Abstract
Type 2 diabetes (T2D) has been known as 'bi-hormonal disorder' since decades ago, the role of glucagon from α-cell has languished whereas β-cell taking center stage. Recently, numerous findings indicate that the defects of glucagon secretion get involve with development and exacerbation of hyperglycemia in T2D. Aberrant α-cell responses exhibit both fasting and postprandial states: hyperglucagonemia contributes to fasting hyperglycemia caused by inappropriate hepatic glucose production, and to postprandial hyperglycemia owing to blunted α-cell suppression. During hypoglycemia, insufficient counter-regulation response is also observed in advanced T2D. Though many debates still remained for exact mechanisms behind the dysregulation of α-cell in T2D, it is clear that the blockade of glucagon receptor or suppression of glucagon secretion from α-cell would be novel therapeutic targets for control of hyperglycemia. Whereas there have not been remarkable advances in developing new class of drugs, currently available glucagon-like peptide-1 and dipeptidyl peptidase-IV inhibitors could be options for treatment of hyperglucagonemia. In this review, we focus on α-cell dysfunction and therapeutic potentials of targeting α-cell in T2D.
Keywords: Diabetes mellitus, type 2; Glucagon; Glucagon-secreting cells; Insulin; Insulin-secreting cells
INTRODUCTION
Pancreatic islets, small islands of endocrine cells in the gland, play critical role in blood glucose homeostasis through producing insulin from β-cells and glucagon from α-cells. Under normal physiology, α- and β-cells in the islet regulate each other reciprocally and thereby systemic glucose levels are maintained within narrow range. About 40 years ago, Unger and Orci [1] suggested "bi-hormonal theory," which presents that relatively or absolutely hypoinsulinemia and relative hyperglucagonemia raise hyperglycemia in type 2 diabetes (T2D). But the role of α-cell has been neglected for a long time whereas β-cell has been centered in the field of diabetic pathophysiology. Many treatment approaches have been focused on insulin deficiency such as insulin injection, stimulation of endogenous insulin production or by improving insulin sensitivity. Though these strategies have been effective in many type 2 diabetic patients, diabetes still remains impasse and need to more efforts for overcoming [2,3]. Recently, the role of α-cell was highlighted again that the excessive glucagon from dysfunctional α-cell was recognized an important therapeutic target in diabetes. This has partly due to the recognition of the glucagon-suppressive effect of incretin hormones glucagon-like peptide-1 (GLP-1) in T2D [4]. In this review, we will focus on the role of α-cell and hyperglucagonemia in the pathogenesis of T2D. Furthermore, the clinical relevance and implications for treatments directed at targeting glucagon secretion will be discussed.
α-CELL FUNCTION AND GLUCAGON SECRETION
Glucagon is a 29-amino acid, 3485-Da peptide hormone released from pancreatic islet α-cells, cleaved by prohormone convertase-2 from proglucagon molecule. Complex mechanism are involved in the regulation of pancreatic α-cell, and glucagon secretion is in response to a variety of nutrient, neural, and hormonal factors [5,6]. Glucagon is well known as the counter-regulatory hormone to insulin, and stimulates hepatic glycogenolysis, gluconeogenesis, fatty acid oxidation and ketogenesis. The main stimulus for islet glucagon release is low blood glucose levels, but amino acids such as L-arginine, activation of autonomic nervous system and gastric inhibitory polypeptide (GIP) also stimulate glucagon secretion [7,8,9,10]. In contrast, high glucose levels, insulin, somatostatin, amylin, and GLP-1 are suppressor of glucagon release [11,12,13,14].
DYSREGULATION OF α-CELL FUNCTION IN TYPE 2 DIABETES
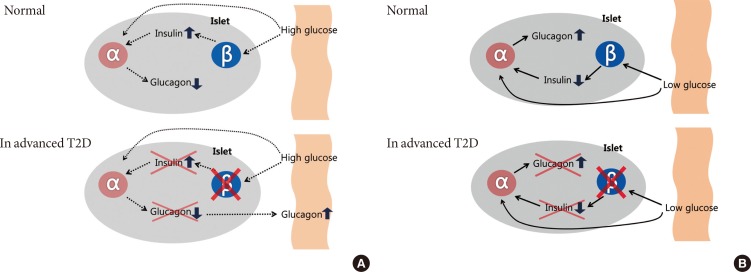
T2D has been considered to as a 'bihormonal disorder' [1,15], which is characterized by a progressive pancreatic islet dysfunction. Similar to β-cell function in the diabetes, there are a number of characteristic dysfunctions in α-cell. Extremely high plasma glucagon concentrations are observed in states of insulin deficiency, such as type 1 diabetes (T1D), advanced T2D or diabetic ketoacidosis [6,16]. In usual case of T2D, plasma glucagon level is often increased and it suggested contributing to the development of glucose toxicity and exacerbation of diabetes [17,18,19,20,21]. Fasting hyperglucagonemia is observed in some but not all T2D patients with moderate glycemic control [22,23,24]. 'Paradoxical' glucagon response to meal is also observed in patients with T2D, which are elevated glucagon levels following a carbohydrate meal and leads to postprandial hyperglycemia [25,26,27]. The response of α-cell to hyperglycemia in diabetes is blunted or vanishing, and plasma glucagon remains inappropriately excessive at comparable blood glucose levels. Moreover, glucagon production is elevated by another stimulus, such as arginine or protein-rich foods, to greater extent in T2D than nondiabetic subjects [23,28]. Even though the mechanisms underlying defects in glucagon secretion are not clear, but the various abnormalities of glucagon secretion have separate implications for glucose homeostasis and those are summarized at Fig. 1.
