Abstract
Control of a viral infection in vivo requires a rapid and efficient cytotoxic-T-lymphocyte response. We demonstrate that lentivirus-mediated introduction of antigen in dendritic cells confers a protective antiviral immunity in vivo in a lymphocytic choriomeningitis virus model. Therefore, lentiviral vectors may be excellent vaccine candidates for viral infections.
The success of an antiviral vaccine depends on its capacity to induce strong and sustained antiviral immunity. While a number of methods are employed to achieve this goal, it is still unclear whether lentiviral vectors are able to induce efficient antiviral immunity in vivo. Introduction of antigen into dendritic cells (DC) by using lentiviral vectors leads to antigen presentation and activation of virus-specific cytotoxic T lymphocytes (CTL) in vitro in human and murine models, as well as in vivo in murine tumor models (4, 5, 7, 10, 20). However, due to rapid viral replication, the control of a viral infection in vivo requires strong T-cell responses, both qualitatively and quantitatively.
The murine model of lymphocytic choriomeningitis virus (LCMV) infection is a well-defined system for studying protective CTL responses in vivo (1, 11, 15, 16, 19, 21). Therefore, this experimental system is appropriate for addressing the question of whether lentiviral vectors are suitable tools for introducing antigen into DC in order to induce protective antiviral immunity in vivo.
Our study demonstrates for the first time that third-generation lentiviral vectors are capable of introducing antigen into DC, which confer a strong protective antiviral immunity in vivo.
Vaccination with LV-GFPUbigp33-transduced DC induces the expansion of antigen-specific T cells in vivo.
We first asked whether LV-GFPUbigp33-transduced DC could prime LCMV glycoprotein-specific CD8+ T cells in vitro and in vivo. LV-GFPUbigp33 is a third-generation lentiviral vector that encodes the transgene GFPUbigp33 (immunodominant epitope of LCMV glycoprotein, positions 33 to 41 [gp33-41], fused with ubiquitin and enhanced green fluorescent protein [GFP] sequences) (17, 18). Bone marrow-derived DC from C57BL/6 mice (IFFA-Credo, L'Arbresle, France) were generated as published previously (20). Briefly, bone marrow cells were differentiated into DC by culturing in medium supplemented with murine granulocyte-macrophage colony stimulating factor (ImmunoKontact, Lugano, Switzerland), interleukin 4, and Flt3-L (R&D, Abingdon, United Kingdom) for 9 days. Third-generation lentiviral vectors were transduced at day 3, after nonadherent cells were discarded from the culture. At day 7, DC were matured by adding 1 μg/ml of lipopolysaccharide (Difco, Detroit, Mich.) in the presence of cytokines.
When DC were transduced with LV-GFPUbigp33 at a multiplicity of infection of 5, the average transduction efficiency for LV-GFPUbigp33 was 18.5% (data not shown). At the same multiplicity of infection the efficiency of transduction of DC with lentiviral vectors encoding GFP was around 35% (20). Importantly, the design of the LV-GFPUbigp33 construct efficiently increases the capacity of transduced DC to stimulate proliferation of the CD8+ T cells from transgenic mice expressing a T-cell receptor specific for LCMV gp33-41 (14). Previously described lentiviral constructs (20), i.e., LV-LCMVgp and LV-gp33IRESGFP, encoding full-length LCMV glycoprotein and a minigene (gp33) internal ribosome entry site GFP, respectively, were less efficient in vitro and in vivo (data not shown; also, see Fig. 2).
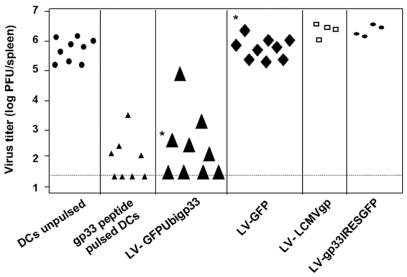
FIG. 2.
Protective antiviral immunity after vaccination with DC transduced with LV-GFPUbigp33 against i.v. LCMV challenge in vivo. Mice were immunized with DC pulsed with gp33-41 peptide or DC transduced with LV-GFPUbigp33, LV-LCMVgp, and LV-gp33IRESGFP. Seven days later, mice were challenged with 200 PFU of LCMV (WE strain). Five days later, LCMV titers were determined in the spleens by using the plaque assay test. The detection limit is represented by the broken line. Results are pooled data from four independent experiments (*, P < 0.0005).
