Abstract
Arguably one of the most important effects of climate change is the potential impact on human health. While this is likely to take many forms, the implications for future transmission of vector-borne diseases (VBDs), given their ongoing contribution to global disease burden, are both extremely important and highly uncertain. In part, this is owing not only to data limitations and methodological challenges when integrating climate-driven VBD models and climate change projections, but also, perhaps most crucially, to the multitude of epidemiological, ecological and socio-economic factors that drive VBD transmission, and this complexity has generated considerable debate over the past 10–15 years. In this review, we seek to elucidate current knowledge around this topic, identify key themes and uncertainties, evaluate ongoing challenges and open research questions and, crucially, offer some solutions for the field. Although many of these challenges are ubiquitous across multiple VBDs, more specific issues also arise in different vector–pathogen systems.
Keywords: climate, climate change, vector-borne diseases, human health, modelling
1. Introduction
There is increasing awareness that levels and layers of complexity are the rule when describing and predicting the impacts of climate variability and change on the transmission of vector-borne diseases (VBDs) [1–5]. Recent work has highlighted that while climate patterns, particularly temperature and rainfall trends (although changing wind patterns could also have important implications for some vectors [6]), can have direct effects on VBD transmission [1,2,4,5,7–13], this influence may be significantly modified by confounding non-climatic factors implicated in human communities [3,4,14–25]. Although climate patterns may govern the potential global distribution of a VBD, the actual magnitude and spatial extent within regions are likely to be governed by a multitude of transmission-related non-climatic factors, including epidemiological, environmental, social, economic and demographic factors [17,25–29]. These studies emphasize that quantifying the impact of climate change on VBDs requires a better understanding of not only the relative effects of these individual variables, but also the combined (and complex) outcomes that interactions between these indirect ecological factors and direct biological influences can have on the vector–pathogen–host relationships that underpin VBD transmission [1,3,4,30]. A more recent emerging trend is also recognition of the role that human reflexivity may play in constraining the reliability of ecological predictions [31,32]; that is, if predictions are made and taken seriously, people may change their actions and behaviour, in turn, making accurate forecasting more difficult. These considerations imply that to better understand the likely impact of climate change on VBDs, it is important to view climate-driven disease systems as complex socio-ecological dynamical systems that emphasize (i) the multi-level causation of disease, (ii) the impact of broad contextual stressor factors that determine transmission at the population level, (iii) the importance of considering nonlinear feedback loops between human activities/reflexivity and global change, and (iv) the hierarchical cross-scale effects of local–global change in disease drivers [33–35].
These considerations, particularly the need for coupling human and natural system dynamics and interactions as implied by the socio-ecological systems paradigm [33–35], clearly present a major challenge to current efforts aimed at reliably quantifying the effects of climate change on VBDs. While, on the one hand, ignoring important non-climatic modifying or confounding factors (such as ecosystem and land-use change, agricultural and other economic practice, urbanization, human migration, health infrastructure and technologies and host demographics and behaviour) will lead to overestimation of the impact of climate change [1,4,22,36], the properties connected with the emergent, adaptive and nonlinear dynamics of human–natural systems will, on the other hand, make predictions of invasion, expansion and response of VBDs in human populations as a result of climate change extremely difficult to achieve [35,37,38]. The complexity deriving from these features constrains the use of simple risk-based ‘reductionist’, linear and equilibrium-based methods, as embodied by regression-based risk modelling methods (even if multivariate in form) and multi-criteria or variable score-based community vulnerability indices [39–41], for both investigating associations of climate variability with VBDs and predicting its effects on future transmission. Such methods provide us with useful frameworks for conceptualizing the links that may operate between climate change and exposure to risk and adaptive capacities of humans in determining the overall vulnerability of a community; however, these complex biological–human interactions mean that they will invariably be less effective for capturing the full range of dynamic behaviours that a VBD system may experience as a result of climate change. This implies a clear need for the development of a more integrated complex systems-based ecological framework that can reliably link climate change, ecosystems, economies and societies with disease dynamics if more reliable analysis and predictions of shifts in VBD transmission dynamics owing to global change are to be achieved [35].
Mathematical models of pathogen population dynamics, by virtue of their ability to provide a quantitative means for integrating and simulating the impacts of multi-factorial and multi-scale disease transmission processes, may offer us a particularly pertinent methodological tool for developing such holistic predictive and investigative frameworks [7–13,19,25,27,42–58]. Recent advances in incorporating the effects of climate, as well as anthropogenic alterations of ecosystems (e.g. via induced changes in vector biodiversity [59], population movements and immunity [60,61], socio-economic development and the effects of public health interventions), with disease transmission [25,62] make these tools even more applicable as quantitative frameworks for capturing the full range of community vulnerabilities to VBD transmission as a result of global climate change. Such models, by careful elaboration and inclusion of nonlinear functional relationships between biological and non-biological social components of disease transmission, may also represent the only means by which the full complexity (emergence, self-organization, points of bifurcation and regime shifts) of the response of a natural–human system to global change may be explored [25,62]. In addition, new data–model assimilation frameworks provide a means for such models to capture and incorporate local social, ecological and climatic conditions, thereby affording the examination of the impacts of these proximate causes within the broader context of global climate change [42,63,64]. This modelling approach thus not only offers the possibility of addressing the dynamic interactions of the various factors involved in transmission, but crucially also the ability to deal with cross-scale interactions and feedbacks that are likely to play major roles in climate-induced changes in transmission [17,35]. In addition, recent work on spatial agent-based modelling frameworks [44,65] offers approaches that allow exploration of how human behaviour and reflexivity may be incorporated with social–economical and natural components at various organizational levels in order to predict likely community vulnerabilities and responses to global climate change. Such individual-based, as well as Monte Carlo-based, ensemble modelling approaches also provide a means of addressing the impacts of uncertainties and stochasticity on predictions, thereby facilitating the generation of outputs in probabilistic terms for use by policy- and decision-makers at various organizational levels [65–68].
2. The role of climate in insect vector–pathogen interactions
(a). The role of temperature in the replication and transmission of major vector-borne diseases
Temperature is well acknowledged to directly impact VBDs in insect hosts; insects are poikilotherms and their internal temperature varies considerably with ambient temperature, greatly affecting their physiology, as well as directly exposing the pathogens they carry to ambient temperatures. Other climatic factors impacting vector–pathogen interactions are those affecting vector susceptibility to infection, including vector physiology and fitness (and thus the ability to fight off infection) and the probability of exposure to infection (such as host preference and biting rate). The impact of environmental factors on different pathogens and vectors is diverse and specific to individual vector–pathogen combinations. This specificity requires tailored parameters for individual vector–pathogen systems to more accurately project the impact of climatic changes on VBD transmission.
(i). Viral vector-borne diseases transmitted by mosquitoes
Viral replication kinetics in insect-cultured cells are dependent on temperature, with viral attachment and cell infection being more efficient at higher temperatures [69]. However, the relationship between temperature and viral replication/transmission dynamics in many biological systems is not straightforward. A number of studies have demonstrated a strong association between temperature and viral replication in mosquito species, with higher temperatures generally leading to shorter extrinsic incubation periods (EIPs), increased infection rates and faster dissemination rates, although these vary considerably in different mosquito/virus combinations. Within the flavivirus genus, yellow fever (YF) infection of Aedes aegypti shows a decreased EIP at higher temperatures, but YF infection of Haemagogus mosquitoes demonstrates lower infection rates at lower temperatures [70]. A recent study, however, found increased YF infection in Aedes albopictus mosquitoes reared at lower temperatures (attributable to impaired RNAi responses in the mosquito), demonstrating further the complexities of downstream temperature effects on immature mosquito life stages [71]. Indeed, the same result also appears to be true for chikungunya virus (CHIKV) infection of Ae. aegypti [71] and Ae. albopictus [72].
Dengue virus (DENV) infection rates are also temperature-dependent, demonstrating increased infection and transmission rates at higher temperatures [73–76], as well as altered infection rates and EIP in response to fluctuating temperatures and diurnal temperature range (DTR). DTRs have been shown to enhance DENV infection rates and reduce EIPs [77] at low temperatures, but decrease infection rates (and not affect DENV EIP) at higher temperatures [78].
Alphaviruses also demonstrate reduced EIPs at higher temperatures, but display variation in the effects on infection and transmission rates. Eastern equine encephalitis virus (EEEV) and West Nile virus (WNV) infection rates in Ae. triseriatus and Culex univittatus are independent of temperature, whereas western equine encephalitis virus (WEEV) infection rates in C. tarsalis decrease at higher temperatures (e.g. 32°C) compared with moderate temperatures (e.g. 25°C) [70]. EEEV, WEEV and WNV all have reduced EIPs at higher temperatures [70,79]. Sindbis virus infection and dissemination rates have been found to be higher in Ae. aegypti mosquitoes after rearing larvae at higher temperatures [80], whereas, in contrast, studies have shown increased CHIKV (and YF) infection rates in larvae raised at lower temperatures [71,72].
Thus, although the overall trend for viral VBDs is for higher temperatures to increase replication rates, decrease EIPs and increase transmission rates, variation in the limited number of examples given here illustrates the need for tailored parametrization of VBD models. To better reflect biological systems, the effects of DTR, temperature effects on vector susceptibility through vector fitness and the impact of temperature variation during mosquito development on vector susceptibility should be considered when assessing climate effects on VBD vector–pathogen interactions.
(ii). Malaria
The transmission cycle of the malaria parasite in mosquito vectors is more complex than that of viruses, including multiple developmental stages and the additional complication of nonlinear density-dependent effects on parasite numbers [81]. Temperature sensitivity of malaria parasites in mosquito hosts has long been established [82–84], with many models using the Detinova curve and monthly average temperatures to describe changes in the parasite EIP with temperature.
More recent work suggests a new temperature sensitivity curve, incorporating the effects of fluctuating daily temperature on development. A series of studies investigating the effect of DTR on parasite development and transmission have shown that DTRs increase rate processes (speed up parasite development) at low mean temperatures and decrease rate processes at higher temperatures [85–88]; thus, using mean temperatures in disease models will underestimate transmission at cooler temperatures and overestimate at warmer temperatures. In addition to DTR affecting parasite development, temperature also has an impact on the proportion of mosquitoes carrying infectious sporozoites, with mosquitoes maintained at higher temperatures demonstrating a lower prevalence of sporozoites in the salivary glands [89]. Thus, at high temperatures, despite a decrease in parasite development time, fewer mosquitoes become infectious and able to transmit the parasite. Another recent study has highlighted another complicating factor in the effects of temperature on parasite transmission, whereby asynchrony between completion of the parasite EIP and occurrence of the next blood meal can develop as temperature changes. This could lead to a delay in parasite transmission, despite a reduction in parasite EIP, as temperatures rise [90], highlighting how thermal effects on both pathogen and its vector must be considered for accurate transmission modelling. Using these new thermal sensitivities, malaria transmission has recently been predicted to peak at 25°C (dramatically lower than previous predictions) and decline significantly above 28°C [91].
In addition to its direct effects on parasite development, temperature can also have a profound effect on vector physiology and immune responses [92]. Temperature has been shown to affect immune responses in a variety of insects, including beetles, crickets, butterflies and Drosophila (fruit flies). Focusing on mosquito vectors, a few studies have investigated the effect of temperature on mosquito immune responses; Suwanchaichinda & Paskewitz [93] showed that the melanization response in Anopheles gambiae progressively decreases as temperatures increase, while Murdock et al. [94] investigated the response of cellular and humoral immunity to changes in temperature. In the latter study, a complex picture emerged, with melanization, phagocytosis and an antimicrobial peptide (AMP) defensin expression peaking at 18°C, whereas nitric oxide synthase expression peaked at 30°C. Expression of a further AMP, cecropin, showed no response to changes in ambient temperature. The study in reference [94] demonstrates how the immune gene profile of a vector at one ambient temperature can be completely altered at a different temperature. This could impact the vectorial capacity of insect vectors of malaria and other pathogens. In [95], it was demonstrated that ambient temperature influences the expression of immune genes that can regulate the intensity of Plasmodium yoelii infection in An. stephensi mosquitoes. Changes in immune responses may alter or temper the effects of temperature on parasite development, although with respect to transmission, these effects are likely to be subtle.
(iii). Lyme disease and other tick-borne diseases
Studies on climate and tick-borne diseases have largely focused on the effect of temperature or climate change on the distribution of the tick hosts (primarily Ixodes scapularis and Ixodes ricinus), with currently little literature documenting tick–pathogen interactions, especially with respect to temperature and environmental conditions.
For Lyme disease transmission, the length of time feeding positively correlates with transmission from infected tick to a mouse host [96], indicating that because climate influences the duration of feeding, it will also affect transmission rates. The EIP of the bacteria is determined by the developmental duration of the immature tick stages, as bacteria are typically acquired during the larvae or nymph stages and transmitted during the nymph or adult stages, respectively [97]. In this respect, the EIP is not dependent on the replication nor developmental kinetics of the pathogen (as seen for viral VBDs and malaria, respectively), but is more directly influenced by vector development and questing behaviour. Studies on the molecular interactions between tick hosts and Lyme disease have shown that temperature changes, stress and pathogen infection lead to the upregulation of heat shock proteins, which contribute to tick survival and possibly reduce infection [98]. To the best of our knowledge, no empirical studies on the effect of temperature on the EIP of Lyme disease, or the probability of transmission, have been carried out.