Fig. 1. Intra-islet insulin & glucagon secretion. Normal (in nondiabetes) and advanced type 2 diabetes (T2D) of the relationship between the inhibitory effects of pancreatic β-cell insulin secretion on pancreatic α-cell glucagon secretion. Normally, an increase in plasma glucose level causes an increase in β-cell insulin secretion that prevents an increase in α-cell glucagon secretion in response to meal. In advanced T2D, however, β-cell failure which is lack of intra-islet signaling result in not only fail to suppress but also an increase in pancreatic α-cell glucagon secretion (A). A decrease in plasma glucose level causes a decrease in β-cell insulin secretion that signals an increase in α-cell glucagon secretion during hypoglycemia. On the other hand, in the advanced T2D, a decrease in plasma glucose cannot cause a decrease in β-cell insulin secretion, and the absence of that signal results in no increase in pancreatic α-cell glucagon secretion during hypoglycemia (B).
Defects of glucagon suppression in T2D exacerbate fasting hyperglycemia
Fasting blood glucose levels is closely linked with abnormal elevation of hepatic glucose productions in T2D, and the excess glucagon output is contribute to the this pathogenesis [22]. Glucagon accounts for 40% to 50% of basal hepatic glucose production [22,29,30,31,32]. Hepatic insulin resistance or severe insulin deficiency mainly causes abnormally high levels of endogenous glucose production, inappropriate glucagon level also largely exacerbate fasting hyperglycemia in diabetes. One potential contributor is a relative increase in ratio of α-cells to β-cells in pancreatic islets owing to a decrease in β-cell mass [33,34], but the α-cell mass is similar to that of nondiabetic individuals [35]. Some, but not all, type 2 diabetic patients have increased fasting glucagon levels that can be 50% greater than nondiabetic subjects [22,24]. Interestingly, some type 2 diabetic patients show apparent α-cell dysfunction without changes of their islet cell composition and failure of adjacent β-cells whose defective insulin secretion [36]. Matsuda et al. [37] has been proposed the possibility that diabetic patients have enhanced sensitivity of hepatic glucose production to glucagon, even though it has not been always consistent [38]. It is important to note that it is in the context of hyperglycemia and hyperinsulinemia (which normally inhibit glucagon production) that the glucagon levels are 'relatively' high to the ambient glucose level, as the glucagon levels might not be increased in absolute terms in the early phase of T2D [39,40]. So the concept of glucagon:insulin ratio has been drawn, a term which depicts the overall islet dysfunction in T2D [6,41].
Inverted glucagon secretion in T2D contribute postprandial high glucose
It is well established that glucagon plays a role in postprandial glucose homeostasis. Plasma glucagon levels after oral glucose or carbohydrate meals are not appropriately suppressed in patients with T2D. After meal ingestions, hepatic glucose production is still remained near fasting levels in diabetic subjects, whereas diminishing abruptly in normal individuals [21], and the failure of glucagon suppression contributes to the postprandial hyperglycemia. According to studies, prandial hyperglucagonemia might be responsible for as much as 50% of the pathological increase in plasma glucose excursions following oral glucose intake in T2D [18,24,42]. There is remarkable evidence that insulin reciprocally regulates α-cell glucagon secretion [43], which raise the possibility that β-cell dysfunction could contribute to α-cell dysfunction. Lack of glucagon suppression by high plasma glucose can exaggerate hyperglycemia under situations of insulin deficiency [44]. It could provide simple explanation for the aberrant glucagon secretion in diabetes and the reason why this is corrected by exogenous insulin administration. The significance of paracrine mechanisms has been highlighted that glucagon secretion is under paracrine control by insulin (the "switch-off" hypothesis) for the architectural proximity between α- and β-cell (Fig. 1). Blockade of insulin signaling with the phosphatidylinositol-3 inhibitor prevents the suppressive effect of high glucose levels to glucagon release from isolated islets [11]. α-Cell specific insulin receptor knockout (alphaIRKO) mice exhibit mild fasting hyperglycemia and markedly high levels of glucose and glucagon secretion upon feeding [45], providing the pivotal role of insulin signaling in regulation of α-cell. Besides paracrine regulation mediated by insulin as well as other factors (such like γ-aminobutyric acid, Zn2+, somatostatin or γ-hydroxybutyric acid [5,46,47,48]), it appears that glucagon secretion is also under intrinsic control (exerted within the α-cell itself) [49]. α-Cells obviously possess the ability to respond to glucose at concentrations too low to elicit insulin or somatostatin secretion, and it involves KATP-channels similar to those found in β-cells. Recent study showed that an inverted glucose regulation of glucagon release observed in T2D might be a result of a minute increase in α-cell KATP-channel activity [50]. In this context, it has been reported that common polymorphism in the KIR 6.2 gene is related to inverted glucagon response, and this gene variant (E23K) is related with an increased risk for diabetes [51]. Some investigators suggested the possibility of that α-cell dysfunction only develops with aging because dysregulated glucagon release is not significant in adolescents with T2D [52,53].
Defective counter regulation in advanced T2D
In contrast with T1D, hypoglycemia is uncommon event-except iatrogenic-in T2D because the physiological and behavioral defenses are valid until late phase. However there is evidence that the counter-regulatory effect of glucagon to hypoglycemia is impaired in subjects with T2D [6,21]. Exact mechanism is not clear, also 'paracrine' and 'intrinsic' control are considered to be related with this condition. Given the findings that β-cell failure precede defects of α-cell response to lowering glucose levels in advanced T2D. It was reported that the degree of α-cell dysfunction is related with the lack of β-cell function in diabetes [54]. Insulin represses glucagon secretion as a pulsatile manner in nondiabetic subject, but this coordination is disrupted in patients with advanced T2D and it could potentially contribute to glucagon dysregulation [55]. In summary, the defect of an increment in glucagon secretion, like the loss of the decrement in insulin secretion, during hypoglycemia is the result of β-cell failure in advanced T2D [56].
