Background: The roles of JLP in CD40 trafficking and signaling in B lymphocytes remain elusive.
Results: JLP deficiency in B lymphocytes resulted in selective diminished CD40 internalization and activation of MAPK and JNK.
Conclusion: JLP-mediated CD40 internalization is essential for downstream JNK and MAPK signaling in B lymphocytes.
Significance: We report a novel role of JLP in the bridging of CD40 internalization and CD40-dependent signaling in B lymphocytes.
Keywords: Cell Signaling, Mitogen-activated Protein Kinase (MAPK), Receptor, Scaffold Protein, Trafficking
Abstract
CD40 expression on the surface of B lymphocytes is essential for their biological function and fate decision. The engagement of CD40 with its cognate ligand, CD154, leads to a sequence of cellular events in B lymphocytes, including CD40 cytoplasmic translocation, a temporal and spatial organization of effector molecules, and a cascade of CD40-induced signal transduction. The JLP scaffold protein was expressed in murine B lymphocytes. Using B lymphocytes from jlp-deficient mice, we observed that JLP deficiency resulted in defective CD40 internalization upon CD154/CD40 engagement. Examination of interactions and co-localization among CD40, JLP, dynein, and Rab5 in B lymphocytes suggested that CD40 internalization is a process of JLP-mediated vesicle transportation that depends on Rab5 and dynein. JLP deficiency also diminished CD40-dependent activation of MAPK and JNK, but not NF-κB. Inhibiting vesicle transportation from the direction of cell periphery to the cell center by a dynein inhibitor (ciliobrevin D) impaired both CD154-induced CD40 internalization and CD40-dependent MAPK activities in B lymphocytes. Collectively, our data demonstrate a novel role of the JLP scaffold protein in the bridging of CD154-triggered CD40 internalization and CD40-dependent signaling in splenic B lymphocytes.
Introduction
CD40 is a member of the TNF receptor superfamily. It is expressed in various immune cells, such as B lymphocytes, dendritic cells, and macrophages. CD154 (a ligand of CD40) is transiently expressed on the surface of activated CD4+ T cells. The CD40-CD154 interaction results in the binding of TNF receptor-associated factors (TRAFs)4 to the CD40 cytoplasmic tail and subsequent activation of MAPK cascades. MAPK signaling triggered by CD40 engagement is pivotal for shaping B lymphocyte function and fate, such as immunoglobulin class switching, memory B lymphocyte development, affinity maturation and germinal center formation, cytokine production, and plasma cell survival (1).
In MAPK signal transduction, scaffold proteins are needed to coordinate the signaling network by organizing discrete components in space and time. JNK-interacting proteins (JIPs) are defined as scaffold proteins in the JNK and p38 signaling pathways. Several members of the JIP family have been discovered: JIP1–4 and JNK-associated leucine zipper protein (JLP), which assemble various components in the JNK and p38 signaling pathways (2). Aside from roles in the control of signaling flow, the JLP scaffold protein is also involved in intracellular molecular trafficking or vesicle transportation (3). JLP has been documented, along with other JIPs, to be important in vesicle or protein transport via its association with the motor protein kinesin or dynein, which is generally required for anterograde or retrograde transport of cargo between the cell center and periphery along microtubules (4, 5).
Upon receptor-ligand engagement, receptor internalization is a common mechanism through which the surface receptor is transported to the cytoplasm to down-regulate receptor density on the cell surface and to initiate downstream signal transduction. Ligand engagement leads to the formation of CD40 lipid rafts on the cell membrane, followed by CD40 internalization and downstream signaling (6–10). Using a gene-silencing approach, we reported previously that the JLP scaffold protein regulated cytoplasmic trafficking of CD40 receptors in dendritic cells (11). However, whether JLP plays a role in regulating CD154-initiated CD40 receptor internalization and downstream signaling in B lymphocytes is unknown. Here, we examined, for the first time, the role of JLP and its underlying mechanism in the regulation of CD154-induced CD40 internalization and MAPK signaling in B lymphocytes.
EXPERIMENTAL PROCEDURES
Animals
Wild-type (jlp+/+) and homozygous (jlp−/−) mice were generated by inbreeding jlp+/− mice, and they were genotyped by RT-PCR and Western blotting as described previously (12). 8–10-week-old weight- and sex-matched jlp+/+ and jlp−/− littermates were used in these experiments. Mice were housed under specific pathogen-free conditions at the Center for Animal Experiments at Wuhan University. All animal experiments were approved by the Animal Ethics Review Board of Wuhan University and performed in accordance with the guidelines of the National Health and Medical Research Council of China.
Reagents
Recombinant mouse CD40 ligand (rCD154) was from R&D Systems (Minneapolis, MN). FITC-conjugated anti-CD19, phycoerythrin-conjugated anti-CD80, allophycocyanin-conjugated anti-CD40, allophycocyanin-conjugated anti-CD86, allophycocyanin-conjugated anti-MHC-II, and rat anti-CD40 (1C10) antibodies and 7-aminoactinomycin D were purchased from BioLegend (San Diego, CA). LPS (Escherichia coli O111:B4) and anisomycin were from Sigma-Aldrich. The dynein inhibitor ciliobrevin D and mouse anti-DIC antibody were from Merck Millipore (Billerica, MA). Anti-ERK, anti-JNK, anti-p38, anti-phospho-ERK (Thr-202/Tyr-204), anti-phospho-JNK (Thr-183/Tyr-185), anti-phospho-p38 (Thr-180/Tyr-182), anti-phospho-IκBα (Ser-32), and anti-phospho-c-Jun (Ser-73) antibodies were purchased from Cell Signaling Technology (Danvers, MA). Rabbit anti-JLP, rabbit anti-CD40, and rabbit anti-dynein intermediate chain (DIC) were purchased from Abcam (Cambridge, MA).
Cell Culture
B lymphocytes (CD19+CD5−) were purified from splenocytes with BD IMag mouse B lymphocyte enrichment set-DM and BD IMag cell separation magnet (BD Biosciences) for negative selection. The purity of the BD IMag-sorted cell populations was ∼88–95%. Sorted B lymphocytes were cultured in complete medium (RPMI 1640 medium supplemented with 10% FCS (Invitrogen) and 1% penicillin/streptomycin (Invitrogen)) at 37 °C in a 5% CO2 incubator. WEHI-279 B cells (American Type Culture Collection, Manassas, VA) were cultured in DMEM supplemented with 10% FCS, 1% penicillin/streptomycin, and 2-mercaptoethanol (Merck Millipore). Plasmids encoding shRNA targeting jlp or mock were described previously (11). Plasmid transfection was carried out using HiPerFect transfection reagent (Qiagen, Hilden, German) following the manufacturer's instructions. HEK293 cells were maintained in complete medium. An empty vector or a vector containing mouse CD154 (InvivoGen) was used in plasmid transfection with X-tremeGENE HP DNA transfection reagent (Roche Diagnostics GmbH, Mannheim, Germany) following the manufacturer's protocol. The efficiency of transfection was verified by flow cytometry analysis of surface CD154 expression in HEK293 cells.
