Abstract
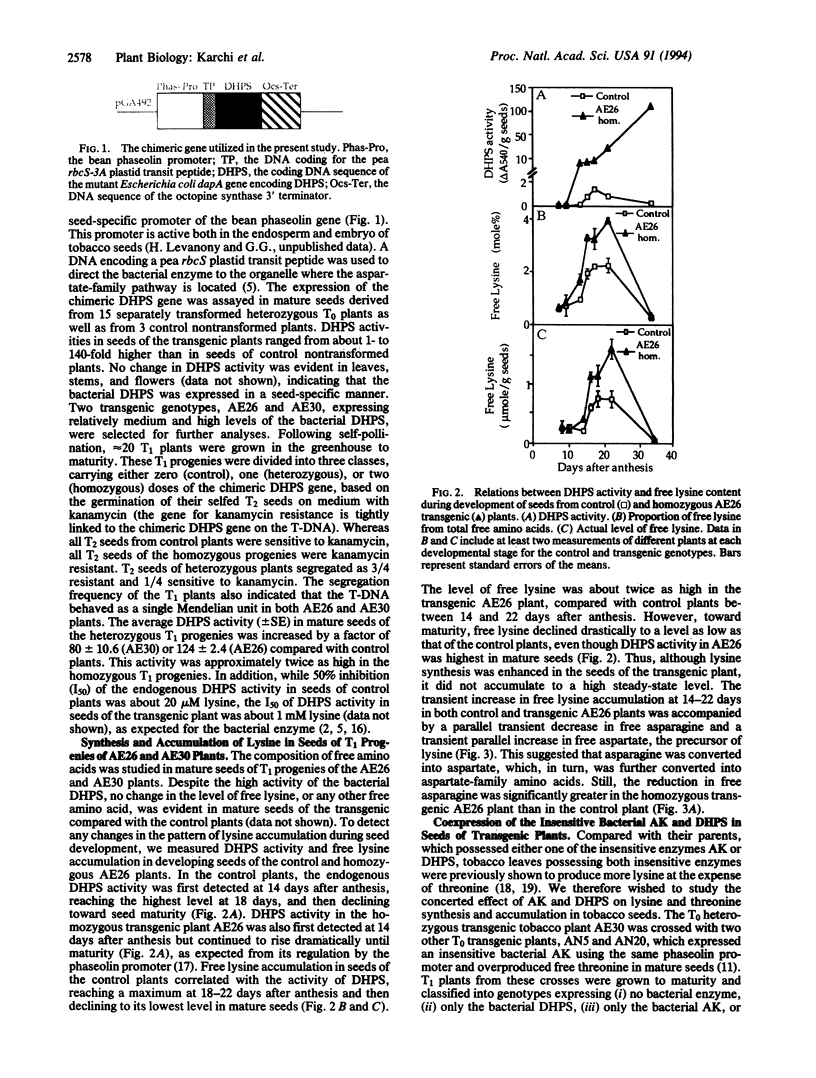
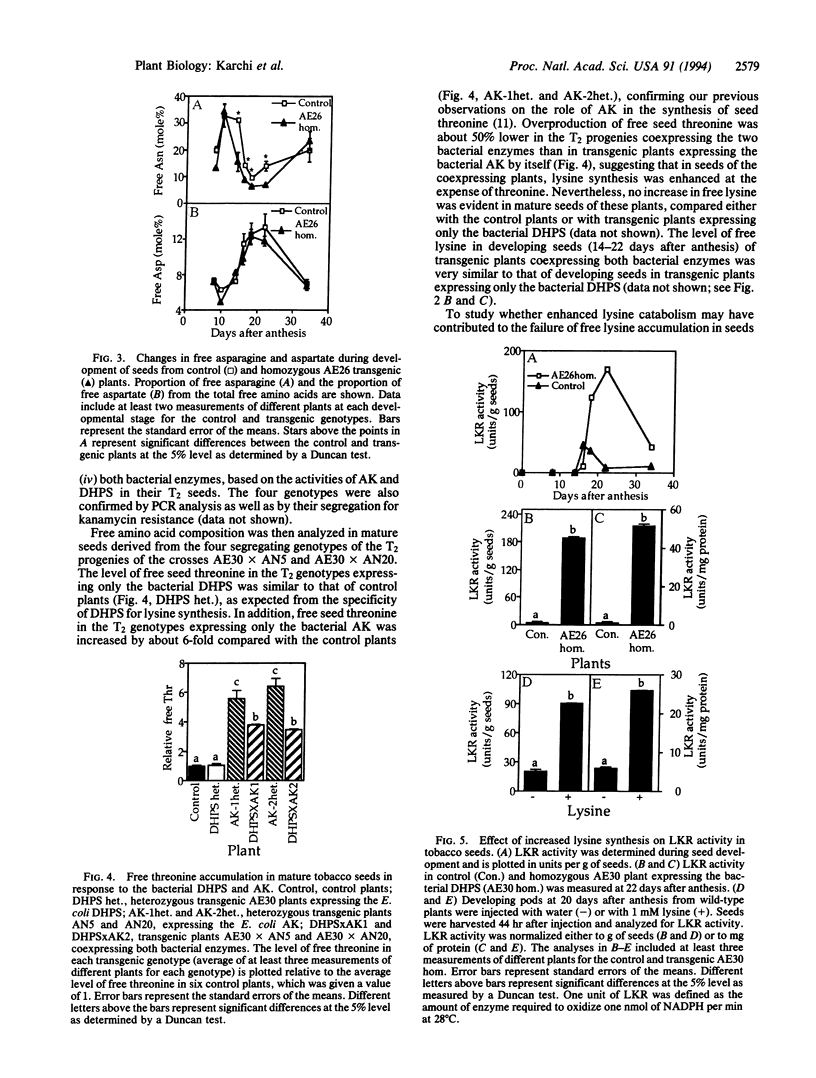
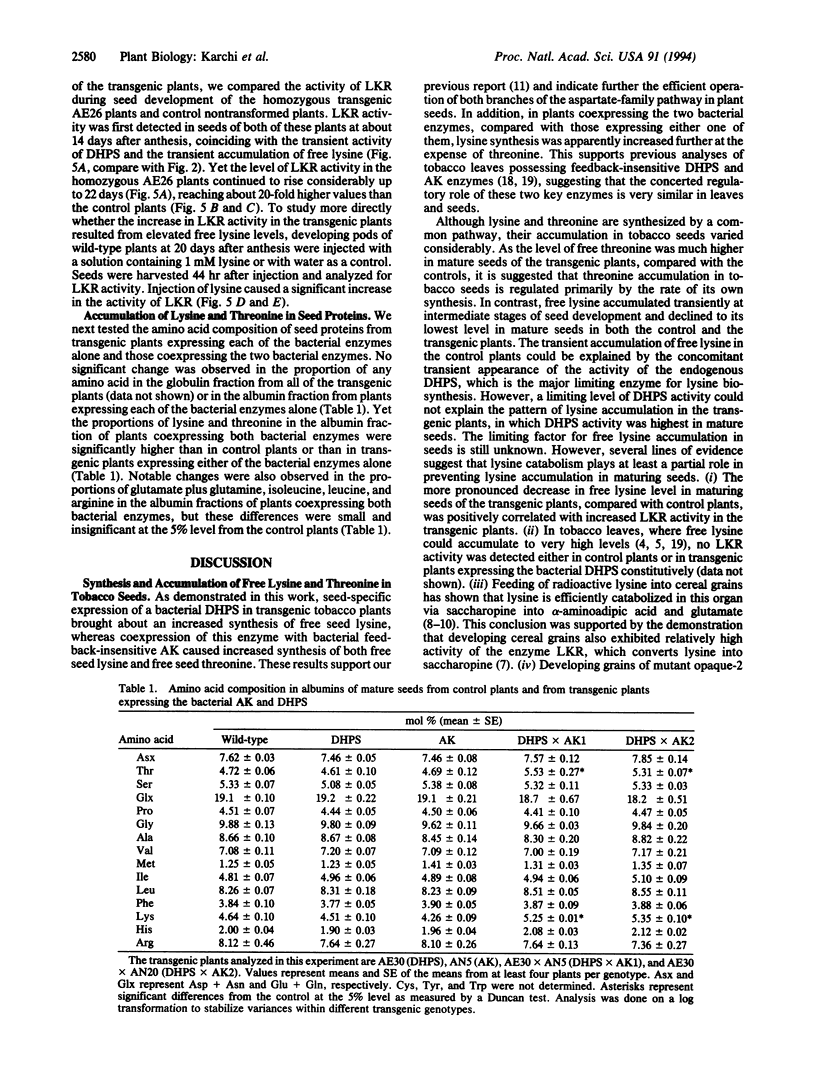
The regulation of synthesis and accumulation of the essential amino acid lysine was studied in seeds of transgenic tobacco plants expressing, in a seed-specific manner, two feedback-insensitive bacterial enzymes: dihydrodipicolinate synthase (EC 4.2.1.52) and aspartate kinase (EC 2.7.2.4). High-level expression of the two bacterial enzymes resulted in only a slight increase in free lysine accumulation at intermediate stages of seed development, while free lysine declined to the low level of control plants toward maturity. To test whether enhanced catabolism may have contributed to the failure of free lysine to accumulate in seeds of transgenic plants, we analyzed the activity of lysine-ketoglutarate reductase (EC 1.5.1.7), an enzyme that catabolizes lysine into saccharopine. In both the control and the transgenic plants, the timing of appearance of lysine-ketoglutarate reductase activity correlated very closely with that of dihydrodipicolinate synthase activity, suggesting that lysine synthesis and catabolism were coordinately regulated during seed development. Notably, the activity of lysine-ketoglutarate reductase was significantly higher in seeds of the transgenic plants than in the controls. Coexpression of both bacterial enzymes in the same plant resulted in a significant increase in the proportions of lysine and threonine in seed albumins. Apparently, the normal low steady-state levels of free lysine and threonine in tobacco seeds may be rate limiting for the synthesis of seed proteins, which are relatively rich in these amino acids.