Abstract
Background and Aims Two ecological strategies of desiccation tolerance exist in plants, constitutive and inducible. Because of difficulties in culturing sporophytes, very little is known about desiccation tolerance in this generation and how desiccation affects sexual fitness.
Methods Cultured sporophytes and vegetative shoots from a single genotype of the moss Aloina ambigua raised in the laboratory were tested for their strategy of desiccation tolerance by desiccating the shoot–sporophyte complex and vegetative shoots at different intensities, and comparing outcomes with those of undried shoot–sporophyte complexes and vegetative shoots. By using a dehardened clonal line, the effects of field, age and genetic variance among plants were removed.
Key Results The gametophyte and embryonic sporophyte were found to employ a predominantly inducible strategy of desiccation tolerance, while the post-embryonic sporophyte was found to employ a moderately constitutive strategy of desiccation tolerance. Further, desiccation reduced sporophyte fitness, as measured by sporophyte mass, seta length and capsule size. However, the effects of desiccation on sporophyte fitness were reduced if the stress occurred during embryonic development as opposed to postembryonic desiccation.
Conclusions The effects of desiccation on dehardened sporophytes of a bryophyte are shown for the first time. The transition from one desiccation tolerance strategy to the other in a single structure or generation is shown for only the second time in plants and for the first time in bryophytes. Finding degrees of inducible strategies of desiccation tolerance in different life phases prompts the formulation of a continuum hypothesis of ecological desiccation tolerance in mosses, where desiccation tolerance is not an either/or phenomenon, but varies in degree along a gradient of ecological inducibility.
Keywords: Bryophyte, moss, Aloina ambigua, Pottiaceae, rate of desiccation, chlorosis, phenology, inducible desiccation tolerance, constitutive desiccation tolerance, sporophyte embryo, sporophyte fitness, dehardening, seta elongation
INTRODUCTION
Desiccation tolerance (DT, also used to abbreviate desiccation-tolerant) is the ability of an organism or structure to tolerate and survive after equilibrating to a relative humidity (RH) of ≤50 % (Alpert and Oliver, 2002; Koster et al., 2010). Ecological DT considers these abilities as applied to whole organisms, structures or generations in plants (sporophytes or gametophytes) and focuses upon survival, reproduction and fitness. There are two prevailing strategies of ecological DT in plants, constitutive DT (CDT) and inducible DT (IDT) (Stark et al., 2013), with the distinction depending upon responses to different rates of desiccation in dehardened plants. These two strategies were formerly known as ‘fully DT’ (constitutive) and ‘modified DT’ (inducible) (Oliver et al., 1998). Organisms and structures that use the CDT strategy can survive drying at any speed and show minimal signs of damage upon rehydration, whereas in IDT species the plant or structure is killed or incurs significant damage when dried rapidly while exhibiting minimal damage when dried slowly.
Sporophyte desiccation tolerance
A wealth of knowledge exists on the mechanisms and responses of plants to vegetative DT. In seed plants, a very small percentage (<1 %) of species exhibits vegetative DT (Proctor and Pence, 2002), and in all cases excepting some ferns and fern allies (Watkins et al., 2007) the strategy employed is IDT (Oliver et al., 1998, 2000). Bryophytes represent a group that is broadly DT in the vegetative (gametophytic) state, with 210 species documented as DT (Wood, 2007) and with the assumption that the majority of bryophytes, comprising 18 000 species (Shaw and Goffinet, 2000), must exhibit a degree of DT because they inhabit microsites that desiccate regularly and exhibit high surface-to-volume ratios that translate into relatively rapid drying (Proctor et al., 2007a). Vegetative DT in bryophytes may be CDT or IDT, with CDT considered more common (Toldi et al., 2009). However, sexual fitness in bryophytes is dependent upon the production of spores through the maturation of the diploid sporophyte, which matures over a period of weeks to years while connected to and physiologically dependent upon the maternal gametophytic shoot (Glime, 2007a). During this developmental period, the sporophyte ‘inevitably spans periods of desiccation’ (Proctor et al., 2007a). Given the critical evolutionary position of the bryophytes with respect to land plants, the dearth of information on the DT of the sporophyte generation is notable.
