Abstract
Improvement in the immune response to influenza virus vaccination in the elderly represents the primary unmet need in influenza virus vaccination. We have shown that topical application of immunostimulating (IS) patches containing heat-labile enterotoxin of Escherichia coli (LT) enhances immune responses to injected vaccines. We extend these findings and show that LT-IS patch application enhances the antibody responses to influenza virus vaccination in both young and aged mice. LT-IS patches markedly increased influenza virus-specific immunoglobulin G (IgG), hemagglutination inhibition antibody, mucosal antibody, and T-cell responses. The magnitude of the immune responses in aged mice receiving an LT-IS patch was equivalent to or greater than that of the immune responses in young mice given vaccine alone. These results suggest that addition of an LT-IS patch may compensate for the deficient immune function seen in the aged in response to influenza virus vaccination. Therefore, use of an LT-IS patch could be a new, safe, and simple immunization strategy that may significantly improve the outcome of influenza virus vaccination in the elderly.
Aging is associated with a decrease in immune system function and remains an important challenge to vaccinologists. The elderly population suffers from an increased incidence of infectious diseases, leading to increased morbidity and mortality. Humans over the age of 65 years are considered at high risk for influenza. During influenza epidemics, the rate of hospitalization in the elderly is high, with up to 90% mortality (1, 43). Influenza virus immunization is an effective (36) yet far from perfect strategy that addresses this widespread annual problem. We and others have explored strategies to improve the immunogenicity of influenza virus vaccinees (25). The use of potent adjuvants and immunization by mucosal routes have been explored to improve the immune responses to influenza virus vaccination (30, 41, 42). Adverse effects may also increase with immune enhancement strategies (15, 37) and highlight the need for a safe but potent strategy for immunostimulation.
A new vaccine delivery system, transcutaneous immunization, using the skin as the site of antigen application, has been shown to be highly effective at inducing strong systemic and mucosal responses (2, 17, 26, 44). This immunization strategy takes advantage of the professional antigen-presenting cells, known as Langerhans cells (LCs), found in the outer layer of the skin, the epidermis. LCs increase their baseline rate of migration out of the epidermis in response to stimuli such as contact sensitizers, inflammatory cytokines, and adjuvants (tumor necrosis factor alpha, interleukin-1β [IL-1β]) (5, 6, 16, 25, 40) and travel to inductive sites of the immune system, which are primarily the draining lymph nodes (DLN). Adjuvants of the ADP-ribosylating exotoxin family, which includes heat-labile enterotoxin of Escherichia coli (LT), and their mutants have been used on the skin and have similar stimulatory effects on LCs (25, 29). These bacterial products have demonstrated very strong adjuvant activity when used for transcutaneous immunization (19, 39) without the side effects observed when they are applied by the oral, intranasal, and parenteral routes (32, 35).
Influenza virus vaccination can protect vaccinees from seasonal infections, yet influenza virus vaccine rates are far from satisfactory in the elderly and can be as low as 17 to 35%, depending on the year and strain (10, 24). Improvement in the immune response to influenza virus vaccination represents the primary unmet need in influenza virus vaccination. In previous studies, we have shown that adjuvants delivered topically to the skin enhance the immune response to injected vaccines (25). This new strategy involves the application of an LT immunostimulating (LT-IS) patch at the anatomical site where vaccine has been administered parenterally, resulting in 10- to 50-fold increases in the influenza virus-specific antibody response. These data demonstrated that targeting the same DLN by injection and LT-IS patch application was essential to obtain significant enhancement of the immune response to the injected antigen, leading to attention to patch placement at the site of immunization (25). Both systemic and mucosal immune responses were augmented, and these data suggested that the addition of an LT-IS patch might be used to enhance protection against influenza virus in the elderly, where the greatest need for immune enhancement may be found.
In the present study, we extended the LT-IS patch study to show that application of an LT-IS patch enhances antibody responses to injected influenza virus vaccines in young, as well as in aged, mice. The results indicate that influenza virus-specific immunoglobulin G (IgG), protective hemagglutination inhibition (HAI) antibodies, and mucosal antibodies were markedly increased with an LT-IS patch. T-cell responses undergirding the antibody responses were also enhanced, as shown by the increased frequency of influenza virus-specific T cells producing IL-4. These data suggest that the use of an LT-IS patch could be a safe and simple immunization strategy that could significantly improve the outcome of influenza virus vaccination in the elderly.
MATERIALS AND METHODS
Immunization.
C57BL/6 female mice (n = 7), 6 to 8 weeks old (young) and 14 months old (aged), were obtained from Taconic Laboratories (Germantown, N.Y.). Aged mice were housed in the animal facility for 4 months before use. Mice were immunized intradermally (i.d.) in the back or intramuscularly (i.m.) in the left thigh muscle with 5 μg of trivalent split influenza virus vaccine containing A/Panama/2007/99, A/New Caledonia/20/99, and B/Johannesburg/15/99 (Aventis Pasteur, Lyon, France). The dorsal caudal surface at the base of the tail (for i.d. groups) and the left leg (for i.m. groups) were shaved 48 h prior to vaccination. Immediately prior to vaccination, the mice were anesthetized by intraperitoneal injection of ketamine-xylazine (30 to 40 μl). The skin was pretreated by hydration with saline-soaked gauze, and the stratum corneum was disrupted by buffing with fine-grade emery paper strips (GE Medical Systems). Following injection, wet patches containing phosphate-buffered saline (PBS) (placebo groups) or LT (Berna Biotech) at 10 and 50 μg were applied to hydrated skin for 18 h.
Lung wash and cell collection.
Three weeks after the third immunization, lung washes were collected as previously described (18). Mice were exsanguinated, the trachea was exposed and transected, a 22-gauge polypropylene tube was inserted, and 0.5 ml of buffer (3 μg of phenylmethylsulfonyl fluoride [Sigma] per ml in PBS) was gently infused to inflate the lungs. The infused material was then withdrawn, centrifuged, and stored at −80°C.
