Abstract
Background
The rate of development of new orphan drugs continues to grow. As a result, reimbursing orphan drugs on an exceptional basis is increasingly difficult to sustain from a health system perspective. An understanding of the value that societies attach to providing orphan drugs at the expense of other health technologies is now recognised as an important input to policy debates.
Objectives
The aim of this work was to scope the social value arguments that have been advanced relating to the reimbursement of orphan drugs, and to locate these within a coherent decision-making framework to aid reimbursement decisions in the presence of limited healthcare resources.
Methods
A scoping review of the peer reviewed and grey literature was undertaken, consisting of seven phases: (1) identifying the research question; (2) searching for relevant studies; (3) selecting studies; (4) charting, extracting and tabulating data; (5) analyzing data; (6) consulting relevant experts; and (7) presenting results. The points within decision processes where the identified value arguments would be incorporated were then located. This mapping was used to construct a framework characterising the distinct role of each value in informing decision making.
Results
The scoping review identified 19 candidate decision factors, most of which can be characterised as either value-bearing or ‘opportunity cost’-determining, and also a number of value propositions and pertinent sources of preference information. We were able to synthesize these into a coherent decision-making framework.
Conclusion
Our framework may be used to structure policy discussions and to aid transparency about the values underlying reimbursement decisions for orphan drugs. These values ought to be consistently applied to all technologies and populations affected by the decision.
Electronic supplementary material
The online version of this article (doi:10.1007/s40273-014-0235-x) contains supplementary material, which is available to authorized users.
Key Points for Decision Makers
| Understanding the value that societies attach to reimbursing orphan drugs at the expense of other health technologies is important. |
| We have scoped the social value arguments advanced in the literature and located these within a coherent framework. This framework may be used to structure policy discussions and to aid transparency regarding the values underlying reimbursement decisions for orphan drugs in the presence of limited healthcare resources. |
| Decision makers should seek to identify which value-bearing factors they deem pertinent to their decision, whose preferences they wish to consider, and what value propositions underpin their decisions. These need to be consistently applied to all technologies and populations affected by the decision: the new orphan drug, any existing therapy for the same disease which will be displaced, and any therapies which will be displaced elsewhere in the system to fund any additional costs of a positive coverage decision. |
Introduction
Since the passage of orphan-drug legislation in the US (1983) and Europe (1999), the rate of development of new orphan drugs has grown rapidly [1, 2]. As a result, there are now a greater number of products available for treating rare diseases than were available two decades ago [3]. For example, more than 400 products have been developed and marketed in the US since 1983 compared with fewer than ten in the previous decade [4]. Quite separately, there have also been advances in personalised medicine, resulting in the division of some diseases into subcategories based on genetic and molecular characteristics. Consequently, diseases once considered ‘common’ have become a collection of individual diseases with smaller prevalence rates, some of which meet the regulatory definitions of rarity. This has significant implications for the licensing and adoption of therapies to treat them [5, 6].
These developments have taken place in an environment in which payers are already facing significant challenges in making coverage decisions for non-orphan disease therapies [7]. Ageing populations, combined with increasingly expensive production costs for many innovative technologies, have led to large and sustained increases in healthcare expenditure. Healthcare budgets have generally increased faster than economies have grown, leading to genuine concerns about affordability in many countries. In response, health systems have established formal mechanisms for making coverage decisions on new health technologies, including drugs [8, 9]. However, stakeholders in these coverage decision processes have expressed criticisms around both the processes and factors considered when deciding whether technologies represent a good investment [10, 11]. These concerns have led policy makers and researchers to attempt to specify the characteristics of good decision processes and to be explicit about the factors considered in arriving at their decisions and their rationale [12].
The growth in both the number and budgetary impact of orphan drugs has accentuated these challenges [13, 14]. Each disease is rare, which hampers the ability to generate high-quality evidence of value. It also leads manufacturers to seek much higher prices to ensure that expected profits are comparable to those provided by treatments for common diseases [15]. However, rare disease diagnoses are increasingly common and reimbursement of orphan drugs on an exceptional basis may no longer be intellectually defensible nor economically sustainable. Furthermore, there is growing recognition of the need to understand the value that societies attach to providing coverage for orphan drugs at the expense of other health technologies as an important input into policy debates in this area.
The objective of this paper was to scope the social value arguments advanced in the academic and policy literature related to the reimbursement of orphan drugs, and to then locate these identified values within a coherent decision-making framework applicable for coverage decisions in the context of a limited healthcare budget.
Methods
To facilitate a structured and transparent approach to identifying the social value arguments advanced in orphan-drug policy debates, we adopted the methods of a scoping review for the discovery component of the study [16]. Since several steps in a scoping review are the same as those in a systematic review, we also followed the PRISMA statement for reporting, where relevant [17]. Drawing upon previous work by the authors on the process of healthcare decision making and decision criteria for coverage decisions in the presence of a fixed budget, we then attempted to locate the points where social values should be incorporated within the decision process [12, 18, 19].
Scoping Review
Our discovery work consisted of seven phases: (1) identifying the research question; (2) searching for relevant studies; (3) selecting studies; (4) charting, extracting and tabulating the data; (5) analyzing the data; (6) consulting relevant experts; and (7) presenting the results [20].
Identifying the Research Question
With input from the team of investigators and collaborators on the Canadian Institutes of Health Research (CIHR) ‘Promoting Rare-disease Innovations through Sustainable Mechanisms’ (PRISM) grant, the following research question was formulated: “What is known about societal values for new therapies for rare and ultra-rare diseases and conditions?” Addressing this question comprised the initial phase of PRISM’s research programme, which aims to develop policy options that optimise access to effective therapies within a sustainable healthcare system [21]. There is no common definition of a rare or ultra-rare disease, nor a shared understanding of what is meant by ‘societal values’. Therefore, to reduce the likelihood of missing relevant studies, a broad approach was adopted. ‘Societal values’ were, in general terms, any statements regarding how healthcare resources should be prioritised to reflect public choices or social preferences. Rare or ultra-rare diseases were any conditions that had been described as such by the respective author(s).
Searching for Relevant Studies
A comprehensive search strategy for identifying published and unpublished papers that met the inclusion criteria (i.e. any type of paper addressing societal values in the context of therapies for rare diseases) was constructed with support from an experienced Research Librarian. Because the goal was to capture any information in this area (including think/conceptual pieces, empirical work, reviews, etc.), search parameters were not limited to a particular study design. However, for feasibility reasons, language and date restrictions were applied (papers appearing in English between January 1990 and October 2013). This date range was deemed sufficient since it spanned the points at which the high costs of therapies for treating rare diseases were recognised as imposing a potential burden upon healthcare systems, sparking discussions around values and their place in determining the legitimacy of reimbursement despite limited evidence of effectiveness. The search strategy, which appears in full detail in Appendix 1 (see electronic supplementary material), was applied to the following databases: PubMed (MEDLINE and non-MEDLINE sources), EMBASE, Web of Science, Scopus, ProQuest, Cochrane Library and EconLit. Citation searches were also performed using the names of authors and journals of relevant papers, and Google Scholar was searched with combinations of keywords for rare diseases, therapies, and values (see Appendix 1 in the electronic supplementary material). For comprehensiveness, reference lists of relevant papers and conference abstracts were manually searched. All of the search results were imported into Reference Manager, and duplicate citations were removed. A detailed breakdown of the number of citations identified through the various information sources is presented in Fig. 1.
Fig. 1.