Effects of bariatric surgery on glucagon secretion
Mounting evidence demonstrates bariatric surgery dramatically induces remission of T2D, weight reduction, favorable metabolic effects, and can prevent of delay incident T2D. Roux-en-Y gastric bypass (RYGB) is the most widely used bariatric procedure, and has a significant impact on glucagon secretion. Most of studies, unexpectedly, showed the postprandial hyperglucagonemia after surgery in patients with T2D as well as nondiabetic subjects [57]. At 1 month after gastric bypass, glucagon levels did not change in the fasting state, but rather increased more in response to oral glucose. It appears independent of incretin hormones, and could be associated with acute neuronal changes. Postprandial hyperglucagonemia was still not suppressed at 6 and 12 months even though fasting glucagon decreased with weight loss [58]. Camastra et al. [59] reported long-term (1 year) effects of RYGB, postprandial peaks of glucose and insulin rapidly increase followed by a sharp drop such like "dumping" pattern in both nondiabetic and diabetic subjects. Whereas fasting glucagon levels dropped in both groups after 1 year, postprandial glucagon significantly increased after bypass surgery, which was related with higher rates of endogenous glucose production during the meal challenge [59]. Glucagon and GLP-1 responses to the meal were heightened at early (15 days) after RYGB, but attenuated at 1 year in another study [60]. Sleeve gastrectomy (SLG), in which the pyloric sphincter is intact, induces favorable effects in glucose homeostasis similar to that with RYGB. Notwithstanding foregut exclusion does not play a role in SLG, there are no differences in the change of glucagon levels between RYGB and SLG [60]. It is not determined the exact mechanism about this 'paradox'; one plausible mechanism is activation of neural pathway by increased glucose levels in the portal vein [57]. Elevated portal, compared with systemic, glycemia enhances the early insulin response and hepatic glucose uptake rather than extrahepatic sites uptake. Further potential mechanisms are overstimulation of glucagon by GIP or GLP-2, and cosecretion of glucagon and GLP-1/GIP by intestinal cells.
ROLE OF GLUCAGON AS A THERAPEUTIC TARGET FOR T2D
Even though many advances in fields of diabetes and new hypoglycemic agents have been developed until recently, most patients with T2D still do not meet the goals. According to above mentioned studies of glucagon metabolism in diabetes, targeting glucagon would be a reasonable therapeutic strategy in T2D. In fact, we already have been used some effective antidiabetic drugs that inhibit glucagon secretion. Recent finding showed that the metformin, recommended as the first choice in current guidelines, suppress hepatic glucagon signaling by decreasing production of cyclic AMP [61]. Both exogenous insulin and sulphonylureas partially inhibit glucagon secretion [62,63], but it is not clear how much this contributes to their treatment effect. There are current available agents for suppression of glucagon according to primary physiological actions of drug: GLP-1 receptor agonists, DPP-IV inhibitors and amylin mimetics (pramlintide) [64]. However, we still need the development of new agents to reduce or block glucagon and this strategy will require a careful balance between beneficial and adverse events.
Glucagon antagonist
There are many experienced studies about the glucagon receptor can be targeted for treatment of T2D [5,65]. With regard to glucose homoeostasis can be disrupted by defective in α-cell function as well as β-cell [28], many researchers have attempted to develop potent and selective glucagon receptor antagonists for over decades [66]. However, there are some concerns about the safety of that approach although antagonism of the glucagon action might be an attractive option [67]. The glucagon response to low plasma glucose levels typically lost in patients with absolute insulin deficiency as discussed above. Similar to this, the blockade of glucagon action or secretion might increase the risk of iatrogenic hypoglycemia in the state of endogenous insulin deficiency such as advanced T2D. Besides there are some limitations of development of theses drug, including a lack of specificity or efficacy, toxicity, potential for induction of immune responses and side effects such as pancreatic α-cell hyperplasia [68]. With the recent significant improvements in drug delivery systems, and the overall patient acceptance of subcutaneous administration of other antidiabetic drugs, such as GLP-1 analogues [69], there is renewed hope for the therapeutic use of peptide-based glucagon receptor antagonists [70,71]. Recent pilot study using two peptide-based glucagon receptor antagonists (desHis1Pro4Glu9-glucagon or desHis1Pro4Glu9Lys12FA-glucagon) can reverse aspects of genetically and dietary-induced obesity-related diabetes in mice [72].
Glucagon-like peptide-1
There is general consensus that therapy using incretin hormone would be effective in part by lowering plasma glucagon levels. GLP-1 is a product of proglucagon that has nearly 50% homology to glucagon [73]. It is mainly synthesized in the intestinal L cells whereas minimally in the α-cell. GLP-1 binds a specific receptor that is distinct from, but related to, the GlcaR (glucagon receptor), and there is minimal cross-reactivity among these two ligand-receptor pairs. Contrary to the action of glucagon, GLP-1 regulates hyperglycemia through a variety of mechanisms: enhancing glucose-dependent insulin secretion from β-cell and reducing the plasma glucagon. GLP-1 administration ameliorate plasma glucagon levels, some questions are still remained as to the mechanism by which this occurs as most studies have not localized the GLP-1 receptor to α-cells [74]. Thus, alternative explanations, such as indirect control through somatostatin or through neural regulation, have been postulated. GLP-1 receptor antagonist administration leads to induce both fasting hyperglycemia and hyperglucagonemia [75,76]. According to these studies, control of α-cell secretion is a physiologic role of GLP-1. Moreover, some study using somatostatin to shut off islet hormone release and exogenous infusions to match the levels seen after GLP-1 administration suggests that the effects of glucagon suppression and insulin stimulation are similar to maintain fasting glucose in patients with T2D [77]. Remarkably, GLP-1 treatment on T1D patients was effective to control hyperglycemia (3 to 4 mM reduction) coincident with a 40% to 50% decrease in glucagon levels [78]. This finding indicate that glucagon suppression is important part of GLP-1 effect on glycemic control. The first clinical trial using a 1-week liraglutide was favorable glucose profile, α- and β-cell function in type 2 diabetic patients, and showed significant reduction of plasma glucagon levels [79]. In summary, GLP-1 based treatment in T2D is optimal choice in the context of islet dysfunction [80].