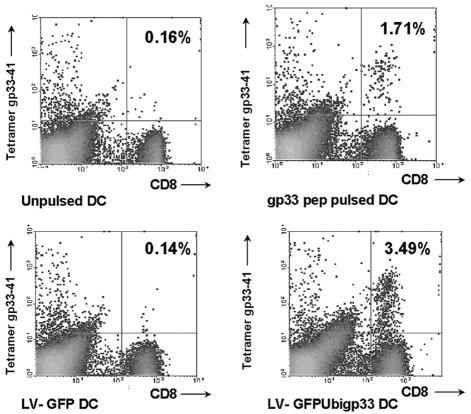
After optimization for lentiviral transduction and antigen presentation, C57BL/6 mice received 105 DC transduced with LV-GFPUbigp33 (normalized for GFP expression) or 105 DC pulsed with LCMV gp33-41 or control DC. Seven days later, peripheral blood mononuclear cells were isolated and analyzed by flow cytometry for the detection of gp33-41 tetramer-positive cells among CD8+ T cells (Fig. 1). LV-GFPUbigp33 induced a mean of 2.01% (standard error of the mean [SEM] = 0.822) tetramer-positive cells among CD8+ T cells in blood, while control LV-GFP-transduced DC gave a mean of 0.2% (SEM = 0.045) (Fig. 1). Corresponding values were 1.26% (SEM = 0.227) for peptide gp33-41-pulsed DC and 0.24% (SEM = 0.059) for unpulsed DC (Fig. 1). Unlike LV-GFPUbigp33, neither LV-LCMVgp nor LV-gp33IRESGFP was able to induce tetramer-positive cells among CD8+ T cells in blood (data not shown).
FIG. 1.
Immunization with DC transduced with lentiviral vectors encoding GFPUbigp33 leads to expansion of antigen-specific CD8+ T cells in vivo. Naïve mice were immunized under the indicated conditions, and peripheral blood mononuclear cells were isolated and subsequently analyzed by flow cytometry for gp33-41 tetramer-positive cells among CD8+ T cells. Results are percentages and are representative of one of at least three animals per group.
Vaccination with LV-GFPUbigp33-transduced DC provides protection against intravenous LCMV infection in vivo.
Next, we tested whether DC transduced with LV-GFPUbigp33 (104 DC) provide CD8-dependent protection against viral challenge with LCMV by the intravenous (i.v.) route. Seven days after vaccination with DC, mice were challenged with LCMV (i.v.). Five days after the challenge, spleens were removed and LCMV titers were determined in spleen homogenates (2, 3). Mice vaccinated with 104 DC transduced with LV-GFPUbigp33 had average LCMV titers that were 4 logs lower than those for mice that received 104 DC encoding an irrelevant protein (GFP) or mice treated with unpulsed DC (104) (P < 0.0005) (Fig. 2). Protection against an i.v. challenge with LCMV was similar in mice vaccinated with 104 DC transduced with LV-GFPUbigp33 and in those vaccinated with 104 DC pulsed with LCMV gp33-41. In contrast, LV-LCMVgp- and LV-gp33IRESGFP-transduced DC were unable to induce protection in mice (Fig. 2).
Vaccination with LV-GFPUbigp33-transduced DC provides complete protection against peripheral LCMV infection in vivo.
We further assessed whether vaccination with DC transduced with lentiviral vectors could protect wild-type C57BL/6 mice against replication of LCMV in the footpad. Protection against viral replication in a peripheral solid organ requires not only a high frequency of LCMV glycoprotein-specific CD8+ memory T cells but also a high-level activation of these memory CD8+ T cells, so that they recirculate through peripheral tissues (8). In this assay, a footpad swelling response is observed in naïve and incompletely protected animals as a consequence of local immune response to LCMV, whereas fully protected animals do not show such a reaction.
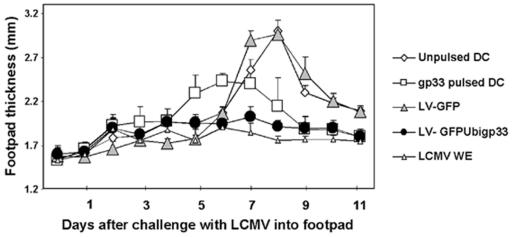
Naïve mice were injected i.v. with 105 DC transduced with LV-GFPUbigp33 (normalized with GFP expression). Seven days later, vaccinated mice were challenged with 103 PFU of LCMV (Armstrong) in the footpads as described previously (6, 9, 12). While 105 DC pulsed with peptide induced swelling on day 4 and 5, 105 DC transduced with LV-gp33IRESGFP caused no detectable footpad swelling (Fig. 3). Control DC or DC transduced with LV-GFP (105) showed maximal swelling at day 7 to 8. The P value for DC transduced with LV-gp33IRESGFP compared with DC transduced with LV-GFP was calculated from day 7 to 10 and was highly significant (P = 0.001). Mice rendered immune by infection with LCMV (200 PFU, WE strain) 7 days prior to footpad infection showed also no detectable footpad swelling (9). Together, these results indicate that DC transduced with LV-gp33IRESGFP confer full protection against peripheral challenge with LCMV (no swelling), while DC pulsed with LCMV gp33-41 incompletely protect the animals (partial swelling on days 5 and 6; P = 0.05 compared with DC transduced with LV-gp33IRESGFP).