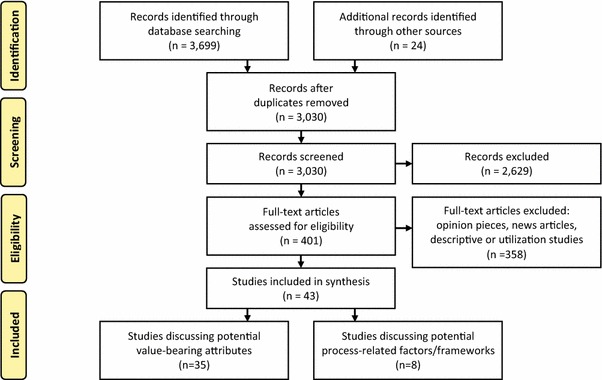
PRISMA flow diagram for the scoping review
Selecting Studies
Inclusion and exclusion criteria were developed at the outset of the review. These were used to create a screening checklist, which was applied to discrete citations or abstracts (where available) by two researchers (TS and DM) independently. Papers addressing both of the following were included: a specific rare or ultra-rare disease, or one or both more broadly; and specific values or factors that should be taken into account during funding deliberations and decision making around treatments for them (inclusion criteria). Studies presenting multi-country comparisons of access to, or utilisation of, specific therapies or centralised drug review processes were excluded (exclusion criteria). The full papers of potentially relevant citations were retrieved for further consideration. Two researchers (MP and TS) independently reviewed full papers using the same criteria and then met to compare findings. Discrepancies were resolved through discussion.
Charting, Extracting and Tabulating the Data
Key pieces of information from papers selected for inclusion in the scoping study were charted by both researchers using a data charting form (similar to a data extraction form used in systematic reviews). Charting involved sifting through and sorting information according to key aspects or concepts [22]. These key aspects or concepts, identified a priori, included author(s), type of paper, country where the paper originated, purpose of the paper, definition of ‘rare’ or ‘ultra-rare’ applied, the types of therapies addressed, factors or values-based statements considered, methods or approaches used (including information sources) to arrive at findings or arguments presented, and conclusions. They formed the common analytical framework applied to papers through the data charting form. This component, which is part of the descriptive analytical method within the narrative review tradition, ensured data were collected in a standard way, enhancing their usefulness [23]. Prior to beginning data extraction, the charting form was pilot-tested on five randomly selected papers (TS and DM). Information from completed forms was entered into tables, with rows representing individual papers and columns representing components of the analytical framework. This was done to assess the nature and distribution of papers comprising the review.
Analyzing the Data
The data were analysed qualitatively using a general inductive approach. This method is commonly applied to research aimed at developing models of the underlying structure of arguments, processes or experiences [24]. Extracted data (raw text from the tables) were read in detail by two researchers (MP and TS) to become familiar with the content and potential themes. Initial coding categories that represented ‘meaning units’ (themes) were then created. Text segments were assigned to one or more of these categories. If a segment was not relevant to the research objectives, no category was assigned. If subthemes emerged within a category, subcategories were created. A subtheme included items such as points of view on how characteristics of a disease or therapy should be valued in decision making. Once all text was coded, subthemes were reduced to avoid overlap or redundancy. The placement of different text segments relative to one another was then considered in order to identify important links between themes. This information was used to map out the themes and subthemes, creating a structure that reflects the relationships between them.
Consulting Relevant Experts
To optimise the usefulness of the review, a consultation exercise was carried out with relevant key stakeholder communities (patients, providers, industry, and government) [25]. The PRISM programme includes a network of individuals from across Canada who represent these communities. Each individual was asked to review the draft results and contribute additional references, as well as insights into factors or arguments that had not been captured or appeared to be incomplete. Feedback received was incorporated into the draft results through a similar approach to that applied to the papers. It was first ‘charted’ using the same analytical framework and then organised by ‘theme’. Where a new ‘theme’ emerged during the consultation, the draft results were re-analysed through an iterative process to ensure that it, or related concepts, had not been missed.
Presenting the Results
To ensure consistency in the approach to reporting information by theme, a template was created and applied to each theme. The template included a description of the theme (e.g. decision-making factor, source of preferences, value proposition), arguments supporting or refuting its role in decision making within the context of therapies for rare diseases, empirical work carried out to inform such arguments (including a comparative analysis of such work, if available, to identify potentially conflicting findings), and a commentary on existing gaps in the evidence base.
Incorporating Social Values Within Coverage Decisions for Orphan Drugs
Building on previous work by the authors on the process of healthcare decision making and decision criteria for coverage decisions in the presence of a fixed budget, points in the decision process where social values would be incorporated were identified [12, 18, 19]. These points were subsequently used to locate the value arguments identified in the scoping review within the decision process.
The mapping of values on to the decision process formed the basis for a framework characterising the distinct role of each value in informing decision making. This included consideration of how each value should be incorporated within the decision problem, how decision makers should engage with issues of value, and how value information can be synthesised with other components of the decision problem to arrive at a coverage decision in a consistent and transparent manner.
Results
Scoping Review
Description of Studies Selected
Using the PRISMA diagram format, Fig. 1 shows the total number of candidate articles through the four phases of the identification and selection process. A total of 3,723 articles were identified, of which 693 were duplicates. Screening of titles and abstracts excluded 2,629 citations, leaving 401 full-text articles for eligibility assessment. After assessment, 43 articles were retained for review and synthesis [3, 12, 14, 15, 18, 26–63]. These articles were either conceptual pieces or empirical studies. Several identified one or more attributes or characteristics around which there may be a social preference, such as the prevalence of disease or the extent to which the disease is life-threatening or chronically debilitating; we labelled these as identified candidate decision factors. Others identified potential sources of preferences or potential value propositions that decision makers might consider when making coverage decisions for treatments for rare diseases. Assessing the strength of opinion or empirical evidence supporting the use of each identified candidate decision factor, preference or value proposition was outside the scope of this paper.
Eight papers included in the review made normative recommendations relating to decision processes for orphan drugs, including institutional considerations, proposals for decision-making committee membership, or procedural justice arguments.
Extraction and Tabulation of the Data
The data extracted from each study is reported in Table S1 (see electronic supplementary material).
Analysis of the Data
A total of 19 identified candidate decision factors were extracted from the 43 studies reviewed. These are summarised in Table S2 (see electronic supplementary material) and described briefly in the following section.
Candidate Decision Factors
Prevalence (Rarity) of the Disease
Fifteen papers discussed the relevance of disease prevalence as a factor to be (or not to be) considered during decision making [18, 28, 31, 34, 37, 38, 43, 48, 50–52, 54, 58, 59, 63]. Several authors questioned whether ‘rarity’ represents a “rational basis for applying a different value to health gain”, and argued that society should place a similar value on a health gain, regardless of whether the beneficiaries have rare or common disorders [18, 37, 52]. The findings of available empirical studies support this position [31]. Survey evidence from Norway found no preference among physicians or the general population for treating patients with rare disorders at the expense of those with common disorders [48]. A Canadian discrete choice experiment found that the probability that participants would prefer funding for a drug was around 30 % higher for common diseases than for rare diseases [31]. The West Midlands Specialist Services Agency in the UK, following lengthy deliberations over its approach to funding orphan drugs, concluded that rarity should not be an overriding factor in any funding decision [52].
Severity (Seriousness) of Disease
Twelve papers considered the relevance of the seriousness or severity of the disorder to decision making around orphan drugs [3, 12, 27, 32, 34, 37, 38, 40, 42, 48, 50, 58]. The authors often indicated that it is socially desirable to prioritise conditions with high disease severity or unmet medical need. According to Siddiqui and Rajkumar, “the seriousness of a cancer diagnosis plays a role in how much cost patients and physicians are willing to bear for modest incremental benefits” [58]. Clarke questioned whether patients should be “denied access to potentially effective new treatments for formerly untreatable and serious diseases only because it is virtually impossible to evaluate the cost-effectiveness of those treatments using conventional criteria” [27]. Proposed frameworks for orphan drugs, as well as actual review bodies, such as the Australian Pharmaceutical Benefits Advisory Committee (PBAC), include gravity of the condition as a consideration during decision making [12, 34, 38].