Flow Cytometry Analysis
Cell surface and intracellular staining was performed according to the manufacturer's instructions (Biolegend). In brief, for surface staining, cells were harvested, washed twice, stained with fluorochrome-conjugated antibody for 30 min on ice in the dark, washed again, and analyzed by flow cytometry. 7-Aminoactinomycin D was added to the samples before acquisition to exclude dead cells. For intracellular staining, Cytofix/Cytoperm and Perm/Wash buffers (BD Biosciences) were used to fix and permeabilize cells, respectively. FACS was performed with a BD Accuri C6 cytometer (BD Biosciences), and data were analyzed using FlowJo software (Tree Star, Ashland, OR).
Western Blotting
Cells (3 × 106) were lysed in CelLytic M supplemented with PMSF and protease inhibitor mixture (Sigma-Aldrich). Protein concentrations of lysed cells were measured by BCA assay (Pierce). Cell extracts were subjected to electrophoresis in a XCell SureLock mini-cell system (Invitrogen). Proteins were then transferred to PVDF membranes (Merck Millipore) that were blocked in TBST (TBS containing 0.1% Tween 20) and 5% skim milk powder for 1 h at room temperature and cultured with the indicated primary antibodies at 4 °C overnight. The next day, membranes were prewashed three times for 5 min with TBST before incubation with IRDye 800CW secondary antibodies (LI-COR Biotechnology, Lincoln, NE) for 1 h at room temperature. After washing three times for 5 min with TBST, membranes were scanned using an Odyssey CLx infrared imaging system (LI-COR Biotechnology), and data were acquired with Odyssey application software. Some protein bands were quantified with Quantity One analysis software (Bio-Rad).
Immunoprecipitation
Cells were lysed, and cell lysates were precleared by adding an appropriate control IgG and Protein G PLUS/Protein A-agarose (Merck Millipore). Precleared lysates were immunoprecipitated by overnight incubation with primary antibodies and subsequent incubation with Protein G PLUS/Protein A-agarose for 2 h. Samples were then separated by SDS-PAGE and analyzed by Western blotting as described above. An isotype-specific IgG-agarose was applied as a negative control in these experiments.
Confocal Microscopy
Purified splenic B lymphocytes were harvested, washed three times with ice-cold PBS, and fixed with 2% paraformaldehyde for 20 min at room temperature. After blocking with blocking buffer (PBS containing 10% donkey serum), samples were incubated with primary antibodies (from rabbit, mouse, or rat) at 4 °C overnight. Alexa Fluor 488- or Alexa Fluor 594-conjugated donkey anti-rabbit, anti-mouse, and anti-rat IgG antibodies (Jackson ImmunoResearch Laboratories, West Grove, PA) were added and incubated for 1 h. After three washings with PBS, cells were visualized using a PerkinElmer UltraVIEW VoX spinning disk confocal microscope system with a ×100 oil immersion objective lens.
Statistical Analysis
SPSS software was used to analyze data, and a two-tailed Student's t test was applied to determine differences between groups. p < 0.05 was considered statistically significant. Data are presented as mean ± S.D.
RESULTS
JLP Deficiency Has No Effect on Splenic Lymphocyte Numbers and Splenic B Cell Phenotypes
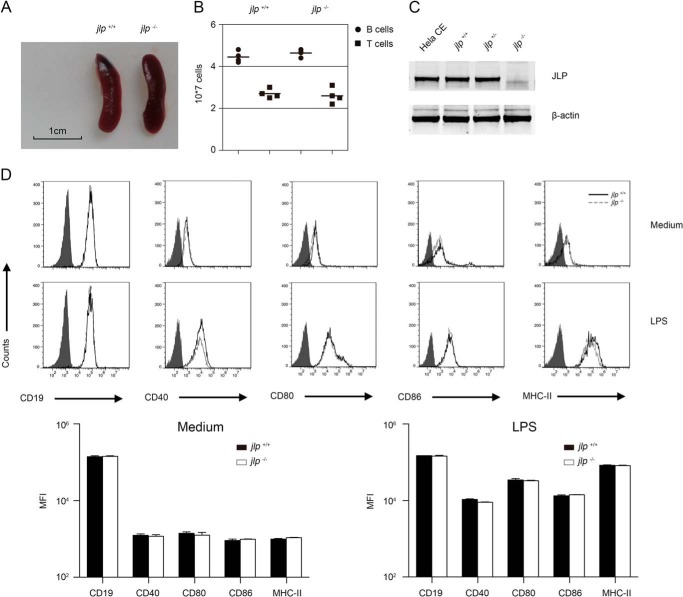
Previous studies suggest that the JLP protein is expressed at moderate levels in the brain, lung, spleen, and ovary and at low levels in the heart, liver, kidney, epididymis, and uterus (12). Thus, JLP deficiency may cause abnormalities in the spleen. To test this, we first examined the spleen volume of both jlp−/− and jlp+/+ mice and observed no significant differences in spleen size and absolute numbers of splenic T and B lymphocytes (Fig. 1, A and B). JLP is expressed in some immortal cell lines and primary dendritic cells (13–15), but its expression in primary B lymphocyte has not yet been reported. Here, we found that JLP proteins are expressed in B lymphocytes (Fig. 1C). To examine whether JLP deficiency affects development of fully differentiated B lymphocytes and LPS-induced activation, we compared the surface expression of CD19, CD40, CD80, CD86, and MHC-II in resting splenic B lymphocytes from jlp+/+ and jlp−/− mice and found the markers to be comparable. Upon LPS stimulation, we observed similar up-regulation of the surface expression of CD19, CD80, CD86, and MHC-II in the activated jlp+/+ and jlp−/− B lymphocytes (Fig. 1D). Of note, no significant difference in surface CD40 expression was observed in the LPS-matured jlp+/+ and jlp−/− B lymphocytes (Fig. 1D).
FIGURE 1.
JLP deficiency has no effect on splenic lymphocyte numbers and B cell phenotypes. A, jlp−/− and jlp+/+ mice had similar spleen volumes. Spleens were obtained from 8-week-old mice, and one representative result is shown. B, analysis of absolute numbers of splenic B lymphocytes (CD19+) and T cells (CD3+) from four pairs of age- and weight-matched jlp+/+ and jlp−/− mice. Horizontal bars are mean values. C, expression of JLP in splenic B lymphocytes from jlp+/+ and jlp−/− mice. Purified splenic B lymphocyte lysates were subjected to SDS-PAGE and analyzed by Western blotting. HeLa cell extract (Hela CE) and actin were used as positive and loading controls, respectively. D, phenotype analysis of splenic B lymphocytes from two mouse genotypes with or without LPS (1 μg/ml) stimulation for 24 h. B lymphocytes were stained with antibodies specific to CD19, CD40, CD80, CD86, and MHC-II. Cells were counted with a flow cytometer, and the histograms represent surface expression of the indicated markers on gated CD19+ B lymphocytes from jlp+/+ (solid black lines) and jlp−/− (dashed gray lines) mice. The filled curves are negative controls. The results from statistical analysis of the mean fluorescence index (MFI) of each marker from three independent experiments are shown. Error bars indicate S.D.
