Abstract
Background and Purpose
Emerging evidence suggests that atrial disease is associated with vascular brain injury in the absence of atrial fibrillation (AF).
Methods
The Cardiovascular Health Study prospectively enrolled community-dwelling adults ≥65 years of age. Among participants who underwent MRI, we examined associations of ECG left atrial abnormality with brain infarcts and leukoaraiosis. P-wave terminal force in lead V1 (PTFV1) was the primary measure of left atrial abnormality; P-wave area and duration were secondary predictors. We excluded participants with AF before or on their index ECG. Primary outcomes were incident infarcts and worsening leukoaraiosis from initial to follow-up scan approximately 5 years later. Secondary outcomes were prevalent infarcts and degree of leukoaraiosis on initial MRI. Relative risk and linear regression models adjusted for vascular risk factors.
Results
Among 3,129 participants with ≥1 scan, each SD increase in PTFV1 was associated with a 0.05-point (95% CI, 0.0003–0.10) higher baseline white matter grade on a 10-point scale. PTFV1 was associated with prevalent infarcts of any type (RR per SD, 1.09; 95% CI, 1.04–1.16), and more so with prevalent non-lacunar infarcts (RR per SD, 1.22; 95% CI, 1.08–1.38). Among 1,839 participants with 2 scans, PTFV1 was associated with worsening leukoaraiosis (RR per SD, 1.09; 95% CI, 1.01–1.18), but not incident infarcts (RR per SD, 1.06; 95% CI, 0.93–1.20). Sensitivity analyses adjusting for incident AF found similar results. P-wave area and duration were not associated with outcomes.
Conclusions
ECG left atrial abnormality is associated with vascular brain injury in the absence of documented AF.
Keywords: Arrhythmia, atrium, ECG, embolism, cerebrovascular disease/stroke
Atrial fibrillation (AF) has long been recognized as a risk factor for vascular brain injury, in both an overt form as ischemic stroke1 and covert as subclinical brain infarction.2 Recent evidence suggests that atrial disease may be related to vascular brain injury even in the absence of AF. Prior studies have found associations between premature atrial contractions or paroxysmal supraventricular tachycardia and ischemic stroke,3–6 even after accounting for diagnoses of AF during follow-up.3,6 P-wave terminal force in lead V1 (PTFV1)—a consistently used marker of left atrial abnormality on a 12-lead electrocardiogram (ECG)7—has been associated with stroke risk,8,9 even in the absence of documented AF.10 Since left atrial ECG abnormality indicates derangements in atrial anatomy and physiology, its association with stroke suggests that left atrial disease may produce a substrate for cardiac thrombus formation and embolization even in the absence of AF. To investigate this hypothesis, we examined the association of left atrial ECG abnormality with subclinical vascular brain injury detected by magnetic resonance imaging (MRI). We examined both covert brain infarcts and the degree of leukoaraiosis, since cerebral white matter disease has also been associated with vascular risk factors.11 Since our hypothesis was that left atrial disease is associated with subclinical cardiac embolism, which typically results in large or cortical infarcts,12 we hypothesized that associations would be stronger with non-lacunar rather than aggregate MRI-defined infarcts.
Methods
Design
The Cardiovascular Health Study (CHS) is a prospective, longitudinal cohort study of community-dwelling men and women ≥65 years of age. In two waves between 1989 and 1993, CHS field centers recruited 5,888 participants from a random sample of people on Medicare eligibility lists in four counties, one each in California, Maryland, North Carolina, and Pennsylvania.13 Participants were invited to return for annual in-person study visits through 1998–1999 and were followed after that time via semiannual telephone calls and use of linked Medicare claims data. Institutional review boards at the University of Washington and each field center approved this study, and all participants provided written informed consent.