Dipeptidyl peptidase-IV inhibitor
Dipeptidyl peptidase-IV (DPP-IV) inhibitor is orally available drug that prolong the activity of GLP-1, as well as other peptides as substrates metabolized by this enzyme. So beneficial effects of DPP-IV inhibitors on glycemic control in T2D have been attributed to GLP-1 and these drugs stimulate insulin secretion and reduce plasma glucagon similar to exogenous GLP-1 receptor agonists. Because changes in plasma glucagon have frequently been more pronounced than changes in insulin during chronic use of DPP-IV inhibitors [67], there has been some tendency to attribute the predominant pharmacologic effect of these drugs to inhibition of α-cells. Recent evidence supports an effect of some DPP-IV inhibitor to improve insulin secretion as well as reduce plasma glucagon [68], which does not support the concept that DPP-IV inhibitors are primarily targeting glucagon in treatment of T2D. Vildagliptin improves glucagon dynamics in patients with T2D: reduced glucagon levels during meal, preserves glucagon counter regulation during hypoglycemia after 4-week treatment in insulin-treated subjects [81].
Taken together, while there has not been significant progress in developing drugs specifically targeting glucagon pathway for use in T2D, there are some evidences that lowering plasma glucagon has beneficial effects on glucose control in patients with T2D. Understanding the mechanisms of the glucagon-suppressing effects of GLP-1 receptor agonists and DPP-IV inhibitors on glucose lowering would provide the potential for targeting glucagon in therapeutics of T2D.
CONCLUSIONS
There are many evidences support inadequate glucagon secretion in the regulation of both fasting and postprandial glucose homeostasis. Owing to α-cell dysfunction, the defects in glucagon secretion is appear that over-secretion when it is not needed and poor production when it is needed. Plasma glucagon concentrations would be abnormally high (even within normal ranges) in patients with T2D, and not normally responsive to usual regulation. Studies in animals and humans implicate inappropriate glucagon signaling in hyperglycemia, and we suggest that normalization of glucagon secretion, or even pharmacologic suppression, could have potent effects on glycemic controls in patients with T2D. Currently available therapies that inhibit glucagon secretion, GLP-1 receptor agonists and DPP-IV inhibitors could be chosen for this reason. However, targeting the specific blockade of glucagon in humans is limited available information. Thus, we suggest that more understanding from basic and clinical research is needed to advance this potentially clinical uses.
ACKNOWLEDGMENTS
This work was supported by the 2014 Yeungnam University Research Grant.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Unger RH, Orci L. The essential role of glucagon in the pathogenesis of diabetes mellitus. Lancet. 1975;1:14–16. doi: 10.1016/s0140-6736(75)92375-2. [DOI] [PubMed] [Google Scholar]
- 2.Moon JS, Ha KS, Yoon JS, Lee HW, Lee HC, Won KC BETA study group. The effect of glargine versus glimepiride on pancreatic beta-cell function in patients with type 2 diabetes uncontrolled on metformin monotherapy: open-label, randomized, controlled study. Acta Diabetol. 2014;51:277–285. doi: 10.1007/s00592-013-0553-z. [DOI] [PubMed] [Google Scholar]
- 3.Defronzo RA. Banting Lecture. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes. 2009;58:773–795. doi: 10.2337/db09-9028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hare KJ, Knop FK, Asmar M, Madsbad S, Deacon CF, Holst JJ, Vilsboll T. Preserved inhibitory potency of GLP-1 on glucagon secretion in type 2 diabetes mellitus. J Clin Endocrinol Metab. 2009;94:4679–4687. doi: 10.1210/jc.2009-0921. [DOI] [PubMed] [Google Scholar]
- 5.Gromada J, Franklin I, Wollheim CB. Alpha-cells of the endocrine pancreas: 35 years of research but the enigma remains. Endocr Rev. 2007;28:84–116. doi: 10.1210/er.2006-0007. [DOI] [PubMed] [Google Scholar]
- 6.Dunning BE, Gerich JE. The role of alpha-cell dysregulation in fasting and postprandial hyperglycemia in type 2 diabetes and therapeutic implications. Endocr Rev. 2007;28:253–283. doi: 10.1210/er.2006-0026. [DOI] [PubMed] [Google Scholar]
- 7.Mitrakou A, Ryan C, Veneman T, Mokan M, Jenssen T, Kiss I, Durrant J, Cryer P, Gerich J. Hierarchy of glycemic thresholds for counterregulatory hormone secretion, symptoms, and cerebral dysfunction. Am J Physiol. 1991;260(1 Pt 1):E67–E74. doi: 10.1152/ajpendo.1991.260.1.E67. [DOI] [PubMed] [Google Scholar]
- 8.Meier JJ, Gallwitz B, Siepmann N, Holst JJ, Deacon CF, Schmidt WE, Nauck MA. Gastric inhibitory polypeptide (GIP) dose-dependently stimulates glucagon secretion in healthy human subjects at euglycaemia. Diabetologia. 2003;46:798–801. doi: 10.1007/s00125-003-1103-y. [DOI] [PubMed] [Google Scholar]