Identifiability of the Beneficiaries of Treatment
In four papers, ‘identifiability’, or the tendency to give preference to ‘visible’ individuals, was discussed as central to definitions of the ‘rule of rescue’ [18, 36, 42, 52]. The authors questioned whether it should be a consideration, raising the notion of opportunity costs to underpin arguments: “it strains credulity to say that the more caring society is the one that sacrifices several anonymous lives in order to save an identifiable one”; and ‘special status’ for orphan drugs “may impose substantial and increasing costs on the healthcare system” and these costs will be borne by “other, unknown patients, with more common diseases who will be unable to access effective and cost effective treatment as a result” [18, 42]. One of the studies also mentioned the outcomes of deliberations by the West Midlands Specialist Services Agency in the UK, which concluded that identifiability should not be an overriding factor in any decision to fund treatment [52].
Extent to Which the Disease is Life-Threatening or Chronically Debilitating
Three papers explicitly addressed the ‘life threatening or chronically debilitating’ nature of a condition, which forms part of the EU’s orphan-drug legislation [42, 56, 59]. The authors discussed ethical arguments for favouring the worst-off, “even when only minor gains can be achieved and the cost is very high” [42]. Pinxten et al. [56] argued that developing and supplying orphan drugs complies with the ‘core biomedical objectives’ of health care because “these patients have urgent, objective medical needs and because their lives are in danger when they do not receive the necessary care … from a biomedical perspective, there are no valid reasons to exclude rare diseases from publicly funded healthcare”.
Evidence of Treatment Efficacy or Effectiveness
Eleven papers discussed the role of evidence of clinical efficacy or effectiveness within the context of orphan drugs [3, 12, 27, 28, 34, 39, 41, 44, 59, 62, 63]. In general, the authors argued that “orphan drugs [should] have to prove effectiveness like any other drug” [62]. Three of the studies presented empirical evidence based upon retrospective analyses of regulatory decisions. The findings were similar: orphan drug trials were more likely to assess disease response rather than overall survival [39, 59, 62]. While some authors have called for more stringent measures of clinical effectiveness to be adopted, others have indicated that it is difficult to evaluate clinical effectiveness “due in part to the nature of rare diseases” [27, 39]. Four of the studies contained proposed decision-making frameworks, all of which included evidence of clinical effectiveness as a criterion [12, 28, 59, 63].
Magnitude of Treatment Benefit
In ten papers, the importance of considering the amount of individual health gain or magnitude of benefit offered by an orphan drug was discussed [26, 29, 31, 32, 38, 42, 50, 57, 58, 60]. The authors suggested that the impact of treatment on life expectancy and quality of life should be taken into account, as well as whether the therapy “remains a symptomatic therapy rather than a cure” [26]. Others argued that the lack of explicit thresholds of clinical benefit contributes to the high cost of drugs, and supported adopting policies similar to the UK’s proposed ‘value-based pricing’ framework, under which drugs demonstrating a greater magnitude of benefit would command higher prices [29]. Other frameworks have also proposed ‘therapeutic benefit’ as a criterion for assessing the value of therapies for rare diseases [38]. Empirical evidence appears to support this view [31, 50].
Availability of Treatment Alternatives
Seven papers addressed the availability of alternative treatments as a consideration during the development of funding decisions for orphan drugs [12, 27, 34, 40, 44, 51, 59]. In general, the lack of disease-modifying treatment options connoted ‘unmet need’, and the authors argued that “it is socially desirable to develop treatments for conditions carrying very high disease severity or having significant unmet medical need irrespective of their rarity”, and that patients should not be denied access to potentially effective new treatments for ‘formerly untreatable’ diseases [27, 40]. Empirical studies have demonstrated that the price of an orphan drug appears to be inversely related to the availability of alternative treatments (i.e. prices are higher where no other options exist) [51]. Proposed frameworks have also incorporated this factor into decision-making criteria [12, 59].
Safety Profile of Treatment
Three papers addressed safety considerations as an important decision-making factor [41, 51, 59]. Two included ‘safety profile’ along with other proposed criteria. However, the third presented empirical work comparing the characteristics of pivotal trials of orphan versus non-orphan drugs for cancer, the findings of which demonstrated that serious adverse event rates were statistically significantly higher in trials of orphan drugs [41].
Innovation Profile of Treatment
In four papers, innovation as a decision-making factor was explored [18, 29, 38, 51]. Some authors questioned whether healthcare systems should pay more than the value of the benefits from a new technology in the hope that a more valuable future technology will be developed (i.e. paying twice for innovation) [18, 29]. Others argued that cost-containment measures, which may be necessary due to the strain that orphan drugs put on national health budgets, will not be productive or appropriate for the long-term development of drugs for rare diseases [51].
Societal Impact of Treatment
The importance of considering the broader impact of orphan drugs on families, and societies as a whole, was discussed in five papers [12, 34, 38, 57, 61]. Concerns over standard methods of assessment, which may not take into account the value of returning patients or carers to work or school, were raised [34]. This point was addressed in two of the proposed funding frameworks for orphan drugs that included societal and familial impact in decision-making criteria [38, 57].
Impact of Treatment upon the Distribution of Health
In six papers, the authors explored the impact of orphan drugs on the distribution of health across competing patient populations [18, 31, 42, 52, 55, 56]. It was argued that debates around whether orphan drugs should receive special status must consider opportunity costs [18]. Opportunity costs present an ethical dilemma which “has to be assessed according to the various existing concepts of distributive justice” [56]. These concepts include equal access, equal resources, and equal outcomes, and they often conflict with one another. For example, if a utilitarian view of distributive justice were adopted, it would be difficult to support the development and supply of orphan drugs. Empirical evidence exploring this issue was limited to one paper. This paper comprised a survey of Norwegian doctors, which found little support for prioritising the treatment of rare diseases, although a preference for allocating resources in accordance with the principle of reserving a small portion of resources for rare disease patients was noted [31]. The authors of two of the papers raised concerns over postcode prescribing and equity in access to orphan drugs across jurisdictions. Different approaches to alleviating these concerns were proposed, including regulation of compassionate access at a multi-jurisdiction (European) level, and assignment of equity weights to quality-adjusted life-years (QALYs) during the assessment of orphan drugs by decision-making bodies [52, 55].
Socioeconomic Policy Objectives
Three papers considered socioeconomic policy objectives in the context of rare diseases [18, 34, 48]. Drummond et al. [34] argued that “it does not make much sense (in terms of efficiency) for the public system to fund or subsidize R&D on orphan drugs and later not reimburse the resulting innovations. This strategy will lead to a waste of R&D resources (if the products are finally not used) and discourage future investment on R&D on orphan drugs”. Meanwhile, McCabe et al. [48] noted that “many healthcare payers have exempted orphan drugs from formal value assessment, arguing that society values equal opportunity for people with rare and common conditions enough to justify the high costs”.
Cost (Price) of Treatment
The price of orphan drugs was discussed in 19 papers [14, 15, 26, 29, 31, 34–36, 40, 43, 45, 46, 48, 50, 51, 53, 54, 57, 58]. Several presented examples of the average per-patient treatment costs, concluding that the prices of orphan drugs “poses a substantial challenge for healthcare systems” and are ‘unsustainable’ [26, 48]. Health insurers cannot, and should not, “be expected to fund, at any price, all effective orphan drugs” [34]. In one paper, the authors attributed the high prices to, in part, “the absence of appropriate benchmarks to gauge whether prices are low, high, or too high relative to expectations” [40]. Their views were echoed in another paper, which stated that “the price usually has very little to do with the drug’s incremental benefit” [43]. Empirical work demonstrated that “awarding orphan designation in itself is associated with higher prices for drugs for rare disease indications” [54].
Budget Impact of Treatment
The relevance of budget impact considerations was discussed in 13 papers [12, 14, 18, 29, 30, 34, 37, 38, 50, 51, 58, 60, 63]. Several authors questioned the need to consider it at all since the budget impact of many orphan drugs is ‘modest’ due to small patient numbers [34]. Others argued that, while the number of patients with a single rare disease is small, there are thousands of these diseases, and industrial and regulatory policies encouraging research and development (R&D) in rare diseases have led to a rapidly growing orphan-drug market. It has been estimated that by 2030 “specialty pharmaceuticals will account for up to 44 % of a plan’s total drug expenditures” [60]. Therefore, budget impact must be considered in funding processes. Budget impact was included as a consideration in three of the papers proposing decision-making frameworks for rare diseases [12, 38, 63].
