Abstract
The expression of maternal behavior in mammals is regulated by the developmental and experiential events over a female’s lifetime. In this review the relationships between the endocrine and neural systems that play key roles in these developmental and experiential that affect both the establishment and maintenance of maternal care are presented. The involvement of the hormones estrogen, progesterone, and lactogens are discussed in the context of ligand, receptor, and gene activity in rodents and to a lesser extent in higher mammals. The roles of neuroendocrine factors, including oxytocin, vasopressin, classical neurotransmitters, and other neural gene products that regulate aspects of maternal care are set forth, and the interactions of hormones with central nervous system mediators of maternal behavior are discussed. The impact of prior developmental factors, including epigenetic events, and maternal experience on subsequent maternal care are assessed over the course of the female’s lifespan. It is proposed that common neuroendocrine mechanisms underlie the regulation of maternal care in mammals.
Keywords: Epigenetics, Estrogens, Genes, Hormone Receptors, Lactogenic Hormones, Maternal Behavior, Maternal Experience, Neural Networks, Neuropeptides, Progesterone
1. Introduction
In recent years our understanding of the relationships between neural and endocrine systems in the regulation of maternal behavior has received considerable research attention. Roles for specific hormones, neurochemicals, and brain regions involved in the induction, maintenance, and retention of maternal care in mammals have been identified. These chemical systems and anatomical substrates are discussed here within the context of development and the female mammal’s reproductive life history.
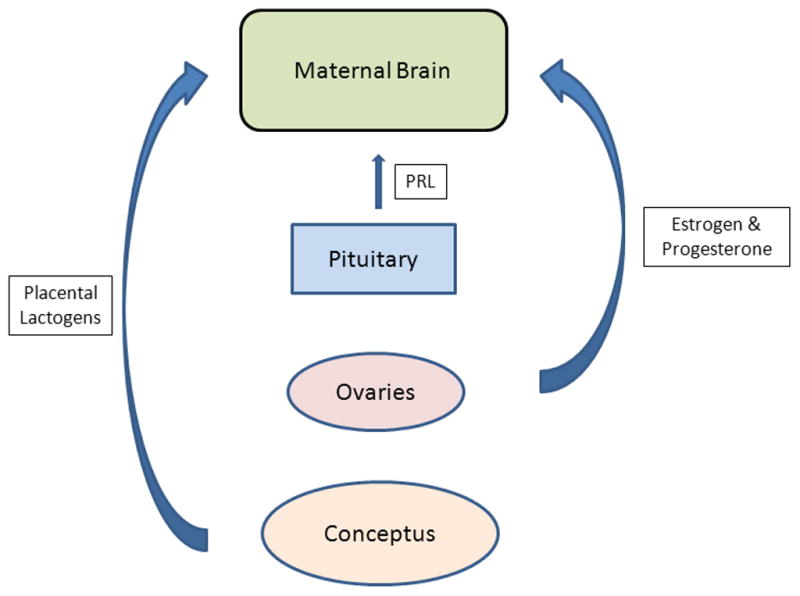
In order to provide an understanding of the relationships between maternal behavior and its biological control, it is important to place the broader players within a conceptual context. Whereas it is commonly considered that the control of maternal behavior has a neuroendocrine basis (Bridges and Byrnes, 1999), it is equally accurate to conceptualize the relationship between the brain and the endocrine system as an endocrine-neurological link. This construct was first brought to my attention by the neuroendocrinologist Dr. Joseph Martin, past Dean of Harvard Medical School, in a seminar he presented in the late 1980s on growth hormone regulation and action. As depicted in the schematic in Figure 1 and elucidated within this review, the actions of hormones from both the pituitary and other peripheral endocrine tissues play a crucial role in feeding back upon sites within the central nervous system, many within the hypothalamus, to regulate the female’s responses to young and related stimuli. Hence, the regulation of maternal behavior is as much of an endocrine-neurological process as it is a strictly neuroendocrine mediated event.
Figure 1.
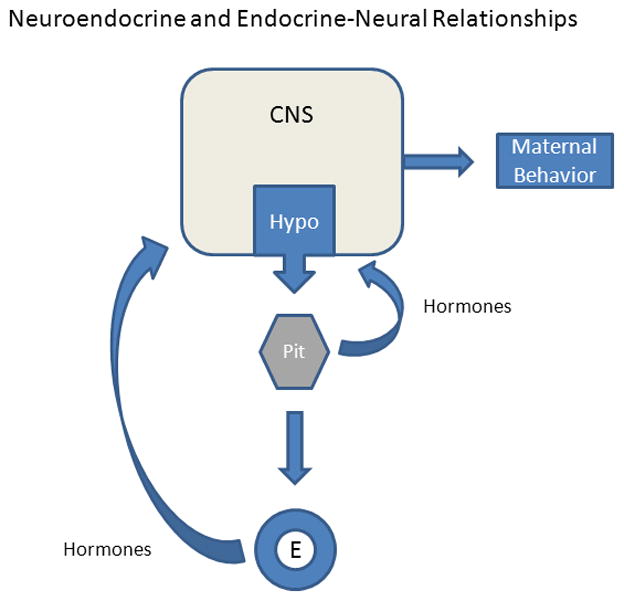
Schematic of the overall relationships between the brain and components of the endocrine system in the regulation of maternal behavior. The actions of hormones secreted by the pituitary (Pit) gland or peripheral endocrine (E) tissues, such as the ovaries, adrenal glands, and placenta, act upon key neural sites within the hypothalamus (Hypo) and other brain regions in the central nervous system (CNS) to stimulate and modulate the expression of maternal behavior. Moreover, intrinsic neurochemical systems within the hypothalamus that are regulated by hormones as well as sensory inputs act centrally to affect maternal care. The actual “neuroendocrine” link between the Hypo and the Pit mediates the release and subsequent actions of neuroactive hormones as part of a feedback system or loop.
The aim of this review is to present the existing framework of the underlying neuroendocrine foundation in the regulation of maternal behavior. Whereas most of the research findings focus on studies in rats and mice, attention to other species, including humans, complements the more expansive data set in rodents. Possible common regulatory mechanisms are considered in light of the normative behavioral responses of the given species.
2. Maternal Behavior
2.1 Maternal Responses
Responses or behaviors displayed by the female that specifically support the development and growth of her offspring constitute a set of responses termed maternal behaviors. The capacity to respond maternally appears to be present throughout development from the pubertal period into adulthood. However, the intensity and incidences of maternal responses are most pronounced beginning at the time of birth. At parturition the new mother displays both an intense interest in the amniotic fluids and the young together with an enhanced motivation to respond positively to her newborn.
What are the maternal responses that emerge at birth and are expressed during the development of her offspring throughout lactation and beyond? In preparation for birth, expectant mothers display increased levels of aggression towards intruders as well as increased nest building (Mayer and Rosenblatt, 1984). At birth most newly parturient mothers ingest amniotic fluid and consume the placenta. These behaviors are thought to provide a source of nutrition for the mother as well as remove a potential olfactory attractant of the afterbirth to predators. The placenta is a rich source of hormones that appear to facilitate lactogenesis and decrease the energetic load on the mother. Maternal responses following birth, in general, can be grouped into two categories, those directed at the young and those indirectly related to the young. These behaviors are listed in Table 1. In rodents, young-directed responses include behaviors such as retrieval, pup-licking and grooming, crouching, and active nursing. Non-directed maternal behaviors include protection of the young or maternal defense, nest building, increased food consumption, i.e. lactational hyperphagia, and diminished anxiety with associated increases in exploratory activity.
Table 1.
Maternal Related-Behaviors
| Parturitional Responses |
| Stimulation of Newborn |
| Consumption of Amniotic Fluid |
| Placentophagia |
| Young Directed Responses |
| Retrieval – Maximizing Contact with Young |
| Licking and Grooming |
| Nursing/Crouching |
| Providing Warmth and Safety/Nest Building |
| Young Related Responses |
| Protection of the Young – Maternal Aggression |
| Increased Food Consumption |
| Reduced Anxiety to Enhance Exploratory Activities |
Maternal responses are set within the behavioral framework of the female. For example, while retrieval is a common behavior displayed by rodents, cats and dogs, in sheep maternal bleats and proximity to young availing access to the utter are characteristic maternal responses. In non-human primates, carrying young and physical closeness are considered important components of maternal care. In humans, visual “en face” contact with babies as well as assessments of attachment, mood state, and olfactory recognition are used as endpoints to measure maternal state. Given the context of behavioral responses across a range of mammals, it makes comparisons of underlying neuroendocrine mechanisms with biological regulators across species challenging. One assumption made in evaluating the neurobiological bases of maternal behavior is that similar, albeit not identical, mechanisms regulate functionally common behavioral responses.
In general, assessment of maternal capacity has typically used measurements of young-directed responses. In rats and mice, for example, latencies and incidences to retrieve and group young in the nest have been utilized to assess the neurobiological capacity of females to display maternal behavior. The basic neurobiological capacity in females to respond in a maternal fashion towards young is present throughout development from the prepubertal period into older age. In prepubertal juvenile female rats maternal-like responses are displayed after exposure to foster young for a day or two (Bridges et al., 1974). Response latencies toward foster young subsequently increase to 5 to 6 days in adulthood (Rosenblatt, 1967), latencies that appear to be maintained throughout adult life in female rats that do not give birth. These response latencies decline during late pregnancy with shortened latencies present prepartum (Slotnick et al, 1973). At birth, the new mother displays a spontaneous set of pup-directed behaviors. Once these behaviors are established, they persist at an enhanced level throughout the remainder of adult life (Bridges 1975; Bridges and Scanlan, 2005; Scanlan et al., 2006).
The majority of studies that have examined the indirect-young associated maternal response of maternal defense or maternal aggression have conducted research using rodent models. Maternal aggression in lactating rats is most pronounced during the first week postpartum (Erskine et al., 1978). When an intruder is introduced into the home cage of the lactating rat, the female displays an increased incidence of a set of responses. This behavior consists of frontal and lateral attacks, rearing, biting, kicking, and upright boxing (Lonstein and Gammie, 2002). Recent studies have further demonstrated that during a second lactation, the intensity of maternal defense in response to a male intruder is greater than that found in age-matched, lactating primiparous dams (Nephew et al., 2010). Although the specific components and incidences of maternal defense may vary, the overall behavioral ethograms are similar across mammals. A second significant shift in non-pup directed behavior associated with pregnancy and lactation involves increases in food consumption together with a temporally related state of leptin resistance (Ladyman and Grattan, 2005). The neurobiological underpinnings of maternal defense and the regulation of energy balance are discussed later in this article.
One perspective that is important to maintain is that the mother’s response to young is contextually driven. That is, her behaviors towards young are dependent upon the stimuli in her immediate environment and involves motor responses that are regulated by alterations in her motivational and reward states that are formed over her lifetime of and a product of the interactions between underlying biological determinants and experiential events. Simplistically stated, at parturition the new mother attends to the immediate set of new stimuli such as amniotic fluid, the placenta, and the newborn in an integrated manner. Moreover, during lactation, for example, general reductions in anxiety that accompany motherhood enhance provisioning for the young and protection. A reduction in anxiety, while not specific for maternal care, when placed within the context of postpartum care, functions as a supporting component of maternal behavior.
2.2 Neurobiological Framework for Maternal Care
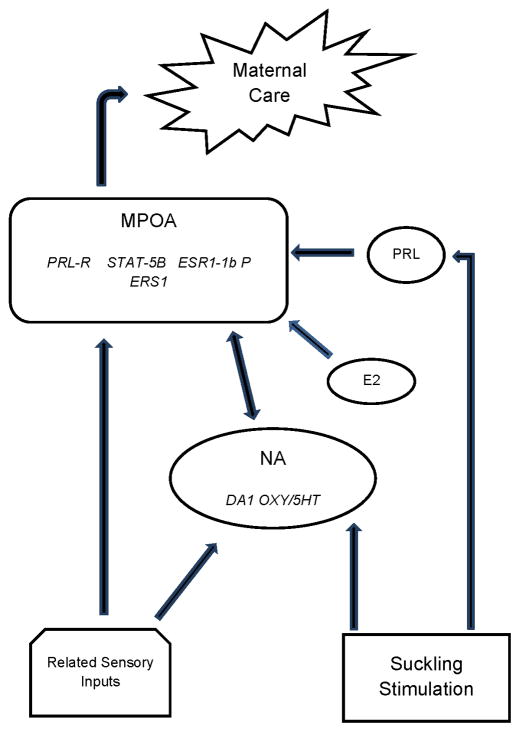
The research of Drs. Michael Numan and Alison Fleming have made major contributions to our understanding of what can be referred to as the maternal neural network. Numan identified the medial preoptic area (MPOA) as a key integrative site where hormones interface with hormone receptors to stimulate the onset of maternal care as well as this area serving as an integral component of ongoing maternal behavior (Numan, 1974; Numan, 2012). Fleming’s research identified a key role for olfaction and its related pathways together with an important involvement of the nucleus accumbens (NA), a brain region associated with mediating reward. in a set of maternal responses, including maternal memory, which in turn can be influenced by cortical inputs (Lee et al., 1999). A schematic representation of a proposed neural network involved in the regulation of maternal care is shown in Figure 2.
Figure 2.
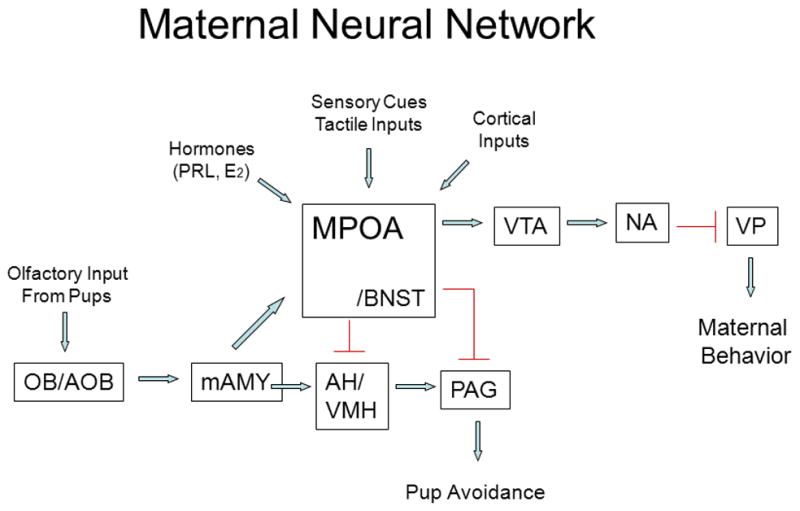
Schematic representation of key neural regions and connections that constitute the maternal neural network. Arrows are stimulatory except that from the AH/VMH to the PAG which is inhibitory. AH, anterior hypothalamus; AOB, accessory olfactory bulb; BNST, bed nucleus of the stria terminalis; mAMY, medial amygdala; MPOA medial preoptic area; NA, nucleus accumbens; OB, olfactory bulb; PAG, periaqueductal gray; VMH, ventromedial hypothalamus; VP, ventral pallidum; VTA, ventral tegmental area. Taken from Bridges, R.S., Nephew, B.N., 2009. Neuroendocrine control: maternal behavior. In Encyclopedia of Neuroscience, volume 6, pp. 333–342, with permission, Elsevier Ltd.
It is noteworthy that the olfactory system plays an important role in the enhanced recognition of young in many, but not all, mammals. Whereas this system in the inexperienced, nulliparous female rat is primarily inhibitory, physiological shifts accompanying pregnancy alter the saliency of young-related sensory cues (Kinsley and Bridges, 1990), resulting in increased levels of maternal care at birth. The projections from the amygdala which receives olfactory cues terminate in a number of hypothalamic sites including the MPOA, anterior hypothalamus (AH), and bed nucleus of the stria terminalis (BNST). Putative inhibitory signals from the amygdala are thought to be dampened at parturition, resulting in a stimulation of maternal care that is processed through these key hypothalamic sites. The outputs from the MPOA to the ventral tegmental area (VTA) are posed to stimulate the mesolimbic dopaminergic projections to reward centers, including the NA. Other projections from the AH/ventromedial hypothalamus (VMH) and MPOA/BNST project to the periaqueductal gray (PAG) to reduce pup avoidance behavior and increase the probability that the female will find the newborn young attractive. A number of hormones and neurochemicals together with sensory cues and cortical inputs converge on this neural network to modulate the expression of maternal care, a network that is altered as a function of both developmental processes and experience.
3. Biochemical Regulation of Maternal Behavior
3.1 Factors Affecting Offspring-Directed Behaviors
The endocrine system through its secretion of hormones during pregnancy plays an important role in stimulating maternal care at parturition in numerous species of mammals (see Figure 3) The dependency on the hormonal stimulation varies, however, as a function of the species and the female’s reproductive history. Hormones appear to play a more obligatory role in rodents and ungulates, whereas the endocrine system’s role appears more modulatory in non-human primates and perhaps women. Moreover, dependency on endocrine stimulation is reduced as the female gains greater reproductive experience, such that multiparous females are less dependent upon hormonal regulation of maternal behavior than are first time mothers.
Figure 3.
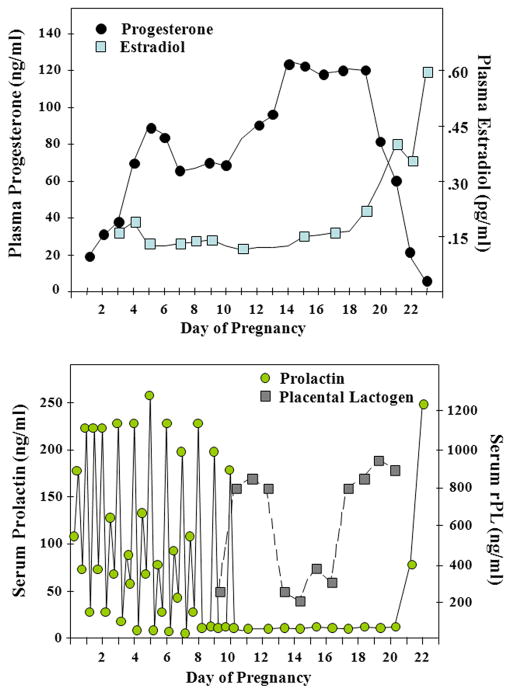
Hormone profiles of estradiol, progesterone, prolactin, and rat placental lactogens during pregnancy in the rat. Adapted from Bridges R.S., 1990. Endocrine regulation of parental behavior in rodents, in Krasnegor, N.A., Bridges, R.S. (Eds.), Mammalian Parenting: Biochemical, Neurobiological, and Behavioral Determinants, Oxford University Press, New York, pp. 93–117, with permission.
