Abstract
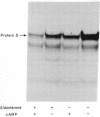
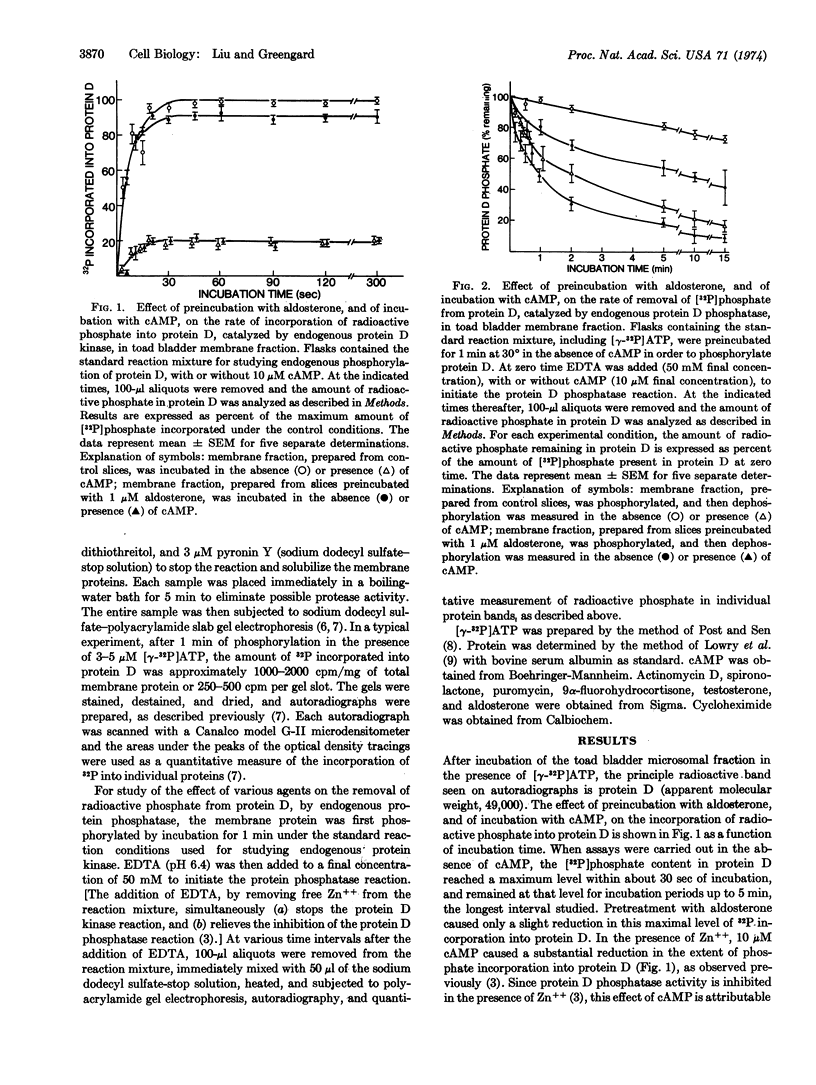
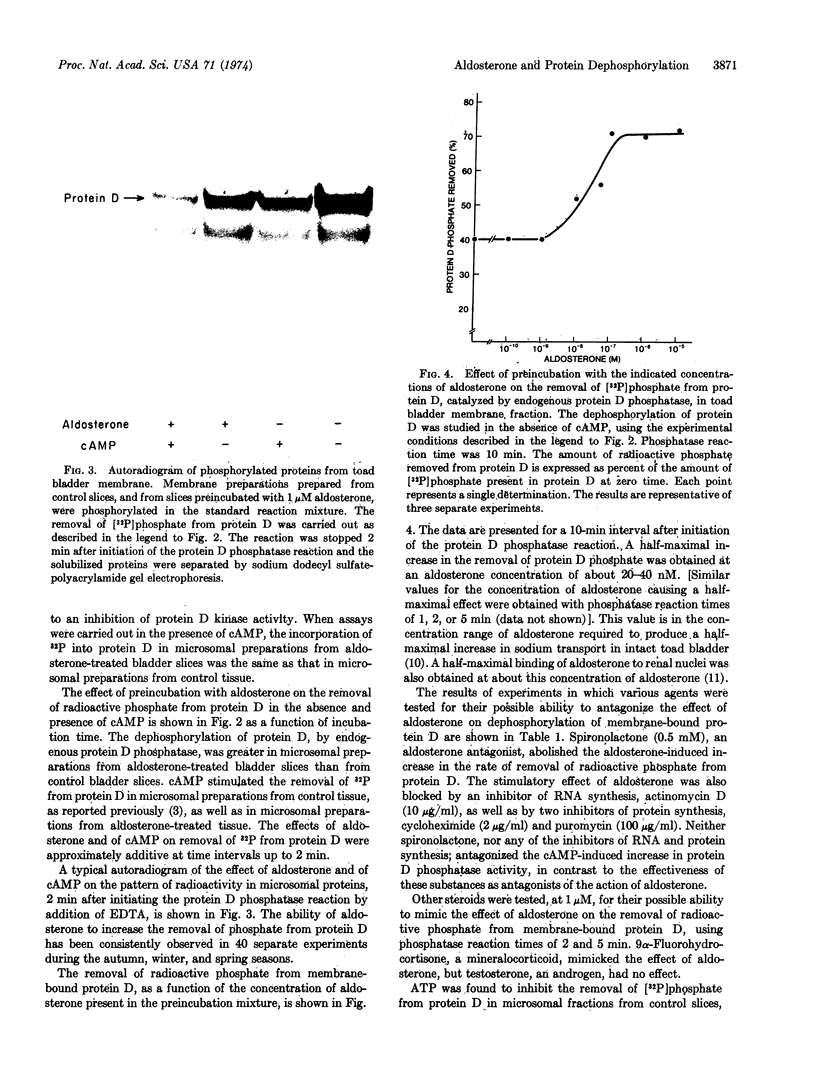
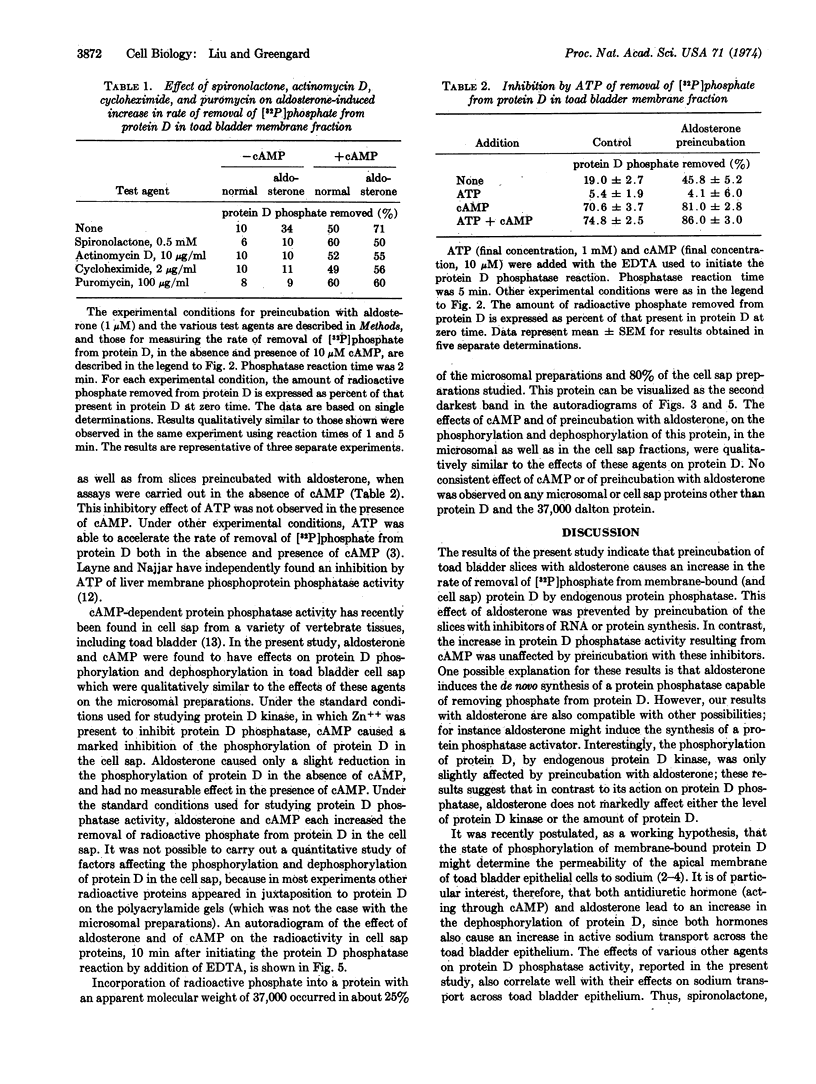
A study has been carried out of the effect of aldosterone on the endogenous phosphorylation and dephosphorylation of membrane-bound and of soluble proteins from toad bladder. Membrane-bound protein D (apparent molecular weight, 49,000), a protein which may possibly be involved in the regulation of sodium transport across the mucosal epithelium of toad bladder, contained a substantial fraction of the radioactive phosphate incorporated into membrane proteins; moreover, it was the only protein to appear consistently in autoradiographs of polyacrylamide gels of phosphorylated membrane proteins. Pretreatment of toad bladder slices with aldosterone caused an increase in the endogenous dephosphorylation of membrane-bound protein D. A half-maximal increase in this dephosphorylation occurred at an aldosterone concentration of 20-40 nM. The increase in protein D phosphatase activity induced by aldosterone was prevented by inhibitors of RNA and protein synthesis as well as by spironolactone, a specific antagonist of aldosterone. The mineralocorticoid, 9α-fluorohydrocortisone, also increased protein D phosphatase activity, but testosterone did not. Aldosterone also increased the removal of [32P]phosphate from protein D in the cell sap. In contrast to the increase in