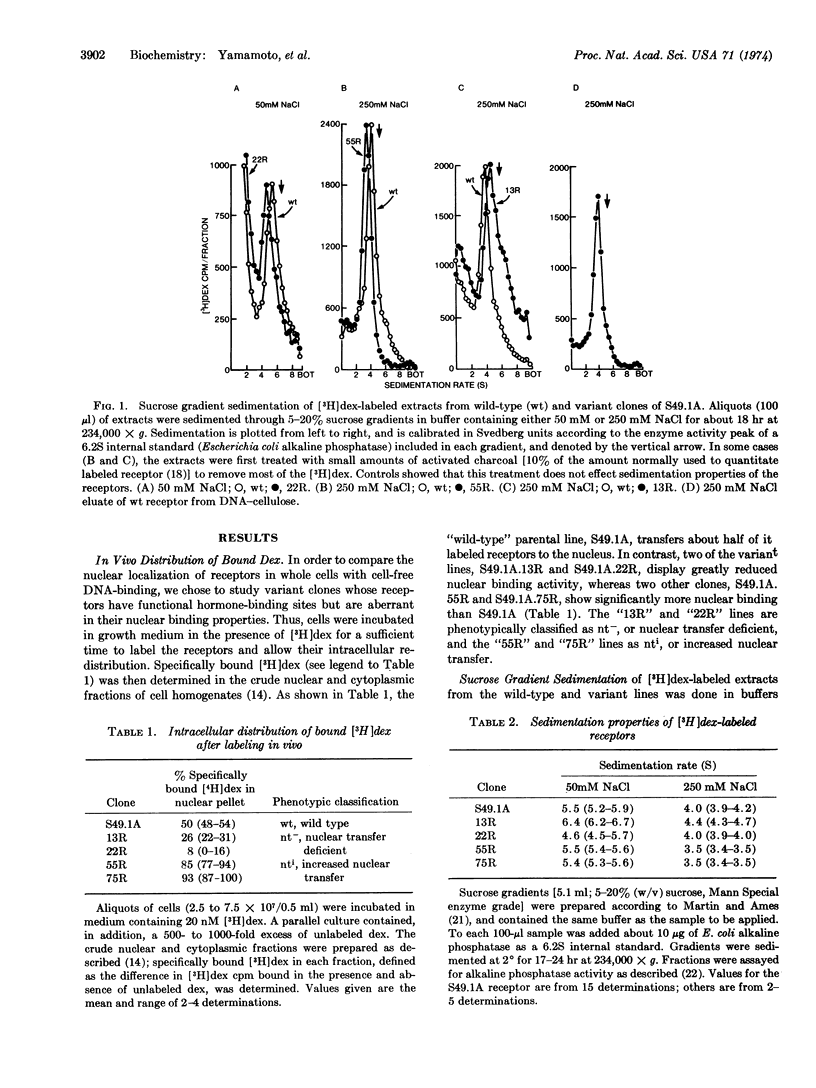
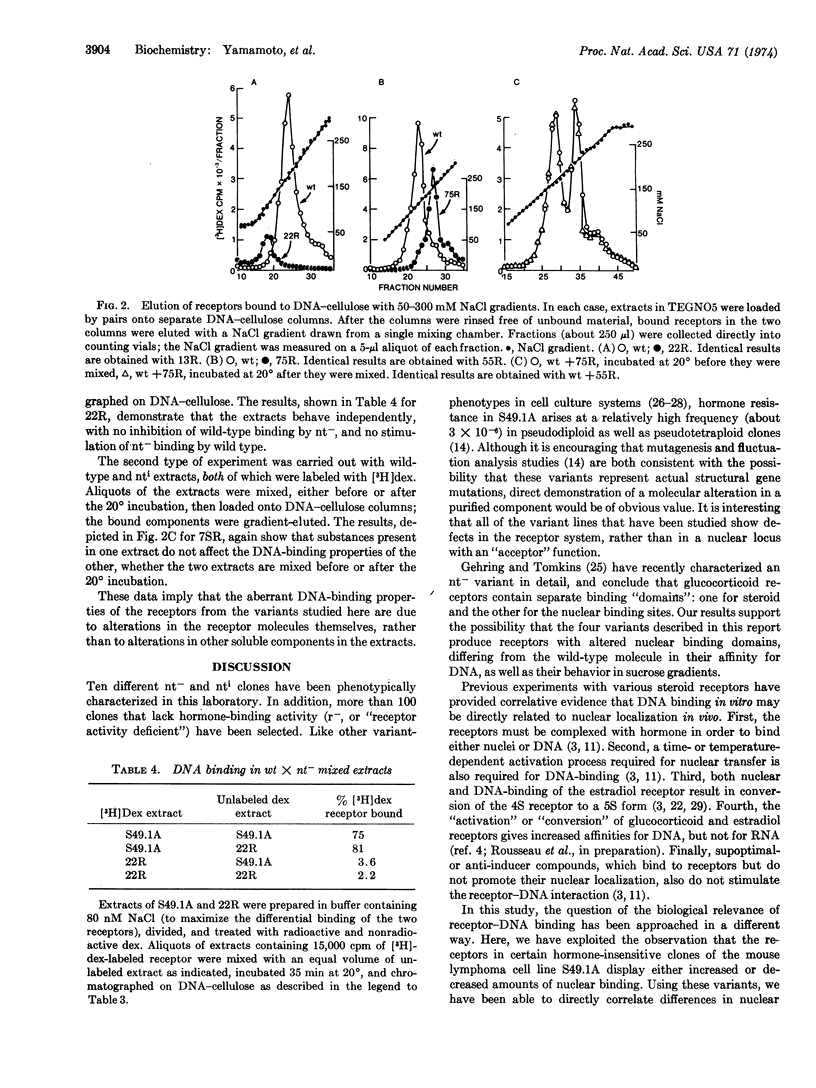
Abstract
Mouse lymphoma tissue culture cells (S49.1A) are normally killed by dexamethasone, a synthetic glucocorticoid hormone. Dexamethasone-resistant clones have been selected from this line, some of which retain the ability to specifically bind dexamethasone. Addition of [3H]dexamethasone to cultures, followed by cell fractionation, reveals that the nuclear transfer of hormone-receptor complexes in some of these variant clones is deficient (nt-), while others show increased nuclear transfer (nti) relative to the parental line. Two independently selected members of each class have been studied here, in an effort to elucidate the molecular determinants involved in the receptor-nucleus interaction in vivo. The labeled receptors in cell-free extracts bind to DNA-cellulose, but only after previous incubation of the extract at 20°, similar to the treatment required for cell-free interaction of receptors with nuclei. More importantly, the apparent DNA-binding affinity of the nt- receptors is lower than the wild type, whereas the nti receptors bind DNA with an affinity higher than the parental molecules. The parallelism of nuclear and DNA binding, together with the observations that the receptors from the variants have sedimentation properties different from the wild-type cells, lead us to conclude that (i) these variants may contain altered receptor molecules and (ii) DNA is probably the primary nuclear binding site for steroid receptors in vivo.
Keywords: tissue culture, altered receptors, sucrose gradients, DNA-cellulose
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baxter J. D., Rousseau G. G., Benson M. C., Garcea R. L., Ito J., Tomkins G. M. Role of DNA and specific cytoplasmic receptors in glucocorticoid action. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1892–1896. doi: 10.1073/pnas.69.7.1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter J. D., Tomkins G. M. Specific cytoplasmic glucocorticoid hormone receptors in hepatoma tissue culture cells. Proc Natl Acad Sci U S A. 1971 May;68(5):932–937. doi: 10.1073/pnas.68.5.932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamness G. C., McGuire W. L. Estrogen receptor in the rat uterus. Physiological forms and artifacts. Biochemistry. 1972 Jun 20;11(13):2466–2472. doi: 10.1021/bi00763a013. [DOI] [PubMed] [Google Scholar]