Based on studies of field-collected gametophytes bearing immature sporophytes, it appears that the sporophyte generation in mosses is more sensitive to desiccation and thermal stresses than the gametophyte (McLetchie and Stark, 2006; Stark et al., 2007). Embryos (embryonic sporophytes) appear to be more susceptible to desiccation, although the precise cause of sporophyte abortions remains undetermined (Stark, 2002, 2005). Embryonic sporophytes of some desert mosses can tolerate long periods of desiccation, and clearly factors such as hardening in field-collected specimens play a prominent role in their survival (Stark et al. 2014). Finding that sporophytic abortions uniformly occur early in development (embryos or early seta elongation phenophases) in two desert species (Stark, 2001, 2005) prompted this inquiry into the possibility that sporophyte DT is phenophase (phenological phase)-dependent. Furthermore, the frequency of abortive sporophytes directly influences sexual fitness, as indicated by abortive sporophytes outnumbering mature sporophytes in studies of Hylocomium splendens (Callaghan et al., 1978) and Syntrichia caninervis (Stark et al., 2000), and abortive sporophytes constituting 38 % of all current cycle sporophytes in Pleurozium schreberi (Longton and Greene, 1969). Sporophyte abortion has been attributed to extrinsic (stress) or intrinsic (resource limitation) causes, reviewed elsewhere (Stark, 2001). Under both conditions (extrinsic, intrinsic), when a sporophyte aborts, it does so at an early phenophase, often embryonic (Longton and Greene, 1969; Stark and Stephenson, 1983; Longton, 1988; Stark et al., 2000).
Value of using deacclimated plants
Using cultured lines (clones) of bryophytes that have been regenerated in the laboratory more than once to assess DT overcomes several of the difficulties encountered regarding the interpretation of results in studies of both mechanistic (cellular) and ecological DT. These problems include genetic variation among shoots, age variance among shoots, seasonal variation effects and, perhaps most importantly, field physiological histories, i.e. field effects. Such field effects may include unknown degrees of hardening to DT that may not necessarily be removed by a hydration period of 24–72 h (Bopp and Werner, 1993; Stark et al., 2014).
Effects of rate of desiccation
The rate at which desiccation proceeds has significant effects on the ability of the bryophyte shoot or protonema to tolerate and repair/reassemble damage incurred by drying. A useful distinction is to consider drying at two rates, slow and rapid, with threshold rates of desiccation being species-specific. When plants or structures are desiccated too rapidly (e.g. in <1 h), several physiological recovery processes are negatively impacted, including microtubule reassembly, which is critical to retaining viability (Pressel and Duckett, 2010). In addition, when exposed to rapid drying, plants exhibit chlorosis, delayed regeneration, reduced vegetative fitness and fewer surviving shoot apices (Schonbeck and Bewley, 1981; Barker et al., 2005; Stark et al., 2011). Compared with slowly dried plants or tissues, rapidly dried plants or tissues are more negatively impacted in measures of rehydration respiration, vacuolar fragmentation, endomembrane structure, cytoskeleton structure, the compensation point of photosynthesis, chlorophyll content, resumption of protein synthesis, polysomal recovery, mRNA synthesis and solute leakage (Bewley, 1995; Pressel and Duckett, 2010). Because ‘firm information on the DT of the developing sporophyte in the common boreal and temperate bryophytes has been almost completely lacking’ (Proctor et al., 2007a), we tested the ecological DT of maturing sporophytes in cultured lines of the moss Aloina ambigua, in the process uncovering the surprising finding that the strategy of DT is dependent upon the phenophase of the sporophyte.
Hypotheses
We hypothesize that (1) the ecological strategy of DT (IDT or CDT) is similar across phenophases of the sporophytes of Aloina; (2) the strategy of DT of the shoots is similar to the strategy of DT of the sporophytes of Aloina; and (3) desiccation stress reduces sexual fitness by reducing sporophyte size and slowing sporophyte development compared with unstressed sporophytes in Aloina.
MATERIALS AND METHODS
Species description
Aloina is a distinctive genus in the Pottiaceae, characterized by infolded succulent leaves bearing upright filaments that cover the costa and part of the lamina. Aloina ambigua is distinguished from other members of the genus by having leaves lacking a hairpoint, leaf bases lacking a differentiated margin of thin-walled hyaline cells, and the costa smooth abaxially (Gallego et al., 1999; Norris and Shevock, 2004; Delgadillo, 2007). The sexual condition is rhizautoicous (male and female shoots connected by rhizoids). This taxon was selected for study due to ease of culturing and its broad distribution in the northern hemisphere.