Lungs were removed, perfused, minced, and dissociated by incubation in digestion medium (RPMI 1640 medium [BioWhittaker, Walkersville, Md.] supplemented with 10% FBS [Invitrogen], 125 U of collagenase [Sigma] per ml, 60 U of hyaluronidase [Sigma] per ml, and 60 U of DNase I [Sigma] per ml) for 1 h at 37°C with constant agitation. Homogenates were filtered, washed, and incubated with Tris-buffered ammonium chloride to lyse erythrocytes.
ELISA.
Influenza virus- and LT-specific IgG and IgA titers were determined in sera and lung washes by enzyme-linked immunosorbent assay (ELISA) on 96-well ELISA plates (Immulon-2HB; Dynex Laboratories) coated overnight with each strain of split influenza virus or with LT. Plates were blocked with 0.5% casein-Tween 20 for 2 h and washed. Samples were serially diluted (twofold) on ELISA plates and incubated for 18 h at 4°C. IgG and IgA were detected by using horseradish peroxidase-conjugated goat anti-mouse IgG (Bio-Rad) and IgA (Zymed) as secondary antibodies and 2,2′-azino-di-(3-ethylbenzthiazolinesulfonic acid) (ABTS) substrate (Kirkegaard & Perry). Antibody titers are reported as the optical density at 405 nm (OD405) or ELISA units, which correspond to the inverse dilution of the serum that yielded an OD405 of 1.0.
HAI antibody titers.
Serum samples from days 0 and 49 were analyzed by John Treanor at the University of Rochester for HAI antibody titers as previously described (28).
Spleen and lymph node cell isolation.
Spleen and lymph node tissues were aseptically removed and pooled, and single-cell suspensions were prepared by gently passing the tissue through sterile 100-μm-mesh screens. For spleen cell suspensions, erythrocytes were lysed with Tris-buffered ammonium chloride and the remaining cells were washed extensively in complete medium (CM), which contains RPMI 1640 medium (BioWhittaker) supplemented with 10% fetal bovine serum (HyClone, Logan, Utah).
ELISPOT.
The ELISPOT assay was used to measure the number of cells producing IL-4. Multiscreen-HA membrane plates (Millipore) were coated with anti-mouse IL-4 (BD Biosciences), incubated for 18 h at 4°C, and blocked with 2% bovine serum albumin in PBS for 1 h at 37°C. Cells isolated from the lungs, spleen, and inguinal lymph nodes were cultured for 18 to 20 h at 37°C in CM alone or in CM containing 10 μg of influenza virus A/Panama or B/Johannesburg split virus vaccine per ml dialyzed against PBS. Spots were resolved as previously described (34).
Statistical analysis.
Two-tailed unpaired Student t tests were used to compare antibody titers in groups, and P < 0.05 was considered significant.
RESULTS
A single immunization with an LT-IS patch significantly improves the responses to injected influenza virus vaccine.
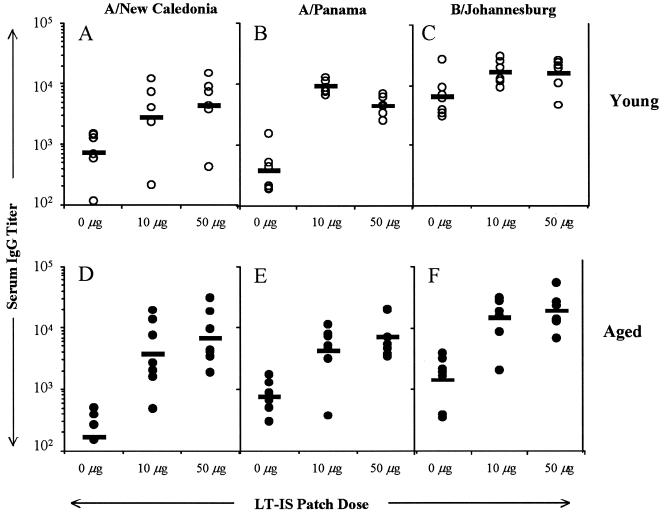
In previous studies, we have shown that LT enhances immune responses to antigen administered by systemic routes if applied in a way that both the injected antigen and the topically applied adjuvant target the same DLN (25). In the present study, we determined whether immune responses after a single injection given to aged mice could be potentiated by LT-IS patch application. Young and aged mice were immunized by i.d. injection of influenza virus vaccine, and patches containing PBS or LT were applied over the injection site. In both age groups, when mice were immunized by i.d. injection with trivalent influenza virus vaccine, the LT-IS patch increased the antibody titers to both the A and B strains of influenza virus (P < 0.05), suggesting that the LT-IS patch may enhance immunity when subjects are naive to the vaccine strain (Fig. 1A to F). Mice immunized similarly with a single dose of influenza virus vaccine by the i.m. route (data not shown) had weaker antibody responses, and the effect of the LT-IS patch was less pronounced than when the i.d. route was used.
FIG. 1.
A single immunization with an LT-IS patch significantly improves the responses to injected influenza virus vaccine. Young (∼2 months old) and aged (18 months old) C57BL/6 mice were immunized with 5 μg of trivalent split influenza virus vaccine (1.7 μg each of the A/New Caledonia, A/Panama, and B/Johannesburg strains) by i.d. injection. Patches containing 25 μl of PBS (0 μg of LT) or 10 or 50 μg of LT were applied to the skin over the injection site immediately after parenteral injection for 18 h. All mice were immunized on study day 0, and serum was collected 2 weeks postimmunization (day 14). Titers of IgG to A/New Caledonia (A and D), A/Panama (B and E), and B/Johannesburg (C and F) in serum were determined by an ELISA method. Individual titers are represented by open circles (young mice) or closed circles (aged mice). The geometric mean of each group is indicated by a horizontal bar. Prebleeding samples had titers of <80 ELISA units for young mice and <230 ELISA units for aged mice.
