Up-regulation of BMP2/4 signaling in trabecular bone and/or stromal cells increases osteoblast-specific marker expression in hyperactive Gja1Jrt/+ osteoblasts and may also increase bone marrow adipogenesis by up-regulation of Pparg2 in the Cx43-deficient Gja1Jrt/+ mouse model.
Abstract
Gja1Jrt/+ mice carry a mutation in one allele of the gap junction protein α1 gene (Gja1), resulting in a G60S connexin 43 (Cx43) mutant protein that is dominant negative for Cx43 protein production of <50% of wild-type (WT) levels and significantly reduced gap junction formation and function in osteoblasts and other Cx43-expressing cells. Previously we reported that Gja1Jrt/+ mice exhibited early-onset osteopenia caused by activation of osteoclasts secondary to activation of osteoblast lineage cells, which expressed increased RANKL and produced an abnormal resorption-stimulating bone matrix high in BSP content. Gja1Jrt/+ mice also displayed early and progressive bone marrow atrophy, with a significant increase in bone marrow adiposity versus WT littermates but no increase in adipose tissues elsewhere in the body. BMP2/4 production and signaling were increased in Gja1Jrt/+ trabecular bone and osteogenic stromal cell cultures, which contributed to the up-regulated expression of osteoblast-specific markers (e.g., Bsp and Ocn) in Gja1Jrt/+ osteoblasts and increased Pparg2 expression in bone marrow–derived adipoprogenitors in vitro. The elevated levels of BMP2/4 signaling in G60S Cx43-containing cells resulted at least in part from elevated levels of cAMP. We conclude that up-regulation of BMP2/4 signaling in trabecular bone and/or stromal cells increases osteoblast-specific marker expression in hyperactive Gja1Jrt/+ osteoblasts and may also increase bone marrow adipogenesis by up-regulation of Pparg2 in the Cx43-deficient Gja1Jrt/+ mouse model.
INTRODUCTION
Gap junctions and hemichannels mediate cellular communication by allowing the passage of small molecules and ions (e.g., ATP, Ca2+, IP3, cAMP) directly between cells and between cells and their extracellular environment, respectively (Loewenstein, 1981; Alexander and Goldberg, 2003). Connexin 43 (Cx43), one member of the large connexin protein family, is the major gap junctional protein found in bone and is expressed by osteoblasts, osteocytes (Civitelli et al., 1993; Donahue et al., 1995), and bone marrow stromal cells (including osteoblast and adipocyte precursors; Dorshkind et al., 1993). Other members of the connexin protein family expressed in bone are Cx45 (Steinberg et al., 1994), Cx46 (Koval et al., 1997; Sanches et al., 2009), and Cx37 (Yamada et al., 2007; Paic et al., 2009), although their expression is much lower than that of Cx43. In bone, Cx43 is important in mediating hormonal and molecular signals (Chung et al., 2006; Plotkin et al., 2008; Zhang et al., 2011), fracture repair (Loiselle et al., 2012), mechanical loading (Grimston et al., 2008, 2012; Zhang et al., 2011), and control of the subpopulation makeup of the stroma (Gonzalez-Nieto et al., 2012).
Cx43 gap junction function is critical to the processes of osteoblast and osteocyte differentiation and activity and bone formation and maintenance and has been studied extensively through the generation of Cx43-knockout (Lecanda et al., 2000), conditionally deleted (Chung et al., 2006; Watkins et al., 2011; Zhang et al., 2011; Bivi et al., 2012; Gonzalez-Nieto et al., 2012), and point-mutation mutant mice (Flenniken et al., 2005; Dobrowolski et al., 2008; Zappitelli et al., 2013) and by overexpression of mutant Cx43 proteins in cell lines (McLachlan et al., 2008). The role of Cx43 in adipocytes and adipogenesis is less well studied. However, it has been reported that functional Cx43 gap junctions are present and required for mitotic expansion and C/EBPβ expression in preadipocytes (Yanagiya et al., 2007) and that levels of Cx43 protein and gap junction formation and function are down-regulated during adipocyte differentiation (Azarnia and Russell, 1985; Umezawa and Hata, 1992; Yeganeh et al., 2012).
In addition to the important role of Cx43 channels in transport of signaling molecules, Cx43 has been shown to interact with intracellular structural and signaling molecules to modulate cellular signaling activities. For instance, Cx43 proteins have been proposed and/or shown to interact with Src kinase to activate ERKs in response to bisphosphonate-mediated cell survival signaling (Plotkin et al., 2002), with β-arrestin in response to PTH survival signaling (Bivi et al., 2011), and with protein kinase Cδ during FGF2 signaling (Niger et al.). Cx43 has also been proposed to physically interact with β-catenin (Ai et al., 2000), although its involvement in Wnt and bone morphogenic protein (BMP) signaling pathways is unknown.
Loss or disruption of Cx43 gap junctions and hemichannels in cells early in the osteogenic lineage has been reported to impair osteoblast differentiation, bone formation, and mineralization activities in various mouse models (Lecanda et al., 2000; Chung et al., 2006; McLachlan et al., 2008; Watkins et al., 2011). However, in Gja1Jrt/+ mice, in which a dominant- negative G60S Cx43 mutation results in a significant (>50%) reduction of Cx43 protein production, phosphorylation, and gap junction formation and function in osteoblasts (McLachlan et al., 2008) and other cell types (Flenniken et al., 2005), we recently showed that osteoblast differentiation and function are not decreased but are instead activated (Zappitelli et al., 2013). In particular, we found that Gja1Jrt/+ osteoblasts overexpress many osteoblast-associated genes, including Bsp, and deposit an abnormal resorption-stimulating bone matrix high in bone sialoprotein (BSP) content. In addition to its novel osteoblast phenotype, Gja1Jrt/+ is the only Cx43 mutant mouse model with a reported change in bone marrow adipogenesis, leading to progressive bone marrow atrophy beginning at 17 wk of age (Flenniken et al., 2005).
We now report that the G60S Cx43 mutation increases the expression level of osteoblast-specific markers in the osteoblasts by up-regulation of BMP2/4 production and signaling and that the increased BMP production by activated osteoblasts and/or stromal cells may also up-regulate Pparg2 (for peroxisome proliferator–activated receptor γ [Pparg2]) expression, leading to increased bone marrow adipogenesis.
RESULTS
The G60S Cx43 mutation concomitantly activates the osteoblast lineage and increases bone marrow adipogenesis in early-onset osteopenic Gja1Jrt/+ mice
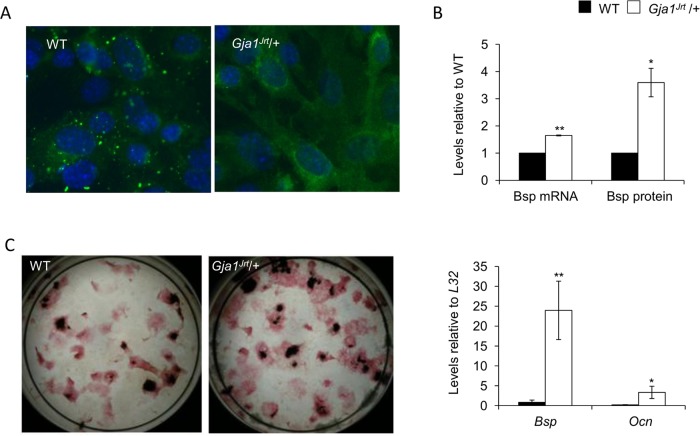
As we previously reported, Gja1Jrt/+ mice, which carry a G60S Cx43 mutation resulting in reduced gap junction formation (Figure 1A) and function, exhibited early-onset osteopenia and changes in the structure and biomechanical properties of bone (Zappitelli et al., 2013). The osteopenic phenotype results from activation of osteoclasts secondary to activation of the osteoblast lineage both in trabecular bone in vivo and bone marrow stromal cultures. We confirmed here that activation of Gja1Jrt/+ osteoblastic cells resulted in increased bone nodule formation, increased osteoblast marker expression (with Bsp being the most highly and significantly up-regulated), and production of an abnormal bone matrix high in BSP content (Figure 1, B and C), which we previously showed stimulates resorption (Zappitelli et al., 2013).