- 9.Rocha DM, Faloona GR, Unger RH. Glucagon-stimulating activity of 20 amino acids in dogs. J Clin Invest. 1972;51:2346–2351. doi: 10.1172/JCI107046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ahren B. Autonomic regulation of islet hormone secretion: implications for health and disease. Diabetologia. 2000;43:393–410. doi: 10.1007/s001250051322. [DOI] [PubMed] [Google Scholar]
- 11.Xu E, Kumar M, Zhang Y, Ju W, Obata T, Zhang N, Liu S, Wendt A, Deng S, Ebina Y, Wheeler MB, Braun M, Wang Q. Intra-islet insulin suppresses glucagon release via GABA-GABAA receptor system. Cell Metab. 2006;3:47–58. doi: 10.1016/j.cmet.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 12.Gedulin BR, Rink TJ, Young AA. Dose-response for glucagonostatic effect of amylin in rats. Metabolism. 1997;46:67–70. doi: 10.1016/s0026-0495(97)90170-0. [DOI] [PubMed] [Google Scholar]
- 13.de Heer J, Rasmussen C, Coy DH, Holst JJ. Glucagon-like peptide-1, but not glucose-dependent insulinotropic peptide, inhibits glucagon secretion via somatostatin (receptor subtype 2) in the perfused rat pancreas. Diabetologia. 2008;51:2263–2270. doi: 10.1007/s00125-008-1149-y. [DOI] [PubMed] [Google Scholar]
- 14.Luft R, Efendic S, Hokfelt T. Somatostatin: both hormone and neurotransmitter? Diabetologia. 1978;14:1–13. doi: 10.1007/BF00429702. [DOI] [PubMed] [Google Scholar]
- 15.Unger RH. Glucagon and insulin: a bihormonal system. Compr Ther. 1976;2:20–26. [PubMed] [Google Scholar]
- 16.Muller WA, Faloona GR, Unger RH. Hyperglucagonemia in diabetic ketoacidosis. Its prevalence and significance. Am J Med. 1973;54:52–57. doi: 10.1016/0002-9343(73)90083-1. [DOI] [PubMed] [Google Scholar]
- 17.Orci L, Baetens D, Rufener C, Amherdt M, Ravazzola M, Studer P, Malaisse-Lagae F, Unger RH. Hypertrophy and hyperplasia of somatostatin-containing D-cells in diabetes. Proc Natl Acad Sci U S A. 1976;73:1338–1342. doi: 10.1073/pnas.73.4.1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dinneen S, Alzaid A, Turk D, Rizza R. Failure of glucagon suppression contributes to postprandial hyperglycaemia in IDDM. Diabetologia. 1995;38:337–343. doi: 10.1007/BF00400639. [DOI] [PubMed] [Google Scholar]
- 19.Larsson H, Ahren B. Islet dysfunction in insulin resistance involves impaired insulin secretion and increased glucagon secretion in postmenopausal women with impaired glucose tolerance. Diabetes Care. 2000;23:650–657. doi: 10.2337/diacare.23.5.650. [DOI] [PubMed] [Google Scholar]
- 20.Ahren B, Larsson H. Impaired glucose tolerance (IGT) is associated with reduced insulin-induced suppression of glucagon concentrations. Diabetologia. 2001;44:1998–2003. doi: 10.1007/s001250100003. [DOI] [PubMed] [Google Scholar]
- 21.Rizza RA. Pathogenesis of fasting and postprandial hyperglycemia in type 2 diabetes: implications for therapy. Diabetes. 2010;59:2697–2707. doi: 10.2337/db10-1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baron AD, Schaeffer L, Shragg P, Kolterman OG. Role of hyperglucagonemia in maintenance of increased rates of hepatic glucose output in type II diabetics. Diabetes. 1987;36:274–283. doi: 10.2337/diab.36.3.274. [DOI] [PubMed] [Google Scholar]
- 23.Unger RH, Aguilar-Parada E, Muller WA, Eisentraut AM. Studies of pancreatic alpha cell function in normal and diabetic subjects. J Clin Invest. 1970;49:837–848. doi: 10.1172/JCI106297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reaven GM, Chen YD, Golay A, Swislocki AL, Jaspan JB. Documentation of hyperglucagonemia throughout the day in nonobese and obese patients with noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab. 1987;64:106–110. doi: 10.1210/jcem-64-1-106. [DOI] [PubMed] [Google Scholar]
- 25.Raskin P, Unger RH. Hyperglucagonemia and its suppression. Importance in the metabolic control of diabetes. N Engl J Med. 1978;299:433–436. doi: 10.1056/NEJM197808312990901. [DOI] [PubMed] [Google Scholar]
- 26.Sherwin RS, Fisher M, Hendler R, Felig P. Hyperglucagonemia and blood glucose regulation in normal, obese and diabetic subjects. N Engl J Med. 1976;294:455–461. doi: 10.1056/NEJM197602262940901. [DOI] [PubMed] [Google Scholar]
- 27.Mitrakou A, Kelley D, Mokan M, Veneman T, Pangburn T, Reilly J, Gerich J. Role of reduced suppression of glucose production and diminished early insulin release in impaired glucose tolerance. N Engl J Med. 1992;326:22–29. doi: 10.1056/NEJM199201023260104. [DOI] [PubMed] [Google Scholar]
- 28.Unger RH. Glucagon physiology and pathophysiology in the light of new advances. Diabetologia. 1985;28:574–578. doi: 10.1007/BF00281991. [DOI] [PubMed] [Google Scholar]