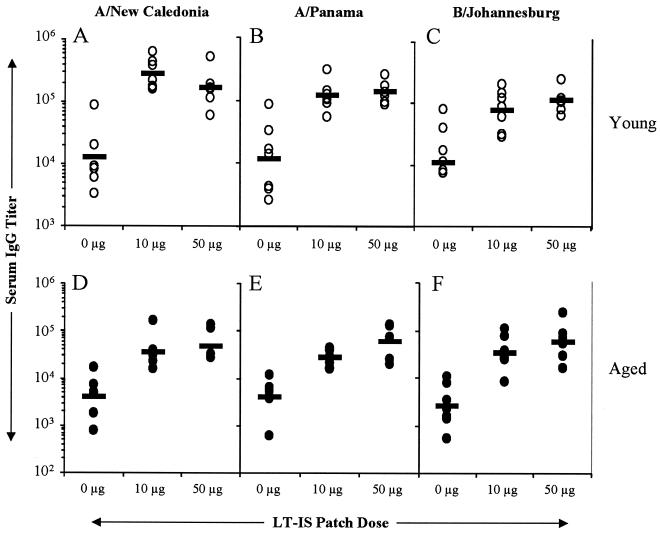
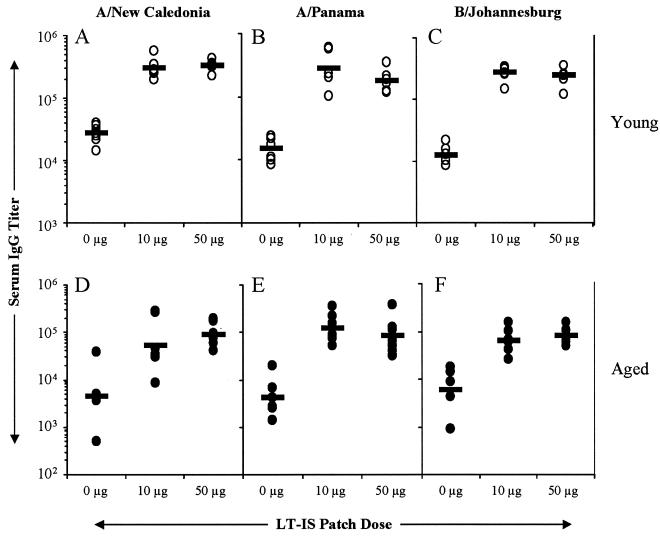
The LT-IS patch significantly enhances responses in aged mice following booster immunizations.
The high rates of immunization and disease exposure in the elderly result in a largely primed population. To determine whether the IS patch could enhance the immune response in primed aged mice, mice in both age groups received three immunizations with trivalent influenza virus vaccine injected i.m. or i.d. and an LT-IS patch or a placebo patch. Influenza virus A- and B-specific serum IgG levels were determined 3 weeks following immunization. The responses induced in both young and aged mice with and without an LT-IS patch were greater than the primary responses. The titers were generally low in aged mice for both the i.m. (Fig. 2D, E, and F) and i.d. (Fig. 3D, E, and F) routes of immunization. The LT-IS patch significantly augmented the responses to all three strains of influenza virus, 10- to 20-fold in young mice (P < 0.01) and 12- to 22-fold in aged mice (P < 0.01; Fig. 2 and 3). Ten micrograms of LT was sufficient to significantly potentiate (P < 0.01) influenza virus A- and B-specific responses in both young and aged mice. Compared to young mice, aged mice immunized with influenza virus by i.m. or i.d. injection and placebo patches had three- to sixfold lower IgG titers for A strains and two- to fourfold lower IgG titers for B strains (Fig. 2 and 3). Overall, the antibody titers in aged mice with the LT-IS patch were higher than in young mice with the placebo patch, suggesting that the LT-IS patch compensated for the deficient immune function in aged mice (Fig. 2 and 3).
FIG. 2.
LT-IS patch enhances secondary responses to influenza virus vaccine delivered by i.m. injection. Young (∼2 months old) and aged (18 months old) C57BL/6 mice were immunized i.m. on days 0, 14, and 28 with 5 μg of trivalent split influenza virus vaccine as indicated in the legend to Fig. 1. Serum was collected, and titers of antibodies to the three strains of influenza virus vaccine were determined by ELISA. Results are reported as described in the legend to Fig. 1.
FIG. 3.
LT-IS patch enhances secondary responses to influenza virus vaccine delivered by i.d. injection. Young (∼2 months old) and aged (18 months old) C57BL/6 mice were immunized i.d. on days 0, 14, and 28 with 5 μg of trivalent split influenza virus vaccine as indicated in the legend to Fig. 1. Serum was collected, and titers of antibodies to the three strains of influenza virus vaccine were determined by ELISA. Results are reported as described in the legend to Fig. 1.
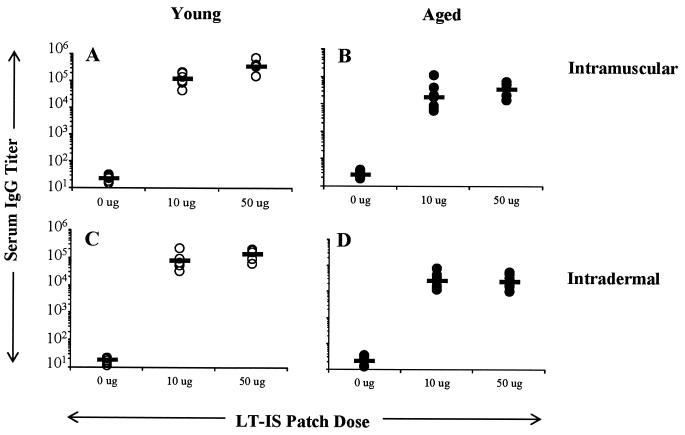
Lower LT-specific responses in aged mice compared to young mice.
