JL. Resistance training in the oldest old: Consequences for muscle strength, fiber types, fiber size, and MHC isoforms. Scand J Med Sci Sports 2007;17:422–430 [DOI] [PubMed] [Google Scholar]
- 366.Lazowski DA, Ecclestone NA, Myers AM, Paterson DH, Tudor-Locke C, Fitzgerald C, Jones G, Shima N, Cunningham DA. A randomized outcome evaluation of group exercise programs in long-term care institutions. J Gerontol A Biol Sci Medical Sci 1999;54:M621–M628 [DOI] [PubMed] [Google Scholar]
- 367.MacRae PG, Asplund LA, Schnelle JF, Ouslander JG, Abrahamse A, Morris C. A walking program for nursing home residents: Effects on walk endurance, physical activity, mobility, and quality of life. J Am Geriatr Soc 1996;44:175–180 [DOI] [PubMed] [Google Scholar]
- 368.McMurdo ME, Rennie LM. Improvements in quadriceps strength with regular seated exercise in the institutionalized elderly. Arch Phys Med Rehab 1994;75:600–603 [PubMed] [Google Scholar]
- 369.McMurdo ME, Johnstone R. A randomized controlled trial of a home exercise programme for elderly people with poor mobility. Age Ageing 1995;24:425–428 [DOI] [PubMed] [Google Scholar]
- 370.Pollock RD, Martin FC, Newham DJ. Whole-body vibration in addition to strength and balance exercise for falls-related functional mobility of frail older adults: A single-blind randomized controlled trial. Clin Rehab 2012;26:915–923 [DOI] [PubMed] [Google Scholar]
- 371.Schnelle JF, Alessi CA, Simmons SF, Al-Samarrai NR, Beck JC, Ouslander JG. Translating clinical research into practice: A randomized controlled trial of exercise and incontinence care with nursing home residents. J Am Geriatr Soc 2002;50:1476–1483 [DOI] [PubMed] [Google Scholar]
- 372.Timonen L, Rantanen T, Makinen E, Timonen TE, Tormakangas T, Sulkava R. Effects of a group-based exercise program on functional abilities in frail older women after hospital discharge. Aging Clin Exp Res 2006;18:50–56 [DOI] [PubMed] [Google Scholar]
- 373.Baker LD, Frank LL, Foster-Schubert K, Green PS, Wilkinson CW, McTiernan A, Cholerton BA, Plymate SR, Fishel MA, Watson GS, Duncan GE, Mehta PD, Craft S. Aerobic exercise improves cognition for older adults with glucose intolerance, a risk factor for Alzheimer's disease. J Alzheimers Dis 2010;22:569–579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Baker LD, Cross DJ, Minoshima S, Belongia D, Watson GS, Craft S. Insulin resistance and Alzheimer-like reductions in regional cerebral glucose metabolism for cognitively normal adults with prediabetes or early type 2 diabetes. Arch Neurol 2011;68:51–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Beck EB, Erbs S, Mobius-Winkler S, Adams V, Woitek FJ, Walther T, Hambrecht R, Mohr FW, Stumvoll M, Bluher M, Schuler G, Linke A. Exercise training restores the endothelial response to vascular growth factors in patients with stable coronary artery disease. Eur J Prev Cardiol 2012;19:412–418 [DOI] [PubMed] [Google Scholar]
- 376.Cassilhas RC, Viana VA, Grassmann V, Santos RT, Santos RF, Tufik S, Mello MT. The impact of resistance exercise on the cognitive function of the elderly. Med Sci Sports Exerc 2007;39:1401–1407 [DOI] [PubMed] [Google Scholar]
- 377.Coelho FM, Pereira DS, Lustosa LP, Silva JP, Dias JM, Dias RC, Queiroz BZ, Teixeira AL, Teixeira MM, Pereira LS. Physical therapy intervention (PTI) increases plasma brain-derived neurotrophic factor (BDNF) levels in non-frail and pre-frail elderly women. Arch Gerontol Geriatr 2012;54:415–420 [DOI] [PubMed] [Google Scholar]
- 378.Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, Kim JS, Heo S, Alves H, White SM, Wojcicki TR, Mailey E, Vieira VJ, Martin SA, Pence BD, Woods JA, McAuley E, Kramer AF. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci USA 2011;108:3017–3022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Ogawa K, Sanada K, Machida S, Okutsu M, Suzuki K. Resistance exercise training-induced muscle hypertrophy was associated with reduction of inflammatory markers in elderly women. Mediators Inflamm 2010;2010:171023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Park J, Nakamura Y, Kwon Y, Park H, Kim E, Park S. The effect of combined exercise training on carotid artery structure and function and vascular endothelial growth factor VEGF in obese older women. Japanese J Phys Fitness Sports Med 2010;59:495–504 [Google Scholar]