Some of the key hormonal changes that accompany pregnancy include increased levels in circulating estradiol, progesterone, and lactogenic hormones (prolactin and placental lactogens). The secretory patterns of these hormones in the rat are illustrated in Figure 4. The role of these hormones in the context of maternal behavior is described here.
Figure 4.
Stimulation of maternal behavior after bilateral infusions of ovine (o) PRL (40 ng/side) into the MPOA region of steroid-primed, nulliparous rats. ** P < 0.01 versus vehicle controls. Taken from Bridges, R.S., Numan, M., Ronsheim, P.M., Mann, P.E., Lupini, C.E., 1990. Central prolactin infusions stimulate maternal behavior in steroid-treated, nulliparous female rats. Proceedings of the National Academy of Sciences USA 87(20), 8003–8007, with permission..
3.1.1 Role of Hormones
The involvement of hormones in the onset of maternal behavior has received research attention based upon the association of the myriad of endocrine changes that occur during pregnancy and at parturition that is associated with the increase and often spontaneous expression of maternal care at birth. Related studies demonstrated both a non-hormonal as well as a humoral basis for stimulation of a rapid onset of maternal care around the time of parturition. An earlier seminal report in rats demonstrated an underlying non-hormonal basis of maternal care (Rosenblatt, 1967). Adult virgin female rats could be induced to display maternal behaviors after approximately 5–7 days of continued pup exposure. Furthermore, this responsiveness could be demonstrated in hypophysectomized subjects. Perhaps, however, one of the more elegant studies that pointed to a humoral basis underlying the rapid onset of maternal behavior at birth was reported a few years later by Terkel who used a parabiotic transfusion system to show that blood transfused from a late pregnant rat to a virgin female recipient resulted in a rapid expression of maternal care in the virgin female recipient towards foster young (Terkel and Rosenblatt, 1972). A similar type of transfusion of blood from a pup-induced maternal virgin to a naïve virgin recipient failed to stimulate maternal care in the virgin recipient (Terkel and Rosenblatt, 1971). Although these investigators did not identify the specific humoral factor in parturient rats responsible for the rapid induction of maternal behavior in recipient subjects, these studies laid the foundation for a series of future studies that were to identify roles for a number of hormones, including estrogens, progesterone, and the lactogenic hormones, prolactin and placental lactogen.
Estrogens
Estradiol-17β (E2) is the most bioactive form of estrogen and plays an important role in the stimulation of maternal behavior in numerous species, including rats, mice, sheep, and possibly non-human primates. Early studies in rats in which estradiol was administered to inexperienced, nulliparous subjects demonstrated that estradiol was an essential component of a so-called maternal pregnancy hormone regimen that consisted of a combination of estradiol, progesterone and prolactin (Moltz et al, 1970; Zarrow et al., 1971). Overall, estrogens play a key role in promoting the induction of maternal care in mammals. In both rodents and sheep estradiol is a key player in stimulating the onset of maternal behavior. Siegel and Rosenblatt (1975a) demonstrated that estradiol benzoate (EB) injections given to primigravid rats whose pregnancies were surgically terminated by a combination of hysterectomy and ovariectomy (HO) on day 16 of gestation resulted in a facilitation of maternal care 48 hours after treatments. Likewise, s.c. injections of virgin rats with higher doses of EB stimulated maternal care towards foster young (Siegel and Rosenblatt, 1975b). Subsequent central studies designed to identify a possible neural site of estradiol stimulation of maternal behavior demonstrated that direct bilateral implants of dilute estradiol into the MPOA of either pregnancy-terminated primigravid rats (Numan et al., 1977) or ovariectomized, virgin rats (Fahrbach and Pfaff, 1986) produced shorter response latencies towards foster test young. The actions of estrogen in promoting maternal care in mice appear to be mediated via the estrogen alpha receptor (Riberio et al., 2012). In sheep, subcutaneous injections of EB enhance maternal responding in non-lactating, multiparous ewes, although it is without effect in inexperienced ewes (Poindron et al., 1988). This latter finding indicated that the actions of estrogens on maternal care can have experiential determinants. Finally, it is noteworthy that the actions of other pro-maternal molecules, i.e. progesterone, prolactin and oxytocin are dependent on concurrent exposure to estrogens (see sections that follow).
Progesterone
Progesterone (P) is a steroid hormone that as the name implies promotes pregnancy or gestation. Circulating progesterone levels are elevated throughout pregnancy and decline either just prior to parturition or at birth. The circulating patterns of P in the rat (Morishige et al., 1973; Bridges, 1984) are illustrated in Figure 4. Progesterone plays important roles in regulating both the expression of maternal behavior as well as lactogenesis. It has been proposed that P has two behavioral functions, first priming the gestating brain to sensitize it to stimuli from young at parturition, and second controlling the timing of increased responsivity. As the duration of P exposure in the gestating rat lengthens, the female becomes more primed to respond maternally to foster rat pups once circulating P levels decline.. This cumulative priming is only expressed once the high circulating levels of P decline. This action is evident both prepartum when both P has declined (Slotnick et al., 1973) as well as after the removal of the ovaries (the primary source of pregnancy in the rat) during the latter stages of gestation (Bridges et al., 1978). Maintaining elevated levels of circulating P after surgical pregnancy termination by HO on day 17 in primigravid rats inhibits the rapid onset of maternal behavior found in HO control subjects (Bridges et al., 1978).
Central studies attempting to localize the site of P’s actions have focused more on the “timing” role rather than the “priming” role of this hormone. Numan (1978), employing a pregnancy-termination HO-EB model, administered P centrally in an effort to identify sites where P might act to inhibit the onset of maternal behavior. Bilateral P treatments directed at the MPOA, VMH, and midbrain tegmentum failed to delay the rapid expression of maternal care towards foster pups. The possibility was raised that P acts at other sites or multiple sites concurrently to inhibit the rapid expression of maternal behavior in the rat. At this time, therefore, sites of P action in regulating the onset of maternal behavior at parturition remain unknown and relatively unexplored.
Other attempts to identify sites of P’s priming action have focused upon the effects of P on neural prolactin receptor expression. This approach provides indirect information as to P’s possible “priming” site of action (Bridges and Hays, 2005). Systemic treatment of animals with P alone for 10 days or with P followed by E2 resulted in reductions in prolactin receptor mRNA expression in the MPOA, indicating that one site of progesterone mediation of maternal care is this brain region. Specifically, the actions of these steroid treatments were more pronounced in the dorsal MPOA, suggesting that this may be one site of P priming of maternal behavior, acting in consort via the prolactin receptor system to mediate the expression of maternal care at parturition.
One mechanism of progesterone’s action is through P receptor mediated ligand-dependent nuclear transcription factors (Mani et al., 1997). Numan et al. (1999) examined this possibility by treating pregnancy-terminated rats with combinations of estradiol, progesterone and the progesterone receptor antagonist RU-486. It was found that treatment with the progesterone receptor antagonist attenuated the blocking actions of progesterone, providing support that the actions of progesterone during late pregnancy in blocking the onset of maternal behavior are mediated by the P receptor. Alternatively, P’s actions may be non-genomically mediated through its conversion to allopregnanolone (Zwain et al., 1999). Using a pregnant rat model, Mann (2006) investigated this possibility by treating primigravid rats during late pregnancy with finasteride, an inhibitor of 5α-reductase that blocks conversion of P to allopregnanolone. The results of this study found that whereas treatment of subjects with finasteride displayed higher levels of anxiety, maternal behavior appeared unaffected. Thus, it appears that the late pregnancy inhibition of maternal behavior by P may not be directly mediated by the neurosteroid allopregnanolone.
In women, the inhibitory actions of progesterone on the myometrium and uterine contractions prior to parturition appear to be mediated by elevated progesterone levels. However, in contrast to most other mammals at the time of birth circulating P levels in women remain elevated (Zakar and Hertelendy, 2007). This would suggest that the behavioral and uterine actions of P in women may not rely on a decline in circulating P, but rather may involve a shift in progesterone receptor (PR) activity. Two forms of the P receptor, PR-A and PR-B, undergo distinctive changes around parturition in women with a shift from PR-B to PR-A dominance (Stjernholm-Vladic et al., 2004; Thijssen, 2005). It is interesting to speculate that an enhancement of maternal interest develops in association with a functional shift in P activity that occurs in women as it does in other mammals. In women this shift in activity may be associated with an increase in the ratio of PR-A to PR-B activity. Further research regarding whether a similar neural mechanism occurs in the brain in P regulation of maternal behavior is needed to help delineate P’s role in regulating the onset of maternal behavior across mammalian species.
Prolactin & Placental Lactogens
The pituitary hormone prolactin (PRL) was first isolated and identified by Oscar Riddle who reported that PRL stimulated maternal behavior in virgin, female rats (Riddle et al., 1935). However, subsequent studies (Lott and Fuchs, 1962; Beach and Wilson, 1963; Baum, 1978) failed to establish a role for PRL in maternal behavior. Research in rabbits, however, provided support for a role for PRL; treatment of pregnant does with an ergot drug that suppresses PRL release interfered with prepartum nest building (Zarrow et al. 1971). A more systematic series of studies in rats during the past 20 years has firmly established a role for PRL (as well as placental lactogens) in the induction of maternal care (Bridges et al., 1985; Bridges et al., 1990; Bridges et al., 1996). These studies are summarized here. This more recent set of experiments assessed the effects of a pregnancy-like steroid hormone regimen consisting of Silastic implants of estradiol and progesterone (Bridges, 1984) on the rate of induction of maternal care in ovariectomized, nulliparous rats that either were hypophysectomized or had intact pituitary glands. The maternal response latencies of the hypophysectomized rats (5–6 days) were unaffected by the steroid treatment, whereas the intact animals displayed significantly reduced latencies of 1–2 days (Bridges et al., 1985). Using a steroid-primed, hypophysectomized, gonadectomized nulliparous preparation, it was demonstrated that the insertion of PRL secreting pituitary implants beneath the kidney capsule instated a rapid onset of maternal behavior towards foster pups that was positively correlated with circulating levels of PRL produced by the implants. Follow-up studies demonstrated that subcutaneous injections of ovine PRL, like the pituitary implants, produced short latencies to initiate maternal care towards foster pups (Bridges et al., 1985), thereby establishing a role for PRL in the induction of maternal behavior in the rat.
Given that hypophysectomy removes multiple hormones and raises questions regarding the general health and suitability of this preparation for use in behavioral studies, we then developed an animal preparation which involved the use of bromocriptine, a dopamine D2 agonist, to suppress endogenous PRL secretion in non-hypophysectomized, steroid-treated nulliparous rats (Bridges & Ronsheim, 1990). The steroid-treatment that was developed and employed in a number of studies included subcutaneous implantation of P-filled Silastic capsules into ovariectomized, nulliparous rats from treatment days 1 to 11. On day 11 the P capsules were removed, a single estradiol-filled Silastic capsule was implanted subcutaneously, and subjects were injected twice daily with bromocriptine throughout behavioral testing that started on day 12 to suppress the estradiol-stimulated rise in plasma PRL. As predicted, treatment with bromocriptine delayed the onset of maternal behavior towards foster young whereas treatment with bromocriptine plus PRL reinstated a rapid onset of maternal care.
Once a clear role for PRL in the induction of maternal care was established, the question arose as to where PRL acted to stimulate the onset of maternal behavior. Work by Walsh et al. (1987) indicated that PRL or a PRL moiety may gain access to the brain through its binding to prolactin receptors in the choroid plexus and the subsequent transport of PRL into the cerebrospinal fluid. These investigators demonstrated that radiolabeled-PRL bound to epithelial cells on the choroid plexus of the cerebral ventricles, thereby bypassing the blood-brain barrier. Subsequent studies using in situ hybridization identified high densities of PRL receptor message in the choroid plexus as well as other neural structures during pregnancy and lactation (Augustine et al., 2003), providing a putative mechanism for PRL entrance from the peripheral circulation into the central nervous system.
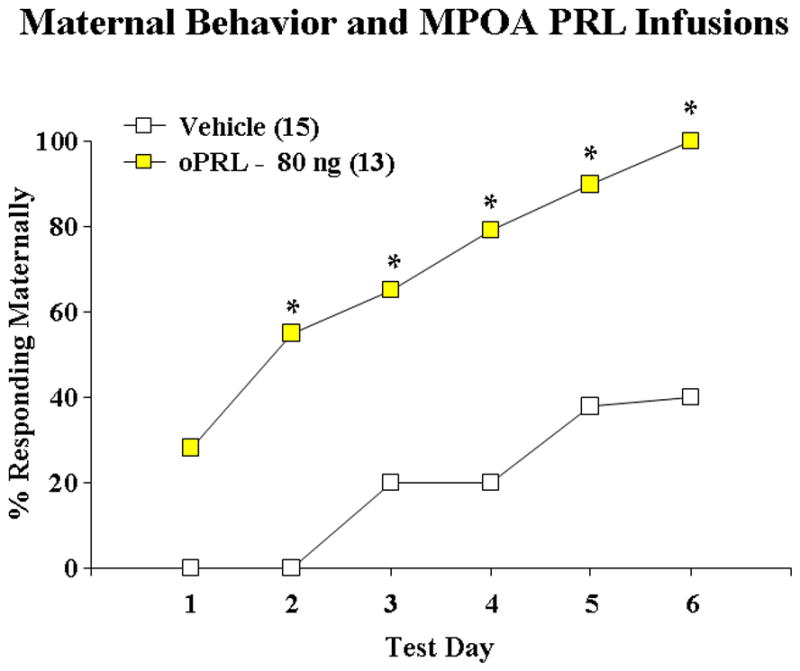
In a series of behavioral studies designed to identify possible central sites of PRL’s actions, it was shown that infusions of PRL into the MPOA of gonadectomized, steroid-treated, bromocriptine injected virgin female rats stimulated a rapid onset of maternal behavior towards foster pups (Bridges et al., 1990; see Figure 5). Latencies of rats infused with 40 ng of oPRL bilaterally averaged less than one day, whereas less than 50% of vehicle-infused controls responded by test day six. The same total dose of oPRL infused into the lateral vehicles failed to stimulate maternal behavior, indicating that the actions of oPRL in the MPOA appear to be site specific. Other central sites of PRL action in the regulation of the establishment of maternal behavior have not been clearly identified. Infusions of PRL into the VMH of rats, however, did reveal a trend towards stimulation (Bridges et al., 1999). Interestingly, the VMH in ring doves has been identified as a site of PRL stimulation of incubation behavior (Buntin, 1996). One other possible site of PRL action, albeit not established, is the PVN. PRL binds to PRL receptors located on OXY cell bodies in the PVN (Kokay et al., 2006) which in turn are linked to the mesolimbic dopamine-nucleus accumbens system (Shahrokh et al., 2010), a neural network shown to mediate a number of aspects of maternal behavior (Li & Fleming, 2003a, 2003b; Parada et al., 2008; Stolzenberg et al., 2007).
Figure 5.
Endocrine inputs that prime the maternal brain. Hormones produced by a range of endocrine structures feedback upon the central nervous system to alter the expression of maternal behavior at parturition. Lactogenic hormones from both the pituitary gland and the developing conceptus appear to act over a prolonged time frame to prime the maternal brain in concert with the rising and falling circulating titers of estradiol and progesterone. The source of the gonadal steroids varies among mammalian species with the conceptus and placenta being more contributory in primates, including humans, than in rodents.
Related endocrine studies have shown that other lactogenic hormones, notably the placental lactogens (PLs), are secreted in large amounts during the second half of gestation in rats (Robertson and Friesen, 1981) as well as in women (Peake et al., 1983). These placental lactogens appear to gain access to the CSF in significant amounts during pregnancy. In female rats, rPL-I and rPL-II, are detected in the CSF from days 12 to pregnancy until parturition (Bridges et al., 1996), whereas in pregnant women hPL is present in the CSF (Peake et al., 1983). Since the PLs are structurally and functionally closely related to PRL as a member of the lactogen family, we asked whether the PLs might play a role in acting on the maternal brain over the course of pregnancy to help stimulate the onset of maternal behavior. Such a mechanism would provide an endocrine communication mechanism between the developing conceptus and the maternal brain. To test this possible behavioral action of PLs, adult ovariectomized, nulliparous rats were treated with the sequential steroid regimen of P followed by E2. Endogenous PRL secretion was blocked by treatment with bromocriptine, and subjects were infused centrally daily for 3 days with either rPL-I or recombinant rPL-II (Bridges et al., 1996). Bilateral infusions of both rPL-I and rPL-II stimulated the onset of maternal behavior reducing latencies from 6 days in vehicle-infused controls to about 2 days in the experimental groups.
These studies clearly demonstrate a role for lactogenic hormones in combination with estrogens and progesterone in the induction of maternal behavior. Figure 5 schematically depicts key endocrine inputs and sources that prime the maternal brain over the course of pregnancy to respond maternally at the time of parturition. The actions of PRL and PLs subsequently were shown to be mediated through the actions of these hormones on the PRL receptor (see also Section 2.3.1.3). A complementary study in female rats reported delays in the rate of onset of maternal care when steroid-primed, nulliparous subjects were given MPOA infusions of the PRL receptor antagonist, S179D-PRL (Bridges et al., 2001). Taken together, these findings provide meaningful support for the involvement of PRL/lactogenic hormones and its receptor system in the stimulation of maternal behavior in the female.
3.1.2 Neurochemical Regulators
A number of neurochemical systems and related molecules have been demonstrated to mediate the expression of the onset of maternal care. Those molecules receiving the greatest attention include the neuropeptides, oxytocin (OT), opioids, and arginine vasopressin (AVP), and the classical neurotransmitters dopamine (DA) norepinephrine (NE), and serotonin (5HT).
Oxytocin
Pedersen and colleagues initially reported a role for oxytocin (OT) as a stimulator of maternal behavior in rats (Pedersen et al., 1982; Pedersen and Boccia, 2003). Intracerebroventricular (ICV) infusions of OT into estrogen-primed adult, virgin female rats significantly shortened the females’ response latencies towards foster pups. OT presumably synthesized within the PVN appears to act centrally to stimulate maternal care. Sites of OT action include the MPOA and ventral tegmental area (VTA; Pedersen et al., 1994). OT’s function may be to enhance maternal motivation to respond to young, possibly by reducing anxiety. However, the effects of increased anxiety is complex and could either promote or interfere with maternal care, depending on the context in which the anxiety appears. OT’s actions are more evident when the subject is tested in a novel environment (Farhbach et al, 1985) or when the subject is partially anosmic (Wamboldt and Insel, 1987). A role for OT system in another affiliative behavior, pair bonding in voles, has complemented studies of maternal care in rats in support of an important role for OT in social behaviors (Young et al., 1998).