- Chasin L. A. The effect of ploidy on chemical mutagenesis in cultured Chinese hamster cells. J Cell Physiol. 1973 Oct;82(2):299–307. doi: 10.1002/jcp.1040820218. [DOI] [PubMed] [Google Scholar]
- Fox T. O., Johnston C. Estradiol receptors from mouse brain and uterus: binding to DNA. Brain Res. 1974 Sep 6;77(2):330–337. doi: 10.1016/0006-8993(74)90797-5. [DOI] [PubMed] [Google Scholar]
- Freed J. J., Mezger-Freed L. Origin of thymidine kinase deficient (TK-) haploid frog cells via an intermediate thymidine transport deficient (TT-) phenotype. J Cell Physiol. 1973 Oct;82(2):199–212. doi: 10.1002/jcp.1040820208. [DOI] [PubMed] [Google Scholar]
- Harris A. W. Differentiated functions expressed by cultured mouse lymphoma cells. I. Specificity and kinetics of cell responses to corticosteroids. Exp Cell Res. 1970 Jun;60(3):341–353. doi: 10.1016/0014-4827(70)90527-6. [DOI] [PubMed] [Google Scholar]
- Harris M. Mutation rates in cells at different ploidy levels. J Cell Physiol. 1971 Oct;78(2):177–184. doi: 10.1002/jcp.1040780204. [DOI] [PubMed] [Google Scholar]
- Higgins S. J., Rousseau G. G., Baxter J. D., Tomkins G. M. Early events in glucocorticoid action. Activation of the steroid receptor and its subsequent specific nuclear binding studied in a cell-free system. J Biol Chem. 1973 Aug 25;248(16):5866–5872. [PubMed] [Google Scholar]
- Jackson V., Chalkley R. The binding of estradiol-17 beta to the bovine endometrial nuclear membrane. J Biol Chem. 1974 Mar 10;249(5):1615–1626. [PubMed] [Google Scholar]
- King R. J., Gordon J. Involvement of DNA in the acceptor mechanism for uterine oestradiol receptor. Nat New Biol. 1972 Dec 6;240(101):185–187. doi: 10.1038/newbio240185a0. [DOI] [PubMed] [Google Scholar]
- Lin S. Y., Riggs A. D. Lac repressor binding to non-operator DNA: detailed studies and a comparison of eequilibrium and rate competition methods. J Mol Biol. 1972 Dec 30;72(3):671–690. doi: 10.1016/0022-2836(72)90184-2. [DOI] [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- Mainwaring W. I., Peterken B. M. A reconstituted cell-free system for the specific transfer of steroid--receptor complexes into nuclear chromatin isolated from the rat ventral prostate gland. Biochem J. 1971 Nov;125(1):285–295. doi: 10.1042/bj1250285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer H. R., Chalkley G. R. Some properties of a nuclear binding site of estradiol. J Mol Biol. 1967 Aug 14;27(3):431–441. doi: 10.1016/0022-2836(67)90049-6. [DOI] [PubMed] [Google Scholar]