Aged mice clearly had reduced influenza virus-specific antibody responses compared to young mice. Previous studies have established that LT elicits immune responses against itself, yet the immune response to LT does not interfere with the immune response to coadministered antigen (39, 44). To confirm the general age-related deficit in the immune response to a second antigen, we looked at LT-specific responses following the third immunization with LT-IS patches with influenza virus immunization. As shown in Fig. 4, aged mice had significantly lower LT IgG titers than did young mice (3- to 11-fold lower, P < 0.01). This suggests that immune responses in aged mice are compromised even if the antigen is highly immunogenic.
FIG. 4.
LT antibody titers are reduced in aged mice compared to responses in young mice. Young and aged C57BL/6 mice were immunized on days 0, 14, and 28 with 5 μg of trivalent split influenza virus vaccine by i.m. (A and B) or i.d. (C and D) injection. Patches containing PBS or LT (10 or 50 μg) were applied over the injection site for ∼18 h. Serum was collected, and titers of antibody to LT were determined by ELISA. Results are reported as described in the legend to Fig. 1. Prebleed samples had titers of <50 ELISA units.
LT-IS patch induces HAI antibodies in aged mice.
Neutralizing antibodies to influenza virus are the best correlate of protection against influenza virus infection in human settings (9, 31). In general, an HAI antibody titer of 40 or greater in humans leads to protection in 50% of vaccinees (14). To determine whether the antibodies produced in young and aged mice might be able to neutralize the virus, sera were tested for titers of HAI antibodies to all three strains of the vaccine. Fifty-seven, 71, and 29% of the young mice immunized i.m. with influenza virus and a placebo patch had titers of HAI antibodies to influenza viruses A/New Caledonia, A/Panama, and B/Johannesburg, respectively, of ≥40 (Table 1). Less than 50% (14% for A/New Caledonia, 43% for A/Panama, and 0% for B/Johannesburg) of the aged mice immunized similarly developed HAI antibody titers of ≥40. For the i.d. route, 100% of the young mice with placebo patches had titers of HAI antibodies to both A strains of ≥40, whereas only 29% of the aged mice had HAI antibody titers of ≥40 (Table 1). An LT-IS patch applied with either i.d. or i.m. injection of influenza virus vaccine elicited HAI antibody titers of ≥40 in 100% of the young and aged mice for influenza viruses A/New Caledonia and A/Panama (Table 1). Similarly, for the B strain, HAI antibody titers were increased significantly by the LT-IS patch (from 14 to 83% in young mice and 60% in aged mice with a 10-μg LT-IS patch; Table 1).
TABLE 1.
Titers of HAI antibodies to influenza A and B viruses
| Influenza virus strain and mouse age (mo) | Amt of LT (μg) | I.m. immunization
|
I.d. immunization
|
||
|---|---|---|---|---|---|
| Mean HAI antibody titera | % Positiveb | Mean HAI antibody titera | % Positiveb | ||
| A/New Caledonia | |||||
| 2 | 50 | 220 | 100 | 747 | 100 |
| 10 | 343 | 100 | 747 | 100 | |
| 0 | 66 | 57 | 91 | 100 | |
| 18 | 50 | 113 | 100 | 153 | 100 |
| 10 | 176 | 100 | 227 | 100 | |
| 0 | 20 | 14 | 36 | 29 | |
| A/Panama | |||||
| 2 | 50 | 880 | 100 | 1,173 | 100 |
| 10 | 914 | 100 | 533 | 100 | |
| 0 | 210 | 71 | 91 | 100 | |
| 18 | 50 | 713 | 100 | 680 | 100 |
| 10 | 680 | 100 | 373 | 100 | |
| 0 | 56 | 43 | 33 | 29 | |
| B/Johannesburg | |||||
| 2 | 50 | 52 | 67 | 220 | 100 |
| 10 | 60 | 71 | 160 | 100 | |
| 0 | 61 | 29 | 14 | 14 | |
| 18 | 50 | 73 | 33 | 43 | 83 |
| 10 | 44 | 60 | 57 | 83 | |
| 0 | 6 | 0 | 11 | 14 | |
Arithmetic mean of seven individual titers 3 weeks after the third immunization.
Percentage of samples for each group with HAI antibody titers of ≥40. Preimmune samples had HAI antibody titers of ≤10 for all three strains.
Mucosal responses in aged mice are enhanced by the LT-IS patch.
