Abstract
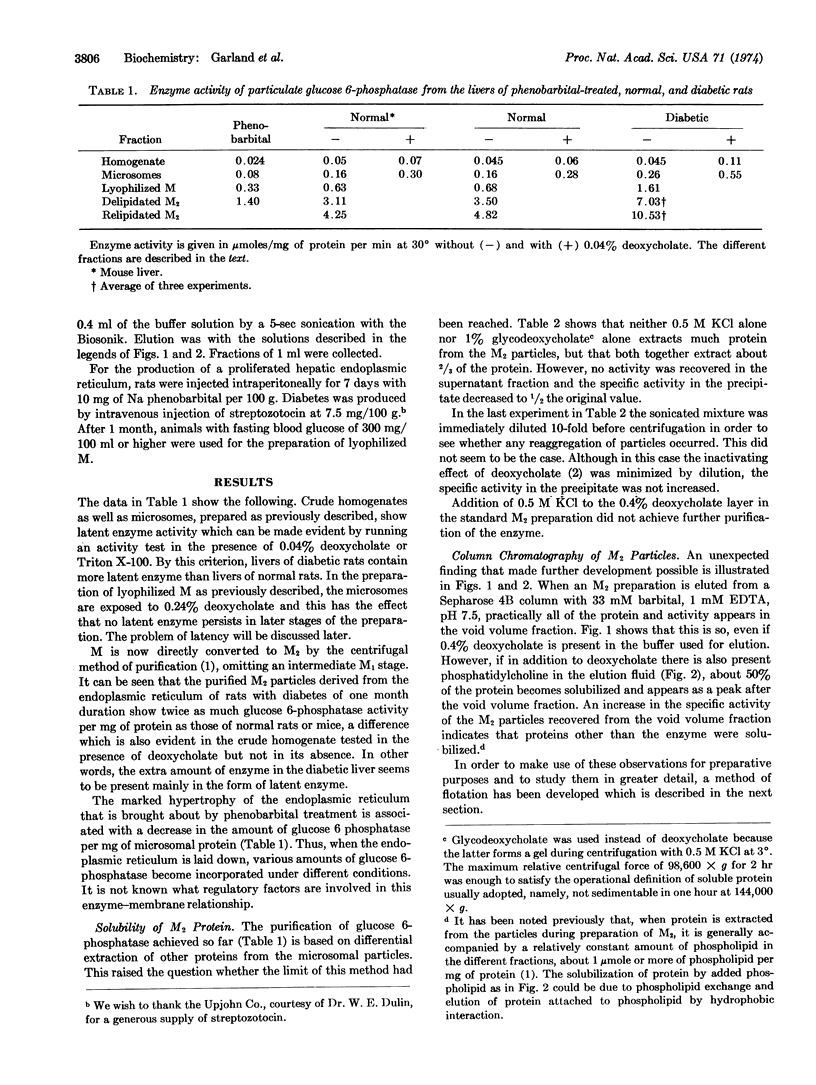
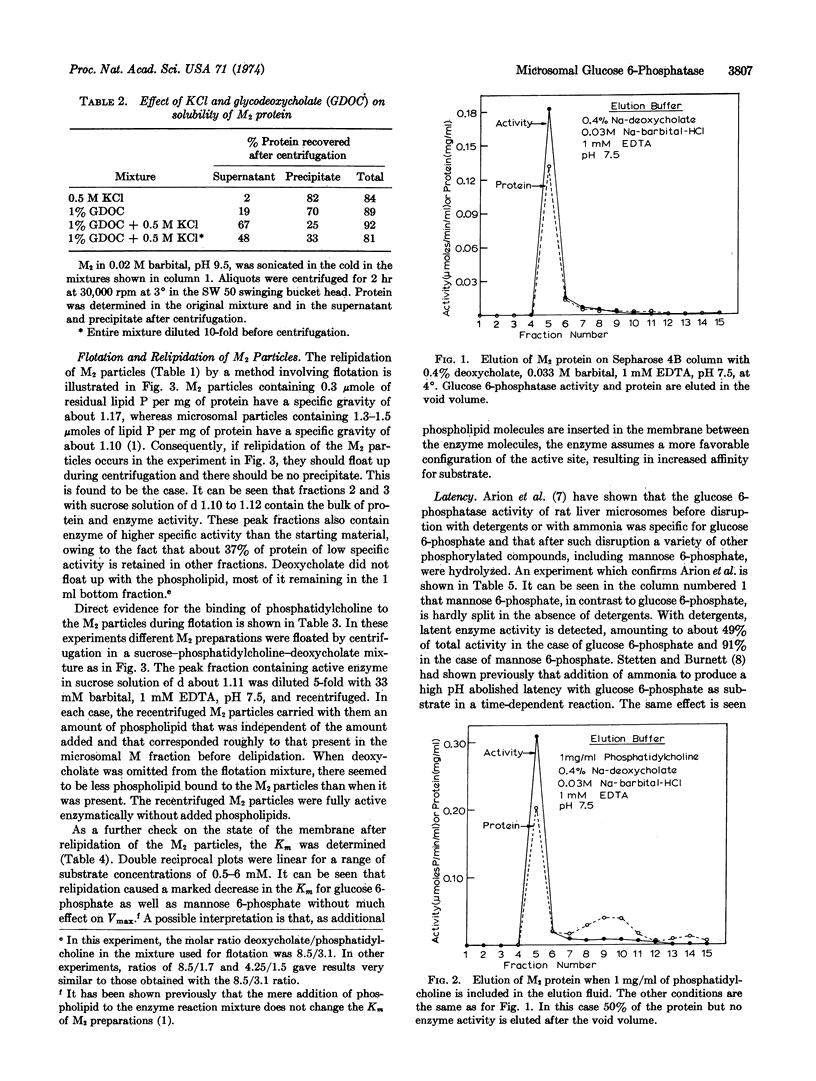
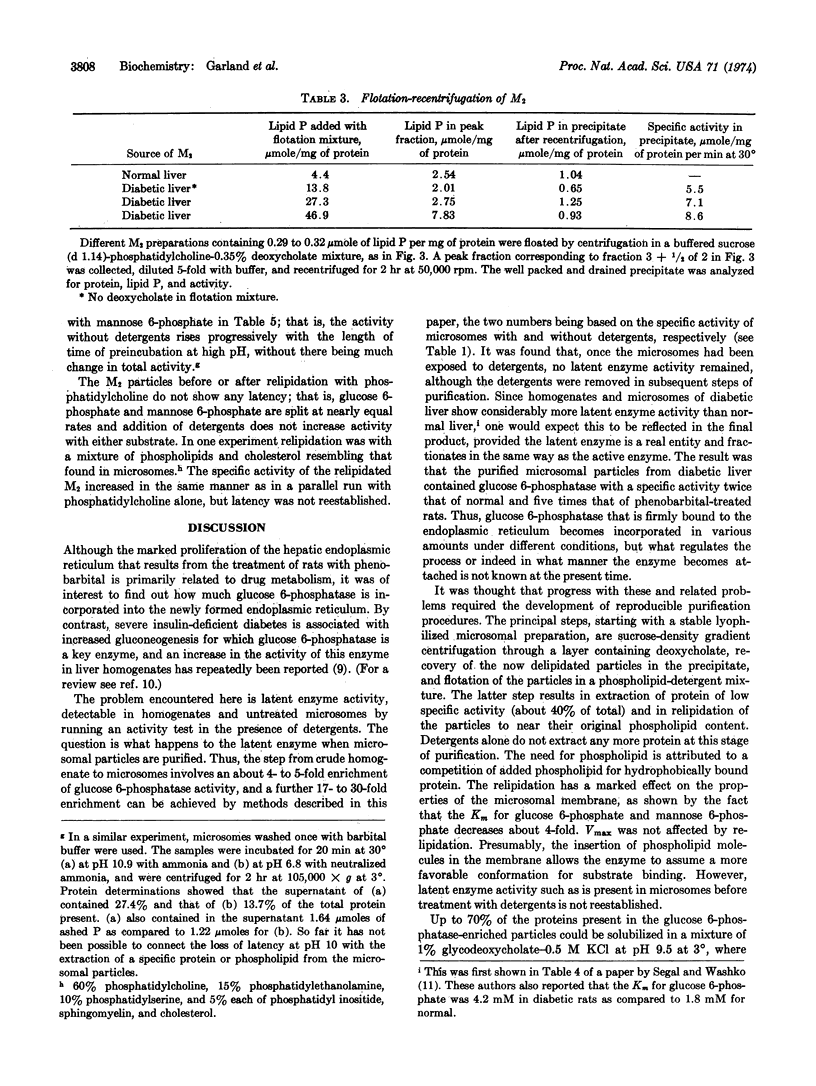
Microsomal particles enriched up to 20-fold in glucose 6-phosphatase activity (as compared to crude microsomal fractions) were prepared from livers of phenobarbital-treated, normal, and diabetic rats by a method involving sucrose-density gradient centrifugation through a layer containing deoxycholate, followed by flotation of the delipidated particles in a phospholipid-detergent mixture. The flotation with phospholipid resulted in the extraction of additional protein, in a corresponding increase in specific activity, and in relipidation of the particles to their original phospholipid content. Detergents alone did not extract any additional protein. Relipidation caused a change in the properties of the microsomal membrane, as indicated by a 4-fold decrease in the Km for glucose 6-phosphate and mannose 6-phosphate. The maximal rate was not affected. The crude homogenate of diabetic rat liver contained more latent enzyme than that of normal rats. The