- 381.Pereira DS, de Queiroz BZ, Miranda AS, Rocha NP, Felicio DC, Mateo EC, Favero M, Coelho FM, Jesus-Moraleida F, Gomes Pereira DA, Teixeira AL, Maximo Pereira LS. Effects of physical exercise on plasma levels of brain-derived neurotrophic factor and depressive symptoms in elderly women—a randomized clinical trial. Arch Phys Med Rehabil 2013;94:1443–1450 [DOI] [PubMed] [Google Scholar]
- 382.Ruiz JR, Gil-Bea F, Bustamante-Ara N, Rodríguez-Romo G, Fiuza-Luces C, Serra-Rexach JA, Cedazo-Minguez A, Lucia A. Resistance training does not have an effect on cognition or related serum biomarkers in nonagenarians: A randomized controlled trial. Int J Sports Med 2014;35:1–7 [DOI] [PubMed] [Google Scholar]
- 383.Schlager O, Giurgea A, Schuhfried O, Seidinger D, Hammer A, Groger M, Fialka-Moser V, Gschwandtner M, Koppensteiner R, Steiner S. Exercise training increases endothelial progenitor cells and decreases asymmetric dimethylarginine in peripheral arterial disease: A randomized controlled trial. Atherosclerosis 2011;217:240–248 [DOI] [PubMed] [Google Scholar]
- 384.Vaughan S, Wallis M, Polit D, Steele M, Shum D, Morris N. The effects of multimodal exercise on cognitive and physical functioning and brain-derived neurotrophic factor in older women: A randomised controlled trial. Age Ageing 2014;43:623–629 [DOI] [PubMed] [Google Scholar]
- 385.Voss MW, Erickson KI, Prakash RS, Chaddock L, Kim JS, Alves H, Szabo A, Phillips SM, Wojcicki TR, Mailey EL, Olson EA, Gothe N, Vieira-Potter VJ, Martin SA, Pence BD, Cook MD, Woods JA, McAuley E, Kramer AF. Neurobiological markers of exercise-related brain plasticity in older adults. Brain Behav Immun 2013;28:90–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Wood RE, Sanderson BE, Askew CD, Walker PJ, Green S, Stewart IB. Effect of training on the response of plasma vascular endothelial growth factor to exercise in patients with peripheral arterial disease. Clin Sci (Lond) 2006;111:401–409 [DOI] [PubMed] [Google Scholar]
- 387.Horsley V, Jansen KM, Mills ST, Pavlath GK. IL-4 acts as a myoblast recruitment factor during mammalian muscle growth. Cell 2003;113:483–494 [DOI] [PubMed] [Google Scholar]
- 388.Lafreniere JF, Mills P, Bouchentouf M, Tremblay JP. Interleukin-4 improves the migration of human myogenic precursor cells in vitro and in vivo. Exp Cell Res 2006;312:1127–1141 [DOI] [PubMed] [Google Scholar]
- 389.Jacquemin V, Butler-Browne GS, Furling D, Mouly V. IL-13 mediates the recruitment of reserve cells for fusion during IGF-1-induced hypertrophy of human myotubes. J Cell Sci 2007;120:670–681 [DOI] [PubMed] [Google Scholar]
- 390.Al-Shanti N, Saini A, Faulkner SH, Stewart CE. Beneficial synergistic interactions of TNF-alpha and IL-6 in C2 skeletal myoblasts—potential cross-talk with IGF system. Growth Factors 2008;26:61–73 [DOI] [PubMed] [Google Scholar]
- 391.Carey AL, Steinberg GR, Macaulay SL, Thomas WG, Holmes AG, Ramm G, Prelovsek O, Hohnen-Behrens C, Watt MJ, James DE, Kemp BE, Pedersen BK, Febbraio MA. Interleukin-6 increases insulin-stimulated glucose disposal in humans and glucose uptake and fatty acid oxidation in vitro via AMP-activated protein kinase. Diabetes 2006;55:2688–2697 [DOI] [PubMed] [Google Scholar]
- 392.van Hall G, Steensberg A, Sacchetti M, Fischer C, Keller C, Schjerling P, Hiscock N, Moller K, Saltin B, Febbraio MA, Pedersen BK. Interleukin-6 stimulates lipolysis and fat oxidation in humans. J Clin Endocrinol Metab 2003;88:3005–3010 [DOI] [PubMed] [Google Scholar]
- 393.Serrano AL, Baeza-Raja B, Perdiguero E, Jardi M, Munoz-Canoves P. Interleukin-6 is an essential regulator of satellite cell-mediated skeletal muscle hypertrophy. Cell Metab 2008;7:33–44 [DOI] [PubMed] [Google Scholar]