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- A simple and general method for transferring genes into plants. Science. 1985 Mar 8;227(4691):1229–1231. doi: 10.1126/science.227.4691.1229. [DOI] [PubMed] [Google Scholar]
- An G. Development of plant promoter expression vectors and their use for analysis of differential activity of nopaline synthase promoter in transformed tobacco cells. Plant Physiol. 1986 May;81(1):86–91. doi: 10.1104/pp.81.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Brochetto-Braga M. R., Leite A., Arruda P. Partial purification and characterization of lysine-ketoglutarate reductase in normal and opaque-2 maize endosperms. Plant Physiol. 1992 Mar;98(3):1139–1147. doi: 10.1104/pp.98.3.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankard V., Ghislain M., Jacobs M. Two Feedback-Insensitive Enzymes of the Aspartate Pathway in Nicotiana sylvestris. Plant Physiol. 1992 Aug;99(4):1285–1293. doi: 10.1104/pp.99.4.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Møller B. L. Lysine Catabolism in Barley (Hordeum vulgare L.). Plant Physiol. 1976 May;57(5):687–692. doi: 10.1104/pp.57.5.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIGAM S. N., McCONNELL W. B. Studies on wheat plants using carbon-14 compounds. XIX. Observations on the metabolism of lysine-C14. Can J Biochem Physiol. 1963 Jun;41:1367–1371. [PubMed] [Google Scholar]
