Abstract
Objective
Family studies and twin studies demonstrate that lower urinary tract symptoms and pelvic organ prolapse are heritable. This review aimed to identify genetic polymorphisms tested for an association with lower urinary tract symptoms or prolapse, and to assess the strength, consistency, and risk of bias among reported associations.
Study Design
PubMed and HuGE Navigator were searched up to May 1, 2014, using a combination of genetic and phenotype key words, including “nocturia,” “incontinence,” “overactive bladder,” “prolapse,” and “enuresis.” Major genetics, urology, and gynecology conference abstracts were searched from 2005 through 2013. We screened 889 abstracts, and retrieved 78 full texts. In all, 27 published and 7 unpublished studies provided data on polymorphisms in or near 32 different genes. Fixed and random effects metaanalyses were conducted using codominant models of inheritance. We assessed the credibility of pooled associations using the interim Venice criteria.
Results
In pooled analysis, the rs4994 polymorphism of the ADRB3 gene was associated with overactive bladder (odds ratio [OR], 2.5; 95% confidence interval [CI], 1.7–3.6; n = 419). The rs1800012 polymorphism of the COL1A1 gene was associated with prolapse (OR, 1.3; 95% CI, 1.0–1.7; n = 838) and stress urinary incontinence (OR, 2.1; 95% CI, 1.4–3.2; n = 190). Other metaanalyses, including those for polymorphisms of COL3A1,LAMC1,MMP1,MMP3, and MMP9 did not show significant effects. Many studies were at high risk of bias from genotyping error or population stratification.
Conclusion
These metaanalyses provide moderate epidemiological credibility for associations of variation in ADRB3 with overactive bladder, and variation of COL1A1 with prolapse. Clinical testing for any of these polymorphisms cannot be recommended based on current evidence.
Key words: genetics, incontinence, lower urinary tract symptoms, overactive bladder, prolapse, systematic review
Female pelvic floor disorders, an umbrella term including urinary incontinence, bladder storage symptoms, and pelvic organ prolapse (POP) are highly prevalent.1,2 Almost one quarter of adult women report at least one clinically meaningful pelvic floor disorder,1,3 with frequent overlap between conditions.4,5 These conditions are associated with a range of comorbidities,6-8 and have a substantial impact on quality of life.9-11 There are strong associations with both age and obesity,12-15 and thus the population burden of these conditions will increase with future demographic shifts.
The existence of inherited risk factors for pelvic floor disorders has been recognized for more than 150 years,16 and there is clear familial aggregation for these conditions. Having an affected first-degree relative with incontinence or prolapse is associated with an approximately 2- to 3-fold increased risk of developing either condition, with effects measurable for all major subtypes of incontinence, and for anterior, apical, and posterior compartment prolapse.17-21 A relevant family history is associated with both earlier onset, and more rapidly progressive symptoms.22,23
Family studies provide limited information on heritability, as they do not control for shared exposure to environmental risk factors. Twin studies have been used to formally quantify the heritability of lower urinary tract symptoms (LUTS) or prolapse. In a sample of 16,886 Swedish twins aged >50 years, heritability was estimated as 41% for stress incontinence surgery, and 43% for prolapse surgery.24 Similarly for twins aged 20-46 years from the same cohort (n = 4550), heritability was estimated as 34% for stress incontinence, 37% for urgency incontinence, and 48% for nocturia.25 Among a cohort of 2336 women enrolled in the Danish Twin Register,26 heritability ranged with age from 42-49% for urgency incontinence, 27-55% for mixed incontinence, and up to 39% for stress incontinence.
Identification of the genetic variants underlying the heritability of these conditions would provide useful markers for clinical risk, prognosis, and treatment response. In addition, however, the insights provided should help explain the pathogenesis of these complex diseases, potentially offering new drug targets and preventative strategies. The aim of this systematic review was therefore to assess which candidate polymorphisms and/or candidate genes had been tested for an association with POP or LUTS in women, and to assess the strength, consistency, and potential for bias among published associations.
Materials and Methods
Eligibility criteria
The review protocol was prospectively registered (PROSPERO 2011:CRD42012001983).27 We prespecified inclusion of both case-control and cross-sectional designs, with both population-based samples and other sampling methods. We included association studies testing for any genetic polymorphism at the nucleotide level, including single-nucleotide polymorphisms (SNPs), deletions, duplications, and copy-number variants, but excluded larger microscopic variants at the karyotype level.
There are no gold standard diagnostic methods for either stress urinary incontinence (SUI) or other LUTS, as these are largely subjective symptomatic diagnoses. For POP, validated staging systems, including POP Quantification, have been widely used, but again there is no universally accepted criterion for diagnosis. We therefore expected to accept diagnostic criteria for LUTS and prolapse as specified within each study. In view of heterogeneity in definitions across studies, we tested for heterogeneity between studies with different criteria in different settings. We accepted definitions based on symptom questionnaires, clinical examination, urodynamics, or other validated assessments. We considered the population of interest as women aged ≥18 years.
Search strategy
We combined searches from PubMed, HuGE Navigator, and an extensive selection of genetic, urological, and urogynecological conference reports. We searched PubMed up to May 1, 2014, without language restrictions, using a combination of genetic and phenotype key words and Medical Subject Headings (MeSH) terms: (polymorphism OR SNP OR CNV OR “copy number variation” OR mutation OR genetic OR chromosome OR VNTR OR InDel OR microsatellite) AND (nocturia OR LUTS OR incontinence OR urgency OR “overactive bladder” OR prolapse OR “Lower Urinary Tract Symptoms”[Mesh] OR “Urinary Incontinence”[MeSH] OR “enuresis”[Mesh] OR “Pelvic Organ Prolapse”[MeSH]) NOT mitral NOT carcinoma[Title] NOT cancer[Title] NOT (animals[mh] NOT humans[mh]).
We searched HuGE Navigator, also through to May 1, 2014, using the following phenotype indexing terms: (“urination disorders” OR “urinary incontinence” OR “pelvic organ prolapse”).
In addition we searched conference abstracts for annual meetings of the American Society of Human Genetics, American Urological Association, American Urogynecologic Society, European Association of Urology, European Society of Human Genetics, International Continence Society, International Urogynecological Association, and Society of Gynecologic Surgeons 2005 through 2013.
Screening and data extraction
We developed standardized data forms for this study, and conducted pilot screening and data extraction training exercises to achieve a high level of consensus between reviewers. All screening and data extraction was then performed independently and in duplicate by methodologically trained reviewers. Reviewers screened study reports by first screening titles and abstracts to select papers for full-text assessment, then screening full-text papers to confirm eligibility of the articles. Screening discrepancies were resolved by adjudication. We hand searched reference lists of all included articles, applying the same standardized screening process. When >1 report was identified for the same association in the same study population, we included the publication with the largest sample size.
We contacted study authors by email, with a reminder after 1 month, for clarifications, additional information about methodology, and additional subgroup analyses where necessary. Data extracted included information on the setting for each study, details of the sampling strategy and sampled populations (age, parity, ethnic/racial composition, and body mass index), the overall sample size and proportion genotyped, the outcome assessments used and phenotypic definitions, the genotyping method employed, and the genotyping quality control applied. Where possible we extracted or requested from authors full genotype frequencies among both cases and controls.
Statistical analysis and risk of bias assessments
For polymorphisms assessed in ≥2 studies for the same phenotype assessed with similar case definitions, we conducted fixed or random effects metaanalyses as appropriate using the Metan28 package (Stata 12.1; StataCorp, College Station, TX). In all cases, we worked from genotype or allele frequencies, rather than using precalculated effect sizes. We did not pool data from studies with mixed male and female samples, unless results stratified by sex were available. We did not pool data from studies with composite case definitions (ie, any urinary incontinence) with those with simple case definitions (ie, SUI). In the absence of a clear rationale supporting any specific model of inheritance, we used the allelic association test/codominant models of inheritance for all polymorphisms. We assessed the credibility of pooled associations using the interim Venice criteria29 (Appendix; Supplementary Figure). We used the I2 statistic as a measure of between study heterogeneity. We recalculated the power of each study, and retested for departure from Hardy-Weinberg equilibrium. We made assessments of risk of bias in phenotype definitions, genotyping, and population stratification. We used the Harbord test of funnel plot asymmetry, and the significance chasing bias test30 to investigate possible reporting biases. Reporting of this review complies with recommendations both of the HuGE Handbook, and the PRISMA statement.31,32
Results
Search outcomes
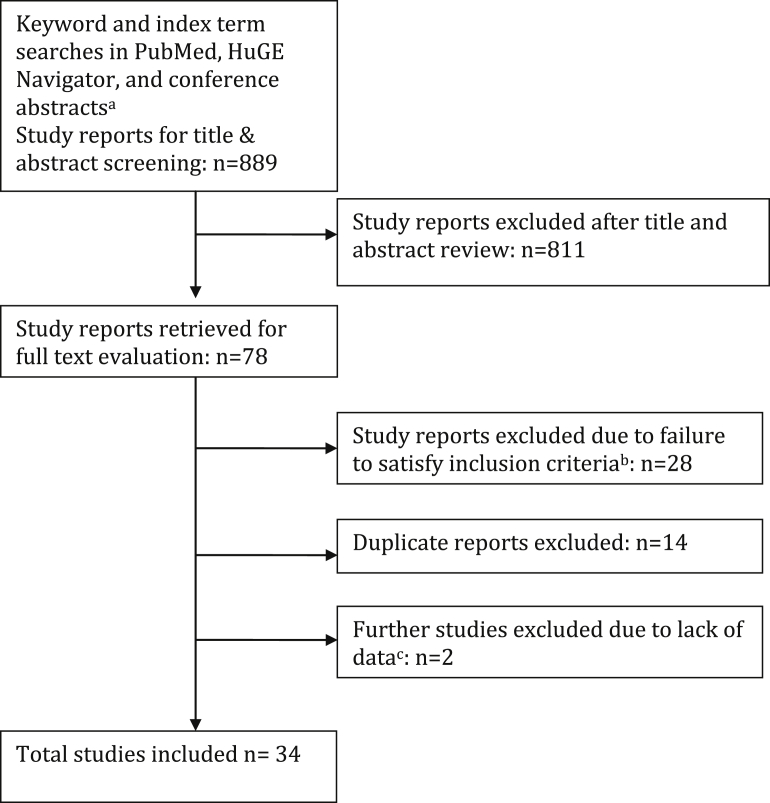
We screened 889 abstracts, and retrieved 78 full texts (Figure 1). In all, 27 published studies and 7 unpublished studies provided data (Table 1) regarding polymorphisms in or near 32 different genes (Supplementary Table 1). Most research interest has focused on variation in genes implicated in extracellular matrix organization and disassembly, with particular focus on collagen and metalloendopeptidase genes (Supplementary Table 2). A number of studies also addressed a variety of steroid hormone receptor genes. All studies investigated POP, SUI, or overactive bladder, with no available data on other individual LUTS.
Figure 1.