Water content and RH
Relative humidity was measured every 10 min during drying using iButtons (Maxim, San Jose, CA, USA) positioned inside lidded (slow drying) and unlidded (rapid drying) Petri dishes (inner diameter 35 mm). For vegetative shoots and sporophytes exposed to a drying event, single shoots or shoot + sporophyte complexes at full turgor were excavated to include rhizoids and associated substrate approximately equivalent in mass to that of the shoot. These were blotted on a chemical wipe for 20 s prior to moving (rapid drying) or moved unblotted (slow drying) onto two-ply filter paper in an unlidded (rapid drying) or lidded (slow drying) Petri dish within a walk-in environmental room set at 50 % RH and 20 °C, with 0 (rapid drying) or 150 (slow drying) µL sterile water pipetted onto the filter paper. The shoot or shoot + sporophyte complex was weighed every 10 min during the first hour (rapid drying) and every 24 h (rapid and slow drying) over a period of 1 (rapid drying) or 4 (slow drying) days. Shoots were subsequently oven-dried (70 °C for 32 h) and weighed directly from the oven to the nearest microgram to determine the dry weight. Water content was determined on a dry weight basis: water content = [shoot mass equilibrated at 50 % RH – d.wt]/d.wt, where d.wt is dry weight). The following structures were analysed for water content for both the rapid drying and the slow drying treatment: vegetative shoots, shoot + sporophyte complexes at late embryo stage (Table 1) and shoot + sporophyte complexes at day 6 of seta elongation (n = 3).
Table 1.
Events marking the beginning of each sporophyte phenophase recognized in laboratory cultures of Aloina ambigua
| Phenophase | Event marking beginning of phenophase |
| Embryo | Fertilization; embryo visible and reaches ∼1–3 mm in length |
| Post-embryonic (seta elongation) | Calyptra formation (rupture of vaginula) and from 1 to 6 days after calyptral rupture |
| Capsule expansion (premeiosis) | Calyptra splits vertically at base |
| Meiosis | Annular and/or proximal opercular region becomes reddish brown |
| Postmeiosis | Theca turns from green to brown |
Culture technique
Shoots from a single specimen (Brinda 2024; Parashant Canyon, Grand Canyon-Parashant National Monument, Mohave County, AZ, USA, 36·155415N, 113·332383W (WGS84), elevation 774 m, 29 June 2007, on soil) were placed in culture and grown using the technique of Horsley et al. (2011), i.e. using locally collected sieved and dry-sterilized sand in 35-mm (inner diameter) plastic Petri dishes placed in a plant growth chamber (model E30B, Percival, Boone, IA, USA) set to a 12-h photoperiod (light 20 °C, dark 8 °C), ∼70 % RH (light), ∼90 % RH (dark), 90–120 µmol m–2 s–1 photosynthetically active radiation (PAR), and watered each week with sterile distilled water (early protonemal growth) and eventually (late protonemal growth and shoots) with 30 % Hoagland’s inorganic nutrient solution (Hoagland and Arnon, 1938). Inside a planted Petri dish, RH is stable near 100 %. All plants used in this experiment were cloned from a single field shoot, and regenerated through subculture to mature shoots at least twice in the laboratory. Microbial contaminants were removed by subculturing. Experimental shoots bearing sporophytes were ∼4–6 months old. Fertilization was effected under normal watering conditions, which thoroughly saturated the medium surface. Embryonic sporophytes were detected using a dissecting microscope at 60× magnification.
Experimental design and desiccation technique
Embryonic and post-embryonic sporophytes were exposed to three treatments while remaining attached to the maternal shoot: (1) an undried transplanted control; (2) a slow drying event of ∼30 h; and (3) a rapid drying event of ∼45 min. During drying, the duration of structures at sub-turgor to full turgor varied and we refer to these differences as different drying rates for convenience (for further details of the technique see Stark et al., 2013). Sporophyte phenophases (sensu Stark, 2001) exposed to these treatments were embryonic, seta elongation at several subintervals (1, 2, 3, 4, 5 and 6 days post-embryonic) and capsule expansion (first day of onset; Table 1). Sporophytes were selected for treatment as they became available. After the medium surface in the immediate vicinity of the shoot had been cleared of other plants, each shoot bearing a sporophyte was gently excavated from the culture using blunt forceps to include a small mass of rhizoids at the base of the shoot roughly equal in diameter and depth to the aboveground shoot, and placed in a droplet of sterile water on a microscope slide. Sixty-four embryos were assessed for each of the three desiccation treatments (total, 192), and these ranged from 1 to 3 mm in length (from the bed of the perichaetium to the base of the archegonial neck), thus spanning the two phenophases early embryo and late embryo. For the post-embryonic treatments, a total of 228 sporophytes in the seta elongation phenophase (76 per treatment) and 36 sporophytes in the capsule expansion phenophase (12 per treatment) were assessed.