Cost Effectiveness of Treatment
The cost effectiveness of treatment was considered in 23 papers [12, 15, 18, 27–30, 34, 38, 39, 42, 43, 48, 50, 52, 53, 56–58, 60–63]. Issues raised fell into one of two categories: appropriateness of standard cost-effectiveness methods in assessments of orphan drugs; and use of conventional cost-effectiveness thresholds to determine the cost effectiveness of orphan drugs. Several authors suggested that standard methodologies of health technology assessments must be updated and tailored to orphan drugs [34, 57]. The application of conventional cost-effectiveness thresholds to coverage decisions has generated significant debate. Some authors argued that ‘cost effectiveness’ should be treated similarly for orphan and non-orphan drugs, and that cost-effectiveness ratios offer an equitable way to guide decision making [43, 52]. Others argued that “a complete restriction on the funding of ultra-orphan drugs is not a practical or realistic solution” [37]. A number of the papers proposing decision-making frameworks included cost effectiveness as a consideration [12, 15, 28, 38, 63].
Feasibility of Diagnosing the Disease
In one paper, the authors argued that funding decisions need to consider whether diagnosis of the rare disease is technically feasible [59]. Not all jurisdictions have the infrastructure, resources, or expertise to accurately diagnose some rare diseases.
Feasibility of Providing Treatment
In one paper, the authors considered the feasibility of treatment as a decision-making criterion [59]. They indicated that specialist training and expertise are often required to ensure patients are appropriately managed.
Industrial and Commercial Policy Considerations
Twelve papers addressed commercial considerations as they relate to the reimbursement of orphan drugs [18, 29, 32, 33, 35, 36, 40, 44, 45, 49, 58, 59]. Some argued that “because of their small market potential, [orphan drugs] are not attractive for pharmaceutical companies to develop and market” [59]. Others questioned this position, arguing that the costs of development for orphan drugs are lower since clinical trials are shorter, regulatory findings are more successful, and Orphan Drug Act benefits such as fee waivers, R&D grants, and tax incentives are available [49]. Citing empirical work, the latter authors argued that “taken together, lower costs, higher rates of regulatory success and parity of revenue-generating potential translate into higher profitability of orphan vs non-orphan drugs”.
Legal Considerations
Two papers raised legal considerations as potential decision-making factors [52, 58]. Siddiqui and Rajkumar considered the implications of the patent system, while Moberly explained that “legal concerns over commercial expectations” contributed towards the UK Department of Health moving commissioning away from the West Midlands Specialized Services Agency [52, 58].
Stakeholder Preferences, Value Propositions, and Institutional Structures
In addition to the 19 candidate decision factors, the review also identified stakeholder preferences, value propositions, and institutional structures as important elements in the reimbursement of orphan drugs.
Stakeholder Preferences
Three sources of preferences that decision makers might consider when making coverage decisions for treatments for rare diseases were identified:
Value Propositions
The following value propositions, comprising statements around how individual or multiple candidate decision factors should be valued or weighed during decision making, were identified:
The ‘rule of rescue’, which supports the non-abandonment—regardless of cost—of identifiable patients with a life-threatening illness if an effective treatment is available. (This addresses ‘identifiability of the beneficiaries of treatment’, ‘severity [seriousness] of disease’, ‘extent to which the disease is life-threatening or chronically debilitating’, and ‘availability of treatment alternatives’, and explicitly excludes ‘cost [price] of treatment’.) [36, 37, 42, 56]
The ‘equity principle’, which argues against special consideration for patients with rare diseases. (This addresses ‘societal impact of treatment’, ‘impact of treatment upon the distribution of health in the population’ and ‘magnitude of treatment benefit’, placing greater weight on the first two factors.) [18, 31, 36, 37, 42, 43, 46]
The ‘rights-based approach’, which proposes that individuals have a right to a decent minimum level of healthcare, implying that treatments for rare diseases should be made available if the respective patients have no other treatment options. (This addresses ‘impact of treatment upon the distribution of health in the population’—defining equity in terms of equal access to treatment—and ‘availability of treatment alternatives’.) [36, 37]
Institutional Structures
Some authors called for a dedicated funding programme for rare diseases and the establishment of an independent body responsible for their assessment. A WHO Orphan Medicines Model List was also proposed as a complement to the existing Model List of Essential Medicines [59].
Integrating the Identified Candidate Decision Factors, Preferences and Value Propositions into a Coherent Decision-Making Framework
Categorising the Identified Candidate Decision Factors
Based on qualitative analyses of discussions related to the 19 candidate decision factors in papers, relationships among them were identified. These were used to group the factors into three categories:
those that determine the opportunity cost of providing coverage for the orphan therapy or its relevant comparators;
those that bear upon the value assigned to the orphan therapy, its comparators, and the opportunity cost of each;
those factors that are neither value-bearing nor determining the opportunity cost, but are, nevertheless, relevant for the decision about whether to provide coverage.
‘Opportunity Cost’-Determining Factors
The ‘opportunity cost’-determining factors identified in the review included:
cost (price) of treatment;
budget impact of treatment.
As described in the papers, the budget impact of treatment is a function of the size of the patient population and the cost of treatment per patient, which, in turn, is a function of the treatment’s purchase price and any other resources required for the safe and effective delivery of the treatment. The larger the budget impact, the greater the opportunity cost when the treatment is covered by the healthcare budget since more treatments will need to be forgone by other patients.
Value-Bearing Factors
The value-bearing factors were further grouped into four non-mutually exclusive categories:
disease-related factors;
technology-related factors;
population-related factors;
socioeconomic-related factors.
Disease-related factors
The disease-related value-bearing factors identified in the review include:
prevalence (rarity) of disease;
severity (seriousness) of disease;
identifiability of the beneficiaries of treatment;
extent to which the disease is life-threatening or chronically debilitating without treatment;
impact of disease upon the distribution of health in the population;
availability of treatment alternatives.
-
2.
Treatment-related factors
The treatment-related value-bearing factors identified in the review include:
evidence of treatment efficacy or effectiveness;
magnitude of treatment benefit;
safety profile of treatment;
innovation profile of treatment;
societal impact of treatment;
impact of treatment upon the distribution of health in the population.
-
3.
Population-related factors
The population-related value-bearing factors identified in the review included:
societal impact of treatment;
impact of treatment upon the distribution of health in the population;
socioeconomic policy objectives.
-
4.
Socioeconomic-related factors
The socioeconomic-related value-bearing factors identified in the review included:
societal impact of treatment;
impact of treatment upon the distribution of health in the population;
socioeconomic policy objectives;
industrial and commercial policy considerations;
legal considerations.
Other Decision Factors
The remaining identified candidate decision factors were neither value-bearing nor ‘opportunity cost’-determining, but were viewed as potentially influencing the decision about whether to provide coverage for an orphan therapy. These included:
feasibility of diagnosing the disease;
feasibility of providing treatment;
cost effectiveness of treatment.
Based on the findings of the review, the feasibility of diagnosing the disease and of providing treatment are regarded as necessary but are not sufficient conditions for the funding of an orphan therapy.
Given the considerable debate in the literature around the cost effectiveness of treatment, careful consideration is required before integrating. This is discussed in further detail later in the article.
Preferences
The results of the scoping review highlighted the diversity of views around the candidate decision factors and how they should be operationalised in coverage decision making. Views often reflected preferences for how healthcare should be allocated across competing patient populations. As noted in several of the papers, those preferences may vary by stakeholder community. Therefore, decision makers may wish to incorporate the preferences of one or more stakeholders when making coverage decisions for orphan therapies. The preferences of patients, physicians and society at large were explicitly identified as possible considerations. However, inferences to input from other stakeholders, such as the members of expert bodies or commercial partners, were made.