Flowchart outlining literature search and article evaluation process
a American Society of Human Genetics, American Urological Association, American Urogynecologic Society, European Association of Urology, European Society of Human Genetics, International Continence Society, International Urogynecological Association, and Society of Gynecologic Surgeons abstracts 2005 through 2014, using online search interfaces and/or full text search of abstract book PDFs; b Includes studies enrolling only men (n = 122), enrolling only children (n = 2), narrative reviews or letters (n = 12), inapplicable phenotype (n = 2), and other study designs including pharmacogenetic studies, gene expression studies, or methylation studies (n = 8); c Authors contacted by email for additional data from 18 studies.
Cartwright. Genetic association studies of LUTS and POP. Am J Obstet Gynecol 2015.
Table 1.
Included studies
| Study | Journal and year | Country | Descent, ethnicity, racea | Gene symbols(s) | Polymorphism(s) dbSNP ID | Case definition | Control definition | Cases genotyped, n | Controls genotyped, n |
|---|---|---|---|---|---|---|---|---|---|
| Allen-Brady et al76 | Obstet Gynecol 2011 | United States, The Netherlands | White and Northern European descent |
LINC0108b ZFAT Intergenic Intergenic Intergenic COL18A1 |
rs1455311 rs1036819 rs430794 rs8027714 rs1810636 rs2236479 |
Surgically treated/recurrent POP with family history | Population controls | 191 | 3036 |
| Campeau et al59 | Neurourol Urodyn 2011 (ICS abstract) | United States | Not stated | MMP1 | rs1144393 rs498186 rs473509 |
Surgically treated POP | Hospital controls “without POP” | 63 | 93 |
| Chen et al55 | Am J Obstet Gynecol 2010 | United States | African American and Caucasian | LAMC1 | rs10911193 rs20563 rs20558 |
POP stage >II | POP stage <II | 165 | 246 |
| Chen et al66 | Int Urogynecol J 2008 | Taiwan | Taiwanese | ESR1 | rs17847075 rs2207647 rs2234693 rs3798577 rs2228480 |
POPQ ≥2 | POPQ <2 | 88 | 153 |
| Chen et al68 | Acta Obstet Gynecol 2009 | Taiwan | Taiwanese | PGR | rs500760 rs484389 |
POPQ ≥2 | POPQ <2 | 87 | 150 |
| Chen et al78 | Am Soc Hum Genet 2013 | United States | African American and Hispanic American | PRCPb | rs2086297 | Symptomatic SUI | No SUI | ≈3343 | ≈8183 |
| Chen et al66 | Int Urogynecol J 2008 | Taiwan | Taiwanese | COL3A1 | rs1800255 rs1801184 |
POPQ ≥2 | POPQ <2 | 84 | 147 |
| Chen et al64 | Eur J Obstet Gynecol 2010 | Taiwan | Taiwanese | MMP9 | rs3918242 rs17576 rs2250889 |
POPQ ≥2 | POPQ <2 | 92 | 152 |
| Chen et al67 | Eur J Obstet Gynecol 2008 | Taiwan | Taiwanese | ESR2 | rs2987983 rs1271572 rs944459 rs1256049 rs1255998 |
POPQ ≥2 | POPQ <2 | 69 | 141 |
| Cho et al45 | Yonsei Med J 2009 | Korea | Korean | COL1A1 | rs1800012 | Surgically treated POPQ ≥3 | POPQ = 0 | 15 | 15 |
| Choy et al69 | ICS abstract 2007 | Hong Kong | Chinese | EDN1 | rs5370 rs10478694 |
POPQ ≥2 | Hospital “normal’’ controls and HapMap Han Chinese controls | 60 (rs5370) and 67 (rs10478694) | 210 |
| Cornu et al70 | World J Urol 2011 | France | Caucasian |
ESR1 CYP17A1 CYP19A1 AR |
rs2234693 rs743572 rs60271534 CAG repeat |
Treated for UI (30 UUI, 107 SUI) | No UI or OAB | 121 | 66 |
| Feiner et al42 | Int Urogynecol J 2009 | Israel | Caucasian or Ashkenazi-Jewish | COL1a1 | rs1800012 | POPQ ≥3 | POPQ <2 | 36 | 36 |
| Ferrari et al44 | Arch Gynecol Obstet 2012 | Italy | Italian |
COL1a1 MMP9 MMP1 MMP3 |
rs1800012 rs3918242 rs1799750 rs3025058 |
POPQ ≥2 | POPQ <2 | 137 | 96 |
| Ferreira et al38 | Am J Obstet Gynecol 2011 | Brazil | White or nonwhite | ADRB3 | rs4994 | Symptomatic OAB without severe SUI | No LUTS | 49 | 169 |
| Ferrell et al75 | Reprod Sci 2009 | United States | African American or Caucasian | LOXL1 | rs16958477 | POP stage ≥II | POP stage <II | 137 | 130 |
| Fu et al56 | J Urol 2009 (AUA abstract) | United States | Not stated |
LAMC1 LOXL1 |
rs10911193 | POP stage ≥III | No POP or UI | 61 | 33 |
| Honda et al37 | Neurourol Urodyn 2014 | Japan | Japanese | ADRb3 | rs4994 | Symptomatic OAB | No OAB | 100 | 101 |
| Jeon et al51 | J Urol 2009 | Korea | Korean | COL3a1 | rs111929073 | POPQ ≥2 | POPQ <2 and no SUI | 36 | 36 |
| Kim et al74 | Eur J Obstet Gynecol Reprod Biol 2014 | Korea | Korean |
GSTM1 GSTT1 GSTP1 |
Null Null rs1695 |
POPQ ≥3 | POPQ <2 | 189 | 156 |
| Kim et al73 | Menopause 2014 | Korea | Korean | PARP1 | rs1136410 | POPQ ≥3 | POPQ <2 | 185 | 155 |
| Lince et al50 | Int Urogynecol J 2014 | The Netherlands | ≈99% Dutch | COL3a1 | rs1800255 | POPQ ≥2 | POPQ <2 | 272 | 82 |
| Martins et al52 | Neurourol Urodyn 2011 | Brazil | White or nonwhite | COL3a1 | rs111929073 | POP stage ≥III | POP stage <II | 107 | 209 |
| Noronha et al71 | J Investig Med 2010 | Brazil | Predominant European/white | HTR2A | rs6313 | Symptomatic UI | Self-reported continent women, and population controls | 68 | 849 |
| Ozbek et al72 | J Obstet Gynaecol Res 2013 | Turkey | Caucasian | LOXL1 | rs2165241 rs3825942 rs1048661 |
Symptomatic SUI | No UI | 93 | 75 |
| Rodrigues et al41 | Int Urogynecol J 2008 | Brazil | White or nonwhite | COL1a1 | rs1800012 | POP stage ≥III | POP stage <II and no SUI | 107 | 209 |
| Romero and Jamison65 | J Pelv Med Surg 2008 | United States | White |
MMP1 MMP2 MMP3 MMP8 MMP9 MMP10 MMP11 TIMP1 TIMP3 |
rs2071230 rs7201 rs679620 rs35866072 rs17576 rs17435959 rs738789 rs4898 rs2016293 |
POPQ ≥3 | POPQ <2 and no UI | 45 | 38 |
| Sioutis et al47 | Int Urogynecol J 2011 | Greece | Greek | COL1a1 | rs1800012 | SUI confirmed with urodynamics and positive pad test, and postmenopausal | Healthy postmenopausal | 45 | 45 |
| Skorupski43 | Int Urogynecol J 2009 (IUGA abstract) | Poland | Polish | COL1a1 | rs1800012 | POPQ ≥2 | POPQ <2 and no UI | 120 | 97 |
| Skorupski et al46 | Am J Obstet Gynecol 2006 | Poland | Polish | COL1a1 | rs1800012 | SUI confirmed with urodynamics and positive pad test | POPQ <2 and no UI | 50 | 50 |
| Skorupski et al61 | Ginekol Polska 2010 | Poland | Polish |
MMP1 MMP3 |
rs1799750 rs3025058 |
POPQ ≥2 | POPQ <2 | 132 | 133 |
| Takeda et al36 | ICS Abstract 2002 | Japan | Japanese |
ADRb3 ADRA1A |
rs4994 rs1048101 |
Any LUTS (includes mixed group of women and men) | No LUTS | 27 | 17 |
| Velez Edwards et al77 | Am Soc Hum Gen 2013 | United States | African American and Hispanic American |
CPEb Intergenic |
rs28573326 rs113518633 |
POP stage ≥I | POP stage = 0 | 1427 | 1274 |
| Vishwajit et al60 | ICS abstract 2009 | United States | Not stated | MMP1 | rs1799750 | SUI with varying POP | Neither SUI nor POP | 40 | 15 |
| Wu et al54 | Am J Obstet Gynecol 2012 | United States | Non-Hispanic white | LAMC1 | rs10911193 rs1413390 rs20558 rs20563 rs10911206 rs2296291 rs12041030 rs12739316 rs3768617 rs2483675 rs10911211 rs41475048 rs1058177 rs12073936 |
POPQ ≥3 | POPQ <2 | 239 | 197 |
| Wu et al63 | Obstet Gynecol 2012 | United States | Non-Hispanic white | MMP9 | rs3918253 rs3918256 rs3918278 rs17576 rs2274755 rs17577 rs2236416 rs3787268 |
POPQ ≥3 | POPQ <2 | 239 | 197 |
AUA, American Urological Association; ICS, International Continence Society; IUGA, International Urogynecological Association; LUTS, lower urinary tract symptoms; OAB, overactive bladder; POP, pelvic organ prolapse; POPQ, Pelvic Organ Prolapse Quantification system; SNP, single-nucleotide polymorphism; SUI, stress urinary incontinence; UI, urinary incontinence; UUI, urge urinary incontinence.
Cartwright. Genetic association studies of LUTS and POP. Am J Obstet Gynecol 2015.
Assessments of descent/ethnicity/race as specified in primary publications, or from additional data from authors, or assumed for countries with low ethnic heterogeneity including Taiwan, Korea, and Japan
Genome-wide significant genes (P <5 × 10-8) reported in genome-wide association study.
Quantitative syntheses were possible for 11 polymorphisms in or near 7 genes: beta 3 adrenoceptor (ADRB3); collagen, type I, alpha 1 (COL1A1); collagen, type 3, alpha 1 (COL3A1); laminin gamma 1 (LAMC1); matrix metalloproteinase-1 (MMP1); matrix metalloproteinase-3 (MMP3); and matrix metalloproteinase-9 (MMP9).