Crimean Congo haemorrhagic fever (CCHF) is caused by a tick-borne virus of the Bunyaviridae family. It has complex enzootic cycles: several putative tick vectors with two- or three-host life cycles. Nothing is known at the molecular level about the interactions between the virus and Hyalomma ticks [97]. This represents a significant research gap that requires attention before any projections can be made on the effect of climate on virus replication and disease transmission.
Another important group of tick-borne pathogens is the emerging rickettsial diseases, with Rocky Mountain spotted fever (Rickettsia rickettsia) being the most severe of the rickettsioses (reviewed in [99]). Emerging in Europe and the Mediterranean are Mediterranean spotted fever (Rickettsia conorii, endemic in the Mediterranean and with occasional cases in northern and central Europe), Rickettsia slovaca (present in France, Slovakia, Hungary and Spain), Rickettsia mongolitimonae (Southern France), Israeli spotted fever, Rickettsia conorii subsp. israelensis (the middle east and Portugal) and R. aeschlimannii (one case in France), among others [100]. A range of tick vectors are involved in transmission and, as for CCHF, very little is known about the interactions between these bacterial pathogens and their invertebrate vectors.
(iv). Leishmaniasis
There have been recent expansions in geographical areas where Leishmaniasis is endemic in both South America and South Europe and changes in climate are one possible contributing factor [101]. Like other vector–pathogen combinations already discussed, ambient temperatures have a clear direct effect on sandfly development, but a less clear effect on range expansion because of the confounding influence of photoperiod on overwintering diapause [101]. However, the role of climate in parasite development has received little attention. One previous study [102] indicated that Leishmania infantum developed better in the digestive tract of phlebotomine sandflies at higher temperatures, whereas another recent study [103] expanded this to examine the effect of temperature on the development of several Leishmania spp., in different sandfly species. The latter study showed that Leishmania parasites developed faster at higher temperatures during the early stages of infection, but that temperature had little effect on the establishment of infection. The montane L. peruviana appeared to show adaptation to cooler temperatures. Differences in development time, blood meal digestion and defaecation were observed for different fly–parasite combinations, again highlighting the need for specific parametrization of transmission models.
(b). Challenges in understanding climate change effects on vector-borne diseases
(i). Incorporating multiple drivers of disease risk
As discussed in §1, spatial and temporal patterns of VBDs are influenced by both ecological and socio-economic factors. Socio-economic conditions can influence transmission risk in a way that complicates our understanding of temperature/climatic influences. Rather than disputing which category of driver is more important [104], we suggest more rigorous exploration of the relative importance of each driver and their interactions. Research integrating ecological and socio-economic factors has begun. For example, a statistical model was developed in [105] to predict the changing global distribution of malaria under climate change and changing per capita gross domestic product (pcGDP) based on the IPCC A1B scenario [106]. This model predicted that increasing pcGDP (a threefold increase by 2050) by itself would strongly reduce the global distribution of malaria in the coming decades, whereas climate change by itself would expand that distribution. Combining predicted changes in pcGDP with climate change, the model predicted that an additional 210 million people will be at risk by 2050. Further evaluations should consider socio-economic and ecological contexts.
GDP is likely to be more important in places where malaria transmission risk is high [107]. If climate change increases the risk of exposure, prior investments in abatement could become inadequate and increased investment in mitigation might be required. It is also likely that temperature gradients are not always collinear with either vector presence or economic gradient, potentially leading to threshold effects. Studies on wildlife diseases could provide an opportunity to tease apart some of these complexities. Wildlife components of disease risk (e.g. zoonotic amplification, persistence and population impacts) are clearly sensitive to temperature (as demonstrated, for instance, with avian malaria in Hawaii [108,109]). In addition, economically driven anthropogenic effects may affect zoonotic VBD dynamics [110], whereas socio-economic factors have also been suggested as driving recent increases in human cases of the monkey malaria Plasmodium knowlesi [111].
(ii). Selecting appropriate metrics of disease risk
The basic reproduction number R0 is frequently used to integrate understanding about vector and pathogen ecology and transmission behaviours. However, the usefulness of R0 compared with other metrics of risk requires further exploration; it is a measure of invasion potential, but not necessarily the best metric for determining possible temporal or spatial changes in disease prevalence or incidence in areas of existing transmission. In this case, measures such as the entomological inoculation rate (EIR), which explicitly capture elements of vector biology, might be a more appropriate risk metric to model as a function of climate change. However, the usefulness of any one metric very much depends on the specifics of the question; trying to determine changes in populations at risk owing to potential climate-induced shifts in disease range, for example, is a very different question from determining future changes in the frequency, timing, size or duration of malaria epidemics at a location characterized by intermittent, seasonal transmission. In general, we would argue that statistical climate models based on the distribution of recorded malaria cases are fraught with problems of interpretation and should be interpreted carefully. The distribution of malaria cases is a complex and poorly understood consequence of ecological, socio-economic and other factors, such that causal relationships are frequently obscured.
(iii). Data availability to parametrize models of disease risk
Arguably the greatest limitation in the development of mechanistic transmission models is our current understanding of the essential empirical relationships between vectors, pathogens and the environment. The low quality and quantity of data available to parametrize models of risk are often not considered in model development or interpretation. Additional experimental research is required to explore and define physiological temperature and moisture constraints for the most important vector species. Strong evidence indicates that temperature variability is important to estimating risk [85–87,112,113], but vital rates are currently estimated from very few data points representing laboratory responses by vectors or pathogens to temperature averages [114], together with inferences based on seasonal occurrence [115]. In addition (and as noted in §2a), estimates of vital rates for specific disease systems, for example, Plasmodium falciparum malaria, are often derived from data on a mix of vector species, whereas endosymbionts such as Wolbachia may be important in the biology and control of certain disease vectors [116] and may exhibit temperature responses [117]. To validate these models, it is important to conduct comparative research on different species within important vector genera. For example, both Ae. aegypti and Ae. albopictus are competent vectors of DENV, with different physiological constraints and distinct transmission potentials. Combining data for these two species to estimate vital rates would not necessarily generate realistic inference. Furthermore, data and research are particularly needed to better understand the influence of environmental factors on key parameters such as the EIP [89], vector competence [94,113], biting behaviour and interactions with infection [118,119], because these have been very under-researched to date.
The enormous variation in EIR that can exist around a given mean seasonal temperature [91,120] remains to be explained, yet is critical to conclusions concerning the role of climate change. Variation in EIR at a given temperature regime could be due to local adaptation [121], control interventions and/or socio-economic factors, differences between vector and/or parasite species complexes across sites, potentially subtle variation in life-history traits such as mosquito population age structure [122], accuracy of (local) temperature estimates or chosen temperature metrics and/or sampling protocols. Both empirical and theoretical studies are needed to explore potential determinants, uncertainties in parameter estimates and consequences for predictive models. Uncertainty in the components of R0 (or other metrics) should be integrated and acknowledged. In addition, many other assumptions are frequently made regarding how biotic interactions (e.g. competition, density dependence and host density) regulate and limit vector populations. Uncertainties owing to these assumptions are rarely considered or acknowledged in risk metrics.
To advance the empirical base from which to derive models, we recommend the following:
(1) Mean seasonal temperatures have been regularly used and might be sufficient for some questions and some settings, but, in general, DTR or similar measures of temporal variability must be included. Off-season temperatures and ranges might also be important in some regions, but an understanding of which temperature metrics are most important during this period is less well developed.
(2) Humidity and evaporation rate influence adult mosquito vital rates, but have received insufficient attention as determinants of VBD risk. The effects of both mean and fluctuating humidity and water vapour pressure, as well as interactive effects with temperature, are often poorly understood.
(3) The timing, frequency and quantity of precipitation are important, but not well understood and are likely to differ for different mosquito species. Total rainfall is likely to have a highly nonlinear effect on mosquito production, with the potential for qualitatively different effects on container-breeding versus seasonal or permanent wetland breeding species.
3. Models of relevant climatological, ecological and epidemiological processes
(a). Types of models
With respect to models of relevance for studying the impact of climate change on VBDs, these fall into the category of either mechanistic or statistical in their construction. Mechanistic (or process-based) models of vectors, disease transmission or climate are those that represent the system using dynamic (often nonlinear) equations, with appropriate scales and interactions, which explicitly capture the relevant physical or biological processes. For behaviours not explicitly resolved, but important to capture, these are parametrized with a diagnostic equation representing the role of the parameter at the resolved scale. The general benefit of these models is that they are able to capture nonlinear and coupled interactions at multiple spatial and temporal scales, but, in the context of accurately modelling VBDs themselves, their reliability depends on possessing a complete picture of all aspects of transmission and being able to accurately translate this into a mathematical representation. In the context of climate change and VBDs, process-based VBD models do not require the assumption of a stationary relationship between determinants of disease and metrics of transmission, which is a considerable advantage on the decadal timescales of global change. Statistical models, on the other hand, represent the relationships between relevant variables (e.g. transmission and climatic factors) from a purely descriptive perspective and their relationship is sometimes assumed to be stochastic (although this can also be built into mechanistic models). They are computationally inexpensive to run (and develop) and are well suited for capturing timeseries and shorter-term (and linear) behaviour for systems with a large set of parameters, but do not attempt to incorporate known mechanistic relationships. Whether predominantly mechanistic or statistical, each model type has benefits and limitations and one possibility is a combination of approaches in order to provide an optimal set of attributes to connect climate with VBD transmission. The coupling of different models produces new complications that need to be addressed carefully; model coupling itself is a mathematical and computational science research problem and although there has been early work to consider hybrid models to maximize the benefit of each for the system under consideration [123], such models will not be considered further here.
(i). Statistical models
The majority of statistical models describing the relationship between VBDs and environmental indicators of climate, climate change, meteorological factors and extreme weather events exploit regression models either adapted to time-series data or, for example, applied to investigate potential future changes in vector distribution [124–126]. Statistical models help to determine the relative contribution of environmental drivers to temporal variations in disease manifestation, support a wide variety of data types, and may offer a platform for developing early-warning systems based on disease and host surveillance [127–129]. Models may differ in their choice of health outcome measure, definitions of exposure or methods of quantifying associations and how different combinations of these components lead to different uncertainties in model estimates. Health outcomes are usually VBD morbidity and mortality, with the latter likely to result from the greatest exposure and/or most vulnerable populations (although the quality of recording, reporting and causal assessment may not be straightforward). Studies of diseases with low fatality may not provide enough cases to achieve the required statistical power. Morbidity outcomes often include a wide range of health conditions and, owing to the repeated nature of potentially observable events, provide a wider range of associations with exposure. The current use of morbidity outcomes is often limited by utilization of medical records, emergency room visits, drug prescription and hospital admissions; these data are typically protected under privacy laws and regulations and thus are frequently difficult to access. Many studies implicitly or explicitly assume that (i) the selected measures of exposure are relevant for the study population, (ii) meteorological and climatological characteristics are reliable proxies for individual exposure and (ii) the selected outcomes are relevant to the selected exposure measure; if violated, a statistical model may be misspecified.
Statistical models based on time-series typically explore the associations between meteorological parameters (most commonly, ambient temperature and rainfall) and health outcomes related to VBDs in selected geographical areas. The classic time-series models and regression models have been widely used to analyse surveillance data [130]. Similar models are also applied to study the health effects of environmental exposures and meteorological conditions [127,131,132]. While illustrating the shape and magnitude of relationships between meteorological parameters and health outcomes, reported results of time-series models are often presented in a non-uniform way, which, in general, complicates comparisons between different studies and inhibits wider generalization. Findings from regression models adapted to counts of health outcomes are typically presented as the change in health outcome per unit change in exposure, or as relative risks/rate ratios and their confidence intervals, to quantify the association between exposure and health outcomes and the degree of uncertainty. For models employing a time-series approach, temporal data resolution dictates the sensitivity of models to rapid change and long-term effects. Daily time-series offer the highest resolution, although weekly or monthly data are the standard for many surveillance systems, which reduces model capability to detect short-term changes. Furthermore, major meteorological episodes may also coincide with social events governed by the local calendar, thus amplifying or dampening the effects of environmental exposures.
Developing an understanding of short-term lags in the effect of exposure and manifestation of the selected health outcome is crucial for correctly capturing true associations [133,134]. These lags might be driven by complex life cycle processes and/or social determinants such as a lack of timely utilization of health care facilities. This aspect is typically handled by including lagged terms in statistical models, although the selection of terms is rarely justified. By the nature of VBDs, seasonal oscillations in health conditions are often observed and manifest via systematic, or repetitive, periodic fluctuations within a predetermined period. Seasonality is characterized by timing (position of extrema on the seasonal curve), magnitude (difference between maxima and minima) and duration. Seasonal patterns of health events measured by their frequency or observed counts per time unit may vary by type of health condition, location and population of interest [128]. In order to account for seasonality, studies may be stratified by summer/winter, warm/cold, dry/wet season or by considering periodic fluctuations. Explicit adjustment for trend, seasonality and other periodic factors may also be applied. Periodicities in meteorological factors and disease incidences are not necessarily aligned nor synchronized; nonetheless, the detected time difference between peak timing of exposure and disease incidence is informative and enables predictions of the potential impacts of environmental drivers on disease manifestation [128].
(ii). Mathematical models
Global atmospheric climate models (such as those within coupled Earth system models used in the latest IPCC report [135]) are typically mechanistic models that resolve the large-scale atmospheric flow field, along with a host of related meteorological variables such as temperature and water vapour, and incorporate a robust treatment of cloud physics parametrizations and chemical tracers [136,137]. These models are configured for interactive coupling to global land surface models that include heterogeneous representations of plant functional types and soil moisture distribution.