FIGURE 1:
The Gja1Jrt mutation activates the osteoblast lineage and alters bone matrix composition. (A) The formation of gap junctional plaques on the surface of Gja1Jrt/+ mouse embryonic fibroblasts was significantly decreased vs. WT cells, whereas intracellular localization of CX43 protein was significantly increased in Gja1Jrt/+ vs. WT cells. (B) Levels of Bsp mRNA and BSP protein were significantly increased in the trabecular bone matrix of Gja1Jrt/+ vs. WT mice. (C) Alkaline phosphatase and VonKossa stained endpoint osteogenic stromal cultures revealed that numbers of CFU-ALP and CFU-O were higher in Gja1Jrt/+ vs. WT cultures. Expression of osteoblast-associated markers Bsp and Ocn at endpoint of culture was significantly increased in stromal cultures isolated from Gja1Jrt/+ vs. WT mice; n ≥ 3. *p < 0.05, **p < 0.01.
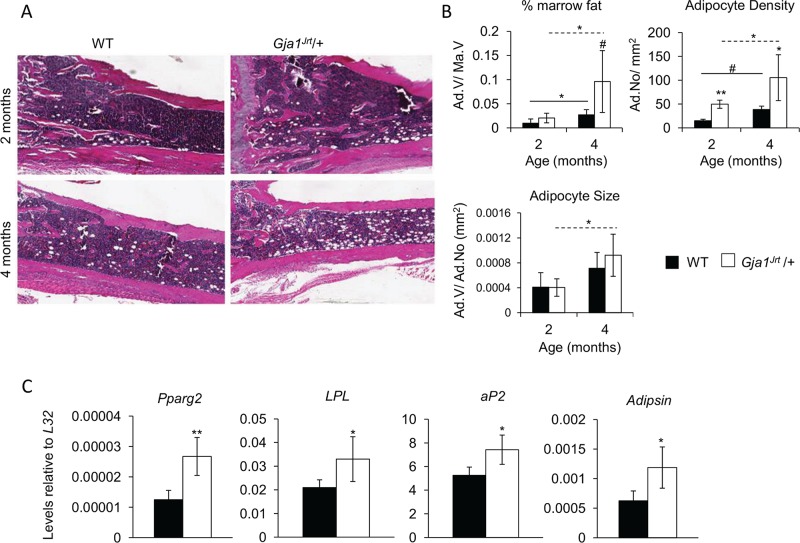
As early as 7 wk of age, Gja1Jrt/+ mice exhibited increased bone marrow atrophy versus wild-type (WT) mice (Figure 2A; Flenniken et al., 2005). Histomorphometry confirmed that whereas adipocyte size (adipocyte volume/adipocyte number) and percentage marrow fat (adipocyte volume/marrow volume) were not significantly different between genotypes, adipocyte density (adipocyte number/tissue volume, mm2) was significantly increased at 2 and 4 mo of age in Gja1Jrt/+ versus WT bone marrow (Figure 2B). Expression of all adipocyte markers tested, including Pparg2, the master adipogenic transcription factor, and downstream adipogenic markers aP2, LPL, and Adipsin, were significantly increased in Gja1Jrt/+ versus WT bone marrow (Figure 2C).
FIGURE 2:
Adipocyte number and activity are increased in Gja1Jrt/+ vs. WT bone marrow. (A) Hematoxylin and eosin–stained tibia bones revealed an increased bone marrow atrophy in Gja1Jrt/+ mice at 2 and 4 mo of age vs. WT littermates. (B) Histomorphometric analysis of the long bones showed a significant increase in adipocyte density at 2 and 4 mo of age. Percentage marrow fat and adipocyte size were unaffected between genotypes; n ≥ 4. (C) Expression of adipocyte-associated markers was significantly increased in Gja1Jrt/+ vs. WT whole bone marrow of 4-mo-old mice; n ≥ 4. Solid and dashed lines indicate significance over time in WT and Gja1Jrt/+ mice, respectively. #p < 0.1, *p < 0.05, **p < 0.01.
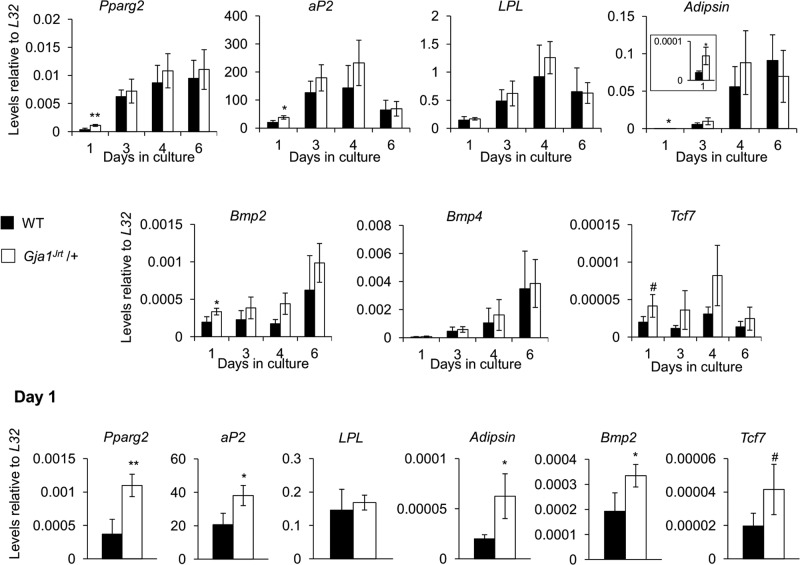
The increase in adipogenesis in Gja1Jrt/+ mice was restricted to the bone marrow, as evidenced by the fact that neither body mass composition (Supplemental Figure S1A) nor expression of adipocyte markers in the epididymal fat pads (Supplemental Figure S1B) was significantly different between Gja1Jrt/+ and WT mice at any age tested. Of note, Gja1Jrt/+ stromal cells cultured under adipogenic conditions displayed significantly increased expression of adipocyte markers at day 1 compared with WT cells but not thereafter (Figure 3); consistent with this, no difference was seen in oil red O staining at endpoint between genotypes (Supplemental Figure S2).
FIGURE 3:
The Gja1Jrt mutation does not affect adipocyte lineage development in vitro. RNA was isolated at four time points throughout proliferation-differentiation in adipogenic stromal cultures derived from 4-mo-old mice. Expression of adipocyte-associated markers Bmp2, Bmp4, and Tcf7 was unchanged between genotypes, except at day 1, when expression of these markers was increased in Gja1Jrt/+ vs. WT adipogenic stromal cultures; n ≥ 3. #p < 0.1, *p < 0.05, **p < 0.01.
The BMP2/4 and Wnt/β-catenin signaling pathways are up-regulated in Gja1Jrt/+ trabecular bone and osteogenic stromal cell cultures, but only BMP2/4 is responsible for the increase in osteoblast-specific gene expression
We next sought to identify which signaling pathway(s) downstream of Cx43 were altered by the G60S Cx43 mutation and might account for the increased expression of markers in the Gja1Jrt/+ osteoblasts. Using a Pathway Finder QPCR array and RNA isolated from Gja1Jrt/+ and WT trabecular bone samples, we identified and selected two candidate pathways for further analysis, the BMP2/BMP4 pathway and the Wnt/β-catenin pathway (based on expression differences of 1.5-fold or greater between genotypes; Table 1). Given the reported ability of β-catenin to interact physically with CX43 (Ai et al., 2000), we first examined the Wnt/β-catenin signaling pathway. Whereas total β-catenin protein was significantly increased in Gja1Jrt/+ versus WT stromal cells, the level of transcriptionally active β-catenin was not (Supplemental Figure S3A). When expression of Axin2 and naked cuticle 1 (Nkd1), two direct targets of β-catenin signaling, was assessed via quantitative PCR, we found that Axin2 was unaffected, but expression of Nkd1 was significantly up-regulated in Gja1Jrt/+ versus WT stromal cells (Supplemental Figure S3B). The inconsistent changes in Wnt/β-catenin target genes suggested that this pathway was not involved in the increased marker expression by Gja1Jrt/+ osteoblasts. This was confirmed by treating WT and Gja1Jrt/+ stromal cells with a Wnt signaling inhibitor, IWP-2. Treatment with 1 μM IWP-2 significantly reduced Wnt/β-catenin signaling in both WT and Gja1Jrt/+ stromal cells, as evidenced by down-regulation of expression of Axin2 (Supplemental Figure S3C); however, IWP-2 treatment had no significant effect on expression of Bsp in either WT or Gja1Jrt/+ stromal cells, and Bsp expression remained higher in Gja1Jrt/+ than in WT stromal cells, regardless of the IWP-2 concentration used (Supplemental Figure S3D).