Given that preferences may vary, when incorporating these into a coherent decision-making framework the preferences of each stakeholder (or stakeholder community) may be considered as representing a unique preference function, , where denotes the stakeholder in question. Within each preference function are a number of arguments, , representing each of the n value-bearing factors. Each stakeholder may place a different weight—which can include zero—on each of these value-bearing factors. For example, a physician might place large weight on the safety profile of a treatment, whereas a patient might place smaller weight on its safety profile but larger weight on the expected magnitude of benefit.
The value that each stakeholder places on any particular treatment—whether that is the orphan therapy being appraised, a comparator, or a treatment forgone by other patients should it be funded—depends upon the weight placed by the stakeholder on each of the value-bearing factors and the extent to which each of these value-bearing factors is relevant to the treatment in question. For example, if a stakeholder places high weight on ‘severity of disease’, then (all else equal) a treatment for patients with more severe disease will be valued more highly by the stakeholder than a treatment for patients with less severe disease. The value placed on treatment by stakeholder is denoted by , where .
Value Propositions
As mentioned above, the scoping review identified three value propositions—the ‘rule of rescue’, the ‘equity principle’, and the ‘rights-based approach’. While this is not an exhaustive list, it provides examples of value propositions that might be considered by decision makers.
Value propositions may be viewed in a similar way to preferences. Each value proposition, , is a unique function, , of the n value-bearing factors, . Each value proposition places different weight on these factors. For example, the ‘rule of rescue’ places relatively large weight on ‘identifiability of the beneficiaries of treatment’ and ‘extent to which the disease is life-threatening or chronically debilitating’, but relatively little weight on ‘impact of treatment upon the distribution of health’. By contrast, the ‘equity principle’ places no weight on ‘identifiability of the beneficiaries of treatment’, nor on ‘prevalence (rarity) of disease’, but a much greater weight upon ‘impact of treatment upon the distribution of health in the population’.
In common with preferences, the value that any particular value proposition assigns to a treatment depends upon the weight placed on each of the value-bearing factors and the extent to which each of these is relevant to the treatment. All else equal, the ‘rights-based approach’ would assign a greater value to an effective treatment if patients have no other treatment options, whereas the ‘equality principle’ would not. The value placed on treatment by value proposition is denoted by , where .
Our Proposed Framework
The above considerations may be used to map out a coherent decision-making framework which incorporates the role that each of the candidate decision factors, preferences and value propositions could play in the decision-making process. This framework is summarised in Fig. 2. The various value-bearing factors are considered in the yellow box at the top-left of the figure. The value placed on any particular treatment by each stakeholder and by each of the various alternative value propositions is a function of these value-bearing factors.
Fig. 2.
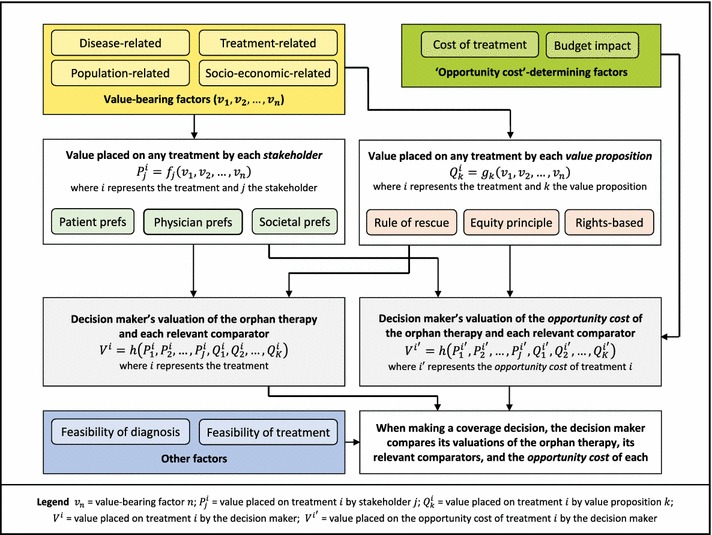
Proposed framework for aiding coverage decisions for orphan therapies. Prefs preferences
The value placed on treatment by the decision maker is a function, , of the value placed on treatment by each stakeholder () and the value placed on treatment by each value proposition (), where is the number of relevant stakeholders and is the number of relevant value propositions. The decision maker determines how much weight (if any) is placed on the values of each stakeholder and each value proposition. These weights may be reflective of the composition of the committee that makes decisions and of the process by which stakeholders are consulted and decisions are made. For example, if patients or advocacy groups are given the opportunity to address the committee prior to the decision, the relative weight assigned to the values of those stakeholders by the decision maker might be increased. The value placed on treatment by the decision maker is denoted by , where .
The decision maker’s valuation of the orphan therapy and each relevant comparator is considered in the grey box to the lower left of Fig. 2. Alongside this, in the grey box to its right, the decision maker also considers its valuation of the opportunity cost of the orphan therapy and each relevant comparator, i.e. its valuation of the treatment(s) that would be forgone by other patients if the orphan therapy (or one of its comparators) were to be funded by the public healthcare system. This opportunity cost is determined by consideration of the ‘opportunity cost’-determining factors listed in the green box at the top-right of the figure. The opportunity cost of treatment is denoted as . The value placed on by the decision maker is , where .
When making a coverage decision, the decision maker compares its valuations of the orphan therapy and each of its relevant comparators, , with its valuation of the opportunity cost of each, . This enables the decision maker to determine the net value of the orphan therapy and each of its comparators, , where . If the net value of the orphan therapy is negative, then it should not be covered by the healthcare system since its value is lower than that of the treatment(s) it is expected to displace. If its net value is positive, but lower than that of one or more relevant comparators, then, again, the orphan therapy should not be funded since greater value can be gained by funding one of its comparators instead. The orphan therapy should only be funded if its net value is positive and greater than that of each relevant comparator.
However, there are other potential decision-bearing factors that the decision maker may wish to consider, listed in the blue box at the bottom-left of Fig. 2. In particular, if diagnosis or treatment of the orphan disease is not feasible, then the orphan therapy should not be covered since the expected value cannot be realised.
Considering the ‘Cost Effectiveness of Treatment’ Within a Decision-Making Framework
The ‘cost effectiveness of treatment’ is the only candidate decision factor identified in the scoping review that is not explicitly considered as a factor within the proposed framework. It requires special consideration for three key reasons:
The cost effectiveness of treatment is a composite of (at least) two other identified candidate decision factors—the cost of treatment, and the effectiveness of treatment. The unit of ‘effectiveness’ used might also be a function of multiple other candidate decision factors; for example, estimation of the QALYs gained with treatment combines consideration of the ‘severity (seriousness) of disease’ and the ‘magnitude of treatment benefit’.
The cost effectiveness of treatment is generally determined using a decision rule, the most common involving the comparison of the incremental cost-effectiveness ratio (ICER) of the treatment to a ‘cost-effectiveness threshold’ [19]. In the context of a budget-constrained healthcare system, this threshold is an estimate of the opportunity cost of funding the treatment (in terms of the units of ‘effectiveness’ forgone elsewhere in the system) [64–66]. Consideration of the cost effectiveness of treatment therefore also incorporates consideration of ‘opportunity cost’-determining factors.
By explicitly incorporating consideration of opportunity cost, cost-effectiveness analysis facilitates comparison of the ‘effectiveness’ of the treatment in question and its comparators to the ‘effectiveness’ of the treatment(s) forgone as a result of their funding. In valuing these, cost-effectiveness analysis typically assumes that the decision maker adopts the ‘equity principle’ as a value proposition—all units of ‘effectiveness’ are valued equally across the treatment, its comparators, and the opportunity cost, regardless of the prevalence (rarity) of disease or the identifiability of the beneficiaries of treatment. Furthermore, the ‘value’ of any treatment considered in a cost-effectiveness analysis is typically determined only in terms of these units of effectiveness—all other value-bearing factors are assumed to have zero weight.