Scaffold Protein JLP Is Required for CD154-induced CD40 Internalization
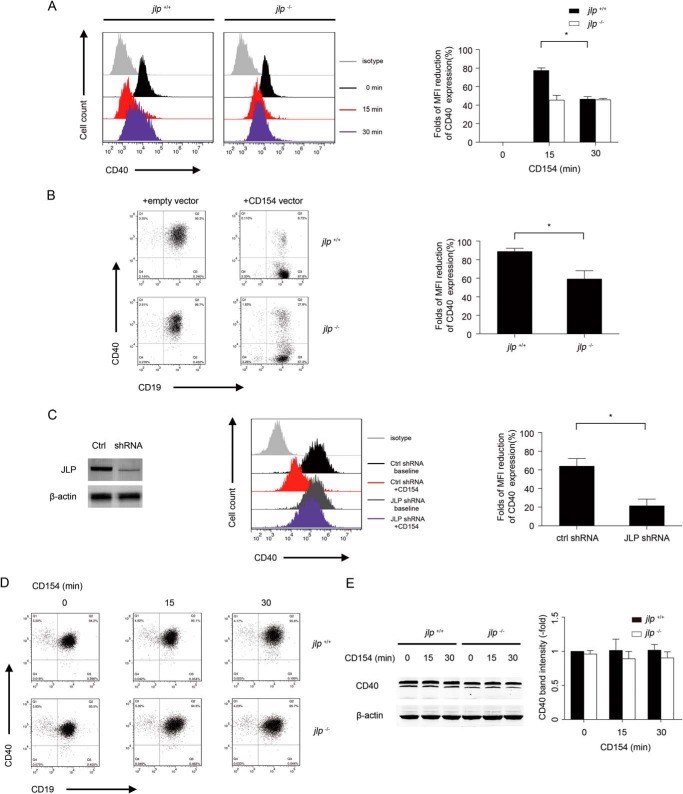
Engagement of the surface CD40 receptor by its cognate ligand CD154 induces CD40 internalization in B lymphocytes and vascular endothelial cells (10, 16–19). The molecular mechanism underlying CD154-induced CD40 internalization has not yet been fully investigated. Using a gene-silencing approach, we demonstrated previously that LPS-stimulated JLP-knockdown dendritic cells were impaired in regulating intracellular CD40 trafficking upon CD40 activation (11). The availability of the JLP-deficient mice allowed us to further examine whether JLP regulation of CD40 internalization is a common mechanism observed in B lymphocytes. As freshly isolated splenic B lymphocytes express low levels of surface CD40 molecules (Fig. 1D), LPS (1 μg/ml) was used to up-regulate surface CD40 expression on B lymphocytes in all of the CD154 stimulation experiments described in the work. We confirmed that addition of CD154 did not interfere with the binding of anti-CD40 antibody to B lymphocytes (data not shown). Upon stimulation of rCD154, surface CD40 expression on the wild-type (jlp+/+) B lymphocytes was rapidly down-regulated within 15 min and recovered at 30 min. In contrast, the surface CD40 down-regulation induced by rCD154 was mitigated in the jlp−/− B lymphocytes (Fig. 2A).
FIGURE 2.
CD40 internalization after CD154 ligation is mediated by the scaffold protein JLP. A, CD40 expression on splenic B lymphocytes with rCD154 stimulation for different times. B lymphocytes from jlp+/+ or jlp−/− mice pre-activated with LPS (1 μg/ml) were stimulated with rCD154 (1 μg/ml) for the indicated times and subsequently subjected to CD19 and CD40 staining. CD40 expression on gated CD19+ B lymphocytes is shown in the histograms. The percentage MFI reduction of CD40 was calculated by dividing the CD40 MFI at the indicated time points by that at 0 min. B, CD40 expression on splenic B lymphocytes co-cultured with CD154-transfected HEK293 cells. Splenic B lymphocytes from jlp+/+ or jlp−/− mice were co-cultured with mock- or CD154-transfected HEK293 cells (HEK293/B cell ratio of 1:50) for 15 min. Cells were harvested and subjected to flow cytometry analysis of CD40 expression. C, CD40 expression on siRNA-transfected WEHI-279 B cells upon rCD154 stimulation. JLP knockdown in WEHI-279 cells with siRNA transfection was confirmed by immunoblot analysis. The percentage MFI reduction of CD40 was calculated by dividing the CD40 MFI after CD154 treatment by that at baseline. Ctrl, control. Total CD40 expression in splenic B lymphocytes at the indicated time points upon soluble CD154 treatment was examined by flow cytometry (D) and immunoblotting (E). For flow cytometry, cells were permeabilized for CD40 staining. The percentage MFI reduction of CD40 expression at the indicated time points is compared with that at 0 min. For immunoblotting, whole-cell lysates were prepared as described above. In the flow cytometry analysis, data from three different experiments were collected and statistically analyzed. In the immunoblot analysis, the indicated band intensity of JLP was normalized by the relevant band intensity of β-actin. Means ± S.D. were collected from at least three independent experiments. Error bars indicate S.D. *, p < 0.05 (Student's t test).
We confirmed this finding further using a jlp gene-silencing approach and CD40L-HEK293 transfectants (which express membrane-bound CD154, a physiologic form of CD154 in CD40 stimulations) in the activation of surface CD40 on B lymphocytes. JLP-specific shRNA was delivered into a mature B cell line (WEHI-279) to knock down endogenous expression of JLP. Impaired CD40 internalization was observed in the JLP-knockdown WEHI-279 B cells upon CD154 engagement (Fig. 2C). To examine the role of JLP in the membrane-bound CD154-induced CD40 internalization in B lymphocytes, purified jlp+/+ and jlp−/− B cells were co-cultured with mock-transfected HEK293 cells or with HEK293 cells transfected with a CD154-expressing vector. We observed strong surface CD40 down-regulation in jlp+/+ B cells after co-culture with the CD154-transfected HEK293 cells. In contrast, only a slight decrease in surface CD40 was detected in jlp−/− B cells (Fig. 2B). Down-regulation of surface CD40 was a result of CD40 internalization because total CD40 expression (both surface and intracellular, determined by flow cytometry analysis and Western blotting) remained stable during CD40 ligation in both jlp+/+ and jlp−/− mice (Fig. 2, D and E).