Participants
The original CHS exclusion criteria were age <65 years, inability to give consent or answer questions without a surrogate, residence in an institutional setting, wheelchair dependence, or active treatment for cancer. For this analysis, we included CHS participants who underwent a brain MRI as part of the study protocol to assess vascular brain injury, including infarcts and leukoaraiosis. We excluded CHS participants who had suffered a clinically recognized stroke before their initial MRI, lacked at least one ECG prior to their initial MRI, were diagnosed with AF before or on their index ECG, or lacked data on any of the model covariates described below. An adjudication committee determined stroke status, as detailed previously.14
Measurements
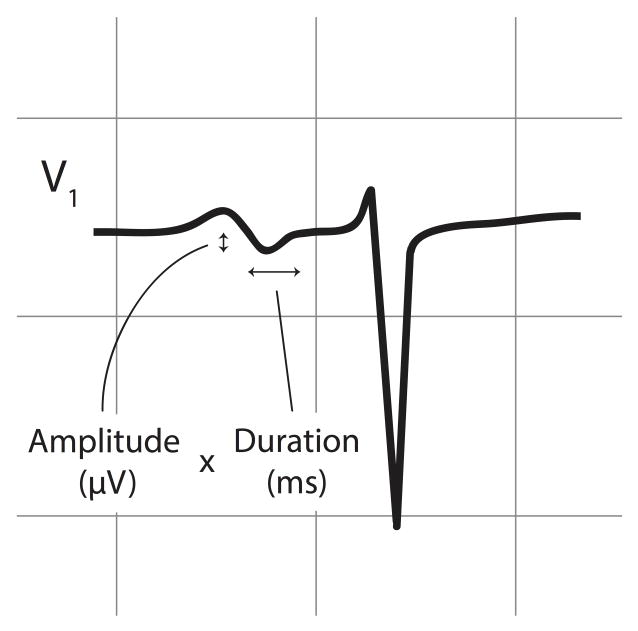
The predictor variables were P-wave measurements derived from digital 12-lead ECGs done at baseline and each annual study visit. We obtained P-wave measurements from the latest ECG prior to the initial MRI. ECGs were obtained on MAC PC ECG machines (Marquette Electronics, Milwaukee, WI) calibrated at 10 mm/mV with a speed of 25 mm/s. P-wave areas, amplitudes, and durations were centrally measured at the Wake Forest Epidemiological Cardiology Research Center using a fully automated program (12-SL, Version 2001, GE Marquette). PTFV1 is the most frequently used ECG criterion for left atrial abnormality, while P-wave area and duration may also be useful.7 Based on this consideration and prior work,8,10 we focused on PTFV1 as the main predictor variable, and also examined maximum P-wave duration and maximum P-wave area. PTFV1 was defined as the duration (ms) of the downward deflection (terminal portion) of the median P-wave in lead V1 multiplied by the absolute value of its amplitude (μV) (Figure 1).15 P-wave areas comprised the sum of the absolute areas of upward and downward P-wave deflections, and were multiplied by 19.52 to harmonize the 12-SL program’s calculations with other machines.8,16
Figure 1.
Illustration of ECG parameters used to calculate P-wave terminal force (PTFV1), defined as the duration of the downward deflection (terminal portion) of the P-wave in lead V1 multiplied by the absolute value of its amplitude.
The outcomes were infarcts and degree of leukoaraiosis on initial (performed in 1991–1994) and follow-up (performed in 1997–1999) MRI scans, as detailed previously.11,17–19 Infarcts were defined as ≥3 mm areas of abnormal signal intensity in a vascular distribution and lacking mass effect, and leukoaraiosis was graded on a 10-point scale, with a white matter grade of 0 representing the least severe and 9 the most severe.11,17 Lacunar infarcts were defined as subcortical infarcts 3 to 20 mm in size, and any other infarcts were considered non-lacunar infarcts. Those with both lacunar and non-lacunar infarcts were classified as having non-lacunar infarcts. The primary outcomes were the presence of any incident infarcts and any worsening of the white matter grade by ≥1 grade among participants who underwent both an initial and follow-up scan. Excluded from all analyses of incident findings were participants who suffered a clinically recognized stroke prior to the follow-up scan; furthermore, participants with any infarcts on the initial scan were excluded from analyses of incident infarcts on the follow-up scan, and those with the highest white matter grade on the initial scan were excluded from analyses of worsening white matter grade. Secondary outcomes were prevalent infarcts and white matter grade on the initial scan.
AF was ascertained by hospital discharge diagnoses, diagnoses of AF from outpatient visits or carrier claims from Medicare data, and ECGs done at annual study exams through 1998–1999.20,21 Additional covariates included age, sex, race, education level, coronary heart disease, congestive heart failure, diabetes, hypertension (defined by systolic blood pressure or anti-hypertension drug therapy), dyslipidemia (defined by high- and low-density lipoprotein and triglyceride levels), smoking status, and NT-proBNP, as detailed previously.11,17–19,22 These variables were determined from the study visit associated with the 12-lead ECG under analysis. If covariates were not collected in that year or were missing, they were carried forward from previous years when available.
Statistical Analysis
We excluded values >99.9th percentile for PTFV1 and P-wave area as clinically implausible outliers. After using generalized additive models to confirm the linearity of associations, and consistent with prior work,10 we evaluated P-wave measurements as linear predictors in standard deviation (SD) increments (calculated on the full sample prior to any exclusions). We displayed the distribution of baseline characteristics by category of PTFV1 (0 and tertiles of values >0).