protein D phosphatase activity, aldosterone had little effect on the phosphorylation of protein D by endogenous protein D kinase. In some experiments, effects of aldosterone and of cAMP, qualitatively similar to those found with protein D, were also observed on the phosphorylation and dephosphorylation of a protein with an apparent molecular weight of 37,000, in both the microsomal and cell sap fractions. No consistent effect of preincubation with aldosterone or of cAMP was observed on any membrane-bound or cell sap protein other than protein D and the 37,000 dalton protein.
Keywords: steroid action, protein phosphorylation, cAMP
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DeLorenzo R. J., Walton K. G., Curran P. F., Greengard P. Regulation of phosphorylation of a specific protein in toad-bladder membrane by antidiuretic hormone and cyclic AMP, and its possible relationship to membrane permeability changes. Proc Natl Acad Sci U S A. 1973 Mar;70(3):880–884. doi: 10.1073/pnas.70.3.880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delorenzo R. J., Greengard P. Activation by adenosine 3':5'-monophosphate of a membrane-bound phosphoprotein phosphatase from toad bladder. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1831–1835. doi: 10.1073/pnas.70.6.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EDELMAN I. S., BOGOROCH R., PORTER G. A. ON THE MECHANISM OF ACTION OF ALDOSTERONE ON SODIUM TRANSPORT: THE ROLE OF PROTEIN SYNTHESIS. Proc Natl Acad Sci U S A. 1963 Dec;50:1169–1177. doi: 10.1073/pnas.50.6.