Thus, the ‘cost-effectiveness of treatment’ (or any measure of the ‘efficiency’ of treatment which combines multiple value-bearing factors, a consideration of opportunity cost, and/or a specific value proposition) should not be incorporated within a coherent decision-making framework as an additional ‘factor’. To do so would amount to a partial double-counting of opportunity cost and effectiveness, the extent of which depends upon the relative weight attached to cost, effectiveness and efficiency. Under specific circumstances, basing reimbursement decisions upon the results of a cost-effectiveness analysis is equivalent to making decisions using the framework proposed. In all other circumstances, including when the decision maker adopts a different value proposition to the ‘equity principle’, or otherwise applies a different value to each treatment than that implied by the measure of ‘effectiveness’ used in the cost-effectiveness analysis, this framework provides an alternative means to informing decisions that is coherent with the principles underlying cost-effectiveness analysis but which allows for a broader account of value than conventional cost-effectiveness analysis. This is because the proposed framework imposes no constraints upon the value-bearing factors that may be considered, the value propositions that may be adopted by the decision maker, or the relative value that may be placed upon any of these, whilst preserving the consideration of opportunity cost that is central to cost-effectiveness analysis.
Summary of Steps Required for Coherent Coverage Decisions
As was highlighted in the results of the scoping review, within a budget-constrained healthcare system, any decision to fund therapy for some patients inevitably imposes an opportunity cost by displacing treatments that would otherwise be provided to other patients. Providing coverage for any therapy (whether for a rare disease or otherwise) is desirable only if the value of doing so is greater than the value of this opportunity cost. Taking all of the findings into account, it may therefore be argued that coherent coverage decisions for orphan drugs require the following steps:
Establish whether the orphan therapy in question has any relevant comparators (treatment alternatives).
Estimate the opportunity cost (i.e. the other treatments expected to be displaced) resulting from providing coverage for the orphan therapy.
Estimate the opportunity cost associated with providing coverage for each relevant comparator.
Determine the value of the orphan therapy and each comparator.
Determine the value of the opportunity cost of the orphan therapy and each comparator.
Calculate the net value of the orphan therapy and each comparator by comparing the value of each with the value of their opportunity cost.
Provide coverage for the orphan therapy only if its net value is positive and exceeds that of each relevant comparator.
Discussion
In this paper we identified social value arguments in published scholarly papers related to the reimbursement of orphan drugs and key linkages among them in order to construct a coherent decision-making framework. Discussions around funding specific orphan drugs and the principles of orphan drug coverage can be characterised as a discussion of values. Advocates of all positions have advanced value-based arguments as to why orphan drugs should or should not be given a special value status in the allocation of limited healthcare resources [11]. However, based on our scoping review, there is ambiguity around what is being valued and from what perspective. Similarly, the values positions implicitly assumed in constructing arguments are often not acknowledged. We have attempted to parse the literature and offer order to the consideration of the value of orphan drugs in the context of healthcare coverage decisions in the presence of limited resources.
To this end, we identified a set of candidate decision factors that authors have proposed should or should not be considered. Some of these are value-bearing factors, which we have characterised as disease-related, treatment-related, population-related, and/or socioeconomic-related. The latter includes legally mandated policy considerations. The remaining factors are not value-bearing but, nonetheless, are important for healthcare coverage decisions, in particular those that determine the opportunity cost of a decision to provide funding. We also identified three potential sources of preferences—those of patients, physicians, and society—and a number of propositions about how values should be incorporated into the decision-making process. The ‘rule of rescue’ proposes that opportunity cost be given a close-to-zero weight when there are identifiable victims facing imminent death or substantial disability. The ‘equity principle’ considers values equally for the beneficiaries of treatment and those bearing the opportunity cost, whilst the ‘rights-based approach’ disregards the issue of opportunity cost entirely in cases where patients have few alternative treatment options.
We propose that decision makers seek to identify which value-bearing factors they deem pertinent to their decision, whose preferences they wish to consider, and what value propositions underpin their decisions. We have identified how these need to be applied consistently to all technologies and populations affected by the decision: the new orphan drug, any existing therapy for the same disease which will be displaced, and any therapies which will be displaced elsewhere in the system to fund any additional costs of a positive coverage decision (the opportunity cost). This approach enables decision makers to arrive at a coverage decision based upon the value of the orphan therapy and its opportunity cost.
In recent years, many published frameworks for making reimbursement decisions on a range of health technologies have used multi-criteria decision analysis (MCDA). The framework we present highlights a number of issues with existing applications of MCDA. For example, a recent paper by Endrei et al. [67] outlines the six major criteria used in the reimbursement of new medical technologies in Hungary: ‘healthcare priorities’, ‘severity of the disease’, ‘equity’, ‘cost effectiveness and quality of life’, ‘aggregated budget impact’, and ‘national and international respect’. Each of these is given a ‘points weight’ that sums to a total of 100. As described above, ‘cost effectiveness’ is a composite of other decision factors. Therefore, its inclusion in an MCDA framework results in ‘double-counting’. Furthermore, cost-effectiveness analysis incorporates an explicit consideration of opportunity cost, which, in a budget-constrained healthcare system, is determined in part by the ‘aggregated budget impact’ of the treatment. It also incorporates an implicit value proposition based upon the ‘equity principle’. The consideration of cost-effectiveness within an MCDA, alongside severity of illness, equity, and aggregated budget impact—where each is assigned a relative weight—becomes invalid. MCDA work conducted by the Vancouver Coastal Health Authority suffers from a similar issue by including ‘efficiency, effectiveness and appropriateness’ among the criteria considered [68]. Within the field of rare diseases, Sussex et al. [69] recently conducted a pilot study of MCDA methods, identifying eight attributes for establishing the value of an orphan medicine. While the authors appropriately excluded consideration of costs or cost effectiveness from these criteria, they note that their approach was intended to “focus on the benefits of [orphan drugs], which can then be compared with net costs, including the price of the [orphan drug] itself”. The framework presented in this paper suggests that another step is required before the ‘benefits’ of an orphan drug can be compared to its net costs—consideration of the opportunity cost resulting from these net costs, and an assessment of the ‘benefits’ forgone as a result. A common theme among these existing implementations of MCDA is that the approach to considering costs appears misplaced. It seems inappropriate to consider costs as an afterthought to compare against the benefits of the treatment in question, or alongside value-bearing factors as an attribute within an MCDA (either as a separate ‘cost’ attribute or embedded within an attribute representing ‘budget impact’, ‘cost effectiveness’ or ‘efficiency’). It seems more appropriate to consider costs as a determinant of the opportunity cost of the treatment. This opportunity cost should then be valued by the decision maker in a manner consistent with the valuation of the treatment and its comparators.
We hope that structuring discussions using this framework might also guide the focus and design of future research to ensure that empirical insights into value arguments around the coverage of treatments for rare diseases meet the needs of decision makers. The recent paper by Linley and Hughes [70] highlights the importance of exploring whether perceived societal values, upon which decision makers have based funding policies, reflect actual societal values; their findings suggest that these often differ. The use of our proposed framework to structure both policy discussions and decisions might aid transparency about the nature of reimbursement decisions for orphan drugs, the values relied upon, and how these values have been implemented.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
This work was supported by an Emerging Team grant from the CIHR for ‘Developing Effective Policies for Managing Technologies for Rare Diseases’, and from a grant provided through the Genome Canada-CIHR 2012 Large-Scale Applied Research Project Competition in Genomics and Personalized Health for ‘PACE-‘Omics: Personalized, Accessible, Cost-effective applications of ‘Omics technologies’. The authors wish to acknowledge the support provided by the Promoting Rare-disease Innovations through Sustainable Mechanisms (PRISM) Collaborators Network. The authors are grateful to Andrea Dunn for her assistance with data extraction, Leigh-Ann Topfer for her guidance and support during the literature search and retrieval phase of this project, and Kerry Nield for her assistance in preparing the manuscript for submission. Mike Paulden, Tania Stafinski, Devidas Menon and Christopher McCabe have no conflicts of interest.
Author contributions
All authors contributed towards the design of the scoping review, the construction of the decision framework, and the writing of the manuscript. Tania Stafinski and Devidas Menon conducted article screening for the scoping review. Mike Paulden and Tania Stafinski were responsible for reviewing each paper, extracting and tabulating data, and identifying the candidate decision factors. Mike Paulden is the overall guarantor for the paper.