We used relative risk regression to examine the associations of P-wave predictors with incident infarcts, prevalent infarcts, and worsening of the white matter grade.22 We used linear regression to examine associations with the white matter grade on the first scan. The final models controlled for age, sex, race, education level, coronary heart disease, congestive heart failure, diabetes, hypertension, dyslipidemia, smoking status, and period of time between the index ECG and initial scan. Since left atrial ECG abnormality is associated with AF,3,8 which in turn has long been known to be associated with brain infarction,1,18,23 we performed additional analyses that adjusted for incident AF diagnoses in the intervening period after the index ECG and before the MRI under analysis (i.e., before the second MRI for analyses of incident findings and before the first MRI for analyses of baseline findings). In additional analyses, we restricted the definition of infarcts to non-lacunar infarcts only. In secondary analyses, we assessed P-wave measurements as binary variables dichotomized at the 95th percentile.
To confirm further that AF did not mediate associations between P-wave predictors and outcomes, we performed sensitivity analyses that entirely excluded participants diagnosed with incident AF after the index ECG and before the MRI under analysis. Lastly, we performed exploratory analyses adjusting for serum NT-proBNP to determine how associations with our electrocardiographic predictors changed after adjusting for this other marker of atrial disease.22
Statistical analyses were performed using Stata 12.1 (StataCorp, TX).
Results
Of the 5,888 participants in the overall CHS cohort, 3,660 underwent an initial MRI scan. Compared with the overall cohort, participants who underwent MRI were significantly younger, had received more education, and suffered fewer medical comorbidities.18 Of these 3,660 participants with MRI data, 3,129 met the other inclusion criteria for our analyses of prevalent findings on the first scan. Those with higher PTFV1 were more likely to be African-American, lack a high school degree, and have hypertension, coronary heart disease, congestive heart failure, or diabetes (Table 1). Of these 3,129 participants, 1,839 underwent a follow-up scan and otherwise met the inclusion criteria for our analyses of incident findings.
Table 1.
Baseline Characteristics of CHS Participants, Stratified by Category of PTFV1*
| Characteristic† | PTFV1 = 0 μV* ms (N = 758) | PTFV1 = 207–2,494 μV* ms (N = 799) | PTFV1 = 2,495–3,780 μV* ms (N = 789) | PTFV1 = 3,781–19,474 μV* ms (N = 778) |
|---|---|---|---|---|
| PTFV1, mean (SD), μV* ms | 0 (0) | 1,786 (471) | 3,088 (367) | 5,436 (1,715) |
| Age, mean (SD), y | 74.5 (5.2) | 74.5 (4.9) | 74.3 (4.8) | 74.8 (5.2) |
| Male | 300 (39.6) | 318 (39.8) | 337 (42.7) | 308 (39.6) |
| African-American | 96 (12.7) | 99 (12.4) | 126 (16.0) | 155 (19.9) |
| High school graduate | 580 (76.5) | 592 (74.1) | 599 (75.9) | 553 (71.1) |
| SBP, mean (SD), mm Hg | 131.9 (19.6) | 134.1 (19.80) | 134.6 (20.5) | 138.1 (22.3) |
| HDL, mean (SD), mg/dL | 54.6 (14.6) | 54.2 (14.5) | 53.6 (14.5) | 54.1 (14.5) |
| LDL, mean (SD), mg/dL | 127.0 (33.7) | 128.7 (32.7) | 128.4 (33.9) | 128.3 (35.2) |
| Coronary heart disease | 119 (15.7) | 126 (15.8) | 133 (16.9) | 188 (24.2) |
| Congestive heart failure | 21 (2.8) | 18 (2.3) | 24 (3.0) | 42 (5.4) |
| Diabetes status: | ||||
| Normal | 601 (80.2) | 597 (75.9) | 586 (75.0) | 571 (74.1) |
| Impaired fasting glucose | 67 (8.9) | 88 (11.2) | 85 (10.9) | 89 (11.5) |
| Diabetes | 81 (10.8) | 102 (13.0) | 110 (14.1) | 111 (14.4) |
| Smoking status: | ||||
| Never | 344 (45.4) | 412 (51.6) | 360 (45.6) | 373 (47.9) |
| Former | 337 (44.5) | 316 (39.5) | 352 (44.6) | 322 (41.4) |
| Current | 77 (10.2) | 71 (8.9) | 77 (9.8) | 83 (10.7) |
Abbreviations: CHS, Cardiovascular Health Study; HDL, high-density lipoprotein; LDL, low-density lipoprotein; PTFV1, P-wave terminal force in lead V1; SBP, systolic blood pressure; SD, standard deviation.