Climate models, like all models that represent physical systems, contain both known and unknown uncertainties of several types including, but not limited to, structural, algorithmic and parametric. Statistical uncertainty has also been identified [138,139], but structural (or systematic) uncertainty, which here refers to the underlying physics and understanding of model behaviour, is less well understood. In large-scale mechanistic models of climate change, the understanding and improvement of climate models through better data collection [140] and reduced spatial and temporal error [141,142], together with an analysis of model sensitivities through sampling algorithms, are emerging areas of interest. Some early work to connect mechanistic models with statistical models and combine information about uncertainty (e.g. stochastic parametrization [143]) has also been undertaken. For climate change models, some of the forecast skill can be attributed to model initialization, as demonstrated with near-term prediction experiments [144].
Numerous mathematical models have been designed and used to quantitatively and qualitatively gain insights into the transmission dynamics and control of VBDs in human populations [145], with the earliest work dating back to the pioneering studies of Ross and Macdonald on malaria [146]; however, only a few of these models have incorporated the effects of climate and/or climate change [9,13,124,147–151]. Recent models have included statistical and stochastic models [16,147,148,152–154], approaches based on compartmental nonlinear ordinary and partial differential equations [9,149,155–157] and nonlinear difference equation models [13,158]. In addition, more complex network models (both static and dynamic), spatially explicit R0 models, agent-based/simulation models and cellular automata models have also been considered [159–161], but their use is not as widespread in this area.
Malaria, by virtue of causing the greatest global burden of disease of all VBDs [162], has dominated modelling studies in the context of climate change impacts on transmission [87,154,156,163–167]. By examining the relationship between malaria and climate in 25 African countries using a semi-parametric economic model, Egbendewe-Mondzozo et al. [16] showed that a marginal change in temperature and precipitation levels would lead to a significant change in the number of malaria cases for most countries considered by the end of the century. Modelling of how the EIP of P. falciparum is expected to vary over time and space across Africa (depending on DTR) was considered in [87]. It was shown in [89] that vector competence tails off at higher temperatures, even though parasite development rate increases. Using a model that incorporates empirically derived nonlinear thermal responses of Anopheles vectors, Mordecai et al. [91] predict that the optimal temperature for malaria transmission is 25°C (and that it significantly decreases beyond 28°C).
Developing more realistic models of the climate-driven nature of Anopheles population dynamics has also increased in recent years; Lunde et al. [156] compared six temperature-dependent mortality models for An. gambiae sensu stricto, whereas White et al. [168] and Parham et al. [158] developed validated models for assessing the effects of environmental variables (including rainfall, wind speed, temperature, relative humidity and density-dependence) on vector abundance by fitting to longitudinal mosquito catch data. Similarly, Beck-Johnson et al. [122] developed a stage-structured, temperature-dependent, deterministic delay-differential equation model to investigate the population dynamics of Anopheles mosquitoes, with a notable finding that, by incorporating the full mosquito life cycle in the model, mosquito abundance is more sensitive to temperature than suggested by studies owing to the strong influence of the juvenile stages (whose vital rates are also temperature-dependent). Recent experimental findings, such as the dependence of adult An. gambiae s.s. life-history parameters on their experiences as juveniles [169] and the significant differences observed by Lyons et al. [170] in temperature-dependent survival and developmental rates in An. gambiae s.s., An. arabiensis and An. funestus populations (the three most important vectors of human malaria in Africa [126]), are also key developments and emphasize the importance of empirical data in developing more reliable parametrization of mechanistic vector, and hence VBD, models.
4. The impact of vector–pathogen–host ecology and behaviour on vector-borne diseases
Independent of the modelling methods employed, certain features of VBDs make efforts to capture them mathematically distinct from epidemiological models of either direct-transmission infectious diseases (such as influenza) or diseases driven primarily by environmental contamination (such as cholera); such features are discussed in this section.
(a). Time scales
One ubiquitous aspect of VBDs that distinguishes their study from efforts to model and gain insights into more general systems within infectious disease epidemiology is the inherent dependence on multiple interacting scales of effect. Each of the individual component biological systems (host, vector and pathogen) suggests its own, implicit temporal scale through the duration of stages/states of development. Mosquito larval development, for example, takes place at timescales varying from days to weeks [171,172], whereas human health interventions such as vaccination programmes may vary from days to weeks to months [173,174]. Modelling efforts must therefore include methods by which to couple the timescales of these processes in biologically relevant ways that capture the dynamics, but without creating artificial restrictions or producing erroneous model artefacts. Capturing these temporal scales successfully is made more complicated by the fact that the duration of life stages/states (excluding disease-specific states) in hosts most often progress unaltered by vector or pathogen, but disease vectors have been shown to be susceptible to alteration in their basic survival, developmental and fecundity rates by the microbes and pathogens they carry [175,176]. Furthermore, pathogen states/stages are predominantly driven by their epidemiological dynamics within and among hosts and vectors and, in some cases, progress to a different developmental stage only upon successful completion of the cycle between vectors and (sometimes multiple species of) hosts [177].
When broadening the scope of questions asked with these models from just the current examination of epidemiological patterns to explore how climate change may alter the behaviour of this entire system in the future, yet another timescale must be incorporated, as the processes of climate change act on time frames of years to centuries or more. While many models make timescale integration among these systems implicit in the values of parameters for continuous interactions, the continuous progress of climate change and the discrete nature of many of these stages make this a potential stumbling point in the accuracy and utility of generalizing the insights from such methods (because conditions are expected to shift), thereby potentially causing the interacting components to affect one another at rates that themselves change with time.
We therefore recommend that models address these issues explicitly, rather than implicitly, selecting rate values that seem to couple the timescales appropriately across systems and choosing timescales based on those factors that are most relevant to the driving question being asked; many of these scales may be interdependent. For the pathogen, we believe three components may be most relevant in determining the timescale most appropriate for that system: replication in the host, duration of persistence in the vector and evolution of the pathogen (which itself may depend on the other two). For the vector, we believe that demographic vital rates (both free from, and under the influence of, the pathogen) and host-biting rates (which are known to drive demographic rates in some systems) will be most relevant. For hosts (especially humans), we believe that demographic rates and the timescales of medical interventions are particularly relevant. The timescale of medical interventions is often difficult to determine and may include: time after introduction until a medical community might detect an outbreak (which will itself depend on the methods of surveillance, which may be more or less sensitive over time and space as an outbreak progresses), time until treatment or prophylaxis is available by either development, production, distribution and/or time until such measures are effective, probability of an outbreak based on the metrics discussed in §2b and the time for implementation and effectiveness of control strategies for vectors (such as spraying). Each of these poses their own challenges in how to best capture them within a mathematical modelling framework, but may be critical in capturing both current VBD dynamics and being able to employ such models to study how climate change may alter these dynamics in the future.
(b). Spatial scales
In dealing with the equally important question of spatial scales, one must decide the level/scale of understanding of climate change required that will be most appropriate to connect with biological features/processes. The assessment of VBD risk may be undertaken on a variety of geographical scales, varying from a village to an entire country, region or globally. The varying geographical and measurement (resolution) scales have immense implications for spatial analysis, such as scaling mismatches (when interpreting events at one scale against data measured at another) [150]. Features to be considered include both climatic (e.g. mean temperature) and meteorological (e.g. humidity or precipitation) features and their associated variability, ecological scales for hosts and vectors, changes in habitat (including both total area and patterns/trends of fluctuation in locations and connectivity) and shifting/expanding regions of infection as it radiates into (potentially) novel areas. For many of these, measurements taken during any state other than the current norm may be of great value for testing how well models perform in predicting the system under perturbation. For that reason, we expect and recommend that extreme weather events (e.g. floods, tsunamis and hurricanes) may provide an unfortunate boon to researchers (and may, for example, cause significant changes to spatio-temporal vector population dynamics), if we are able to gather relevant metrics in their wake.
(c). The impact of pathogen/host interactions on disease dynamics and evolution
Pathogen/host interactions are key factors in the evolution of infectious diseases. External or environmental phenomena impact behavioural, physiological, reproductive and ecological characteristics of individuals and populations. Among the components that may be altered and play a significant role in disease transmission are the pathogen life cycle and evolution processes, host susceptibility to infection, within-host/pathogen competition (if infections involve more than one strain or coinfection with multiple pathogens is likely), pathogen resistance to treatment, characteristics of the vector life cycle (potentially altered by pathogen-induced changes in behaviour and/or reproductive physiology), physiologically induced or purposefully adopted behavioural patterns of the host that alter (either increasing or decreasing) risks of exposure to infection, social/cultural/economic/behavioural factors in (human) host compliance with public health efforts and vector feeding behaviour as exemplified by preferential feeding patterns.
(d). Data gaps
Parametrization is a key challenge in modelling complex biological systems and awareness of the limitations in both the modelling framework and data quality (and availability) is an important requirement. Given that a model is a response to a certain set of precise, relevant and well-formulated questions about the phenomena under consideration, key concerns are, for example, the quality and type of empirical measurements necessary to parametrize the model, the identification and sensitivity of parameters to measurement error (and other uncertainties) and the availability and quality of empirical data needed (but published elsewhere). Data may be roughly classified into a few categories related to the source. Here, we propose the following, which is intended only as a rough guide to organize the complex set of variables that are, in fact, interrelated: ecological field measurements relevant to both vectors and hosts, laboratory experiments to investigate single-system and multi-system (i.e. one or more components of pathogen, vector and host) effects and rates, economic variables (e.g. land use, economic indices), sociological (e.g. education level, human movement, transportation), medical (e.g. reporting, vaccination, hospital availability) and political (e.g. border issues).
In the context of VBDs and climate/climate change, there are certain well-defined measurement and data needs that are still lacking or require replication because of inherent technical difficulties in their acquisition; these include good knowledge of basic population dynamic parameters (such as population densities) and the number of intermediate and alternative hosts, vector competence for the main vector species involved in disease transmission (including all component parameters in the definition of competence), efficacy and cost of prevention measures (e.g. bednets/vaccines, prophylactics, vector repellents), meteorological indices and associated temporal patterns, habitat fragmentation changes over time, latency and cross-immune time spans in diseases that involve multiple pathogen strains and the impact of health metrics on disease progression in vectors and hosts. Other examples are also discussed in §2a,b.
5. Potential climate risk assessment framework for assessing vector-borne diseases risk
As our understanding of the climate system and ability to model future scenarios of climate change have improved and as society has become increasingly aware of the costs and benefits of using climate information to better manage climate-related risks, there has been a rapid increase in demand for climate data, future projections and assessment tools to enable appropriate climate risk management decisions to be made. As discussed above, the characteristics and behaviour of VBDs typically vary across space and time and among species. They are influenced by multiple direct and indirect forcings and complex interactions with the environment, pathogen and host. Given such complexity, it is clear that climate is only one of many influences on such systems. Thus, in order to manage the risks that VBDs pose to humans as a result of climate change, it is important to assess the potential impacts within the wider context of the current risks of VBDs on humans, key (climate and other) drivers and interactions affecting VBDs in humans and potential adaptation and decision support options to reduce and manage the risks [178].
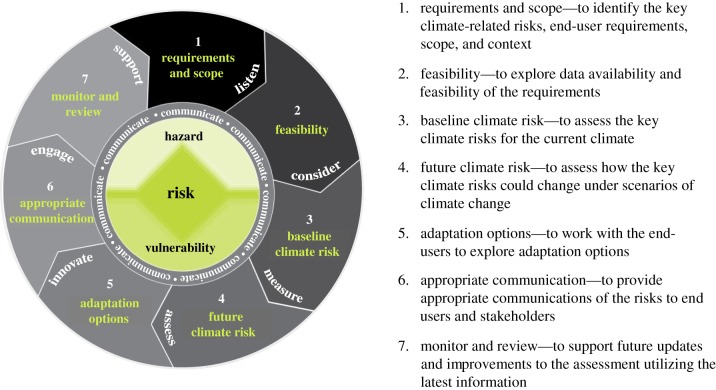
A variety of conceptual frameworks have been developed to guide the assessment of climate change risks for a range of applications [179–182]. Many of these are generic and flexible enough to be applied to climate change and VBD risks in humans. Here, we highlight one particular framework, namely the Climate Impacts and Risk assessment Framework (CIRF; http://www.metoffice.gov.uk/publicsector/hazardmanager/CIRF) and demonstrate its potential application to guide the systematic assessment of VBD risks. The CIRF (figure 1) is a seven-step iterative process used by the UK Meteorological Office to guide the assessment and management of weather- and climate-related risks. Important features of the CIRF are the looped structure to encourage continuous cycles of improvement and the emphasis on frequent communication among researchers, intermediaries and end-users throughout each of the seven steps. In the CIRF, risk is considered to be a combination of multiple hazards (typically environmental factors, e.g. climate) and vulnerabilities (typically exposure or social factors/age). A brief overview of the key features and relevant guidance for each of the steps is provided below.
Figure 1.
Schematic of the climate impacts and risk assessment framework (CIRF). (Online version in colour.)
(a). Step 1: requirements and scope
At the outset of any climate-related risk assessment, it is important to understand the requirements, scope and context for the assessment. This typically involves a literature review to summarize current understanding and communications with various information providers (such as scientists and/or communication experts) and information users (such as end-users and stakeholders). Early engagement of end-users and stakeholders involved in the management of risks identified is vital in order to comprehend their requirements, understand the scope of the assessment and encourage best use of the outputs and recommendations. This often requires specialists in communication [183], employing various methods, including surveys, interviews, workshops, focus groups, working groups, presentations, displays and online feedback [184].