Vasopressin
Studies that examine the role of the neuropeptide arginine vasopressin (AVP) in the induction of maternal behavior are limited. In early research, Pedersen reported that central ICV treatment of virgin female rats with AVP potentiated a more rapid rate of onset of maternal care towards foster young (Pedersen et al., 1982). The role of AVP in maternal care has focused more on its role in maternal aggression (see Section 3.3). Additional studies of the involvement of AVP in pup-directed maternal care is warranted, especially given the established role of AVP in pair bonding in voles (see Section 4).
Opioids
Opposing roles for opioids in maternal care have been proposed, including a stimulatory role (Panksepp et al., 1994) as well as inhibitory or disruptive role (Bridges and Grimm, 1982). Opiates were initially proposed to facilitate the maternal bond (Panksepp et al., 1994), whereas studies in rats treated with morphine at the end of pregnancy found that morphine treatment rendered the females less responsive to young (Bridges and Grimm, 1982). Examination of the disruptive actions of opioids found that central administration of morphine to the MPOA, but not the VMH, disrupted maternal behavior in female rats, an action reversed by concurrent treatment with the opiate antagonist, naloxone (Rubin and Bridges, 1984). Morphine’s disruptive effects on maternal care appeared to be through the mu opiate receptor as treatment with delta and kappa agonists failed to interfere with ongoing maternal behavior (Mann et al., 1991), while central MPOA infusions of the mu opiate receptor agonist β-endorphin blocked ongoing maternal care (Mann and Bridges, 1992). Interestingly, with increasing reproductive experience, the central disruptive effects of β-endorphin are reduced, suggestive of the development of some form of opiate tolerance with repeated births.
Although opioids have the ability to interfere with maternal care, it is a fair question to ask what their physiological roles are. A related study in lactating rats suggests that opioids may function during lactation to modulate the duration of nursing (Byrnes et al., 2000). Systemic treatment with naloxone shortened nursing bout duration. Another study indicated that opiates may act with the midbrain to modulate the amount of time spent by a lactating rat hunting for food versus staying in the nest (Sukikara et al., 2007). This latter action appears to be influenced by opiate exposure during late gestation and involves a desensitization of the endogenous opiate receptor system (Cruz et al., 2010).
Classical Neurotransmitters and Maternal Behavior
Dopamine
The neurotransmitter dopamine (DA) has been the most systematically studied neurotransmitter implicated in both the induction and maintenance of maternal care in mammals. Early pharmacological depletion studies using the drug 6-hydroxydopamine that lesioned the VTA or NA interfered with the onset and maintenance of maternal behavior in the rat (Hansen et al., 1991). Also, increased DA release in to the NAcc is associated with increases in mother-pup interactions (Champagne et al., 2004) and appears to modulate the onset of maternal behavior (Numan et al., 2005). In studies in rats DA acting through the DA D1 receptor in the NAcc has been shown to stimulate the onset of maternal care (Stolzenberg et al., 2007). Likewise, pharmacological blockage of DA D1 receptors around the time of parturition resulted in the absence or deficits in maternal care postpartum and/or later in life (Byrnes, et al., 2002; Parada et al., 2008). Other studies have demonstrated that blockade of DA receptors impairs maternal behavior (Pereira & Ferreira, 2006; Silva, Bernardi & Felicio, 2001), an action that can be reversed with DA agonist treatment (Giordano, Johnson & Rosenblatt, 1990). In accordance with the stimulatory effects of DA on maternal care, infusions of the neurotoxin picrotoxin into the VTA that stimulates DA activity within the VTA-NA system shortened maternal response latencies in virgin female rats exposed to foster pups (Byrnes et al., 2011). It is worth noting that DA antagonist action at the MPOA or NAcc as well as severing connections between these neural regions cause similar impairments of maternal behavior (Miller & Lonstein, 2005; Numan & Callahan, 1980, Numan & Smith, 1984). These anatomical studies provide valuable support for the importance of the VTA-NA-MPOA DA system as modulators of maternal care. Given the established role of DA within the mesolimbic reward systems (Berridge & Robinson, 1998), it is feasible and likely that the actions of DA serve to promote the rewarding stimulus properties of the young to the mother.
Whereas a role for the DA D1 receptor in maternal care is firmly established, the involvement of the DA D2 receptor has received less attention. Yet, like the DA D1 receptor, pharmacological blockage of the DA D2 receptor with clebopride around the time of birth severely impacts maternal care by inhibiting the expression of maternal care. Interestingly, while blocking the DA D2 receptor at birth interferes with maternal care immediately postpartum, it fails to prevent the establishment of maternal memory (Byrnes et al., 2002). Genetic studies in which the dopamine transporter (DAT) gene is deleted while enhancing DA tone, increasing locomotor activity and impairing lactational performance only resulted in slight disruptions of maternal care and retrieval (Morice et al., 2004; Spielewoy et al., 2000). Given the potential breath of neural effects of DA gene deletions, use of this and related genetic null mutation models to study a fairly complex motivated behavior has distinct limitations.
Norepinephrine
Important evidence for the involvement of norepinephrine (NE) in maternal care comes to a large extent from studies in sheep. Destruction of the NA inputs (Pissonnier et al., 1985) or blockade of β-adrenergic receptors in the olfactory bulb (Levy et al, 1990) prevented olfactory learning of offspring recognition in the ewe. Measurements of the release of NE within the olfactory bulb around the time of parturition in ewes revealed that both NE and acetylcholine (ACh) increase at the time of birth with this effect being dependent upon the reproductive history of the ewe; multiparous, but not primiparous mothers, display increased amounts of transmitter release (Levy et al., 1993). The role of NE in maternal care in rats is more equivocal as early pharmacological studies demonstrated deficits in maternal behavior in female rats treated with NE antagonists (Rosenberg et al., 1977; Steele et al., 1979). Likewise, treatment of rats with noradrenergic alpha-2 receptor antagonists when infused into either the bed nucleus of the stria terminalis or medial preoptic area interferes with normal maternal care (Smith et al., 2012), whereas null mutation studies in which the gene for the enzyme dopamine-β-hydroxylase (DHH) which converts dopamine to norepinephrine was deleted found that mice homozygous for the DBH null mutation exhibited deficits both in placentophagia and pup retrieval (Thomas and Palmiter, 1997).. In contrast, surgical transection of the ascending noradrenergic pathways of rats that partially deleted hypothalamic NE did not eliminate maternal care, although it did affect the quality of nest building and lactational performance (Bridges et al., 1982). It thus appears that the relative tone of the noradrenergic system affects maternal care, affecting selected components of maternal care.
Serotonin
Examination of the role of serotonin (5-HT) in maternal behavior is limited, yet 5HT is a feasible candidate for involvement in maternal care. An early study reported by Barofsky et al. (1983b) reported a role for the median raphe and 5HT in maternal care in rats. Neurotoxic lesions of the median raphe produced deficits in postpartum care and pup-oriented responses. In an accompanying report (Barofsky et al., 1983a), neurotoxic lesions of the dorsal raphe impaired suckling-induced prolactin secretion and lactational performance. Given the important role of 5-HT in lactation via its role in prolactin regulation, a more thorough evaluation of serotoninergic regulation of maternal care is warranted. Gene knockout models have also indicated that deletion of tryptophan hydroxylase (TH), an enzyme that is essential for serotonin production results in poorer maternal care, including pup retrieval, in postpartum mice (Alenina et al., 2009). The effects of this gene deletion on maternal care, however, need to be weighed in terms of specificity, since mice with null mutations of THs displayed a range of modifications in other behavioral and physiological phenotypes.
In a related study, the involvement of and relationships between the neurochemicals oxytocin and serotonin in social preference in mice (Dölen et al., 2013), another form of affiliative behavior, was examined. In an elegant set of experiments these investigators demonstrated that the social facilitation associated with increased social preference in mice was mediated by serotoninergic input from the midbrain dorsal raphe to the nucleus accumbens where oxytocin-induced synaptic plasticity required the activation of 5-HT1B receptors. Whereas this finding was framed within the context of autism spectrum disorders, this neurochemical system may more generally reflect a wide range of attachment responses including maternal care. It is of interest that in the maternal lactating female that suckling stimuli appears to stimulate prolactin release through an ascending sensory/neuroendocrine pathway that includes the dorsal raphe (Tindal and Knaggs, 1977). It is feasible that suckling input from the dorsal raphe stimulates both prolactin secretion via connections to the TIP39 peptide system in the thalamus (Cservenák et al., 2010) as well through dorsal raphe which communicates rewarding stimuli from the young at the level of the nucleus accumbens.
Parathyroid Hormone 2 Receptor (PTH2 receptor)
Recent studies have identified a role for a 39 residue tuberoinfundibular peptide (TIP39) in the rat that binds to the parathyroid 2 receptor in both the MPOA and arcuate region in regulating suckling-induced prolactin secretion (Cservenák et al., 2010) and more recently maternal motivation (Cservenák et al., 2013). Retrograde studies by these investigators have identified that TIP39 producing cells that project to the arcuate region and medial preoptic area are located in the posterior intralaminar complex of the thalamus. These findings are particularly of interest, since it establishes a link between the major sensory region of the brain, the thalamus, and neuroendocrine and behavioral sites within the hypothalamus.
Galanin
Galanin is a neuropeptide consisting of 29–30 amino acids that is widely distributed throughout the brain and body. Although notably present in olfactory structures, the GAL gene is also expressed in the MPOA of both female and male mice. Examination of the possible role of galanin in parental behavior in mice provided support for this neuropeptide in parental care (Wu et al., 2014). Whereas this study focused more on paternal than maternal behavior, bilateral injections of recombinant adeno-associated virus expressing Cre-dependent diphtheria toxin A fragment eliminated about 60% of MPOA Gal+ cells and resulted in reductions in retrieval behavior and increases in pup-directed aggression in virgin female mice. The authors proposed that MPOA Gal+ cells constitute an essential neuronal populations for maternal care in virgin females. It would be of interest to determine whether similar effects can be identified in lactating subjects and whether optogenetic activation of MPOA Gal+ neurons suppress attack and promote maternal care as has been found in males (Wu et al., 2014). The interactions of Gal neurons with other candidate molecules, i.e. estradiol, prolactin and oxytocin, also merits exploration.
3.2 Neuroendocrine Regulation of Non-Offspring Directed Maternal Care – Maternal Aggression
Maternal aggression increases during pregnancy in rats (Mayer and Rosenblatt, 1984; see review by Lonstein and Gammie, 2002) and reaches its highest level during the early postpartum period (Erksine et al., 1978). Likewise, in mice maternal aggression is elevated during pregnancy and early lactation (Svare, 1990). The expression of maternal aggression towards an intruder which functions to protect the young appears to be dependent on shifts in sensory processing rather than endocrine control, since hypophysectomy of day 5 postpartum rats does not reduce the expression of maternal aggression (Erskine et al., 1980). Reproductive experience also affects the degree of aggression as both in rats and mice; multiparous, lactating dams display higher levels of maternal aggression than do age-matched, first time or primiparous mothers (Svare, 1990; Nephew et al., 2009).
The neurobiological control of maternal aggression has received increased research attention in recent years. Roles for neuropeptides as well as neurotransmitters have been identified together with key neural sites and networks that mediate the expression of maternal aggression (see Lonstein and Gammie, 2002). Neurochemicals implicated in maternal aggression include GABA, AVP, oxytocin, corticotrophin-releasing hormone (CRH), and nitrous oxide (NO).
Evidence for the roles of oxytocin and AVP are somewhat equivocal. Specifically, findings on the possible role of AVP appear to relate to the animal model studied. Bosch and colleagues report that in rats selected for high anxiety-related behavior (HABs) AVP promotes maternal aggression (Bosch et al., 2008). These subjects also have increased expression of the AVP gene in the PVN, an area implicated in maternal aggression (Lonstein and Gammie, 2003). Treatment of day 5 lactating rats with an AVP receptor antagonist, V1a-A, reduced the incidence of attacks. Intracerebroventricular (ICV) infusions of low anxiety behavior (LAB) dams with AVP, in contrast, reduced maternal aggression in rats and enhances pup-oriented maternal care in mice (Kessler et al., 2010). Stidies on the role of AVP on maternal aggression using standard Sprague-Dawley rats not selected for high or low levels of anxiety yielded conflicting results. ICV infusions of AVP reduced maternal aggression in lactating rats, while treatments with an AVP receptor antagonist increased maternal aggression during early lactation (Nephew and Bridges 2008b). AVP treatment similarly reduced maternal aggression in the more aggressive multiparous lactating rat model (Nephew et al., 2009; Nephew et al., 2010). The disparity in findings between research groups may reflect the animal models employed and thus the behavioral differences in findings. In general, however, it appears that AVP can be considered a pro-affiliative regulator during the lactational period, promoting both care towards the young and when perturbed enhancing the level of maternal defense. Additional studies, however, are needed to clarify the interactions between anxiety and AVP actions in this social context as a function of the female’s anxiety status.
The role of oxytocin in maternal aggression has received somewhat limited examination. In general, oxytocin is thought to increase the level of maternal aggression as infusions of OT into the central nucleus of the amygdala increases maternal aggression (Bosch et al., 2012). OT concentrations increase in the central amygdala of HAB rats when exposed to an intruder (Bosch et al., 2008). Blocking OT activity in the HAB rats with infusions of an OT antagonist into the PVN or central amygdala reduces maternal aggression, whereas OT infusions into the PVN increase maternal aggression (Bosch et al., 2008). In contrast, lesions of the PVN, a primary source of OT, as well as knocking out the OT gene fail to affect maternal aggression in rodents. Thus, it appears that enhanced central OT activity during lactation functions to increase social awareness of the dam, allowing her to recognize social threats more effectively, heightening her response to a threat such as when an intruder comes into proximity to the maternal nest and young.
The neuropeptide CRH and nitrous oxide (NO) also has been implicated in the regulation of maternal aggression in rodents. ICV infusions of CRH in lactating mice reduce maternal attacks (Gammie et al., 2004), possibly through the CRH-2 receptor (Gammie et al., 2005) and may be associated with the reductions in anxiety associated with the lactational state (Lonstein, 2007). NO itself appears to stimulate maternal aggression as reflected in the decline of maternal aggression in nitric oxide synthase deficient mice (Gammie and Nelson, 1999). In addition, treatment of lactating rats with the NO synthase antagonist, L-NAME, reduced maternal aggression during early lactation (Popeski and Woodside, 2004). The possible interactions between NO and CRH remain to be identified as well as how they molecules may interact with more established regulators of maternal aggression such as AVP and OT. Finally, the role of the GABAergic system in maternal aggression merits study in light of GABA’s role in regulating the intensity of postpartum anxiety (see Lonstein, 2007). It has been shown, for example, that GABA receptor antagonist injections into the VMH or medial amygdala reduce the number of attacks, while at low doses treatment with benzodiazepine agonists increase maternal aggression (Hansen and Ferreira, 1986).
4. Genetic Modulation of Motherhood
The contributions of nature and nurture in the regulation of maternal behavior have gained a greater focus with the emergence of modern molecular genetics and more detailed behavioral analyses of maternal care. The predominance of research on the involvement of specific genes in maternal care has been conducted in mice and to a lesser extent in rats (Kuroda et al., 2011). The approach of these studies has been to draw upon previously data that have either identified roles for certain hormones and neurotransmitters in maternal care or have serendipitously identified alterations in parental care in animals bearing specific gene deletions.
4.1 Role of Endocrine-Related Genes in Maternal Care
The effects of deletions of genes and their receptors of endocrine regulators of maternal behavior have provided a tool to verify or examine the roles of specific hormonal systems in maternal care. Studies primarily conducted in mice explored the previously established roles of the gonadal steroids, estradiol and progesterone, as well those of prolactin and oxytocin noted in Section 3. The results of these studies are as follows.
Estrogens
The stimulatory actions of estradiol on maternal behavior appear to be mediated through the estrogen receptor alpha (ERα or ESR1). Null mutations of ERS1 in male mice result in deficits in paternal care (Ogawa et al., 1998). Likewise, silencing ESR1 expression in mice using siRNA injected directly into the MPOA reduced pup care, but surprisingly failed to affect maternal aggression towards a male intruder (Ribeiro et al., 2012; see Figure 6). Prolonged silencing of the ERS1 apparently did not prevent adequate maternal care as female mice were able to maintain their litters and presumably lactate in spite of MPOA ERS1 silencing. Epigenetic studies indicate that the 1b promoter region of the ERS1 gene within the MPOA mediates such modifications associated with maternal experience (Champagne et al., 2006), further supporting a key role for ESR1 in maternal care. The epigenetic actions of maternal behavior on offspring MPOA ERS1 levels are sensitive to maternal care experienced prior to day 10 postnatally, but not after that time (Peña et al., 2013). In sheep ESR1 expression in the hypothalamus and medial amygdala are altered in experienced ewes. ERα cell density is increased in ewes during a subsequent pregnancy relative to an initial one (Meurisse et al., 2005). These changes are evident during late pregnancy in the MPOA, PVN, supraoptic nucleus, and medial amygdala. Likewise, in situ hybridization studies in rats have demonstrated increased ERS1 cell numbers in similar hypothalamic and limbic brain regions as a function of prior reproductive experience, i.e. pregnancy and lactation (Byrnes et al., 2009). It is of interest as proposed by Pfaff et al. (2011) that reproductive behaviors more generally, i.e. sexual and maternal behaviors, involve an estrogen-receptor mediated mechanism. Given the recent creation of ERS1 null mutant rats (Rumi et al., 2014), in the near future an examination of the role of the ERS1 in rats will also be possible to study the role of ERS1 gene deletion in both the onset of maternal care in pup-exposed nulliparous subjects as well as in the activation of maternal memory.
Figure 6.