TABLE 1:
Results of the Mouse Signal Transduction Pathway Finder RT2 Profiler PCR Array.
| Gene symbol | Gene name | Expression, fold change vs. WT |
|---|---|---|
| Bmp2 | Bone morphogenetic protein 2 | 2.33 |
| Bmp4 | Bone morphogenetic protein 4 | 3.00 |
| Ccl2 | Chemokine (C-C motif) ligand 2 | −1.56 |
| Cdkn2a | Cyclin-dependent kinase inhibitor 2A | −1.58 |
| Cxcl1 | Chemokine (C-X-C motif) ligand 1 | −2.53 |
| Egr1 | Early growth response 1 | 1.79 |
| Fn1 | Fibronectin 1 | −2.02 |
| Hhip | Hedgehog-interacting protein | 2.79 |
| Il1a | Interleukin 1α | 1.65 |
| Il4ra | Interleukin 4 receptor α | −1.75 |
| Mmp10 | Matrix metallopeptidase 10 | −1.91 |
| Pparg | Peroxisome proliferator–activated receptor γ | 2.53 |
| Selp | Selectin, platelet | −3.82 |
| Tcf7 | Transcription factor 7, T-cell specific | 3.21 |
| Tfrc | Transferrin receptor | 1.69 |
| Pmepa1 | Prostate transmembrane protein, androgen induced 1 | −1.56 |
| Vcam1 | Vascular cell adhesion molecule 1 | 1.54 |
| Vegfa | Vascular endothelial growth factor A | 1.52 |
| Wisp1 | WNT1-inducible signaling pathway protein 1 | 1.89 |
Genes of interest were identified as those whose expression was changed 1.5-fold or greater in Gja1Jrt/+ vs. WT samples. RNA was isolated from trabecular bone of 2-mo-old WT and Gja1Jrt/+ mice; n = 2, and each sample was the combination of n ≥ 2 independent biological samples.
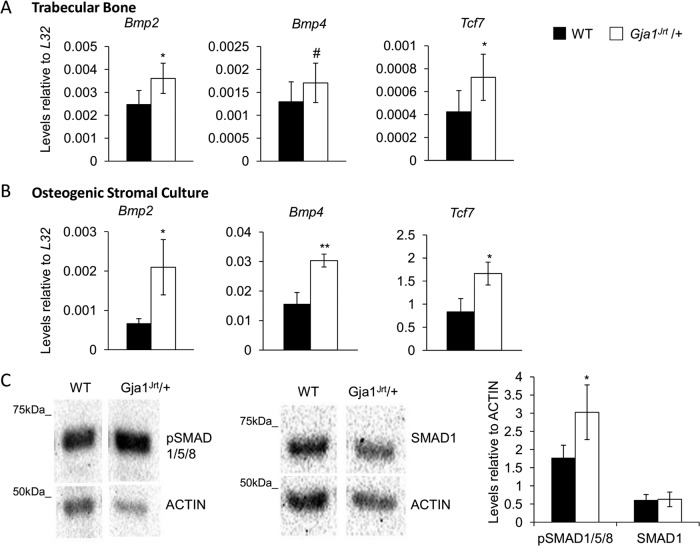
Therefore we next examined the BMP2/4 signaling pathway and confirmed that expression of Bmp2, Bmp4, and Tcf7 (for transcription factor 7 [Tcf7]) was significantly increased in RNA isolated from both Gja1Jrt/+ trabecular bone (Figure 4A) and osteogenic stromal cell cultures (Figure 4B) versus WT specimens. BMP2/4 signaling, determined by immunoblotting for phosphorylated SMAD1/5/8 (pSMAD1/5/8) proteins, was also significantly increased in Gja1Jrt/+ versus WT stromal cell cultures. Levels of SMAD1 were unaffected between genotypes (Figure 4C).
FIGURE 4:
BMP2/4 signaling is increased in Gja1Jrt/+ in vivo and in vitro. Expression of Bmp2, Bmp4, and Tcf7 was increased in Gja1Jrt/+ vs. WT (A) trabecular bone at 4 mo of age (n ≥ 8) and in (B) osteogenic stromal cultures at confluence (n ≥ 3). (C) Levels of pSMAD1/5/8 were significantly increased in Gja1Jrt/+ vs. WT confluent osteogenic stromal cultures. Levels of SMAD1 were unchanged. One representative blot is shown; n ≥ 4. #p < 0.1, *p < 0.05, **p < 0.01.
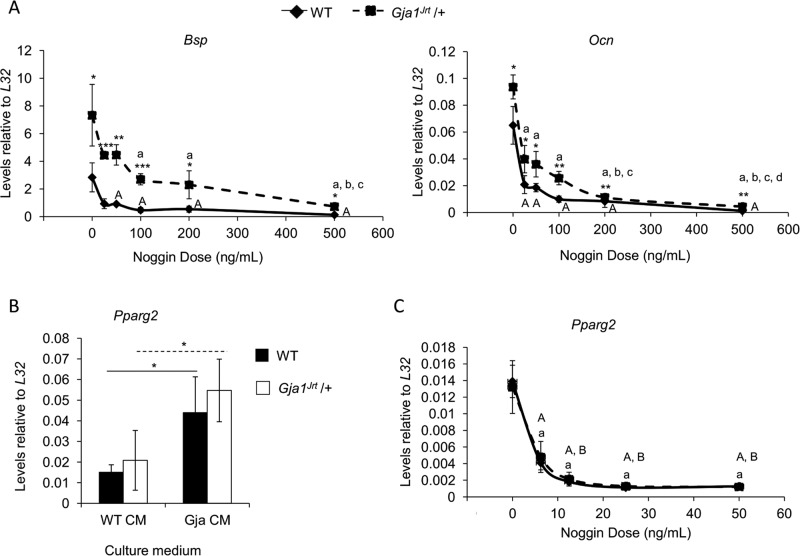
To determine whether the up-regulated marker expression in Gja1Jrt/+ osteoblasts resulted directly from up-regulated BMP2/4 signaling, we treated osteogenic stromal cell cultures with Noggin, a BMP2/4-signaling inhibitor. The dose of Noggin required for either half (ID50) or maximal knockdown of both Bsp and Ocn (for osteocalcin [Ocn]) expression was higher in Gja1Jrt/+ than WT cells (e.g., ID50 = 12.5–25 ng/ml Noggin in WT cells vs. ID50 = 100–200 ng/ml Noggin in Gja1Jrt/+ cells; Figure 5A). The significant reduction in pSMAD1/5/8 levels confirmed that Noggin treatment knocked down BMP2/4 signaling in cells of both genotypes (Supplemental Figure S4).
FIGURE 5:
Up-regulated BMP2/4 signaling is responsible for the increased osteoblast marker expression and the increased Pparg2 expression in bone marrow–derived adipocytes and adipogenic precursors in Gja1Jrt/+ vs. WT mice. (A) The dose of Noggin required for either half (ID50) or maximal knockdown of both Bsp and Ocn expression was higher in Gja1Jrt/+ vs. WT osteogenic stromal cells. One representative experiment is shown, and samples were run in triplicate; n = 3. (B) Both WT and Gja1Jrt/+ adipogenic stromal cells grown in the presence of Gja1Jrt/+ conditioned medium expressed higher levels of Pparg2 vs. those grown with the addition of WT conditioned medium; n ≥ 3. Solid and dashed lines indicate significant differences between cells cultured in either conditioned medium situation in WT and Gja1Jrt/+ cells, respectively. (C) Expression of Pparg2 declined significantly when cells of either genotype (cultured under adipogenic conditions and with the addition of Gja1Jrt/+ conditioned medium) were treated with Noggin; n = 5. Asterisks indicate significance between genotypes at that dosage concentration; *p < 0.05, **p < 0.01, and ***p < 0.001. Capital letters indicate significance between WT samples; lowercase letters indicate significance between Gja1Jrt/+ samples; letters are assigned in alphabetical order according to the dosages (e.g., significant difference vs. dose 0 is denoted A in WT and a in Gja1Jrt/+). p < 0.05 was considered statistically significant.