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Fanestil D. D., Edelman I. S. Characteristics of the renal nuclear receptors for aldosterone. Proc Natl Acad Sci U S A. 1966 Sep;56(3):872–879. doi: 10.1073/pnas.56.3.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanestil D. D., Edelman I. S. On the mechanism of action of aldosterone on sodium transport: effects of inhibitors of RNA and of protein synthesis. Fed Proc. 1966 May-Jun;25(3):912–916. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Orloff J., Handler J. The role of adenosine 3',5'-phosphate in the action of antidiuretic hormone. Am J Med. 1967 May;42(5):757–768. doi: 10.1016/0002-9343(67)90093-9. [DOI] [PubMed] [Google Scholar]
- PORTER G. A., EDELMAN I. S. THE ACTION OF ALDOSTERONE AND RELATED CORTICOSTEROIDS ON SODIUM TRANSPORT ACROSS THE TOAD BLADDER. J Clin Invest. 1964 Apr;43:611–620. doi: 10.1172/JCI104946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHARP G. W., LEAF A. BIOLOGICAL ACTION OF ALDOSTERONE IN VITRO. Nature. 1964 Jun 20;202:1185–1188. doi: 10.1038/2021185a0. [DOI] [PubMed] [Google Scholar]
- Ueda T., Maeno H., Greengard P. Regulation of endogenous phosphorylation of specific proteins in synaptic membrane fractions from rat brain by adenosine 3':5'-monophosphate. J Biol Chem. 1973 Dec 10;248(23):8295–8305. [PubMed] [Google Scholar]