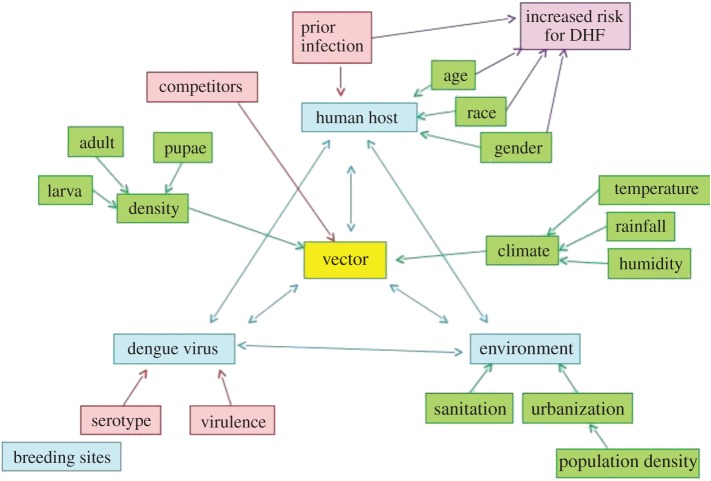
At the end of this step, those involved in the assessment should have developed a broad understanding of the key weather- and climate-related risks involved and the main drivers and interactions influencing those risks. Constructing a system diagram is one useful way to identify the drivers and interactions involved in a specific risk. Figure 2 illustrates one such diagram, focusing on the risk of DENV transmission in humans across the Eastern Mediterranean and Middle East (EMME) region. This is constructed around the disease triangle (host, pathogen, environment; [185]), including the vector, and identifies the main drivers and influences affecting the suitability of the environment, potential for transmission and susceptibility of the human host to DENV.
Figure 2.
System diagram showing the key requirements for understanding the risk of dengue virus transmission in humans across the Eastern Mediterranean and Middle East (EMME) region (pink) and the linkages between drivers, hosts and potential indicators for monitoring. DHF, Dengue haemorrhagic fever. (Online version in colour.)
Many of the key influences and interactions affecting VBDs of humans are highlighted earlier in this review, together with the complexities involved in considering how interactions between climate change, biological, economic and social factors occur and may combine to influence future VBD transmission patterns [178,186–189].
(b). Step 2: feasibility
This step involves scoping of the datasets, models, techniques and communications that would be needed to meet the requirements (detailed in Step 1). The main aim here is to assess if the specified requirements can be met with available data, knowledge and technology. This step also provides opportunity for major uncertainties, caveats, assumptions and gaps in knowledge and data to be identified and discussed with all end-users, stakeholders and information/data providers. In terms of VBD risk, many examples exist of background on gaps, uncertainties and emerging areas of understanding on risk from a range of VBDs that would be useful for this step [187,188,190–192]. Sections 2 and 4 consider appropriate metrics for monitoring VBD risk, considerations regarding appropriate spatial scales (see also Proestos et al. [193]) and limitations in modelling risk, whereas §3 also provides a useful overview on the advantages and limitations of different modelling paradigms for assessing VBD risk.
(c). Step 3: baseline climate risk
Prior to assessing the risks associated with future climate change, it is necessary to provide a baseline of the current risks. This may involve quantitative assessment and validation using historic records, appropriate models (see §3) and ensemble techniques for quantifying uncertainty and/or qualitative assessment based on, for example, expert elicitation [194]. For VBD risk, a wide range of models (statistical and mathematical), incorporating various environmental and other constraints, are available for this purpose. For example, prior to exploring future climate change projections of dengue risk, Rogers [189] uses disease and vector databases, together with a relatively high-resolution historic dataset of monthly climate variables, to provide baseline global climate risk maps for dengue, exploring uncertainties through use of multiple dengue or vector species models and spatial resolution considerations.
(d). Step 4: future climate risk
Future potential climate risks should be assessed relative to the baseline risk in Step 3 using comparable metrics, models and uncertainty measures (where appropriate). Such an assessment requires suitable ‘what-if’ scenarios of future potential changes in climate and other key drivers of risk, such as population and land-use changes. These may be tailored for the specific requirement (e.g. to study the implications of a specific adaptation option; see Step 5) or, more typically, standard scenarios of greenhouse gas and socio-economic changes in the future, such as the special report on emissions scenarios (SRES) [106] or representative concentration pathways [195], are used to drive global climate or earth system models.
In this issue, Rogers [189] uses climate outputs from a single global climate model, HadCM3, driven by a range of SRES emissions scenarios, as climate determinants for estimates of future dengue risks, whereas Levi et al. [196] consider how climate warming may affect the phenology of ticks and Campbell et al. [197] analyse how the distribution of two key disease vectors of dengue and chikungunya may be affected by potential climatic changes. As spatial resolution can be an important influence of VBD risk estimates, studies [187,192,193] have, for example, used climate data from relatively high-resolution regional climate models for their various VBD risk projections.
(e). Step 5: adaptation options
Decision support for end-users and other stakeholders is a key feature of risk management and integral to the CIRF. This step is intended to further focus the results of the climate risk assessments, possibly through specific analyses of adaptation scenarios in Step 5, in order to help end-users evaluate the costs and benefits of potential adaptation options and facilitate practical decision-making.
(f). Step 6: appropriate communication
Communicating and disseminating risk information can be very challenging. Although communications along the ‘climate information chains' between information providers and end-users are important to avoid misunderstandings and respond to changing needs throughout the CIRF, this step provides a focus for considering what form of communicating climate risk information would be most appropriate for the requirements (identified in Step 1). This may take the form of short-term (minutes to seasons) early-warning systems (via web, television, radio, text or email alerts) and real-time information updates, or longer-term baseline and future potential vulnerability, hazard or combined risk maps [178,189]. Both short- and longer-term communications are useful to help manage VBD risks. For example, decision-makers should be encouraged to include risk maps for mosquito vectors within their strategies, as these would provide useful guidance for managing the potential for vector establishment, proliferation and potential activity periods.
(g). Step 7: monitor and review
The final step in the CIRF encourages ongoing monitoring and reviewing of procedures, enabling continual improvements to be made based on the most up-to-date data, tools and techniques. The importance of monitoring and appropriate communication tools for managing VBD risk is highlighted by the WHO and partner agencies through one of their key programme aims, namely to promote climate risk management through improved surveillance, meteorologically informed early warning systems and spatial risk mapping [178].
The iterative risk management framework outlined here provides us a flexible structure to guide the assessment of risks in a logical order, but where steps may be omitted if irrelevant to the specific focus. It is well suited to address the calls made by Hoberg & Brooks [198] for more proactive and evolutionary risk management of emerging infectious diseases and may be combined with more responsive disaster risk management activities to coordinate both reactive and proactive approaches to managing VBD and other interacting risks.
6. Conclusion
The next generation of risk assessment methods should ideally take into account the complexity of VBD transmission dynamics, including the effects of broader societal contexts in which pathogen transmission occurs, if more reliable evaluations of the effects of climate change on VBDs are to be undertaken. Although mathematical models can provide us with powerful tools for incorporating and examining the impact of linked biological and societal variables on transmission as a result of climate change, several challenges need to first be overcome.
The first regards the mismatch of spatial scales of available climate prediction and socio-economic data with the more locally scaled ecological and biological variables underlying the typically focal transmission of VBDs [1,4,17,35,199]. Although regional climate models may overcome some of the problems connected with scale [200], it is possible that there is a lower limit to the spatial resolution of reliable climate and social data (typically available down to only district-level scales in most countries) available for analysis, which will limit the application of models at finer resolutions. A key need is therefore estimation of the error that such data aggregation will induce into model predictions and what this implies for the use of dynamic modelling approaches for evaluating the impacts of future climate change on transmission. This also includes identifying the most appropriate spatial resolution for applying such frameworks. Currently, disease modelling is still largely based on single interacting species frameworks, which ignore the complex interdependence between all diseases observed within a population at a certain time. In addition, while recent progress has been made in more realistically quantifying and modelling the effects of climate (for example, fluctuating daily temperatures) on pathogen transmission, extensive research gaps remain in our understanding of the effect of climate on the interactions between VBDs and their invertebrate hosts. This is particularly true for tick-borne diseases. Existing research has demonstrated that these interactions are complex, often nonlinear and vector–pathogen specific, highlighting the need for further empirical work in order to improve the parametrization of VBD transmission models.
Thus, a second need is to develop models that take account of multiple co-occurring diseases and how climate change may affect co-transmission patterns [17]. While several workers have developed models of VBD transmission via several vector hosts and have shown how ecosystem change-induced alterations to vector biodiversity may influence pathogen transmission [201–203], more direct coupling of climate variables with vector population dynamics, as well as with within-host interactions with other infections, is yet to be systematically undertaken.
A third major need is for more information on how best to address cross-scale issues. Here, exploration of the concept of panarchy and how such ecological thinking and methods (which focus on how fast and slow, small and big events and processes across regions may transform socio-ecological systems through evolution, adaptation and societal learning [204,205]) can be incorporated into disease modelling frameworks will prove illuminating and potentially transformative.
Finally, as always, there is a major need to gain a better understanding of the pathways and functional forms through which human activities, particularly deforestation, road building, transportation, urbanization, irrigation, dam building and agricultural extensions and culture, can impact disease transmission processes. This knowledge will be crucial to the development of better models describing how societal change may accentuate or dampen the effects of climate change on VBD transmission. Such activities must also include the effects of public health interventions, eco-evolutionary response by pathogens, vectors and hosts to climate- and human-induced ecological change and the impact of human reflexivity in response to perceived or predicted threats and risks. These challenges may appear daunting, but we suggest that with increased computational power, advances in climate modelling, the development of new ecological theories of cross-scaled climate-dependent dynamics and increased publication of multi-sectoral data on human economic and public health interventions, these difficulties may be overcome and the next generation of modelling frameworks will be able to make considerable advances on this important global issue.
Acknowledgements
P.E.P. thanks the Centre for Health Economics and Medicines Evaluation at Bangor University for funding during the early phases of developing this paper.
Authors' contributions
P.E.P. led the design, coordination and management of this paper, as well as writing the first draft. All authors made substantial contributions to the conception, design and analysis of content in individual sections, with the contributions of P.E.P., F.A., K.J.E., N.F., H.G., A.G., S.L.D., S.L., R.E.M., E.N.N., R.O., P.D.R., M.B.T. and J.V.-H. arising as the result of discussions at the first meeting of the National Institute for Mathematical and Biological Synthesis Working Group on ‘Climate Change and VBDs' from 3 to 5 December 2013 in Knoxville, Tennessee. All authors provided edits for intellectual content during the development and drafting phases of this manuscript. All authors gave final approval for publication.
Funding statement
D.H.'s contribution was supported by the Joint UK DECC/Defra Met Office Hadley Centre Climate Programme (GA01101). Some of the authors (P.E.P., F.A., K.J.E., N.F., H.G., A.G., S.L.D., S.L., R.E.M., E.N.N., R.O., P.D.R., M.B.T. and J.V.-H.) are grateful to the National Institute for Mathematical and Biological Synthesis (NIMBioS) for funding the Working Group on ‘Climate Change and Vector-borne Diseases’. NIMBioS is an Institute sponsored by the National Science Foundation, the U.S. Department of Homeland Security, and the U.S. Department of Agriculture through NSF Award #EF-0832858, with additional support from The University of Tennessee, Knoxville. Some of this work was performed at the U.S. Department of Homeland Security CCICADA Center at Rutgers University and at Oak Ridge National Laboratory, which is supported by the Office of Science of the U.S. Department of Energy under Contract No. DE-AC05-00OR22725. E.M. acknowledges the support of the Eck Institute for Global Health, University of Notre Dame for funding to develop a portion of this work.
Competing interests
We have no competing interests.