diabetic rats also yielded purified microsomal particles of specific glucose 6-phosphatase activity twice that of normal and five times that of phenobarbital-treated rats, indicating that some regulatory mechanism exists for the incorporation of different amounts of the enzyme into the endoplasmic reticulum.
Keywords: enzyme enrichment, membrane effects, latency, diabetes, phenobarbital
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arion W. J., Carlson P. W., Wallin B. K., Lange A. J. Modifications of hydrolytic and synthetic activities of liver microsomal glucose 6-phosphatase. J Biol Chem. 1972 Apr 25;247(8):2551–2557. [PubMed] [Google Scholar]
- Cori C. F., Garland R. C., Chang H. W. Purification of particulate glucose-6-phosphatase. Biochemistry. 1973 Jul 31;12(16):3126–3130. doi: 10.1021/bi00740a029. [DOI] [PubMed] [Google Scholar]
- Garland R. C., Cori C. F. Separation of phospholipids from glucose-6-phosphatase by gel chromatography. Specificity of phospholipid reactivation. Biochemistry. 1972 Dec 5;11(25):4712–4718. doi: 10.1021/bi00775a012. [DOI] [PubMed] [Google Scholar]
- Gluecksohn-Waelsch S., Schiffman M. B., Thorndike J., Cori C. F. Complementation studies of lethal alleles in the mouse causing deficiencies of glucose-6-phosphatase, tyrosine aminotransferase, and serine dehydratase. Proc Natl Acad Sci U S A. 1974 Mar;71(3):825–829. doi: 10.1073/pnas.71.3.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MOSBACH E. H., KALINSKY H. J., HALPERN E., KENDALL F. E. Determination of deoxycholic and cholic acids in bile. Arch Biochem Biophys. 1954 Aug;51(2):402–410. doi: 10.1016/0003-9861(54)90495-6. [DOI] [PubMed] [Google Scholar]
- SEGAL H. L., WASHKO M. E. Studies of liver glucose 6-phosphatase. III. Solubilization and properties of the enzyme from normal and diabetic rats. J Biol Chem. 1959 Aug;234(8):1937–1941. [PubMed] [Google Scholar]
- Stetten M. R., Burnett F. F. Activation of rat-liver microsomal glucose 6-phosphatase, inorganic pyrophosphatase and inorganic pyrophosphate-glucose phosphotransferase by hydroxyl ion. Biochim Biophys Acta. 1966 Nov 15;128(2):344–350. doi: 10.1016/0926-6593(66)90181-0. [DOI] [PubMed] [Google Scholar]