Up-regulated BMP2/4 production by Gja1Jrt/+ osteogenic stromal cell cultures increases adipocyte gene expression in adipogenic stromal cultures
As summarized so far, adipocyte density and volume were increased in the bone marrow but not elsewhere in the body (e.g., fat pads, trunk) of Gja1Jrt/+ mice, suggesting that the increase was dependent on factors in the marrow microenvironment. The fact that increased expression of adipocyte-associated genes was seen only at early times, that is, day 1, but not thereafter in Gja1Jrt/+ versus WT stromal cell cultures also supported the possibility that cells other than adipocytes or their endogenous factors in the marrow microenvironment were responsible for the increased adipogenesis. Previously, we showed that mature endosteal osteoblasts are dislodged from bone surfaces when bone marrow is flushed from long bones, and they remain viable for only short periods of time in culture (Malaval et al., 1994). We therefore hypothesized that it is the hyperactive Gja1Jrt/+ osteoblasts that are responsible, potentially through their increased production of BMP2/4, for increased marrow adipogenesis in Gja1Jrt/+ mice. The increased Bmp2/4 expression and signaling in Gja1Jrt/+ versus WT osteogenic stromal cell cultures at confluence (Figure 4, B and C) supports this possibility. To test this hypothesis further, we cultured stromal cells under adipogenic conditions but further supplemented with addition of either WT or Gja1Jrt/+ osteogenic stromal cell–conditioned medium. The expression of Pparg2 was significantly increased when cells of either genotype were grown in the presence of Gja1Jrt/+ conditioned medium versus those supplemented with WT conditioned medium (Figure 5B). To determine whether this increase was due to increased BMP2/4 in the Gja1Jrt/+ conditioned medium, we next treated adipogenic stromal cells supplemented with Gja1Jrt/+ conditioned medium with Noggin for 48 h. In cultures supplemented with Gja1Jrt/+ conditioned medium, treatment with 6.25 ng/ml Noggin significantly reduced the expression of Pparg2 in both WT and Gja1Jrt/+ adipogenic cells (Figure 5C).
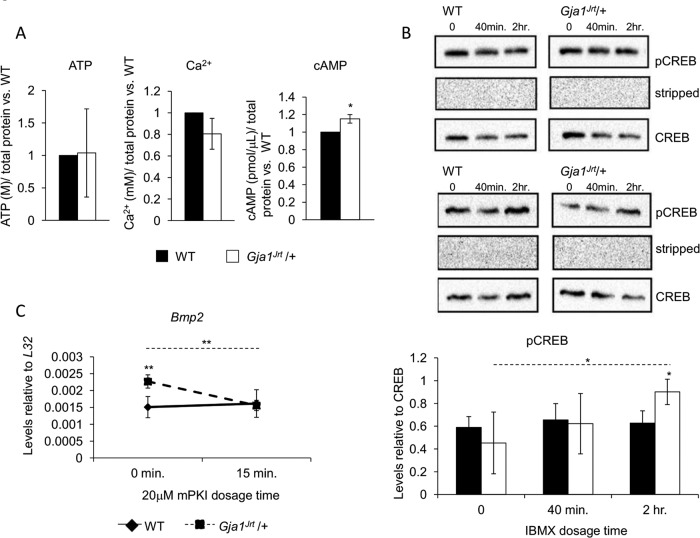
Increased levels of cAMP contribute to the up-regulation of BMP2/4 signaling in Gja1Jrt/+ versus WT osteogenic stromal cell cultures
To establish the link between decreased G60S Cx43 channel function and the increased activity of Gja1Jrt/+ osteoblasts including increased BMP2/4 production, we next compared the intracellular concentrations of several molecules and ions known to be transported through Cx43 channels in WT and Gja1Jrt/+ stromal cells. Whereas intracellular levels of ATP and Ca2+ were not different between genotypes, cAMP levels were significantly increased in Gja1Jrt/+ versus WT stromal cells cultured under osteogenic conditions (Figure 6A). To determine whether cAMP signaling was up-regulated, we quantified phosphorylation of cAMP-responsive element–binding protein (CREB), a cAMP-responsive transcription factor; given the relatively rapid decay of cAMP and low basal levels of pCREB/CREB, we performed the assay in the presence of 3-isobutyl-1-methylxanthine (IBMX), a nonselective phosphodiesterase inhibitor, which inhibits cAMP breakdown, thereby amplifying any differences in levels of cAMP and its targets between genotypes. Levels of pCREB/CREB increased significantly in Gja1Jrt/+ cells after 2 h of IBMX treatment, whereas no change in cAMP levels was detectable in WT cells; of note, pCREB/CREB levels were also significantly increased in Gja1Jrt/+ versus WT stromal cells at 2 h of IBMX treatment (Figure 6B). To determine whether increased cAMP signaling in Gja1Jrt/+ cells was the mechanism behind the increased BMP2/4 production, we next treated WT and Gja1Jrt/+ stromal cells with an inhibitor of cAMP signaling and assessed Bmp2 expression. Myristoylated cAMP-dependent protein kinase inhibitor (mPKI) knocks down cAMP signaling by interfering with the activation of protein kinase A (Ashby and Walsh, 1972, 1973). Treatment with mPKI had no significant effect on Bmp2 expression in WT stromal cells but knocked down Bmp2 expression in Gja1Jrt/+ cells to WT levels (Figure 6C).
FIGURE 6:
Intracellular levels of cAMP and cAMP signaling are increased in Gja1Jrt/+ vs. WT osteogenic stromal cells. (A) Intracellular levels of cAMP were significantly increased in confluent Gja1Jrt/+ vs. WT osteogenic stromal cells. Intracellular levels of ATP and Ca2+ were unchanged; n ≥ 6. Levels of the signaling molecules and ions were normalized to WT levels. (B) Basal levels of pCREB/CREB were unaffected in osteogenic stromal cells isolated from 4-mo- old mice. However, treatment of cells with 1 mM IBMX for at least 2 h resulted in increased levels of pCREB/CREB in Gja1Jrt/+ vs. WT cells. Two representative blots; n = 3. (C) Bmp2 expression decreased when Gja1Jrt/+ osteogenic stromal cells were treated with 20 μM mPKI, a cAMP signaling inhibitor, for 15 min. Treatment of WT cells with mPKI had no effect on Bmp2 expression. One representative experiment; n ≥ 4. Solid and dashed lines indicate significant differences over time in WT and Gja1Jrt/+ mice, respectively. *p < 0.05, **p < 0.01.
DISCUSSION
G60S Cx43-containing cells exhibit significantly reduced levels of total CX43 protein, gap junction formation, and gap junction coupling between cells (Flenniken et al., 2005; McLachlan et al., 2008), which result in an early-onset osteopenic phenotype in Gja1Jrt/+ mice due to activation of osteoclast bone resorption by production of an abnormal resorption stimulating bone matrix, high in BSP, as well as changes in receptor activator of nuclear factor-κB ligand (RANKL)–osteoprotegerin (OPG) signaling, both of which arise from activation of osteoblast lineage cells (Zappitelli et al., 2013). We now report that the activation (i.e., up-regulated Bsp and Ocn expression) of the osteoblast lineage results from increased BMP2/4 production and signaling. Increased BMP2/4 also increases Pparg2 expression to promote bone marrow adipogenesis in Gja1Jrt/+ versus WT mice. Our data also suggest that the increased production of BMP2/4 is due to reduced gap junction intercellular communication and consequent build-up of intracellular cAMP and its downstream signaling in Gja1Jrt/+ osteogenic stromal cells.
Increased BMP2/4 signaling results in up-regulated osteoblast-specific marker expression in Gja1Jrt/+ osteoblasts
Gja1Jrt/+ mice, like other Cx43 mutant mice exhibiting reduced gap junction formation and function, are osteopenic, but the osteopenia results not from decreased osteogenesis and osteoblast activity, but from osteoblast hyperactivity, which activates osteoclasts (Zappitelli et al., 2013). We show here that BMP2/4 production and signaling are significantly increased in Gja1Jrt/+ versus WT trabecular bone and/or osteogenic stromal cells both in vivo and in vitro. The knockdown of expression of up-regulated genes such as Bsp and Ocn in Gja1Jrt/+ stromal cell cultures to WT levels by Noggin treatment confirmed that the up-regulation of gene expression in Gja1Jrt/+ osteoblasts is at least partly, if not entirely, due to up-regulated levels of BMP2/4. Whether the increase in BMP2/4 production is directly responsible for all of the up-regulated osteoblast activities (i.e., not only increased osteoblast-specific gene expression, but also production of an abnormal bone matrix due to increased BSP incorporation and changes in RANKL-OPG production), which result in activation of osteoclasts and therefore the early-onset osteopenic phenotype of Gja1Jrt/+ mice, remains to be determined, for example, by knocking down BMP2/4 signaling in Gja1Jrt/+ mice. We also cannot exclude the possibility that other intrinsic defects or disrupted signaling molecules or pathways may be responsible for or contribute to the activation of the Gja1Jrt/+ osteoblasts.