References
- 1.Lafferty KD. 2009. The ecology of climate change and infectious diseases. Ecology 90, 888–900. ( 10.1890/08-0079.1) [DOI] [PubMed] [Google Scholar]
- 2.Rohr JR, Dobson AP, Johnson PT, Kilpatrick AM, Paull SH, Raffel TR, Ruiz-Moreno D, Thomas MB. 2011. Frontiers in climate change—disease research. Trends Ecol. Evol. 26, 270–277. ( 10.1016/j.tree.2011.03.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sutherst RW. 2004. Global change and human vulnerability to vector-borne diseases. Clin. Microbiol. Rev. 17, 136–173. ( 10.1128/CMR.17.1.136-173.2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tabachnick WJ. 2010. Challenges in predicting climate and environmental effects on vector-borne disease episystems in a changing world. J. Exp. Biol. 213, 946–954. ( 10.1242/jeb.037564) [DOI] [PubMed] [Google Scholar]
- 5.Rodó X, et al. 2013. Climate change and infectious diseases: can we meet the needs for better prediction? Clim. Change 118, 625–640. ( 10.1007/s10584-013-0744-1) [DOI] [Google Scholar]
- 6.Garms R, Walsh JF, Davies JB. 1979. Studies on the reinvasion of the onchocerciasis control programme in the Volta River Basin by Simulium damnosum s.I. with emphasis on the south-western areas. Tropenmed. Parasitol. 30, 345–362. [PubMed] [Google Scholar]
- 7.Paaijmans KP, Imbahale SS, Thomas MB, Takken W. 2010. Relevant microclimate for determining the development rate of malaria mosquitoes and possible implications of climate change. Malar. J. 9, 196 ( 10.1186/1475-2875-9-196) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parham PE, Michael E. 2010. Modelling climate change and malaria transmission. Adv. Exp. Med. Biol. 673, 184–199. ( 10.1007/978-1-4419-6064-1_13) [DOI] [PubMed] [Google Scholar]
- 9.Parham PE, Michael E. 2010. Modeling the effects of weather and climate change on malaria transmission. Environ. Health Perspect. 118, 620–626. ( 10.1289/ehp.0901256) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang HM, Macoris ML, Galvani KC, Andrighetti MT, Wanderley DM. 2009. Assessing the effects of temperature on dengue transmission. Epidemiol. Infect. 137, 1179–1187. ( 10.1017/S0950268809002052) [DOI] [PubMed] [Google Scholar]
- 11.Yang HM, Macoris ML, Galvani KC, Andrighetti MT, Wanderley DM. 2009. Assessing the effects of temperature on the population of Aedes aegypti, the vector of dengue. Epidemiol. Infect. 137, 1188–1202. ( 10.1017/S0950268809002040) [DOI] [PubMed] [Google Scholar]
- 12.Martens WJ. 1998. Health impacts of climate change and ozone depletion: an ecoepidemiologic modeling approach. Environ. Health Perspect. 106(Suppl. 1), 241–251. ( 10.1289/ehp.98106s1241) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoshen MB, Morse AP. 2004. A weather-driven model of malaria transmission. Malar. J. 3, 32 ( 10.1186/1475-2875-3-32) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bezirtzoglou C, Dekas K, Charvalos E. 2011. Climate changes, environment and infection: facts, scenarios and growing awareness from the public health community within Europe. Anaerobe 17, 337–340. ( 10.1016/j.anaerobe.2011.05.016) [DOI] [PubMed] [Google Scholar]
- 15.Brisbois BW, Ali SH. 2010. Climate change, vector-borne disease and interdisciplinary research: social science perspectives on an environment and health controversy. Ecohealth 7, 425–438. ( 10.1007/s10393-010-0354-6) [DOI] [PubMed] [Google Scholar]
- 16.Egbendewe-Mondzozo A, Musumba M, McCarl BA, Wu X. 2011. Climate change and vector-borne diseases: an economic impact analysis of malaria in Africa. Int. J. Environ. Res. Public Health 8, 913–930. ( 10.3390/ijerph8030913) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Froment A. 2009. Biodiversity and health: the place of parasitic and infectious diseases. In Biodiversity change and human health (eds Sala Osvaldo E., Meyerson Laura A., Parmesan Camille.), pp. 211–227. Washington, DC: Island Press. [Google Scholar]
- 18.Hay SI, Guerra CA, Tatem AJ, Atkinson PM, Snow RW. 2005. Urbanization, malaria transmission and disease burden in Africa. Nat. Rev. Microbiol. 3, 81–90. ( 10.1038/nrmicro1069) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martens WJ, Slooff R, Jackson EK. 1997. Climate change, human health, and sustainable development. Bull. World Health Organ. 75, 583–588. [PMC free article] [PubMed] [Google Scholar]
- 20.Mills JN, Gage KL, Khan AS. 2010. Potential influence of climate change on vector-borne and zoonotic diseases: a review and proposed research plan. Environ. Health Perspect. 118, 1507–1514. ( 10.1289/ehp.0901389) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qi Q, Guerra CA, Moyes CL, Elyazar IR, Gething PW, Hay SI, Tatem AJ. 2012. The effects of urbanization on global Plasmodium vivax malaria transmission. Malar. J. 11, 403 ( 10.1186/1475-2875-11-403) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reiter P. 2001. Climate change and mosquito-borne disease. Environ. Health Perspect. 109(Suppl. 1), 141–161. ( 10.2307/3434853) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tatem AJ, Gething PW, Smith DL, Hay SI. 2013. Urbanization and the global malaria recession. Malar. J. 12, 133 ( 10.1186/1475-2875-12-133) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tusting LS, Willey B, Lucas H, Thompson J, Kafy HT, Smith R, Lindsay SW. 2013. Socioeconomic development as an intervention against malaria: a systematic review and meta-analysis. Lancet 382, 963–972. ( 10.1016/S0140-6736(13)60851-X) [DOI] [PubMed] [Google Scholar]
- 25.Yang HM, Ferreira MU. 2000. Assessing the effects of global warming and local social and economic conditions on the malaria transmission. Rev. Saude Publ. 34, 214–222. ( 10.1590/S0034-89102000000300002) [DOI] [PubMed] [Google Scholar]
- 26.Martens P, Moser SC. 2001. Health impacts of climate change. Science 292, 1065–1066. ( 10.1126/science.292.5519.1065) [DOI] [PubMed] [Google Scholar]
- 27.McMichael AJ, Woodruff RE. 2005. Detecting the health effects of environmental change: scientific and political challenge. Ecohealth 2, 1–3. ( 10.1007/s10393-004-0152-0) [DOI] [Google Scholar]
- 28.Casman EA, Dowlatabadi H. (eds). 2002. The contextual determinants of malaria. Resources for the future. Washington, DC: Routledge. [Google Scholar]
- 29.Tol RS, Dowlatabadi H. 2001. Vector-borne diseases, development and climate change. Integr. Assess. 2, 173–181. ( 10.1023/A:1013390516078) [DOI] [Google Scholar]
- 30.Lafferty KD. 2009. Calling for an ecological approach to studying climate change and infectious diseases. Ecology 90, 932–933. ( 10.1890/08-1767.1) [DOI] [PubMed] [Google Scholar]
- 31.Funtowicz SO, Ravetz JR. 1992. Three types of risk assessment and the emergence of post-normal science. In Social theories of risk (eds Krimsky Sheldon, Golding Dominic.), pp. 251–274. Wesport, CT: Praeger Publishers. [Google Scholar]
- 32.Funtowicz SO, Ravetz JR. 1994. Uncertainty, complexity and post-normal science. Environ. Toxicol. Chem. 13, 1881–1885. ( 10.1002/etc.5620131203) [DOI] [Google Scholar]
- 33.Horwitz P, Wilcox BA. 2005. Parasites, ecosystems and sustainability: an ecological and complex systems perspective. Int. J. Parasitol. 35, 725–732. ( 10.1016/j.ijpara.2005.03.002) [DOI] [PubMed] [Google Scholar]
- 34.Wilcox BA, Gubler DJ. 2005. Disease ecology and the global emergence of zoonotic pathogens. Environ. Health Prev. Med. 10, 263–272. ( 10.1007/BF02897701) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cumming G. 2005. Ecology in global scenarios. Ecosyst. Hum. Well-being 45, 45–70. [Google Scholar]
- 36.Reiter P. 2008. Climate change and mosquito-borne disease: knowing the horse before hitching the cart. Rev. Sci. Tech. 27, 383–398. [PubMed] [Google Scholar]
- 37.Soskolne CL, Broemling N. 2002. Eco-epidemiology: on the need to measure health effects from global change. Glob. Change Hum. Health 3, 58–66. ( 10.1023/A:1019692414126) [DOI] [Google Scholar]
- 38.Xun WW, Khan AE, Michael E, Vineis P. 2010. Climate change epidemiology: methodological challenges. Int. J. Public Health 55, 85–96. ( 10.1007/s00038-009-0091-1) [DOI] [PubMed] [Google Scholar]
- 39.Adger WN. 2006. Vulnerability. Glob. Environ. Change 16, 268–281. ( 10.1016/j.gloenvcha.2006.02.006) [DOI] [Google Scholar]
- 40.Wu S, Pan T, He S. 2012. Climate change risk research: a case study on flood disaster risk in China. Adv. Clim. Change Res. 3, 92–98. ( 10.3724/SP.J.1248.2012.00092) [DOI] [Google Scholar]
- 41.Turner BL, et al. 2003. A framework for vulnerability analysis in sustainability science. Proc. Natl Acad. Sci. USA 100, 8074–8079. ( 10.1073/pnas.1231335100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Beven KJ. 2004. Towards environmental models of everywhere: advances in modelling and data assimilation. Hydrology: science and practice for the 21st century. In Proc. British Hydrological Society Int. Conf. Imperial College, London, July 2004 (eds Webb B, Arnell N, Onof C, McIntyre N, Gurney R, Kirby C.), pp. 244–250. London, UK: British Hydrological Society. [Google Scholar]
- 43.Bomblies A, Duchemin JB, Eltahir EA. 2009. A mechanistic approach for accurate simulation of village scale malaria transmission. Malar. J. 8, 223 ( 10.1186/1475-2875-8-223) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bouden M, Moulin B, Gosselin P. 2008. The geosimulation of West Nile virus propagation: a multi-agent and climate sensitive tool for risk management in public health. Int. J. Health Geogr. 7, 35 ( 10.1186/1476-072X-7-35) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chaves LF, Kaneko A, Pascual M. 2009. Random, top-down, or bottom-up coexistence of parasites: malaria population dynamics in multi-parasitic settings. Ecology 90, 2414–2425. ( 10.1890/08-1022.1) [DOI] [PubMed] [Google Scholar]
- 46.Chikaki E, Ishikawa H. 2009. A dengue transmission model in Thailand considering sequential infections with all four serotypes. J. Infect. Dev. Ctries. 3, 711–722. ( 10.3855/jidc.616) [DOI] [PubMed] [Google Scholar]
- 47.Chiyaka C, Garira W, Dube S. 2009. Effects of treatment and drug resistance on the transmission dynamics of malaria in endemic areas. Theor. Popul. Biol. 75, 14–29. ( 10.1016/j.tpb.2008.10.002) [DOI] [PubMed] [Google Scholar]
- 48.Coutinho FA, Burattini MN, Lopez LF, Massad E. 2006. Threshold conditions for a non-autonomous epidemic system describing the population dynamics of dengue. Bull. Math. Biol. 68, 2263–2282. ( 10.1007/s11538-006-9108-6) [DOI] [PubMed] [Google Scholar]
- 49.Cruz-Pacheco G, Esteva L, Montano-Hirose JA, Vargas C. 2005. Modelling the dynamics of West Nile virus. Bull. Math. Biol. 67, 1157–1172. ( 10.1016/j.bulm.2004.11.008) [DOI] [PubMed] [Google Scholar]
- 50.de Castro Medeiros LC, Castilho CA, Braga C, de Souza WV, Regis L, Monteiro AM. 2011. Modeling the dynamic transmission of dengue fever: investigating disease persistence. PLoS. Negl. Trop. Dis. 5, e942 ( 10.1371/journal.pntd.0000942) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gu W, Killeen GF, Mbogo CM, Regens JL, Githure JI, Beier JC. 2003. An individual-based model of Plasmodium falciparum malaria transmission on the coast of Kenya. Trans. R. Soc. Trop. Med. Hyg. 97, 43–50. ( 10.1016/S0035-9203(03)90018-6) [DOI] [PubMed] [Google Scholar]
- 52.Morin CW, Comrie AC. 2010. Modeled response of the West Nile virus vector Culex quinquefasciatus to changing climate using the dynamic mosquito simulation model. Int. J. Biometeorol. 54, 517–529. ( 10.1007/s00484-010-0349-6) [DOI] [PubMed] [Google Scholar]
- 53.Otero M, Schweigmann N, Solari HG. 2008. A stochastic spatial dynamical model for Aedes aegypti. Bull. Math. Biol. 70, 1297–1325. ( 10.1007/s11538-008-9300-y) [DOI] [PubMed] [Google Scholar]
- 54.Otero M, Solari HG. 2010. Stochastic eco-epidemiological model of dengue disease transmission by Aedes aegypti mosquito. Math. Biosci. 223, 32–46. ( 10.1016/j.mbs.2009.10.005) [DOI] [PubMed] [Google Scholar]
- 55.Pinho STR, Ferreira CP, Esteva L, Barreto FR, Morato e Silva VC, Teixeira MGL. 2010. Modelling the dynamics of dengue real epidemics. Phil. Trans. R. Soc. A 368, 5679–5693. ( 10.1098/rsta.2010.0278) [DOI] [PubMed] [Google Scholar]
- 56.Ruiz D, Poveda G, Velez ID, Quinones ML, Rua GL, Velasquez LE, Zuluaga JS. 2006. Modelling entomological–climatic interactions of Plasmodium falciparum malaria transmission in two Colombian endemic–regions: contributions to a national malaria early warning system. Malar. J. 5, 66 ( 10.1186/1475-2875-5-66) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schaeffer B, Mondet B, Touzeau S. 2008. Using a climate-dependent model to predict mosquito abundance: application to Aedes (Stegomyia) africanus and Aedes (Diceromyia) furcifer (Diptera: Culicidae). Infect. Genet. Evol. 8, 422–432. ( 10.1016/j.meegid.2007.07.002) [DOI] [PubMed] [Google Scholar]
- 58.Ye Y, Hoshen M, Kyobutungi C, Louis VR, Sauerborn R. 2009. Local scale prediction of Plasmodium falciparum malaria transmission in an endemic region using temperature and rainfall. Glob. Health Action 2 ( 10.3402/gha.v2i0.1923) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Laporta GZ, Lopez de Prado PI, Kraenkel RA, Coutinho RM, Sallum MA. 2013. Biodiversity can help prevent malaria outbreaks in tropical forests. PLoS Negl. Trop. Dis. 7, e2139 ( 10.1371/journal.pntd.0002139) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dobson A. 2009. Climate variability, global change, immunity, and the dynamics of infectious diseases. Ecology 90, 920–927. ( 10.1890/08-0736.1) [DOI] [PubMed] [Google Scholar]