ADRB3
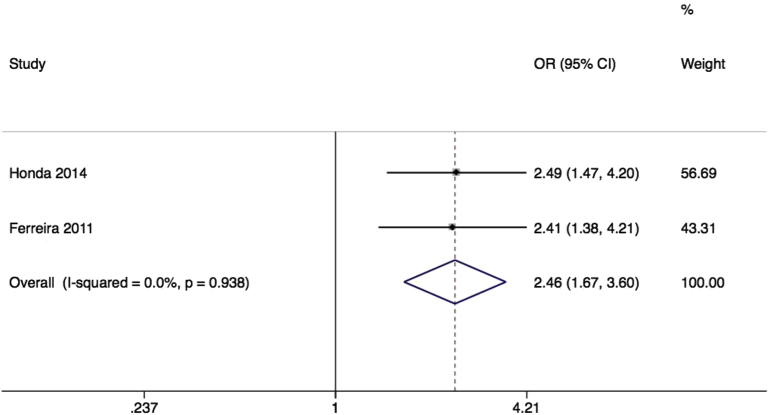
Variation in the beta-3 adrenoceptor, particularly of the rs4994 SNP, also known as Trp64Arg, has been extensively investigated in association with obesity, type 2 diabetes mellitus, and other metabolic syndrome phenotypes. The beta-3 adrenoceptor is highly expressed in bladder, and mediates detrusor muscle relaxation.33 A beta-3 adrenoceptor agonist has recently been approved for treatment of overactive bladder symptoms.34,35 One conference abstract,36 and 2 published papers37,38 provided relevant information on the common rs4994 missense mutation, of which 2 could be included in metaanalysis. In the initial report, in a heterogeneous Japanese sample of 13 men and 31 women, with diverse urological pathologies including neurogenic bladder and benign prostatic hyperplasia, the rs4994 SNP was not associated with LUTS (odds ratio [OR], 1.20; 95% confidence interval [CI], 0.32–4.47).36 Results were not available stratified by sex, and could not be included in quantitative synthesis. Subsequent reports used larger samples of Japanese women,37 and Brazilian women38 (Table 1), and looked specifically at the overactive bladder phenotype, finding a large effect size (pooled OR, 2.46; 95% CI, 1.67–3.60) (Figure 2), with no heterogeneity. Despite a lack of information about genotyping quality control (QC), and some risk of population stratification, this large effect size confers some protection from bias, providing Venice grading BBB, or moderate epidemiological credibility (Table 2).
Figure 2.
Forest plot of rs4994 SNP of ADRB3 and overactive bladder
Forest plot of studies37,38 reporting associations between rs4994 single-nucleotide polymorphism (SNP)* of beta 3 adrenoceptor gene and overactive bladder. *RefSNP alleles C/T. Plot presented as risk associated with minor allele C.
CI, confidence interval; OR, odds ratio.
Cartwright. Genetic association studies of LUTS and POP. Am J Obstet Gynecol 2015.
Table 2.
Interim Venice assessments of epidemiological credibility for each metaanalysis
| Gene | SNP | Phenotype | Studies, n | Sample with minor allelea | Pooled OR | I2 % | Deviation from HWEb | Proteus effect | Harbord test P value | Funnel plot | Genotyping QC | Risk of population stratification | Venice rating | Overall credibility |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ADRB3 | rs4994 | OAB | 2 | 136 | 2.46 | 0.0 | None | None | n/a | n/a | Not reported | Yes38c | BBB | Moderate |
| COL1A1 | rs1800012 | SUI | 2 | 92 | 2.09 | 0.0 | Yes46 | None | n/a | n/a | Not reported | Low | CBC | Weak |
| POP | 4 | 249 | 1.33 | 0.0 | None | None | .88 | Symmetric | Not reported | Yes41,42c | BBB | Moderate | ||
| COL3A1 | rs1800255 | POP | 2 | 257 | 1.19 | 0.0 | None | Yes | n/a | n/a | Not reported49/appropriate50 | None | BCB | Weak |
| rs111929073 | POP | 2 | 115 | 0.56 | 83.7 | None | None | n/a | n/a | Not reported | Yes52c | BCB | Weak | |
| LAMC1 | rs10911193 | POP | 4 | 218 | 1.12 | 0.0 | None | None | .97 | Symmetric | Appropriate54,55/not reported56 | Low | BCB | Weak |
| rs20563 | POP | 3 | 525 | 1.12 | 0.0 | None | None | .86 | Symmetric | Appropriate | Low | BCA | Weak | |
| rs20558 | POP | 3 | 551 | 1.12 | 0.0 | None | None | .93 | Symmetric | Appropriate | Low | BCA | Weak | |
| MMP1 | rs1799750 | POP | 2 | 234 | 0.83 | 74.9 | Yes61 | Yes | n/a | n/a | Not reported | Low | BCC | Weak |
| SUI | 2 | 150 | 0.88 | 3.4 | None | None | n/a | n/a | Not reported | Yes60c | BCC | Weak | ||
| MMP3 | rs3025058 | POP | 2 | 381 | 1.11 | 0.0 | Yes61 | None | n/a | n/a | Not reported | Low | BCC | Weak |
| MMP9 | rs3918242 | POP | 2 | 99 | 1.25 | 0.0 | None | None | n/a | n/a | Not reported | Low | CCC | Weak |
| rs17576 | POP | 3 | 473 | 1.05 | 68.9 | None | None | .72 | Symmetric | Not reported57/appropriate54 | Low | BCB | Weak |
Three-letter code corresponds to A through C ratings of amount of evidence, its consistency, and its protection from bias (Supplementary Figure).
HWE, Hardy Weinberg Equilibrium; OAB, overactive bladder; OR, odds ratio; POP, pelvic organ prolapse; QC, quality control; SNP, single-nucleotide polymorphism; SUI, stress urinary incontinence.
Cartwright. Genetic association studies of LUTS and POP. Am J Obstet Gynecol 2015.
Pooled sample size of participants with minor allele
Checked in controls and whole population, and metaanalysis rechecked excluding studies with significant departure
Studies each include populations with mixed descent groups without reported adjustment.
COL1A1
rs1800012 also known as the Sp1-binding site polymorphism of collagen, type I, alpha 1, modifies transcription factor binding and gene expression. It has been most extensively studied in association with osteoporosis, where the minor allele is modestly associated with reduced bone mineral density and increased fracture risk.39 Collagen, type I, alpha 1 is a major structural component of the vaginal epithelium and endopelvic fascia. The available data on gene and protein expression in pelvic tissue from women with prolapse or stress incontinence are heterogeneous but suggest increased COL1A1 expression with reduced type 1 collagen content.40 Seven studies provided data on the rs1800012 SNP in association with either POP or stress incontinence, of which 6 could be included in quantitative syntheses.
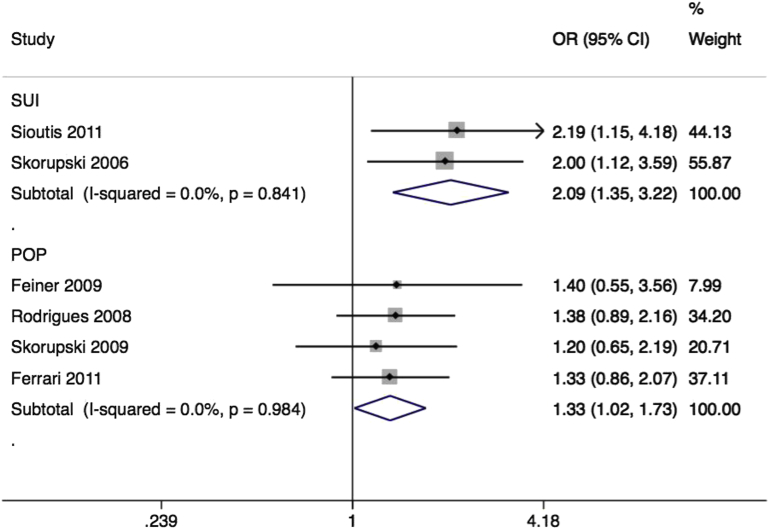
Five studies reported associations of rs1800012 with anatomical POP in Brazilian,41 Israeli,42 Polish,43 Italian,44 and Korean45 populations (Table 1). The Korean study found only the wild type GG allele among all 30 participants, and could not be included in quantitative synthesis. Despite each individual study being underpowered, the pooled effect size for the remaining 4 studies was significant (OR, 1.33; 95% CI, 1.02–1.73) (Figure 3) with low inconsistency. With limited information about genotyping QC, and a possible risk of population stratification in 2 samples,41,42 we considered that bias could not be fully excluded, providing Venice grading BBB, or moderate epidemiological credibility (Table 2).
Figure 3.
Forest plot of studies of rs1800013 SNP of COL1A1
Forest plot of studies41-44,46-47 reporting associations between rs1800012 single-nucleotide polymorphism (SNP)* of collagen type 1 alpha 1 gene and either stress urinary incontinence (SUI) or pelvic organ prolapse (POP). *RefSNP alleles G/T. Plot presented as risk associated with minor allele T.
CI, confidence interval; OR, odds ratio.
Cartwright. Genetic association studies of LUTS and POP. Am J Obstet Gynecol 2015.
Two studies of Polish46 and Greek47 women reported associations of the same polymorphism with stress incontinence, in both cases using a combined symptomatic and objectively measured case definition. The pooled effect size was large (OR, 2.09; 95% CI, 1.35–3.22) (Figure 3) with no heterogeneity (I2 = 0%). There was significant deviation from Hardy-Weinberg equilibrium in one sample,46 suggesting significant potential for bias. However, exclusion of this study would not change the result. With high risk of bias the Venice grading was CBC, or weak epidemiological credibility (Table 2).