To date, no other studies have attributed the osteopenic bone phenotypes of Cx43 mutants to increased BMP2/4 signaling, although osteoblast hyperactivity has also not been reported in other mouse models with decreased Cx43 gap junction formation and function. However, Gja1Jrt/+ mice also exhibit all the classical features of oculodentodigital dysplasia (ODDD), including syndactyly (Flenniken et al., 2005). Alterations in BMP2 levels have been reported to underlie common phenotypic features of ODDD, although in contrast to the activation we report in Gja1Jrt/+ cells, others have shown that disruption of Cx43 gap junction function causes a reduction in BMP2 signaling. For instance, Dobrowolski et al. (2009) showed that the syndactyly phenotype of the Cx43+/G138R and Cx43−/− mice arises from decreased interdigital apoptosis due to decreased SHH and BMP2, along with subsequent increase in FGF signaling due to increased FGF4 and FGF8. Kim et al. (2005) showed that disruption of Cx43 by antisense-oligonucleotides caused increased Shh and decreased Bmp2 expression levels during fungiform papillae development. Clearly, disruption of Cx43 gap junction coupling can lead to alterations of morphogens such as BMP2, but differences may arise due to the specific and diverse effects of the various mutations on gap junction and hemichannel formation and function (Laird, 2014).
Altered Cx43 gap junction and hemichannel formation and functioning can vary significantly, depending on the location and type of Cx43 point mutation (Shibayama et al., 2005; Dobrowolski et al., 2007). For instance, the H194P Cx43 mutation inhibits gap junction coupling but has no effect on hemichannel activity, whereas the G138R and G143S mutants reduce gap junction coupling but increase hemichannel activity (Dobrowolski et al., 2007). The effect of the mutation on the differing channels is important, since hemichannels and gap junctions exhibit distinct specificity with regard to molecules that they transport and the functions that they perform (reviewed in Jiang et al., 2007). The effect of the G60S Cx43 mutation on hemichannel activity remains to be determined, but alterations in formation, transport, and/or degradation of small second messenger molecules that are transported across Cx43 channels, such as cAMP (as discussed later), suggest that changes in signaling by hemichannels may play a role in the up-regulation of BMP production and the hyperactive phenotype of the Gja1Jrt/+ osteoblasts.
Whereas the increase in BMP2/4 signaling is directly responsible for the increased expression of osteoblast-specific markers in hyperactive Gja1Jrt/+ osteoblasts, our data for the Wnt/β-catenin inhibitor IWP-2 indicate that the up-regulation of Wnt/β-catenin signaling is not. However, the inconsistent and variable changes in the Wnt/β-catenin pathway that we report in the Gja1Jrt/+ mice is consistent with recently published data by Bivi et al. (2013), who reported increased expression of (total) β-catenin protein and some Wnt target genes (e.g., Axin2) when Cx43 is disrupted in osteocytes (in bones of DMP1Cre;Cx43fl/fl mice and in the MLO-Y4 osteocytic cell line). At the same time, however, the expression of other Wnt/β-catenin target genes (e.g., cyclin D1 and Smad6) and Wnt-mediated transcription (assessed via a TCF/Lef1-luciferase reporter assay) were unaffected in Cx43-silenced MLO-Y4 cells (Bivi et al., 2013). These results, along with the data we present here, suggest that the accumulation of β-catenin protein in Cx43-deficient cells may not lead to increased Wnt/β-catenin–mediated transcription; instead, elevated levels of total β-catenin in Cx43 mutant mice may be involved in other cellular processes, such as mediating the responsiveness of cells to mechanical stimulation (i.e., enhancing the responsiveness of “primed” Cx43-deficient cells to mechanical stimulation) or to other signals/factors, independently of classical Wnt/β-catenin transcription. As such, it will be interesting to determine whether sensitivity to mechanical loading or unloading in the Gja1Jrt/+ mice is altered as a result of the increased β-catenin protein levels.
Increased bone marrow adipogenesis in Gja1Jrt/+ mice
Whether the Gja1Jrt/+ mouse bone marrow adipocyte phenotype is a direct consequence of altered Cx43 function in adipogenic cells and/or is indirect through other cell types is not yet clear. Adipogenesis and expression of adipocyte markers are increased in Gja1Jrt/+ bone marrow but not at other anatomical sites in Gja1Jrt/+ mice. Similarly, several adipocyte markers and BMP2 were increased at very early times in cultures of Gja1Jrt/+ versus WT stromal cells but not later, and no increase in adipogenesis was detectable in these cultures. We therefore hypothesized that the up-regulated production of BMP2 and BMP4 in Gja1Jrt/+ trabecular bone and osteogenic stromal cell cultures is responsible for the increased marrow adipogenesis in vivo and in stromal cultures, respectively. The fact that Noggin abrogated the increased expression of Pparg2 in adipogenic stromal cells treated with Gja1Jrt/+ osteogenic stromal cell–conditioned medium is consistent with such a hypothesis. Similarly, no other Cx43 mutant mouse models have been described to have an adipocyte phenotype or an increase in bone marrow adipogenesis, either because the marrow has not been screened in other models or, more likely, reflecting the unique activated osteoblast phenotype seen in the Gja1Jrt/+ model with its associated up-regulated BMP production. Thus no increase in adipogenesis was described in the global G138R mutant, Cx43+/G138R, or the conditional deletion and conditional G138R mutant, DM1Cre;Cx43−/fl and DM1Cre;Cx43+/fl(G138R), mice, whose osteoblasts are dysfunctional but not hyperactive as in the Gja1Jrt/+ mice (Dobrowolski et al., 2008; Watkins et al., 2011).
It is also worth noting that early in adipogenesis in vitro, Cx43 is highly phosphorylated and localized in the plasma membrane of 3T3-L1 cells (Azarnia and Russell, 1985) and H-1/A stromal cells (Umezawa and Hata, 1992), and functional gap junctions are required in these early stages (Yanagiya et al., 2007). However, as preadipocytes differentiate into mature adipocytes, the level of Cx43 protein declines via proteasomal degradation, an effect reported to be essential for the development of mature adipocytes (Yeganeh et al., 2012). In Gja1Jrt/+ mice, however, the reduction in Cx43 expression and gap junction function appears to have no effect on adipogenesis in epididymal fat pads or elsewhere, except in the bone marrow, where our data suggest that the adipogenic consequences of Cx43 deficiency are indirect and via osteoblasts and possibly other BMP2/4-producing cells. The data suggest that only a low level of gap junctional communication is necessary for the early differentiation stages of adipogenesis, levels commensurate with the low residual gap junction communication seen in Gja1Jrt/+ cells, or that other pathways, such as an enhanced BMP2/4 pathway, can compensate for low Cx43. However, we cannot entirely rule out a direct action of the G60S Cx43 mutation on bone marrow adipocyte precursors, which may behave phenotypically differently than adipocytes in other tissues. Future experiments will be aimed at assessing the direct effects of the germline G60S Cx43 mutation in bone marrow–derived adipocyte precursors and testing more rigorously the link between increased BMP2/4 production and increased bone marrow adipogenesis in Gja1Jrt/+ mice.
Increased intracellular cAMP in G60S Cx43 osteogenic stromal cells plays a role in increased BMP2/4 production
To uncover a link between the G60S Cx43 mutant channels and the up-regulation of BMP2/4 production by Gja1Jrt/+ cells, we investigated intracellular levels of several signaling molecules and ions known to be released via Cx43 channels. Although our results revealed only a small increase in levels of intracellular cAMP, a second messenger signaling molecule known to pass through Cx43 gap junctions (Lawrence et al., 1978), such small changes can have profound impacts on cell activities. Consistent with this, treatment with the cAMP-dependent protein kinase inhibitor mPKI reduced the elevated levels of Bmp2 expression in Gja1Jrt/+ cells to WT levels, suggesting that increased cAMP signaling was at least partly responsible for the up-regulation of Bmp2 expression. We cannot exclude the possibility that the transport of other small second messenger molecules, which we did not test, were also affected by the G60S Cx43 mutation and involved in the up-regulation of BMP2/4 production and/or the expression of downstream osteoblast markers in Gja1Jrt/+ cells. Further analysis of the G60S Cx43 mutation on hemichannel and gap junction channel conductance, permeability, pore size, and specificity are underway.