Baseline characteristics are displayed by category of PTFV1:: 0 and tertiles of values >0. A value of 0 reflects the absence of a downward deflection of the P-wave in lead V1.
Data are presented as number (%) unless otherwise specified.
In primary analyses limited to participants with two scans, 17.1% had new infarcts and 27.4% had worsening of the white matter grade on the repeat scan. Each SD increase in PTFV1 (2160 μV*ms) was associated with worsening white matter grade (RR per SD, 1.09; 95% CI, 1.01–1.18). This association did not substantially change after adjusting for incident AF or NT-proBNP, or excluding patients with incident AF. PTFV1 was not associated with incident infarcts, including incident non-lacunar infarcts (Table 2).
Table 2.
ECG P-Wave Measurements (in Standard Deviation Units) and Risk of Vascular Brain Injury
| Outcome | PTFV1 | P-Wave Duration | P-Wave Area |
|---|---|---|---|
| Incident infarcts* | |||
| Any infarcts | |||
| Model 1† | 1.07 (0.94–1.21) | 1.01 (0.88–1.16) | 1.00 (0.88–1.14) |
| Model 2‡ | 1.06 (0.93–1.20) | 1.01 (0.88–1.16) | 1.00 (0.88–1.14) |
| Model 3§ | 1.05 (0.93–1.19) | 1.00 (0.87–1.15) | 1.00 (0.88–1.14) |
| Non-lacunar infarcts | |||
| Model 1 | 1.16 (0.94,1.42) | 1.07 (0.86,1.34) | 1.09 (0.89,1.33) |
| Model 2 | 1.12 (0.91,1.39) | 1.01 (0.80,1.27) | 1.08 (0.88,1.32) |
| Model 3 | 1.13 (0.91,1.40) | 1.01 (0.80,1.26) | 1.08 (0.89,1.32) |
| Worsening white matter grade* | |||
| Model 1 | 1.10 (1.02–1.19) | 1.04 (0.95–1.14) | 1.05 (0.97–1.15) |
| Model 2 | 1.09 (1.01–1.18) | 1.04 (0.95–1.13) | 1.03 (0.95–1.12) |
| Model 3 | 1.09 (1.01–1.18) | 1.03 (0.94–1.13) | 1.03 (0.95–1.12) |
| Prevalent infarcts* | |||
| Any infarcts | |||
| Model 1 | 1.12 (1.06–1.19) | 1.05 (0.99–1.11) | 1.06 (1.00–1.12) |
| Model 2 | 1.09 (1.04–1.16) | 1.02 (0.97–1.08) | 1.05 (0.99–1.11) |
| Model 3 | 1.09 (1.04–1.15) | 1.02 (0.96–1.08) | 1.05 (0.99–1.11) |
| Non-lacunar infarcts | |||
| Model 1 | 1.27 (1.13,1.43) | 1.13 (0.98,1.29) | 1.13 (0.99,1.29) |
| Model 2 | 1.22 (1.08,1.38) | 1.08 (0.94,1.24) | 1.11 (0.97,1.27) |
| Model 3 | 1.22 (1.08,1.37) | 1.07 (0.94,1.23) | 1.11 (0.97,1.27) |
| Baseline white matter grade|| | |||
| Model 1 | 0.07 (0.02–0.12) | 0.00 (−0.05–0.05) | 0.04 (−0.01–0.09) |
| Model 2 | 0.05 (0.0003–0.10) | −0.02 (−0.06–0.03) | 0.04 (−0.02–0.09) |
| Model 3 | 0.05 (0.0005–0.10) | −0.02 (−0.06–0.03) | 0.04 (−0.02–0.09) |
Abbreviations: ECG, electrocardiogram; PTFV1, P-wave terminal force in lead V1.
Results are reported as the relative risk of the outcome associated with a 1-standard deviation increase in the predictor (95% confidence interval).
Adjusted for age- sex- race- and education level.
Adjusted for variables in Model 1 plus coronary heart disease, congestive heart failure, hypertension, diabetes mellitus, dyslipidemia, smoking status, and time from ECG to the initial magnetic resonance imaging (MRI) scan.
Adjusted for variables in Model 2 plus incident atrial fibrillation diagnosed after the ECG and before the MRI under analysis.
Results are reported as the change in white matter grade for a 1-standard deviation higher level of the predictor (95% confidence interval).
