Abstract
The ability of the γ134.5 protein to suppress the PKR response plays a crucial role in herpes simplex virus pathogenesis. In this process, the γ134.5 protein associates with protein phosphatase 1 to form a large complex that dephosphorylates eIF-2α and thereby prevents translation shutoff mediated by PKR. Accordingly, γ134.5 null mutants are virulent in PKR-knockout mice but not in wild-type mice. However, γ134.5 deletion mutants, with an extragenic compensatory mutation, inhibit PKR activity but remain avirulent, suggesting that the γ134.5 protein has additional functions. Here, we show that a substitution of the γ134.5 gene with the NS1 gene from influenza A virus renders viral resistance to interferon involving PKR. The virus replicates as efficiently as wild-type virus in SK-N-SH and CV-1 cells. However, in mouse 3T6 cells, the virus expressing the NS1 protein grows at an intermediate level between the wild-type virus and the γ134.5 deletion mutant. This decrease in growth, compared to that of the wild-type virus, is due not to an inhibition of viral protein synthesis but rather to a block in virus release or egress. Virus particles are predominantly present in the nucleus and cytoplasm. Notably, deletions in the amino terminus of the γ134.5 protein lead to a significant decrease in virus growth in mouse 3T6 cells, which is independent of eIF-2α dephosphorylation. In correlation, a series of deletions in the amino-terminal domain impair nuclear as well as cytoplasmic egress. These results indicate that efficient viral replication depends on the γ134.5 functions required to prevent the PKR response and to facilitate virus egress in the different stages during virus infection.
Herpes simplex viruses (HSV) are human pathogens responsible for a variety of diseases, including localized mucocutanous infection, encephalitis, and disseminated disease (49). Following primary infection, HSV establishes a latent infection or lytic infection in which viruses undergo transcription, replication, assembly, and egress. While many viral factors are involved in this complex process, the γ134.5 protein has been demonstrated to be a critical determinant of virus infection (18). Several lines of evidence indicate that the γ134.5 protein contributes to HSV virulence in vivo (18, 34, 35, 46, 48). HSV type 1 (HSV-1) mutants that fail to express the γ134.5 protein are incapable of multiplying and causing encephalitis in experimental animal models (18, 35, 48). Similar phenotypes have been observed for HSV-2 mutants lacking the γ134.5 gene (34, 38).
The precise roles of the γ134.5 protein in HSV infection are not fully understood. In HSV-infected cells, the double-stranded RNA-dependent protein kinase (PKR) is activated to phosphorylate the α subunit of translation initiation factor 2 (eIF-2α) (17, 19). This leads to the translation arrest and subsequent inhibition of viral replication (19). As a way to evade the host response, the γ134.5 protein recruits cellular protein phosphatase 1 (PP1), forming a high-molecular-weight complex that dephosphorylates eIF-2α (28, 29). Studies indicate that dephosphorylation of eIF-2α facilitated by the γ134.5 protein is linked to viral resistance to alpha/beta interferon (14, 31). Consistent with these findings, the γ134.5 null mutant is virulent in PKR-knockout mice but not in wild-type mice (18, 32, 48). Paradoxically, a γ134.5 null mutant with a secondary mutation in the US11 promoter region inhibits PKR activity but nevertheless remains avirulent (11, 39, 40). The virus is cleared a few days after ocular infection in experimental mice (47). Moreover, a γ134.5 null mutant with an additional mutation in the other regions of the viral genome partially restores virulence (10).
The γ134.5 gene is located in the inverted repeats of the HSV genome flanking the unique long sequence and is present in two copies per genome (1, 21, 22). In HSV-1, the γ134.5 gene encodes a protein of 263 amino acids consisting of an amino-terminal domain, a linker region of three-amino-acid repeats (Ala-Thr-Pro), and a carboxyl-terminal domain (21). The triplet repeats are a constant feature of the γ134.5 protein in HSV-1, but the number of repeats varies among different strains (6, 21). The number of triplet repeats in the γ134.5 protein appears to affect the ability of HSV to invade the central nervous system from the peripheral tissue (6, 37). However, the triplet repeats are not present in the γ134.5 protein of HSV-2 (38). The carboxyl terminus of the γ134.5 protein consists of a PP1-binding domain and an effector domain, both of which are essential to antagonize the antiviral activity of PKR (12, 15, 28). This portion of the protein is homologous to the corresponding domains of the growth arrest and DNA damage response protein GADD34 and a virulence factor, NL/I14L, of the African swine fever virus (25, 33, 50, 51). Currently, the biological function of the amino-terminal domain of the γ134.5 protein is unknown. Published data suggest that mutations in this region affect neurovirulence, but this domain itself is not sufficient to confer virulence (2, 18).
Previous studies indicated that the γ134.5 protein of HSV-1(F) accumulates both in the nucleus and in the cytoplasm during virus infection (1). In agreement with this observation, the γ134.5 protein is found in both the nucleus and the cytoplasm when expressed alone in mammalian cells (13, 36). Deletion analysis showed that the γ134.5 protein bears nuclear import and export signals that direct shuttling of the γ134.5 protein between the cytoplasm, nucleus, and nucleolus (13). A proposed model is that this dynamic process is required for the different activities performed by the γ134.5 protein during viral infection (13).
Accumulating evidence suggests that the γ134.5 protein is a multifunctional protein. In mouse 3T6 cells (8), a γ134.5 deletion mutant derived from the HSV-1 (17) strain is defective in egress, although the way by which the γ134.5 protein is involved is unknown. Notably, the growth of the γ134.5 null mutant is severely impaired in resting 3T6 cells but less so in actively dividing cells (7). Like its cellular homologue GADD34, the carboxyl-terminal domain of the γ134.5 protein complexes with PCNA (proliferating cell nuclear antigen), a nuclear protein involved in DNA replication and cell cycle regulation (9). In addition, the γ134.5 protein inhibits autophagy (42). Recent experiments showed that the triplet repeats of the γ134.5 protein are implicated in glycoprotein processing in infected cells (6, 37). The γ134.5 protein also blocks the surface expression of major histocompatibility complex class II molecules in HSV-infected cells, which is thought to inhibit the functions of CD4+ T cells (45). In HSV-infected cells, eIF-2α dephosphorylation mediated by the γ134.5 protein is coupled with viral resistance to interferon but is not sufficient for efficient viral replication (16). The observations that the γ134.5 protein has different activities are intriguing, but contributions of these activities to HSV infection are not yet clear.
The present study was undertaken to dissect the functions of the γ134.5 protein during HSV infection. Here, we report that a critical function of the γ134.5 protein is to block translation shutoff mediated by PKR. In addition, the γ134.5 protein facilitates virus egress or release late in infection. Evidence is presented that the amino terminus of the γ134.5 protein contributes to virus egress that is independent of the function required to prevent the PKR response. These results indicate that efficient HSV infection depends on different functions of the γ134.5 protein during productive infection.
MATERIALS AND METHODS
Cells and viruses.
Vero, 143tk−, SK-N-SH, CV-1, and mouse embryo fibroblast (MEF) 3T6 cell lines were obtained from the American Type Culture Collection and propagated in Dulbecco's modified Eagle's medium supplemented with 5% (Vero) or 10% (143tk−, SK-N-SH, CV-1, and MEF 3T6) fetal bovine serum.
HSV-1(F) is a prototype HSV-1 strain used in these studies (24). In recombinant virus R3616, a 1-kb fragment from the coding region of the γ134.5 gene was deleted (18). In recombinant virus H9813, codons encoding Val193 and Phe195 of the γ134.5 gene were replaced with those encoding Glu and Leu, respectively (15). In recombinant viruses R4002, R931, R908, and R909, the sequences of the γ134.5 gene encoding amino acids 1 to 30, 30 to 72, 72 to 106, and 106 to 146 were deleted, respectively (20).
To construct recombinant virus JL0253R, the plasmid pJL0203 was transformed into an Escherichia coli RR1 strain that harbored a wild-type HSV-bacterial artificial chromosome (BAC) by electrophoration (30). HSV-BAC is derived from HSV-1(F) with an insertion of miniF in the tk locus (a gift from Brian Horsburgh and Frank Tafaro). After a 2-h incubation at 30°C in Luria-Bertani (LB) broth, the bacteria were plated on zeocin-chloramphenicol (CHL) (25 and 20 μg/ml, respectively) plates and incubated overnight at 43°C for integration. Several clones were picked up and diluted serially in LB broth, plated on CHL-5% sucrose LB plates, and incubated at 30°C overnight. The sucrose-CHL-resistant clones were screened by PCR. The primers used for this purpose were OligBH0018 (CCACCCCGGCACGCTCTCTGTCTC) and OligBH0020 (TATAGCGCGGCTCCTGCCATCGTC), which are specific to the γ134.5 gene at nucleotides −25 and +886, respectively. Additional primers used were OligMC0203 (TCAAGCTTTCAGGTAGATTGCTTTCT) and OligMC0204 (TTCTCATTACTGCTTCTCCAAGCGA), which are specific to the NS1 gene at nucleotides +45 and +652, respectively. The positive clones were used to prepare HSV-BAC DNA with the QIAGEN plasmid purification kit. Viral DNA was transfected into Vero cells by using Lipofectamine reagent (Invitrogen). Virus was harvested 3 to 4 days after transfection and amplified on Vero cells. To restore the thymidine kinase (tk) gene, recombinant viral DNA was transfected along with plasmid pRB4867 containing the tk gene into Vero cells (15). The recombinant progeny was selected and purified on 143tk− cells overlaid with HAT medium (0.1 mM sodium hypoxanthine, 0.4 μM aminopterin, 16 μM thymidine). Preparation of viral stock and titration of infectivity were carried out on Vero cells.