Of importance, we and others have found no evidence of changes in or compensation by Cx45 (gene or protein expression levels; unpublished data) in Cx43-mutant ODDD mouse models to account for alteration in levels of BMP2/4 molecules (i.e., up-regulated BMP2/4 levels in Gja1Jrt/+ mice or down-regulated BMP2 levels in Cx43+/G138R mice; Dobrowolski et al., 2008). This likely reflects the inability of connexin family members to adequately compensate for one another, since the channels have different molecular and ionic permeabilities (Steinberg et al., 1994; Veenstra et al., 1994; Koval et al., 1995), serving different functions as cells differentiate (Lecanda et al., 2000). Alternatively, however, elevated levels of Cx45 protein have been proposed to partially compensate for loss of Cx43 in Cx43−/− neonatal calvaria osteoblasts (Lecanda et al., 2000); it is possible that compensatory mechanisms by other connexins can be influenced on the basis of whether Cx43 is entirely deleted, as in the knockout, or simply mutated, as in the G60S or G138R mutants.
In summary, we report the novel findings that up-regulation of BMP2/4 signaling in trabecular bone and/or stromal cells increases osteoblast-specific marker expression in hyperactive Gja1Jrt/+ osteoblasts and may also increase bone marrow adipogenesis by up-regulation of Pparg2 in the Cx43-deficient Gja1Jrt/+ mouse model. We also report that increased cAMP signaling may promote the up-regulated production of BMP2 and BMP4 signaling molecules by Gja1Jrt/+ cells.
MATERIALS AND METHODS
Animals studies
Gja1Jrt/+ mice were generated as previously described (Zappitelli et al., 2013). The studies reported here were done on male Gja1Jrt/+ and WT littermates between 2 and 4 mo of age. All experimental procedures were performed in accordance with protocols approved by the Canadian Council on Animal Care and the University of Toronto Faculty of Medicine and Pharmacy Animal Care Committee.
Body composition
Dual energy x-ray absorptiometry (PIXImus; Lunar, Madison, WI) was used to measure body composition (percentage fat, lean tissue mass) on the whole body (excluding the head).
Immunocytochemistry
Mouse embryonic fibroblasts cultured on glass coverslips were permeabilized in 1% Triton X-100 in phosphate-buffered saline (PBS), washed in PBS, blocked in 2% fetal bovine serum (FBS)–PBS, incubated with rabbit anti-Cx43 (Invitrogen, Carlsbad, CA), diluted 1:200 in PBS at room temperature for 2 h, washed in PBS, incubated in Alexa Fluor goat anti-rabbit immunoglobulin G (Molecular Probes, Eugene, OR), washed in PBS, and then stained with 1 mg/ml Hoechst 33258 diluted 1:1000 in PBS for 5 min at room temperature. Coverslips were mounted and stored at 4°C overnight. Cells were imaged on a Leitz Dialux 20 microscope with fluorescence and camera attachments (Leitz, Rockleigh, NJ).
Histochemistry
The right femur fixed in 4% paraformaldehyde was embedded in a mixture of methyl methacrylate and glycolmethacrylate, and 5-μm sections were stained with hematoxylin and eosin (Carson, 1990). Images were captured and analyzed using a Bioquant Osteoimager and Bioquant Osteo 2012 (Bioquant Image Analysis, Nashville, TN).
Isolation of bone marrow cells
Bone marrow cells were isolated from resected tibia and femora using a modification of a previously published method (Scutt et al., 2004). Cells were plated in α-MEM supplemented with 10% heat-inactivated FBS and antibiotics (100 μg/ml penicillin, 1 μg/ml streptomycin, 50 μg/ml gentamicin, 250 ng/ml Fungizone; standard medium) at 1 × 106 nucleated cells/35-mm dish.
Osteogenic assay
. After 3 d, the medium was changed to differentiation medium (standard medium supplemented with 50 μg/ml ascorbic acid and 10 mM β-glycerophosphate).
Adipogenic assay.
After 3 d, the medium was changed to differentiation medium (standard medium with 50 μg/ml ascorbic acid and 10−5 M thiazolidinedione [BRL 49653]).
Conditioned medium.
Conditioned medium was collected from osteogenic stromal cell cultures at confluence after 24–48 h of conditioning. The conditioned medium was then used at 50:50 with fresh adipogenic medium.
Inhibitor studies in stromal cells
Stromal cells were cultured in standard medium with 50 μg/ml ascorbic acid until day 6 (matrix-forming time point). Cells were then treated for 24 h with 0.1 or 1 μM IWP-2 (I0536; Sigma-Aldrich, St. Louis, MO) in dimethyl sulfoxide (DMSO) or vehicle (DMSO) alone. RNA was isolated as described later.
Noggin
Osteogenic cells.
Stromal cells were cultured in standard osteogenic differentiation medium with vehicle (20 μg/ml acetic acid in 0.1% BSA-PBS) or 25, 50, 100, 200, or 500 ng/ml Noggin (ab50156; Abcam, Cambridge, MA). Cells were cultured until vehicle-treated wells contained mineralized nodules, and RNA was then isolated as described later.
Adipogenic cells.
Stromal cells were cultured in standard adipogenic differentiation medium for 24 h and then treated with vehicle (20 μg/ml acetic acid in 0.1% BSA-PBS) or 25, 50, or 200 ng/ml Noggin for 48 h. RNA was isolated as described later.
3-Isobutyl-1-methylxanthine
Stromal cells were cultured in standard medium with 50 μg/ml ascorbic acid to confluence, serum starved (α-MEM with 0.5% heat-inactivated FBS and antibiotics) overnight, and treated with 1 mM IBMX (I5879; Sigma-Aldrich) for 40 min, 2 h or 4 h. RNA and protein were isolated as described later.
mPKI (14–22)
Osteogenic cells.
Stromal cells were cultured in standard medium with 50 μg/ml ascorbic acid until confluence. Cells were then treated with vehicle (water) or 5, 10, or 20 μM mPKI (PHZ1202; Life Technologies, Carlsbad, CA) for 24 h, and RNA was collected.
Adipogenic cells.
Stromal cells were cultured in standard adipogenic differentiation medium for 48 h and then treated with vehicle (water) or 5, 10, or 20 μM mPKI for 24 h, and RNA was collected.
Quantitative reverse transcription PCR
Total RNA was isolated from bone, bone marrow, and cell cultures using TriReagent (Sigma-Aldrich) and reverse transcribed using Superscript II (Invitrogen) and random hexamers. cDNA was combined with 0.5 μM each of the forward and reverse primers (Supplemental Table S1) and iQ SYBR Green Supermix and run in the MyIQ Real-Time PCR system (Bio-Rad, Hercules, CA). Raw data were analyzed with PCR Miner (Zhao and Fernald, 2005) and normalized using the internal control transcript for ribosomal protein L32.
SA Biosciences Mouse Signal Transduction PathwayFinder PCR Array
RNA was isolated from trabecular bone samples. Sample preparation and RNA isolation were performed using TriReagent (Sigma-Aldrich) and SA Biosciences qPCR-Grade RNA isolation kit (Qiagen, Venlo, Netherlands) following manufacturer's instructions. The Mouse Signal Transduction Pathway Finder RT2 Profiler PCR Array (Qiagen) was used following the manufacturer's instructions.
Protein isolation from bone and stromal culture and Western blotting
Long bones cleaned of surrounding tissue, epiphysis, and bone marrow were cut slightly below the growth plate to separate trabecular bone and washed in PBS. Stromal culture plates were washed with PBS. Proteins were extracted in cell lysis buffer as previously described (Thomas et al., 2004). Protein extracts (30 μg) underwent immunoblotting with antibodies of interest (Supplemental Table S2); actin was used as a loading control. Western blots were developed using chemiluminescence, imaged with Bio-Rad ChemiDoc-XRS+, and analyzed using Image Lab software (Bio-Rad).