References
- 1.Haffner ME. Adopting orphan drugs—two dozen years of treating rare diseases. N Engl J Med. 2006;354:445–447. doi: 10.1056/NEJMp058317. [DOI] [PubMed] [Google Scholar]
- 2.Braun MM, Farag-El-Massah S, Xu K, Coté TR. Emergence of orphan drugs in the United States: a quantitative assessment of the first 25 years. Nat Rev Drug Discov. 2010;9:519–522. doi: 10.1038/nrd3160. [DOI] [PubMed] [Google Scholar]
- 3.Dunoyer M. Accelerating access to treatments for rare diseases. Nature. 2011;10:475–476. doi: 10.1038/nrd3493. [DOI] [PubMed] [Google Scholar]
- 4.Food and Drug Administration. Developing products for rare diseases and conditions. Available from: http://www.fda.gov/ForIndustry/DevelopingProductsforRareDiseasesConditions/default.htm. Accessed 13 Nov 2014.
- 5.Food and Drug Administration. Orphan drug regulations. Washington DC; 2013. Available from: http://www.gpo.gov/fdsys/pkg/FR-2013-06-12/pdf/2013-13930.pdf. Accessed 13 Nov 2014.
- 6.Salari K, Watkins H, Ashley EA. Personalized medicine: Hope or hype? Eur Heart J. 2012;33:1564–1570. doi: 10.1093/eurheartj/ehs112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reeves A, McKee M, Basu S, Stuckler D. The political economy of austerity and healthcare: cross-national analysis of expenditure changes in 27 European nations 1995–2011. Health Policy. 2014;115:1–8. doi: 10.1016/j.healthpol.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 8.Stafinski T, Menon D, Davis C, McCabe C. Role of centralized review processes for making reimbursement decisions on new health technologies in Europe. Clin Outcomes Res. 2011;3:117–186. doi: 10.2147/CEOR.S14407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stafinski T, Menon D, Philippon DJ, McCabe C. Health technology funding decision-making processes around the world: the same, yet different. Pharmacoeconomics. 2011;29:475–495. doi: 10.2165/11586420-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 10.Simoens S. Pricing and reimbursement of orphan drugs: the need for more transparency. Orphanet J Rare Dis. 2011;6:42. doi: 10.1186/1750-1172-6-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Drummond M, Towse A. Orphan drugs policies: a suitable case for treatment. Eur J Health Econ. 2014;15:335–40. [DOI] [PubMed]
- 12.Stafinski T, Menon D, McCabe C, Philippon DJ. To fund or not to fund: development of a decision-making framework for the coverage of new health technologies. Pharmacoeconomics. 2011;29:771–780. doi: 10.2165/11539840-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 13.Hutchings A, Schey C, Dutton R, Achana F, Antonov K. Estimating the budget impact of orphan drugs in Sweden and France 2013–2020. Orphanet J Rare Dis. 2014;9:22. doi: 10.1186/1750-1172-9-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schey C, Milanova T, Hutchings A. Estimating the budget impact of orphan medicines in Europe: 2010–2020. Orphanet J Rare Dis. 2011;27:62. doi: 10.1186/1750-1172-6-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hughes-Wilson W, Palma A, Schuurman A, Simoens S. Paying for the Orphan Drug System: break or bend? Is it time for a new evaluation system for payers in Europe to take account of new rare disease treatments? Orphanet J Rare Dis. 2012;7:74. doi: 10.1186/1750-1172-7-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mays N, Roberts E, Popay J. Synthesising research evidence. In: Fulop N, Allen P, Clarke A, Black N, editors. Methods for studying the delivery and organisation of health services. London: Routledge; 2001: p. 188–220.
- 17.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McCabe C, Claxton K, Tsuchiya A. Orphan drugs and the NHS: should we value rarity? BMJ. 2005;331:1016–9. [DOI] [PMC free article] [PubMed]
- 19.McCabe C, Claxton K, Culyer AJ. The NICE cost-effectiveness threshold: what it is and what that means. Pharmacoeconomics. 2008;26:733–744. doi: 10.2165/00019053-200826090-00004. [DOI] [PubMed] [Google Scholar]
- 20.Arksey H, O’Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. 2005;8:19–32. doi: 10.1080/1364557032000119616. [DOI] [Google Scholar]
- 21.PRISM Group. Promoting rare-disease innovations through sustainable mechanisms (PRISM). 2014. Available from: http://www.prismfive.org. Accessed 13 Nov 2014.
- 22.Ritchie J, Spencer L. Qualitative data analysis for applied policy research. In: Bryman A, Burgess RG, editors. Anaylsing qualitative data. London: Routledge; 1994. p. 173–94.
- 23.Pawson R. Evidence-based policy: in search of a method. Evaluation. 2002;8:157–181. doi: 10.1177/1358902002008002512. [DOI] [Google Scholar]
- 24.Bryman A, Burgess R. Analyzing qualitative data. London: Routledge; 1994. p. 232. [Google Scholar]
- 25.Oliver S. Making research more useful: integrating different perspectives and different methods. In: Oliver S, Peersman G, editors. Useful Research for Effective Health Promotion. Buckingham: Open University Press; 2001. pp. 167–179. [Google Scholar]
- 26.Barrett P, Alagely A, Topol E. Cystic fibrosis in an era of genomically guided therapy. Hum Mol Genet. 2012;21:R66–R71. doi: 10.1093/hmg/dds345. [DOI] [PubMed] [Google Scholar]
- 27.Clarke JT. Is the current approach to reviewing new drugs condemning the victims of rare diseases to death? A call for a national orphan drug review policy. Can Med Assoc J. 2006;174:189–190. doi: 10.1503/cmaj.050706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clarke J, Bell C, Coyle D, Stevenson H, Evans G, Martin M, et al. A policy framework for funding drugs for rare diseases. Value Health. 2009;12(7):A243. [DOI] [PubMed]
- 29.Claxton K, Briggs A, Buxton MJ, Culyer AJ, McCabe C, Walker S, et al. Value based pricing for NHS drugs: an opportunity not to be missed? BMJ. 2008;336:251–4. [DOI] [PMC free article] [PubMed]
- 30.Denis A, Mergaert L, Fostier C, Cleemput I, Simoens S. Budget impact analysis of orphan drugs in Belgium: estimates from 2008 to 2013. J Med Econ. 2010;13:295–301. doi: 10.3111/13696998.2010.491427. [DOI] [PubMed] [Google Scholar]
- 31.Desser AS. Prioritizing treatment of rare diseases: a survey of preferences of Norwegian doctors. Soc Sci Med. 2013;94:56–62. [DOI] [PubMed]
- 32.Dickson P, Pariser A, Groft S, Ishihara R, McNeil D, Tagle D, et al. Research challenges in central nervous system manifestations of inborn errors of metabolism. Mol Genet Metab. 2011;102:326–338. doi: 10.1016/j.ymgme.2010.11.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Drakulich A. Global healthcare on the ground: NIH aims to help treat 200 rare diseases. Pharm Technol. 2011;35:22. [Google Scholar]
- 34.Drummond MF, Wilson DA, Kanavos P, Ubel P, Rovira J. Assessing the economic challenges posed by orphan drugs. Int J Technol Assess Health Care. 2007;23:36–42. doi: 10.1017/S0266462307051550. [DOI] [PubMed] [Google Scholar]
- 35.Garattini S. Time to revisit the orphan drug law. Eur J Clin Pharmacol. 2012;68:113. doi: 10.1007/s00228-011-1115-3. [DOI] [PubMed] [Google Scholar]
- 36.Gupta S. Rare diseases : Canada’s “research orphans”. Open Med. 2012;6:23–27. [PMC free article] [PubMed] [Google Scholar]
- 37.Hughes D, Tunnage B, Yeo S. Drugs for exceptionally rare diseases: do they deserve special status for funding? QJM Int J Med. 2005;98:829–836. doi: 10.1093/qjmed/hci128. [DOI] [PubMed] [Google Scholar]
- 38.Hutchings A, Ethgen O, Schmitt C, Rollet P. Defining elements of value for rare disease treatments. Value Health. 2012;15(4):A31.