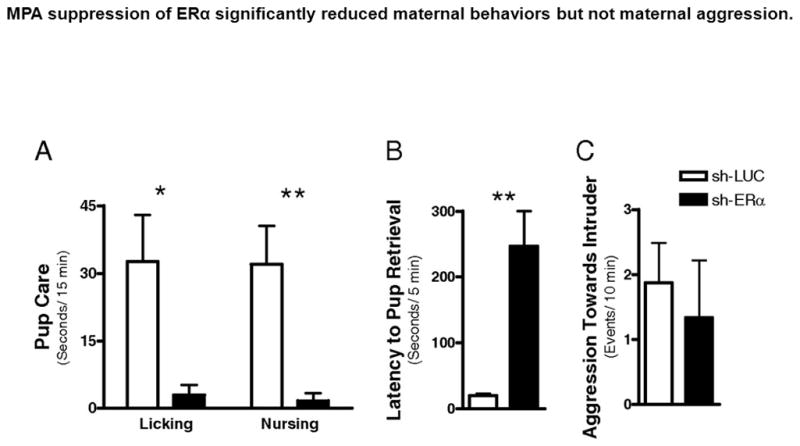
MPOA suppression of ERS1 significantly reduced maternal behaviors but not maternal aggression in lactating mice. (A) Pup care. (B) Latency to pup retrieval. (C) Aggressive behaviors toward male intruder. Values represent mean ± SEM. *P <0.05, ** P<0.01. Taken from Ribeiro, A.C., Musatov, S., Shteyler, A., Simanduyev, S., Arrieta-Cruz, I., Ogawa, S., Pfaff, D.W., 2012. siRNA silencing of estrogen receptor expression specifically in the medial preoptic area neurons abolishes maternal care in female mice. Proceedings of the National Academy of Sciences USA 109, 16324–16329, with permission.
Progesterone
Progesterone is secreted in large amounts by the corpora lutea of the ovaries or the placenta in the gestating mammal. In rodents the corpora lutea are the primary source of progesterone, whereas in primates the placenta is the primary source of progesterone. Progesterone, like estradiol, is essential for pregnancy maintenance; interfering with progesterone production or its action results in a rapid termination of pregnancy. This factor makes it unfeasible to use a pregnant model to assess the role of genetic knockouts to examine the role of progesterone in maternal care. However, Horton and colleagues (2008) have evaluated the role of the progesterone receptor (PR) in male parental care. Using male C57BL/6 mice in which there was a null mutation of the PR gene (Lydon et al., 1995), it was found that PRKO males displayed higher levels of paternal care towards foster young than did the wild type controls (Horton et al., 2008). Similar studies in female mice have not been conducted to determine if the removal of the PR in females also potentiates maternal responsiveness. One can speculate, however, that selective silencing of the PR gene might stimulate maternal care in the female, mimicking the prepartum decline in circulating progesterone which unmasks the maternal potential that develops over the course of pregnancy (Bridges et al., 1978).
Prolactin
Genetic manipulations of the prolactin gene and the prolactin receptor gene have further validated the previously noted role of prolactin in maternal care (Lucas et la., 1998) as well as raised questions (Horseman et al., 1997; Dorshkind and Horseman, 2000) regarding the exclusive involvement of the PRL gene in stimulating maternal care. First, null mutation of the PRL receptor in female mice provided support for a role for the prolactin system in maternal behavior (Lucas et al., 1998). In a series of experiments, the responsiveness of mice with null mutation of the PRL-R, heterozygotes for the PRL-R, and wild type (WT) mice were tested for responsiveness towards foster young. Latencies to retrieve pups were significantly longer in mice homozygous for the PRL-R deletion, while the responses for the heterozygotes were intermediate between the complete knockouts and WT controls. In a related study (Ormandy et al., 1997), mice heterozygotes for the long form of the PRL-R were bred. These females were able to maintain pregnancy, but were deficient in lactational performance postpartum. When these females were re-mated and gave birth to a second litter, their lactational performance was significantly improved, indicating that repeated reproductive experience may enhance the effectiveness of the existing lactogenic system and the neuroendocrine system that regulates lactation. The existence of enhanced endocrine efficiency as a function of prior reproductive experience is supported by related studies in rats that demonstrate increased neural sensitivity to prolactin activation of its receptor system (Anderson et al., 2006). Moreover, the neural STAT-5 transduction pathways that mediate the actions of PRL on its neural receptor system appear to be up-regulated in experienced mothers. Thus, one must consider the possibility of altered endocrine regulation, i.e. neuroendocrine plasticity, in assessing the role of neural and endocrine factors in the expression of maternal care over the lifespan of the female.
Examination of the effects of PRL gene deletion itself on maternal behavior by Horseman et al. (1997) failed to detect any apparent deficits in maternal care in mice. The validity of this finding has to be viewed in light of a somewhat limited behavioral analysis. Reexamination of the role of the PRL gene is warranted to confirm these findings under more stringent behavioral test conditions. Given that the PRL-R can mediate the expression of maternal care, the possible stimulation of the PRL receptor by other lactogenic hormones or by an independent non-ligand mechanism should also be considered as alternative mechanisms involved in activation of the neural lactogenic receptor system. Other lactogens or lactogenic moieties, such as placental lactogens, bind to the PRL receptor and presumably activate neurons with PRL receptors (Freemark et al., 1996).
Oxytocin
A number of studies have examined the effects of null mutations of the oxytocin gene as well as the oxytocin receptor gene (Nishimori et al., 1996; Young et al., 1996). What emerges from these studies is that in knockout mice neither oxytocin nor its receptor appears essential for the full expression of maternal behavior. Postpartum KO mice, while lacking the oxytocin-mediated milk ejection response, appeared to display fairly normal levels of maternal care. However, modest deficits in maternal care emerged when the oxytocin deficient mice were tested in a novel test environment (Pedersen et al. 2006). Likewise, modest deficiencies in pup retrieval latencies were found in OXY-R knockout mice (Takayanagi et al., 2005). Kuroda et al. (2011) have suggested that shifts in anxiety and responses to novel environments may underlie shifts in maternal care in oxytocin KO preparations, since OT regulates related behavioral responses such as social recognition, anxiety, and coping with stress. This concept is consistent with early findings of Fahrbach et al. (1985) and Wambolt and Insel (1987) who demonstrated that oxytocin’s actions on maternal behavior were conditionally dependent upon the test environment; oxytocin appeared to overcome increased anxiety associated with testing in a novel cage.
One conclusion drawn from knock-out studies is that there may be no single obligatory molecular mediator of maternal care. Rather, the various endocrine factors contribute to the sensory awareness of the female, in a way tuning her focus to the sensory characteristics of the young. It would seem to be a disadvantage from an evolutionary perspective to rely exclusively on a single hormone or neurochemical for the stimulation of such a critical behavior. This is not to say that these molecules are not important, but rather that redundancy and compensations in regulatory processes exist to maximize survival of offspring and the species.
4.2 Other Genes Affecting Maternal Care
Roles for a number of other genes have been reported to affect the expression of maternal behavior. One of the genes identified in earlier studies that affected maternal care in mice was the oncogene FosB (Brown et al., 1996); the deletion of FosB resulted in severe deficits in postpartum care in mice, including failure to eat the placentas and show interest in the newborns. Another gene which when deleted or knocked out produced a range of deficits in mice was the gene for CD38, a transmembrane glycoprotein that regulates OT release within the brain (Jin et al., 2007). Deletion of CD38 in mice result in more maternal care and more general severe social behavior deficits, some similar to those associated with autism (Salmina et al., 2010). A third chemical system that may mediate social responsiveness and maternal care is the endocannabinoid system (ECS), a system activated via CB1 receptors (Schechter et al., 2012). Blocking CB1 receptors in postpartum mice interfered with pup-oriented maternal care. The relationship between the ECS and related maternal mediators is unknown at this time.
A broader genetic approach to elucidate the neurobiological bases of motherhood involves asking what genes ae up or down regulated as a function of parturition and lactation (Akbari et al., 2013). Microarray analyses of brain regions found significant effects of pup exposure and parity on the expression of dopamine and glucocorticoid-related genes, the mu-1 opiate receptor, and the GABA and serotonin receptor genes in the MPOA. In the mAMY, genes related to the glucocorticoid family were likewise affected by parity. This approach can help identify candidate genes, both known and unknown, which may participate in the regulation of maternal behavior. More causal follow-up studies are merited to determine the precise involvement of these genes and their products.
5. Developmental Programming of Maternal Care
The expression of maternal care is molded by physiological and behavioral events over the course of the female’s development - starting during the perinatal period and continuing throughout adulthood (see Figure 7). The programming of maternal care is discussed here within the framework of early organizational actions of hormones on the so-called “maternal capacity” as well as a function of maternal experience as an adult. Within this latter framework, the long-term effects of reproductive experience on motherhood as well as epigenetic influences on maternal behavior are presented.
Figure 7.
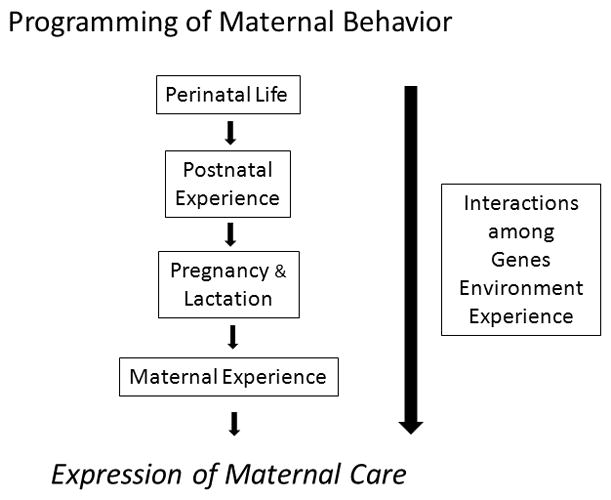
The developmental programming of maternal behavior begins during prenatal life, continues postnatally, and throughout adult development. Genetic factors together with epigenetic and experiential behavioral inputs modify the expression of maternal care over the female’s lifespan. The types and extent of maternal experience can enhance or interfere with maternal care with repeated parenting bouts generally strengthen maternal proficiency.
5.1 Perinatal Programming of Maternal Behavior
Exposure of females to elevated levels of androgens during the perinatal period can interfere with that female’s responsiveness to young in adulthood. Studies in rats have shown that exposure to testosterone postnatally and subsequently treated with a pregnancy-like hormone regimen in adulthood reduced retrieval behavior in a novel T-maze test (Bridges et al., 1973) as well as female typical maternal responses in the home cage (Quadagno et al., 1972). Neonatal castration of males rendered the genetic males more female-like in terms of their responses to foster young and increased their responsiveness to a pregnancy-like hormone regimen. In a related study, the proximity of a female fetus to one or two male fetuses alters the maternal responsiveness of that female when she becomes an adult. Contiguity of a female fetus to male fetuses increases the likelihood that the female will display reductions in maternal care as an adult (Kinsley et al., 1986; vom Saal and Bronson, 1978). That is, females developing in utero between two males display somewhat attenuated maternal care as an adult, presumably due to greater exposure to circulating androgens. The importance of these findings is that the pattern of hormone secretion and possible alterations during gestation may modify the female’s capacity to display maternal care later in her life.
5.2 Experiential Effects on Maternal Behavior
There is a cliché that states “once a mother, always a mother.” It turns out that there is a great deal of scientific truth to this statement. Females that have given birth and cared for their young typically display heightened levels of responsiveness upon subsequent exposure to young whether following another birth or independent of the physiological states of pregnancy and parturition. The role of maternal experience has been examined extensively in the rat. Research conducted in the laboratories of Dr. Alison Fleming and Dr. Robert Bridges, the present author, has demonstrated the impact and neural bases associated with experiential components of maternal care. Specifically, rats giving birth the first time, i.e. primiparous subjects, require only the parturitional experience of interacting with their newborn to consolidate what has been termed “maternal memory” that can be expressed weeks to months later. When these females are exposed to foster pups 3–4 weeks after last exposure to young, their latencies to re-induced maternal behavior is significantly shorter than those of females never exposed to young (Bridges, 1975) or caesarean sectioned just prior to the expected date of birth and not given pup exposure after surgery (Bridges, 1977). This retention of maternal behavior following parturition is long lasting and persists at least 80 days after the maternal experience postpartum (Scanlan et al., 2006) Moreover, it appears that the duration of the postpartum experience does not affect the rate of reinduction of maternal behavior 25 days later as reinduction latencies of primiparous rats given 1, 4 or 9 days of postpartum experience displayed similar reinduction latencies 25 days after last pup exposure (Cohen and Bridges, 1981). Likewise, neither actually suckling of young postpartum nor is the presence of ovarian hormones essential for the establishment of maternal memory during the periparturitional period (Bridges, 1975).
The neural sites identified to date that are involved in the establishment or consolidation of maternal memory include two important neural sites, the shell region of the nucleus accumbens (NAs) and the medial amygdala (mAMY). A significant body of research reported by Lee, Li and Fleming (Lee et al., 1999; Li and Fleming, 2003b) demonstrated a role for the NA, particularly the NAs, in the establishment of maternal memory in parturient rats. Likewise, blocking protein synthesis at the time of parturition following intracerebroventricular infusions of cyclohexamide also interfered with the consolidation of maternal memory (Fleming et al., 1990). Pharmacological studies have indicated a role for the dopamine D2 receptor (Byrnes et al., 2002; Parada et al., 2008) and possibly an involvement of the D1 receptor (Parada et al., 2008) in the consolidation of maternal memory. It is interesting to postulate that a shift in dopamine sensitivity with an associated modulation of maternal care results from reproductive experience, as previously parous rats display enhanced behavioral sensitivity to dopaminergic stimulation (Byrnes et al., 2001). These studies point to an involvement of the ventral tegmental area and the mesolimbic dopaminergic system in the consolidation of maternal memory. An involvement of AVP also acting within the mAMY in the consolidation of maternal memory was more recently reported (Nephew and Bridges, 2008a). When primiparous rats were chronically infused bilaterally with an AVP receptor antagonist during the periparturitional period, their latencies to respond maternally to foster young 10 days later was delayed relative to vehicle controls. These data indicate that AVP may act as a pro-affiliative neuropeptide within the context of maternal memory. Possible interactions between the NAs and mAMY as well as other neural regions within the context of maternal memory have not been investigated. It is noted that the neurobiological events mediating the expression of maternal memory weeks following prior maternal experience, the so-called reactivation phase, have received limited attention and are the subject of future studies. Investigations should determine whether similar neurochemical systems and regions mediate both the consolidation of maternal memory postpartum and the subsequent expression of the memory, behavioral reactivation, later during development. It is of interest how the established changes in PRL receptor expression and PRL responsiveness that accompany reproductive experience (Anderson et al., 2006) impact both the expression of maternal memory and the enhancement of maternal care accompanying multiparity.
The effects of prior maternal experience on subsequent responsivity to pups are not exclusive to the parturient subject. Adult virgin female rats can be induced to display maternal behavior (Rosenblatt, 1967) independent of the states of pregnancy, birth and lactation. When adult virgin rats are first induced to show maternal care with foster pups and then retested for maternal memory weeks later, these females display reductions in response latencies of about 2 to 3 days (Cohen and Bridges, 1981; Scanlan et al., 2006). However, the latencies of the reinduced virgin female rats, while significantly reduced, remain significantly longer than primiparous subjects tested under similar conditions. Thus, experience shortens latencies, but does so more effectively in the parous, so-called “biological” female.
Comparisons of the behavioral and neurochemical responses of re-induced, behaviorally experienced virgin and parous rats have revealed that whereas the behaviors of the reinduced subjects appear similar in the home cage and in a T-maze pup retrieval a paradigm, their responsiveness on the elevated plus maze (EPM), which is used to measure anxiety varied when subjects were tested with pups placed in a container beyond the reach of an open arm of the maze (Scanlan et al., 2006). The reinduced parous subjects spent more time in open arms than did the reinduced virgin subjects (see Figure 8). When the brains of these subjects were examined for cFos protein, increased cFos protein was found in the cortical and medial amygdala of pup-exposed parous subjects, whereas no differences in cFos protein were evident in either the MPOA or NA core or shell. In a related study the numbers of glial fibrillary acidic protein (GFAP) cells were quantified in selected brain regions of primiparous and multiparous rats (Featherstone et al., 2000). GFAP is a significant component of the astrocytic cytoskeleton. Multiparous subjects were found to have a greater number of GFAP staining cells in the MPOA that that found in primiparous dams. In contrast, the number of GFAP cells present in the medial amygdala and habenula were reduced with increasing parity. It thus appears that parity and repeated parity result in differential responsiveness towards pup stimuli in selected neural regions implicated in maternal behavior as well as changes in the neuroanatomical substrate that may regulate these behavioral changes.
Figure 8.
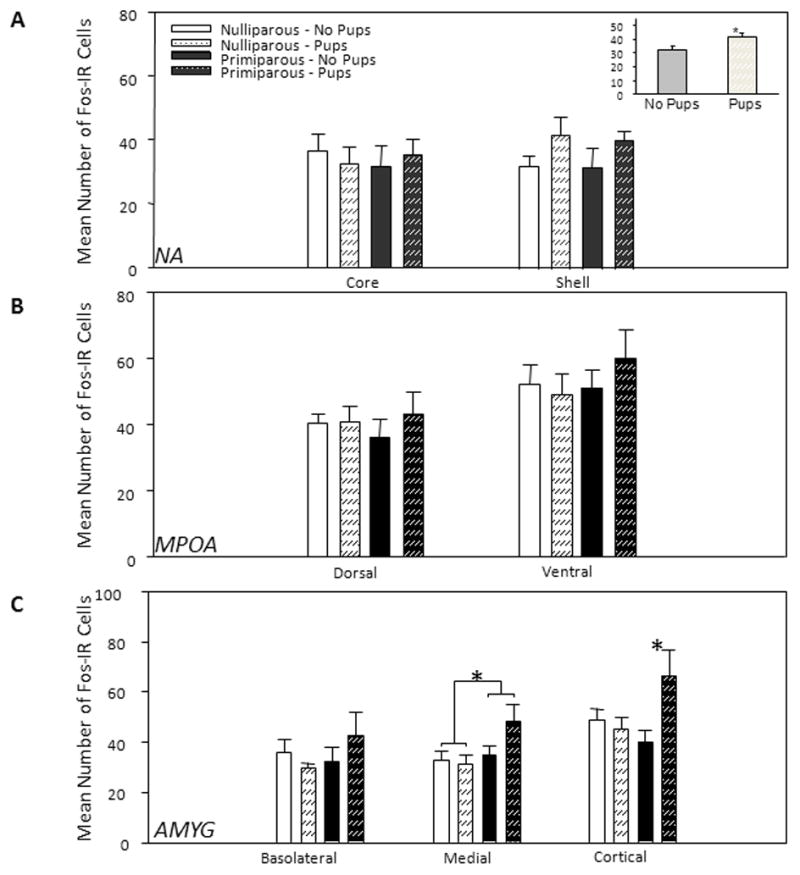
Mean (± SEM) number of Fos-IR nuclei activated in response to the elevated plus-maze test in different brain regions. N=6 per group. A: Nucleus accumbens (NA). Inset: Main effect of pups in the shell, *p = .036 (one-tailed test). B: Medial preoptic area (MPOA); no significant differences were detected. C: Amygdala (AMYG) - Interaction between reproductive experience and pup exposure in cortical AMYG and main effect of parity in medial AMYG. *p < .05. IR = immunoreactivity. Taken from Scanlan, V.F., Byrnes, E.M., Bridges, R.S., 2006. Reproductive experience and activation of maternal memory. Behavioral Neuroscience 120, 676–686, with permission.