Plasmids.
Plasmid pCAGGS-PR8 NS1 SAM contains the EcoRI-XhoI fragment encoding the NS1 protein of influenza A (PR8) virus (43). Plasmid pRB143 contains a BamHI S fragment of HSV-1(F) in the BamHI site of pBR322. To construct plasmid pKY0102, a BamHI S fragment from pRB143 was cloned into the BamHI site of pBluescript SK. To construct pKY0103, the BstEII-BspEI fragment in the γ134.5 gene was replaced with a polylinker (oligonucleotide GTAACCAGTAACTT and its complement, CCGGAAGTACTG) containing an ScaI site. Plasmid pKY0104 was constructed by ligating the BamHI fragment of pKY0103 into the BamHI site of pKO5y. To construct pKY0140, the BglII-DraIII/Klenow fragment from pCDNA3 (Invitrogen) was cloned into the ScaI/Klenow site pKY0104. As a result, a cytomegalovirus promoter was inserted into pKY0140. To construct pJL0203, the EcoRI-XhoI fragment encoding the NS1 protein was isolated from pCAGGS-PR8 NS1 SAM and cloned into the EcoRI-XhoI sites of pKY0140.
Southern blot analysis.
Vero cells were infected with viruses at 10 PFU per cell. At 18 h after infection, cells were harvested and resuspended in ice-cold Tris-EDTA buffer (pH 7.8) containing NP-40 (0.5%) and RNase A (50 μg/ml). The cytoplasmic fraction was collected and treated with proteinase K (0.5 mg/ml) for 30 min at 37°C. Viral DNAs were prepared and subjected to restriction digestions, electrophoretic separation in agarose gels, transfer to nitrocellulose membranes, and hybridization with the 32P-labeled DNA fragments as described previously (20). Autoradiographic images were obtained by exposure to Kodak X-ray film.
Virus growth assay.
Monolayers of SK-N-SH, CV-1, or MEF 3T6 cells were infected with viruses at either 0.01 or 10 PFU per cell. After adsorption for 2 h, the monolayers were overlaid with Dulbecco's modified Eagle's medium and incubated at 37°C. At 24 or 48 h postinfection, samples were harvested, and viruses, released by three cycles of freezing and thawing, were titrated on Vero cells.
Immunoblotting.
Virus-infected cells were washed, harvested, and solubilized in disruption buffer containing 50 mM Tris-HCl (pH 7.0), 5% 2-mercaptoethanol, 2% sodium dodecyl sulfate, and 2.75% sucrose. Samples were then sonicated, boiled, subjected to electrophoresis on denaturing 12% polyacrylamide gels, transferred to nitrocellulose membranes, blocked with 5% nonfat milk, and reacted with a selected primary antibody. The membranes were rinsed in phosphate-buffered saline and reacted with donkey anti-rabbit immunoglobulin conjugated to horseradish peroxidase. Protein bands were detected by enhanced chemiluminescence (Amersham Pharmacia Biotech Inc.). The primary antibodies used include anti-γ134.5 antibody, anti-HSV-1 antibody (Dako Corporation), anti-eIF2α antibody, anti-eIF2α ser51p antibody (Cell Signaling Technology), anti-glycoprotein C (gC) antibody, anti-gD antibody (a gift from Gary Cohen and Roselyn Eisenberg), and anti-NS1 antibody (4).
Interferon assay.
Monolayers of Vero cells grown to 80% confluency were either untreated or pretreated with human leukocyte alpha interferon (1,000 U/ml; Sigma) for 20 h. Cells were then infected with viruses at 0.05 PFU per cell and incubated at 37°C. At 48 h after infection, cells were harvested, and virus yields were determined on Vero cells (14).
Electron microscopy analysis.
Monolayers of MEF 3T6 cells were infected with viruses at 0.5 PFU per cell in 35-mm dishes. At 24 h postinfection, samples were first fixed in 4% glutaraldehyde with 100 mM phosphate buffer (pH 6.8 to 7.2), fixed in 1% osmium tetroxide in phosphate buffer, dehydrated in a series of 50, 70, 85, 95, and 100% ethanol, and embedded in LX112 resin (Ladd Research Industries). Samples were removed from the petri dishes and remounted on aluminum stubs. The ultrathin sections were cut with a Leica Ultracut UCT, placed on 200 mesh copper grids, and stained with uranyl acetate and lead citrate. Grids were viewed with a Joel 1220 transmission electron microscope at 80 kV. Images were taken at various magnifications with a digital charge-coupled device (CCD) camera (Software digital micrograph; Gatan Inc.).
RESULTS
Construction of a recombinant virus in which the γ134.5 gene is replaced with the NS1 gene of influenza A virus.
Studies demonstrated that the γ134.5 protein of HSV-1 is essential to promote viral virulence in vivo (18, 35, 48). Although the protein antagonizes the antiviral effect of interferon mediated by PKR (14, 19, 29, 32), its precise roles in HSV infection remain unresolved. This is partly attributed to the fact that the γ134.5 protein appears to have multiple functions. In order to dissect the functions of the γ134.5 protein, we sought to develop a model system. By design, we constructed a recombinant virus, JL0253R, in which the γ134.5 gene is replaced by the NS1 gene of influenza A virus (Fig. 1). The objective was to assess to what extent the NS1 protein complements the γ134.5 protein in the context of HSV infection. The NS1 protein was chosen because it is an extensively characterized viral protein that blocks interferon response by preventing the activation of PKR (5, 27, 44).
FIG. 1.
(A) Schematic representation of the genome structure of HSV-1 and its derivatives. The two covalently linked components of HSV-1 DNA, L and S, each consist of unique sequences, UL and US, respectively, flanked by inverted repeats (41). The reiterated sequences flanking UL, designated as ab and b′ a′, are each 9 kbp in size, whereas the repeats flanking US, designated a′ c′ and ca, are 6.3 kbp in size. The location of the γ134.5 gene is shown in the expanded portions of the inverted repeat sequences b and b′. Since the b sequence is repeated in an inverted orientation, there are two copies of the γ134.5 gene per genome. HSV-1(F) is the prototype strain used in our laboratory (24). In R3616, the coding region between BstEII-StuI sites of the γ134.5 gene is deleted (21). In recombinant virus JL0253R, the γ134.5 gene is replaced with the influenza A virus NS1 gene (43), which is driven by a cytomegalovirus promoter. Restriction site designations are as follows: N, NcoI; Be, BstEII; S, SacI; and St, StuI. (B) Autoradiographic images of viral DNAs. Vero cells were infected with the indicated viruses at 10 PFU per cell. At 18 h postinfection, cells were harvested, and viral DNA was prepared and then digested with either BamHI, EcoRI and XhoI, or BstEII and DraIII. Samples were electrophoretically separated on 0.8% agarose gels and transferred to a nitrocellulose membrane. The tk gene was detected by hybridization to a 32P-labeled BamHI Q fragment of HSV-1. Similarly, the NS1 gene was probed with a 32P-labeled NS1 fragment spanning nucleotides 45 to 652, and the γ134.5 gene was probed with a 32P-labeled γ134.5 fragment spanning nucleotides 345 to 579. (C) Expression of the γ134.5 protein and the NS1 protein. Vero cells were either mock infected or infected with the indicated viruses at 10 PFU per cell. At 18 h postinfection, the cells were harvested and subjected to electrophoresis, transferred to a nitrocellulose membrane, and reacted with either anti-NS1 antibody or anti-γ134.5 antibody.
