Abstract
Adenovirus 2 mRNAs synthesized in productive infection were assigned to specific regions of the genome by hybridization to unique fragments of viral DNA. Radioactive viral RNA synthesized early or late in infection was first fractionated by polyacrylamide gel electrophoresis. Eluted RNAs were then tested for complementary hybrid formation with each of the six fragments of adenovirus 2 DNA generated by cleavage with endonuclease R·R1.
Early RNA species migrating as 13S, 19S, and 20S RNAs were identified as transcription products of fragments A, B, and D, respectively. In addition to the 13S RNA transcribed from A fragment DNA, 13S RNA also hybridized to the D and E fragment DNAs; 11S RNA annealed to both A and B fragments. The RNA that hybridized to fragment C DNA was heterogeneous in size.
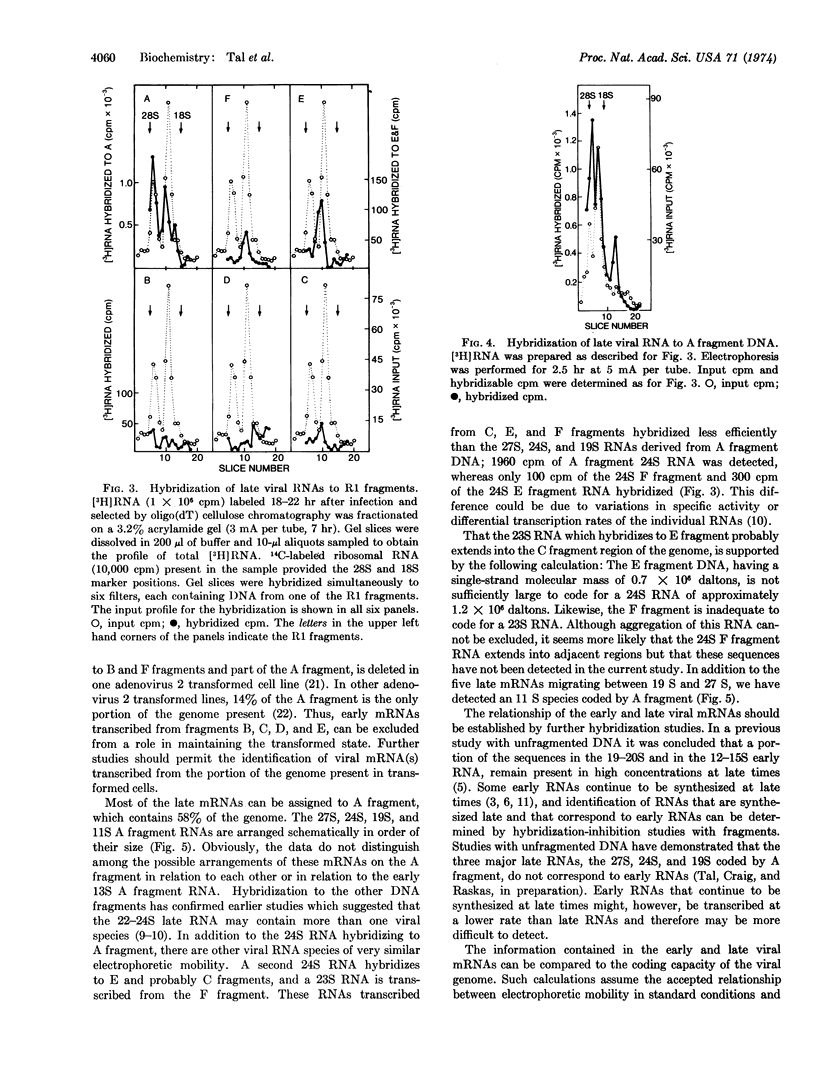
Viral RNA synthesized late in infection included 27S, 24S, 19S, and 11S size classes, all of which annealed to A fragment DNA. Additional RNA migrating as 24S hybridized to E and C fragment DNA, and 23S RNA annealed to F fragment DNA.
The results of the hybridizations of size fractionated RNAs with viral DNA fragments enabled formation of a partial map of viral mRNAs with respect to the adenovirus 2 genome. Some of the viral RNAs may represent transcripts which overlap R1 cleavage sites, because in at least three instances hybridization revealed viral RNAs which have the same electrophoretic mobility and anneal to fragments that are contiguous on the genome.