Many female mammals give birth more than once or multiple times. Studies of the effects of multiple births indicate that a cumulative effect or some additive component is associated with multiparty. Some of the early work in rats reported that multiparous mothers were more responsive to their young than were primiparous mothers (Moltz and Robbins, 1965; Moltz et al., 1966). Likewise, in sheep, multiparous ewes are more likely to adopt an alien lamb than either nulliparous or primiparous ewes (Lévy and Poindron, 1987). Similarly, multiparous sows are more likely to care for their piglets than primiparous sows that display higher incidences of cannibalism. In primates, Ruppenthal et al. (1976) reported that repeated births are associated with a decrease in abusive or neglect behaviors and an increase in caregiving responses in females that had themselves been raised under deprived conditions. Neuroimaging studies in women demonstrate increased activation or BOLD responses by fMRI in brain regions associated with maternal care (Swain et al., 2007; Swain et al., 2011). Studies of the effects of multiparity in general, however, are limited and merit further study.
5.3 Epigenetic Influences on Maternal Care
Recent and exciting research findings during the past decade have established that maternal care is not only influenced by the female’s genotype and exposure to endocrine and neurochemical factors, but also is affected by the experience that the female receives from her own mother during early life which is subsequently reflected in modifications in the nurtured female’s genetic expression (Champagne, 2012). A number of studies have investigated the heritable alterations in gene expression caused by “epigenetic” mechanisms independent of changes in DNA sequence. Such alterations include modifications in DNA methylation and histone acetylation brought about by experiential events which result in a stable inherited phenotype, but no change in DNA sequence. A well characterized epigenetic influence on maternal care emerged from investigations in the laboratory of Dr. Michael Meaney at McGill University (Francis et al., 1999; Meaney, 2001; Champagne et al., 2001, 2003; Weaver et al., 2004a, 2004b). Meaney and colleagues examined the maternal behaviors of lactating rats and documented the normal distribution pattern of licking and grooming behaviors displayed by the dams towards their litters. Females were scored for frequencies of licking and grooming (LG). This behavioral analysis defined the population distribution of LG of mothers. Subgroups of mothers that occupied the extremes of the total population distribution were then examined. Groups of mothers at the upper and lower portions of frequency distributions were placed into sub-populations, and their neurobiological states were characterized. It was shown that the quality of mother-offspring interactions affected DNA methylation and histone acetylation patterns (Meaney and Szyf, 2005; Weaver et al., 2004a). In female offspring of high LG mothers, increased estrogen sensitivity in the MPOA was found when the offspring reached adulthood. In addition, these females displayed increased levels of maternal behaviors toward their own pups following birth (Champagne et al., 2001; 2003). Likewise, as pups, female offspring of high LG mothers display increased mRNA and protein of the estrogen receptor alpha in the MPOA than that found in the low LG offspring (Champagne et al., 2006; see Figure 9). This change is accompanied by increases in DNA methylation in the 1B promoter region of the ERS1 gene of the low LG offspring and a reduction in transcriptional factor, STAT5a, possibly resulting in a functional increase in silencing of this gene with its associated behavioral output. The plasticity of the maternal care dependent MPOA estrogen receptor systems appears to be conferred upon the offspring prior to postnatal day 10 as MPOA ESR1 receptor levels are sensitive to the maternal care experienced during early postnatal life, but not after day 10 of life experienced (Peña et al., 2013).
Figure 9.
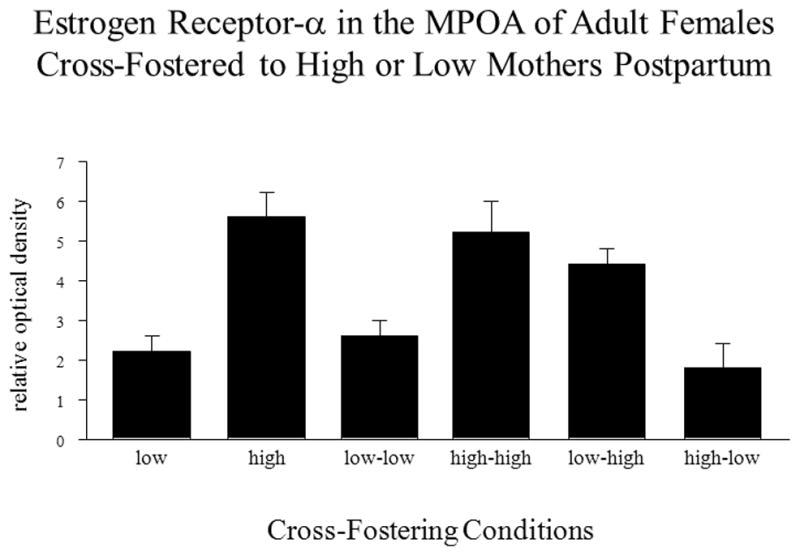
Effects of maternal care of young on their subsequent expression of ERα (ESR1) mRNA in the MPOA as adults. Females cross-fostered from a high or low licking-grooming (LG) mother to a low LG mother, had equivalent levels of ERα mRNA and had less ER expression in the MPOA than females cross-fostered from a high or low LG to a high LG mother. The original mother is designated on the left and the cross fostered mother on the right of hyphenated terms, i.e. low-low. Adapted with Permission from Champagne, F.A., Weaver, I.C.G., Diorio, J., Dymov, S., Szyf, M., Meaney, M., 2006. Maternal care associated with methylation of the estrogen receptor-α1b promoter and estrogen receptor-α expression in the medial preoptic area of female offspring. Endocrinology 147, 2909–2915, with permission from The Endocrine Society.
Other research has explored the involvement of histone acetylation in experience-related changes in maternal care. Acetylation of histones is associated with an enhancement of gene activity and may be a conduit for epigenetic modifications of behavior. In a study in female mice, subjects were treated in their drinking water with sodium butyrate that enhances histone acetylation and both behaviors and gene expression in neural tissues was measured. Maternal care was promoted in sodium butyrate-treated mice (see Figure 10) as was the expression of estrogen receptor β, oxytocin, and CREB binding protein in the MPOA (Stolzenberg et al., 2012). The specific role of these changes in protein and peptide expression in the MPOA remain to be delineated, but may provide a link to the enhanced maternal care resulting from maternal experience. Other research from our laboratory demonstrated a similar facilitation of maternal care in maternal induction latencies in inexperienced, nulliparous female rats after treatment with sodium butyrate (Gleason et al., unpublished findings). Sodium butyrate-treated rats displayed shorter induction latencies to respond to foster pups than did the salinity-balanced controls. Together, these findings indicate that increases in histone acetylation may result in enhanced levels of maternal care. Finally, one must also keep in mind that methylation and acetylation effects may be reversible and may not permanently alter aspects of maternal behavior.
Figure 10.
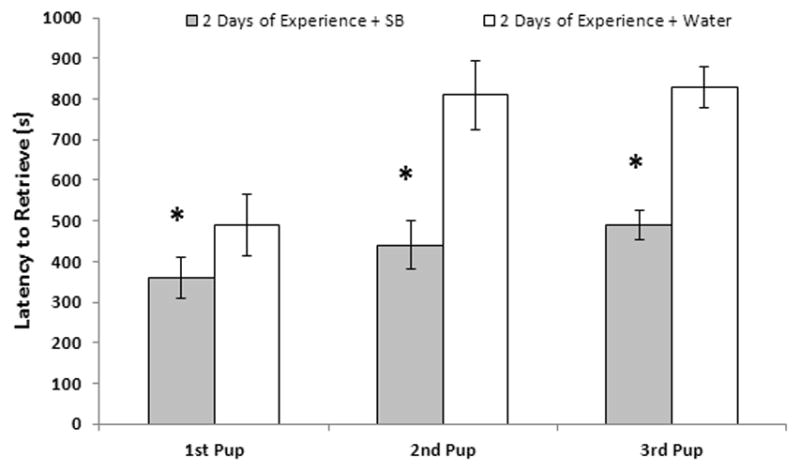
Effects of treatment of mice with the histone deacetylase inhibitor, sodium butyrats (SB), on maternal responsiveness in a novel T-maze after 2 days (2h/day) of maternal experience 24 h after last pup exposure (N = 7/group). Values represent mean ± SEM latencies in seconds (s) to retrieve each of three test pups from the T-maze. * P < 0.05. Adapted with permission from Stolzenberg, D.S., Stevens, J.S., Rissman, E.F., 2012. Experience-facilitated improvements in pup retrieval: evidence for an epigenetic effect. Hormones and Behavior 62, 128–135, Elsevier Press, New York.
5.4 Gestational Programming of Maternal Care – Possible Involvement of Neurogenesis
In addition to the identification of epigenetic effects on maternal behavior and the actions of hormones as stimulators of maternal care, related developmental events can impact the quality of maternal and associated postpartum responses in the mother. Larsen and Grattan (2010) proposed a role for prolactin during early pregnancy in modulating the postpartum expression of maternal care and anxiety in mice. Partial suppression of PRL secretion from days 2–4 of pregnancy with the dopamine agonist, bromocriptine, resulted in elevations in anxiety postpartum and deficiencies in retrieval behavior when treated females were tested in a novel environment, but not their home cage (See Figure 11). Examination of gestational neurogenesis in these groups revealed that neurogenesis in the subventricular zone (SVZ) which normally increases in mice during pregnancy (Shingo et al., 2004) was suppressed in subjects that were bromocriptine-treated (Larsen and Grattan, 2010). These findings indicate that events, i.e. stress, that may suppress PRL secretion during early pregnancy might affect or impair neurogenesis which in turn results in an enhancement in postpartum anxiety and poorer postpartum maternal care. Although suppression of neurogenesis was associated with deficits in maternal behavior in female mice (Larsen and Grattan, 2010), the precise relationship between neurogenesis in the induction or maintenance of maternal care is not firmly established. However, in nulliparous rats the onset of maternal behavior induced by repeated pup exposure is associated with an increase in neurogenesis in the SVZ (Furuta and Bridges, 2009). Whether the actual induction of maternal behavior is caused by the increase in neurogenesis or whether animals that displayed increases in neurogenesis also have higher levels of neurogenesis is unknown. In a related study in rats, treatment of pregnant rats during early gestation with bromocriptine resulted in some impairments in maternal care postpartum when subjects were tested in a large, novel cage (Price and Bridges, 2014). These findings provide further support for the multiple actions of PRL in the regulation of maternal care with PRL affecting maternal care throughout pregnancy, birth and perhaps later in life by programming a range of maternal responses (i.e. postpartum reductions in anxiety; Lonstein, 2007). In related studies in sheep, a shift in neural cell proliferation was found in sheep during pregnancy with an apparent down-regulation present in the subventricular zone, dentate gyrus and the main olfactory bulb around the time of parturition (Brus et al., 2010). These authors suggest that these changes in cell proliferation may participate in the facilitation of olfactory memory associated with the birth of young. Moreover, they proposed that the new neurons identified in the hippocampus 3 months postpartum may function to maintain maternal care during this developmental period and help promote offspring survival (Brus et al., 2013).
Figure 11.
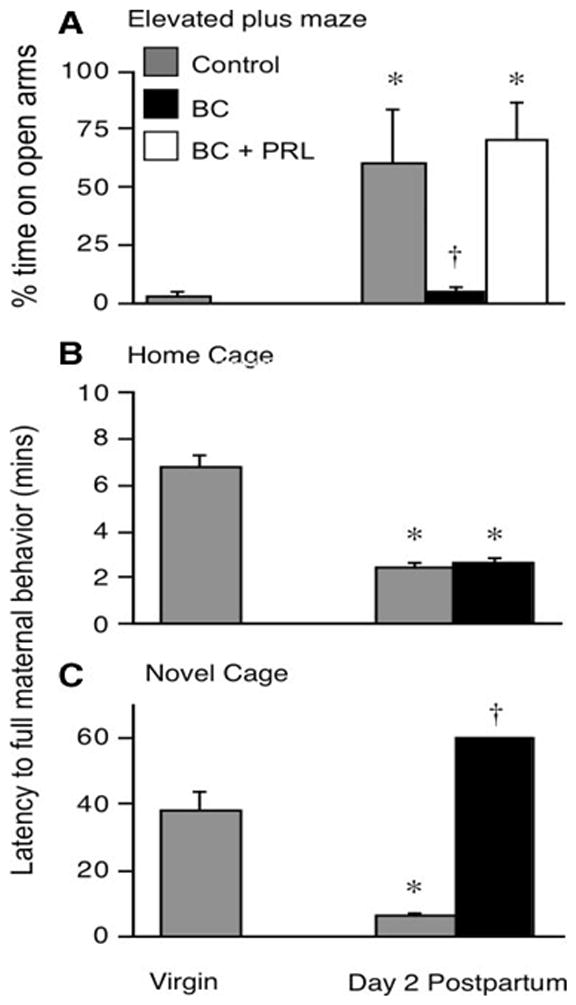
Suppression of prolactin during early pregnancy was associated with subsequent postpartum anxiety and impaired maternal behavior. Mice treated with bromocriptine (BC) during early pregnancy spent less time in the open arms of the elevated plus maze, reflecting increased anxiety (panel A). This effect was reversed with concurrent prolactin treatment during early pregnancy. When tested in both the home cage (panel B) and a novel environment (panel C) differences in maternal care emerged in novel cage testing. Mice treated with BC during early pregnancy displayed impaired maternal care when compared with non-BC treated subjects. Taken from Larsen, C.M., Grattan, D.R., 2010. Prolactin-induced mitogenesis in the subventricular zone of the maternal brain during early pregnancy is essential for normal postpartum behavioral responses in the mother. Endocrinology 151, 3805–3814, with permission from The Endocrine Society.
6. Neuroendocrine Aspects of Human Mothering
The results of animal studies have in part led to the evaluation of the possible involvement of hormones and presumably the neuroendocrine system in maternal care in humans. Putative candidates examined in the context of maternal attitude, depression and attachment have focused upon hormones with known roles in non-human animals as well as related molecules. Specifically, Fleming’s group examined the relationships between both the steroid hormones, estradiol and progesterone, and cortisol in maternal affect (Fleming et al., 1997a; Fleming et al., 1997b; Barrett & Fleming, 2011). Whereas, in their initial study, pregnancy hormones were not related to the growth of attachment to the infant across pregnancy, the pattern of change in the ratio of estradiol to progesterone from early to late gestation reflected postpartum attachment feelings. The hormonal state was found to relate to the mothers’ mood state. Interestingly, mothers with high estradiol to progesterone ratios exhibited more depression and anxiety. Postpartum mothers with low estrogen to progesterone ratios displayed high feelings of attachment. Moreover, mothers who showed an increase in the estrogen to progesterone ratio from the fifth and seven months to the ninth month of pregnancy had higher attachment feelings than those with smaller or no changes in ratios. These findings indicate that the relative concentrations of these two key steroid hormones affect maternal care in a context and species specific manner.
In a second study (Fleming et al., 1997b), the possible relationship between cortisol and maternal responsiveness was examined in women. It is noted that animal studies had not identified a central involvement of corticoids in maternal care. However, when salivary samples from human mothers were measured together with a battery of behavioral tests, it was shown that first-time, new mothers with higher cortisol levels were more attracted to their own baby’s body odor and able to recognize their own infants’ odors. Correlations were not found between testosterone or progesterone levels in maternal saliva and the mother’s ability to identify infant odors. Hence, presumed activation of the hypothalamo-pituitary adrenal (HPA) axis, a system required for milk production, appears to play a role in this aspect of maternal care and infant attachment.
More recent studies have explored the possible role of oxytocin in maternal attachment in women (Levine et al., 2007; Feldman et al., 2010; Feldman, 2012). Non-primate animal studies have provided a substantive basis for the exploration of a possible relationship between oxytocin and social/maternal care in women (see recent review; Rilling & Young, 2014). Feldman and colleagues (2010) examined the relationship between plasma and salivary oxytocin and affectionate contact displayed towards their infants. It was found that human mothers who provided high levels of affectionate contact showed an oxytocin increase following mother-infant interactions, but no such increase was detected in mothers showing low levels of affectionate contact. As noted by Feldman (2012), research on the role of OT in maternal care relies on peripheral assessments of OT in plasma, saliva, urine and CSF, molecular genetic correlates that assess variations in the oxytocin receptor gene, and intranasal administration of OT to parents. Comparisons of plasma OT in pregnant versus single women indicated that plasma OT was higher in pregnant women during the proposed time of maternal bond formation (Gordon et al., 2008). Moreover, the amount of OT during the first trimester of pregnancy was able to predict the extent of postpartum maternal care, suggesting that an extended duration of OT exposure during pregnancy may help set up enhanced maternal care postpartum. Genetic analyses in association with behavioral assessments have also shed light on the possible role of OXTR in maternal attachment. Reduced plasma OT and risk alleles on the OXTR and CD38 genes were associated with reduced frequencies of parental affectionate touch (Feldman, 2012). Efforts to directly determine the effects of OT treatment on maternal mood state have mostly been conducted in males (Gordon et al., 2010; Feldman, 2012; Weisman et al., 2013). A recent imaging study in women, however, reported that intranasal oxytocin treatment in response to infant crying produced an activation in the amygdala, a neural region associated with anxiety an aversion as well as implicated in the neural mediation of maternal care in rodents (Fleming et al., 1980), and increased fMRI activation in brain areas associated with empathy (Riem et al., 2011).
It is worth noting that each of these methodological approaches, while providing potential supportive evidence for a role for OT in mothering, remain limited due to the lack of direct physiological causal evidence. However, when combined, these approaches are able to provide supportive evidence for a role for OT in aspects of maternal care. As in much of research, direct causality is the standard for demonstrating a definitive role of any molecule or gene in an established discrete, behavioral response.
Future studies of oxytocin as well as other candidate molecules are needed to unravel the neurobiological basis of human parental behavior and its associated dysfunctional states, such as postpartum depression. Progress during the past decades has provided a substantive basis for elucidation of such states and possible treatments. However, it will be crucial to understand key regulators of candidate molecules in order to view the neuroendocrine regulation of maternal care as an integrated and multifactorial system. There likely is not a single “key” substance that accounts for this complex behavior. Rather there are most likely multiple players in this interactive system.