FIG. 3.
Protective antiviral immunity after vaccination with DC transduced with LV-GFPUbigp33 against peripheral LCMV challenge in vivo. Mice were immunized with DC pulsed with gp33-41 peptide or DC transduced with LV-GFPUbigp33 and challenged with 103 PFU of LCMV (Armstrong) in the hind footpads. CTL-mediated footpad swelling reaction was monitored daily. Results are pooled data for three or four animals per group (six to eight footpads), plus standard deviations.
Our study firmly establishes that lentiviral vector-mediated transduction of DC leads to induction of antiviral immunity in vivo, against both an i.v. and a peripheral viral challenge in a LCMV model. The LCMV mouse model is a well-established system for studying the efficiency of a candidate vaccine in vivo (1, 11, 15, 16, 19, 21).
Our vaccination protocol with DC transduced with lentiviral vectors encoding LCMV gp33-41 and injected i.v. induced no footpad swelling in response to viral challenge (i.e., viral elimination) in peripheral tissue (i.e., the footpad), while peptide-pulsed DC induced an early and reduced footpad swelling in our experiments. Mullins et al. (13), using peptide-pulsed DC injected i.v., were also unable to confer protective immunity to subcutaneously implanted tumors. It thus appears that lentiviral transduction of antigen into DC may generate a more potent vaccine than pulsing DC with peptide in vivo.
Therefore, lentiviral vectors should be considered good candidates for vaccines against acute and chronic infections with viruses such as human immunodeficiency virus and hepatitis B or C virus, either by direct injection or after loading on dendritic cells.
Acknowledgments
We thank C. Esslinger, H. R. MacDonald, and F. Lévy for the GFPUbigp33 construct (Ludwig Institute for Cancer Research, Epalinges, Switzerland) and Didier Trono for critical comments.
This work was supported by the Geneva Cancer League and by Swiss National Science Foundation grant 3345-67200.01 to V.P. and grants 32-65238.01 and 3200-067973.02 to C.H. V.P. is the recipient of a “Professor SNF” position (PP00A-68785).
REFERENCES
- 1.Ahmed, R., B. D. Jamieson, and D. D. Porter. 1987. Immune therapy of a persistent and disseminated viral infection. J. Virol. 61:3920-3929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bachmann, M. F., T. M. Kundig, H. Hengartner, and R. M. Zinkernagel. 1997. Protection against immunopathological consequences of a viral infection by activated but not resting cytotoxic T cells: T cell memory without “memory T cells”? Proc. Natl. Acad. Sci. USA 94:640-645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Battegay, M., S. Cooper, A. Althage, J. Banziger, H. Hengartner, and R. M. Zinkernagel. 1991. Quantification of lymphocytic choriomeningitis virus with an immunological focus assay in 24- or 96-well plates. J. Virol. Methods 33:191-198. (Errata, 35:115, 1991, and 38:263, 1992.) [DOI] [PubMed] [Google Scholar]
- 4.Dyall, J., J. B. Latouche, S. Schnell, and M. Sadelain. 2001. Lentivirus-transduced human monocyte-derived dendritic cells efficiently stimulate antigen-specific cytotoxic T lymphocytes. Blood 97:114-121. [DOI] [PubMed] [Google Scholar]
- 5.Esslinger, C., L. Chapatte, D. Finke, I. Miconnet, P. Guillaume, F. Levy, and H. R. MacDonald. 2003. In vivo administration of a lentiviral vaccine targets DCs and induces efficient CD8+ T cell responses. J. Clin. Investig. 111:1673-1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fung-Leung, W. P., T. M. Kundig, R. M. Zinkernagel, and T. W. Mak. 1991. Immune response against lymphocytic choriomeningitis virus infection in mice without CD8 expression. J. Exp. Med. 174:1425-1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gruber, A., J. Kan-Mitchell, K. L. Kuhen, T. Mukai, and F. Wong-Staal. 2000. Dendritic cells transduced by multiply deleted HIV-1 vectors exhibit normal phenotypes and functions and elicit an HIV-specific cytotoxic T-lymphocyte response in vitro. Blood 96:1327-1333. [PubMed] [Google Scholar]