COL3A1
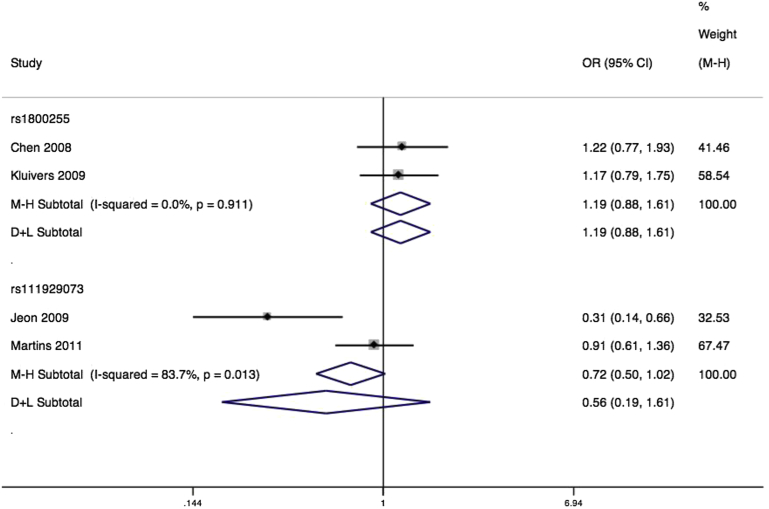
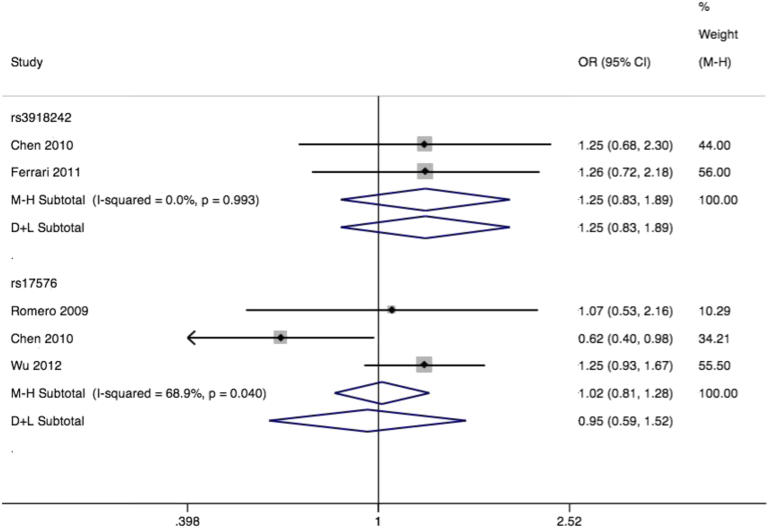
A large number of mutations in collagen, type 3, alpha 1 have been associated with vascular Ehlers-Danlos syndrome. Inconsistent evidence suggests that urinary incontinence and prolapse may be prevalent among women with Ehlers-Danlos.48 Collagen, type 3 has a particular function in tissue repair, and is typically overexpressed in pelvic tissues from women with prolapse.40 We identified studies testing associations with 2 missense variants rs1800255 and rs111929073, as well as 1 synonymous SNP rs1801184. Both missense variants had been tested in 2 studies, and therefore could be combined in quantitative syntheses. Separate Taiwanese49 and Dutch50 studies found a nonsignificant pooled association between rs1800255 and anatomic prolapse (OR, 1.19; 95% CI, 0.88–1.61) (Figure 4), with no heterogeneity (Table 2).
Figure 4.
Forest plot of COL3A1 SNPs and prolapse
Forest plot of studies49-52 reporting associations between rs1800255* and rs111929073* single-nucleotide polymorphisms (SNPs) of collagen type 3, alpha 1 gene and pelvic organ prolapse with either fixed or random effects models**. *For both SNPs RefSNP alleles A/G. Plot presented as risk associated with minor allele A. **Mantel-Haenszel fixed effects model (M-H)/DerSimonian and Laird random effects model (D+L).
CI, confidence interval; OR, odds ratio.
Cartwright. Genetic association studies of LUTS and POP. Am J Obstet Gynecol 2015.
For rs111929073, separate Korean51 and Brazilian52 samples demonstrated a nonsignificant pooled effect (OR, 0.56; 95% CI, 0.19–1.61) (Figure 4) with high heterogeneity (I2 = 83.7%, P < .01). Case definitions were similar for the 2 studies, making this an unlikely source of heterogeneity. The primary Korean study had suggested a large protective effect of the minor allele, and the heterogeneity between studies might instead be explained by differences in populations, or a simple Proteus effect.
LAMC1
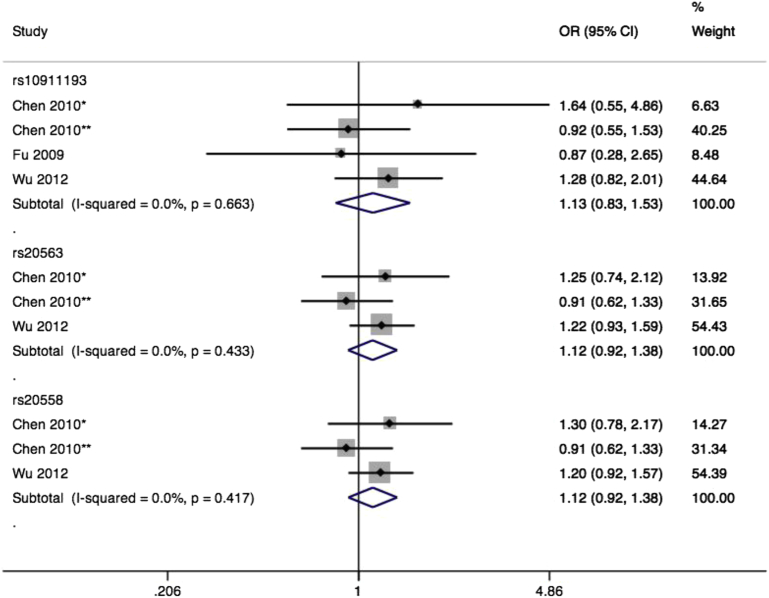
Laminin gamma 1 is 1 of 3 kinds of laminin chain that combine to make different laminin isoforms. These extracellular matrix glycoproteins are an important constituent of basement membranes, with roles in cell adhesion and migration. LAMC1 was initially proposed as a candidate gene for prolapse in a linkage study of 9 individuals from a family affected by early-onset severe prolapse.53 We identified 3 further studies all from the United States that attempted to replicate this initial report of an association with rs10911193,54-56 with all 3 including testing of additional SNPs (Table 1).
All 3 individual studies found no association for rs10911193, with a nonsignificant pooled effect (OR, 1.13; 95% CI, 0.83–1.53) (Figure 5) and no heterogeneity. There was no evidence of small study bias or publication bias. Genotyping QC was generally well documented for these studies, and population stratification appropriately accounted for. Two of the studies54,55 provided further data on rs20563 and rs20558, 2 missense SNPs in near perfect linkage disequilibrium, but again with nonsignificant pooled effects (both OR, 1.12; 95% CI, 0.92–1.38) (Figure 5) and no heterogeneity.
Figure 5.
Forest plot of LAMC1 SNPs and prolapse
Forest plot of studies54-56 reporting associations among rs10911193, rs20563, and rs20558 single-nucleotide polymorphisms (SNPs) of laminin gamma 1 gene and pelvic organ prolapse. *African American subsample. **White subsample. rs10911193 RefSNP alleles C/T. Plot presented as risk associated with minor allele T. rs20563 RefSNP alleles A/G. Plot presented as risk associated with minor allele A. rs20558 RefSNP alleles C/T. Plot presented as risk associated with minor allele C.
CI, confidence interval; OR, odds ratio.
Cartwright. Genetic association studies of LUTS and POP. Am J Obstet Gynecol 2015.
MMP1
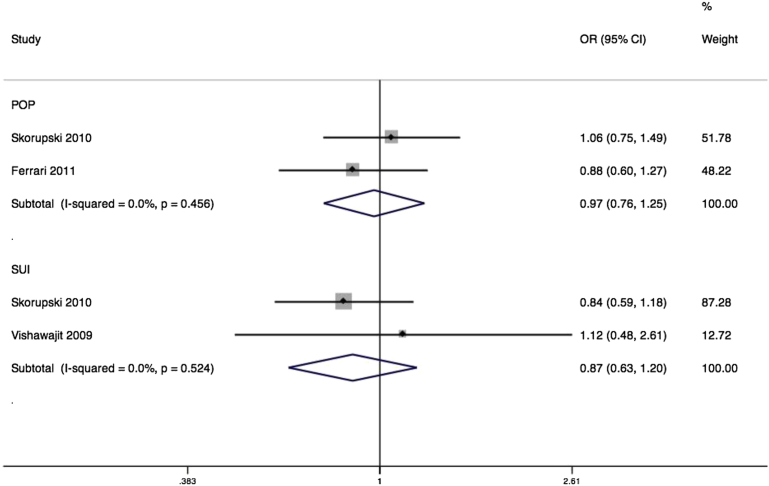
Matrix metalloproteinase-1, also known as interstitial collagenase, is one of a number of enzymes that cleave collagen type 1. The MMP1 gene is up-regulated in pelvic tissues of women with prolapse.40 Common variants of this gene have been extensively studied in association with chronic obstructive pulmonary disease,57 cardiovascular disease,58 and a number of cancers including of lung, colon, and breast. We identified 2 unpublished studies from the United States,59,60 and 2 published studies of Polish61,62 and Italian44 samples assessing associations between MMP1 variants and stress incontinence or prolapse. Of these, 2 studies reported on rs1799750 in association with prolapse,44,61 with a nonsignificant pooled effect (OR, 0.97; 95% CI, 0.76–1.25) (Figure 6) with no heterogeneity. One of the 2 studies included demonstrated marked deviation from Hardy-Weinberg equilibrium, and exclusion of this study would however leave a single eligible study with a nonsignificant association (OR, 0.88; 95% CI, 0.60–1.27).44 For the 2 studies testing associations with SUI,60,61 the pooled effect was again nonsignificant (OR, 0.87; 95% CI, 0.63–1.20), with no heterogeneity.
Figure 6.
Forest plot of rs1799750 SNP of MMP1
Forest plot of studies44,60,61 reporting associations between rs1799750* single-nucleotide polymorphism (SNP) of matrix metalloproteinase 1 (MMP1) gene and either stress urinary incontinence (SUI) or pelvic organ prolapse (POP) with either fixed or random effects models. ∗RefSNP Alleles -/G. Plot presented as risk associated with minor deletion allele.
CI, confidence interval; OR, odds ratio.
Cartwright. Genetic association studies of LUTS and POP. Am J Obstet Gynecol 2015.
MMP3
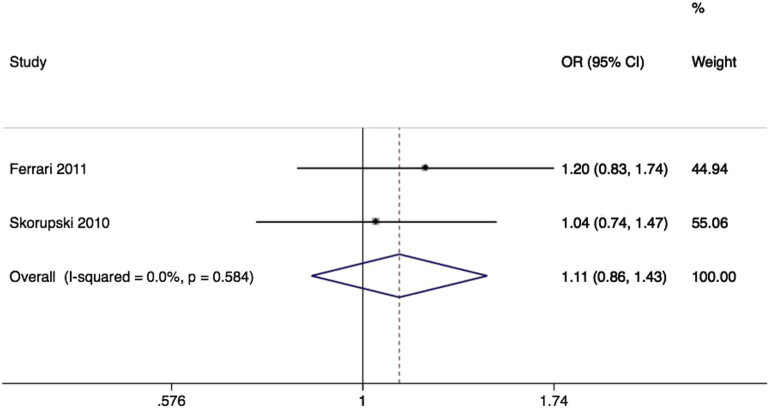
Matrix metalloproteinase-3, also known as stromelysin-1, is an enzyme that degrades a number of extracellular matrix components including collagen type 3 and elastin. Similarly to MMP1, its common variants have received most research attention in association with cardiovascular disease,58 and a number of cancers. We identified 2 studies again of women of European descent,44,61,62 both testing associations of rs3025058, known as the 5A/6A promoter InDel, with prolapse. The pooled effect was again nonsignificant (OR, 1.11; 95% CI, 0.86–1.43) (Figure 7) with no heterogeneity.
Figure 7.
Forest plot of rs3025058 SNP of MMP3 and prolapse
Forest plot of studies6,44 reporting associations between rs3025058* single-nucleotide polymorphism (SNP) of matrix metalloproteinase 3 gene and pelvic organ prolapse. *RefSNP Alleles -/T. Plot presented as risk associated with minor deletion allele.