JLP Interacts with CD40 during CD40 Internalization
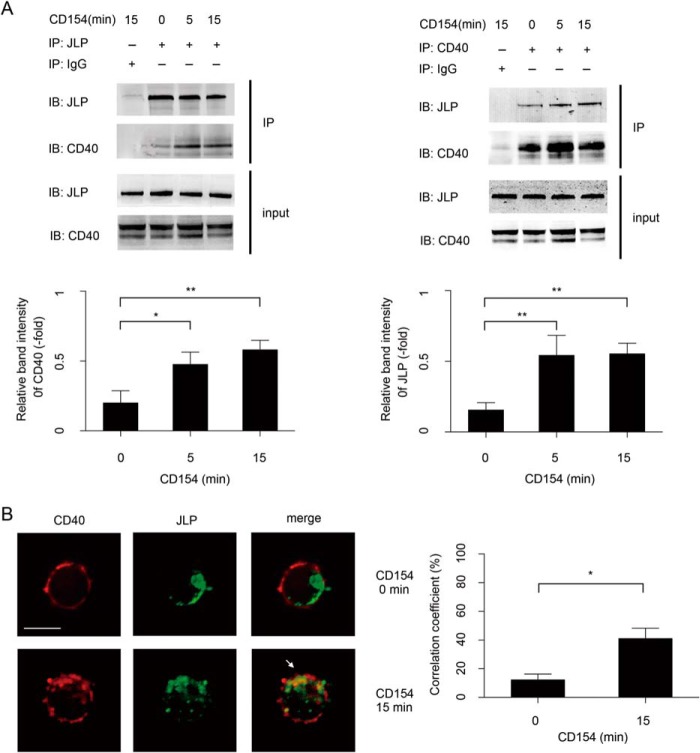
We next examined whether JLP proteins associate with CD40 in the internalization process. Using anti-CD40 and anti-JLP antibodies in co-immunoprecipitation experiments, we observed that there was a basal level of JLP and CD40 association before rCD154 stimulation. Upon addition of rCD154, the association of JLP and CD40 was increased by 2.2-fold at 5 min and by 2.7-fold at 15 min as determined by immunoprecipitation of JLP (Fig. 3A, left panel) or by 4.2-fold at 5 min and by 4.3-fold at 15 min as determined by immunoprecipitation of CD40 (Fig. 3A, right panel). Confocal microscopy also confirmed the co-localization of CD40 with JLP in the intracellular vesicles after B lymphocytes were stimulated with rCD154 for 15 min (Fig. 3B). These results demonstrate that CD40-CD154 engagement promotes the formation of CD40-JLP complexes in stimulated B lymphocytes.
FIGURE 3.
JLP interacts with CD40 in B lymphocytes. A, cell extracts of splenic B lymphocytes from wild-type mice treated with rCD154 at the indicated time points were first incubated overnight with specific primary antibodies and then analyzed with the indicated antibody by co-immunoprecipitation. Input was 10% of total cell extracts, and immunoglobulin G was used as a negative control. Data are representative of three independent experiments. The indicated band intensity of immunoprecipitation (IP) was normalized to the relevant band intensity of the input. Means ± S.D. were calculated from three independent experiments. Error bars indicate S.D. *, p < 0.05; **, p < 0.01. IB, immunoblot. B, fluorescence microscopy of B lymphocytes from wild-type mice. Cells were untreated (0 min) or were treated with rCD154 for 15 min and then labeled with the indicated primary antibody followed by a fluorescence-conjugated secondary antibody. The white arrow indicates areas of overlap. Scale bar = 5 μm. Experiments were performed in triplicate. The co-localization correlation coefficients of CD40 and JLP were collected from three separate experiments and statistically analyzed using UltraVIEW software. *, p < 0.05 (Student's t test).
CD40 Internalization Depends on Both JLP and Dynein
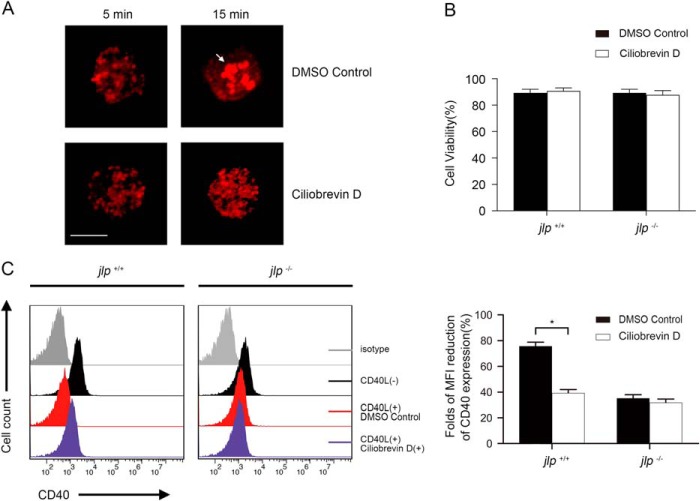
Vesicle transportation is involved in various cell biological activities, including receptor internalization and endocytosis. Motor proteins are needed to drive vesicle complexes to move along cellular microtubules. Kinesin and dynein are two major superfamilies of motor proteins identified to date and are required for anterograde or retrograde transport of cargos between the cell center and periphery along these microtubules (20). Dynein transports cargos from the cell periphery to the cell center with the help of an accessory complex, dynactin (5, 21). To investigate whether dynein is required for CD40 internalization in B lymphocytes, we studied the effects of ciliobrevin D, a recently described dynein-specific inhibitor (22–24), on the CD40 internalization induced by CD154 engagement. A previous study reported that dynein inhibition in B lymphocytes resulted in severe impairment of the net movement of antigen (anti-IgM) toward the center (25). We observed a similar effect in the ciliobrevin D-treated B lymphocytes. As shown in Fig. 4A, anti-IgM monoclonal antibody stimulation induced significant antigen intracellular gathering in a dimethyl sulfoxide control but not in the presence of ciliobrevin D, thus confirming the efficiency of ciliobrevin D in dynein inhibition. No cytotoxicity effects was observed in jlp+/+ and jlp−/− cells upon treatment with ciliobrevin D at 50 μm for 60 min (Fig. 4B). Surface CD40 down-regulation caused by rCD154 engagement was largely decreased in the ciliobrevin D-treated jlp+/+ splenic B lymphocytes. In contrast, ciliobrevin D pretreatment did not affect surface CD40 expression upon rCD154 stimulation in the jlp−/− splenic B lymphocytes (Fig. 4C). These data demonstrate that dynein is involved in CD40 internalization by CD154 ligation in B lymphocytes.
FIGURE 4.
CD40 internalization in B lymphocytes is mediated via a JLP- and dynein-dependent process. A, efficiency of ciliobrevin D treatment in dynein inhibition. Purified splenic B lymphocytes were pretreated with a dimethyl sulfoxide (DMSO) control or ciliobrevin D for 30 min. Cells were then stimulated with rat anti-mouse IgM monoclonal antibody (antigen, 2 μg/ml) for 5 and 15 min, respectively, and stained for fluorescence-conjugated donkey anti-rat secondary antibody before visualization with a microscope. The arrow indicates antigen gathering. Experiments were performed in triplicate, and representative results are shown. Scale bar = 5 μm. B, ciliobrevin D treatment caused no cytotoxicity in splenic B lymphocytes. Cells were pretreated with ciliobrevin D under the indicated conditions, and cell viability was assessed by trypan blue staining. The quantitation provided combines three independent experiments. C, purified splenic B lymphocytes from jlp+/+ and jlp−/− mice were first activated with LPS (1 μg/ml) for 24 h. Activated B lymphocytes were then left untreated (red) or were pretreated with ciliobrevin D (50 μm) for 30 min (purple), with or without further CD154 cross-linking for 15 min (black). Cells were washed three times with binding buffer to exclude residual rCD154, stained with anti-CD19 and anti-CD40 antibodies, and analyzed by flow cytometry. Negative controls (gray) were stained with an irrelevant isotype-specific antibody. The histograms show surface expression of CD40 on gated CD19+ B lymphocytes. One representative experimental result of three is shown. The CD40 MFI for each indicated group was calculated by flow cytometry. Means ± S.D. were calculated from three independent experiments. Error bars indicate S.D. *, p < 0.05 (Student's t test).