In secondary analyses of participants with at least an initial scan, 28.4% had prevalent infarcts of any type, and 7.1% had prevalent non-lacunar infarcts. Higher PTFV1 was associated with prevalent infarcts of any type (RR per SD, 1.09; 95% CI, 1.04–1.16), and even more so with prevalent non-lacunar infarcts (RR per SD, 1.22; 95% CI, 1.08–1.38). These associations did not substantially change after adjusting for NT-proBNP or for incident AF occurring after the index ECG and before the MRI scan, or excluding patients with incident AF. Each SD increase in PTFV1 was associated with a 0.05-point (95% CI, 0.0003–0.10) higher baseline white matter grade. This association did not change appreciably when adjusting for or excluding patients with incident AF, although the association was no longer significant after adjusting for NT-proBNP (0.04 higher white matter grade for each SD increase in PTFV1; 95% CI, −0.01–0.10). PTFV1 dichotomized at the 95th percentile was associated with prevalent infarcts of any type (RR, 1.52; 95% CI, 1.26–1.82) and prevalent non-lacunar infarcts (RR, 1.65; 95% CI, 1.06–2.56), but not with the other outcomes.
P-wave area, duration, and the other dichotomized P-wave measurements were not significantly associated with baseline or incident MRI findings (Table 2).
Discussion
In this large, prospective cohort study, PTFV1—an established ECG marker of left atrial abnormality—was significantly associated with prevalent MRI-defined infarcts, especially non-lacunar infarcts as compared with infarcts of any type. These associations held regardless of whether or not we adjusted for NT-proBNP and incident diagnoses of AF. PTFV1 was also associated with baseline and worsening white matter disease grade, but was not associated with incident infarcts.
These findings expand on recent data regarding the relationships between ECG markers of atrial disease and vascular brain injury. Among a prospective cohort of participants enrolled in the Multi-Ethnic Study of Atherosclerosis (MESA), greater PTFV1 on the baseline 12-lead ECG was significantly associated with a higher risk of subsequent ischemic stroke, even after adjusting for incident AF.10 PTFV1 was more strongly associated with incident stroke than with incident AF, making it less likely that subclinical AF predominantly mediated the link between PTFV1 and stroke.10 Two other studies, one of which was focused primarily on prediction of AF, have also demonstrated an association between PTFV1 and stroke, although these analyses did not adjust for AF when examining this association.8,9 Our present results provide further evidence that PTFV1 is associated with brain infarcts in the absence of documented AF, and novel evidence that PTFV1 is associated with leukoaraiosis.
Our results should be considered in light of the limitations of this study. First, participants did not undergo continuous heart-rhythm monitoring to detect subclinical AF, and we cannot exclude the possibility that subclinical AF largely mediates the relationship between PTFV1 and vascular brain injury. However, if this were the case, the addition of incident AF to our models would be expected to change the strength of association between PTFV1 and our outcomes, whereas the associations we found were substantially the same regardless of whether or not we included incident AF diagnoses. Furthermore, even if undetected subclinical AF mediates some of the associations between markers of atrial cardiopathy and vascular brain injury, as a practical matter these measurements are easier and less costly to obtain than prolonged heart-rhythm monitoring. Nevertheless, it will be important to conduct further studies of the association between PTFV1 and vascular brain injury in patients undergoing continuous heart-rhythm monitoring. Second, given the modest strength of association between PTFV1 and our outcomes, as well as the multiple predictors and outcomes tested, these results may simply represent chance findings. This may be less likely because our results are consistent with findings of an association between PTFV1 and stroke in a separate population-based cohort enrolled in the MESA study.10 Third, MRIs were performed using earlier-generation machines that are likely to have been less sensitive than current scanners. This would be expected to result in nondifferential misclassification bias that would have attenuated the associations between P-wave measurements and outcomes. In addition, the CHS participants who underwent MRI scans tended to be younger and healthier than the overall cohort, which probably further reduced the associations between ECG markers and vascular brain injury. Furthermore, given a lack of side-by-side imaging review, we were unable to reliably identify new infarcts on follow-up scans among participants with prevalent infarcts on the initial scans, and therefore had to exclude participants with prevalent infarcts from analyses of incident infarcts. This resulted in a lower-risk sample for our analyses of incident infarcts, which would be expected to further reduce associations and may partly explain the lack of association between PTFV1 and incident infarcts. Future studies with larger sample sizes may be better able to assess the relationship between PTFV1 and incident infarcts. Fifth, inclusion of detailed imaging data about the left atrium may allow a more complete assessment of the relationship between left atrial dysfunction and vascular brain injury.