CI, confidence interval; OR, odds ratio.
Cartwright. Genetic association studies of LUTS and POP. Am J Obstet Gynecol 2015.
MMP9
Matrix metalloproteinase-9, also known as 92-kDa type IV collagenase, degrades collagen type 4 and type 5. Some evidence suggests increased activation of MMP9 in pelvic tissues from women with prolapse.63 Like MMP1 and MMP3, its common polymorphisms have been linked to chronic obstructive pulmonary disease,57 cardiovascular disease,58 and some cancers. We identified 4 studies of Italian,44 Taiwanese,64 and white US63,65 samples, assessing 10 different polymorphisms in association with prolapse. Three studies contributed to a metaanalysis of the rs17576 missense polymorphism. The pooled effect was nonsignificant (OR, 1.02; 95% CI, 0.81–1.28) (Figure 8) but with significant heterogeneity (I2 = 68.9%, P = .04). Case definitions were similar for the 3 studies, making this an unlikely source of heterogeneity. All studies demonstrated Hardy-Weinberg equilibrium, and we judged a low risk of population stratification. The single study among Asian women64 suggested a narrowly significant effect (OR, 0.62; 95% CI, 0.40–0.98), while subgroup analysis of the 2 white US samples showed no pooled effect (OR, 1.22; 95% CI, 0.93–1.60). Two studies contributed to metaanalysis of rs3918242, with a nonsignificant effect (OR, 1.25; 95% CI, 0.83–1.89) (Figure 8) and no heterogeneity.
Figure 8.
Forest plot of MM9 SNPs and prolapse
Forest plot of studies44,63-65 reporting associations between rs3918242* and rs17576** single-nucleotide polymorphisms (SNPs) of matrix metalloproteinase 9 gene and pelvic organ prolapse with either fixed or random effects models⌘. *rs3918242 RefSNP alleles C/T. Plot presented as risk associated with minor allele T. **rs17576 RefSNP alleles A/G. Plot presented as risk associated with minor allele A. ⌘Mantel-Haenszel fixed effects model (M-H)/DerSimonian and Laird random effects model (D+L).
CI, confidence interval; OR, odds ratio.
Cartwright. Genetic association studies of LUTS and POP. Am J Obstet Gynecol 2015.
Publication bias and selective analysis
Each metaanalysis included at most 4 studies or subgroups, providing low power for conventional measures of funnel plot asymmetry. The Harbord test demonstrated no evidence of small study bias or publication bias (all P > .1). We applied the significance chasing bias test,30 to look for further evidence of publication bias or selective outcome reporting. This exploratory test is used to detect an excess of significant results, either within a single metaanalysis, or in a whole domain of research. In common with other tests of publication bias, P < .1 is usually taken as the threshold for significance. We applied the test across each of the 13 metaanalyses conducted individually, and for the 13 considered together as 1 domain. Given the power of the individual studies to detect the observed pooled effect sizes in each metaanalysis, across the domain as a whole we expected 6.61 statistically significant studies, and observed 7 significant studies in our own prespecified reanalyses using the allelic test (P = .87). However, primary publications applied a variety of analytic techniques, and from the set of studies included in metaanalysis we observed 11 studies reporting statistically significant results in their own analyses (P = .14), typically using alternative models of inheritance. These findings are suggestive primarily of selective analysis, rather than publication bias. Individual metaanalyses again provided limited power for this test, but possible bias was most apparent in the quantitative synthesis of association of prolapse with the rs17576 SNP of MMP9 (P = .11).
Genes and/or polymorphisms reported in a single study
Among the included studies, some had assessed associations with polymorphisms for which no replication has been reported. Statistically significant associations have been suggested between prolapse and the rs2228480 polymorphism of ESR1, the estrogen receptor alpha66; between prolapse and certain haplotypes of ESR2, the estrogen receptor beta67; between prolapse and the rs484389 polymorphism of PGR, the progesterone receptor68; between prolapse and the rs10478694 polymorphism of EDN1, endothelin 169; between incontinence and the CAG copy number variant of AR, the androgen receptor70; between incontinence and the rs6313 polymorphism of HTR2A, the serotonin 2A receptor71; between stress incontinence and both the rs2165241 and rs1048661 variants of LOX-L1, lysyloxidaselike-172; between the rs1136410 polymorphism of poly-ADP ribose polymerase (PARP)73 and prolapse; and finally between the rs1695 polymorphism of glutathione S-transferase pi (GSTP1) and prolapse.74 We found only 1 published study reporting entirely nonsignificant results,75 further suggesting a high probability of selective outcome reporting or publication bias for this field of study as a whole. Following the Venice recommendations,28 we a priori assigned all nominally significant but unreplicated associations weak epidemiological credibility. Three genome-wide association studies (GWAS) have now been reported for incontinence or prolapse.76-78 Of note, none of these suggested candidates for prolapse or incontinence, including both those from single studies, as well as those included in metaanalyses, were identified in these genome-wide analyses. Across the 3 GWAS, SNPs at 9 independent loci have reached genome-wide significance (P < 5 × 10-8) (Table 1) in discovery cohorts, although replication of these candidate loci has not been demonstrated.
Comment
Strengths and limitations
The strengths of this review include a comprehensive search of both published and unpublished studies, applying explicit criteria to potentially eligible studies, and employing standardized, piloted data forms for data collection, guided by written instructions, and an unbiased assessment and synthesis of reported associations. We followed a prespecified data analysis plan, and contacted authors for clarifications and additional data.
Among the challenges faced in this review was the inclusion of studies with varying diagnostic criteria. There may be considerable disparity between symptomatic and objective findings for both LUTS and prolapse, and despite long-standing efforts for standardization2 diagnostic criteria are not widely agreed upon. Despite this caution, we found that the literature had used largely concordant definitions. From the prolapse studies, 2 studies had used a prolapse case definition based on need for surgical treatment, but all others used an accepted anatomic staging system, typically POP Quantification. There was also little variation in cutoffs for significant prolapse, with almost all studies considering prolapse stage 0 or stage 1 as normal/control. Both overactive bladder studies included in metaanalysis used a combination of self-reported symptoms, with 3-day bladder diary for diagnosis. Similarly both SUI studies included in metaanalysis used a combination of cystometry and pad testing for diagnosis. This is reflected in a lack of heterogeneity in most metaanalyses. Regardless of the presence of statistical heterogeneity, there remains potential for bias toward the null from heterogeneity in case definitions.
It is evident that overactive bladder in particular may have multiple underlying causes,79,80 and these syntheses may therefore include participants with diverse underlying etiologies for their symptoms. The largest metaanalyses possible still include <1000 participants in total, and therefore provide adequate power only for associations with large effect size (approximately OR, ≤0.6 or OR, ≥1.8). It is both likely that smaller effect sizes have been missed in these syntheses, and highly probable that polymorphisms with larger effect sizes are still to be discovered.
Future work
Future advances are likely within the context of GWAS using large-scale population-based cohorts phenotyped for these conditions. The discovery of further causative variants should both help to explain the complex pathophysiology of these conditions, and provide potentially a route to effective prevention and treatment.
Conclusions
Family and twin studies have provided convincing evidence for genetic predisposition to incontinence, prolapse, and overactive bladder, with genetic variation contributing up to half of population phenotypic variability. These metaanalyses provide moderate epidemiological credibility for associations of variation in ADRB3 with overactive bladder, and COL1A1 with prolapse. As for all complex diseases, these 2 currently identified polymorphisms explain a tiny fraction of that phenotypic variation. The widespread availability of direct-to-consumer testing means that some patients may present with questions about the implications of these polymorphisms. However, testing for any of these SNPs cannot be recommended based on current evidence. Nevertheless, clinicians and researchers should be aware of the putative risks associated with these SNPs, and the uncertainty regarding potential biases in the primary studies. In the future, genetic counseling may play one part of advice about risks of mode of delivery, and may help target women for primary or secondary prevention. Currently, clinicians should continue to use a family history of prolapse or incontinence as a simple marker of future risk, with clearly documented interactions with modifiable risk factors such as vaginal childbirth and obesity.
Footnotes
This study was supported by grants from the International Continence Society and the United Kingdom Medical Research Council (grant no. G1100377). Neither body had any involvement in the analysis or preparation of the manuscript. The work of K.A.O.T. was supported by unrestricted grants from the Suomen Kulttuurirahasto, the Finnish Medical Foundation, Jane and Aatos Erkko Foundation, and Sigrid Jusélius Foundation. The work of M-R.J. was supported by an unrestricted grant from the Suomen Akatemia. The work of P.B. and V.K. is supported by the National Institute for Health Research Biomedical Research Center, based at Imperial College Healthcare National Health Service Trust and Imperial College London.
C.C. and V.K. are speakers, consultants, and paid investigators for Astellas Pharma, Allergan, and Pfizer and C.C. for Recordati as well. The other authors report no conflict of interest.
Cite this article as: Cartwright R, Kirby AC, Tikkinen KAO, et al. Systematic review and metaanalysis of genetic association studies of urinary symptoms and prolapse in women. Am J Obstet Gynecol 2015;212:199.e1-24.
Appendix
Supplementary Figure.

Summary of interim Venice guideline ratings of credibility of genetic associations
Strong credibility for association requires AAA rating. Any B rating confers maximum moderate credibility, while any C rating confers weak credibility.
Cartwright. Genetic association studies of LUTS and POP. Am J Obstet Gynecol 2015.
Adapted and abridged from Ioannidis et al.29
Supplementary Table 1.