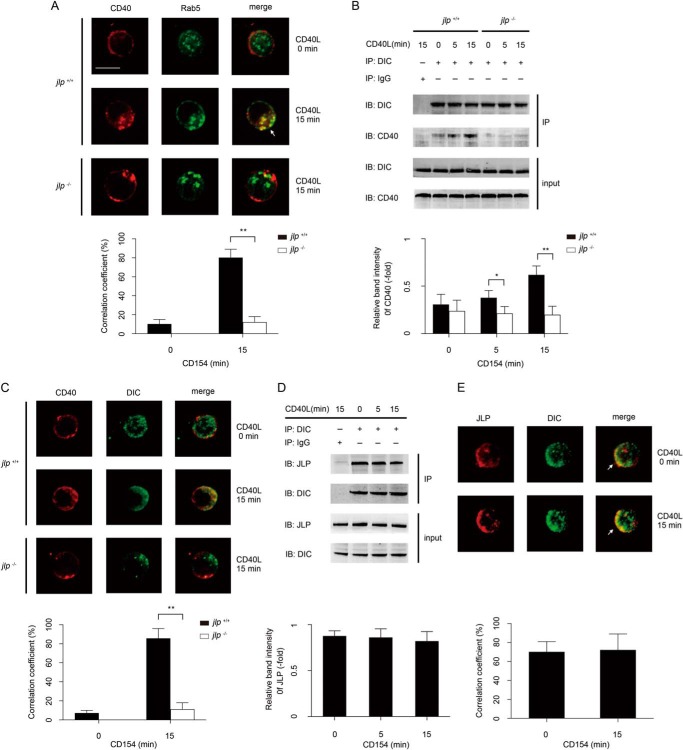
Association among CD40, Rab5, JLP, and Dynein during CD40 Internalization
Rab proteins are important in recruiting specific motors to organellar membranes or adaptor/scaffold proteins. Among these Rab molecules, Rab5 is reported to be responsible for receptor transportation from the plasma membrane to early endosomes (26, 27). To further identify the molecular mechanism of CD40 internalization in B lymphocytes, we investigated interactions and co-localizations among CD40, Rab5, JLP, and dynein in jlp+/+ and jlp−/− B lymphocytes.
We first examined the localization of CD40 and Rab5 in B lymphocytes during CD154-induced CD40 internalization. Under normal conditions, no significant co-localization of CD40 and Rab5 can be observed because CD40 and Rab5 are located in different cellular compartments. However, 15 min after CD40-CD154 engagement, substantial co-localization of CD40 and Rab5 in the same subcellular compartment was observed in the cytoplasm of jlp+/+ B lymphocytes. In contrast, CD40 ligation in jlp−/− B lymphocytes did not lead to detectable CD40 and Rab5 co-localization (Fig. 5A).
FIGURE 5.
JLP deficiency impairs association among CD40, Rab5, and dynein during CD40 engagement. A, confocal microscopy was used to visualize co-localization of CD40 and Rab5 in splenic B lymphocytes from jlp+/+ and jlp−/− mice. B lymphocytes were left untreated (0 min) or were treated with rCD154 (1 μg/ml) for 15 min. Cells were then stained with antibody specific to the appropriate molecules. The co-localization correlation coefficients of CD40 and Rab5 from jlp+/+ (black bars) and jlp−/− (white bar) B lymphocytes were collected from three separate experiments and statistically analyzed using UltraVIEW software. **, p < 0.01 (Student's t test). CD40 immunoprecipitated with anti-dynein antibody in jlp+/+ and jlp−/− mouse splenic B lymphocytes (B), and JLP immunoprecipitated with anti-dynein antibody in cell extracts of splenic B lymphocytes from wild-type mice (D). Cells were treated as described above and then immunoprecipitated. Input was 10% of total cell extracts. IgG served as a negative control. The indicated band intensity of immunoprecipitation (IP) was normalized to the relevant band intensity of the input. Means ± S.D. were calculated from three independent experiments. Error bars indicate S.D. *, p < 0.05; **, p < 0.01 (Student's t test). IB, immunoblot. The co-localization of CD40 (red) and dynein (green) in jlp+/+ and jlp−/− splenic B lymphocytes (C) or JLP (red) and dynein (green) in wild-type splenic B lymphocytes (E) was visualized using a confocal microscope. B lymphocytes were left untreated (0 min) or were treated with rCD154 (1 μg/ml) for 15 min. Cells were then stained with a primary antibody, followed by a fluorescence-conjugated secondary antibody. The white arrows indicate areas of overlap. Scale bar = 5 μm. Experiments were performed three times, and similar images were obtained. The co-localization correlation coefficients of the indicated molecules were collected from three separate experiments and statistically analyzed. **, p < 0.01 (Student's t test).
Immunoprecipitation of proteins from lysates of jlp+/+ B lymphocytes revealed a constitutive association between CD40 and DIC without CD40 engagement. This association was strongly enhanced at 15 min after rCD154 addition. In contrast, a weak association between CD40 and DIC was detected in jlp−/− B lymphocytes despite CD40 ligation (Fig. 5B). Confocal microscopy confirmed a JLP-dependent CD40-DIC association because co-localization between CD40 and DIC could be initiated by rCD154 only in jlp+/+ B lymphocytes, but not in jlp−/− B lymphocytes (Fig. 5C). Also, we confirmed an association between dynein and JLP in B lymphocytes by immunoprecipitation and confocal microscopy, and this association was not altered by CD40 ligation (Fig. 5, D and E). We found that upon CD154 engagement, internalized CD40 was recruited together with JLP, dynein, and Rab5 in the same subcellular compartment.