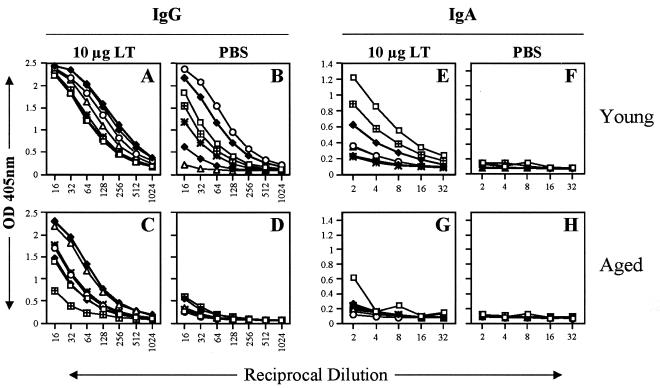
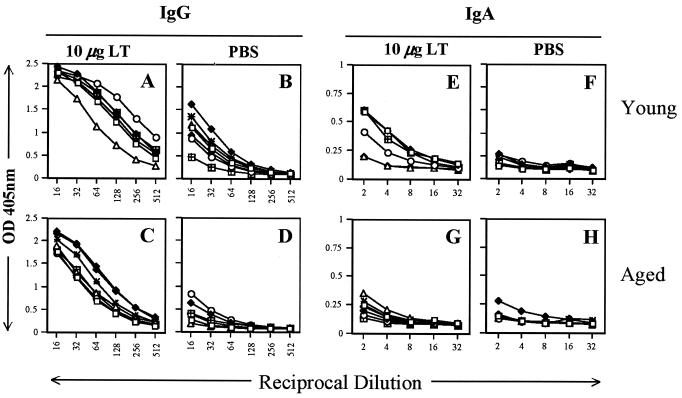
Mucosal antibodies can play an important role in protection of the respiratory tract against influenza virus infection (27), and previous data suggested that skin immunization could result in mucosal responses (2, 19, 21, 22, 44). To characterize pulmonary antibody responses, ELISAs for influenza viruses B/Johannesburg (Fig. 5) and A/Panama (Fig. 6) were performed with lung washes collected from mice immunized with influenza virus vaccine by the i.m. route and a placebo or LT-IS patch. Aged mice immunized with influenza virus vaccine i.m. and a placebo patch had very low to unmeasurable influenza virus-specific IgG responses (Fig. 5 and 6D). In contrast, young mice immunized by injection and a placebo patch alone generated mucosal IgG (Fig. 5 and 6B), and the responses were augmented by LT-IS patch application (Fig. 5 and 6A). In aged mice, application of an LT-IS patch generated strong influenza virus-specific lung IgG responses (Fig. 5 and 6C). IgA responses were not detected in aged and young mice immunized without an LT patch (Fig. 5 and 6F and H). Application of an LT-IS patch induced IgA responses in young mice but only very weak responses in aged mice (Fig. 5 and 6E and G).
FIG. 5.
Mucosal responses to influenza virus B/Johannesburg are significantly enhanced by an LT-IS patch. Young and aged mice were immunized as described in the legend to Fig. 1, with trivalent influenza virus vaccine by i.m. injection. A patch containing PBS (0 μg) or 10 μg of LT was applied over the site of the i.m. injection. Lung washes were collected 3 weeks after the third immunization and analyzed for B/Johannesburg-specific IgG (A to D) and IgA (E to H) by ELISA. Young and aged naive mouse lung washes had nearly undetectable levels of IgG and IgA (OD, <0.1).
FIG. 6.
Mucosal responses to influenza virus A/Panama are significantly enhanced by an LT-IS patch. Young and aged mice were immunized as described in the legend to Fig. 5. Lung washes were collected 3 weeks after the third immunization and analyzed for A/Panama-specific IgG (A to D) and IgA (E to H) by ELISA. Young and aged naive mouse lung washes had nearly undetectable levels of IgG and IgA (OD, <0.1).
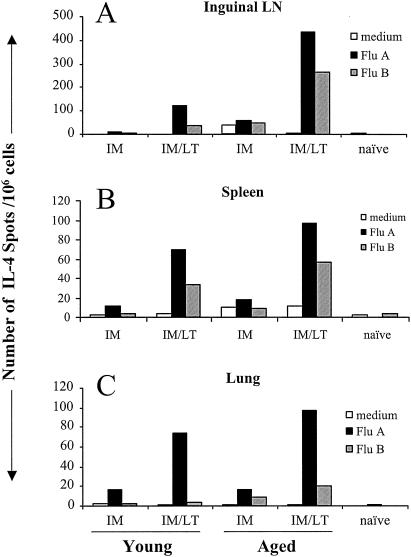
LT-IS patch increases the number of IL-4-producing cells.
Antibody responses were clearly enhanced by an LT-IS patch in young mice, as well as in aged mice. Th2 responses help to stimulate B cells to produce antibodies (11). To determine whether Th2 responses were upregulated by the LT-IS patch, we measured the frequency of cells producing IL-4 in the lungs, spleen, and inguinal lymph nodes after the third immunization. In all three organs, few cells produced IL-4 following i.m. injection alone in both young and aged mice (Fig. 7). With LT-IS patch application, the frequency of cells was greatly increased in aged and young mice. In a previous study (24), the increase in IL-4 correlated with a predominant IgG1 isotype seen with an LT-IS patch (the IgG1/IgG2a ratio was 107 with an LT-IS patch of 5 μg). IL-4-producing cell levels were greater in response to the A/Panama strain than in response to the B/Johannesburg strain, particularly in the lungs. More IL-4-producing cells were detected in the spleens, inguinal lymph nodes, and lungs of aged mice immunized with influenza virus and an LT patch, indicating increased Th2 help in this age group than in young mice.
FIG. 7.
LT-IS patch increases the number of IL-4-producing cells. Young and aged mice were immunized as described in the legend to Fig. 1. Three weeks after the third immunization, single-cell suspensions were prepared from the spleens, inguinal lymph nodes (LN), and lungs and measured in an ELISPOT assay for the frequency of IL-4-secreting cells when cultured in the presence of CM or CM containing 10 μg of influenza virus A/Panama (Flu A) or influenza virus B/Johannesburg (Flu B) per ml. The number of IL-4-producing cells per 106 cells was plotted.
DISCUSSION
This study shows that an LT-IS patch may compensate for the deficient immune function seen in aged mice in response to influenza virus vaccination. The results clearly indicated that serum antibody responses were significantly increased by LT-IS patch application, and neutralizing antibodies (the principal correlate for protection and a marker used for vaccine licensure) (12) were produced in 100% of the aged mice given LT-IS patches. Mucosal antibodies that were not detectable after injection alone were induced following LT-IS patch application. Consistent with the improvement in antibody responses, the number of IL-4-producing cells was significantly enhanced, suggesting the presence of strong Th2 help for B cells to produce antibodies.