- 39.Joppi R, Bertele’ V, Garattini S. Orphan drugs, orphan diseases. The first decade of orphan drug legislation in the EU. Eur J Clin Pharmacol. 2013;69:1009–1024. doi: 10.1007/s00228-012-1423-2. [DOI] [PubMed] [Google Scholar]
- 40.Kanavos P, Nicod E. What is wrong with orphan drug policies? Suggestions for ways forward. Value Health. 2012;15:1182–1184. doi: 10.1016/j.jval.2012.08.2202. [DOI] [PubMed] [Google Scholar]
- 41.Kesselheim AS, Myers JA, Avorn J. Characteristics of clinical trials to support approval of orphan vs nonorphan drugs for cancer. JAMA. 2011;305:2320–2326. doi: 10.1001/jama.2011.769. [DOI] [PubMed] [Google Scholar]
- 42.Largent EA, Pearson SD. Which orphans will find a home? The rule of rescue in resource allocation for rare diseases. Hastings Cent Rep. 2012;42:27–34. doi: 10.1002/hast.12. [DOI] [PubMed] [Google Scholar]
- 43.Laupacis A. Evidence and values: requirements for public reimbursement of drugs for rare diseases: a case study in oncology. Can J Clin Pharmacol. 2009;16:e282–e284. [PubMed] [Google Scholar]
- 44.Liang BA, Mackey T. Reforming off-label promotion to enhance orphan disease treatment. Science. 2010;327:273–274. doi: 10.1126/science.1181567. [DOI] [PubMed] [Google Scholar]
- 45.Luisetti M, Balfour-Lynn IM, Johnson SR, Miravitlles M, Strange C, Trapnell BC, et al. Perspectives for improving the evaluation and access of therapies for rare lung diseases in Europe. Respir Med. 2012;106:759–768. doi: 10.1016/j.rmed.2012.02.016. [DOI] [PubMed] [Google Scholar]
- 46.Matthews J, Glass L. The effect of market-based economic factors on the adoption of orphan drugs across multiple countries. Ther Innov Regul Sci. 2013;47:226–234. doi: 10.1177/2168479012471945. [DOI] [PubMed] [Google Scholar]
- 47.Mavris M, Le Cam Y. Involvement of patient organisations in research and development of orphan drugs for rare diseases in Europe. Mol Syndromol. 2012;3:237–242. doi: 10.1159/000342758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McCabe C, Stafinski T, Menon D. Is it time to revisit orphan drug policies? BMJ. 2010;341:c4777. doi: 10.1136/bmj.c4777. [DOI] [PubMed] [Google Scholar]
- 49.Meekings KN, Williams CSM, Arrowsmith JE. Orphan drug development: an economically viable strategy for biopharma R&D. Drug Discov Today. 2012;17:660–664. doi: 10.1016/j.drudis.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 50.Mentzakis E, Stefanowska P, Hurley J. A discrete choice experiment investigating preferences for funding drugs used to treat orphan diseases: an exploratory study. Health Econ Policy Law. 2011;6:405–433. doi: 10.1017/S1744133110000344. [DOI] [PubMed] [Google Scholar]
- 51.Michel M, Toumi M. Access to orphan drugs in Europe: current and future issues. Expert Rev Pharmacoecon Outcomes Res. 2012;12:23–29. doi: 10.1586/erp.11.95. [DOI] [PubMed] [Google Scholar]
- 52.Moberly T. Rationing and access to orphan drugs. Pharm J. 2005;275:569–570. [Google Scholar]
- 53.Owen A, Spinks J, Meehan A, Robb T, Hardy M, Kwasha D, et al. A new model to evaluate the long-term cost effectiveness of orphan and highly specialised drugs following listing on the Australian Pharmaceutical Benefits Scheme: the Bosentan Patient Registry. J Med Econ. 2008;11:235–243. doi: 10.3111/13696990802034525. [DOI] [PubMed] [Google Scholar]
- 54.Picavet E, Dooms M, Cassiman D, Simoens S. Drugs for rare diseases: influence of orphan designation status on price. Appl Health Econ Health Policy. 2011;9:275–279. doi: 10.2165/11590170-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 55.Picavet E, Cassiman D, Simoens S. Evaluating and improving orphan drug regulations in Europe: a Delphi policy study. Health Policy. 2012;108:1–9. doi: 10.1016/j.healthpol.2012.08.023. [DOI] [PubMed] [Google Scholar]
- 56.Pinxten W, Denier Y, Dooms M, Cassiman J, Dierickx K. A fair share for the orphans: ethical guidelines for a fair distribution of resources within the bounds of the 10-year-old European Orphan Drug Regulation. J Med Ethics. 2012;38:148–153. doi: 10.1136/medethics-2011-100094. [DOI] [PubMed] [Google Scholar]
- 57.Prevot J, Watters D. HTA’s and access to rare diseases therapies: the view from the PID community. Pharm Policy Law. 2011;11:177–181. [Google Scholar]
- 58.Siddiqui M, Rajkumar SV. The high cost of cancer drugs and what we can do about it. Mayo Clin Proc. 2012;87:935–943. doi: 10.1016/j.mayocp.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stolk P, Willemen MJC, Leufkens HGM. Rare essentials: drugs for rare diseases as essential medicines. Bull World Health Organ. 2006;84:745–751. doi: 10.2471/BLT.06.031518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sullivan SD. The promise of specialty pharmaceuticals: are they worth the price? J Manag Care Pharm. 2008;14:S3–S6. doi: 10.18553/jmcp.2008.14.S4-A.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Valverde J-L. Editorial. Pharm Policy Law. 2011;13:115–116. [Google Scholar]
- 62.Wild C, Hintringer K, Nachtnebel A. Orphan drugs in oncology. Pharm Policy Law. 2011;13:223–232. [Google Scholar]
- 63.Winquist E, Bell CM, Clarke JTR, Evans G, Martin J, Sabharwal M, et al. An evaluation framework for funding drugs for rare diseases. Value Health. 2012;15:982–986. doi: 10.1016/j.jval.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 64.Culyer A, McCabe C, Briggs A, Claxton K, Buxton M, Akehurst R, et al. Searching for a threshold, not setting one: the role of the National Institute for Health and Clinical Excellence. J Health Serv Res Policy. 2007;12:56–58. doi: 10.1258/135581907779497567. [DOI] [PubMed] [Google Scholar]
- 65.Claxton K, Paulden M, Gravelle H, Brouwer W, Culyer AJ. Discounting and decision making in the economic evaluation of health-care technologies. Health Econ. 2011;20:2–15. doi: 10.1002/hec.1612. [DOI] [PubMed] [Google Scholar]
- 66.Claxton K, Martin S, Soares M, Rice N, Spackman E, Hinde S, et al. Methods for the estimation of the NICE cost effectiveness threshold. York: Centre for Health Economics, York University; 2013. Report No. 81.
- 67.Endrei D, Molics B, Ágoston I. Multicriteria decision analysis in the reimbursement of new medical technologies: real-world experiences from Hungary. Value Health. 2014;17(4):487–9. [DOI] [PubMed]
- 68.Mitton C, Dionne F, Damji R, Campbell D, Bryan S. Difficult decisions in times of constraint: criteria based resource allocation in the Vancouver Coastal Health Authority. BMC Health Serv Res. 2011;11:169. doi: 10.1186/1472-6963-11-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sussex J, Rollet P, Garau M, Schmitt C, Kent A, Hutchings A. A pilot study of multicriteria decision analysis for valuing orphan medicines. Value Health. 2013;16:1163–1169. doi: 10.1016/j.jval.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 70.Linley WG, Hughes DA. Societal views on nice, cancer drugs fund and value-based pricing criteria for prioritising medicines: a cross-sectional survey of 4118 adults in Great Britain. Health Econ. 2013;22:948–964. doi: 10.1002/hec.2872. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