Functional annotation of investigated genes
| Official gene symbol | Gene name(s) | No. of studies included | Molecular function(s) |
|---|---|---|---|
| ADRA1A | Adrenergic, alpha-1A-, receptor | 1 | Adrenoceptor activity Alpha-adrenergic receptor activity Alpha1-adrenergic receptor activity Amine receptor activity |
| ADRB3 | Adrenergic, beta-3-, receptor | 3 | Adrenoceptor activity Beta-adrenergic receptor activity Amine receptor activity Beta-3-adrenergic receptor activity Adrenergic receptor binding Beta-3 adrenergic receptor binding Identical protein binding Protein homodimerization activity Amine binding Alcohol binding Protein dimerization activity Epinephrine binding Norepinephrine binding |
| AR | Androgen receptor | 1 | DNA binding Transcription factor activity Steroid hormone receptor activity Ligand-dependent nuclear receptor activity Androgen receptor activity Steroid binding Androgen binding Zinc ion binding Lipid binding Transcription activator activity Transcription regulator activity Hormone binding Ion binding Cation binding Sequence-specific DNA binding Metal ion binding Transition metal ion binding Protein dimerization activity |
| COL1A1 | Collagen, type I, alpha 1 | 7 | Structural molecule activity Extracellular matrix structural constituent Growth factor binding Identical protein binding Platelet-derived growth factor binding, |
| COL3A1 | Collagen, type III, alpha 1 | 4 | Integrin binding Structural molecule activity Extracellular matrix structural constituent Growth factor binding Protein complex binding SMAD binding Platelet-derived growth factor binding |
| COL18A1 | Collagen, type XVIII, alpha 1 | 1 | Structural molecule activity Extracellular matrix structural constituent Zinc ion binding Ion binding Cation binding Metal ion binding Transition metal ion binding |
| CPE | Carboxypeptidase E | 1 | Metallocarboxypeptidase activity Zinc ion binding |
| CYP17A1 | Cytochrome P450, family 17, subfamily A, polypeptide 1 | 1 | Steroid 17-alpha-monooxygenase activity Iron ion binding Steroid hydroxylase activity Electron carrier activity Oxygen binding Heme binding Ion binding Cation binding Metal ion binding Tetrapyrrole binding Transition metal ion binding |
| CYP19A1 | Cytochrome P450, family 19, subfamily A, polypeptide 1 | 1 | Iron ion binding Electron carrier activity Oxidoreductase activity Oxygen binding Heme binding Ion binding Cation binding Metal ion binding Tetrapyrrole binding Transition metal ion binding Aromatase activity |
| EDN1 | Endothelin 1 | 1 | Hormone activity Bombesin receptor binding Endothelin A receptor binding Endothelin B receptor binding |
| ESR1 | Estrogen receptor 1 | 1 | DNA binding Transcription factor activity Steroid hormone receptor activity Ligand-dependent nuclear receptor activity Steroid binding Zinc ion binding Lipid binding Promoter binding Nitric-oxide synthase regulator activity Estrogen receptor activity Transcription regulator activity Estrogen response element binding Hormone binding Ion binding Cation binding Sequence-specific DNA binding Metal ion binding transition Metal ion binding Protein N-terminus binding |
| ESR2 | Estrogen receptor 2 (ER beta) | 1 | DNA binding Transcription factor activity Steroid hormone receptor activity Transcription cofactor activity Transcription coactivator activity Ligand-dependent nuclear receptor activity Steroid binding transcription factor binding Zinc ion binding Lipid binding Transcription activator activity Estrogen receptor activity Transcription regulator activity Receptor regulator activity Receptor inhibitor activity Hormone binding Ion binding Cation binding Sequence-specific DNA binding Metal ion binding Transition metal ion binding Receptor antagonist activity |
| GSTM1 | Glutathione S-transferase mu 1 | 1 | Glutathione transferase activity |
| GSTP1 | Glutathione S-transferase pi 1 | 1 | Glutathione transferase activity |
| GSTT1 | Glutathione S-transferase theta 1 | 1 | Glutathione transferase activity |
| HTR2A | 5-Hydroxytryptamine (serotonin) receptor 2A | 1 | Serotonin receptor activity Amine receptor activity Protein complex binding Amine binding Serotonin binding |
| LAMC1 | Laminin, gamma 1 (formerly LAMB2) | 3 | Structural molecule activity Extracellular matrix structural constituent, |
| LOXL1 | Lysyl oxidase-like 1 | 3 | Copper ion binding Oxidoreductase activity Ion binding Cation binding Metal ion binding Transition metal ion binding |
| MMP1 | Matrix metallopeptidase 1 (interstitial collagenase) | 5 | Endopeptidase activity Metalloendopeptidase activity Calcium ion binding Peptidase activity Metallopeptidase activity Zinc ion binding Ion binding Cation binding Metal ion binding Transition metal ion binding Peptidase activity Acting on L-amino acid peptides |
| MMP10 | Matrix metallopeptidase 10 (stromelysin 2) | 1 | Endopeptidase activity Metalloendopeptidase activity Calcium ion binding Peptidase activity Metallopeptidase activity Zinc ion binding Ion binding Cation binding Metal ion binding Transition metal ion binding Peptidase activity Acting on L-amino acid peptides |
| MMP11 | Matrix metallopeptidase 11 (stromelysin 3) | 1 | Endopeptidase activity Metalloendopeptidase activity Calcium ion binding Peptidase activity Metallopeptidase activity Zinc ion binding Ion binding Cation binding Metal ion binding Transition metal ion binding Peptidase activity Acting on L-amino acid peptides |
| MMP2 | Matrix metallopeptidase 2 (gelatinase A, 72-kDa gelatinase, 72-kDa type IV collagenase) | 1 | Endopeptidase activity Metalloendopeptidase activity Calcium ion binding Peptidase activity Metallopeptidase activity Zinc ion binding Ion binding Cation binding Metal ion binding Transition metal ion binding Peptidase activity Acting on L-amino acid peptides |
| MMP3 | Matrix metallopeptidase 3 (stromelysin 1, progelatinase) | 3 | Endopeptidase activity Metalloendopeptidase activity Calcium ion binding Peptidase activity Metallopeptidase activity Zinc ion binding Ion binding Cation binding Metal ion binding Transition metal ion binding Peptidase activity Acting on L-amino acid peptides |
| MMP8 | Matrix metallopeptidase 8 (neutrophil collagenase) | 1 | Endopeptidase activity Metalloendopeptidase activity Calcium ion binding Peptidase activity Metallopeptidase activity Zinc ion binding Ion binding Cation binding Metal ion binding Transition metal ion binding Peptidase activity Acting on L-amino acid peptides |
| MMP9 | Matrix metallopeptidase 9 (gelatinase B, 92-kDa gelatinase, 92-kDa type IV collagenase) | 4 | Endopeptidase activity Metalloendopeptidase activity Calcium ion binding Peptidase activity Metallopeptidase activity Zinc ion binding Ion binding Cation binding Metal ion binding Transition metal ion binding Peptidase activity Acting on L-amino acid peptides |
| PARP1 | Poly (ADP-ribose) polymerase 1 | 1 | Telomere maintenance DNA repair Transcription Chromosome organization |
| PGR | Progesterone receptor | 1 | DNA binding transcription factor activity Steroid hormone receptor activity Ligand-dependent nuclear receptor activity Steroid binding Zinc ion binding Lipid binding Transcription regulator activity Ion binding Cation binding Sequence-specific DNA binding Metal ion binding Transition metal ion binding Protein N-terminus binding |
| PRCP | Prolylcarboxypeptidase (angiotensinase C) | 1 | Carboxypeptidase activity Serine hydrolase activity |
| TIMP1 | TIMP metallopeptidase inhibitor 1 | 1 | Enzyme inhibitor activity, Endopeptidase inhibitor activity Metalloendopeptidase inhibitor activity Metalloenzyme regulator activity Peptidase inhibitor activity Metalloenzyme inhibitor activity |
| TIMP3 | TIMP metallopeptidase inhibitor 3 | 1 | Enzyme inhibitor activity Endopeptidase inhibitor activity Metalloendopeptidase inhibitor activity Metalloenzyme regulator activity Peptidase inhibitor activity Metalloenzyme inhibitor activity |
| ZFAT | Zinc finger and AT hook domain containing | 1 | DNA binding Zinc ion binding Ion binding Cation binding Metal ion binding Transition metal ion binding |
Functions assigned from http://david.abcc.ncifcrf.gov.
Cartwright. Genetic association studies of LUTS and POP. Am J Obstet Gynecol 2015.
Supplementary Table 2.
Overrepresented Gene Ontology biological processes among investigated genes
| Biological process | GO term | Description | Genes |
|---|---|---|---|
| 1 | GO:0030574 | Collagen catabolic process | MMP1 MMP2 MMP3 MMP8 MMP9 MMP10 MMP11 |
| 2 | GO:0005578 | Proteinaceous extracellular matrix | LOXL1 MMP1 MMP2 MMP3 MMP8 MMP9 MMP10 MMP11 TIMP1 |
| 3 | GO:0006508 | Proteolysis | CPE MMP1 MMP2 MMP3 MMP8 MMP9 MMP10 MMP11 PRCP |
| 4 | GO:0008152 | Metabolic process | CPE GSTM1 MMP2 MMP8 MMP3 GSTP1 MMP1 MMP10 MMP9 |
| 5 | GO:0005576 | Extracellular region | COL1A1 COL3A1 CPE EDN1 ESR2 LAMC1 LOXL1 MMP1 MMP2 MMP3 MMP8 MMP9 MMP10 MMP11 TIMP1 COL18A1 |
| 6 | GO:0007267 | Cell-cell signalling | PGR AR ADRA1A ESR2 EDN1 |
| 7 | GO:0005615 | Extracellular space | AR COL1A1 COL3A1 EDN1 LAMC1 LOXL1 MMP2 MMP3 MMP8 MMP9 MMP10 COL18A1 |
| 8 | GO:0008270 | Zinc ion binding | ESR1 ESR2 MMP1 MMP2 MMP3 MMP8 MMP9 MMP10 MMP11 PGR ZFAT |
All processes significant at P < .01, analysis using http://webclu.bio.wzw.tum.de/profcom/. Overrepresented Gene Ontology: http://www.geneontology.org.
Cartwright. Genetic association studies of LUTS and POP. Am J Obstet Gynecol 2015.