- 61.Eggo RM, Cauchemez S, Ferguson NM. 2011. Spatial dynamics of the 1918 influenza pandemic in England, Wales and the United States. J. R. Soc. Interface 8, 233–243. ( 10.1098/rsif.2010.0216) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bonds MH, Keenan DC, Rohani P, Sachs JD. 2010. Poverty trap formed by the ecology of infectious diseases. Proc. R. Soc. B 277, 1185–1192. ( 10.1098/rspb.2009.1778) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.LaDeau SL, Glass GE, Hobbs NT, Latimer A, Ostfeld RS. 2011. Data-model fusion to better understand emerging pathogens and improve infectious disease forecasting. Ecol. Appl. 21, 1443–1460. ( 10.1890/09-1409.1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Luo Y, Ogle K, Tucker C, Fei S, Gao C, LaDeau S, Clark JS, Schimel DS. 2011. Ecological forecasting and data assimilation in a data-rich era. Ecol. Appl. 21, 1429–1442. ( 10.1890/09-1275.1) [DOI] [PubMed] [Google Scholar]
- 65.Bankes SC. 2002. Agent-based modeling: a revolution? Proc. Natl Acad. Sci. USA 99(Suppl. 3), 7199–7200. ( 10.1073/pnas.072081299) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gao C, Wang H, Weng E, Lakshmivarahan S, Zhang Y, Luo Y. 2011. Assimilation of multiple data sets with the ensemble Kalman filter to improve forecasts of forest carbon dynamics. Ecol. Appl. 21, 1461–1473. ( 10.1890/09-1234.1) [DOI] [PubMed] [Google Scholar]
- 67.Ruiz D, Brun C, Connor SJ, Omumbo JA, Lyon B, Thomson MC. 2014. Testing a multi-malaria-model ensemble against 30 years of data in the Kenyan highlands. Malar. J. 13, 206 ( 10.1186/1475-2875-13-206) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Smith T, Ross A, Maire N, Chitnis N, Studer A, Hardy D, Brooks A, Penny M, Tanner M. 2012. Ensemble modeling of the likely public health impact of a pre-erythrocytic malaria vaccine. PLoS Med. 9, e1001157 ( 10.1371/journal.pmed.1001157) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vancini R, Wang G, Ferreira D, Hernandez R, Brown DT. 2013. Alphavirus genome delivery occurs directly at the plasma membrane in a time- and temperature-dependent process. J. Virol. 87, 4352–4359. ( 10.1128/JVI.03412-12) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Turell MJ, Rossi CA, Bailey CL. 1985. Effect of extrinsic incubation temperature on the ability of Aedes taeniorhynchus and Culex pipiens to transmit rift valley fever virus. Am. J. Trop. Med. Hyg. 34, 1211–1218. [DOI] [PubMed] [Google Scholar]
- 71.Adelman ZN, et al. 2013. Cooler temperatures destabilize RNA interference and increase susceptibility of disease vector mosquitoes to viral infection. PLoS Negl. Trop. Dis. 7, e2239 ( 10.1371/journal.pntd.0002239) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Westbrook CJ, Reiskind MH, Pesko KN, Greene KE, Lounibos LP. 2010. Larval environmental temperature and the susceptibility of Aedes albopictus Skuse (Diptera: Culicidae) to chikungunya virus. Vector Borne Zoonotic Dis. 10, 241–247. ( 10.1089/vbz.2009.0035) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Watts DM, Burke DS, Harrison BA, Whitmire RE, Nisalak A. 1987. Effect of temperature on the vector efficiency of Aedes aegypti for dengue 2 virus. Am. J. Trop. Med. Hyg. 36, 143–152. [DOI] [PubMed] [Google Scholar]
- 74.Rohani A, Wong YC, Zamre I, Lee HL, Zurainee MN. 2009. The effect of extrinsic incubation temperature on development of dengue serotype 2 and 4 viruses in Aedes aegypti (L.). Southeast Asian J. Trop. Med. Public Health 40, 942–950. [PubMed] [Google Scholar]
- 75.Halstead SB. 2008. Dengue virus–mosquito interactions. Annu. Rev. Entomol. 53, 273–291. ( 10.1146/annurev.ento.53.103106.093326) [DOI] [PubMed] [Google Scholar]
- 76.Barbazan P, Guiserix M, Boonyuan W, Tuntaprasart W, Pontier D, Gonzalez JP. 2010. Modelling the effect of temperature on transmission of dengue. Med. Vet. Entomol. 24, 66–73. ( 10.1111/j.1365-2915.2009.00848.x) [DOI] [PubMed] [Google Scholar]
- 77.Carrington LB, Lambrechts L, Scott TW. 2013. Fluctuations at a low mean temperature accelerate dengue virus transmission by Aedes aegypti. PLoS Negl. Trop. Dis. 7, e2190 ( 10.1371/journal.pntd.0002190) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lambrechts L, Paaijmans KP, Fansiri T, Carrington LB, Kramer LD, Thomas MB, Scott TW. 2011. Impact of daily temperature fluctuations on dengue virus transmission by Aedes aegypti. Proc. Natl Acad. Sci. USA 108, 7460–7465. ( 10.1073/pnas.1101377108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Reisen WK, Fang Y, Martinez VM. 2006. Effects of temperature on the transmission of west nile virus by Culex tarsalis (Diptera: Culicidae). J. Med. Entomol. 43, 309–317. ( 10.1603/0022-2585(2006)043[0309:EOTOTT]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 80.Muturi EJ, Alto BW. 2011. Larval environmental temperature and insecticide exposure alter Aedes aegypti competence for arboviruses. Vector Borne Zoonotic Dis. 11, 1157–1163. ( 10.1089/vbz.2010.0209) [DOI] [PubMed] [Google Scholar]
- 81.Sinden RE, et al. 2007. Progression of Plasmodium berghei through Anopheles stephensi is density-dependent. PLoS Pathog. 3, e195 ( 10.1371/journal.ppat.0030195) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Boyd MF. 1949. Epidemiology: factors related to the definitive host. In Malariology: a comprehensive survey of all aspects of this group of diseases from a global standpoint (ed. Boyd MF.), pp. 608–697. Philadelphia, PA: W.B. Saunders Company. [Google Scholar]
- 83.Macdonald G. 1957. The epidemiology and control of malaria. London, UK: Oxford University Press. [Google Scholar]
- 84.Dentinova TS. 1962. Age-grouping methods in diptera of medical importance. Geneva, Switzerland: World Health Organisation. [PubMed] [Google Scholar]
- 85.Paaijmans KP, Blanford S, Bell AS, Blanford JI, Read AF, Thomas MB. 2010. Influence of climate on malaria transmission depends on daily temperature variation. Proc. Natl Acad. Sci. USA 107, 15 135–15 359. ( 10.1073/pnas.1006422107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Paaijmans KP, Read AF, Matthew TB. 2009. Understanding the link between malaria risk and climate. Proc. Natl Acad. Sci. USA 106, 13 844–13 849. ( 10.1073/pnas.0903423106). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Blanford JI, Blanford S, Crane RG, Mann ME, Paaijmans KP, Schreiber KV, Thomas MB. 2013. Implications of temperature variation for malaria parasite development across Africa. Sci. Rep. 3, 1300 ( 10.1038/srep01300) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhao X, Chen F, Feng Z, Li X, Zhou XH. 2014. Characterizing the effect of temperature fluctuation on the incidence of malaria: an epidemiological study in south-west China using the varying coefficient distributed lag non-linear model. Malar. J. 13, 192 ( 10.1186/1475-2875-13-192) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Paaijmans KP, Blanford S, Chan BH, Thomas MB. 2012. Warmer temperatures reduce the vectorial capacity of malaria mosquitoes. Biol. Lett. 8, 465–468. ( 10.1098/rsbl.2011.1075) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Paaijmans KP, Cator LJ, Thomas MB. 2013. Temperature-dependent pre-bloodmeal period and temperature-driven asynchrony between parasite development and mosquito biting rate reduce malaria transmission intensity. PLoS ONE 8, e55777 ( 10.1371/journal.pone.0055777) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mordecai EA, et al. 2013. Optimal temperature for malaria transmission is dramatically lower than previously predicted. Ecol. Lett. 16, 22–30. ( 10.1111/ele.12015) [DOI] [PubMed] [Google Scholar]
- 92.Murdock CC, Paaijmans KP, Cox-Foster D, Read AF, Thomas MB. 2012. Rethinking vector immunology: the role of environmental temperature in shaping resistance. Nat. Rev. Microbiol. 10, 869–876. ( 10.1038/nrmicro2900) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Suwanchaichinda C, Paskewitz SM. 1998. Effects of larval nutrition, adult body size, and adult temperature on the ability of Anopheles gambiae (Diptera: Culicidae) to melanize sephadex beads. J. Med. Entomol. 35, 157–161. [DOI] [PubMed] [Google Scholar]
- 94.Murdock CC, Paaijmans KP, Bell AS, King JG, Hillyer JF, Read AF, Thomas MB. 2012. Complex effects of temperature on mosquito immune function. Proc. R. Soc. B 279, 3357–3366. ( 10.1098/rspb.2012.0638) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Murdock CC, Blanford S, Luckhart S, Thomas MB. 2014. Ambient temperature and dietary supplementation interact to shape mosquito vector competence for malaria. J. Insect Physiol. 67C, 37–44. ( 10.1016/j.jinsphys.2014.05.020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Crippa M, Rais O, Gern F, Gern L. 2002. Investigations on the mode and dynamics of transmission and infectivity of Borrelia burgdorferi sensu stricto and Borrelia afzelii in Ixodes ricinus ticks. Vector Borne Zoonotic Dis. 2, 3–9. ( 10.1089/153036602760260724) [DOI] [PubMed] [Google Scholar]
- 97.Estrada-Pena A, Ayllon N, de la Fuente J. 2012. Impact of climate trends on tick-borne pathogen transmission. Front. Physiol. 3, 64 ( 10.3389/fphys.2012.00064) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Villar M, et al. 2010. Expression of heat shock and other stress response proteins in ticks and cultured tick cells in response to Anaplasma spp. infection and heat shock. Int. J. Proteomics 2010, 657261 ( 10.1155/2010/657261) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Parola P, Paddock CD, Raoult D. 2005. Tick-borne rickettsioses around the world: emerging diseases challenging old concepts. Clin. Microbiol. Rev. 18, 719–756. ( 10.1128/CMR.18.4.719-756.2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Oteo JA, Portillo A. 2012. Tick-borne rickettsioses in Europe. Ticks Tick Borne Dis 3, 271–278. ( 10.1016/j.ttbdis.2012.10.035) [DOI] [PubMed] [Google Scholar]
- 101.Ready PD. 2013. Biology of phlebotomine sand flies as vectors of disease agents. Annu. Rev. Entomol. 58, 227–250. ( 10.1146/annurev-ento-120811-153557) [DOI] [PubMed] [Google Scholar]
- 102.Rioux JA, Aboulker JP, Lanotte G, Killick-Kendrick R, Martini-Dumas A. 1985. Ecology of leishmaniasis in the south of France. 21. Influence of temperature on the development of Leishmania infantum Nicolle, 1908 in Phlebotomus ariasi Tonnoir 1921. Experimental study]. Ann. Parasitol. Hum. Comp. 60, 221–229. [DOI] [PubMed] [Google Scholar]
- 103.Hlavacova J, Votypka J, Volf P. 2013. The effect of temperature on Leishmania (Kinetoplastida: Trypanosomatidae) development in sand flies. J. Med. Entomol. 50, 955–958. ( 10.1603/ME13053) [DOI] [PubMed] [Google Scholar]
- 104.Gething PW, Smith DL, Patil AP, Tatem AJ, Snow RW, Hay SI. 2010. Climate change and the global malaria recession. Nature 465, 342–345. ( 10.1038/nature09098) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Béguin A, Hales S, Rocklöv J, Åström C, Louis VR, Sauerborn R. 2011. The opposing effects of climate change and socio-economic development on the global distribution of malaria. Glob. Environ. Change 21, 1209–1214. ( 10.1016/j.gloenvcha.2011.06.001) [DOI] [Google Scholar]
- 106.Nakicenovic N, Swart R. 2000. Special report on emissions scenarios (eds Nakicenovic Nebojsa, Swart Robert.), p. 612 Cambridge, UK: Cambridge University Press. [Google Scholar]
- 107.Paaijmans KP, Thomas MB. 2011. Health: wealth versus warming. Nat. Clim. Change 1, 349–350. ( 10.1038/nclimate1234) [DOI] [Google Scholar]
- 108.Freed LA, Cann RL. 2013. Vector movement underlies avian malaria at upper elevation in Hawaii: implications for transmission of human malaria. Parasitol. Res. 112, 3887–3895. ( 10.1007/s00436-013-3578-x) [DOI] [PubMed] [Google Scholar]
- 109.Freed LA, Cann RL, Goff ML, Kuntz WA, Bodner GR. 2005. Increase in avian malaria at upper elevation in Hawai'i. The Condor 107, 753–764. ( 10.1650/7820.1) [DOI] [Google Scholar]
- 110.Walsh J, Molyneux D, Birley M. 1993. Deforestation: effects on vector-borne disease. Parasitology 106, S55–S75. ( 10.1017/S0031182000086121) [DOI] [PubMed] [Google Scholar]
- 111.Singh B, Daneshvar C. 2013. Human infections and detection of Plasmodium knowlesi. Clin. Microbiol. Rev. 26, 165–184. ( 10.1128/CMR.00079-12) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Paaijmans KP, Heinig RL, Seliga RA, Blanford JI, Blanford S, Murdock CC, Thomas MB. 2013. Temperature variation makes ectotherms more sensitive to climate change. Glob. Change Biol. 19, 2373–2380. ( 10.1111/gcb.12240) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Murdock CC, Moller-Jacobs LL, Thomas MB. 2013. Complex environmental drivers of immunity and resistance in malaria mosquitoes. Proc. R. Soc. B 280, 20132030 ( 10.1098/rspb.2013.2030) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mordecai EA. 2012. Soil moisture and fungi affect seed survival in California grassland annual plants. PLoS ONE 7, e39083 ( 10.1371/journal.pone.0039083) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cailly P, Tran A, Balenghien T, L'Ambert G, Toty C, Ezanno P. 2012. A climate-driven abundance model to assess mosquito control strategies. Ecol. Model. 227, 7–17. ( 10.1016/j.ecolmodel.2011.10.027) [DOI] [Google Scholar]