Current ECG machines do not routinely report PTFV1, and its values show a moderate degree of measurement-to-measurement variation,24 thereby limiting its immediate clinical value as a risk marker for vascular brain injury. However, these technical issues are likely to be surmountable with further clinical development of this marker, and in the meantime, PTFV1 may serve as an important research tool in the ongoing quest to better understand cryptogenic strokes and vascular brain injury. Many cryptogenic strokes are suspected to arise from occult cardiac embolism.25 Since PTFV1 has long been established as a marker of increased left atrial pressure and hypertrophy,26,27 associations between PTFV1 and brain infarcts independent of documented AF suggest that atrial cardiopathy may cause embolism in the absence of AF. Furthermore, AF has been previously associated with periventricular white matter disease independent of other vascular risk factors,28 possibly due to the cerebral hypoperfusion seen in AF.29 Our finding of an association between PTFV1 and white matter disease suggests that atrial dysfunction might be a marker of cerebral hypoperfusion even in the absence of AF. This possibility would be consistent with the recent finding that left atrial volume and function correlate with white matter disease even in the absence of AF.30 The associations between left atrial abnormality on ECG and vascular brain injury on MRI may also reflect shared vascular risk factors that were not fully adjusted for in our models, although the seemingly stronger association we found for non-lacunar rather than lacunar infarcts supports some degree of a causal link between atrial cardiopathy and vascular brain injury.
Future work may be able to identify some combination of ECG markers such as PTFV1, echocardiographic measurements of left atrial size and function,31,32 and serum biomarkers such as NT-proBNP33,34 that predict the risk of vascular brain injury better than simply the presence or absence of apparent AF. Therefore, further confirmation and delineation of atrial cardiopathy as a risk factor for vascular brain injury may help to advance efforts to prevent stroke.
Acknowledgments
Funding Sources
This research was funded by grant K23NS082367 (Kamel) from the National Institute of Neurological Disorders and Stroke. This research was supported by contracts HHSN268201200036C, HHSN268200800007C, N01HC55222, N01HC85079, N01HC85080, N01HC85081, N01HC85082, N01HC85083, N01HC85086, N01HC15103, and grant U01HL080295 from the National Heart, Lung, and Blood Institute, with additional contribution from the National Institute of Neurological Disorders and Stroke. Additional support was provided by R01AG023629 from the National Institute on Aging. A full list of principal Cardiovascular Health Study investigators and institutions can be found at CHS-NHLBI.org. Roche Diagnostics provided funding and laboratory reagents for the NT pro-BNP assay.
Footnotes
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Disclosures
Dr. Elkind reports serving as a consultant for BMS-Pfizer Partnership, Daiichi-Sankyo, Janssen Pharmaceuticals, and Boehringer-Ingelheim on the subject of antithrombotic therapy for AF; and for Biotelemetry on the subject of cardiac monitoring for paroxysmal AF. The other authors report no conflicts of interest.
References
- 1.Wolf PA, Dawber TR, Thomas HE, Jr, Kannel WB. Epidemiologic assessment of chronic atrial fibrillation and risk of stroke: the Framingham study. Neurology. 1978;28:973–977. doi: 10.1212/wnl.28.10.973. [DOI] [PubMed] [Google Scholar]
- 2.Das RR, Seshadri S, Beiser AS, Kelly-Hayes M, Au R, Himali JJ, et al. Prevalence and correlates of silent cerebral infarcts in the Framingham offspring study. Stroke. 2008;39:2929–2935. doi: 10.1161/STROKEAHA.108.516575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Binici Z, Intzilakis T, Nielsen OW, Kober L, Sajadieh A. Excessive supraventricular ectopic activity and increased risk of atrial fibrillation and stroke. Circulation. 2010;121:1904–1911. doi: 10.1161/CIRCULATIONAHA.109.874982. [DOI] [PubMed] [Google Scholar]