The mechanism by which the LT-IS patch exerts its immunostimulatory effect is not fully understood. However, previous studies have shown that topical application of LT admixed with fluorescein isothiocyanate increases the number of activated CD11c- and fluorescein isothiocyanate-positive cells in the DLN (25, 29). Studies using direct labeling of LT have confirmed that LT-activated LCs migrate directly to the DLN (25). It has also been demonstrated that LT potentiates the immune responses to injected antigen only under the condition that both the antigen and adjuvant target the same lymph nodes (25). The immune enhancement of the injected vaccine may be the result of bystander activation of cells in the DLN or increased expression of inflammatory cytokines and antigen-presenting cell costimulatory molecules. Inflammatory stimuli induce a rapid and transient boost of major histocompatibility complex (MHC) class II synthesis and increase the half-life of these molecules, resulting in large numbers of long-lived peptide-loaded MHC class II molecules capable of stimulating T cells even after several days (3). Similarly, bacteria can increase the stabilization of MHC class I molecules, multiplying their half-life by threefold and leading to efficient presentation in the DLN (38). An LT-IS patch increases the antigen-processing capacity of LCs and thereby accelerates peptide-MHC presentation to T cells (25). Taken together, the data show that LT has a profound IS impact on the antigen-presenting capacity of LCs, increasing phenotypic and functional characteristics important for priming and boosting of immune responses to vaccines.
Although protection against and recovery from influenza virus infection involves both humoral and cell-mediated responses, it is generally accepted that an HAI antibody titer of ≥40 could be expected to confer protection in 50% of elderly subjects with this titer (14). Unfortunately, antibody responses to influenza virus in elderly persons show reduced levels of both IgG and IgA antibodies, delayed peak antibody titers, and shorter maintenance of antibody titers (8, 13, 23). Studies with rodents indicate that during aging a progressively increasing proportion of nonfunctional cells results in decreased T-cell proliferation, IL-2 production, and cytotoxicity (33). Our study clearly indicates a significant decrease in antibody titers and HAI antibody levels in aged mice compared to those in young mice. T-cell responses, as indicated by influenza virus-specific IL-4-producing cells, were not apparently reduced in aged mice. The LT-IS patch significantly increased the number of IL-4-producing cells. Although IL-4-producing CD4+ T cells do not clear influenza virus, their presence is required for antibody production (11) and can also contribute to the lung consolidation that is characteristic of influenza virus pneumonia (43). Further studies may specifically address the role of influenza virus-specific CD8+ T cells in viral clearance and the effect of LT-IS patches on this process. Although topical delivery of LT appears to target the epidermal LCs, the immune deficit in aged mice does not appear to be due to deficits in the skin immune system, as the deficit is seen after both parenteral and topical immunizations and the topical application of LT has significant IS effects. This issue needs further study as well, as there is a decreased epidermal LC density and migratory capacity of LCs in aged mouse skin (7).
Influenza virus vaccination is important for protection against the annual attack of influenza virus, most notably in the elderly, where high rates of influenza-related death and morbidity occur (4, 36). Enhancement of vaccine efficacy in the elderly remains the most important unmet need for influenza virus vaccination. Influenza virus vaccine response rates are low, and the poor response to vaccination is common knowledge among influenza specialists. Adjuvants act as potent immune stimulators that may decrease the gap between normal adult immune responses and those of the elderly. Given that the epidermis is equipped with potent immune cells, LCs, and that LT can be used as an efficient activator of resident LCs (20, 25), we found that simple topical placement of an adjuvant-laden patch on the skin resulted in enhanced influenza virus-specific responses in aged mice. The LT-IS patch enhanced the immune response to injected influenza virus vaccine, and by using adjuvant the magnitude of the serum responses was equivalent to or greater than that of the immune responses of young mice given the vaccine alone. Taken together, these data suggest that the LT-IS patch may address the principal unmet need in influenza virus vaccination of the elderly.
Acknowledgments
We thank Vernisha Simmons for excellent technical assistance and Wanda Hardy for preparation of the manuscript.
REFERENCES
- 1.Barker, W. H., and J. P. Mullooly. 1980. Impact of epidemic type A influenza in a defined adult population. Am. J. Epidemiol. 112:798-811. [DOI] [PubMed] [Google Scholar]
- 2.Berry, L. J., D. K. Hickey, K. A. Skelding, S. Bao, A. M. Rendina, P. M. Hansbro, C. M. Gockel, and K. W. Beagley. 2004. Transcutaneous immunization with combined cholera toxin and CpG adjuvant protects against Chlamydia muridarum genital tract infection. Infect. Immun. 72:1019-1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cella, M., A. Engering, V. Pinet, J. Pieters, and A. Lanzavecchia. 1997. Inflammatory stimuli induce accumulation of MHC class II complexes on dendritic cells. Nature 388:782-787. [DOI] [PubMed] [Google Scholar]
- 4.Crocetti, E., S. Arniani, F. Bordoni, G. Maciocco, M. Zappa, and E. Buiatti. 2001. Effectiveness of influenza vaccination in the elderly in a community in Italy. Eur. J. Epidemiol. 17:163-168. [DOI] [PubMed] [Google Scholar]
- 5.Cumberbatch, M., M. Bhushan, R. J. Dearman, I. Kimber, and C. E. Griffiths. 2003. IL-1β-induced Langerhans' cell migration and TNF-α production in human skin: regulation by lactoferrin. Clin. Exp. Immunol. 132:352-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cumberbatch, M., R. J. Dearman, R. W. Groves, C. Antonopoulos, and I. Kimber. 2002. Differential regulation of epidermal Langerhans cell migration by interleukins (IL)-1α and IL-1β during irritant- and allergen-induced cutaneous immune responses. Toxicol. Appl. Pharmacol. 182:126-135. [DOI] [PubMed] [Google Scholar]
- 7.Cumberbatch, M., R. J. Dearman, and I. Kimber. 2002. Influence of ageing on Langerhans cell migration in mice: identification of a putative deficiency of epidermal interleukin-1β. Immunology 105:466-477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.D'Alessio, D. J., P. M. Cox, Jr., and E. C. Dick. 1969. Failure of inactivated influenza vaccine to protect an aged population. JAMA 210:485-489. [PubMed] [Google Scholar]
- 9.Davies, J. R., and E. A. Grilli. 1989. Natural or vaccine-induced antibody as a predictor of immunity in the face of natural challenge with influenza viruses. Epidemiol. Infect. 102:325-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Bruijn, I. A., E. J. Remarque, C. M. Jol-van der Zijde, M. J. van Tol, R. G. Westendorp, and D. L. Knook. 1999. Quality and quantity of the humoral immune response in healthy elderly and young subjects after annually repeated influenza vaccination. J. Infect. Dis. 179:31-36. [DOI] [PubMed] [Google Scholar]
- 11.DeFranco, A. L. 1999. B-lymphocyte activation, p. 225-261. In W. E. Paul (ed.), Fundamental immunology, 4th ed. Lippincott-Raven, Philadelphia, Pa.