7. Summary and Perspective
Our present understanding of the relationships between the brain and endocrine system in the regulation of maternal care has made significant advances during the past two decades. Now that involvements for specific hormones and transmitters, e.g., estrogen, prolactin, oxytocin, have been firmly established, future challenges include identification of the interactions of candidate regulators of maternal care over time and experience and a more complete delineation of a maternal neural network. Both developmental and epigenetic studies will assist in fostering an understanding of those factors that bring about both adaptive and maladaptive maternal responses at a given time in the female’s life history. By understanding normative aspects of the neuroendocrine and endocrine-neural regulation of maternal care, a better comprehension of the role of identified systems underlying conditions such as postpartum blues and depression should emerge. Use of animal models in understanding the neurobiology of maternal care will also identify neural systems that more generally mediate affiliative responses and help in the evaluation of the translational relevance of such models. A comprehension of the biological commonalities of processes that regulate maternal care among female mammals and affiliative-related disorders, such as post-partum depression, autism, and drug addiction should lead to a better understanding of the evolutionary foundation of affiliative responses more broadly.
Whereas a completely comprehensive depiction of a neuroendocrine-neural network model is indeed complicated, one representation of the relationships between key players in experience-based modifications in maternal care has emerged that is shown here (see Figure 12). As noted earlier, maternal experience brings about enhancements of maternal care well into later adulthood independent of an ongoing postpartum state. It is proposed here that two key sets of neurochemical/neuroendocrine changes occur within the MPOA, a central link in the maternal neural network (Numan 2012), and in the NA, a key neural site that mediates social reward (Dölen et al., 2013). In the MPOA, the changes include increased expression of the prolactin receptor and its signal transduction pathway (STAT5b) and an increase in neural sensitivity to PRL stimulation (Anderson et al., 2006). Maternal care is also associated with an increase in the expression of the estrogen receptor promoter ESR1-1b along with its ability to bind STAT5b that stimulates this promoter (Champagne et al., 2006), and an actual increase in MPOA neurons that contain ERS1 (Byrnes et al., 2009). These changes may render the female more sensitive to estrogen which can stimulate maternal care (Siegel and Rosenblatt, 1975a, 1975b; Fahrbach and Pfaff, 1986; Ribeiro et al., 2012). Likewise, the neural activity of the NA may become modified by social reward (Dölen et al, 2013) through serotoninergic inputs from the dorsal raphe that synapse on NA oxytocin neurons. Hence, this key social reward center which likely is stimulated both by experience and subsequent suckling stimuli provide an important motivational link for the concurrent activation of the MPOA. The involvement of NA dopamine may also be a candidate for experientially-induced neuroplasticity as the DA D1 receptor has been shown to mediate the initial induction of maternal care in postpartum mother rats (Stozenberg et al., 2007) and may be modified by experience. The developmental interplay between experience and the neuroendocrine mediation of maternal care is a crucial perspective that must be considered when addressing the regulation of maternal behavior. Integration of these considerations will assist in providing a clearer understanding of the neural, neuroendocrine and endocrine interplay in this behavior that is critical for mammalian parenting and species survival.
Figure 12.
Model depicting key neural and neuroendocrine relationships associated with experience-enhanced maternal care. Key modifications occur within the MPOA and NA as a function of prior maternal experience which result in enhanced responsiveness to pups. These changes include increased expression of the PRL-R, STAT5B and ESR1 1b promoter within the MPOA that result in increased activity and responsiveness of the ESR1 system which in turn increases the saliency of sensory stimuli. Likewise, there appears to be an increase in the social reward value of stimuli from young mediated by the NA oxytocinergic and dopaminergic systems. It is hypothesized that this change in social reward results in part from an increase in serotoninergic activity on oxytocin neurons in the NA. Interestingly, the activity within these systems in the MPOA and NA are both affected by suckling stimuli via the suckling afferent pathway (Tindal & Knaggs, 1977). Suckling stimuli are processed through the dorsal raphe by serotonergic inputs to the NA (Dölen et al., 2013) and indirectly through suckling afferents via a thalamic relay to the MPOA (Cservenak et al., 2010) that also increase prolactin release. Together, it is postulated that these experience-induced changes in neurochemical sensitivity render the maternal neural network more sensitive to neurotropic stimulation of maternal care by the lactogenic and estrogen receptor systems.
Highlights.
The onset of maternal care at birth results from the interactions of multiple hormones and central neurochemicals.
Ongoing maternal care is more dependent of the mother’s neurobiological status than her endocrine state.
The maternal brain is plastic, undergoing numerous changes as a result of prior maternal experience.
Animal studies of maternal and affiliative behaviors have identified neural systems that appear common to all mammals.
Perturbations of maternal neural systems may account for postpartum mood disorders that impact effective maternal care.
Acknowledgments
Thanks are extended to Dr. Phyllis Mann for assistance in preparation of the figures and editorial comments. This contribution resulted in part from the long term support of research by the National Institute of Child Health and Human Development at the NIH, specifically PHS grants HD 019789 and HD 039895 awarded to RSB.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akbari EM, Shams S, Belay HT, Kaiguo M, Razak Z, Kent CF, Westwood T, Sokolowski MB, Fleming AS. The effects of parity and maternal behavior on gene expression in the medial preoptic area and the medial amygdala in postpartum and virgin female rats: a microarray study. Behavioral Neuroscience. 2013;127:913–922. doi: 10.1037/a0034884. [DOI] [PubMed] [Google Scholar]
- Alenina N, Kikic D, Todiras M, Mosienko V, Qadri F, Plehm R, Boye P, Villanovitch L, Sohr R, Tenner K, Hortnagl H, Bader M. Growth retardation and altered autonomic control in mice lacking brain serotonin. Proceedings of the National Academy of Science USA. 2009;106:10332–10337. doi: 10.1073/pnas.0810793106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson GM, Grattan DR, van der Ancker W, Bridges RS. Reproductive experience increases prolactin responsiveness in the medial preoptic area and arcuate nucleus of female rats. Endocrinology. 2006;147:4688–4694. doi: 10.1210/en.2006-0600. [DOI] [PubMed] [Google Scholar]
- Augustine RA, Kokay IC, Andrews ZB, Ladyman SR, Grattan DR. Quantitation of prolactin receptor mRNA in the maternal rat brain during pregnancy and lactation. Journal of Molecular Endocrinology. 2003;31:221–232. doi: 10.1677/jme.0.0310221. [DOI] [PubMed] [Google Scholar]
- Baum MJ. Failure of pituitary implants to facilitate the onset of maternal behavior in ovariectomized virgin rats. Physiology and Behavior. 1978;20:87–90. doi: 10.1016/0031-9384(78)90207-x. [DOI] [PubMed] [Google Scholar]
- Barofsky AL, Taylor J, Massari VJ. Dorsal raphe-hypothalamic projections provide the stimulatory serotonergic input to suckling-induced prolactin release. Endocrinology. 1983a;113:1894–1903. doi: 10.1210/endo-113-5-1894. [DOI] [PubMed] [Google Scholar]
- Barofsky AL, Taylor J, Tizabi Y, Kumar R, Jones-Quartey K. Specific neurotoxin lesions of median raphe serotonergic neurons disrupt maternal behavior in the lactating rat. Endocrinology. 1983b;113:1884–1893. doi: 10.1210/endo-113-5-1884. [DOI] [PubMed] [Google Scholar]
- Barrett J, Fleming AS. Annual research review: All mothers are not created equal: neural and psychobiological perspectives on mothering and the importance of individual differences. Journal of Child Psychology and Psychiatry. 2011;52:368–397. doi: 10.1111/j.1469-7610.2010.02306.x. [DOI] [PubMed] [Google Scholar]
- Beach FA, Wilson JR. Effects of prolactin, progesterone and estrogen on reactions of nonpregnant rats to foster young. Psychological Reports. 1963;13:231–239. [Google Scholar]
- Berridge KC, Robinson TE. What is the role of dopamine in reward: Hedonic impact, reward learning, or incentive salience? Brain Research Reviews. 1998;28:309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- Bosch OJ, Oliver J, Neumann ID. Brain vasopressin is an important regulator of maternal behavior independent of dams’ trait anxiety. Proceedings of the National Academy of Sciences USA. 2008;105:17139–17144. doi: 10.1073/pnas.0807412105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch OJ, Neumann ID. Both oxytocin and vasopressin are mediators of maternal care and aggression in rodents: from central release to sites of action. Hormones and Behavior. 2012;61:293–303. doi: 10.1016/j.yhbeh.2011.11.002. [DOI] [PubMed] [Google Scholar]
- Bridges RS. Long-term effects of pregnancy and parturition upon maternal responsiveness in the rat. Physiology and Behavior. 1975;14:245–249. doi: 10.1016/0031-9384(75)90028-1. [DOI] [PubMed] [Google Scholar]
- Bridges RS. Parturition: its role in the long-term retention of maternal behavior in the rat. Physiology and Behavior. 1977;18:487–490. [Google Scholar]
- Bridges RS. A quantitative analysis of the roles of dosage, sequence, and duration of estradiol and progesterone exposure in the regulation of maternal behavior in the rat. Endocrinology. 1984;114:930–940. doi: 10.1210/endo-114-3-930. [DOI] [PubMed] [Google Scholar]
- Bridges RS, Byrnes EM. Neuroendocrinology in Physiology and Medicine. The Humana Press, Inc; Totawa, NJ: 1999. Neuroendocrine regulation of maternal behavior; pp. 301–315. [Google Scholar]
- Bridges RS, Clifton DK, Sawyer CH. Postpartum luteinizing hormone release and maternal behavior in the rat after late-gestational depletion of hypothalamic norepinephrine. Neuroendocrinology. 1982;34:286–291. doi: 10.1159/000123314. [DOI] [PubMed] [Google Scholar]
- Bridges RS, DiBiase R, Loundes DD, Doherty PC. Prolactin stimulation of maternal behavior in female rats. Science. 1985;227:782–784. doi: 10.1126/science.3969568. [DOI] [PubMed] [Google Scholar]
- Bridges RS, Grimm CT. Reversal of morphine disruption of maternal behavior by concurrent treatment with the opiate antagonist naloxone. Science. 1982;218:166–168. doi: 10.1126/science.7123227. [DOI] [PubMed] [Google Scholar]
- Bridges RS, Hays LE. Steroid-induced alterations in mRNA expression of the long-form of the prolactin receptor in the medial preoptic area of female rats: effects of exposure to a pregnancy-like regimen of progesterone and estradiol. Molecular Brain Research. 2005;140:10–16. doi: 10.1016/j.molbrainres.2005.06.011. [DOI] [PubMed] [Google Scholar]
- Bridges RS, Mann PE, Coppeta JS. Hypothalamic involvement in the regulation of maternal behavior in the rat: inhibitory roles for the ventromedial, dorsal and anterior hypothalamic areas. J Neuroendocrinology. 1999;11:259–266. doi: 10.1046/j.1365-2826.1999.00322.x. [DOI] [PubMed] [Google Scholar]
- Bridges RS, Numan M, Ronsheim PM, Mann PE, Lupini CE. Central prolactin infusions stimulate maternal behavior in steroid-treated, nulliparous female rats. Proc Natl Acad Sci USA. 1990;87(20):8003–8007. doi: 10.1073/pnas.87.20.8003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges RS, Rigero BA, Byrnes EM, Yang L, Walker AM. Central infusions of the recombinant human prolactin antagonist, S179D-PRL, delay the onset of maternal behavior in steroid-primed, nulliparous female rats. Endocrinology. 2001;142:730–739. doi: 10.1210/endo.142.2.7931. [DOI] [PubMed] [Google Scholar]
- Bridges RS, Robertson MC, Shiu RPC, Friesen HG, Stuer AM, Mann PE. Endocrine communication between conceptus and mother: placental lactogen stimulation of maternal behavior. Neuroendocrinology. 1996;64:57–64. doi: 10.1159/000127098. [DOI] [PubMed] [Google Scholar]
- Bridges RS, Ronsheim PM. Prolactin (PRL) regulation of maternal behavior in rats: bromocriptine treatment delays and PRL promotes the rapid onset of behavior. Endocrinology. 1990;126:837–848. doi: 10.1210/endo-126-2-837. [DOI] [PubMed] [Google Scholar]
- Bridges RS, Rosenblatt JS, Feder HH. Serum progesterone concentrations and maternal behavior in rats after pregnancy termination: behavioral stimulation following progesterone withdrawal and inhibition by progesterone treatment. Endocrinology. 1978;102:258–267. doi: 10.1210/endo-102-1-258. [DOI] [PubMed] [Google Scholar]
- Bridges RS, Scanlan VF. Maternal memory in adult, nulliparous rats: effects of testing interval on the retention of maternal behavior. Developmental Psychobiology. 2005;14:13–18. doi: 10.1002/dev.20038. [DOI] [PubMed] [Google Scholar]
- Bridges RS, Zarrow MX, Goldman BD, Denenberg VH. A developmental study of maternal responsiveness in the rat. Physiology and Behavior. 1974;12:149–151. doi: 10.1016/0031-9384(74)90082-1. [DOI] [PubMed] [Google Scholar]
- Bridges RS, Zarrow MX, Goldman BD, Denenberg VH. The role of neonatal androgen in the expression of hormonally induced maternal responsiveness in the rat. Hormones and Behavior. 1973;4:315–322. [Google Scholar]
- Brown JR, Ye H, Bronson RT, Dikkes P, Greenberg ME. A defect in nurturing in mice lacking the immediate early gene fosB. Cell. 1996;86:297–309. doi: 10.1016/s0092-8674(00)80101-4. [DOI] [PubMed] [Google Scholar]
- Brus M, Meurisse M, Franceschini I, Keller M, Lévy F. Evidence for cell proliferation in the sheep brain and its down-regulation by parturition and interactions with the young. Hormones and Behavior. 2010;58:737–746. doi: 10.1016/j.yhbeh.2010.07.006. [DOI] [PubMed] [Google Scholar]
- Brus M, Meurisse M, Gheusi G, Keller M, Liedo PM, Lévy F. Dynamics of olfactory and hippocampal neurogenesis in adult sheep. Journal of Comparative Neurology. 2013;52:169–188. doi: 10.1002/cne.23169. [DOI] [PubMed] [Google Scholar]
- Buntin JD. Neural and hormonal control of parental behavior in birds. Advances in the Study of Behavior. 1996;25:161–213. [Google Scholar]
- Byrnes EM, Babb JA, Bridges RS. Differential expression of oestrogen receptor α following reproductive experience in young and middle-aged female rats. Journal of Neuroendocrinology. 2009;21:550–557. doi: 10.1111/j.1365-2826.2009.01874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrnes EM, Byrnes JJ, Bridges RS. Increased sensitivity of dopamine systems following reproductive experience in rats. Pharmacology, Biochemistry and Behavior. 2001;68:481–489. doi: 10.1016/s0091-3057(01)00449-x. [DOI] [PubMed] [Google Scholar]
- Byrnes EM, Rigero BA, Bridges RS. Opioid receptor antagonism during early lactation results in the increased duration of nursing bouts. Physiology & Behavior. 2000;70:211–216. doi: 10.1016/s0031-9384(00)00231-6. [DOI] [PubMed] [Google Scholar]
- Byrnes EM, Rigero BA, Bridges RS. Dopamine antagonists during parturition disrupt maternal care and the retention of maternal behavior in rats. Pharmacology, Biochemistry and Behavior. 2002;73:869–875. doi: 10.1016/s0091-3057(02)00941-3. [DOI] [PubMed] [Google Scholar]
- Byrnes JJ, Gleason ED, Schoen MF, Carini L, Lovelock DF, Byrnes EM, Bridges RS. Accelerated maternal responding after intra-VTA pertussis toxin treatment. Behavioural Brain Research. 2011;223:322–328. doi: 10.1016/j.bbr.2011.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne F. Interplay between social experiences and the genome: epigenetic consequences for behavior. Advances in Genetics. 2012;77:33–57. doi: 10.1016/B978-0-12-387687-4.00002-7. [DOI] [PubMed] [Google Scholar]
- Champagne F, Chretien P, Stevenson CW, Zhang TY, Gratton A, Meaney MJ. Variations in nucleus accumbens dopamine associated with individual differences in maternal behavior in the rat. Journal of Neuroscience. 2004;24:4113–4123. doi: 10.1523/JNEUROSCI.5322-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne F, Diorio J, Sharma S, Meaney MJ. Variations in maternal care in the rat are associated with differences in estrogen-related changes in oxytocin receptor levels. Proceedings of the National Academy fo Sciences USA. 2001;98:12736–12741. doi: 10.1073/pnas.221224598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne F, Weaver ICG, Diorio J, Dymov S, Szyf M, Meaney M. Maternal care associated with methylation of the estrogen receptor-α1b promoter and estrogen receptor-α expression in the medial preoptic area of female offspring. Endocrinology. 2006;147:2909–2915. doi: 10.1210/en.2005-1119. [DOI] [PubMed] [Google Scholar]