- 394.Nielsen AR, Hojman P, Erikstrup C, Fischer CP, Plomgaard P, Mounier R, Mortensen OH, Broholm C, Taudorf S, Krogh-Madsen R, Lindegaard B, Petersen AM, Gehl J, Pedersen BK. Association between interleukin-15 and obesity: Interleukin-15 as a potential regulator of fat mass. J Clin Endocrinol Metab 2008;93:4486–4493 [DOI] [PubMed] [Google Scholar]
- 395.Quinn LS, Anderson BG. Interleukin-15, IL-15 receptor-alpha, and obesity: Concordance of laboratory animal and human genetic studies. J Obes 2011;2011:456347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Carbo N, Lopez-Soriano J, Costelli P, Busquets S, Alvarez B, Baccino FM, Quinn LS, Lopez-Soriano FJ, Argiles JM. Interleukin-15 antagonizes muscle protein waste in tumour-bearing rats. Br J Cancer 2000;83:526–531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.Quinn LS, Strait-Bodey L, Anderson BG, Argiles JM, Havel PJ. Interleukin-15 stimulates adiponectin secretion by 3T3-L1 adipocytes: Evidence for a skeletal muscle-to-fat signaling pathway. Cell Biol Int 2005;29:449–457 [DOI] [PubMed] [Google Scholar]
- 398.Broholm C, Laye MJ, Brandt C, Vadalasetty R, Pilegaard H, Pedersen BK, Scheele C. LIF is a contraction-induced myokine stimulating human myocyte proliferation. J Appl Physiol 2011;111:251–259 [DOI] [PubMed] [Google Scholar]
- 399.Kurek J, Bower J, Romanella M, Austin L. Leukaemia inhibitory factor treatment stimulates muscle regeneration in the mdx mouse. Neurosci Lett 1996;212:167–170 [DOI] [PubMed] [Google Scholar]
- 400.Spangenburg EE, Booth FW. Leukemia inhibitory factor restores the hypertrophic response to increased loading in the LIF(−/−) mouse. Cytokine 2006;34:125–130 [DOI] [PubMed] [Google Scholar]
- 401.White JD, Bower JJ, Kurek JB, Austin L. Leukemia inhibitory factor enhances regeneration in skeletal muscles after myoblast transplantation. Muscle Nerve 2001;24:695–697 [DOI] [PubMed] [Google Scholar]
- 402.Hittel DS, Axelson M, Sarna N, Shearer J, Huffman KM, Kraus WE. Myostatin decreases with aerobic exercise and associates with insulin resistance. Med Sci Sports Exerc 2010;42:2023–2029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Palsgaard J, Brons C, Friedrichsen M, Dominguez H, Jensen M, Storgaard H, Spohr C, Torp-Pedersen C, Borup R, De Meyts P, Vaag A. Gene expression in skeletal muscle biopsies from people with type 2 diabetes and relatives: Differential regulation of insulin signaling pathways. PLoS One 2009;4:e6575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Constantin D, McCullough J, Mahajan RP, Greenhaff PL. Novel events in the molecular regulation of muscle mass in critically ill patients. J Physiol 2011;589:3883–3895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Yarasheski KE, Bhasin S, Sinha-Hikim I, Pak-Loduca J, Gonzalez-Cadavid NF. Serum myostatin-immunoreactive protein is increased in 60–92 year old women and men with muscle wasting. J Nutr Health Aging 2002;6:343–348 [PubMed] [Google Scholar]
- 406.McPherron AC, Lee SJ. Suppression of body fat accumulation in myostatin-deficient mice. J Clin Invest 2002;109:595–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Koltai E, Szabo Z, Atalay M, Boldogh I, Naito H, Goto S, Nyakas C, Radak Z. Exercise alters SIRT1, SIRT6, NAD and NAMPT levels in skeletal muscle of aged rats. Mech Ageing Dev 2010;131:21–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Romacho T, Azcutia V, Vazquez-Bella M, Matesanz N, Cercas E, Nevado J, Carraro R, Rodriguez-Manas L, Sanchez-Ferrer CF, Peiro C. Extracellular PBEF/NAMPT/visfatin activates pro-inflammatory signalling in human vascular smooth muscle cells through nicotinamide phosphoribosyltransferase activity. Diabetologia 2009;52:2455–2463 [DOI] [PubMed] [Google Scholar]
- 409.Cheng Q, Dong W, Qian L, Wu J, Peng Y. Visfatin inhibits apoptosis of pancreatic beta-cell line, MIN6, via the mitogen-activated protein kinase/phosphoinositide 3-kinase pathway. J Mol Endocrinol 2011;47:13–21 [DOI] [PubMed] [Google Scholar]
- 410.Li Y, Zhang Y, Dorweiler B, Cui D, Wang T, Woo CW, Brunkan CS, Wolberger C, Imai S, Tabas I. Extracellular Nampt promotes macrophage survival via a nonenzymatic interleukin-6/STAT3 signaling mechanism. J Biol Chem 2008;283:34833–34843 [DOI] [PMC free article] [PubMed] [Google Scholar]