Statistical analysis
Results are presented as mean ± SD. Experiments were repeated at least three times with independent biological samples. Statistical analysis was performed using Prism 4.0 software (GraphPad, La Jolla, CA). One-way analysis of variance was used to determine longitudinal significance in dosage experiments. Unpaired t test was used for direct comparisons between mutant and WT parameters; paired t test was used for comparisons within genotypes (e.g., changes over treatment time); n values presented are independent biological samples.
Supplementary Material
Acknowledgments
We thank members of the Centre for Modeling Human Disease (www.cmhd.ca), particularly Celeste Owen, for their support; Ralph Zirngibl and Marco Cardelli from the Aubin lab for support and helpful discussions; the Center for Bone and Periodontal Research (www.bone.mcgill.ca) and Feryal Sarraf from the Faculty of Dentistry for expert technical assistance; and Liliana Attisano and Jane Mitchell for providing reagents and discussion. This work was supported by a Canadian Institutes of Health Research operating grant (FRN 69198 to J.E.A.), as well as scholarship support from the government of Ontario through the Ontario Graduate Scholarship (T.Z.), the Queen Elizabeth II-GSST (T.Z.), and the Department of Medical Biophysics, University of Toronto (T.Z.). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Abbreviations used:
- BMP
bone morphogenic protein
- BSP
bone sialoprotein
- CREB
cAMP response element–binding protein
- Cx43
connexin 43
- IBMX
3-isobutyl-1-methylxanthine
- mPKI
myristoylated cAMP-dependent protein kinase inhibitor
- Ocn
osteocalcin
- OPG
osteoprotegerin
- Pparg2
peroxisome proliferator–activated receptor γ
- RANKL
receptor activator of nuclear factor-κB ligand
- Tcf7
transcription factor 7
- WT
wild type.
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E14-06-1136) on January 7, 2015.
Study design and conduct: T.Z., F.C., and J.E.A. Data collection: T.Z. and F.C. Data analysis: T.Z. and F.C. Data interpretation: T.Z., F.C., and J.E.A. Drafting the manuscript: T.Z. and J.E.A. Revising manuscript content and approving final version of manuscript: T.Z., F.C., and J.E.A. T.Z., F.C., and J.E.A. take responsibility for the integrity of the data analysis.
REFERENCES
- Ai Z, Fischer A, Spray DC, Brown AM, Fishman GI. Wnt-1 regulation of connexin43 in cardiac myocytes. J Clin Invest. 2000;105:161–171. doi: 10.1172/JCI7798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander DB, Goldberg GS. Transfer of biologically important molecules between cells through gap junction channels. Curr Med Chem. 2003;10:2045–2058. doi: 10.2174/0929867033456927. [DOI] [PubMed] [Google Scholar]
- Ashby CD, Walsh DA. Characterization of the interaction of a protein inhibitor with adenosine 3',5'-monophosphate-dependent protein kinases. I. Interaction with the catalytic subunit of the protein kinase. J Biol Chem. 1972;247:6637–6642. [PubMed] [Google Scholar]
- Ashby CD, Walsh DA. Characterization of the interaction of a protein inhibitor with adenosine 3',5'-monophosphate-dependent protein kinases. II. Mechanism of action with the holoenzyme. J Biol Chem. 1973;248:1255–1261. [PubMed] [Google Scholar]
- Azarnia R, Russell TR. Cyclic AMP effects on cell-to-cell junctional membrane permeability during adipocyte differentiation of 3T3-L1 fibroblasts. J Cell Biol. 1985;100:265–269. doi: 10.1083/jcb.100.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bivi N, Lezcano V, Romanello M, Bellido T, Plotkin LI. Connexin43 interacts with betaarrestin: a pre-requisite for osteoblast survival induced by parathyroid hormone. J Cell Biochem. 2011;112:2920–2930. doi: 10.1002/jcb.23208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bivi N, Nelson MT, Faillace ME, Li J, Miller LM, Plotkin LI. Deletion of Cx43 from osteocytes results in defective bone material properties but does not decrease extrinsic strength in cortical bone. Calcif Tissue Int. 2012;91:215–224. doi: 10.1007/s00223-012-9628-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bivi N, Pacheco-Costa R, Brun LR, Murphy TR, Farlow NR, Robling AG, Bellido T, Plotkin LI. Absence of Cx43 selectively from osteocytes enhances responsiveness to mechanical force in mice. J Orthop Res. 2013;31:1075–1081. doi: 10.1002/jor.22341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson FL. Histotechnology: A Self-Instructional Text. Chicago, IL: American Society for Clinical Pathology Press; 1990. [Google Scholar]
- Chung DJ, Castro CH, Watkins M, Stains JP, Chung MY, Szejnfeld VL, Willecke K, Theis M, Civitelli R. Low peak bone mass and attenuated anabolic response to parathyroid hormone in mice with an osteoblast-specific deletion of connexin43. J Cell Sci. 2006;119:4187–4198. doi: 10.1242/jcs.03162. [DOI] [PubMed] [Google Scholar]
- Civitelli R, Beyer EC, Warlow PM, Robertson AJ, Geist ST, Steinberg TH. Connexin43 mediates direct intercellular communication in human osteoblastic cell networks. J Clin Invest. 1993;91:1888–1896. doi: 10.1172/JCI116406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrowolski R, Hertig G, Lechner H, Worsdorfer P, Wulf V, Dicke N, Eckert D, Bauer R, Schorle H, Willecke K. Loss of connexin43-mediated gap junctional coupling in the mesenchyme of limb buds leads to altered expression of morphogens in mice. Hum Mol Genet. 2009;18:2899–2911. doi: 10.1093/hmg/ddp227. [DOI] [PubMed] [Google Scholar]
- Dobrowolski R, Sasse P, Schrickel JW, Watkins M, Kim JS, Rackauskas M, Troatz C, Ghanem A, Tiemann K, Degen J, et al. The conditional connexin43G138R mouse mutant represents a new model of hereditary oculodentodigital dysplasia in humans. Hum Mol Genet. 2008;17:539–554. doi: 10.1093/hmg/ddm329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrowolski R, Sommershof A, Willecke K. Some oculodentodigital dysplasia-associated Cx43 mutations cause increased hemichannel activity in addition to deficient gap junction channels. J Membr Biol. 2007;219:9–17. doi: 10.1007/s00232-007-9055-7. [DOI] [PubMed] [Google Scholar]
- Donahue HJ, McLeod KJ, Rubin CT, Andersen J, Grine EA, Hertzberg EL, Brink PR. Cell-to-cell communication in osteoblastic networks: cell line-dependent hormonal regulation of gap junction function. J Bone Miner Res. 1995;10:881–889. doi: 10.1002/jbmr.5650100609. [DOI] [PubMed] [Google Scholar]
- Dorshkind K, Green L, Godwin A, Fletcher WH. Connexin-43-type gap junctions mediate communication between bone marrow stromal cells. Blood. 1993;82:38–45. [PubMed] [Google Scholar]
- Flenniken AM, Osborne LR, Anderson N, Ciliberti N, Fleming C, Gittens JE, Gong XQ, Kelsey LB, Lounsbury C, Moreno L, et al. A Gja1 missense mutation in a mouse model of oculodentodigital dysplasia. Development. 2005;132:4375–4386. doi: 10.1242/dev.02011. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Nieto D, Li L, Kohler A, Ghiaur G, Ishikawa E, Sengupta A, Madhu M, Arnett J, Santho R, Dunn SK, et al. Connexin-43 in the osteogenic BM niche regulates its cellular composition and the bidirectional traffic of hematopoietic stem cells and progenitors. Blood. 2012;119:5144–5154. doi: 10.1182/blood-2011-07-368506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimston SK, Brodt MD, Silva MJ, Civitelli R. Attenuated response to in vivo mechanical loading in mice with conditional osteoblast ablation of the connexin43 gene (Gja1) J Bone Miner Res. 2008;23:879–886. doi: 10.1359/JBMR.080222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimston SK, Watkins MP, Brodt MD, Silva MJ, Civitelli R. Enhanced periosteal and endocortical responses to axial tibial compression loading in conditional connexin43 deficient mice. PLoS One. 2012;7:e44222. doi: 10.1371/journal.pone.0044222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang JX, Siller-Jackson AJ, Burra S. Roles of gap junctions and hemichannels in bone cell functions and in signal transmission of mechanical stress. Front Biosci. 2007;12:1450–1462. doi: 10.2741/2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JY, Cho SW, Lee MJ, Hwang HJ, Lee JM, Lee SI, Muramatsu T, Shimono M, Jung HS. Inhibition of connexin 43 alters Shh and Bmp-2 expression patterns in embryonic mouse tongue. Cell Tissue Res. 2005;320:409–415. doi: 10.1007/s00441-005-1091-y. [DOI] [PubMed] [Google Scholar]