As illustrated in Fig. 1A, the BstEII-BspEI fragment containing the γ134.5 gene was replaced with the cDNA fragment of the NS1 gene from influenza A virus in the recombinant virus JL0253R. This was done by homologous recombination using the BAC system as described in Materials and Methods. The BAC plasmid inserted in the tk gene was removed by cotransfection of viral DNA and a plasmid containing the tk gene into Vero cells. The recombinant progeny JL0253R was then selected on 143tk− cells overlaid with medium containing hypoxathine-aminopterin-thymidine. To verify the virus construct, Southern blot analysis was carried out after BamHI, BstEII, and DraIII digestion of viral DNAs (Fig. 1B). As expected, HSV-1(F), R3616, and JL0253R yielded a 3-kb BamHI Q fragment containing the tk gene (Fig. 1B, lanes 1 to 3). In addition, JL0253R produced a 720-bp EcoRI-XhoI fragment containing the NS1 gene (lane 3), and HSV-1(F) yielded a 526-bp BstEII-DraIII fragment representing the γ134.5 gene (lane 1). To examine protein expression, Western blot analysis was performed by using anti-NS1 and anti-γ134.5 antibodies, respectively. The results in Fig. 1C show that in virus-infected cells, HSV-1(F) expressed the γ134.5 protein, whereas the recombinant virus JL0253R produced the NS1 protein (lanes 2 and 4). The γ134.5 protein was not detected in cells infected with either JL0253R (lane 4) or R3616, which lacks the γ134.5 gene (lane 3).
Substitution of the γ134.5 gene with the NS1 gene does not affect HSV response to alpha interferon.
Since the γ134.5 protein is involved in HSV resistance to interferon (14), we examined whether a substitution of the γ134.5 gene with the NS1 gene had any effect in this process. Specifically, monolayers of Vero cells were untreated or pretreated with alpha interferon (1,000 U/ml) to induce the antiviral state. Cells were then infected with the indicated viruses, and virus yields were determined 48 h after infection. As seen in Fig. 2, in the absence of interferon, HSV-1(F) replicated to a titer of 8.7 × 108 PFU/ml and R3616 reached a titer of 2.6 × 107 PFU/ml. Similarly, JL0253R replicated to a titer of 1.6 × 108 PFU/ml. When cells were pretreated with interferon, replication of HSV-1(F) decreased slightly (fourfold), with a titer at 2.4 × 108 PFU/ml. Due to a deletion of the γ134.5 gene, replication of R3616 decreased dramatically to a titer of 2.4 ×105 PFU/ml, exhibiting an interferon-sensitive phenotype. Under this condition, JL0253R still replicated efficiently, reaching a titer of 9.3 × 107 PFU/ml. Thus, like wild-type HSV-1(F), the recombinant virus JL0253R is capable of blocking the antiviral action of alpha interferon. We conclude from this experiment that the NS1 protein functions to confer viral resistance to interferon when expressed in the context of the HSV genome.
FIG. 2.
Viral response to alpha interferon. Monolayers of Vero cells were either untreated or pretreated with human leukocyte alpha interferon (1,000 U/ml; Sigma) for 20 h. Cells were then infected with viruses at 0.05 PFU per cell and incubated at 37°C. At 48 h postinfection, cells were harvested, and virus yields were determined on Vero cells (14). Data represent the average from three independent experiments, with the standard deviation indicated.
Viruses lacking the γ134.5 gene exhibit differential growth defects in mammalian cell lines.
Based on the above analysis, we evaluated the growth properties of JL0253R in human neuroblastoma SK-N-SH cells and African monkey kidney CV-1 cells. These cell lines are restrictive to the γ134.5 deletion mutant due to the shutoff of protein synthesis triggered by viral DNA synthesis (19). In this series of experiments, monolayers of cells were infected with HSV-1(F), R3616, or JL0253 at 0.01 PFU, and virus yields were measured. The results in Fig. 3A show that in SK-N-SH cells, HSV-1(F) replicated to a titer of 2.2 × 106 PFU/ml 24 h after infection. This virus maintained an efficient growth, reaching a titer of 3.8 ×107 PFU/ml 48 h after infection. As expected, R3616 replicated poorly, with a titer of 3.7 × 103 PFU/ml at 24 h and 4 × 104 PFU/ml at 48 h. The decrease in virus growth correlated with the inability of R3616 to prevent the shutoff of protein synthesis (17). Over the same growth period, JL0253R replicated as efficiently as did wild-type HSV-1(F), with titers reaching 2.6 × 106 PFU/ml at 24 h and 3.8 × 107 PFU/ml at 48 h. Virtually identical growth patterns were also seen for these viruses in CV-1 cells (Fig. 3B). Further analysis showed similar phenotypes in mouse fibroblast 10T1/2 cells infected with HSV-1(F), R3616, or JL0253R (data not shown). Therefore, the growth property of the recombinant virus JL0253R is indistinguishable from that of wild-type HSV-1 (F) in these cell lines.
FIG. 3.
Growth properties of wild-type HSV-1(F), the γ134.5 deletion mutant, and the recombinant virus expressing the NS1 protein. Confluent monolayers of SK-N-SH (A), CV-1 (B), and MEF 3T6 (C) cells were infected with HSV-1(F), R3616, or JL0253R at 0.01 PFU per cell and incubated at 37°C. Viruses were harvested at 24 and 48 h postinfection. Samples were freeze-thawed three times and titrated on Vero cells at 37°C. As a parallel experiment, confluent monolayers of MEF 3T6 cells were also infected at 10 PFU per cell (D). Viruses were harvested at 24 h postinfection and titrated as described above. The data represent an average from three independent experiments, and the error bars indicate standard deviations. MOI, multiplicity of infection.
To further examine the requirement of the γ134.5 protein in HSV infection, we also measured virus growth patterns in MEF 3T6 cells. Monolayers of cells were infected with HSV-1(F), R3616, or JL0253R at 0.01 PFU per cell, and virus yields were then determined. Figure 3C shows that wild-type HSV-1(F) replicated to a high titer of 1.9 × 107 PFU/ml at 24 h postinfection. It continued to maintain efficient replication at 48 h after infection, with a titer of 4.7 × 107 PFU/ml. R3616 replicated poorly, with a titer of 7 × 101 PFU/ml at 24 h postinfection, which then increased to a titer of 3.2 × 103 PFU/ml at 48 h postinfection. Surprisingly, JL0253R replicated to a titer of only 2.6 × 103 PFU/ml at 24 h, which then increased to 8.5 ×104 PFU/ml at 48 h after infection. The growth of this virus was approximately 500-fold lower than that of HSV-1(F) but was 26- to 37-fold greater than that of R3616. The modest increase for JL0253R was consistently observed in several independent experiments. These results indicated that the NS1 protein only partially complements the functions of the γ134.5 protein required for viral growth in MEF 3T6 cells.
Based on the unique growth phenotype of JL0253R in MEF 3T6 cells, we tested whether the virus grew similarly at a high multiplicity of infection. For this purpose, monolayers of MEF 3T6 cells were infected with viruses at 10 PFU per cell. At 24 h after infection, virus titers were determined. As shown in Fig. 3D, HSV-1(F) replicated to a titer of 1.1 × 107 PFU/ml, whereas R3616 replicated to a titer of 1.2 × 105 PFU/ml. Under this condition, replication of R3616 was 100-fold less than that of HSV-1 (F), which may result from a block that inhibited viral replication. Unlike R3616, JL0253R reached a titer of 6.3 × 106 PFU/ml, which is close to that of HSV-1(F), with a virus yield only 1.7-fold lower than that for HSV-1(F). These results suggest that once infection is initiated, JL0253R is able to complete its replication cycle within an infected cell, albeit at a slightly lower level than that of HSV-1(F).
The different growth patterns between JL0253R and R3616 suggest that JL0253R, but not R3616, is able to overcome the PKR response in 3T6 cells. To address this issue, viral protein synthesis in 3T6 cells was examined by Western blot analysis using anti-HSV antibodies. As indicated in Fig. 4A, a high level of viral protein was detected in cells infected with HSV-1(F) or JL0253R. A slightly lower level of viral protein synthesis in JL0253R correlated with the 1.7-fold decrease in viral growth at a high multiplicity of infection (Fig. 3D). In sharp contrast, little or no viral protein was detected in 3T6 cells either mock infected or infected with R3616. Immunoblot analysis indicated that levels of eIF-2α were comparable in mock-infected and virus-infected cells. However, a significant amount of phosphorylated eIF-2α was seen only in cells infected with R3616. Obviously, HSV-1(F) as well as JL0253R prevented the translation shutoff mediated by PKR in 3T6 cells, whereas R3616 did not. Collectively, these data suggest that the marked decrease in virus growth associated with R3616 resulted from the translation block mediated by PKR. However, the growth defect associated with JL0253R, derived from a defect(s) after viral protein translation, could be virus release or egress.
FIG. 4.
(A) Synthesis of viral proteins in virus-infected MEF 3T6 cells. Confluent monolayers of MEF 3T6 cells were either mock infected or infected with the indicated viruses at 10 PFU per cell. At 16 h postinfection, cells were harvested, solubilized, subjected to polyacrylamide gel analysis, transferred to a nitrocellulose sheet, and reacted with polyclonal antibodies against whole HSV-1 antigens (Dako Corporation). (B) Phosphorylation state of eIF-2α. The same membrane described above (A) was stripped and probed with antibodies against eIF-2α and phosphorylated eIF-2α (Cell Signaling Technology). The positions of eIF-2α and phosphorylated eIF-2α are shown on the right.