- 4.Chong BH, Pong V, Lam KF, Liu S, Zuo ML, Lau YF, et al. Frequent premature atrial complexes predict new occurrence of atrial fibrillation and adverse cardiovascular events. Europace. 2012;14:942–947. doi: 10.1093/europace/eur389. [DOI] [PubMed] [Google Scholar]
- 5.Engstrom G, Hedblad B, Juul-Moller S, Tyden P, Janzon L. Cardiac arrhythmias and stroke: increased risk in men with high frequency of atrial ectopic beats. Stroke. 2000;31:2925–2929. doi: 10.1161/01.str.31.12.2925. [DOI] [PubMed] [Google Scholar]
- 6.Kamel H, Elkind MS, Bhave PD, Navi BB, Okin PM, Iadecola C, et al. Paroxysmal supraventricular tachycardia and the risk of ischemic stroke. Stroke. 2013;44:1550–1554. doi: 10.1161/STROKEAHA.113.001118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hancock EW, Deal BJ, Mirvis DM, Okin P, Kligfield P, Gettes LS, et al. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram. J Am Coll Cardiol. 2009;53:992–1002. doi: 10.1016/j.jacc.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 8.Soliman EZ, Prineas RJ, Case LD, Zhang ZM, Goff DC., Jr Ethnic distribution of ECG predictors of atrial fibrillation and its impact on understanding the ethnic distribution of ischemic stroke in the Atherosclerosis Risk in Communities (ARIC) study. Stroke. 2009;40:1204–1211. doi: 10.1161/STROKEAHA.108.534735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kohsaka S, Sciacca RR, Sugioka K, Sacco RL, Homma S, Di Tullio MR. Electrocardiographic left atrial abnormalities and risk of ischemic stroke. Stroke. 2005;36:2481–2483. doi: 10.1161/01.STR.0000185682.09981.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kamel H, Soliman EZ, Heckbert SR, Kronmal RA, Longstreth WT, Jr, Nazarian S, et al. P-wave morphology and the risk of incident ischemic stroke in the Multi-Ethnic Study of Atherosclerosis. Stroke. 2014;45:2786–2788. doi: 10.1161/STROKEAHA.114.006364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Longstreth WT, Jr, Arnold AM, Beauchamp NJ, Jr, Manolio TA, Lefkowitz D, Jungreis C, et al. Incidence, manifestations, and predictors of worsening white matter on serial cranial magnetic resonance imaging in the elderly: the Cardiovascular Health Study. Stroke. 2005;36:56–61. doi: 10.1161/01.STR.0000149625.99732.69. [DOI] [PubMed] [Google Scholar]
- 12.Ringelstein EB, Koschorke S, Holling A, Thron A, Lambertz H, Minale C. Computed tomographic patterns of proven embolic brain infarctions. Ann Neurol. 1989;26:759–765. doi: 10.1002/ana.410260612. [DOI] [PubMed] [Google Scholar]
- 13.Fried LP, Borhani NO, Enright P, Furberg CD, Gardin JM, Kronmal RA, et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1:263–276. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 14.Longstreth WT, Jr, Bernick C, Fitzpatrick A, Cushman M, Knepper L, Lima J, et al. Frequency and predictors of stroke death in 5,888 participants in the Cardiovascular Health Study. Neurology. 2001;56:368–375. doi: 10.1212/wnl.56.3.368. [DOI] [PubMed] [Google Scholar]
- 15.Soliman EZ, Alonso A, Misialek JR, Jain A, Watson KE, Lloyd-Jones DM, et al. Reference ranges of PR duration and P-wave indices in individuals free of cardiovascular disease: The Multi-Ethnic Study of Atherosclerosis (MESA) J Electrocardiol. 2013;46:702–706. doi: 10.1016/j.jelectrocard.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dewhurst M, Adams P. Regarding article “Ethnic distribution of electrocardiographic predictors of atrial fibrillation and its impact on understanding the ethnic distribution of ischemic stroke in the atherosclerosis risk in communities study (ARIC)”. Stroke. 2011;42:e19. doi: 10.1161/STROKEAHA.110.592097. author reply e20. [DOI] [PubMed] [Google Scholar]
- 17.Longstreth WT, Jr, Dulberg C, Manolio TA, Lewis MR, Beauchamp NJ, Jr, O’Leary D, et al. Incidence, manifestations, and predictors of brain infarcts defined by serial cranial magnetic resonance imaging in the elderly: the Cardiovascular Health Study. Stroke. 2002;33:2376–2382. doi: 10.1161/01.str.0000032241.58727.49. [DOI] [PubMed] [Google Scholar]
- 18.Longstreth WT, Jr, Manolio TA, Arnold A, Burke GL, Bryan N, Jungreis CA, et al. Clinical correlates of white matter findings on cranial magnetic resonance imaging of 3301 elderly people. The Cardiovascular Health Study. Stroke. 1996;27:1274–1282. doi: 10.1161/01.str.27.8.1274. [DOI] [PubMed] [Google Scholar]