- Koval M, Geist ST, Westphale EM, Kemendy AE, Civitelli R, Beyer EC, Steinberg TH. Transfected connexin45 alters gap junction permeability in cells expressing endogenous connexin43. J Cell Biol. 1995;130:987–995. doi: 10.1083/jcb.130.4.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koval M, Harley JE, Hick E, Steinberg TH. Connexin46 is retained as monomers in a trans-Golgi compartment of osteoblastic cells. J Cell Biol. 1997;137:847–857. doi: 10.1083/jcb.137.4.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird DW. Syndromic and non-syndromic disease-linked Cx43 mutations. FEBS Lett. 2014;588:1339–1348. doi: 10.1016/j.febslet.2013.12.022. [DOI] [PubMed] [Google Scholar]
- Lawrence TS, Beers WH, Gilula NB. Transmission of hormonal stimulation by cell-to-cell communication. Nature. 1978;272:501–506. doi: 10.1038/272501a0. [DOI] [PubMed] [Google Scholar]
- Lecanda F, Warlow PM, Sheikh S, Furlan F, Steinberg TH, Civitelli R. Connexin43 deficiency causes delayed ossification, craniofacial abnormalities, and osteoblast dysfunction. J Cell Biol. 2000;151:931–944. doi: 10.1083/jcb.151.4.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewenstein WR. Junctional intercellular communication: the cell-to-cell membrane channel. Physiol Rev. 1981;61:829–913. doi: 10.1152/physrev.1981.61.4.829. [DOI] [PubMed] [Google Scholar]
- Loiselle AE, Paul EM, Lewis GS, Donahue HJ. Osteoblast and osteocyte-specific loss of Connexin43 results in delayed bone formation and healing during murine fracture healing. J Orthop Res. 2012;31:147–154. doi: 10.1002/jor.22178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaval L, Modrowski D, Gupta AK, Aubin JE. Cellular expression of bone-related proteins during in vitro osteogenesis in rat bone marrow stromal cell cultures. J Cell Physiol. 1994;158:555–572. doi: 10.1002/jcp.1041580322. [DOI] [PubMed] [Google Scholar]
- McLachlan E, Plante I, Shao Q, Tong D, Kidder GM, Bernier SM, Laird DW. ODDD-linked Cx43 mutants reduce endogenous Cx43 expression and function in osteoblasts and inhibit late stage differentiation. J Bone Miner Res. 2008;23:928–938. doi: 10.1359/jbmr.080217. [DOI] [PubMed] [Google Scholar]
- Niger C, Hebert C, Stains JP. Interaction of connexin43 and protein kinase C-delta during FGF2 signaling. BMC Biochem. 11:14. doi: 10.1186/1471-2091-11-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paic F, Igwe JC, Nori R, Kronenberg MS, Franceschetti T, Harrington P, Kuo L, Shin DG, Rowe DW, Harris SE, Kalajzic I. Identification of differentially expressed genes between osteoblasts and osteocytes. Bone. 2009;45:682–692. doi: 10.1016/j.bone.2009.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotkin LI, Lezcano V, Thostenson J, Weinstein RS, Manolagas SC, Bellido T. Connexin 43 is required for the anti-apoptotic effect of bisphosphonates on osteocytes and osteoblasts in vivo. J Bone Miner Res. 2008;23:1712–1721. doi: 10.1359/JBMR.080617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotkin LI, Manolagas SC, Bellido T. Transduction of cell survival signals by connexin-43 hemichannels. J Biol Chem. 2002;277:8648–8657. doi: 10.1074/jbc.M108625200. [DOI] [PubMed] [Google Scholar]
- Sanches DS, Pires CG, Fukumasu H, Cogliati B, Matsuzaki P, Chaible LM, Torres LN, Ferrigno CR, Dagli ML. Expression of connexins in normal and neoplastic canine bone tissue. Vet Pathol. 2009;46:846–859. doi: 10.1354/vp.08-VP-0263-S-FL. [DOI] [PubMed] [Google Scholar]
- Scutt A, Beier N, Fittschen C. EMD273316 & EMD95833, type 4 phosphodiesterase inhibitors, stimulate fibroblastic-colony formation by bone marrow cells via direct inhibition of PDE4 and the induction of endogenous prostaglandin synthesis. BMC Pharmacol. 2004;4:10. doi: 10.1186/1471-2210-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibayama J, Paznekas W, Seki A, Taffet S, Jabs EW, Delmar M, Musa H. Functional characterization of connexin43 mutations found in patients with oculodentodigital dysplasia. Circ Res. 2005;96:e83–91. doi: 10.1161/01.RES.0000168369.79972.d2. [DOI] [PubMed] [Google Scholar]
- Steinberg TH, Civitelli R, Geist ST, Robertson AJ, Hick E, Veenstra RD, Wang HZ, Warlow PM, Westphale EM, Laing JG, et al. Connexin43 and connexin45 form gap junctions with different molecular permeabilities in osteoblastic cells. EMBO J. 1994;13:744–750. doi: 10.1002/j.1460-2075.1994.tb06316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas T, Telford D, Laird DW. Functional domain mapping and selective trans-dominant effects exhibited by Cx26 disease-causing mutations. J Biol Chem. 2004;279:19157–19168. doi: 10.1074/jbc.M314117200. [DOI] [PubMed] [Google Scholar]
- Umezawa A, Hata J. Expression of gap-junctional protein (connexin 43 or alpha 1 gap junction) is down-regulated at the transcriptional level during adipocyte differentiation of H-1/A marrow stromal cells. Cell Struct Funct. 1992;17:177–184. doi: 10.1247/csf.17.177. [DOI] [PubMed] [Google Scholar]
- Veenstra RD, Wang HZ, Beyer EC, Brink PR. Selective dye and ionic permeability of gap junction channels formed by connexin45. Circ Res. 1994;75:483–490. doi: 10.1161/01.res.75.3.483. [DOI] [PubMed] [Google Scholar]
- Watkins M, Grimston SK, Norris JY, Guillotin B, Shaw A, Beniash E, Civitelli R. Osteoblast connexin43 modulates skeletal architecture by regulating both arms of bone remodeling. Mol Biol Cell. 2011;22:1240–1251. doi: 10.1091/mbc.E10-07-0571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada Y, Ando F, Shimokata H. Association of candidate gene polymorphisms with bone mineral density in community-dwelling Japanese women and men. Int J Mol Med. 2007;19:791–801. [PubMed] [Google Scholar]
- Yanagiya T, Tanabe A, Hotta K. Gap-junctional communication is required for mitotic clonal expansion during adipogenesis. Obesity (Silver Spring) 2007;15:572–582. doi: 10.1038/oby.2007.547. [DOI] [PubMed] [Google Scholar]
- Yeganeh A, Stelmack GL, Fandrich RR, Halayko AJ, Kardami E, Zahradka P. Connexin 43 phosphorylation and degradation are required for adipogenesis. Biochim Biophys Acta. 2012;1823:1731–1744. doi: 10.1016/j.bbamcr.2012.06.009. [DOI] [PubMed] [Google Scholar]
- Zappitelli T, Chen F, Moreno L, Zirngibl RA, Grynpas M, Henderson JE, Aubin JE. The G60S connexin 43 mutation activates the osteoblast lineage and results in a resorption-stimulating bone matrix and abrogation of old age-related bone loss. J Bone Miner Res. 2013;28:2400–2413. doi: 10.1002/jbmr.1965. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Paul EM, Sathyendra V, Davison A, Sharkey N, Bronson S, Srinivasan S, Gross TS, Donahue HJ. Enhanced osteoclastic resorption and responsiveness to mechanical load in gap junction deficient bone. PLoS One. 2011;6:e23516. doi: 10.1371/journal.pone.0023516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S, Fernald RD. Comprehensive algorithm for quantitative real-time polymerase chain reaction. J Comput Biol. 2005;12:1047–1064. doi: 10.1089/cmb.2005.12.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.