- 116.Hoffmann A, et al. 2011. Successful establishment of Wolbachia in Aedes populations to suppress dengue transmission. Nature 476, 454–457. ( 10.1038/nature10356) [DOI] [PubMed] [Google Scholar]
- 117.Pintureauand B, Bolland P. 2001. A Trichogramma species showing a better adaptation to high temperature than its symbionts. Biocontrol Sci. Technol. 11, 13–20. ( 10.1080/09583150020029691) [DOI] [Google Scholar]
- 118.Cator LJ, George J, Blanford S, Murdock CC, Baker TC, Read AF, Thomas MB. 2013. ‘Manipulation’ without the parasite: altered feeding behaviour of mosquitoes is not dependent on infection with malaria parasites. Proc. R. Soc. B 280, 20130711 ( 10.1098/rspb.2013.0711) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Cator LJ, Lynch PA, Read AF, Thomas MB. 2012. Do malaria parasites manipulate mosquitoes? Trends Parasitol. 28, 466–470. ( 10.1016/j.pt.2012.08.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hay SI, Rogers DJ, Toomer JF, Snow RW. 2000. Annual Plasmodium falciparum entomological inoculation rates (EIR) across Africa: literature survey, internet access and review. Trans. R. Soc. Trop. Med. Hyg. 94, 113–127. ( 10.1016/S0035-9203(00)90246-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sternberg ED, Thomas MB. 2014. Local adaptation to temperature and the implications for vector-borne diseases. Trends Parasitol. 30, 115–122. ( 10.1016/j.pt.2013.12.010) [DOI] [PubMed] [Google Scholar]
- 122.Beck-Johnson LM, Nelson WA, Paaijmans KP, Read AF, Thomas MB, Bjornstad ON. 2013. The effect of temperature on Anopheles mosquito population dynamics and the potential for malaria transmission. PLoS ONE 8, e79276 ( 10.1371/journal.pone.0079276) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Nabout JC, Caetano JM, Ferreira RB, Teixeira IR, de Freitas A, Sueli M. 2012. Using correlative, mechanistic and hybrid niche models to predict the productivity and impact of global climate change on maize crop in Brazil. Natureza & Conservacao 10, 177–183. ( 10.4322/natcon.2012.034) [DOI] [Google Scholar]
- 124.Rogers DJ, Randolph SE. 2000. The global spread of malaria in a future, warmer world. Science 289, 1763–1766. ( 10.1126/science.289.5478.391b) [DOI] [PubMed] [Google Scholar]
- 125.Craig M, Snow R, Le Sueur D. 1999. A climate-based distribution model of malaria transmission in sub-Saharan Africa. Parasitol. Today 15, 105–111. ( 10.1016/S0169-4758(99)01396-4) [DOI] [PubMed] [Google Scholar]
- 126.Sinka ME, et al. 2010. The dominant Anopheles vectors of human malaria in Africa, Europe and the Middle East: occurrence data, distribution maps and bionomic précis. Parasit. Vectors 3, 117 ( 10.1186/1756-3305-3-117) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Goto K, Kumarendran B, Mettananda S, Gunasekara D, Fujii Y, Kaneko S. 2013. Analysis of effects of meteorological factors on dengue incidence in Sri Lanka using time series data. PLoS ONE 8, e63717 ( 10.1371/journal.pone.0063717) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Silal SP, Barnes KI, Kok G, Mabuza A, Little F. 2013. Exploring the seasonality of reported treated malaria cases in Mpumalanga, South Africa. PLoS ONE 8, e76640 ( 10.1371/journal.pone.0076640) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chabot-Couture G, Nigmatulina K, Eckhoff P. 2014. An environmental data set for vector-borne disease modeling and epidemiology. PLoS ONE 9, e94741 ( 10.1371/journal.pone.0094741) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhang X, Zhang T, Young AA, Li X. 2014. Applications and comparisons of four time series models in epidemiological surveillance data. PLoS ONE 9, e88075 ( 10.1371/journal.pone.0088075) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gharbi M, Quenel P, Gustave J, Cassadou S, La Ruche G, Girdary L, Marrama L. 2011. Time series analysis of dengue incidence in Guadeloupe, French West Indies: forecasting models using climate variables as predictors. BMC Infect. Dis. 11, 166 ( 10.1186/1471-2334-11-166) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Briët OJ, Amerasinghe PH, Vounatsou P. 2013. Generalized seasonal autoregressive integrated moving average models for count data with application to malaria time series with low case numbers. PLoS ONE 8, e65761 ( 10.1371/journal.pone.0065761) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hartley DM, Barker CM, Le Menach A, Niu T, Gaff HD, Reisen WK. 2012. Effects of temperature on emergence and seasonality of West Nile virus in California. Am. J. Trop. Med. Hyg. 86, 884–894. ( 10.4269/ajtmh.2012.11-0342) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Paz S, et al. 2013. Permissive summer temperatures of the 2010 European West Nile fever upsurge. PLoS ONE 8, e56398 ( 10.1371/journal.pone.0056398) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Stocker TF, et al. 2013. Climate change 2013: the physical science basis. In Intergovernmental Panel on Climate Change, Working Group I Contribution to the IPCC Fifth Assessment Report (AR5). New York, NY: Cambridge University Press. [Google Scholar]
- 136.Neale RB, Richter J, Park S, Lauritzen PH, Vavrus SJ, Rasch PJ, Zhang M. 2013. The mean climate of the community atmosphere model (CAM4) in forced SST and fully coupled experiments. J. Clim. 26, 5150–5168. ( 10.1175/JCLI-D-12-00236.1) [DOI] [Google Scholar]
- 137.Hewitt H, Copsey D, Culverwell I, Harris C, Hill R, Keen A, McLaren AJ, Hunke EC. 2011. Design and implementation of the infrastructure of HadGEM3: the next-generation Met office climate modelling system. Geosci. Model. Dev. 4, 223–253. ( 10.5194/gmd-4-223-2011) [DOI] [Google Scholar]
- 138.Murphy JM, Sexton DM, Barnett DN, Jones GS, Webb MJ, Collins M, Stainforth DA. 2004. Quantification of modelling uncertainties in a large ensemble of climate change simulations. Nature 430, 768–772. ( 10.1038/nature02771) [DOI] [PubMed] [Google Scholar]
- 139.Palmer T, Shutts G, Hagedorn R, Doblas-Reyes F, Jung T, Leutbecher M. 2005. Representing model uncertainty in weather and climate prediction. Annu. Rev. Earth Planet. Sci. 33, 163–193. ( 10.1146/annurev.earth.33.092203.122552) [DOI] [Google Scholar]
- 140.Penner JE, et al. 1994. Quantifying and minimizing uncertainty of climate forcing by anthropogenic aerosols. Bull. Am. Meteorol. Soc. 75, 375–400. () [DOI] [Google Scholar]
- 141.Taylor M, Tribbia J, Iskandarani M. 1997. The spectral element method for the shallow water equations on the sphere. J. Comput. Phys. 130, 92–108. ( 10.1006/jcph.1996.5554) [DOI] [Google Scholar]
- 142.Jia J, Hill JC, Evans KJ, Fann GI, Taylor MA. 2013. A spectral deferred correction method applied to the shallow water equations on a sphere. Mon. Weather Rev. 141, 3435–3449. ( 10.1175/MWR-D-12-00048.1) [DOI] [Google Scholar]
- 143.Wilks DS. 2008. Effects of stochastic parametrization on conceptual climate models. Phil. Trans. R. Soc. A 366, 2477–2490. ( 10.1098/rsta.2008.0005) [DOI] [PubMed] [Google Scholar]
- 144.Doblas-Reyes FJ, et al. 2013. Initialized near-term regional climate change prediction. Nat. Commun. 4, 1715 ( 10.1038/ncomms2704) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Reiner RC, Jr, et al. 2013. A systematic review of mathematical models of mosquito-borne pathogen transmission: 1970–2010. J. R. Soc. Interface 10, 20120921 ( 10.1098/rsif.2012.0921) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Smith DL, Battle KE, Hay SI, Barker CM, Scott TW, McKenzie FE. 2012. Ross, Macdonald, and a theory for the dynamics and control of mosquito-transmitted pathogens. PLoS Pathog. 8, e1002588 ( 10.1371/journal.ppat.1002588) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Jackson MC, Johansen L, Furlong C, Colson A, Sellers KF. 2010. Modelling the effect of climate change on prevalence of malaria in western Africa. Stat. Neerlandica 64, 388–400. ( 10.1111/j.1467-9574.2010.00453.x) [DOI] [Google Scholar]
- 148.Koenraadt C, Githeko A, Takken W. 2004. The effects of rainfall and evapotranspiration on the temporal dynamics of Anopheles gambiae ss and Anopheles arabiensis in a Kenyan village. Acta Trop. 90, 141–153. ( 10.1016/j.actatropica.2003.11.007) [DOI] [PubMed] [Google Scholar]
- 149.Lou Y, Zhao X. 2010. A climate-based malaria transmission model with structured vector population. SIAM J. Appl. Math. 70, 2023–2044. ( 10.1137/080744438) [DOI] [Google Scholar]
- 150.Martens P, Thomas C. 2005. Climate change and malaria risk: complexity and scaling. Frontis 9, 3–14. [Google Scholar]
- 151.Patz JA, Olson SH. 2006. Malaria risk and temperature: influences from global climate change and local land use practices. Proc. Natl Acad. Sci. USA 103, 5635–5636. ( 10.1073/pnas.0601493103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Hay SI, Cox J, Rogers DJ, Randolph SE, Stern DI, Shanks GD, Myers MF, Snow RW. 2002. Climate change and the resurgence of malaria in the East African highlands. Nature 415, 905–909. ( 10.1038/415905a) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Thomson MC, Mason SJ, Phindela T, Connor SJ. 2005. Use of rainfall and sea surface temperature monitoring for malaria early warning in Botswana. Am. J. Trop. Med. Hyg. 73, 214–221. [PubMed] [Google Scholar]
- 154.Zhou G, Minakawa N, Githeko AK, Yan G. 2004. Association between climate variability and malaria epidemics in the East African highlands. Proc. Natl Acad. Sci. USA 101, 2375–2380. ( 10.1073/pnas.0308714100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Lou Y, Zhao X. 2010. The periodic Ross–Macdonald model with diffusion and advection. Appl. Anal. 89, 1067–1089. ( 10.1080/00036810903437804) [DOI] [Google Scholar]
- 156.Lunde TM, Bayoh MN, Lindtjorn B. 2013. How malaria models relate temperature to malaria transmission. Parasit. Vectors 6, 20 ( 10.1186/1756-3305-6-20) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Pascual M, Ahumada J, Chaves L, Rodo X, Bouma M. 2006. Malaria resurgence in the East African highlands: temperature trends revisited. Proc. Natl Acad. Sci. USA 103, 5829–5834. ( 10.1073/pnas.0508929103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Parham PE, Pople D, Christiansen-Jucht C, Lindsay S, Hinsley W, Michael E. 2012. Modeling the role of environmental variables on the population dynamics of the malaria vector Anopheles gambiae sensu stricto. Malar. J. 11, 271 ( 10.1186/1475-2875-11-271) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Depinay JO, et al. 2004. A simulation model of African Anopheles ecology and population dynamics for the analysis of malaria transmission. Malar. J. 3, 29 ( 10.1186/1475-2875-3-29) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ermert V, Fink AH, Jones AE, Morse AP. 2011. Development of a new version of the Liverpool Malaria Model. I. Refining the parameter settings and mathematical formulation of basic processes based on a literature review. Malar. J. 10, 35 ( 10.1186/1475-2875-10-35) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Hartemink N, Vanwambeke SO, Heesterbeek H, Rogers D, Morley D, Pesson B, Davies C, Mahamdallie S, Ready P. 2011. Integrated mapping of establishment risk for emerging vector-borne infections: a case study of canine leishmaniasis in southwest France. PLoS ONE 6, e20817 ( 10.1371/journal.pone.0020817) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Murray CJ, et al. 2013. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the global burden of disease study 2010. Lancet 380, 2197–2223. ( 10.1016/S0140-6736(12)61689-4) [DOI] [PubMed] [Google Scholar]
- 163.Ebi KL, Hartman J, Chan N, Mcconnell J, Schlesinger M, Weyant J. 2005. Climate suitability for stable malaria transmission in Zimbabwe under different climate change scenarios. Clim. Change 73, 375–393. ( 10.1007/s10584-005-6875-2) [DOI] [Google Scholar]
- 164.Jaenisch T, Patz J. 2002. Assessment of associations between climate and infectious diseases: a comparison of the reports of the intergovernmental panel on climate change (IPCC), the National Research Council (NRC), and United States Global Change Research Program (USGCRP). Glob. Change Hum. Health 3, 67–72. ( 10.1023/A:1019625332705) [DOI] [Google Scholar]
- 165.Ndiaye O, et al. 2001. Variations climatiques et mortalité attribuée au paludisme dans la zone de Niakhar, Sénégal, de 1984 à 1996. Cahiers d’études et de recherches francophones/Santé 11, 25–33. [PubMed] [Google Scholar]
- 166.Wandiga SO, et al. 2010. Vulnerability to epidemic malaria in the highlands of Lake Victoria basin: the role of climate change/variability, hydrology and socio-economic factors. Clim. Change 99, 473–497. ( 10.1007/s10584-009-9670-7) [DOI] [Google Scholar]
- 167.Yang HM. 2001. A mathematical model for malaria transmission relating global warming and local socioeconomic conditions. Rev. Saude Publ. 35, 224–231. ( 10.1590/S0034-89102001000300002) [DOI] [PubMed] [Google Scholar]