- 12.European Agency for the Evaluation of Medicinal Products. 1997. CPMP note for guidance on harmonisation of requirements for influenza vaccines. Human Medicines Evaluation Unit, European Agency for the Evaluation of Medicinal Products, London, England.
- 13.Feery, B. J., M. G. Evered, and E. I. Morrison. 1979. Different protection rates in various groups of volunteers given subunit influenza virus vaccine in 1976. J. Infect. Dis. 139:237-241. [DOI] [PubMed] [Google Scholar]
- 14.Fukuda, K., R. A. Levandowski, C. B. Bridges, and N. J. Cox. 2004. Inactivated influenza vaccines, p. 339-370. In S. A. Plotkin and W. A. Orenstein (ed.), Vaccines, 4th ed. Elsevier, Inc., Philadelphia, Pa.
- 15.Gasparini, R., T. Pozzi, E. Montomoli, E. Fragapane, F. Senatore, M. Minutello, and A. Podda. 2001. Increased immunogenicity of the MF59-adjuvanted influenza vaccine compared to a conventional subunit vaccine in elderly subjects. Eur. J. Epidemiol. 17:135-140. [DOI] [PubMed] [Google Scholar]
- 16.Geissmann, F., M. C. Dieu-Nosjean, C. Dezutter, J. Valladeau, S. Kayal, M. Leborgne, N. Brousse, S. Saeland, and J. Davoust. 2002. Accumulation of immature Langerhans cells in human lymph nodes draining chronically inflamed skin. J. Exp. Med. 196:417-430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Glenn, G. M., R. T. Kenney, S. A. Hammond, and L. R. Ellingsworth. 2003. Transcutaneous immunization and immunostimulant strategies. Immunol. Allergy Clin. N. Am. 23:787-813. [DOI] [PubMed] [Google Scholar]
- 18.Glenn, G. M., T. Scharton-Kersten, R. Vassell, C. P. Mallett, T. L. Hale, and C. R. Alving. 1998. Transcutaneous immunization with cholera toxin protects mice against lethal mucosal toxin challenge. J. Immunol. 161:3211-3214. [PubMed] [Google Scholar]
- 19.Glenn, G. M., T. Scharton-Kersten, R. Vassell, G. R. Matyas, and C. R. Alving. 1999. Transcutaneous immunization with bacterial ADP-ribosylating exotoxins as antigens and adjuvants. Infect. Immun. 67:1100-1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Glenn, G. M., D. N. Taylor, X. Li, S. Frankel, A. Montemarano, and C. R. Alving. 2000. Transcutaneous immunization: a human vaccine delivery strategy using a patch. Nat. Med. 6:1403-1406. [DOI] [PubMed] [Google Scholar]
- 21.Gockel, C. M., S. Bao, and K. W. Beagley. 2000. Transcutaneous immunization induces mucosal and systemic immunity: a potent method for targeting immunity to the female reproductive tract. Mol. Immunol. 37:537-544. [DOI] [PubMed] [Google Scholar]
- 22.Godefroy, S., L. Goestch, H. Plotnicky-Gilquin, T. N. Nguyen, D. Schmitt, M. J. Staquet, and N. Corvaia. 2003. Immunization onto shaved skin with a bacterial enterotoxin adjuvant protects mice against respiratory syncytial virus (RSV). Vaccine 21:1665-1671. [DOI] [PubMed] [Google Scholar]
- 23.Govaert, T. M., M. J. Sprenger, G. J. Dinant, K. Aretz, N. Masurel, and J. A. Knottnerus. 1994. Immune response to influenza vaccination of elderly people: a randomized double-blind placebo-controlled trial. Vaccine 12:1185-1189. [DOI] [PubMed] [Google Scholar]
- 24.Gross, P. A., A. W. Hermogenes, H. S. Sacks, J. Lau, and R. A. Levandowski. 1995. The efficacy of influenza vaccine in elderly persons: a meta-analysis and review of the literature. Ann. Intern. Med. 123:518-527. [DOI] [PubMed] [Google Scholar]
- 25.Guebre-Xabier, M., S. A. Hammond, D. E. Epperson, J. Yu, L. Ellingsworth, and G. M. Glenn. 2003. Immunostimulant patch containing heat-labile enterotoxin from Escherichia coli enhances immune responses to injected influenza virus vaccine through activation of skin dendritic cells. J. Virol. 77:5218-5225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guerena-Burgueno, F., E. R. Hall, D. N. Taylor, F. J. Cassels, D. A. Scott, M. K. Wolf, Z. J. Roberts, G. V. Nesterova, C. R. Alving, and G. M. Glenn. 2002. Safety and immunogenicity of a prototype enterotoxigenic Escherichia coli vaccine administered transcutaneously. Infect. Immun. 70:1874-1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haan, L., W. R. Verweij, M. Holtrop, R. Brands, G. J. van Scharrenburg, A. M. Palache, E. Agsteribbe, and J. Wilschut. 2001. Nasal or intramuscular immunization of mice with influenza subunit antigen and the B subunit of Escherichia coli heat-labile toxin induces IgA- or IgG-mediated protective mucosal immunity. Vaccine 19:2898-2907. [DOI] [PubMed] [Google Scholar]