Virus release is decreased in MEF 3T6 cells infected with viruses lacking the γ134.5 gene.
To analyze the growth defect associated with JL0253R, we determined yields of viruses that remained associated with cells and that were released into the medium. In this experiment, monolayers of MEF 3T6 cells were infected with HSV-1(F), R3616, or JL0253R at 0.5 PFU per cell. At 24 h after infection, viruses associated with the cells or in the medium were collected and measured for infectivity on Vero cells. The data in Table 1 show that for HSV-1(F)-infected cells, the total virus yield was 8.93 × 107 PFU/ml. Among these infectious virus particles, 63% were found in the cell body and 37% were present in the medium. As expected, for cells infected with R3616, the overall virus yield was low, with a titer of 1.73 × 104 PFU/ml. There was approximately a 1,000-fold drop in virus production compared to that of HSV-1(F). Moreover, a larger fraction (80%) was associated with the cells, and a smaller fraction (20%) was detected in the medium. Virus particles released into the medium were reduced by 17% in relation to HSV-1(F). Interestingly, in cells infected with JL0253R, the total virus yield was 8.25 × 106 PFU/ml, which was close to that for HSV-1(F). However, most of the virus particles (90%) were associated with cells, and a very small portion (10%) was released into the medium. There was a 27% decrease in virus release compared to HSV-1(F). Therefore, despite the quantitative difference, virus release was less efficient in cells infected with JL0253R or R3616. However, the overall virus production for JL0253R was about 400-fold greater than that for R3616. This result is concordant with the ability of JL0253R to overcome eIF-2α phosphorylation mediated by PKR.
TABLE 1.
Yields of secreted and cell-associated infectious virionsa
| Virus | No. of virion particles (%)
|
||
|---|---|---|---|
| Cell-free particles | Cell-associated particles | Total | |
| HSV-1(F) | (3.20 ± 1.1) × 107 (37) | (5.73 ± 2.8) × 107 (63) | (8.93 ± 3.7) × 107 (100) |
| R3616 | (3.67 ± 2.0) × 103 (20) | (1.37 ± 0.4) × 104 (80) | (1.73 ± 0.6) × 104 (100) |
| JL0253R | (7.83 ± 1.5) × 105 (10) | (7.47 ± 2.3) × 106 (90) | (8.25 ± 2.2) × 106 (100) |
Confluent monolayers of MEF 3T6 cells were infected with HSV-1(F), R3616, and JL0253R at 0.5 PFU per cell and incubated at 37°C. At 24 h postinfection, cell-associated viruses and viruses in the supernatant were collected separately and titrated on Vero cells. The data represent an average from three independent experiments, with standard deviations indicated.
Virus particles are predominantly present within MEF 3T6 cells infected with viruses lacking the γ134.5 gene.
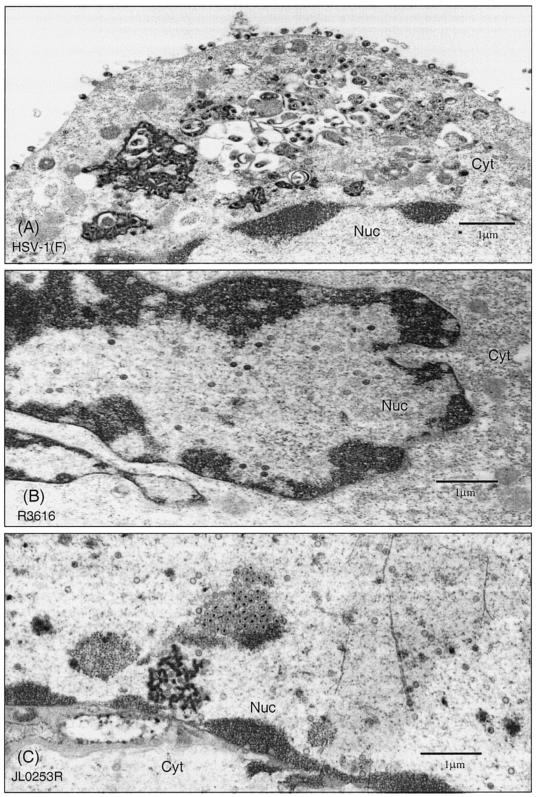
As JL0253R is less efficient in virion release than HSV-1(F), we further examined the distribution of virus particles within the infected 3T6 cells. Cells were infected with HSV-1(F), R3616, or JL0253R and fixed 24 h after infection. Samples were prepared for thin sections and examined by electron microscopic analysis. As presented in Fig. 5, in cells infected with HSV-1(F), a large number of enveloped virions were observed both in the cytoplasmic vesicles and in the extracellular space (Fig. 5A). The significant number of virus particles seen on the cell surface suggests efficient viral production and maturation. In cells infected with R3616, there was a drastic decrease in overall virus particle production. In addition, virus particles were predominantly seen in the nucleus (Fig. 5B). In some cases, the virus particles were localized to areas close to the nuclear membrane. Very few virus particles were found in the cytoplasm or in the extracellular space. This phenotype is similar to that observed for the γ134.5 deletion mutant derived from HSV-1(17+) (8). Importantly, in cells infected with JL0253R, there were a large number of virus particles being produced. Nevertheless, these virus particles were mainly confined to the nucleus (Fig. 5C). Some particles were in close proximity to the nuclear membrane, whereas others formed an aggregate or cluster. To quantitate the distribution of virus particles, 15 to 20 cells were examined for each virus. Locations and numbers of virus particles were enumerated, and the data are summarized in Table 2. In HSV-1(F)-infected cells, 23% of virus particles were in the nucleus, 30% were in the perinuclear region or cytoplasm, and 47% were in the extracellular space. In R3616-infected cells, 48% of virus particles were in the nucleus, 40% were in the perinuclear region or cytoplasm, and 12% were in the extracellular space. Thus, in the absence of the γ134.5 gene, a significant fraction of virus particles were trapped in the nucleus or cytoplasm. Similarly, in cells infected with JL0253R, a large fraction of virus particles were retained in the nucleus or cytoplasm, with 62% of particles in the nucleus, 26% in the perinuclear region or cytoplasm, and only 13% in the extracellular space. Therefore, substitution of the γ134.5 protein with the NS1 protein did not promote virus release or egress, suggesting that an additional function(s) of the γ134.5 protein is required for this process in MEF 3T6 cells.
FIG. 5.
Cellular distribution of virus particles in MEF 3T6 cells. Confluent monolayers of MEF 3T6 cells were infected with HSV-1(F), R3616, or JL0253R at 0.5 PFU per cell. At 24 h postinfection, cells were first fixed in 4% glutaraldehyde in 100 mM phosphate buffer (pH 6.8 to 7.2) and then fixed in 1% osmium tetroxide. Cells were dehydrated in ethanol, embedded in LX112 resin, and stained with uranyl acetate and lead citrate. Thin sections were prepared and viewed with a Joel 1220 transmission electron microscope at 80 kV. Images were captured with a Gatan digital CCD camera. (A) HSV-1(F)-infected MEF 3T6 cells. (B) R3616-infected MEF 3T6 cells. (C) JL0253R-infected MEF 3T6 cells. Scale bars are shown in each panel. Abbreviations: Nuc, nuclear region; Cyt, cytoplasm.
TABLE 2.
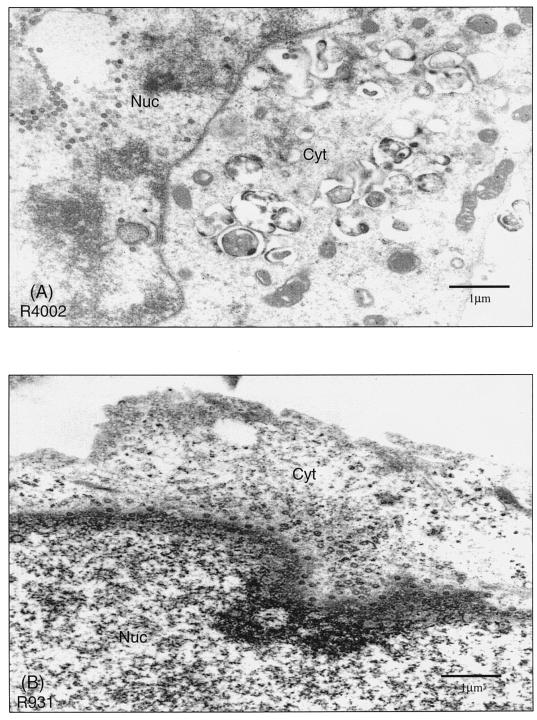
Distribution of virions in MEF 3T6 cells
| Virus | Avg no. (%) of particles/cell ina:
|
||
|---|---|---|---|
| Nucleus | Perinuclear region/cytoplasm | Extracellular space | |
| HSV-1(F) | 58 (23) | 5/67 (30) | 113 (47) |
| R3616 | 29 (48) | 17/7 (40) | 7 (12) |
| JL0253R | 62 (61) | 3/24 (26) | 13 (13) |
| R4002 | 87 (55) | 2/32 (22) | 37 (23) |
| R931 | 10 (9) | 78/12 (83) | 8 (8) |
| R908 | 11 (10) | 80/8 (85) | 5 (5) |
| R909 | 38 (24) | 3/47 (32) | 69 (44) |
Virus particles present in the nucleus, perinuclear region, and cytoplasm and on the cell surface were counted in electron micrographs of at least 15 to 20 randomly sampled MEF 3T6 cells infected with the indicated viruses. The numbers represent the average number of virus particles per cellular compartment, and the numbers in the parentheses denote the percentage of virus particles in the different sections of a cell.