Keywords: early viral RNA, late viral RNA, restriction enzymes
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burger H., Doerfler W. Intracellular forms of adenovirus DNA. 3. Integration of the DNA of adenovirus type 2 into host DNA in productively infected cells. J Virol. 1974 May;13(5):975–992. doi: 10.1128/jvi.13.5.975-992.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig E. A., Raskas H. J. Effect of cycloheximide on RNA metabolism early in productive infection with adenovirus 2. J Virol. 1974 Jul;14(1):26–32. doi: 10.1128/jvi.14.1.26-32.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawid I. B., Chase J. W. Mitochondrial RNA in Xenopus laevis. II. Molecular weights and other physical properties of mitochondrial ribosomal and 4 s RNA. J Mol Biol. 1972 Jan 28;63(2):217–231. doi: 10.1016/0022-2836(72)90371-3. [DOI] [PubMed] [Google Scholar]
- Fujinaga K., Green M. Mechanism of viral carcinogenesis by DNA mammalian viruses. VII. Viral genes transcribed in adenovirus type 2 infected and transformed cells. Proc Natl Acad Sci U S A. 1970 Feb;65(2):375–382. doi: 10.1073/pnas.65.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREEN M., PINA M. BIOCHEMICAL STUDIES ON ADENOVIRUS MULTIPLICATION, VI. PROPERTIES OF HIGHLY PURIFIED TUMORIGENIC HUMAN ADENOVIRUSES AND THEIR DNA. Proc Natl Acad Sci U S A. 1964 Jun;51:1251–1259. doi: 10.1073/pnas.51.6.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M. Oncogenic viruses. Annu Rev Biochem. 1970;39:701–756. doi: 10.1146/annurev.bi.39.070170.003413. [DOI] [PubMed] [Google Scholar]
- Lindberg U., Persson T., Philipson L. Isolation and characterization of adenovirus messenger ribonucleic acid in productive infection. J Virol. 1972 Nov;10(5):909–919. doi: 10.1128/jvi.10.5.909-919.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas J. J., Ginsberg H. S. Synthesis of virus-specific ribonucleic acid in KB cells infected with type 2 adenovirus. J Virol. 1971 Aug;8(2):203–214. doi: 10.1128/jvi.8.2.203-214.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas J. J., Ginsberg H. S. Transcription and transport of virus-specific ribonucleic acids in African green monkey kidney cells abortively infected with type 2 adenovirus. J Virol. 1972 Dec;10(6):1109–1117. doi: 10.1128/jvi.10.6.1109-1117.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire P. M., Swart C., Hodge L. D. Adenovirus messenger RNA in mammalian cells: failure of polyribosome association in the absence of nuclear cleavage. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1578–1582. doi: 10.1073/pnas.69.6.1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulder C., Delius H. Specificity of the break produced by restricting endonuclease R 1 in Simian virus 40 DNA, as revealed by partial denaturation mapping. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3215–3219. doi: 10.1073/pnas.69.11.3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons J. T., Gardner J., Green M. Biochemical studies on adenovirus multiplication, XIX. Resolution of late viral RNA species in the nucleus and cytoplasm. Proc Natl Acad Sci U S A. 1971 Mar;68(3):557–560. doi: 10.1073/pnas.68.3.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons J. T., Green M. Biochemical studies on adenovirus multiplication. 18. Resolution of early virus-specific RNA species in Ad 2 infected and transformed cells. Virology. 1971 Jul;45(1):154–162. doi: 10.1016/0042-6822(71)90122-x. [DOI] [PubMed] [Google Scholar]
- Pettersson U., Mulder C., Deluis H., Sharp P. A. Cleavage of adenovirus type 2 DNA into six unique fragments by endonuclease R-RI. Proc Natl Acad Sci U S A. 1973 Jan;70(1):200–204. doi: 10.1073/pnas.70.1.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philipson L., Wall R., Glickman G., Darnell J. E. Addition of polyadenylate sequences to virus-specific RNA during adenovirus replication. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2806–2809. doi: 10.1073/pnas.68.11.2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reijnders L., Sloof P., Sival J., Borst P. Gel electrophoresis of RNA under denaturing conditions. Biochim Biophys Acta. 1973 Oct 26;324(3):320–333. doi: 10.1016/0005-2787(73)90278-5. [DOI] [PubMed] [Google Scholar]
- Sharp P. A., Pettersson U., Sambrook J. Viral DNA in transformed cells. I. A study of the sequences of adenovirus 2 DNA in a line of transformed rat cells using specific fragments of the viral genome. J Mol Biol. 1974 Jul 15;86(4):709–726. doi: 10.1016/0022-2836(74)90348-9. [DOI] [PubMed] [Google Scholar]
- Sharp P. A., Sugden B., Sambrook J. Detection of two restriction endonuclease activities in Haemophilus parainfluenzae using analytical agarose--ethidium bromide electrophoresis. Biochemistry. 1973 Jul 31;12(16):3055–3063. doi: 10.1021/bi00740a018. [DOI] [PubMed] [Google Scholar]
- Thomas D. C., Green M. Biochemical studies on adenovirus multiplication, xi. Evidence of a cytoplasmic site for the synthesis of viral-coded proteins. Proc Natl Acad Sci U S A. 1966 Jul;56(1):243–246. doi: 10.1073/pnas.56.1.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tibbetts C., Pettersson U., Johansson K., Philpson L. Relationship of mRNA from productively infected cells to the complementary strands of adenovirus type 2 DNA. J Virol. 1974 Feb;13(2):370–377. doi: 10.1128/jvi.13.2.370-377.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall R., Philipson L., Darnell J. E. Processing of adenovirus specific nuclear RNA during virus replication. Virology. 1972 Oct;50(1):27–34. doi: 10.1016/0042-6822(72)90342-x. [DOI] [PubMed] [Google Scholar]