- Milgrom E., Atger M., Baulieu E. E. Acidophilic activation of steroid hormone receptors. Biochemistry. 1973 Dec 4;12(25):5198–5205. doi: 10.1021/bi00749a029. [DOI] [PubMed] [Google Scholar]
- O'Malley B. W., Spelsberg T. C., Scharder W. T., Chytil F., Steggles A. W. Mechanisms of interaction of a hormone--receptor complex with the genome of a eukaryotic target cell. Nature. 1972 Jan 21;235(5334):141–144. doi: 10.1038/235141a0. [DOI] [PubMed] [Google Scholar]
- O'Malley B. W., Toft D. O., Sherman M. R. Progesterone-binding components of chick oviduct. II. Nuclear components. J Biol Chem. 1971 Feb 25;246(4):1117–1122. [PubMed] [Google Scholar]
- Puca G. A., Sica V., Nola E. Identification of a high affinity nuclear acceptor site for estrogen receptor of calf uterus. Proc Natl Acad Sci U S A. 1974 Mar;71(3):979–983. doi: 10.1073/pnas.71.3.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousseau G. G., Baxter J. D., Tomkins G. M. Glucocorticoid receptors: relations between steroid binding and biological effects. J Mol Biol. 1972 Jun 14;67(1):99–115. doi: 10.1016/0022-2836(72)90389-0. [DOI] [PubMed] [Google Scholar]
- Sibley C. H., Tomkins G. M. Isolation of lymphoma cell variants resistant to killing by glucocorticoids. Cell. 1974 Aug;2(4):213–220. doi: 10.1016/0092-8674(74)90013-0. [DOI] [PubMed] [Google Scholar]
- Sibley C. H., Tomkins G. M. Mechanisms of steroid resistance. Cell. 1974 Aug;2(4):221–227. doi: 10.1016/0092-8674(74)90014-2. [DOI] [PubMed] [Google Scholar]
- Stancel G. M., Leung K. M., Gorski J. Estrogen receptors in the rat uterus. Multiple forms produced by concentration-dependent aggregation. Biochemistry. 1973 May 22;12(11):2130–2136. doi: 10.1021/bi00735a018. [DOI] [PubMed] [Google Scholar]
- Toft D. The interaction of uterine estrogen receptors with DNA. J Steroid Biochem. 1972 Apr;3(3):515–522. doi: 10.1016/0022-4731(72)90098-2. [DOI] [PubMed] [Google Scholar]
- Williams D., Gorski J. Kinetic and equilibrium analysis of estradiol in uterus: a model of binding-site distribution in uterine cells. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3464–3468. doi: 10.1073/pnas.69.11.3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K. R., Alberts B. M. In vitro conversion of estradiol-receptor protein to its nuclear form: dependence on hormone and DNA. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2105–2109. doi: 10.1073/pnas.69.8.2105. [DOI] [PMC free article] [PubMed] [Google Scholar]