Glycoproteins are processed in cells infected with wild-type virus or virus expressing the NS1 protein of influenza A virus.
Recent studies showed a connection between the γ134.5 protein and processing of gC and gD (6, 37). This process is thought to influence virion maturation and egress (6). To characterize whether the substitution of the γ134.5 gene with the NS1 gene affected glycoprotein processing, monolayers of CV-1, SK-N-SH, and MEF 3T6 cells were mock infected or infected with HSV-1(F), R3616, or JL0253R. At 16 h postinfection, lysates of cells were processed for Western blot analysis by using anti-gC antibody or anti-gD antibody. Figure 6A shows that, like HSV-1(F), JL0253R virus yielded both immature (84 kDa) and mature (116 kDa) gC in all infected cells, which is indicative of efficient glycoprotein processing. Similarly, gD was also efficiently processed in virus-infected cells, with the 52- and 55-kDa bands representing immature and mature forms, respectively. However, R3616 expressed little gC or gD, which resulted from a block in viral protein synthesis due to the deletion of the γ134.5 gene. These data indicate that the defect in virion maturation or egress in 3T6 cells infected with JL0253R is not linked to the processing of viral gC and gD.
FIG. 6.
Processing of gC and gD in virus-infected cells. (A) Confluent monolayers of MEF 3T6, CV-1, and SK-N-SH cells were either mock infected or infected with the indicated viruses at 10 PFU per cell. At 16 h postinfection, cells were harvested, solubilized, subjected to polyacrylamide gel analysis, transferred to a nitrocellulose membrane, and reacted with anti-gC antibody. The positions of mature (116 kDa) and premature (84 kDa) forms are indicated on the right. (B) The same membrane described above (A) was stripped and incubated with anti-gD antibody. The positions of mature (55 kDa) and premature (52 kDa) forms are indicated on the right.
Deletions in the amino-terminal domain of the γ134.5 protein reduce viral growth that is independent of eIF-2α dephosphorylation in MEF 3T6 cells.
Previous studies demonstrated that the carboxyl terminus of the γ13.5 protein is essential to prevent the shutoff of protein synthesis mediated by PKR (19, 28), but the function of the amino-terminal domain is unknown. To address whether the amino terminus is required for virus growth in MEF 3T6 cells, we analyzed the growth properties of a panel of γ134.5 mutants. These mutants have either a series of deletions spanning the region from amino acids 1 to 146 or site-specific mutations in the carboxyl terminus of the γ134.5 protein (15, 20). Cells were infected with viruses at 0.01 PFU per cell, and virus yields were determined at 24 h postinfection. The data in Fig. 7 show that wild-type HSV-1(F) grew to a titer of 1.1 × 107 PFU/ml, whereas the γ134.5 deletion mutant R3616 reached a titer of only 7.7 × 101 PFU/ml. A deletion of the entire γ134.5 gene severely hindered viral replication, with a 105-fold decrease in virus growth. Mutant H9813, which has Val193Glu and Phe195Leu substitutions in the PP1-interacting motif of the γ134.5 protein, grew poorly, with a titer reaching only 2.6 × 102 PFU/ml, which is similar to that for R3616. Hence, a defect that disrupts the ability of the γ134.5 protein to block the antiviral action of interferon involving PKR results in a block in viral replication. Importantly, mutants R4002 (with a deletion of the region of the γ134.5 protein containing amino acids 1 to 30 [Δ1-30aa]), R931 (Δ30-72aa), and R908 (Δ72-106aa) grew more efficiently than R3616, with titers reaching 1.5 ×104 to 5.5 × 104 PFU/ml. However, these mutants also displayed a growth defect compared to HSV-1(F). There was an approximately 1,000-fold decrease in virus titer for these mutants. In addition, mutant R909 (Δ106-146aa) exhibited a moderate decrease (25-fold) in virus growth. These results suggest that the region containing amino acids 1 to 146 is crucial for efficient viral growth in MEF 3T6 cells.
FIG. 7.
(A) Growth of γ134.5 deletion mutants in MEF 3T6 cells. Confluent monolayers of MEF 3T6 cells were infected with viruses at 0.01 PFU per cell and incubated at 37°C. Twenty-four hours postinfection, viruses were harvested, freeze-thawed three times, and titrated on Vero cells at 37°C. The data represent an average from three independent experiments, and the error bars indicate standard deviations. HSV-1(F) is a wild-type virus, whereas R3616 lacks the γ134.5 gene (18, 24). H9813 has Val193Glu and Phe195Leu substitutions in the PP1-binding motif of the γ134.5 protein (15). R4002 (Δ1-30aa), R931 (Δ30-72aa), R908 (Δ72-106aa), and R909 (Δ106-146aa) have a series of deletions in the amino terminus consisting of amino acids 1 to 146 (20). (B) Synthesis of viral polypeptides in virus-infected MEF 3T6 cells. Confluent monolayers of MEF 3T6 cells were either mock infected or infected with the indicated viruses at 10 PFU per cell. At 16 h postinfection, cells were harvested and processed for immunoblot analysis with antibodies against whole HSV-1 antigens (Dako Corporation). (C) Phosphorylation state of eIF-2α. Confluent monolayers of MEF 3T6 cells were either mock infected or infected with the indicated viruses at 10 PFU per cell. At 16 h postinfection, cells were harvested and processed for immunoblot analysis with antibodies against eIF-2α and phosphorylated eIF-2α (Cell Signaling Technology). The positions of eIF-2α and phosphorylated eIF-2α are shown on the right.
To explore the basis for the phenotype described above, we analyzed viral protein synthesis in 3T6 cells. Monolayers of cells were either mock infected or infected with the indicated viruses, and cell lysates were processed for immunoblot analysis by using anti-HSV antibodies. The data in Fig. 7B show that viral proteins were produced in cells infected with HSV-1(F) but not in cells infected with R3616 or H9813, which correlated well with viral growth properties shown in Fig. 7A. Notably, in cells infected with R4002, R931, R908, or R909, viral proteins were produced at a level comparable to that seen for HSV-1(F). Further analysis of the phosphorylation state of eIF-2α indicated that eIF-2α was phosphorylated in cells either mock infected or infected with R3616 or H9813. A low level of phosphorylated eIF-2α in mock-infected cells represents background. Thus, failure to produce viral proteins in cells infected with R3616 or H9813 resulted from the antiviral action of PKR. Importantly, eIF-2α remained nonphosphorylated in cells infected with HSV-1(F), R4002, R931, R908, or R909. This correlated well with efficient synthesis of viral proteins in cells infected with these viruses. As R4002, R931, R908, and R909 were able to inhibit the activity of PKR, the growth defects associated with these viruses in MEF 3T6 cells are due to a step after viral protein production (Fig. 7A). These phenotypes paralleled those seen in cells infected with JL0253R, in which the γ134.5 gene was substituted with the NS1 gene.
Deletions in the amino terminus of the γ134.5 protein impair nuclear as well as cytoplasmic egress in MEF 3T6 cells.