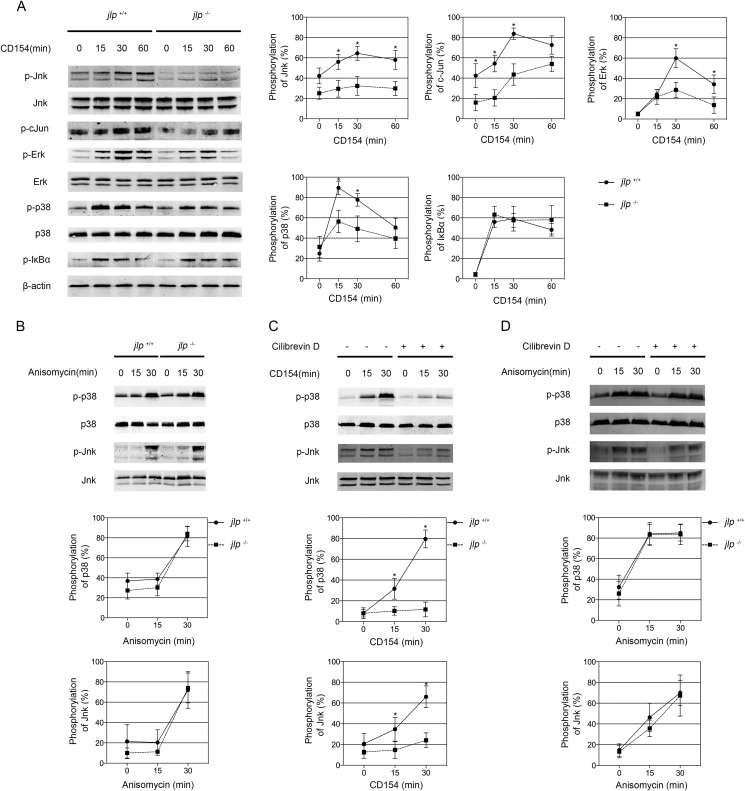
Effects of JLP Deficiency on the CD40-initiated Signaling Pathway in B Lymphocytes
In B lymphocytes, CD40 engagement leads to activation of the ERK, JNK, p38, and NF-κB signaling pathways (28–30), which are coordinated by several scaffold proteins (31, 32). JLP has been reported to potentially interact with diverse effector components involved in JNK and p38 MAPK signaling (2). We analyzed the effects of JLP deficiency on these signaling pathways in B lymphocytes. We found that CD154-induced phosphorylation of JNK and c-Jun was impaired in jlp−/− B lymphocytes compared with jlp+/+ B lymphocytes. There was slightly less CD154-induced ERK activation and moderately less p38 MAPK activation in JLP-defective B lymphocytes compared with jlp+/+ B lymphocytes. However, no substantial difference in phosphorylated IκBα was observed between the two genotypic B lymphocytes (Fig. 6A).
FIGURE 6.
JLP deficiency selectively attenuates CD154-triggered activation of the MAPK (but not NF-κB) signaling pathway in B lymphocytes. A, purified splenic B lymphocytes from jlp+/+ and jlp−/− mice were left untreated (0 min) or were treated with rCD154 (1 μg/ml) at the indicated time points. Cells were harvested, and whole-cell lysates were electrophoresed and analyzed for total ERK, p38, JNK, and relevant phosphorylated (p) forms of ERK, p38, JNK, c-Jun, and IκBα by immunoblotting. Equal protein loading was controlled by actin staining. B, purified splenic B lymphocytes from jlp+/+ and jlp−/− mice were stimulated with anisomycin (250 ng/ml) at the indicated time points. Whole-cell extracts were analyzed by immunoblotting as described above. Wild-type B lymphocytes were pretreated with or without ciliobrevin D (50 μm) for 30 min and left unstimulated (0 min) or stimulated with rCD154 (C) or anisomycin (D) at 15 min and 30 min. Cells were then harvested, and the extracts were analyzed by immunoblotting as described above. Expression of the phosphorylated molecules was normalized to the indicated total molecule expression or β-actin. Means ± S.D. were collected from at least three independent experiments. Error bars indicate S.D. *, p < 0.05 (two-tailed Student's t test).
Because JLP functions as a scaffold protein that may coordinate interactions between MAPK modules, weakened JNK and p38 MAPK signaling in jlp-deficient B lymphocytes may not be CD40-specific. To confirm its specific role in CD40 signaling, we cultured B lymphocytes in the presence of anisomycin, a specific agonist targeting the p38 and JNK signaling pathways. We observed that phosphorylation of JNK and p38 in the anisomycin-stimulated jlp−/− B lymphocytes was comparable with that in the jlp+/+ B lymphocytes (Fig. 6B). Furthermore, blocking CD154-induced CD40 cytoplasmic movement by treatment with ciliobrevin D prevented CD154-induced JNK and p38 activation in B lymphocytes (Fig. 6C). There was no difference in the activation of JNK and p38 signaling in the anisomycin-stimulated B lymphocytes pretreated with or without ciliobrevin D (Fig. 6D), indicating a specific upstream signaling flow of MAPK activation triggered by CD40 engagement and anisomycin may exist.
DISCUSSION
The engagement of CD40 by its ligand (CD154) leads to CD40 clustering in membrane rafts (7, 8), TRAF recruitment to lipid rafts (16, 17), CD40 cytoplasmic translocation (16, 17), and subsequent signal initiation and transduction (33) in B lymphocytes. Upon ligation of CD40-CD154, effector molecules are finely organized both temporally and spatially via scaffold proteins. However, to our knowledge, the details of scaffold protein-mediated CD40 trafficking and signaling are still poorly understood. Here, we investigated the role of the scaffold protein JLP in CD154-triggered CD40 internalization and subsequent CD40-dependent signaling pathways, including MAPK and NF-κB, in primary murine B lymphocytes. We observed that JLP deficiency causes defective CD40 internalization upon CD154 engagement. Through analysis of interactions and co-localizations among CD40, JLP, dynein, and Rab5 in B lymphocytes, we found that CD40 internalization is a form of JLP-mediated vesicle transportation that depends on Rab5 and dynein. JLP deficiency diminishes CD40-dependent activation of the MAPK (but not NF-κB) signaling pathway, and JNK activity is the most severely affected. The effect of JLP deficiency in signaling pathways is CD40-specific because the p38 and JNK pathways can be activated by a p38- and JNK-specific agonist (anisomycin) in jlp-deficient B lymphocytes.
Intracellular synthesized CD40 proteins are separated from the Golgi complex and transported to the cell membrane as a surface receptor. There, surface CD40 can translocate to the cytoplasm upon engagement with its ligand and initiate various cellular responses. We observed that CD40 endocytosis is halted more obviously than its recycling to the surface in the jlp-deficient B lymphocytes. However, we cannot rule out the possibility that the impaired CD40 recycling in the JLP-deficient cells is due to less surface CD40 being internalized into the cytoplasm. We also demonstrated that JLP deficiency cannot completely abolish CD40 internalization, indicating that other JIP molecules may be also involved in CD40 internalization (34–38). In fact, JIP1 and JIP3 have been confirmed to bind dynactin to regulate cargo or organellar retrograde trafficking (39–41). We speculate that CD40 cytoplasmic translocation in B lymphocytes occurs through multiple JIP-dependent mechanisms.