References
- 1.Nygaard I., Barber M.D., Burgio K.L. Prevalence of symptomatic pelvic floor disorders in US women. JAMA. 2008;300:1311–1316. doi: 10.1001/jama.300.11.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haylen B.T., De Ridder D., Freeman R.M. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Neurourol Urodyn. 2010;29:4–20. doi: 10.1002/nau.20798. [DOI] [PubMed] [Google Scholar]
- 3.Burgio K.L., Matthews K.A., Engel B.T. Prevalence, incidence and correlates of urinary incontinence in healthy, middle-aged women. J Urol. 1991;146:1255–1259. doi: 10.1016/s0022-5347(17)38063-1. [DOI] [PubMed] [Google Scholar]
- 4.Lawrence J.M., Lukacz E.S., Nager C.W., Hsu J.-W.Y., Luber K.M. Prevalence and co-occurrence of pelvic floor disorders in community-dwelling women. Obstet Gynecol. 2008;111:678–685. doi: 10.1097/AOG.0b013e3181660c1b. [DOI] [PubMed] [Google Scholar]
- 5.Coyne K.S., Matza L.S., Kopp Z.S. Examining lower urinary tract symptom constellations using cluster analysis. BJU Int. 2008;101:1267–1273. doi: 10.1111/j.1464-410X.2008.07598.x. [DOI] [PubMed] [Google Scholar]
- 6.Danforth K.N., Townsend M.K., Lifford K., Curhan G.C., Resnick N.M., Grodstein F. Risk factors for urinary incontinence among middle-aged women. Am J Obstet Gynecol. 2006;194:339–345. doi: 10.1016/j.ajog.2005.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pal L., Hailpern S.M., Santoro N.F. Association of pelvic organ prolapse and fractures in postmenopausal women. Menopause. 2008;15:59–66. doi: 10.1097/gme.0b013e3181151444. [DOI] [PubMed] [Google Scholar]
- 8.Coyne K.S., Kaplan S.A., Chapple C.R. Risk factors and comorbid conditions associated with lower urinary tract symptoms: EpiLUTS. BJU Int. 2009;103(Suppl):24–32. doi: 10.1111/j.1464-410X.2009.08438.x. [DOI] [PubMed] [Google Scholar]
- 9.Sampselle C.M., Harlow S.D., Skurnick J., Brubaker L., Bondarenko I. Urinary incontinence predictors and life impact in ethnically diverse perimenopausal women. Obstet Gynecol. 2002;100:1230–1238. doi: 10.1016/s0029-7844(02)02241-x. [DOI] [PubMed] [Google Scholar]
- 10.van der Vaart C.H., de Leeuw J.R.J., Roovers J.-P.W.R., Heintz A.P.M. Measuring health-related quality of life in women with urogenital dysfunction: the urogenital distress inventory and incontinence impact questionnaire revisited. Neurourol Urodyn. 2003;22:97–104. doi: 10.1002/nau.10038. [DOI] [PubMed] [Google Scholar]
- 11.Coyne K.S., Sexton C.C., Kopp Z.S., Ebel-Bitoun C., Milsom I., Chapple C. The impact of overactive bladder on mental health, work productivity and health-related quality of life in the UK and Sweden: results from EpiLUTS. BJU Int. 2011;108:1459–1471. doi: 10.1111/j.1464-410X.2010.10013.x. [DOI] [PubMed] [Google Scholar]
- 12.Anger J.T., Saigal C.S., Litwin M.S., Urologic Diseases of America Project The prevalence of urinary incontinence among community dwelling adult women: results from the National Health and Nutrition Examination Survey. J Urol. 2006;175:601–604. doi: 10.1016/S0022-5347(05)00242-9. [DOI] [PubMed] [Google Scholar]
- 13.Hannestad Y.S., Rortveit G., Daltveit A.K., Hunskaar S. Are smoking and other lifestyle factors associated with female urinary incontinence? The Norwegian EPINCONT Study. BJOG. 2003;110:247–254. [PubMed] [Google Scholar]
- 14.Miedel A., Tegerstedt G., Maehle-Schmidt M., Nyrén O., Hammarström M. Nonobstetric risk factors for symptomatic pelvic organ prolapse. Obstet Gynecol. 2009;113:1089–1097. doi: 10.1097/AOG.0b013e3181a11a85. [DOI] [PubMed] [Google Scholar]
- 15.Vaughan C.P., Johnson T.M., Ala-Lipasti M.A. The prevalence of clinically meaningful overactive bladder: bother and quality of life results from the population-based FINNO study. Eur Urol. 2011;59:629–636. doi: 10.1016/j.eururo.2011.01.031. [DOI] [PubMed] [Google Scholar]
- 16.Thompson H. On irritability of the bladder. Lancet. 1854;63:637–638. [Google Scholar]
- 17.Diokno A.C., Brock B.M., Herzog A.R., Bromberg J. Medical correlates of urinary incontinence in the elderly. Urology. 1990;36:129–138. doi: 10.1016/0090-4295(90)80211-5. [DOI] [PubMed] [Google Scholar]
- 18.Hannestad Y.S., Lie R.T., Rortveit G., Hunskaar S. Familial risk of urinary incontinence in women: population based cross sectional study. BMJ. 2004;329:889–891. doi: 10.1136/bmj.329.7471.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buchsbaum G.M., Duecy E.E. Incontinence and pelvic organ prolapse in parous/nulliparous pairs of identical twins. Neurourol Urodyn. 2008;27:496–498. doi: 10.1002/nau.20555. [DOI] [PubMed] [Google Scholar]
- 20.Slieker-ten Hove M.C.P., Pool-Goudzwaard A.L., Eijkemans M.J.C., Steegers-Theunissen R.P.M., Burger C.W., Vierhout M.E. Symptomatic pelvic organ prolapse and possible risk factors in a general population. Am J Obstet Gynecol. 2009;200:184.e1–184.e7. doi: 10.1016/j.ajog.2008.08.070. [DOI] [PubMed] [Google Scholar]
- 21.Lince S.L., van Kempen L.C., Vierhout M.E., Kluivers K.B. A systematic review of clinical studies on hereditary factors in pelvic organ prolapse. Int Urogynecol J. 2012;23:1327. doi: 10.1007/s00192-012-1704-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gontard von A., Heron J., Joinson C. Family history of nocturnal enuresis and urinary incontinence: results from a large epidemiological study. J Urol. 2011;185:2303–2306. doi: 10.1016/j.juro.2011.02.040. [DOI] [PubMed] [Google Scholar]
- 23.Jack G.S., Nikolova G., Vilain E., Raz S., Rodríguez L.V. Familial transmission of genitovaginal prolapse. Int Urogynecol J. 2005;17:498–501. doi: 10.1007/s00192-005-0054-x. [DOI] [PubMed] [Google Scholar]
- 24.Altman D., Forsman M., Falconer C., Lichtenstein P. Genetic influence on stress urinary incontinence and pelvic organ prolapse. Eur Urol. 2008;54:918–922. doi: 10.1016/j.eururo.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 25.Wennberg A.-L., Altman D., Lundholm C. Genetic influences are important for most but not all lower urinary tract symptoms: a population-based survey in a cohort of adult Swedish twins. Eur Urol. 2011;59:1032–1038. doi: 10.1016/j.eururo.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rohr G., Kragstrup J., Gaist D., Christensen K. Genetic and environmental influences on urinary incontinence: a Danish population-based twin study of middle-aged and elderly women. Acta Obstet Gynecol Scand. 2004;83:978–982. doi: 10.1111/j.0001-6349.2004.00635.x. [DOI] [PubMed] [Google Scholar]
- 27.Cartwright R, Mangera A, Kirby A, et al. PROSPERO: a systematic review of candidate gene association studies of lower urinary tract symptoms and pelvic organ prolapse in women. Available at: http://www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42012001983. Accessed Oct. 4, 2013.
- 28.Harris R.J., Bradburn M.J., Deeks J.J. Metan: fixed- and random-effects meta-analysis. Stata J. 2008;8:3–28. [Google Scholar]
- 29.Ioannidis J.P., Boffetta P., Little J. Assessment of cumulative evidence on genetic associations: interim guidelines. Int J Epidemiol. 2007;37:120–132. doi: 10.1093/ije/dym159. [DOI] [PubMed] [Google Scholar]
- 30.Ioannidis J.P.A., Trikalinos T.A. An exploratory test for an excess of significant findings. Clin Trials. 2007;4:245–253. doi: 10.1177/1740774507079441. [DOI] [PubMed] [Google Scholar]
- 31.Little J, Higgins J, Bray M, Ioannidis J, Khoury M. The HuGENet HuGE review handbook, version 1.0, 2006. Available at: http://www.med.uottawa.ca/public-health-genomics/web/assets/documents/huge_review_handbook_v1_0.pdf. Accessed Sept. 11, 2014.
- 32.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. Ann Int Med. 2009;151:264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 33.Aizawa N., Homma Y., Igawa Y. Effects of mirabegron, a novel β3-adrenoceptor agonist, on primary bladder afferent activity and bladder microcontractions in rats compared with the effects of oxybutynin. Eur Urol. 2012;62:1165–1173. doi: 10.1016/j.eururo.2012.08.056. [DOI] [PubMed] [Google Scholar]
- 34.Khullar V., Amarenco G., Angulo J.C. Efficacy and tolerability of mirabegron, a β(3)-adrenoceptor agonist, in patients with overactive bladder: results from a randomized European-Australian phase 3 trial. Eur Urol. 2013;63:283–295. doi: 10.1016/j.eururo.2012.10.016. [DOI] [PubMed] [Google Scholar]
- 35.Chapple C.R., Kaplan S.A., Mitcheson D. Randomized double-blind, active-controlled phase 3 study to assess 12-month safety and efficacy of mirabegron, a β(3)-adrenoceptor agonist, in overactive bladder. Eur Urol. 2013;63:296–305. doi: 10.1016/j.eururo.2012.10.048. [DOI] [PubMed] [Google Scholar]
- 36.Takeda M, Araki I, Kamiyama M, Takihana Y, Tanabe N. Single nucleotide polymorphism of alpha1a and beta3-adrenoceptors in urological patients with and without micturition symptoms–possible mechanism for hyperactivity of adrenergic nerve and tailor-made medicine. Presented as a poster at: the International Continence Society Annual Meeting; Aug. 30, 2002; Heidelberg, Germany.