To investigate the role of the amino-terminal domain of the γ134.5 protein, we further analyzed the localization of virus particles in cells infected with HSV-1(F), R3616 (Δγ134.5), R4002 (Δ1-30aa), R931 (Δ30-72aa), R908 (Δ72-106aa), or R909 (Δ106-146aa). MEF 3T6 cells infected with viruses were processed for thin sections and examined by electron microscopic analysis 24 h after infection. As expected, in cells infected with HSV-1(F), virus particles were evident not only in the cytoplasm but also in the extracellular space. In cells infected with R3616, there was a drastic decrease in overall virus particle production. In addition, virus particles were predominantly seen in the nucleus (data not shown). Notably, in cells infected with R4002, virus particles were confined mainly to the nucleus (Fig. 8A). Although a significant number of virus particles were present in infected cells, very few were found in the cytoplasmic vesicles or in the extracellular space. This mutant displayed a defect in nucleocapsid transit from the nucleus to the cytoplasm. However, the most striking observation was that in cells infected with R931 or R908, a majority of the virus particles were trapped in the cytoplasm (Fig. 8B and C). A large number of these particles were in areas close to or associated with the outer nuclear membrane. Virus particles were rarely seen in the nucleus or on the cell surface (Fig. 8B and C). It seems that R931 and R908 were capable of budding into the cytoplasm from the nucleus but incapable of reaching the cell surface. In cells infected with R909, the distribution pattern of virus particles was indistinguishable from that for HSV-1(F) (Fig. 8D), but with a slight decrease in virus particle number. To quantitate the observed differences, the subcellular distribution of virions was counted in 15 to 20 cells for each virus. As summarized in Table 2, in the absence of the γ134.5 gene, a significant fraction (48%) of virus particles were trapped in the nucleus. A similar phenotype was seen with R4002 (55%), indicating a block or delay in virus budding from the nucleus to the cytoplasm. In contrast, in cells infected with R931 or R908, more than 80% of virus particles accumulated in the cytoplasm, whereas less than 10% were on the cell surface. This finding indicates that these mutants have a defect in egress from the cytoplasm to the cell surface. These results correlated with those seen in the virus growth assay for the mutants described above (Fig. 7A). The distribution of R909 seems similar to that of HSV-1(F). Given that this mutant exhibited a moderate growth defect (Fig. 7A), it is likely that the small difference in egress may not be detectable under this experimental condition. Collectively, these data suggest that the amino-terminal domain of the γ134.5 protein acts to facilitate virus egress at two steps. While the region containing amino acids 1 to 30 is required for nuclear egress, the region spanning amino acids 30 to 106 is crucial for cytoplasmic egress.
FIG. 8.
Intracellular distribution of virions in cells infected with γ134.5 mutants with deletions in the amino-terminal domain. Confluent monolayers of MEF 3T6 cells in 35-mm dishes were infected with the indicated viruses at 0.5 PFU per cell. At 24 h postinfection, cells were harvested and processed for electron microscopic analysis as described in Materials and Methods. Digital images were taken at various magnifications with a Gatan digital CCD camera. R4002-infected MEF 3T6 cells (A), R931-infected MEF 3T6 cells (B), R908-infected MEF 3T6 cells (C), and R909-infected MEF 3T6 cells (D) are shown. Scale bars are shown in each picture. Nuc, nuclear region; Cyt, cytoplasm.
DISCUSSION
Several studies have shown that the γ134.5 protein is essential to promote virulence in experimental animal models (18, 34, 35, 46, 48). It functions to inhibit the PKR response in HSV infection (14, 17, 19, 28, 29). Importantly, γ134.5 null mutants are virulent in PKR-knockout mice but not in wild-type mice (18, 32). However, γ134.5 null mutants, with an extragenic mutation, are capable of blocking PKR activity but nonetheless remain avirulent or partially virulent in vivo (10, 40). One hypothesis to reconcile these findings is that the γ134.5 protein has additional functions required for virus infection or virulence. In the present study, we report that efficient virus replication requires the γ134.5 functions that promote viral protein synthesis and virus release or egress in the different stages of the virus life cycle.
The cell line-dependent virus growth distinguishes the functions of the γ134.5 protein in virus infection. The recombinant virus expressing the NS1 protein was resistant to the antiviral action of interferon. The virus was replication competent in both SK-N-SH and CV-1 cells, which are nonpermissive to the γ134.5 deletion mutants because of the PKR response (19). Thus, the γ134.5 protein and the NS1 protein are functionally interchangeable in antagonizing interferon response involving PKR. Surprisingly, in MEF 3T6 cells, the virus expressing the NS1 protein displayed a completely different phenotype. At a low multiplicity of infection, its growth was intermediate between the wild-type virus and the γ134.5 deletion mutant, which would seemingly result from a defect in viral DNA replication or spread from infected cells. However, as the virus expressing the NS1 protein grew efficiently at a high multiplicity of infection where all cells are infected initially, it is unlikely that viral DNA replication is inhibited. Two lines of evidence support this argument. First, viral polypeptides were produced at comparable levels in MEF 3T6 cells infected with either wild-type virus or virus expressing the NS1 protein (Fig. 4A). Second, gC (a γ2 gene), whose expression depends strictly on viral DNA replication, was fully expressed in cells infected with the virus expressing the NS1 protein (Fig. 6A). These results are consistent with previous findings that a deletion of the γ134.5 gene from HSV-1 has no effect on viral DNA replication. The fact that the virus expressing the NS1 protein blocked eIF-2α phosphorylation indicates that the growth defect seen for the virus stems from a block after viral protein synthesis.
It is of interest that the γ134.5 deletion mutant exhibited a severe defect in SK-N-SH, CV-1, and MEF 3T6 cells. The impaired growth in SK-N-SH and CV-1 cells is attributable to the shutoff of protein translation induced by virus infection (19), but the growth defect in MEF 3T6 cells has been suggested to result from a depletion of cellular factors involved in virus replication (7). Brown and colleagues and Harland et al. reported that the γ134.5 protein interacts with PCNA, a cellular protein required for DNA replication and cell cycle control (9, 26). The interaction between the two proteins was postulated to release cells from growth arrest and facilitate viral replication in HSV-infected MEF 3T6 cells (9). While these observations are interesting, the PKR response in MEF 3T6 cells has not been examined. In this regard, it is notable that viral proteins were barely detectable in MEF 3T6 cells infected with the γ134.5 deletion mutant (Fig. 4A and 6A). Moreover, eIF-2α is phosphorylated in MEF 3T6 cells infected with the γ134.5 deletion mutant only. These results suggest that the antiviral activity mediated by PKR is operational in MEF 3T6 cells. This conclusion is further supported by the evidence that amino acid substitutions in the PP1-binding domain of the γ134.5 protein caused a severe defect in viral growth, which paralleled viral translation shutoff and eIF-2α phosphorylation (Fig. 7). Given that viral DNA replication triggers the shutoff of protein synthesis approximately 6 h after infection (19), the ability of the γ134.5 protein to counteract the PKR response seems to be essential during the early phase of virus infection. Thus, the restricted growth pattern of the γ134.5 deletion mutant in MEF 3T6 cells resulted partly from a block in viral protein synthesis mediated by PKR.
Previous studies indicated that a γ134.5 deletion mutant derived from the HSV-1(17+) strain is defective in egress (8). However, whether this block in egress is secondary to a defect in synthesis of other viral late proteins has not been resolved. In MEF 3T6 cells infected with the γ134.5 deletion mutant, there is not only a dramatic decrease in virus production but also a decrease in virus release. Thus, it is difficult to assess the specific involvement of egress in productive infection. We have resolved this problem and extended the previous finding by constructing a recombinant virus in which the γ134.5 gene was substituted with the NS1 gene of influenza A virus. As noted above, the substitution of γ134.5 with NS1 restored the function required to antagonize the PKR response but partially rescued virus growth in MEF 3T6 cells. The virus expressing the NS1 protein yielded more virus particles than the γ134.5 deletion mutant in MEF 3T6 cells, but only a smaller fraction was released (Table 1). These results are strengthened by electron microscopic analysis of virus-infected 3T6 cells, where more virus particles are trapped in the nucleus or cytoplasm in cells infected with the γ134.5 deletion mutant or the recombinant virus expressing the NS1 protein than in wild-type HSV-1(F). It is apparent that inhibition of the PKR response early in infection is not sufficient for efficient productive infection. An additional function(s) of the γ134.5 protein is required to promote virus release or egress in MEF 3T6 cells.
Clinical HSV-1 isolates involved in a block in glycoprotein processing and a limited virion release have been described previously (6, 23). During HSV infection, glycoproteins are integrated into the envelope within the endoplasmic reticulum and processed from the high-mannose precursor form to the sialyated mature form as the virus is released. A comparison of the γ134.5 proteins from clinical HSV-1 isolates suggested that a large plaque-producing variant is efficient in virus release that correlates with efficient processing of glycoproteins (6, 37). Unexpectedly, the recombinant virus expressing the NS1 protein is competent in the processing of glycoproteins (gC and gD), yet the virus displayed decreased virus release or egress. The basis for this is unclear, and additional experiments are needed to resolve this issue.