Several signaling pathways can be activated in response to CD40 engagement in B lymphocytes (33). Activation of the Akt (but not MAPK or NF-κB) signaling pathway has been documented to be dependent on CD40-lipid raft association, which is independent of any signaling events (42). The formation of a signaling complex containing CD40, TRAFs, and other components is required but not sufficient in CD40-dependent MAPK and NF-κB activation, emphasizing the importance of cytoplasmic translocation of the signaling complex (43). This two-stage signaling mechanism can explain the spatial and temporal separation of MAPKs and NF-κB, but the molecular details regarding how these two stages are linked is unclear. In this study, we elucidated an essential role of the scaffold protein JIP in bridging signaling complex translocation and downstream signal transduction. Previous studies show that TRAF2/3 is critical for CD40-induced JNK activation, but p38 MAPK and NF-κB are activated via multiple TRAF-dependent processes (17, 44). Also, TRAF2 or TRAF3 may have a structural function in mediating CD40 receptor internalization (17). In this study, we found that CD40-induced activation of ERK, p38, and JNK (but not NF-κB) is impaired in jlp-deficient B cells, which is consistent with previous findings showing that CD40 triggers different signal pathways via distinct molecular mechanisms. In addition, we have provided further details regarding JLP-mediated CD40 trafficking and JNK activation. With JLP deficiency, CD154-triggered CD40 internalization is severely limited, and CD40-induced activation of ERK, p38, and JNK, but not NF-κB, is impaired to various extents. Thus, different scaffold proteins are responsible for tethering CD40, TRAFs, and relevant downstream signaling kinases together to trigger different signaling events. Among these scaffold proteins, JLP may be critical for trafficking of a signaling complex containing CD40 and TRAF2/3. Whether JLP associates with TRAF2/3 directly or indirectly and whether other JIPs interact with other TRAFs to facilitate CD40-triggered signaling responses coordinated in B lymphocytes need to be examined further in future investigations.
In conclusion, we analyzed the role of the JLP scaffold protein in CD40 receptor internalization and subsequent signaling pathway activation in B lymphocytes. We described, for the first time, a putative molecular mechanism of CD40 receptor translocation and CD40-dependent signal transduction coordinated by the JLP scaffold protein. This study highlights further the exciting roles of scaffold proteins in the immune regulation of B lymphocytes.
This work was supported by National Natural Science Foundation of China Grants 81172793 and 81370800, Chongqing Science and Technology Community of Chongqing City Grant 2011BB5028, the Independent Research Project of Wuhan University, and National Key Technology R&D Program of China Grant 2011BAI10B04 (to H. W.) and by Canadian Institutes of Health Research/Manitoba Health Research Council Regional Partnership Program Operating Grant 109761 (to S. K. P. K.).
- TRAF
- TNF receptor-associated factor
- JIP
- JNK-interacting protein
- JLP
- JNK-associated leucine zipper protein
- rCD154
- recombinant CD154
- DIC
- dynein intermediate chain
- MFI
- mean fluorescence index.
REFERENCES
- 1. Graham J. P., Arcipowski K. M., Bishop G. A. (2010) Differential B-lymphocyte regulation by CD40 and its viral mimic, latent membrane protein 1. Immunol. Rev. 237, 226–248 [DOI] [PubMed] [Google Scholar]
- 2. Lee C. M., Onésime D., Reddy C. D., Dhanasekaran N., Reddy E. P. (2002) JLP: a scaffolding protein that tethers JNK/p38MAPK signaling modules and transcription factors. Proc. Natl. Acad. Sci. U.S.A. 99, 14189–14194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ikonomov O. C., Fligger J., Sbrissa D., Dondapati R., Mlak K., Deeb R., Shisheva A. (2009) Kinesin adapter JLP links PIKfyve to microtubule-based endosome-to-trans-Golgi network traffic of furin. J. Biol. Chem. 284, 3750–3761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Verhey K. J., Hammond J. W. (2009) Traffic control: regulation of kinesin motors. Nat. Rev. Mol. Cell Biol. 10, 765–777 [DOI] [PubMed] [Google Scholar]
- 5. Roberts A. J., Kon T., Knight P. J., Sutoh K., Burgess S. A. (2013) Functions and mechanics of dynein motor proteins. Nat. Rev. Mol. Cell Biol. 14, 713–726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pham L. V., Tamayo A. T., Yoshimura L. C., Lo P., Terry N., Reid P. S., Ford R. J. (2002) A CD40 signalosome anchored in lipid rafts leads to constitutive activation of NF-κB and autonomous cell growth in B cell lymphomas. Immunity 16, 37–50 [DOI] [PubMed] [Google Scholar]
- 7. Grassmé H., Jendrossek V., Bock J., Riehle A., Gulbins E. (2002) Ceramide-rich membrane rafts mediate CD40 clustering. J. Immunol. 168, 298–307 [DOI] [PubMed] [Google Scholar]
- 8. Grassmé H., Bock J., Kun J., Gulbins E. (2002) Clustering of CD40 ligand is required to form a functional contact with CD40. J. Biol. Chem. 277, 30289–30299 [DOI] [PubMed] [Google Scholar]
- 9. Reyes-Moreno C., Sharif-Askari E., Girouard J., Léveillé C., Jundi M., Akoum A., Lapointe R., Darveau A., Mourad W. (2007) Requirement of oxidation-dependent CD40 homodimers for CD154/CD40 bidirectional signaling. J. Biol. Chem. 282, 19473–19480 [DOI] [PubMed] [Google Scholar]
- 10. Chen Y., Chen J., Xiong Y., Da Q., Xu Y., Jiang X., Tang H. (2006) Internalization of CD40 regulates its signal transduction in vascular endothelial cells. Biochem. Biophys. Res. Commun. 345, 106–117 [DOI] [PubMed] [Google Scholar]
- 11. Wang H., Zhao C., Zhang M., Lee C. M., Reddy E. P., Kung S. K. (2013) A novel role of the scaffolding protein JLP in tuning CD40-induced activation of dendritic cells. Immunobiology 218, 835–843 [DOI] [PubMed] [Google Scholar]
- 12. Iwanaga A., Wang G., Gantulga D., Sato T., Baljinnyam T., Shimizu K., Takumi K., Hayashi M., Akashi T., Fuse H., Sugihara K., Asano M., Yoshioka K. (2008) Ablation of the scaffold protein JLP causes reduced fertility in male mice. Transgenic Res. 17, 1045–1058 [DOI] [PubMed] [Google Scholar]
- 13. Kashef K., Lee C. M., Ha J. H., Reddy E. P., Dhanasekaran D. N. (2005) JNK-interacting leucine zipper protein is a novel scaffolding protein in the Gα13 signaling pathway. Biochemistry 44, 14090–14096 [DOI] [PubMed] [Google Scholar]
- 14. Kashef K., Xu H., Reddy E. P., Dhanasekaran D. N. (2006) Endodermal differentiation of murine embryonic carcinoma cells by retinoic acid requires JLP, a JNK-scaffolding protein. J. Cell. Biochem. 98, 715–722 [DOI] [PubMed] [Google Scholar]