- 37.Honda K., Yamaguchi O., Nomiya M. Association between polymorphism of beta3-adrenoceptor gene and overactive bladder. Neurourol Urodyn. 2014;33:400–402. doi: 10.1002/nau.22476. [DOI] [PubMed] [Google Scholar]
- 38.Ferreira C.E., Fonseca A.M., Silva I.D., Girao M.J., Sartori M.G., Castro R.A. The relationship between the Trp 64 Arg polymorphism of the beta 3-adrenoceptor gene and idiopathic overactive bladder. Am J Obstet Gynecol. 2011;205:82.e10–82.e14. doi: 10.1016/j.ajog.2011.02.052. [DOI] [PubMed] [Google Scholar]
- 39.Jin H., Evangelou E., Ioannidis J.P.A., Ralston S.H. Polymorphisms in the 5′ flank of COL1A1 gene and osteoporosis: meta-analysis of published studies. Osteoporos Int. 2010;22:911–921. doi: 10.1007/s00198-010-1364-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen B., Yeh J. Alterations in connective tissue metabolism in stress incontinence and prolapse. J Urol. 2011;186:1768–1772. doi: 10.1016/j.juro.2011.06.054. [DOI] [PubMed] [Google Scholar]
- 41.Rodrigues A.M., Girão M.J.B.C., da Silva I.D.C.G., Sartori M.G.F., Martins K de F., Castro R de A. COL1A1 Sp1-binding site polymorphism as a risk factor for genital prolapse. Int Urogynecol J. 2008;19:1471–1475. doi: 10.1007/s00192-008-0662-3. [DOI] [PubMed] [Google Scholar]
- 42.Feiner B., Fares F., Azam N., Auslender R., David M., Abramov Y. Does COLIA1 SP1-binding site polymorphism predispose women to pelvic organ prolapse? Int Urogynecol J. 2009;20:1061–1065. doi: 10.1007/s00192-009-0895-9. [DOI] [PubMed] [Google Scholar]
- 43.Skorupski P. Does polymorphism of the gene encoding alpha-1 chain of collagen type 1 influence the risk of pelvic organ prolapse? Int Urogynecol J. 2009;20:s243–s244. [Google Scholar]
- 44.Ferrari M.M., Rossi G., Biondi M.L., Viganò P., Dell’Utri C., Meschia M. Type I collagen and matrix metalloproteinase 1, 3 and 9 gene polymorphisms in the predisposition to pelvic organ prolapse. Arch Gynecol Obstet. 2012;285:1581–1586. doi: 10.1007/s00404-011-2199-9. [DOI] [PubMed] [Google Scholar]
- 45.Cho H.J., Jung H.J., Kim S.K., Choi J.R., Cho N.H., Bai S.W. Polymorphism of a COLIA1 gene Sp1 binding site in Korean women with pelvic organ prolapse. Yonsei Med J. 2009;50:564–568. doi: 10.3349/ymj.2009.50.4.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Skorupski P., Król J., Starega J., Adamiak A., Jankiewicz K., Rechberger T. An alpha-1 chain of type I collagen Sp1-binding site polymorphism in women suffering from stress urinary incontinence. Am J Obstet Gynecol. 2006;194:346–350. doi: 10.1016/j.ajog.2005.07.034. [DOI] [PubMed] [Google Scholar]
- 47.Sioutis D., Economou E., Lambrinoudaki I., Tsamadias V., Creatsa M., Liapis A. Sp1 collagen I A1 polymorphism in women with stress urinary incontinence. Int Urogynecol J. 2011;22:835–839. doi: 10.1007/s00192-011-1372-9. [DOI] [PubMed] [Google Scholar]
- 48.Lammers K., Lince S.L., Spath M.A. Pelvic organ prolapse and collagen-associated disorders. Int Urogynecol J. 2012;23:313–319. doi: 10.1007/s00192-011-1532-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen H.Y., Chung Y.W., Lin W.Y., Wang J.C., Tsai F.J., Tsai C.H. Collagen type 3 alpha 1 polymorphism and risk of pelvic organ prolapse. Int J Gynaecol Obstet. 2008;103:55–58. doi: 10.1016/j.ijgo.2008.05.031. [DOI] [PubMed] [Google Scholar]
- 50.Lince S.L., van Kempen L.C., Dijkstra J.R., IntHout J., Vierhout M.E., Kluivers K.B. Collagen type III alpha 1 polymorphism (rs1800255, COL3A1 2209 G>A) assessed with high-resolution melting analysis is not associated with pelvic organ prolapse in the Dutch population. Int Urogynecol J. 2014;25:1237–1242. doi: 10.1007/s00192-014-2385-y. [DOI] [PubMed] [Google Scholar]
- 51.Jeon M.J., Chung S.M., Choi J.R., Jung H.J., Kim S.K., Bai S.W. The relationship between COL3A1 exon 31 polymorphism and pelvic organ prolapse. J Urol. 2009;181:1213–1216. doi: 10.1016/j.juro.2008.11.027. [DOI] [PubMed] [Google Scholar]
- 52.Martins K de F., de Jármy-DiBella Z.I.K., da Fonseca A.M.R.M. Evaluation of demographic, clinical characteristics, and genetic polymorphism as risk factors for pelvic organ prolapse in Brazilian women. Neurourol Urodyn. 2011;30:1325–1328. doi: 10.1002/nau.21066. [DOI] [PubMed] [Google Scholar]
- 53.Nikolova G., Lee H., Berkovitz S. Sequence variant in the laminin gamma1 (LAMC1) gene associated with familial pelvic organ prolapse. Hum Genet. 2007;120:847–856. doi: 10.1007/s00439-006-0267-1. [DOI] [PubMed] [Google Scholar]
- 54.Wu J.M., Visco A.G., Grass E.A. Comprehensive analysis of LAMC1 genetic variants in advanced pelvic organ prolapse. Am J Obstet Gynecol. 2012;206:447.e1–447.e6. doi: 10.1016/j.ajog.2012.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen C., Hill L.D., Schubert C.M., Strauss J.F., Matthews C.A. Is laminin gamma-1 a candidate gene for advanced pelvic organ prolapse? Am J Obstet Gynecol. 2010;202:505.e1–505.e5. doi: 10.1016/j.ajog.2010.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fu R., Hagstrom S., Daneshgari F. Mutation screen of lysyl oxidase-like-1 and laminin gamma 1 variant in patients with advanced pelvic organ prolapse. J Urol. 2009;181:481. [Google Scholar]
- 57.Chen L., Wang T., Liu L., Shen Y., Wan C., Wen F. Matrix metalloproteinase-9 -1562C/T promoter polymorphism confers risk for COPD: a meta-analysis. PLoS One. 2013;8 doi: 10.1371/journal.pone.0060523. e60523-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li M., Shi J., Fu L., Wang H., Zhou B., Wu X. Genetic polymorphism of MMP family and coronary disease susceptibility: a meta-analysis. Gene. 2012;495:36–41. doi: 10.1016/j.gene.2011.12.025. [DOI] [PubMed] [Google Scholar]
- 59.Campeau L, Gorbachinsky I, Stancill J, Rohazinski J, Andersson KE. Characterization of SNPs within the MMP-1 promotor region in women with and without POP. Presented as a poster at: the International Continence Society Annual Meeting; Aug. 31, 2011; Glasgow, United Kingdom.
- 60.Vishwajit S, Rohozinski J, Badlani G, Andersson K-E. Association of MMP1 promoter variant with stress urinary incontinence and pelvic organ prolapse in women. Presented as a poster at: the International Continence Society Annual Meeting; Oct. 3, 2009; San Francisco, CA.
- 61.Skorupski P., Miotla P., Jankiewicz K., Rechberger T. MMP-1 and MMP-3 gene encoding polymorphism and the risk of the development of pelvic organ prolapse and stress urinary incontinence. Ginekol Pol. 2010;81:594–599. [PubMed] [Google Scholar]
- 62.Skorupski P., Jankiewicz K., Miotła P., Marczak M. The polymorphisms of the MMP-1 and the MMP-3 genes and the risk of pelvic organ prolapse. Int Urogynecol J. 2013;24:1033–1038. doi: 10.1007/s00192-012-1970-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wu J.M.J., Visco A.G.A., Grass E.A.E. Matrix metalloproteinase-9 genetic polymorphisms and the risk for advanced pelvic organ prolapse. Obstet Gynecol. 2012;120:587–593. doi: 10.1097/AOG.0b013e318262234b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen H.Y., Lin W.Y., Chen Y.H., Chen W.C., Tsai F.J., Tsai C.H. Matrix metalloproteinase-9 polymorphism and risk of pelvic organ prolapse in Taiwanese women. Eur J Obstet Gynecol Reprod Biol. 2010;149:222–224. doi: 10.1016/j.ejogrb.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 65.Romero A., Jamison M. Are single nucleotide polymorphisms associated with pelvic organ prolapse? J Pelv Med Surg. 2008;14:37–43. [Google Scholar]
- 66.Chen H.Y., Chung Y.W., Lin W.Y., Chen W.C., Tsai F.J., Tsai C.H. Estrogen receptor alpha polymorphism is associated with pelvic organ prolapse risk. Int Urogynecol J. 2008;19:1159–1163. doi: 10.1007/s00192-008-0603-1. [DOI] [PubMed] [Google Scholar]
- 67.Chen H.Y., Wan L., Chung Y.W., Chen W.C., Tsai F.J., Tsai C.H. Estrogen receptor beta gene haplotype is associated with pelvic organ prolapse. Eur J Obstet Gynecol Reprod Biol. 2008;138:105–109. doi: 10.1016/j.ejogrb.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 68.Chen H.Y., Chung Y.W., Lin W.Y., Chen W.C., Tsai F.J., Tsai C.H. Progesterone receptor polymorphism is associated with pelvic organ prolapse risk. Acta Obstet Gynecol Scand. 2009;88:835–838. doi: 10.1080/00016340902822073. [DOI] [PubMed] [Google Scholar]
- 69.Choy KW, Wong ASW, Cheon WC, et al. Genetic association study in women with pelvic organ prolapse. Presented as a poster at: the International Continence Society Annual Meeting; Aug. 24, 2007; Rotterdam, The Netherlands.
- 70.Cornu J.N., Merlet B., Cussenot O. Genetic susceptibility to urinary incontinence: implication of polymorphisms of androgen and estrogen pathways. World J Urol. 2011;29:239–242. doi: 10.1007/s00345-010-0585-8. [DOI] [PubMed] [Google Scholar]
- 71.Noronha J.A., Schwanke C.H., Machado D.C. Association between T102C polymorphism of serotonin 2A receptor gene and urinary incontinence in older women. J Investig Med. 2010;58:32–37. doi: 10.2310/JIM.0b013e3181c04760. [DOI] [PubMed] [Google Scholar]
- 72.Ozbek E., Polat E.C., Ozcan L., Otunctemur A., Emrence Z., Ustek D. TT polymorphism in rs2165241 and rs1048661 region in lysyl oxidase like-1 gene may have a role in stress urinary incontinence physiopathology. J Obstet Gynaecol Res. 2013;39:237–242. doi: 10.1111/j.1447-0756.2012.01942.x. [DOI] [PubMed] [Google Scholar]
- 73.Kim J.Y., Kim E.J., Jeon M.J., Kim H., Moon Y.J., Bai S.W. Association between the poly(ADP-ribose) polymerase-1 gene polymorphism and advanced pelvic organ prolapse. Menopause. 2014;21:177–181. doi: 10.1097/GME.0b013e3182976f1f. [DOI] [PubMed] [Google Scholar]
- 74.Kim J.Y., Kim E.J., Jeon M.J., Kim R., Lee M.W., Kim S.W. Association between susceptibility to advanced pelvic organ prolapse and glutathione S-transferase P1 Ile105Val polymorphism. Eur J Obstet Gynecol Reprod Biol. 2014;175:205–208. doi: 10.1016/j.ejogrb.2014.01.028. [DOI] [PubMed] [Google Scholar]
- 75.Ferrell G., Lu M., Stoddard P. A single nucleotide polymorphism in the promoter of the LOXL1 gene and its relationship to pelvic organ prolapse and preterm premature rupture of membranes. Reprod Sci. 2009;16:438–446. doi: 10.1177/1933719108330567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Allen-Brady K., Cannon-Albright L., Farnham J.M. Identification of six loci associated with pelvic organ prolapse using genome-wide association analysis. Obstet Gynecol. 2011;118:1345–1353. doi: 10.1097/AOG.0b013e318236f4b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Velez Edwards DR, Ward RM, Giri A, et al. Trans-ethnic GWAS of pelvic organ prolapse among African American and Hispanic postmenopausal women of the Women's Health Initiative. Presented as a poster at: the American Society of Human Genetics Annual Meeting; Oct. 25, 2013; Boston, MA.
- 78.Chen C, Rajkovic A, Park A, Heiss G, Hendrix S, Franceschini N. Uncovering loci associated with urinary incontinence in African and Hispanic American women. Presented as a poster at: the American Society of Human Genetics Annual Meeting; Oct. 25, 2013; Boston, MA.
- 79.Herschorn S. Overactive bladder: symptom complex or separate entity? Can Urol Assoc J. 2011;5(Suppl):S152–S154. doi: 10.5489/cuaj.11210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tikkinen K.A., Auvinen A. Does the imprecise definition of overactive bladder serve commercial rather than patient interests? Eur Urol. 2012;61:746–748. doi: 10.1016/j.eururo.2011.12.013. [DOI] [PubMed] [Google Scholar]