- Champagne F, Weaver ICG, Diorio J, Sharma S, Meaney M. Natural variations in maternal care are associated with estrogen receptor α expression and estrogen sensitivity in the medial preoptic area. Endocrinology. 2003;144:4720–4724. doi: 10.1210/en.2003-0564. [DOI] [PubMed] [Google Scholar]
- Cohen J, Bridges RS. Retention of maternal behavior in nulliparous and primiparous rats: effects of duration of previous maternal experience. Journal of Comparative and Physiological Psychology. 1981;95:450–459. [Google Scholar]
- Cruz AM, Maiorka PC, Canteras NS, Sukikara MH, Felicio LF. Morphine treatment during pregnancy modulates behavioral selection in lactating rats. Physiology and Behavior. 2010;101:40–44. doi: 10.1016/j.physbeh.2010.04.013. [DOI] [PubMed] [Google Scholar]
- Cservenak M, Bodnar I, Usdin TB, Palkovits M, Nagy GM, Dobolyi A. Tuberoinfundibular peptide of 39 residues is activated during lactation and participates in the suckling-induced prolactin release in rat. Endocrinology. 2010;151:5830–5840. doi: 10.1210/en.2010-0767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cservenak M, Szabo ER, Bodnar I, Leko A, Palkovits M, Nagy GM, Usdin TB, Dobolyi A. Thalamic neuropeptide mediating the effects of nursing on lactation and maternal motivation. Psychoneuroendocrinology. 2013 doi: 10.1016/j.psyneuen.2013.09.004. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dölen G, Darvishzadeh A, Huang KW, Malenka RC. Social reward requires coordinated activity of nucleus accumbens oxytocin and serotonin. Nature. 2013;501:179–186. doi: 10.1038/nature12518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorshkind E, Horseman ND. The roles of prolactin, growth hormone, insulin-like growth factor-I, and thyroid hormones in lymphocyte development and function: insights from genetic models of hormone and hormone receptor deficiency. Endocrine Reviews. 2000;21:292–312. doi: 10.1210/edrv.21.3.0397. [DOI] [PubMed] [Google Scholar]
- Erskine MS, Barfield RJ, Goldman BD. Intraspecific fighting during late pregnancy and lactation in rats and effects of litter removal. Behavioral Biology. 1978;23:206–218. doi: 10.1016/s0091-6773(78)91814-x. [DOI] [PubMed] [Google Scholar]
- Erskine MS, Barfield RJ, Goldman BD. Postpartum aggression in rats: I. Effects of hypophysectomy. Journal of Comparative and Physiological Psychology. 1980;94:484–494. doi: 10.1037/h0077686. [DOI] [PubMed] [Google Scholar]
- Fahrbach SE, Morrell JI, Pfaff DW. Possible role of endogenous oxytocin in estrogen-facilitated maternal behavior in rats. Neuroendocrinology. 1985;40:526–532. doi: 10.1159/000124125. [DOI] [PubMed] [Google Scholar]
- Fahrbach SE, Pfaff DW. Effect of preoptic region implants of dilute estradiol on the maternal behavior of ovariectomized, nulliparous rats. Hormones and Behavior. 1986;20:354–363. doi: 10.1016/0018-506x(86)90043-7. [DOI] [PubMed] [Google Scholar]
- Featherstone RE, Fleming AS. Plasticity in the maternal circuit: effects of experience and partum condition on brain astrocyte number in female rats. Behavioral Neuroscience. 2000;114:158–172. doi: 10.1037//0735-7044.114.1.158. [DOI] [PubMed] [Google Scholar]
- Feldman R. Oxytocin and social affiliation in humans. Hormones and Behavior. 2012;61:380–391. doi: 10.1016/j.yhbeh.2012.01.008. [DOI] [PubMed] [Google Scholar]
- Feldman R, Gordon I, Schneiderman I, Weisman O, Zagoory-Sharon O. Natural variations in maternal and paternal care are associated with systematic changes in oxytocin following parent-infant contact. Psychoneuroendocrinology. 2010;35:1133–1141. doi: 10.1016/j.psyneuen.2010.01.013. [DOI] [PubMed] [Google Scholar]
- Fleming AS, Cheung US, Barry M. Cycloheximide blocks the retention of maternal experience in postpartum rats. Behavioral and Neural Biology. 1990;53:64–73. doi: 10.1016/0163-1047(90)90814-m. [DOI] [PubMed] [Google Scholar]
- Fleming AS, Ruble D, Krieger H, Wong PY. Hormonal and experiential correlates of maternal responsiveness during pregnancy and the puerperium in human mothers. Hormones and Behavior. 1997a;31:145–158. doi: 10.1006/hbeh.1997.1376. [DOI] [PubMed] [Google Scholar]
- Fleming AS, Steiner M, Corter C. Cortisol, hedonics, and maternal responsiveness in human mothers. Hormones and Behavior. 1997b;32:85–98. doi: 10.1006/hbeh.1997.1407. [DOI] [PubMed] [Google Scholar]
- Fleming AS, Vaccarino F, Luebke C. Amygdaloid inhibition of maternal behavior in the nulliparous female rat. Physiology and Behavior. 1980;25:731–743. doi: 10.1016/0031-9384(80)90377-7. [DOI] [PubMed] [Google Scholar]
- Francis DD, Diorio J, Liu D, Meaney MJ. Nongenomic transmission across generations in maternal behavior and stress responses in the rat. Science. 1999;286:1155–1158. doi: 10.1126/science.286.5442.1155. [DOI] [PubMed] [Google Scholar]
- Freemark M, Driscoll P, Andrews J, Kelly PA, Boyster M. Ontogenesis of prolactin receptor gene expression in the rat olfactory system: potential roles for lactogenic hormones in olfactory development. Endocrinology. 1996;137:934–942. doi: 10.1210/endo.137.3.8603606. [DOI] [PubMed] [Google Scholar]
- Furuta M, Bridges RS. Effects of maternal behavior induction and pup exposure on neurogenesis in adult, virgin female rats. Brain Research Bulletin. 2009;80:408–413. doi: 10.1016/j.brainresbull.2009.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gammie SC, Hasen NS, Stevenson SA, Bale BL, D’Anna KD. Elevated stress sensitivity in corticotropin-releasing factor receptor 2 deficient mice decreases maternal, but not intermale aggression. Behavioral Brain Research. 2005;160:169–177. doi: 10.1016/j.bbr.2004.11.026. [DOI] [PubMed] [Google Scholar]
- Gammie SC, Negron A, Newman SM, Rhodes JS. Corticotropin-releasing factor inhibits maternal aggression in mice. Behavioral Neuroscience. 2004;118:805–814. doi: 10.1037/0735-7044.118.4.805. [DOI] [PubMed] [Google Scholar]
- Gammie SC, Nelson RJ. Maternal aggression is reduced in neuronal nitric oxide synthase-deficient mice. Journal of Neuroscience. 1999;19:8027–8035. doi: 10.1523/JNEUROSCI.19-18-08027.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano AL, Johnson AE, Rosenblatt JS. Haloperidol-induced disruptions of retrieval behavior and reversal with apomorphine in lactating rats. Physiology and Behavior. 1990;48:211–214. doi: 10.1016/0031-9384(90)90288-f. [DOI] [PubMed] [Google Scholar]
- Gordon I, Zagoory-Sharon O, Leckman JF, Felman R. Oxytocin and the development of parenting in humans. Biological Psychiatry. 2010;68:377–382. doi: 10.1016/j.biopsych.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon I, Zagoory-Sharon O, Schneiderman I, Leckman JF, Weller A, Feldman R. Oxytocin and cortisol in romantically unattached young adults; associations with bonding and psychological distress. Psychophysiology. 2008;45:349–352. doi: 10.1111/j.1469-8986.2008.00649.x. [DOI] [PubMed] [Google Scholar]
- Hansen S, Harthon C, Wallin E, Lofberg L, Svensson K. The effects of 6-OHDA-induced dopamine depletions in the ventral and dorsal striatum on maternal and sexual behavior in the female rat. Pharmacology, Bioochemistry and Behavior. 1991;39:71–79. doi: 10.1016/0091-3057(91)90399-m. [DOI] [PubMed] [Google Scholar]
- Hansen S, Ferreira A. Effects of bicuculline infusions in the ventromedial hypothalamus and amygdaloid complex on food intake and affective behavior in mother rats. Behavioral Neuroscience. 1986;100:410–415. doi: 10.1037//0735-7044.100.3.410. [DOI] [PubMed] [Google Scholar]
- Horton TH, Schneider JS, Jiminez MA, Levine JE. Fathers behaving badly: the role of progesterone receptors. In: Bridges RS, editor. Neurobiology of the Parental Brain. Academic Press; New York, NY: 2008. pp. 449–460. [Google Scholar]
- Horseman ND, Zhao W, Montecino-Rodriguez E, Tanaka M, Nakashima K, Engle SJ, Smith F, Markoff E, Dorshkind K. Defective mammopoiesis, but normal hematopoiesis, in mice with a targeted disruption of the prolactin gene. The EMBO Journal. 1997;16:6926–6935. doi: 10.1093/emboj/16.23.6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin D, Liu HX, Hirai H, Torashima T, Nagai T, Lopatina O, Shnayder NA, Yamada K, Noda M, Seike T, Fujita K, Takasawa S, Yokoyama S, Koizumi K, Shiraishi Y, Tanaka S, Hashii M, Yoshihara T, Higashida K, Islam MS, Yamada N, Hayashi K, Noguchi N, Kato I, Okamoto H, Matsushima A, Salmina A, Munesue T, Shimizu N, Mochida S, Asano M, Higashida H. CD 38 is critical for social behaviour by regulating oxytocin secretion. Nature. 2007;446:41–45. doi: 10.1038/nature05526. [DOI] [PubMed] [Google Scholar]
- Kessler MS, Bosch OJ, Bunck M, Landgraf R, Neumann ID. Maternal care differs in mice bred for high vs. low trait anxiety: impact of brain vasopressin and cross-fostering. Social Neuroscience. 2011;6:156–168. doi: 10.1080/17470919.2010.495567. [DOI] [PubMed] [Google Scholar]
- Kinsley CH, Miele J, Ghiraldi L, Konen C, Svare B. Intrauterine position modulates maternal behaviors in female mice. Physiology and Behavior. 1986;36:793–799. doi: 10.1016/0031-9384(86)90434-8. [DOI] [PubMed] [Google Scholar]
- Kokay IC, Bull PM, Davis RL, Ludwig M, Grattan DR. Expression of the long form of the prolactin receptor in magnocellular oxytocin neurons is associated with specific prolactin regulation of oxytocin neurons. Am J Regul Integr Comp Physiol. 2006;290:1216–1225. doi: 10.1152/ajpregu.00730.2005. [DOI] [PubMed] [Google Scholar]
- Kuroda KO, Tachikawa K, Yoshida S, Tsuneoka Y, Numan M. Neuromolecular basis of parental behavior in laboratory mice and rats: With special emphasis on technical issues of using mouse genetics. Neuro-Psychopharmacology and Biological Psychiatry. 2011;35:1205–1231. doi: 10.1016/j.pnpbp.2011.02.008. [DOI] [PubMed] [Google Scholar]
- Ladyman SR, Grattan DR. Suppression of leptin receptor messenger ribonucleic acid and leptin responsiveness in the ventromedial nucleus of the hypothalamus during pregnancy in the rat. Endocrinology. 2005;146:3868–3874. doi: 10.1210/en.2005-0194. [DOI] [PubMed] [Google Scholar]
- Larsen CM, Grattan DR. Prolactin-induced mitogenesis in the subventricular zone of the maternal brain during early pregnancy is essential for normal postpartum behavioral responses in the mother. Endocrinology. 2010;151:3805–3814. doi: 10.1210/en.2009-1385. [DOI] [PubMed] [Google Scholar]
- Lee A, Li M, Watchus J, Fleming AS. Neuroanatomical basis of maternal memory in postpartum rats: selective role for the nucleus accumbens. Behavioral Neuroscience. 1999;113:523–538. doi: 10.1037//0735-7044.113.3.523. [DOI] [PubMed] [Google Scholar]
- Levine A, Zagoory-Sharon O, Feldman R, Weller A. Oxytocin during pregnancy and early postpartum: individual patterns and maternal-fetal attachment. Peptides. 2007;28:1162–1169. doi: 10.1016/j.peptides.2007.04.016. [DOI] [PubMed] [Google Scholar]
- Lévy F, Gervais R, Kinderman U, Orgeur P. Importance of β-noradrenergic receptors in the olfactory bulb of sheep for recognition of lambs. Behavioral Neuroscience. 1990;104:464–469. doi: 10.1037//0735-7044.104.3.464. [DOI] [PubMed] [Google Scholar]
- Lévy F, Guevara-Guzman R, Hinton MR, Kendrick KM, Keverne EB. Effects of parturition and maternal experience on noradrenaline and acetylcholine release in the olfactory bulb of sheep. Behavioral Neuroscience. 1993;107:662–668. doi: 10.1037//0735-7044.107.4.662. [DOI] [PubMed] [Google Scholar]
- Lévy F, Poindron P. The importance of amniotic fluids for the establishment of maternal behaviour in experienced and non-experienced ewes. Animal Behaviour. 1987;35:1188–1192. [Google Scholar]
- Li M, Fleming AS. The nucleus accumbens shell is critical for normal expression of pup-retrieval in postpartum female rats. Behavioural Brain Research. 2003a;145:99–111. doi: 10.1016/s0166-4328(03)00135-9. [DOI] [PubMed] [Google Scholar]
- Li M, Fleming AS. Differential involvement of nucleus accumbens shell and core subregions in maternal memory in postpartum female rats. Behavioral Neuroscience. 2003b;117:426–445. doi: 10.1037/0735-7044.117.3.426. [DOI] [PubMed] [Google Scholar]
- Lonstein JS. Regulation of anxiety during the postpartum period. Frontiers in Neuroendocrinology. 2007;28:115–141. doi: 10.1016/j.yfrne.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Lonstein JS, Gammie SC. Sensory, hormonal, and neural control of maternal aggression in laboratory rodents. Neuroscience and Biobehavioral Reviews. 2002;26:869–888. doi: 10.1016/s0149-7634(02)00087-8. [DOI] [PubMed] [Google Scholar]
- Lott DL, Fuchs SS. Failure to induce retrieving by sensitization or the injection of prolactin. Journal of Comparative and Physiological Psychology. 1962;65:111–113. [Google Scholar]
- Lucas BK, Ormandy CJ, Binart N, Bridges RS, Kelly PA. Null mutation of the prolactin receptor gene produces a defect in maternal behavior. Endocrinology. 1998;139:4102–4107. doi: 10.1210/endo.139.10.6243. [DOI] [PubMed] [Google Scholar]
- Lydon JP, DeMayo FJ, Funk CR, Mani SK, Hughes AR, Montgomery CA, Jr, Shyamala G, Conneely OM, O’Malley BW. Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes and Development. 1995;9:2266–2278. doi: 10.1101/gad.9.18.2266. [DOI] [PubMed] [Google Scholar]
- Mani SK, Blaustein JD, O’Malley BW. Progesterone receptor function from a behavioral perspective. Hormones and Behavior. 1997;31:244–255. doi: 10.1006/hbeh.1997.1393. [DOI] [PubMed] [Google Scholar]
- Mann PE. Finasteride delays the onset of maternal behavior in primigravid rats. Physiology and Behavior. 2006;88:333–338. doi: 10.1016/j.physbeh.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Mann PE, Bridges RS. Neural and endocrine sensitivities to opioids decline as a function of multiparity in rats. Brain Research. 1992;580:241–248. doi: 10.1016/0006-8993(92)90950-e. [DOI] [PubMed] [Google Scholar]
- Mann PE, Kinsley CH, Bridges RS. Opioid receptor subtype involvement in maternal behavior in lactating rats. Neuroendocrinology. 1991;53:487–492. doi: 10.1159/000125762. [DOI] [PubMed] [Google Scholar]
- Mayer AD, Rosenblatt JS. Prepartum changes in maternal responsiveness and nest-defense in Rattus norvegicus. Journal of Comparative Psychology. 1984;98:177–188. [PubMed] [Google Scholar]
- Meaney MJ. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annual Review of Neuroscience. 2001;24:1161–1192. doi: 10.1146/annurev.neuro.24.1.1161. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Szyf M. Maternal care as a model for experience-dependent chromatin plasticity? Trends in Neuroscience. 2005;28:456–463. doi: 10.1016/j.tins.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Meurisse M, Gonzalez A, Delsol G, Caba M, Levy F, Poindron P. Estradiol receptor-α expression in hypothalamic and limbic regions of ewes is influenced by physiological state and maternal experience. Hormones and Behavior. 2005;48:34–43. doi: 10.1016/j.yhbeh.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Miller SM, Lonstein JS. Dopamine D1 and D2 receptor antagonism in the preoptic area produces different effects on maternal behavior in lactating rats. Behavioral Neuroscience. 2005;119:1072–1083. doi: 10.1037/0735-7044.119.4.1072. [DOI] [PubMed] [Google Scholar]
- Moltz H, Lubin M, Leon M, Numan M. Hormonal induction of maternal behavior in the ovariectomized nulliparous rat. Physiology and Behavior. 1970;5:1373–1377. doi: 10.1016/0031-9384(70)90122-8. [DOI] [PubMed] [Google Scholar]
- Moltz H, Robbins D. Maternal behavior of primiparous and multiparous rats. Journal of Comparative and Physiological Psychology. 1965;60:417–421. doi: 10.1037/h0022565. [DOI] [PubMed] [Google Scholar]
- Moltz H, Robbins D, Parks M. Caesarean delivery and maternal behavior of primiparous and multiparous rats. Journal of Comparative Physiological Psychology. 1966;61:455–460. doi: 10.1037/h0023263. [DOI] [PubMed] [Google Scholar]
- Morice E, Denis C, Giros B, Nosten-Bertrand M. Phenotypic expression of the targeted null-mutation in the dopamine transporter gene varies as a function of the genetic background. European Journal of Neuroscience. 2004;20:120–126. doi: 10.1111/j.1460-9568.2004.03465.x. [DOI] [PubMed] [Google Scholar]
- Morishige WK, Pepe GJ, Rothchild I. Serum luteinizing hormone, prolactin and progesterone levels during pregnancy in the rat. Endocrinology. 1973;92:1527–1530. doi: 10.1210/endo-92-5-1527. [DOI] [PubMed] [Google Scholar]
- Nephew BC, Bridges RS. Arginine vasopressin V1a receptor antagonist impairs maternal memory in rats. Physiology and Behavior. 2008a;95:182–186. doi: 10.1016/j.physbeh.2008.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nephew BC, Bridges RS. Central actions of arginine vasopressin and V1a receptor antagonist on maternal aggression, maternal behavior, and grooming in lactating rats. Pharmacology, Biochemistry and Behavior. 2008b;91:77–83. doi: 10.1016/j.pbb.2008.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nephew BC, Bridges RS, Lovelock DF, Byrnes EM. Enhanced maternal aggression and associated changes in neuropeptide gene expression in multiparous rats. Behavioral Neuroscience. 2009;123:949–957. doi: 10.1037/a0016734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nephew BC, Byrnes EM, Bridges RS. Vasopressin mediates enhanced offspring protection in multiparous rats. Neuropharmacology. 2010;58:102–106. doi: 10.1016/j.neuropharm.2009.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimori K, Young LJ, Guo Q, Wang Z, Insel TR, Matzuk MM. Oxytocin is required for nursing but is not essential for parturition or reproductive behavior. Proceedings of the National Academy of Sciences USA. 1996;93:11699–16704. doi: 10.1073/pnas.93.21.11699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numan M. Medial preoptic area and maternal behavior in the female rat. Journal of Comparative and Physiological Psychology. 1974;87:746–759. doi: 10.1037/h0036974. [DOI] [PubMed] [Google Scholar]