- 29.Del Prato S, Castellino P, Simonson DC, DeFronzo RA. Hyperglucagonemia and insulin-mediated glucose metabolism. J Clin Invest. 1987;79:547–556. doi: 10.1172/JCI112846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liljenquist JE, Mueller GL, Cherrington AD, Keller U, Chiasson JL, Perry JM, Lacy WW, Rabinowitz D. Evidence for an important role of glucagon in the regulation of hepatic glucose production in normal man. J Clin Invest. 1977;59:369–374. doi: 10.1172/JCI108649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brand CL, Jorgensen PN, Knigge U, Warberg J, Svendsen I, Kristensen JS, Holst JJ. Role of glucagon in maintenance of euglycemia in fed and fasted rats. Am J Physiol. 1995;269(3 Pt 1):E469–E477. doi: 10.1152/ajpendo.1995.269.3.E469. [DOI] [PubMed] [Google Scholar]
- 32.Gelling RW, Du XQ, Dichmann DS, Romer J, Huang H, Cui L, Obici S, Tang B, Holst JJ, Fledelius C, Johansen PB, Rossetti L, Jelicks LA, Serup P, Nishimura E, Charron MJ. Lower blood glucose, hyperglucagonemia, and pancreatic alpha cell hyperplasia in glucagon receptor knockout mice. Proc Natl Acad Sci U S A. 2003;100:1438–1443. doi: 10.1073/pnas.0237106100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yoon KH, Ko SH, Cho JH, Lee JM, Ahn YB, Song KH, Yoo SJ, Kang MI, Cha BY, Lee KW, Son HY, Kang SK, Kim HS, Lee IK, Bonner-Weir S. Selective beta-cell loss and alpha-cell expansion in patients with type 2 diabetes mellitus in Korea. J Clin Endocrinol Metab. 2003;88:2300–2308. doi: 10.1210/jc.2002-020735. [DOI] [PubMed] [Google Scholar]
- 34.Rahier J, Goebbels RM, Henquin JC. Cellular composition of the human diabetic pancreas. Diabetologia. 1983;24:366–371. doi: 10.1007/BF00251826. [DOI] [PubMed] [Google Scholar]
- 35.Henquin JC, Rahier J. Pancreatic alpha cell mass in European subjects with type 2 diabetes. Diabetologia. 2011;54:1720–1725. doi: 10.1007/s00125-011-2118-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Unger RH, Orci L. Paracrinology of islets and the paracrinopathy of diabetes. Proc Natl Acad Sci U S A. 2010;107:16009–16012. doi: 10.1073/pnas.1006639107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Matsuda M, Defronzo RA, Glass L, Consoli A, Giordano M, Bressler P, Delprato S. Glucagon dose-response curve for hepatic glucose production and glucose disposal in type 2 diabetic patients and normal individuals. Metabolism. 2002;51:1111–1119. doi: 10.1053/meta.2002.34700. [DOI] [PubMed] [Google Scholar]
- 38.Nielsen MF, Wise S, Dinneen SF, Schwenk WF, Basu A, Rizza RA. Assessment of hepatic sensitivity to glucagon in NIDDM: use as a tool to estimate the contribution of the indirect pathway to nocturnal glycogen synthesis. Diabetes. 1997;46:2007–2016. doi: 10.2337/diab.46.12.2007. [DOI] [PubMed] [Google Scholar]
- 39.Dunning BE, Foley JE, Ahren B. Alpha cell function in health and disease: influence of glucagon-like peptide-1. Diabetologia. 2005;48:1700–1713. doi: 10.1007/s00125-005-1878-0. [DOI] [PubMed] [Google Scholar]
- 40.Lund A, Bagger JI, Christensen M, Knop FK, Vilsboll T. Glucagon and type 2 diabetes: the return of the alpha cell. Curr Diab Rep. 2014;14:555. doi: 10.1007/s11892-014-0555-4. [DOI] [PubMed] [Google Scholar]
- 41.Unger RH. Role of glucagon in the pathogenesis of diabetes: the status of the controversy. Metabolism. 1978;27:1691–1709. doi: 10.1016/0026-0495(78)90291-3. [DOI] [PubMed] [Google Scholar]
- 42.Shah P, Vella A, Basu A, Basu R, Schwenk WF, Rizza RA. Lack of suppression of glucagon contributes to postprandial hyperglycemia in subjects with type 2 diabetes mellitus. J Clin Endocrinol Metab. 2000;85:4053–4059. doi: 10.1210/jcem.85.11.6993. [DOI] [PubMed] [Google Scholar]
- 43.Bansal P, Wang Q. Insulin as a physiological modulator of glucagon secretion. Am J Physiol Endocrinol Metab. 2008;295:E751–E761. doi: 10.1152/ajpendo.90295.2008. [DOI] [PubMed] [Google Scholar]
- 44.Shah P, Basu A, Basu R, Rizza R. Impact of lack of suppression of glucagon on glucose tolerance in humans. Am J Physiol. 1999;277(2 Pt 1):E283–E290. doi: 10.1152/ajpendo.1999.277.2.E283. [DOI] [PubMed] [Google Scholar]
- 45.Kawamori D, Kurpad AJ, Hu J, Liew CW, Shih JL, Ford EL, Herrera PL, Polonsky KS, McGuinness OP, Kulkarni RN. Insulin signaling in alpha cells modulates glucagon secretion in vivo. Cell Metab. 2009;9:350–361. doi: 10.1016/j.cmet.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wendt A, Birnir B, Buschard K, Gromada J, Salehi A, Sewing S, Rorsman P, Braun M. Glucose inhibition of glucagon secretion from rat alpha-cells is mediated by GABA released from neighboring beta-cells. Diabetes. 2004;53:1038–1045. doi: 10.2337/diabetes.53.4.1038. [DOI] [PubMed] [Google Scholar]
- 47.Ishihara H, Maechler P, Gjinovci A, Herrera PL, Wollheim CB. Islet beta-cell secretion determines glucagon release from neighbouring alpha-cells. Nat Cell Biol. 2003;5:330–335. doi: 10.1038/ncb951. [DOI] [PubMed] [Google Scholar]