The present study shows that the amino-terminal domain of the γ134.5 protein is required for efficient virus replication and egress. Previous studies indicated that deletions in the amino terminus of the γ134.5 protein do not affect HSV response to interferon involving PKR (14). Similarly, in MEF 3T6 cells infected with the amino-terminal deletion mutants, viral protein synthesis was not significantly different from that of wild-type virus, and eIF-2α was not phosphorylated. However, deletions in the amino terminus of the γ134.5 protein resulted in a decrease in virus growth in MEF 3T6 cells. Relevant to these observations are two interesting phenotypes. First, a deletion of the region containing amino acids 1 to 30 from the γ134.5 protein led to an increased accumulation of capsid in the nucleus. It is notable that this portion of the protein contains a nucleolar import signal, which determines the nucleocytoplasmic shuttling of the γ134.5 protein (13). As the γ134.5 protein is a virion component (26), it is possible that this cis element is required to direct virus egress from the nucleus to the cytoplasm. Second, a deletion of the region encoding either amino acids 30 to 72 or 72 to 106 from the γ134.5 protein inhibited cytoplasmic egress. Thus, it is conceivable that this region may represent a functional domain required for cytoplasmic transport of virions to the extracellular space. Work is in progress to test these hypotheses.
Lastly, it should be pointed out that γ134.5 mutants exhibit deficient virus growth or egress in mouse 3T6 cells but not in other cells, for example, SK-N-SH and Vero cells. We speculate that the mouse 3T6 cell line may lack a cellular factor that is critical for egress of γ134.5 mutants. Alternatively, this cell line may express an inhibitor that may act to block virus egress during HSV infection. It remains to be established whether γ134.5 mutants are defective in virus egress in human cell lines. Nevertheless, our experimental results appear to correlate with the pathogenesis of HSV infection. Previous studies suggested that mutations in the amino-terminal domain of the γ134.5 protein affect neurovirulence in a mouse model, but this domain is not sufficient to confer virulence (2, 3, 18). Consistent with these observations, the γ134.5 null mutants, with secondary mutations in other regions of the viral genome, block the translation shutoff mediated by PKR. However, these mutants remain highly attenuated in vivo (10, 40). Collectively, this study and others suggest that the γ134.5 protein has an additional function(s) required for viral virulence. The fact that γ134.5 mutants are defective in virus egress suggests that efficient virus egress mediated by the γ134.5 protein is crucial for the pathogenesis of HSV infection.
Acknowledgments
We thank Peter Palese for plasmid and anti-NS1 antibody, Bernard Roizman for HSV-1 strains, and Gary Cohen and Roselyn Eisenberg for anti-gC and anti-gD antibodies.
This work is supported by grants AI 46665 (B.H.) from the National Institute of Allergy and Infectious Diseases.
REFERENCES
- 1.Ackermann, M., J. Chou, M. Sarmiento, R. A. Lerner, and B. Roizman. 1986. Identification by antibody to a synthetic peptide of a protein specified by a diploid gene located in the terminal repeats of the L component of herpes simplex virus genome. J. Virol. 58:843-850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andreansky, S., L. Soroceanu, E. R. Flotte, J. Chou, J. M. Markert, G. Y. Gillespie, B. Roizman, and R. J. Whitley. 1997. Evaluation of genetically engineered herpes simplex viruses as oncolytic agents for human malignant brain tumors. Cancer Res. 57:1502-1509. [PubMed] [Google Scholar]
- 3.Andreansky, S. S., B. He, G. Y. Gillespie, L. Soroceanu, J. Markert, J. Chou, B. Roizman, and R. J. Whitley. 1996. The application of genetically engineered herpes simplex viruses to the treatment of experimental brain tumors. Proc. Natl. Acad. Sci. USA 93:11313-11318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Basler, C. F., X. Wang, E. Muhlberger, V. Volchkov, J. Paragas, H. D. Klenk, A. Garcia-Sastre, and P. Palese. 2000. The Ebola virus VP35 protein functions as a type I IFN antagonist. Proc. Natl. Acad. Sci. USA 97:12289-12294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bergmann, M., A. Garcia-Sastre, E. Carnero, H. Pehamberger, K. Wolff, P. Palese, and T. Muster. 2000. Influenza virus NS1 protein counteracts PKR-mediated inhibition of replication. J. Virol. 74:6203-6206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bower, J. R., H. Mao, C. Durishin, E. Rozenbom, M. Detwiler, D. Rempinski, T. L. Karban, and K. S. Rosenthal. 1999. Intrastrain variants of herpes simplex virus type 1 isolated from a neonate with fatal disseminated infection differ in the ICP34.5 gene, glycoprotein processing, and neuroinvasiveness. J. Virol. 73:3843-3853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown, S. M., J. Harland, A. R. MacLean, J. Podlech, and J. B. Clements. 1994. Cell type and cell state determine differential in vitro growth of non-neurovirulent ICP34.5-negative herpes simplex virus types 1 and 2. J. Gen. Virol. 75:2367-2377. [DOI] [PubMed] [Google Scholar]
- 8.Brown, S. M., A. R. MacLean, J. D. Aitken, and J. Harland. 1994. ICP34.5 influences herpes simplex virus type 1 maturation and egress from infected cells in vitro. J. Gen. Virol. 75:3679-3686. [DOI] [PubMed] [Google Scholar]
- 9.Brown, S. M., A. R. MacLean, E. A. McKie, and J. Harland. 1997. The herpes simplex virus virulence factor ICP34.5 and the cellular protein MyD116 complex with proliferating cell nuclear antigen through the 63-amino-acid domain conserved in ICP34.5, MyD116, and GADD34. J. Virol. 71:9442-9449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cassady, K. A., M. Gross, G. Y. Gillespie, and B. Roizman. 2002. Second-site mutation outside of the US10-12 domain of Δγ134.5 herpes simplex virus 1 recombinant blocks the shutoff of protein synthesis induced by activated protein kinase R and partially restores neurovirulence. J. Virol. 76:942-949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cassady, K. A., M. Gross, and B. Roizman. 1998. The second-site mutation in the herpes simplex virus recombinants lacking the γ134.5 genes precludes shutoff of protein synthesis by blocking the phosphorylation of eIF-2α. J. Virol. 72:7005-7011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cerveny, M., S. Hessefort, K. Yang, G. Cheng, M. Gross, and B. He. 2003. Amino acid substitutions in the effector domain of the γ134.5 protein of herpes simplex virus 1 have differential effects on viral response to interferon-α. Virology 307:290-300. [DOI] [PubMed] [Google Scholar]
- 13.Cheng, G., M. E. Brett, and B. He. 2002. Signals that dictate nuclear, nucleolar, and cytoplasmic shuttling of the γ134.5 protein of herpes simplex virus type 1. J. Virol. 76:9434-9445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheng, G., M. E. Brett, and B. He. 2001. Val193 and Phe195 of the γ134.5 protein of herpes simplex virus 1 are required for viral resistance to interferon α/β. Virology 290:115-120. [DOI] [PubMed] [Google Scholar]
- 15.Cheng, G., M. Gross, M.-E. Brett, and B. He. 2001. AlaArg motif in the carboxyl terminus of the γ134.5 protein of herpes simplex virus type 1 is required for the formation of a high-molecular-weight complex that dephosphorylates eIF-2α. J. Virol. 75:3666-3674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheng, G., K. Yang, and B. He. 2003. Dephosphorylation of eIF-2α mediated by the γ134.5 protein of herpes simplex virus type 1 is required for viral response to interferon but is not sufficient for efficient viral replication. J. Virol. 77:10154-10161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chou, J., J.-J. Chen, M. Gross, and B. Roizman. 1995. Association of a Mr 90,000 phosphoprotein with protein kinase PKR in cells exhibiting enhanced phosphorylation of translation initiation factor eIF-2α and premature shutoff of protein synthesis after infection with γ134.5− mutants of herpes simplex virus 1. Proc. Natl. Acad. Sci. USA 92:10516-10520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chou, J., E. R. Kern, R. J. Whitley, and B. Roizman. 1990. Mapping of herpes simplex virus-1 neurovirulence to γ134.5, a gene nonessential for growth in culture. Science 250:1262-1266. [DOI] [PubMed] [Google Scholar]