- 168.White MT, Griffin JT, Churcher TS, Ferguson NM, Basáñez M, Ghani AC. 2011. Modelling the impact of vector control interventions on Anopheles gambiae population dynamics. Parasit. Vectors 4, 153 ( 10.1186/1756-3305-4-153) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Christiansen-Jucht C, Parham PE, Saddler A, Koella JC, Basanez MG. 2014. Temperature during larval development and adult maintenance influences the survival of Anopheles gambiae s.s. Parasit. Vectors 7, 489 ( 10.1186/s13071-014-0489-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Lyons CL, Coetzee M, Chown SL. 2013. Stable and fluctuating temperature effects on the development rate and survival of two malaria vectors, Anopheles arabiensis and Anopheles funestus. Parasit. Vectors 6, 104 ( 10.1186/1756-3305-6-104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Rueda L, Patel K, Axtell R, Stinner R. 1990. Temperature-dependent development and survival rates of Culex quinquefasciatus and Aedes aegypti (Diptera: Culicidae). J. Med. Entomol. 27, 892–898. [DOI] [PubMed] [Google Scholar]
- 172.Lyimo E, Takken W, Koella J. 1992. Effect of rearing temperature and larval density on larval survival, age at pupation and adult size of Anopheles gambiae. Entomol. Exp. Appl. 63, 265–271. ( 10.1111/j.1570-7458.1992.tb01583.x) [DOI] [Google Scholar]
- 173.Grais RF, de Radiguès X, Dubray C, Fermon F, Guerin PJ. 2006. Exploring the time to intervene with a reactive mass vaccination campaign in measles epidemics. Epidemiol. Infect. 134, 845–849. ( 10.1017/S0950268805005716) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Grais RF, Conlan A, Ferrari M, Djibo A, Le Menach A, Bjørnstad O, Grenfell BT. 2008. Time is of the essence: exploring a measles outbreak response vaccination in Niamey, Niger. J. R. Soc. Interface 5, 67–74. ( 10.1098/rsif.2007.1038) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Weiss B, Aksoy S. 2011. Microbiome influences on insect host vector competence. Trends Parasitol. 27, 514–522. ( 10.1016/j.pt.2011.05.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Niebylski ML, Peacock MG, Schwan TG. 1999. Lethal effect of Rickettsia rickettsiion on its tick vector (Dermacentor andersoni). Appl. Environ. Microbiol. 65, 773–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Bray R, Garnham P. 1982. The life-cycle of primate malaria parasites. Br. Med. Bull. 38, 117–122. [DOI] [PubMed] [Google Scholar]
- 178.Campbell-Lendrum D, Manga L, Bagayoko M, Sommerfeld J. 2015. Climate change and vector-borne diseases: what are the implications for public health research and policy? Phil. Trans. R. Soc. B 370, 20130552 ( 10.1098/rstb.2013.0552) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Parry M, Carter T. 1996. Climate impact and adaptation assessment: the IPCC method. Routledge. [Google Scholar]
- 180.Jones RN. 2001. An environmental risk assessment/management framework for climate change impact assessments. Nat. Hazards 23, 197–230. ( 10.1023/A:1011148019213) [DOI] [Google Scholar]
- 181.Willows RI, Connell RK. (eds). 2003. Climate adaptation: risk, uncertainty and decision-making. Oxford, UK: UKCIP Technical Report. [Google Scholar]
- 182.Taubenböck H, Post J, Roth A, Zosseder K, Strunz G, Dech S. 2008. A conceptual vulnerability and risk framework as outline to identify capabilities of remote sensing. Nat. Hazards Earth Syst. Sci. 8, 409–420. ( 10.5194/nhess-8-409-2008) [DOI] [Google Scholar]
- 183.Jones GE, Garforth C. 1997. The history, development, and future of agricultural extension. In Improving agricultural extension: a reference manual (ed. Swanson Burton E., Bentz Robert P., Sofranko Andrew J.), pp. 3–12. Rome, Italy: Food and Agriculture Organization of the United Nations. [Google Scholar]
- 184.Martínez R, et al. 2012. Improving climate risk management at local level: techniques, case studies, good practices and guidelines for World Meteorological Organization members. In Risk management—current issues and challenges (ed. Banaitiene Nerija.). Rijeka, Croatia: InTech; ( 10.5772/51554) [DOI] [Google Scholar]
- 185.Jeger MJ, Pautasso M. 2008. Plant disease and global change–the importance of long-term data sets. New Phytol. 177, 8–11. ( 10.1111/j.1469-8137.2007.02312.x) [DOI] [PubMed] [Google Scholar]
- 186.Parham PE, Hughes DA. 2015. Climate influences on the cost-effectiveness of vector-based interventions against malaria in elimination scenarios. Phil. Trans. R. Soc. B 370, 20130557 ( 10.1098/rstb.2013.0557) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Medone P, Ceccarelli S, Parham PE, Figuera A, Rabinovich JE. 2015. The impact of climate change on the geographical distribution of two vectors of Chagas disease: implications for the force of infection. Phil. Trans. R. Soc. B 370, 20130560 ( 10.1098/rstb.2013.0560) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Paz S. 2015. Climate change impacts on West Nile virus transmission in a global context. Phil. Trans. R. Soc. B 370, 20130561 ( 10.1098/rstb.2013.0561) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Rogers DJ. 2015. Dengue: recent past and future threats. Phil. Trans. R. Soc. B 370, 20130562 ( 10.1098/rstb.2013.0562) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Egizi A, Fefferman NH, Fonseca DM. 2015. Evidence that implicit assumptions of ‘no evolution’ of disease vectors in changing environments can be violated on a rapid timescale. Phil. Trans. R. Soc. B 370, 20140136 ( 10.1098/rstb.2014.0136) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Ostfeld RS, Brunner JL. 2015. Climate change and Ixodes tick-borne diseases of humans. Phil. Trans. R. Soc. B 370, 20140051 ( 10.1098/rstb.2014.0051) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Cheke RA, et al. 2015. Potential effects of warmer worms and vectors on onchocerciasis transmission in West Africa. Phil. Trans. R. Soc. B 370, 20130559 ( 10.1098/rstb.2013.0559) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Proestos Y, Christophides GK, Ergüler K, Tanarhte M, Waldock J, Lelieveld J. 2015. Present and future projections of habitat suitability of the Asian tiger mosquito, a vector of viral pathogens, from global climate simulation. Phil. Trans. R. Soc. B 370, 20130554 ( 10.1098/rstb.2013.0554) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Gale P, et al. 2010. Assessing the impact of climate change on vector-borne viruses in the EU through the elicitation of expert opinion. Epidemiol. Infect. 138, 214–225. ( 10.1017/S0950268809990367) [DOI] [PubMed] [Google Scholar]
- 195.Van Vuuren DP, et al. 2011. The representative concentration pathways: an overview. Clim. Change 109, 5–31. ( 10.1007/s10584-011-0148-z) [DOI] [Google Scholar]
- 196.Levi T, Keesing F, Oggenfuss K, Ostfeld RS. 2015. Accelerated phenology of blacklegged ticks under climate warming. Phil. Trans. R. Soc. B 370, 20130556 ( 10.1098/rstb.2013.0556) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Campbell LP, Luther C, Moo-Llanes D, Ramsey JM, Danis-Lozano R, Peterson AT. 2015. Climate change influences on global distributions of dengue and chikungunya virus vectors. Phil. Trans. R. Soc. B 370, 20140135 ( 10.1098/rstb.2014.0135) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Hoberg EP, Brooks DR. 2015. Evolution in action: climate change, biodiversity dynamics and emerging infectious disease. Phil. Trans. R. Soc. B 370, 20130553 ( 10.1098/rstb.2013.0553) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Lambin EF, Tran A, Vanwambeke SO, Linard C, Soti V. 2010. Pathogenic landscapes: interactions between land, people, disease vectors, and their animal hosts. Int. J. Health Geogr. 9, 54 ( 10.1186/1476-072X-9-54) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Feser F, Rockel B, von Storch H, Winterfeldt J, Zahn M. 2011. Regional climate models add value to global model data: a review and selected examples. Bull. Am. Meteorol. Soc. 92, 1181–1192. ( 10.1175/2011BAMS3061.1) [DOI] [Google Scholar]
- 201.Johnson PT, Thieltges DW. 2010. Diversity, decoys and the dilution effect: how ecological communities affect disease risk. J. Exp. Biol. 213, 961–970. ( 10.1242/jeb.037721) [DOI] [PubMed] [Google Scholar]
- 202.Ostfeld RS, Keesing F. 2000. Biodiversity series: the function of biodiversity in the ecology of vector-borne zoonotic diseases. Can. J. Zool. 78, 2061–2078. ( 10.1139/z00-172) [DOI] [Google Scholar]
- 203.Swaddle JP, Calos SE. 2008. Increased avian diversity is associated with lower incidence of human West Nile infection: observation of the dilution effect. PLoS ONE 3, e2488 ( 10.1371/journal.pone.0002488) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Holling CS. 1996. Cross-scale morphology, geometry, and dynamics of ecosystems. In Ecosystem management, pp. 351–423. Berlin, Germany: Springer. [Google Scholar]
- 205.Gunderson LH. 2001. Panarchy: understanding transformations in human and natural systems. Washington, DC: Island Press. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Arguably the greatest limitation in the development of mechanistic transmission models is our current understanding of the essential empirical relationships between vectors, pathogens and the environment. The low quality and quantity of data available to parametrize models of risk are often not considered in model development or interpretation. Additional experimental research is required to explore and define physiological temperature and moisture constraints for the most important vector species. Strong evidence indicates that temperature variability is important to estimating risk [85–87,112,113], but vital rates are currently estimated from very few data points representing laboratory responses by vectors or pathogens to temperature averages [114], together with inferences based on seasonal occurrence [115]. In addition (and as noted in §2a), estimates of vital rates for specific disease systems, for example, Plasmodium falciparum malaria, are often derived from data on a mix of vector species, whereas endosymbionts such as Wolbachia may be important in the biology and control of certain disease vectors [116] and may exhibit temperature responses [117]. To validate these models, it is important to conduct comparative research on different species within important vector genera. For example, both Ae. aegypti and Ae. albopictus are competent vectors of DENV, with different physiological constraints and distinct transmission potentials. Combining data for these two species to estimate vital rates would not necessarily generate realistic inference. Furthermore, data and research are particularly needed to better understand the influence of environmental factors on key parameters such as the EIP [89], vector competence [94,113], biting behaviour and interactions with infection [118,119], because these have been very under-researched to date.
The enormous variation in EIR that can exist around a given mean seasonal temperature [91,120] remains to be explained, yet is critical to conclusions concerning the role of climate change. Variation in EIR at a given temperature regime could be due to local adaptation [121], control interventions and/or socio-economic factors, differences between vector and/or parasite species complexes across sites, potentially subtle variation in life-history traits such as mosquito population age structure [122], accuracy of (local) temperature estimates or chosen temperature metrics and/or sampling protocols. Both empirical and theoretical studies are needed to explore potential determinants, uncertainties in parameter estimates and consequences for predictive models. Uncertainty in the components of R0 (or other metrics) should be integrated and acknowledged. In addition, many other assumptions are frequently made regarding how biotic interactions (e.g. competition, density dependence and host density) regulate and limit vector populations. Uncertainties owing to these assumptions are rarely considered or acknowledged in risk metrics.
To advance the empirical base from which to derive models, we recommend the following:
(1) Mean seasonal temperatures have been regularly used and might be sufficient for some questions and some settings, but, in general, DTR or similar measures of temporal variability must be included. Off-season temperatures and ranges might also be important in some regions, but an understanding of which temperature metrics are most important during this period is less well developed.
(2) Humidity and evaporation rate influence adult mosquito vital rates, but have received insufficient attention as determinants of VBD risk. The effects of both mean and fluctuating humidity and water vapour pressure, as well as interactive effects with temperature, are often poorly understood.
(3) The timing, frequency and quantity of precipitation are important, but not well understood and are likely to differ for different mosquito species. Total rainfall is likely to have a highly nonlinear effect on mosquito production, with the potential for qualitatively different effects on container-breeding versus seasonal or permanent wetland breeding species.