- 15. Takaesu G., Kang J. S., Bae G. U., Yi M. J., Lee C. M., Reddy E. P., Krauss R. S. (2006) Activation of p38α/β MAPK in myogenesis via binding of the scaffold protein JLP to the cell surface protein Cdo. J. Cell Biol. 175, 383–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hostager B. S., Catlett I. M., Bishop G. A. (2000) Recruitment of CD40 and tumor necrosis factor receptor-associated factors 2 and 3 to membrane microdomains during CD40 signaling. J. Biol. Chem. 275, 15392–15398 [DOI] [PubMed] [Google Scholar]
- 17. Manning E., Pullen S. S., Souza D. J., Kehry M., Noelle R. J. (2002) Cellular responses to murine CD40 in a mouse B cell line may be TRAF dependent or independent. Eur. J. Immunol. 32, 39–49 [DOI] [PubMed] [Google Scholar]
- 18. Chen J., Chen L., Wang G., Tang H. (2007) Cholesterol-dependent and -independent CD40 internalization and signaling activation in cardiovascular endothelial cells. Arterioscler. Thromb. Vasc. Biol. 27, 2005–2013 [DOI] [PubMed] [Google Scholar]
- 19. Anolik J., Looney R. J., Bottaro A., Sanz I., Young F. (2003) Down-regulation of CD20 on B cells upon CD40 activation. Eur. J. Immunol. 33, 2398–2409 [DOI] [PubMed] [Google Scholar]
- 20. Gennerich A., Vale R. D. (2009) Walking the walk: how kinesin and dynein coordinate their steps. Curr. Opin. Cell Biol. 21, 59–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Caviston J. P., Holzbaur E. L. (2006) Microtubule motors at the intersection of trafficking and transport. Trends Cell Biol. 16, 530–537 [DOI] [PubMed] [Google Scholar]
- 22. Firestone A. J., Weinger J. S., Maldonado M., Barlan K., Langston L. D., O'Donnell M., Gelfand V. I., Kapoor T. M., Chen J. K. (2012) Small-molecule inhibitors of the AAA+ ATPase motor cytoplasmic dynein. Nature 484, 125–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kikkawa M. (2013) Big steps toward understanding dynein. J. Cell Biol. 202, 15–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Clippinger A. J., Alwine J. C. (2012) Dynein mediates the localization and activation of mTOR in normal and human cytomegalovirus-infected cells. Genes Dev. 26, 2015–2026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schnyder T., Castello A., Feest C., Harwood N. E., Oellerich T., Urlaub H., Engelke M., Wienands J., Bruckbauer A., Batista F. D. (2011) B cell receptor-mediated antigen gathering requires ubiquitin ligase Cbl and adaptors Grb2 and Dok-3 to recruit dynein to the signaling microcluster. Immunity 34, 905–918 [DOI] [PubMed] [Google Scholar]
- 26. Zerial M., McBride H. (2001) Rab proteins as membrane organizers. Nat. Rev. Mol. Cell Biol. 2, 107–117 [DOI] [PubMed] [Google Scholar]
- 27. Jordens I., Marsman M., Kuijl C., Neefjes J. (2005) Rab proteins, connecting transport and vesicle fusion. Traffic 6, 1070–1077 [DOI] [PubMed] [Google Scholar]
- 28. Li Y. Y., Baccam M., Waters S. B., Pessin J. E., Bishop G. A., Koretzky G. A. (1996) CD40 ligation results in protein kinase C-independent activation of ERK and JNK in resting murine splenic B cells. J. Immunol. 157, 1440–1447 [PubMed] [Google Scholar]
- 29. Sakata N., Patel H. R., Terada N., Aruffo A., Johnson G. L., Gelfand E. W. (1995) Selective activation of c-Jun kinase mitogen-activated protein kinase by CD40 on human B cells. J. Biol. Chem. 270, 30823–30828 [DOI] [PubMed] [Google Scholar]
- 30. Sutherland C. L., Heath A. W., Pelech S. L., Young P. R., Gold M. R. (1996) Differential activation of the ERK, JNK, and p38 mitogen-activated protein kinases by CD40 and the B cell antigen receptor. J. Immunol. 157, 3381–3390 [PubMed] [Google Scholar]
- 31. Blonska M., Lin X. (2011) NF-κB signaling pathways regulated by CARMA family of scaffold proteins. Cell Res. 21, 55–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shaw A. S., Filbert E. L. (2009) Scaffold proteins and immune-cell signalling. Nat. Rev. Immunol. 9, 47–56 [DOI] [PubMed] [Google Scholar]
- 33. Rickert R. C., Jellusova J., Miletic A. V. (2011) Signaling by the tumor necrosis factor receptor superfamily in B-cell biology and disease. Immunol. Rev. 244, 115–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bowman A. B., Kamal A., Ritchings B. W., Philp A. V., McGrail M., Gindhart J. G., Goldstein L. S. (2000) Kinesin-dependent axonal transport is mediated by the Sunday Driver (SYD) protein. Cell 103, 583–594 [DOI] [PubMed] [Google Scholar]
- 35. Verhey K. J., Meyer D., Deehan R., Blenis J., Schnapp B. J., Rapoport T. A., Margolis B. (2001) Cargo of kinesin identified as JIP scaffolding proteins and associated signaling molecules. J. Cell Biol. 152, 959–970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nguyen Q., Lee C. M., Le A., Reddy E. P. (2005) JLP associates with kinesin light chain 1 through a novel leucine zipper-like domain. J. Biol. Chem. 280, 30185–30191 [DOI] [PubMed] [Google Scholar]
- 37. Muresan Z., Muresan V. (2005) c-Jun NH2-terminal kinase-interacting protein-3 facilitates phosphorylation and controls localization of amyloid-β precursor protein. J. Neurosci. 25, 3741–3751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kelkar N., Standen C. L., Davis R. J. (2005) Role of the JIP4 scaffold protein in the regulation of mitogen-activated protein kinase signaling pathways. Mol. Cell. Biol. 25, 2733–2743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cavalli V., Kujala P., Klumperman J., Goldstein L. S. (2005) Sunday Driver links axonal transport to damage signaling. J. Cell Biol. 168, 775–787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Arimoto M., Koushika S. P., Choudhary B. C., Li C., Matsumoto K., Hisamoto N. (2011) The Caenorhabditis elegans JIP3 protein UNC-16 functions as an adaptor to link kinesin-1 with cytoplasmic dynein. J. Neurosci. 31, 2216–2224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Fu M. M., Holzbaur E. L. (2013) JIP1 regulates the directionality of APP axonal transport by coordinating kinesin and dynein motors. J. Cell Biol. 202, 495–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nadiri A., Polyak M. J., Jundi M., Alturaihi H., Reyes-Moreno C., Hassan G. S., Mourad W. (2011) CD40 translocation to lipid rafts: signaling requirements and downstream biological events. Eur. J. Immunol. 41, 2358–2367 [DOI] [PubMed] [Google Scholar]
- 43. Matsuzawa A., Tseng P. H., Vallabhapurapu S., Luo J. L., Zhang W., Wang H., Vignali D. A., Gallagher E., Karin M. (2008) Essential cytoplasmic translocation of a cytokine receptor-assembled signaling complex. Science 321, 663–668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Häcker H., Tseng P. H., Karin M. (2011) Expanding TRAF function: TRAF3 as a tri-faced immune regulator. Nat. Rev. Immunol. 11, 457–468 [DOI] [PubMed] [Google Scholar]