- 19.Longstreth WT, Jr, Bernick C, Manolio TA, Bryan N, Jungreis CA, Price TR. Lacunar infarcts defined by magnetic resonance imaging of 3660 elderly people: the Cardiovascular Health Study. Arch Neurol. 1998;55:1217–1225. doi: 10.1001/archneur.55.9.1217. [DOI] [PubMed] [Google Scholar]
- 20.Psaty BM, Manolio TA, Kuller LH, Kronmal RA, Cushman M, Fried LP, et al. Incidence of and risk factors for atrial fibrillation in older adults. Circulation. 1997;96:2455–2461. doi: 10.1161/01.cir.96.7.2455. [DOI] [PubMed] [Google Scholar]
- 21.Piccini JP, Hammill BG, Sinner MF, Jensen PN, Hernandez AF, Heckbert SR, et al. Incidence and prevalence of atrial fibrillation and associated mortality among Medicare beneficiaries, 1993–2007. Circ Cardiovasc Qual Outcomes. 2012;5:85–93. doi: 10.1161/CIRCOUTCOMES.111.962688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patton KK, Ellinor PT, Heckbert SR, Christenson RH, DeFilippi C, Gottdiener JS, et al. N-terminal pro-B-type natriuretic peptide is a major predictor of the development of atrial fibrillation: the Cardiovascular Health Study. Circulation. 2009;120:1768–1774. doi: 10.1161/CIRCULATIONAHA.109.873265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kalantarian S, Stern TA, Mansour M, Ruskin JN. Cognitive impairment associated with atrial fibrillation: a meta-analysis. Ann Intern Med. 2013;158:338–346. doi: 10.7326/0003-4819-158-5-201303050-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Snyder ML, Soliman EZ, Whitsel EA, Gellert KS, Heiss G. Short-term repeatability of electrocardiographic P wave indices and PR interval. J Electrocardiol. 2014;47:257–263. doi: 10.1016/j.jelectrocard.2013.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hart RG, Diener HC, Coutts SB, Easton JD, Granger CB, O’Donnell MJ, et al. Embolic strokes of undetermined source: the case for a new clinical construct. Lancet Neurol. 2014;13:429–438. doi: 10.1016/S1474-4422(13)70310-7. [DOI] [PubMed] [Google Scholar]
- 26.Morris JJ, Jr, Estes EH, Jr, Whalen RE, Thompson HK, Jr, McIntosh HD. P-wave analysis in valvular heart disease. Circulation. 1964;29:242–252. doi: 10.1161/01.cir.29.2.242. [DOI] [PubMed] [Google Scholar]
- 27.Alpert MA, Munuswamy K. Electrocardiographic diagnosis of left atrial enlargement. Arch Intern Med. 1989;149:1161–1165. [PubMed] [Google Scholar]
- 28.de Leeuw FE, de Groot JC, Oudkerk M, Kors JA, Hofman A, van Gijn J, et al. Atrial fibrillation and the risk of cerebral white matter lesions. Neurology. 2000;54:1795–1801. doi: 10.1212/wnl.54.9.1795. [DOI] [PubMed] [Google Scholar]
- 29.Lavy S, Stern S, Melamed E, Cooper G, Keren A, Levy P. Effect of chronic atrial fibrillation on regional cerebral blood flow. Stroke. 1980;11:35–38. doi: 10.1161/01.str.11.1.35. [DOI] [PubMed] [Google Scholar]
- 30.Russo C, Jin Z, Liu R, Iwata S, Tugcu A, Yoshita M, et al. LA volumes and reservoir function are associated with subclinical cerebrovascular disease: the CABL (Cardiovascular Abnormalities and Brain Lesions) study. JACC Cardiovasc Imaging. 2013;6:313–323. doi: 10.1016/j.jcmg.2012.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Benjamin EJ, D’Agostino RB, Belanger AJ, Wolf PA, Levy D. Left atrial size and the risk of stroke and death. The Framingham Heart Study. Circulation. 1995;92:835–841. doi: 10.1161/01.cir.92.4.835. [DOI] [PubMed] [Google Scholar]
- 32.Barnes ME, Miyasaka Y, Seward JB, Gersh BJ, Rosales AG, Bailey KR, et al. Left atrial volume in the prediction of first ischemic stroke in an elderly cohort without atrial fibrillation. Mayo Clin Proc. 2004;79:1008–1014. doi: 10.4065/79.8.1008. [DOI] [PubMed] [Google Scholar]
- 33.Longstreth WT, Jr, Kronmal RA, Thompson JL, Christenson RH, Levine SR, Gross R, et al. Amino terminal pro-B-type natriuretic peptide, secondary stroke prevention, and choice of antithrombotic therapy. Stroke. 2013;44:714–719. doi: 10.1161/STROKEAHA.112.675942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Folsom AR, Nambi V, Bell EJ, Oluleye OW, Gottesman RF, Lutsey PL, et al. Troponin T, N-Terminal pro-B-type natriuretic peptide, and incidence of stroke: the atherosclerosis risk in communities study. Stroke. 2013;44:961–967. doi: 10.1161/STROKEAHA.111.000173. [DOI] [PMC free article] [PubMed] [Google Scholar]