- 48.Li C, Liu C, Nissim I, Chen J, Chen P, Doliba N, Zhang T, Nissim I, Daikhin Y, Stokes D, Yudkoff M, Bennett MJ, Stanley CA, Matschinsky FM, Naji A. Regulation of glucagon secretion in normal and diabetic human islets by gamma-hydroxybutyrate and glycine. J Biol Chem. 2013;288:3938–3951. doi: 10.1074/jbc.M112.385682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rorsman P, Salehi SA, Abdulkader F, Braun M, MacDonald PE. K(ATP)-channels and glucose-regulated glucagon secretion. Trends Endocrinol Metab. 2008;19:277–284. doi: 10.1016/j.tem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 50.Zhang Q, Ramracheya R, Lahmann C, Tarasov A, Bengtsson M, Braha O, Braun M, Brereton M, Collins S, Galvanovskis J, Gonzalez A, Groschner LN, Rorsman NJ, Salehi A, Travers ME, Walker JN, Gloyn AL, Gribble F, Johnson PR, Reimann F, Ashcroft FM, Rorsman P. Role of KATP channels in glucose-regulated glucagon secretion and impaired counterregulation in type 2 diabetes. Cell Metab. 2013;18:871–882. doi: 10.1016/j.cmet.2013.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tschritter O, Stumvoll M, Machicao F, Holzwarth M, Weisser M, Maerker E, Teigeler A, Haring H, Fritsche A. The prevalent Glu23Lys polymorphism in the potassium inward rectifier 6.2 (KIR6.2) gene is associated with impaired glucagon suppression in response to hyperglycemia. Diabetes. 2002;51:2854–2860. doi: 10.2337/diabetes.51.9.2854. [DOI] [PubMed] [Google Scholar]
- 52.Elder DA, Prigeon RL, Wadwa RP, Dolan LM, D'Alessio DA. Beta-cell function, insulin sensitivity, and glucose tolerance in obese diabetic and nondiabetic adolescents and young adults. J Clin Endocrinol Metab. 2006;91:185–191. doi: 10.1210/jc.2005-0853. [DOI] [PubMed] [Google Scholar]
- 53.Tfayli H, Bacha F, Gungor N, Arslanian S. Islet cell antibody-positive versus-negative phenotypic type 2 diabetes in youth: does the oral glucose tolerance test distinguish between the two? Diabetes Care. 2010;33:632–638. doi: 10.2337/dc09-0305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fukuda M, Tanaka A, Tahara Y, Ikegami H, Yamamoto Y, Kumahara Y, Shima K. Correlation between minimal secretory capacity of pancreatic beta-cells and stability of diabetic control. Diabetes. 1988;37:81–88. doi: 10.2337/diab.37.1.81. [DOI] [PubMed] [Google Scholar]
- 55.Menge BA, Gruber L, Jorgensen SM, Deacon CF, Schmidt WE, Veldhuis JD, Holst JJ, Meier JJ. Loss of inverse relationship between pulsatile insulin and glucagon secretion in patients with type 2 diabetes. Diabetes. 2011;60:2160–2168. doi: 10.2337/db11-0251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Segel SA, Paramore DS, Cryer PE. Hypoglycemia-associated autonomic failure in advanced type 2 diabetes. Diabetes. 2002;51:724–733. doi: 10.2337/diabetes.51.3.724. [DOI] [PubMed] [Google Scholar]
- 57.Salehi M, D'Alessio DA. Going with the flow: adaptation of beta-cell function to glucose fluxes after bariatric surgery. Diabetes. 2013;62:3671–3673. doi: 10.2337/db13-1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bose M, Teixeira J, Olivan B, Bawa B, Arias S, Machineni S, Pi-Sunyer FX, Scherer PE, Laferrere B. Weight loss and incretin responsiveness improve glucose control independently after gastric bypass surgery. J Diabetes. 2010;2:47–55. doi: 10.1111/j.1753-0407.2009.00064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Camastra S, Muscelli E, Gastaldelli A, Holst JJ, Astiarraga B, Baldi S, Nannipieri M, Ciociaro D, Anselmino M, Mari A, Ferrannini E. Long-term effects of bariatric surgery on meal disposal and beta-cell function in diabetic and nondiabetic patients. Diabetes. 2013;62:3709–3717. doi: 10.2337/db13-0321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nannipieri M, Baldi S, Mari A, Colligiani D, Guarino D, Camastra S, Barsotti E, Berta R, Moriconi D, Bellini R, Anselmino M, Ferrannini E. Roux-en-Y gastric bypass and sleeve gastrectomy: mechanisms of diabetes remission and role of gut hormones. J Clin Endocrinol Metab. 2013;98:4391–4399. doi: 10.1210/jc.2013-2538. [DOI] [PubMed] [Google Scholar]
- 61.Miller RA, Chu Q, Xie J, Foretz M, Viollet B, Birnbaum MJ. Biguanides suppress hepatic glucagon signalling by decreasing production of cyclic AMP. Nature. 2013;494:256–260. doi: 10.1038/nature11808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Raskin P, Aydin I, Unger RH. Effect of insulin on the exaggerated glucagon response to arginine stimulation in diabetes mellitus. Diabetes. 1976;25:227–229. doi: 10.2337/diab.25.3.227. [DOI] [PubMed] [Google Scholar]