- Perl A., Shaul O., Galili G. Regulation of lysine synthesis in transgenic potato plants expressing a bacterial dihydrodipicolinate synthase in their chloroplasts. Plant Mol Biol. 1992 Aug;19(5):815–823. doi: 10.1007/BF00027077. [DOI] [PubMed] [Google Scholar]
- Sengupta-Gopalan C., Reichert N. A., Barker R. F., Hall T. C., Kemp J. D. Developmentally regulated expression of the bean beta-phaseolin gene in tobacco seed. Proc Natl Acad Sci U S A. 1985 May;82(10):3320–3324. doi: 10.1073/pnas.82.10.3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaul O., Galili G. Concerted regulation of lysine and threonine synthesis in tobacco plants expressing bacterial feedback-insensitive aspartate kinase and dihydrodipicolinate synthase. Plant Mol Biol. 1993 Nov;23(4):759–768. doi: 10.1007/BF00021531. [DOI] [PubMed] [Google Scholar]
- Sodek L., Wilson C. M. Incorporation of leucine-14C and lysine-14C into protein in the developing endosperm of normal and opaque-2 corn. Arch Biochem Biophys. 1970 Sep;140(1):29–38. doi: 10.1016/0003-9861(70)90006-8. [DOI] [PubMed] [Google Scholar]
- Williamson J. D., Galili G., Larkins B. A., Gelvin S. B. The synthesis of a 19 kilodalton zein protein in transgenic petunia plants. Plant Physiol. 1988 Dec;88(4):1002–1007. doi: 10.1104/pp.88.4.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yugari Y., Gilvarg C. The condensation step in diaminopimelate synthesis. J Biol Chem. 1965 Dec;240(12):4710–4716. [PubMed] [Google Scholar]