- 19.Chou, J., and B. Roizman. 1992. The γ134.5 gene of herpes simplex virus 1 precludes neuroblastoma cells from triggering total shutoff of protein synthesis characteristic of programed cell death in neuronal cells. Proc. Natl. Acad. Sci. USA 89:3266-3270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chou, J., and B. Roizman. 1994. Herpes simplex virus 1 γ134.5 gene function, which blocks the host response to infection, maps in the homologous domain of the genes expressed during growth arrest and DNA damage. Proc. Natl. Acad. Sci. USA 91:5247-5251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chou, J., and B. Roizman. 1990. The herpes simplex virus 1 gene for ICP34.5, which maps in inverted repeats, is conserved in several limited-passage isolates but not in strain 17syn+. J. Virol. 64:1014-1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chou, J., and B. Roizman. 1986. The terminal a sequence of the herpes simplex virus genome contains the promoter of a gene located in the repeat sequences of the L component. J. Virol. 57:629-637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dick, J. W., and K. S. Rosenthal. 1995. A block in glycoprotein processing correlates with small plaque morphology and virion targetting to cell-cell junctions for an oral and an anal strain of herpes simplex virus type-1. Arch. Virol. 140:2163-2181. [DOI] [PubMed] [Google Scholar]
- 24.Ejercito, P. M., E. D. Kieff, and B. Roizman. 1968. Characterization of herpes simplex virus strains differing in their effects on social behaviour of infected cells. J. Gen. Virol. 2:357-364. [DOI] [PubMed] [Google Scholar]
- 25.Goatley, L. C., M. B. Marron, S. C. Jacobs, J. M. Hammond, J. E. Miskin, C. C. Abrams, G. L. Smith, and L. K. Dixon. 1999. Nuclear and nucleolar localization of an African swine fever virus protein, I14L, that is similar to the herpes simplex virus-encoded virulence factor ICP34.5. J. Gen. Virol. 80:525-535. [DOI] [PubMed] [Google Scholar]
- 26.Harland, J., P. Dunn, E. Cameron, J. Conner, and S. M. Brown. 2003. The herpes simplex virus (HSV) protein ICP34.5 is a virion component that forms a DNA-binding complex with proliferating cell nuclear antigen and HSV replication proteins. J. Neurovirol. 9:477-488. [DOI] [PubMed] [Google Scholar]
- 27.Hatada, E., S. Saito, and R. Fukuda. 1999. Mutant influenza viruses with a defective NS1 protein cannot block the activation of PKR in infected cells. J. Virol. 73:2425-2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.He, B., M. Gross, and B. Roizman. 1998. The γ134.5 protein of herpes simplex virus 1 has the structural and functional attributes of a protein phosphatase 1 regulatory subunit and is present in a high molecular weight complex with the enzyme in infected cells. J. Biol. Chem. 273:20737-20743. [DOI] [PubMed] [Google Scholar]
- 29.He, B., M. Gross, and B. Roizman. 1997. The γ134.5 protein of herpes simplex virus 1 complexes with protein phosphatase 1α to dephosphorylate the α subunit of the eukaryotic translation initiation factor 2 and preclude the shutoff of protein synthesis by double-stranded RNA-activated protein kinase. Proc. Natl. Acad. Sci. USA 94:843-848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Horsburgh, B. C., M. M. Hubinette, D. Qiang, M. L. MacDonald, and F. Tufaro. 1999. Allele replacement: an application that permits rapid manipulation of herpes simplex virus type 1 genomes. Gene Ther. 6:922-930. [DOI] [PubMed] [Google Scholar]
- 31.Leib, D. A., T. E. Harrison, K. M. Laslo, M. A. Machalek, N. J. Moorman, and H. W. Virgin. 1999. Interferons regulate the phenotype of wild-type and mutant herpes simplex viruses in vivo. J. Exp. Med. 189:663-672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leib, D. A., M. A. Machalek, B. R. Williams, R. H. Silverman, and H. W. Virgin. 2000. Specific phenotypic restoration of an attenuated virus by knockout of a host resistance gene. Proc. Natl. Acad. Sci. USA 97:6097-6101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lord, K. A., B. Hoffman-Liebermann, and D. A. Liebermann. 1990. Sequence of MyD116 cDNA: a novel myeloid differentiation primary response gene induced by IL6. Nucleic Acids Res. 18:2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.MacLean, A., L. Robertson, E. McKay, and S. M. Brown. 1991. The RL neurovirulence locus in herpes simplex virus type 2 strain HG52 plays no role in latency. J. Gen. Virol. 72:2305-2310. [DOI] [PubMed] [Google Scholar]
- 35.MacLean, A. R., M. ul-Fareed, L. Robertson, J. Harland, and S. M. Brown. 1991. Herpes simplex virus type 1 deletion variants 1714 and 1716 pinpoint neurovirulence-related sequences in Glasgow strain 17+ between immediate early gene 1 and the ′a' sequence. J. Gen. Virol. 72:631-639. [DOI] [PubMed] [Google Scholar]
- 36.Mao, H., and K. S. Rosenthal. 2002. An N-terminal arginine-rich cluster and a proline-alanine-threonine repeat region determines the cellular localization of the herpes simplex virus type-1 ICP34.5 protein and its ligand, protein phosphatase 1. J. Biol. Chem. 277:11423-11431. [DOI] [PubMed] [Google Scholar]
- 37.Mao, H., and K. S. Rosenthal. 2003. Strain-dependent structural variants of herpes simplex virus type 1 ICP34.5 determine viral plaque size, efficiency of glycoprotein processing, and viral release and neuroinvasive disease potential. J. Virol. 77:3409-3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McGeoch, D. J., C. Cunningham, G. McIntyre, and A. Dolan. 1991. Comparative sequence analysis of the long repeat regions and adjoining parts of the long unique regions in the genomes of herpes simplex viruses types 1 and 2. J. Gen. Virol. 72:3057-3075. [DOI] [PubMed] [Google Scholar]
- 39.Mohr, I., and Y. Gluzman. 1996. A herpesvirus genetic element which affects translation in the absence of the viral GADD34 function. EMBO J. 15:4759-4766. [PMC free article] [PubMed] [Google Scholar]
- 40.Mohr, I., D. Sternberg, S. Ward, D. Leib, M. Mulvey, and Y. Gluzman. 2001. A herpes simplex virus type 1 γ34.5 second-site suppressor mutant that exhibits enhanced growth in cultured glioblastoma cells is severely attenuated in animals. J. Virol. 75:5189-5196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sheldrick, P., and N. Berthelot. 1975. Inverted repetitions in the chromosome of herpes simplex virus. Cold Spring Harb. Symp. Quant. Biol. 39:667-678. [DOI] [PubMed] [Google Scholar]
- 42.Tallóczy, Z., W. Jiang, H. W. Virgin IV, D. A. Leib, D. Scheuner, R. J. Kaufman, E.-L. Eskelinen, and B. Levine. 2002. Regulation of starvation- and virus-induced autophagy by the eIF2α kinase signaling pathway. Proc. Natl. Acad Sci. USA 99:190-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Talon, J., C. M. Horvath, R. Polley, C. F. Basler, T. Muster, P. Palese, and A. García-Sastre. 2000. Activation of interferon regulatory factor 3 is inhibited by the influenza A virus NS1 protein. J. Virol. 74:7989-7996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tan, S. L., and M. G. Katze. 1998. Biochemical and genetic evidence for complex formation between the influenza A virus NS1 protein and the interferon-induced PKR protein kinase. J. Interferon Cytokine Res. 18:757-766. [DOI] [PubMed] [Google Scholar]
- 45.Trgovcich, J., D. Johnson, and B. Roizman. 2002. Cell surface major histocompatibility complex class II proteins are regulated by the products of the γ134.5 and UL41 genes of herpes simplex virus 1. J. Virol. 76:6974-6986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Valyi-Nagy, T., M. U. Fareed, J. S. O'Keefe, R. M. Gesser, A. R. MacLean, S. M. Brown, J. G. Spivack, and N. W. Fraser. 1994. The herpes simplex virus type 1 strain 17+ gamma 34.5 deletion mutant 1716 is avirulent in SCID mice. J. Gen. Virol. 75:2059-2063. [DOI] [PubMed] [Google Scholar]
- 47.Ward, S. L., D. Scheuner, J. Poppers, R. J. Kaufman, I. Mohr, and D. A. Leib. 2003. In vivo replication of an ICP34.5 second-site suppressor mutant following corneal infection correlates with in vitro regulation of eIF2α phosphorylation. J. Virol. 77:4626-4634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Whitley, R. J., E. R. Kern, S. Chatterjee, J. Chou, and B. Roizman. 1993. Replication, establishment of latency, and induced reactivation of herpes simplex virus γ134.5 deletion mutants in rodent models. J. Clin. Investig. 91:2837-2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Whitley, R. J., and B. Roizman. 2001. Herpes simplex virus infections. Lancet 357:1513-1518. [DOI] [PubMed] [Google Scholar]
- 50.Zhan, Q., K. A. Lord, I. Alamo, Jr., M. C. Hollander, F. Carrier, D. Ron, K. W. Kohn, B. Hoffman, D. A. Liebermann, and A. J. Fornace, Jr. 1994. The gadd and MyD genes define a novel set of mammalian genes encoding acidic proteins that synergistically suppress cell growth. Mol. Cell. Biol. 14:2361-2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zsak, L., Z. Lu, G. F. Kutish, J. G. Neilan, and D. L. Rock. 1996. An African swine fever virus virulence-associated gene NL-S with similarity to the herpes simplex virus ICP34.5 gene. J. Virol. 70:8865-8871. [DOI] [PMC free article] [PubMed] [Google Scholar]