- Numan M. Progesterone inhibition of maternal behavior in the female rat. Journal of Comparative and Physiological Psychology. 1978;87:746–759. doi: 10.1037/h0036974. [DOI] [PubMed] [Google Scholar]
- Numan M. Maternal behavior: neural circuits, stimulus valence, and motivational processes. Parenting, Science and Practice. 2012;12:105–114. [Google Scholar]
- Numan M, Callahan EC. The connections of the medial preoptic region and maternal behavior in the rat. Physiology and Behavior. 1980;25:653–665. doi: 10.1016/0031-9384(80)90367-4. [DOI] [PubMed] [Google Scholar]
- Numan M, Numan MJ, Pliakou N, Stolzenberg DS, Mullins OJ, Murphy JM, Smith CD. The effects of D1 or D2 receptor antagonism in the medial preoptic area, ventral pallidum, or nucleus accumbens on maternal retrieval response and other aspects of maternal behavior in rats. Behavioral Neuroscience. 2005;119:1588–1604. doi: 10.1037/0735-7044.119.6.1588. [DOI] [PubMed] [Google Scholar]
- Numan M, Rosenblatt JS, Komisauruk BR. Medial preoptic area and onset of maternal behavior in the rat. Journal of Comparative Physiological and Psychology. 1977;91:146–164. doi: 10.1037/h0077304. [DOI] [PubMed] [Google Scholar]
- Numan M, Smith HG. Maternal behavior in rats: Evidence for the involvement of preoptic projections to the ventral tegmental area. Behavioral Neuroscience. 1984;98:712–727. doi: 10.1037//0735-7044.98.4.712. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Eng V, Taylor J, Lubahn DB, Korach KS, Pfaff DW. Roles of estrogen receptor-α gene expression in reproduction-related behaviors in female mice. Endocrinology. 1998;139:5070–5081. doi: 10.1210/endo.139.12.6357. [DOI] [PubMed] [Google Scholar]
- Ormandy CJ, Camus A, Barra J, Damotte D, Lucas B, Buteau H, Edery M, Brousse N, Babinet C, Binart N, Kelly PA. Null mutation of the prolactin receptor gene produces multiple reproductive defects in the mouse. Genes and Development. 1997;11:167–178. doi: 10.1101/gad.11.2.167. [DOI] [PubMed] [Google Scholar]
- Panksepp J, Nelson E, Siviy S. Brain opioids and mother-infant social motivation. Acta Paediatrica Supplement. 1994;397:40–46. doi: 10.1111/j.1651-2227.1994.tb13264.x. [DOI] [PubMed] [Google Scholar]
- Parada M, King S, Li M, Fleming A. The roles of accumbal dopamine D1 and D2 receptors in maternal memory in rats. Behavioral Neuroscience. 2008;122:368–376. doi: 10.1037/0735-7044.122.2.368. [DOI] [PubMed] [Google Scholar]
- Peake GT, Buckman MT, Davis LE, Standefer J. Pituitary and placentally derived hormones in cerebrospinal fluid during normal human pregnancy. Journal of Clinical Endocrinology and Metabolism. 1983;56:46–52. doi: 10.1210/jcem-56-1-46. [DOI] [PubMed] [Google Scholar]
- Pederson CA, Asher JA, Monroe YL, Prange AJ., Jr Oxytocin induces maternal behavior in virgin female rats. Science. 1982;216:648–650. doi: 10.1126/science.7071605. [DOI] [PubMed] [Google Scholar]
- Pedersen C, Boccia M. Oxytocin antagonism alters rat dams’ oral grooming and upright posturing over pups. Physiology & Behavior. 2003;80:233–241. doi: 10.1016/j.physbeh.2003.07.011. [DOI] [PubMed] [Google Scholar]
- Pedersen CA, Caldwell JD, Walker C, Ayers G, Mason GA. Oxytocin activates the postpartum onset of rat maternal behavior in the ventral tegmental and medial preoptic areas. Behavioral Neuroscience. 1994;108:1163–1171. doi: 10.1037//0735-7044.108.6.1163. [DOI] [PubMed] [Google Scholar]
- Pedersen CA, Vadlamudi SV, Boccia M, Amico JA. Maternal behavior deficits in nulliparous oxytocin knockout mice. Genes Brain and Behavior. 2006;5:274–281. doi: 10.1111/j.1601-183X.2005.00162.x. [DOI] [PubMed] [Google Scholar]
- Peña CJ, Neugut D, Champagne FA. Developmental timing of the effects of maternal care on gene expression and epigenetic regulation of hormone receptor levels in female rats. Endocrinology. 2013;154:4340–4351. doi: 10.1210/en.2013-1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira M, Ferreira A. Demanding pups improve maternal behavior impairments in sensitized and haloperidol-treated lactating female rats. Behavioral Brain Research. 2006;175:139–148. doi: 10.1016/j.bbr.2006.08.013. [DOI] [PubMed] [Google Scholar]
- Pissonier D, Thiéry JC, Fabre-Nys C, Poindron P, Keverne EB. The importance of olfactory bulb noradrenalin for maternal recognition in sheep. Physiology and Behavior. 1985;35:361–364. doi: 10.1016/0031-9384(85)90309-9. [DOI] [PubMed] [Google Scholar]
- Poindron P, Levy F, Krehbiel D. Genital, olfactory and endocrine interactions in the development of maternal behaviour in the parturient ewe. Psychoneuroendocrinology. 1988;13:99–125. doi: 10.1016/0306-4530(88)90009-1. [DOI] [PubMed] [Google Scholar]
- Popeski N, Woodside B. Central nitric oxide synthase inhibition disrupts maternal behavior in the rat. Behavioral Neuroscience. 2004;118:1305–1316. doi: 10.1037/0735-7044.118.6.1305. [DOI] [PubMed] [Google Scholar]
- Price AK, Bridges RS. The effects of bromocriptine treatment during early pregnancy on postpartum maternal behaviors in rats. Developmental Psychobiology. 2014;56:4131–1437. doi: 10.1002/dev.21224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quadagno DM, Rockwell J. The effect of gonadal hormones in infancy on maternal behavior in the adult rat. Hormones and Behavior. 1972;3:55–62. doi: 10.1016/0018-506x(72)90007-4. [DOI] [PubMed] [Google Scholar]
- Riddle O, Lahr EL, Bates RW. Maternal behavior induced in virgin rats by prolactin. Proceedings of the Society for Experimental Biology and Medicine. 1935;32:730–734. [Google Scholar]
- Ribeiro AC, Musatov S, Shteyler A, Simanduyev S, Arrieta-Cruz I, Ogawa S, Pfaff DW. siRNA silencing of estrogen receptor expression specifically in the medial preoptic area neurons abolishes maternal care in female mice. Proceedings of the National Academy of Sciences USA. 2012;109:16324–16329. doi: 10.1073/pnas.1214094109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riem MME, Bakersman-Kranenburg MJ, Pieper S, Tops M, Boksem MAS, Vermeiren RRJM, van IJzendoorn MH, Rombouts SARB. Oxytocin modulates amygdala, insula, and inferior frontal gyrus responses to infant crying: a randomized controlled trial. Biological Psychiatry. 2011;70:291–297. doi: 10.1016/j.biopsych.2011.02.006. [DOI] [PubMed] [Google Scholar]
- Rilling JK, Young LJ. The biology of mammalian parenting and its effect on offspring social development. Science. 2014;345:771–776. doi: 10.1126/science.1252723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson MC, Friesen HG. Two forms of placental lactogen revealed by radioimmunoassay. Endocrinology. 1981;108:2388–2390. doi: 10.1210/endo-108-6-2388. [DOI] [PubMed] [Google Scholar]
- Rosenberg P, Halaris A, Moltz H. Effects of central norepinephrine depletion on the initiation and maintenance of maternal behavior in the rat. Pharmacology, Biochemistry and Behavior. 1977;6:21–24. doi: 10.1016/0091-3057(77)90155-1. [DOI] [PubMed] [Google Scholar]
- Rosenblatt JS. Nonhormonal basis of maternal behavior in the rat. Science. 1967;156:1512–1513. doi: 10.1126/science.156.3781.1512. [DOI] [PubMed] [Google Scholar]
- Rubin BS, Bridges RS. Disruption of ongoing maternal responsiveness in rats by central administration of morphine sulfate. Brain Research. 1984;307:91–97. doi: 10.1016/0006-8993(84)90464-5. [DOI] [PubMed] [Google Scholar]
- Rumi MAK, Dhakal P, Kubota K, Chakraborty D, Lei T, Larson MA, Wolfe MW, Roby KF, Vivian JL, Soares MJ. Generation of ESR1 knockout rats using zinc finger nuclease-mediated genome editing. Endocrinology. 2014;155:1991–1999. doi: 10.1210/en.2013-2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruppenthal GC, Arling GL, Harlow HF, Sackett GP, Suomi SJ. A 10-year perspective of motherless-mother behavior. Journal of Abnormal Psychology. 1976;85:341–349. doi: 10.1037//0021-843x.85.4.341. [DOI] [PubMed] [Google Scholar]
- Salmina AB, Lopatina O, Ekimova MV, Mikhutkina SV, Higashida H. CD 38/cyclic ADP-ribose system: a new player for oxytocin secretion and regulation of social behaviour. Journal of Neuroendocrinology. 2010;22:380–392. doi: 10.1111/j.1365-2826.2010.01970.x. [DOI] [PubMed] [Google Scholar]
- Scanlan VF, Byrnes EM, Bridges RS. Reproductive experience and activation of maternal memory. Behavioral Neuroscience. 2006;120:676–686. doi: 10.1037/0735-7044.120.3.676. [DOI] [PubMed] [Google Scholar]
- Schechter M, Pinhasov A, Weller A, Fride E. Blocking the postpartum mouse dam’s CB1 receptors impairs maternal behavior as well as offspring development and their adult social-emotional behavior. Behavioural Brain Research. 2012;226:481–492. doi: 10.1016/j.bbr.2011.10.016. [DOI] [PubMed] [Google Scholar]
- Shahrokh DK, Zhang T-Y, Diorio J, Gratton A, Meaney MJ. Oxytocin-dopamine interactions mediate variations in maternal behavior in the rat. Endocrinology. 2010;151:2276–2286. doi: 10.1210/en.2009-1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shingo T, Gregg C, Enwere E, Fujiwawa H, Hassam R, Geary C, Cross JC, Weiss S. Pregnancy-stimulated neurogenesis in the adult female forebrain. Science. 2004;299:117–120. doi: 10.1126/science.1076647. [DOI] [PubMed] [Google Scholar]
- Siegel HI, Rosenblatt JS. Hormonal basis of hysterectomy-induced maternal behavior during pregnancy in the rat. Hormones and Behavior. 1975a;6:211–222. doi: 10.1016/0018-506x(75)90008-2. [DOI] [PubMed] [Google Scholar]
- Siegel HI, Rosenblatt JS. Estrogen-induced maternal behavior in hysterectomized-ovariectomized virgin rats. Physiology and Behavior. 1975b;14:465–471. doi: 10.1016/0031-9384(75)90012-8. [DOI] [PubMed] [Google Scholar]
- Silva MR, Bernardi MM, Felicio LF. Effects of dopamine receptor antagonists on ongoing maternal behavior in rats. Pharmacology, Biochemistry and Behavior. 2001;68:461–468. doi: 10.1016/s0091-3057(01)00471-3. [DOI] [PubMed] [Google Scholar]
- Slotnick BM, Carpenter ML, Fusco R. Initiation of maternal behavior in pregnant nulliparous rats. Hormones and Behavior. 1973;4:53–59. [Google Scholar]
- Smith CD, Holschback MA, Olsewicz J, Lonstein JS. Effects of noradrenergic alpha-2 receptor antagonism or noradrenergic lesions in the ventral bed nucleus of the stria terminalis and medial preoptic area on maternal care in female rats. Psychopharmacology. 2012;224:263–276. doi: 10.1007/s00213-012-2749-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielewoy C, Roubert C, Hamon M, Nosten-Bertrand M, Betancur C, Giros B. Behavioural disturbances associated with hyperdopaminergia in dopamine-transporter knockout mice. Behavioral Pharmacology. 2000;11:279–290. doi: 10.1097/00008877-200006000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele MK, Rowland D, Moltz H. Initiation of maternal behavior in the rat: possible involvement of limbic norepinephrine. Pharmacology, Biochemistry and Behavior. 1979;11:123–130. doi: 10.1016/0091-3057(79)90001-7. [DOI] [PubMed] [Google Scholar]
- Stjernholm-Vladic Y, Wang H, Stygar D, Ekman G, Sahlin L. Differential regulation of the progesterone receptor A and B in the human uterine cervix at parturition. Gynecol Endocrinol. 2004;18:41–46. doi: 10.1080/09513590310001651777. [DOI] [PubMed] [Google Scholar]
- Stolzenberg D, McKenna J, Keough S, Hancock R, Numan MJ, Numan M. Dopamine D1 receptor stimulation of the nucleus accumbens or the medial preoptic area promotes the onset of maternal behavior in pregnancy-terminated rats. Behavioral Neuroscience. 2007;121:907–919. doi: 10.1037/0735-7044.121.5.907. [DOI] [PubMed] [Google Scholar]
- Stolzenberg DS, Stevens JS, Rissman EF. Experience-facilitated improvements in pup retrieval: evidence for an epigenetic effect. Hormones and Behavior. 2012;62:128–135. doi: 10.1016/j.yhbeh.2012.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukikara MH, Platero MD, Canteras NS, Felicio LF. Opiate regulation of behavioral selection during lactation. Pharmacology, Biochemistry and Behavior. 2007;87:315–320. doi: 10.1016/j.pbb.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Svare BB. Mammalian Parenting:Biochemical, Neurobiological and Behavioral Determinants. Oxford University Press; New York, NY: 1990. Maternal aggression: hormonal, genetic, and developmental determinants; pp. 118–132. [Google Scholar]
- Swain JE, Kim P, Ho SS. Neuroendocrinology of parental response to baby-cry. Journal of Neuroendocrinology. 2011;23:1036–1041. doi: 10.1111/j.1365-2826.2011.02212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain JE, Leckman JF, Mayes LC, Feldman R, Hoyt E, Kang H, Kim P, Schultz RT. Baby cry and picture activations of parent brains vary with gender, experience, and dyadic relationship. Biological Psychiatry. 2007;61:S103. [Google Scholar]
- Takayanagi Y, Yoshida M, Bielsky IF, Ross HE, Kawamata M, Onaka T, Yanagisawa T, Kimura T, Matzuk MM, Young LJ, Nishimori K. Pervasive social deficits, but normal parturition, in oxytocin receptor deficient mice. Proceedings of the National Academy of Sciences USA. 2005;102:16096–16101. doi: 10.1073/pnas.0505312102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terkel J, Rosenblatt JS. Aspects of nonhormonal maternal behavior in the rat. Hormones and Behavior. 1971;2:161–171. [Google Scholar]
- Terkel J, Rosenblatt JS. Humoral factors underlying maternal behavior at parturition: cross transfusion between freely moving rats. Journal of Comparative and Physiological Psychology. 1972;80:365–371. doi: 10.1037/h0032965. [DOI] [PubMed] [Google Scholar]
- Thijssen JHH. Progesterone receptors in the human uterus and their possible role in parturition. Journal of Steroid Biochemistry and Molecular Biology. 2005;97:397–400. doi: 10.1016/j.jsbmb.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Thomas SA, Palmiter RD. Impaired maternal behavior in mice lacking norepinephrine and epinephrine. Cell. 1997;91:583–592. doi: 10.1016/s0092-8674(00)80446-8. [DOI] [PubMed] [Google Scholar]
- Tindal JS, Knaggs GS. Pathways in the forebrain of the rat concerned with the release of prolactin. Brain Research. 1977;119:211–221. doi: 10.1016/0006-8993(77)90101-9. [DOI] [PubMed] [Google Scholar]
- Vom saal FS, Bronson FH. In utero proximity of female mouse fetuses to males: effect on reproductive performance during later life. Biology of Reproduction. 1978;19:842–853. doi: 10.1095/biolreprod19.4.842. [DOI] [PubMed] [Google Scholar]
- Walsh RJ, Slaby FJ, Posner BI. A receptor-mediated mechanism for the transport of prolactin from blood to cerebrospinal fluid. Endocrinology. 1987;120:1846–1850. doi: 10.1210/endo-120-5-1846. [DOI] [PubMed] [Google Scholar]
- Wamboldt MZ, Insel TR. The ability of oxytocin to induce short latency maternal behavior is dependent on peripheral anosmia. Behavioral Neuroscience. 1987;101:439–441. doi: 10.1037//0735-7044.101.3.439. [DOI] [PubMed] [Google Scholar]
- Weaver IC, Cervoni N, Champagne FA, D’Alessio AC, Sharma S, Secki JR, Dymov S, Szyf M, Meaney MJ. Epigenetic programming by maternal behavior. Nature Neuroscience. 2004a;7:847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- Weaver IC, Diorio J, Secki JR, Szyr M, Meaney MJ. Early environmental regulation of hippocampal glucocorticoid receptor gene expression: characterization of intracellular mediators and potential genomic trigger sites. Annals of the New York Academy of Science. 2004b;1024:182–212. doi: 10.1196/annals.1321.099. [DOI] [PubMed] [Google Scholar]
- Weisman O, Delaherche E, Rondeau M, Chetouani M, Cohen D, Feldman R. Oxytocin shapes parental motion during father-infant interaction. Biology Letters. 2013;9:20130828. doi: 10.1098/rsbl.2013.0828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Autry AE, Bergan JF, Watabe-Uchida M, Dulac CG. Galanin neurons in the medial preoptic area govern parental behaviour. Nature. 2014;509:325–330. doi: 10.1038/nature13307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young LJ, Wang Z, Insel TR. Neuroendocrine bases of monogamy. Trends in Neuroscience. 1998;21:71–75. doi: 10.1016/s0166-2236(97)01167-3. [DOI] [PubMed] [Google Scholar]
- Young WS, III, Shepard E, Amico J, Hennighausen L, Wagner KU, LaMarca ME, McKinney C, Ginns EI. Deficiency in mouse oxytocin prevents milk ejection, but not fertility or parturition. Journal of Neuroendocrinology. 1996;8:847–853. doi: 10.1046/j.1365-2826.1996.05266.x. [DOI] [PubMed] [Google Scholar]
- Zakar T, Hertelendy F. Progesterone withdrawal: key to parturition. American Journal of Obstetrics and Gynecology. 2007;196:289–296. doi: 10.1016/j.ajog.2006.09.005. [DOI] [PubMed] [Google Scholar]
- Zarrow MX, Gandelman R, Denenberg VH. Prolactin: is it an essential hormone for maternal behavior in mammals? Hormones and Behavior. 1971;2:343–354. [Google Scholar]
- Zwain IH, Yen SS. Neurosteroidogenesis in astrocytes, oligodendrocytes, and neurons of cerebral cortex of rat brain. Endocrinology. 1999;140:3843–3852. doi: 10.1210/endo.140.8.6907. [DOI] [PubMed] [Google Scholar]