- 63.Pfeifer MA, Halter JB, Judzewitsch RG, Beard JC, Best JD, Ward WK, Porte D., Jr Acute and chronic effects of sulfonylurea drugs on pancreatic islet function in man. Diabetes Care. 1984;7(Suppl 1):25–34. [PubMed] [Google Scholar]
- 64.Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, Peters AL, Tsapas A, Wender R, Matthews DR. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2015;38:140–149. doi: 10.2337/dc14-2441. [DOI] [PubMed] [Google Scholar]
- 65.Jiang G, Zhang BB. Glucagon and regulation of glucose metabolism. Am J Physiol Endocrinol Metab. 2003;284:E671–E678. doi: 10.1152/ajpendo.00492.2002. [DOI] [PubMed] [Google Scholar]
- 66.Johnson DG, Goebel CU, Hruby VJ, Bregman MD, Trivedi D. Hyperglycemia of diabetic rats decreased by a glucagon receptor antagonist. Science. 1982;215:1115–1116. doi: 10.1126/science.6278587. [DOI] [PubMed] [Google Scholar]
- 67.Cryer PE. Minireview: glucagon in the pathogenesis of hypoglycemia and hyperglycemia in diabetes. Endocrinology. 2012;153:1039–1048. doi: 10.1210/en.2011-1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bagger JI, Knop FK, Holst JJ, Vilsboll T. Glucagon antagonism as a potential therapeutic target in type 2 diabetes. Diabetes Obes Metab. 2011;13:965–971. doi: 10.1111/j.1463-1326.2011.01427.x. [DOI] [PubMed] [Google Scholar]
- 69.Russell S. Incretin-based therapies for type 2 diabetes mellitus: a review of direct comparisons of efficacy, safety and patient satisfaction. Int J Clin Pharm. 2013;35:159–172. doi: 10.1007/s11096-012-9729-9. [DOI] [PubMed] [Google Scholar]
- 70.Irwin N, Franklin ZJ, O'Harte FP. desHis(1)Glu(9)-glucagon-[mPEG] and desHis(1)Glu(9)(Lys(3)(0)PAL)-glucagon: long-acting peptide-based PEGylated and acylated glucagon receptor antagonists with potential antidiabetic activity. Eur J Pharmacol. 2013;709:43–51. doi: 10.1016/j.ejphar.2013.03.041. [DOI] [PubMed] [Google Scholar]
- 71.O'Harte FP, Franklin ZJ, Rafferty EP, Irwin N. Characterisation of structurally modified analogues of glucagon as potential glucagon receptor antagonists. Mol Cell Endocrinol. 2013;381:26–34. doi: 10.1016/j.mce.2013.07.014. [DOI] [PubMed] [Google Scholar]
- 72.O'Harte FP, Franklin ZJ, Irwin N. Two novel glucagon receptor antagonists prove effective therapeutic agents in high-fat-fed and obese diabetic mice. Diabetes Obes Metab. 2014;16:1214–1222. doi: 10.1111/dom.12360. [DOI] [PubMed] [Google Scholar]
- 73.Drucker DJ. The biology of incretin hormones. Cell Metab. 2006;3:153–165. doi: 10.1016/j.cmet.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 74.Gromada J, Rorsman P. New insights into the regulation of glucagon secretion by glucagon-like peptide-1. Horm Metab Res. 2004;36:822–829. doi: 10.1055/s-2004-826169. [DOI] [PubMed] [Google Scholar]
- 75.D'Alessio DA, Vogel R, Prigeon R, Laschansky E, Koerker D, Eng J, Ensinck JW. Elimination of the action of glucagon-like peptide 1 causes an impairment of glucose tolerance after nutrient ingestion by healthy baboons. J Clin Invest. 1996;97:133–138. doi: 10.1172/JCI118380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schirra J, Sturm K, Leicht P, Arnold R, Goke B, Katschinski M. Exendin(9-39)amide is an antagonist of glucagon-like peptide-1(7-36)amide in humans. J Clin Invest. 1998;101:1421–1430. doi: 10.1172/JCI1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hare KJ, Vilsboll T, Asmar M, Deacon CF, Knop FK, Holst JJ. The glucagonostatic and insulinotropic effects of glucagon-like peptide 1 contribute equally to its glucose-lowering action. Diabetes. 2010;59:1765–1770. doi: 10.2337/db09-1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Creutzfeldt WO, Kleine N, Willms B, Orskov C, Holst JJ, Nauck MA. Glucagonostatic actions and reduction of fasting hyperglycemia by exogenous glucagon-like peptide I(7-36) amide in type I diabetic patients. Diabetes Care. 1996;19:580–586. doi: 10.2337/diacare.19.6.580. [DOI] [PubMed] [Google Scholar]
- 79.Degn KB, Juhl CB, Sturis J, Jakobsen G, Brock B, Chandramouli V, Rungby J, Landau BR, Schmitz O. One week's treatment with the long-acting glucagon-like peptide 1 derivative liraglutide (NN2211) markedly improves 24-h glycemia and alpha- and beta-cell function and reduces endogenous glucose release in patients with type 2 diabetes. Diabetes. 2004;53:1187–1194. doi: 10.2337/diabetes.53.5.1187. [DOI] [PubMed] [Google Scholar]
- 80.Meier JJ. GLP-1 receptor agonists for individualized treatment of type 2 diabetes mellitus. Nat Rev Endocrinol. 2012;8:728–742. doi: 10.1038/nrendo.2012.140. [DOI] [PubMed] [Google Scholar]
- 81.Farngren J, Persson M, Schweizer A, Foley JE, Ahren B. Glucagon dynamics during hypoglycaemia and food-re-challenge following treatment with vildagliptin in insulin-treated patients with type 2 diabetes. Diabetes Obes Metab. 2014;16:812–818. doi: 10.1111/dom.12284. [DOI] [PubMed] [Google Scholar]