- 28.Halperin, S. A., B. Smith, T. Mabrouk, M. Germain, P. Trepanier, T. Hassell, J. Treanor, R. Gauthier, and E. L. Mills. 2002. Safety and immunogenicity of a trivalent, inactivated, mammalian cell culture-derived influenza vaccine in healthy adults, seniors, and children. Vaccine 20:1240-1247. [DOI] [PubMed] [Google Scholar]
- 29.Hammond, S. A., M. Guebre-Xabier, J. Yu, and G. M. Glenn. 2001. Transcutaneous immunization: an emerging route of immunization and potent immunostimulation strategy. Crit. Rev. Ther. Drug Carrier Syst. 18:503-526. [PubMed] [Google Scholar]
- 30.Higgins, D. A., J. R. Carlson, and G. Van Nest. 1996. MF59 adjuvant enhances the immunogenicity of influenza vaccine in both young and old mice. Vaccine 14:478-484. [DOI] [PubMed] [Google Scholar]
- 31.Hirota, Y., M. Kaji, S. Ide, J. Kajiwara, K. Kataoka, S. Goto, and T. Oka. 1997. Antibody efficacy as a keen index to evaluate influenza vaccine effectiveness. Vaccine 15:962-967. [DOI] [PubMed] [Google Scholar]
- 32.Michetti, P., C. Kreiss, K. L. Kotloff, N. Porta, J. L. Blanco, D. Bachmann, M. Herranz, P. F. Saldinger, I. Corthesy-Theulaz, G. Losonsky, R. Nichols, J. Simon, M. Stolte, S. Ackerman, T. P. Monath, and A. L. Blum. 1999. Oral immunization with urease and Escherichia coli heat-labile enterotoxin is safe and immunogenic in Helicobacter pylori-infected adults. Gastroenterology 116:804-812. [DOI] [PubMed] [Google Scholar]
- 33.Miller, R. A. 1996. Aging and the immune response, p. 355-392. In E. Schneider and J. Rowe (ed.), Handbook of the biology of aging. Academic Press, San Diego, Calif.
- 34.Murali-Krishna, K., J. D. Altman, M. Suresh, D. J. Sourdive, A. J. Zajac, J. D. Miller, J. Slansky, and R. Ahmed. 1998. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity 8:177-187. [DOI] [PubMed] [Google Scholar]
- 35.Mutsch, M., W. Zhon, P. Rhodes, M. Bopp, R. T. Chen, T. Linder, C. Spyr, and R. Steffen. 2004. Use of the inactivated intranasal influenza vaccine and the risk of Bell’s palsy in Switzerland. N. Engl. J. Med. 350:896-903. [DOI] [PubMed]
- 36.Nichol, K. L., J. Nordin, J. Mullooly, R. Lask, K. Fillbrandt, and M. Iwane. 2003. Influenza vaccination and reduction in hospitalizations for cardiac disease and stroke among the elderly. N. Engl. J. Med. 348:1322-1332. [DOI] [PubMed] [Google Scholar]
- 37.Pittman, P. R., G. Kim-Ahn, D. Y. Pifat, K. Coonan, P. Gibbs, S. Little, J. G. Pace-Templeton, R. Myers, G. W. Parker, and A. M. Friedlander. 2002. Anthrax vaccine: immunogenicity and safety of a dose-reduction, route-change comparison study in humans. Vaccine 20:1412-1420. [DOI] [PubMed] [Google Scholar]
- 38.Rescigno, M., S. Citterio, C. Thery, M. Rittig, D. Medaglini, G. Pozzi, S. Amigorena, and P. Ricciardi-Castagnoli. 1998. Bacteria-induced neo-biosynthesis, stabilization, and surface expression of functional class I molecules in mouse dendritic cells. Proc. Natl. Acad. Sci. USA 95:5229-5234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scharton-Kersten, T., J. Yu, R. Vassell, D. O'Hagan, C. R. Alving, and G. M. Glenn. 2000. Transcutaneous immunization with bacterial ADP-ribosylating exotoxins, subunits, and unrelated adjuvants. Infect. Immun. 68:5306-5313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schuler, G., and R. M. Steinman. 1985. Murine epidermal Langerhans cells mature into potent immunostimulatory dendritic cells in vitro. J. Exp. Med. 161:526-546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Treanor, J. J., H. R. Mattison, G. Dumyati, A. Yinnon, S. Erb, D. O'Brien, R. Dolin, and R. F. Betts. 1992. Protective efficacy of combined live intranasal and inactivated influenza A virus vaccines in the elderly. Ann. Intern. Med. 117:625-633. [DOI] [PubMed] [Google Scholar]
- 42.Walker, R. I. 1994. New strategies for using mucosal vaccination to achieve more effective immunization. Vaccine 12:387-400. [DOI] [PubMed] [Google Scholar]
- 43.Webster, R. G. 2000. Immunity to influenza in the elderly. Vaccine 18:1686-1689. [DOI] [PubMed] [Google Scholar]
- 44.Yu, J., F. Cassels, T. Scharton-Kersten, S. A. Hammond, A. Hartman, E. Angov, B. Corthesy, C. Alving, and G. Glenn. 2002. Transcutaneous immunization using colonization factor and LT induces correlates of protective immunity for enterotoxigenic Escherichia coli. Infect. Immun. 70:1056-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]