- 8.Kundig, T. M., M. F. Bachmann, S. Oehen, U. W. Hoffmann, J. J. Simard, C. P. Kalberer, H. Pircher, P. S. Ohashi, H. Hengartner, and R. M. Zinkernagel. 1996. On the role of antigen in maintaining cytotoxic T-cell memory. Proc. Natl. Acad. Sci. USA 93:9716-9723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ludewig, B., S. Oehen, F. Barchiesi, R. A. Schwendener, H. Hengartner, and R. M. Zinkernagel. 1999. Protective antiviral cytotoxic T cell memory is most efficiently maintained by restimulation via dendritic cells. J. Immunol. 163:1839-1844. [PubMed] [Google Scholar]
- 10.Metharom, P., K. A. Ellem, C. Schmidt, and M. Q. Wei. 2001. Lentiviral vector-mediated tyrosinase-related protein 2 gene transfer to dendritic cells for the therapy of melanoma. Hum. Gene Ther. 12:2203-2213. [DOI] [PubMed] [Google Scholar]
- 11.Moskophidis, D., F. Lechner, H. Pircher, and R. M. Zinkernagel. 1993. Virus persistence in acutely infected immunocompetent mice by exhaustion of antiviral cytotoxic effector T cells. Nature 362:758-761. [DOI] [PubMed] [Google Scholar]
- 12.Moskophidis, D., and F. Lehmanngrube. 1989. Virus-induced delayed-type hypersensitivity reaction is sequentially mediated by CD8+ and CD4+ T lymphocytes. Proc. Natl. Acad. Sci. USA 86:3291-3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mullins, D. W., S. L. Sheasley, R. M. Ream, T. N. Bullock, Y. X. Fu, and V. H. Engelhard. 2003. Route of immunization with peptide-pulsed dendritic cells controls the distribution of memory and effector T cells in lymphoid tissues and determines the pattern of regional tumor control. J. Exp. Med. 198:1023-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ohashi, P. S., S. Oehen, K. Buerki, H. Pircher, C. T. Ohashi, B. Odermatt, B. Malissen, R. M. Zinkernagel, and H. Hengartner. 1991. Ablation of “tolerance” and induction of diabetes by virus infection in viral antigen transgenic mice. Cell 65:305-317. [DOI] [PubMed] [Google Scholar]
- 15.Oldstone, M. B. 2002. Biology and pathogenesis of lymphocytic choriomeningitis virus infection. Curr. Top. Microbiol. Immunol. 263:83-117. [DOI] [PubMed] [Google Scholar]
- 16.Oldstone, M. B., P. Blount, P. J. Southern, and P. W. Lampert. 1986. Cytoimmunotherapy for persistent virus infection reveals a unique clearance pattern from the central nervous system. Nature 321:239-243. [DOI] [PubMed] [Google Scholar]
- 17.Valmori, D., V. Dutoit, D. Lienard, F. Lejeune, D. Speiser, D. Rimoldi, V. Cerundolo, P. Y. Dietrich, J. C. Cerottini, and P. Romero. 2000. Tetramer-guided analysis of TCR beta-chain usage reveals a large repertoire of melan-A-specific CD8+ T cells in melanoma patients. J. Immunol. 165:533-538. [DOI] [PubMed] [Google Scholar]
- 18.Valmori, D., U. Gileadi, C. Servis, P. R. Dunbar, J. C. Cerottini, P. Romero, V. Cerundolo, and F. Levy. 1999. Modulation of proteasomal activity required for the generation of a cytotoxic T lymphocyte-defined peptide derived from the tumor antigen MAGE-3. J. Exp. Med. 189:895-906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Volkert, M., and J. H. Larsen. 1965. Immunological tolerance to viruses. Prog. Med. Virol. 7:160-207. [PubMed] [Google Scholar]
- 20.Zarei, S., F. Leuba, J. F. Arrighi, C. Hauser, and V. Piguet. 2002. Transduction of dendritic cells by antigen-encoding lentiviral vectors permits antigen processing and MHC class I-dependent presentation. J. Allergy Clin. Immunol. 109:988-994. [DOI] [PubMed] [Google Scholar]
- 21.Zinkernagel, R. M., and P. C. Doherty. 1974. Restriction of in vitro T cell-mediated cytotoxicity in lymphocytic choriomeningitis within a syngeneic or semiallogeneic system. Nature 248:701-702. [DOI] [PubMed] [Google Scholar]