Slowly dried sporophytes (shoots with attached sporophytes) were placed in a Petri dish containing two-ply filter paper (Whatman No. 1 cut to the inner circumference of the Petri dish and the filter paper equilibrated at 50 % RH for at least 24 h prior to use). These plants were fully hydrated and had free water on their surfaces in addition to the water added to the filter paper. For this treatment, the filter paper was pre-moistened by pipette with 150 µL of sterile distilled water. The sporophyte was positioned such that the embryo or seta was not contacting the filter paper in order to limit physical damage (only the rhizoid mat touched the filter paper). The Petri dish was lidded (but not sealed) and placed under dim light (2–4 µmol m–2 s–1) in an environmental control room set at 50 % RH and 20 °C. Drying time was ∼30 h, and the sporophytes were allowed to equilibrate at 50 % RH for an additional 15–20 h to reach constant mass at 50 % RH. At this point the shoot–sporophyte complex was rehydrated by gently planting in pre-moistened substrate in a Petri dish within a jar at the recovery settings given above (section Culture technique). Sporophytes were watered daily with sterile distilled water as needed.
The rapidly dried sporophytes (shoots with attached sporophytes) were handled in the same manner as the slowly dried sporophytes, except they were first blotted dry for ∼20 s on a chemical wipe and then placed directly on dry two-ply filter paper, and the Petri dish was unlidded. Drying time was 30–45 min, with the plants given an additional 20–24 h equilibration period to reach constant mass at 50 % RH. Sporophytes were rehydrated, planted and allowed to recover as described above for slowly dried sporophytes.
Controls were transplanted without drying into a Petri dish containing locally collected pre-moistened sand medium (under the same conditions as described above in the section Culture technique); the Petri dish was placed in a lidded glass jar (height 7 cm, diameter 6 cm) allowing room for sporophytes to lengthen, with a few drops of sterile water in the base of the jar (to maintain RH near 100 %), and the jar was then placed in the growth chamber at the recovery settings described above (section Culture technique). Additional gametophyte controls were included to assess leaf damage from vegetative shoots (no attached sporophytes) as undried, slowly dried and rapidly dried (treated in the same manner as the sporophytes); these allowed comparison between leaf damage exhibited by shoots having attached sporophytes with those lacking sporophytes and also subjected to undried, slow drying and rapid drying treatments, and were selected as the nearest vegetative shoot (shoot without an attached sporophyte and not usually expressing sex) to a sporophytic shoot selected for the experiment.
Response variables
Leaf damage was assessed on day 7 (post-rehydration or, for controls, post-planting) by observing the shoots at 60× magnification and classifying each leaf as entirely chlorophyllose (value = 1), partially chlorotic (value = 0·5) or entirely chlorotic (value = 0). In order to be classed as entirely chlorophyllose, both the lamina and the lamellar filaments had to be undamaged (chlorophyllose). The percentage of chlorophyllose leaves was calculated from these values. If a lower leaf was obscured by upper leaves it was not scored. Such visual estimates of leaf damage correlate well to chlorophyll fluorescence parameters (Greenwood and Stark, 2014). The time course of sporophyte development was assessed daily using the sporophyte phenophases of Stark (2001). The events marking the transition to each phenophase are given in Table 1. If an observation day was missed during a phenophase change, the data point was not used. Assessment of survival of the sporophyte was based on its ability to progress to meiosis; if the sporophyte did not resume development and reach meiosis, it was classified as abortive. Sporophyte size was assessed using dry mass and image analysis software on mature sporophytes. Two weeks after the last sporophyte in a Petri dish had become postmeiotic, the bottle containing the Petri dish was unlidded and the sporophytes were allowed to dry in the growth chamber under the normal diurnal cycle for a period of 1 week. The sporophytes were then moved into the dark in a laboratory cabinet at ∼20–50 % RH and stored until analysis. Each sporophyte was then removed from the Petri dish and hydrated briefly, the calyptra was removed with forceps, the lower shoot leaves were removed with a straight-edge cut at the apex of the vaginula, and the sporophyte was placed into a micropacket, oven-dried at 70 °C for at least 48 h and weighed to the nearest microgram immediately out of the oven, and the following measurements were taken using ImageJ software on the dried plants (photographing the sporophyte and measuring using the software): seta length, theca length (the capsule excluding the operculum but including the apophysis) and theca width (at its widest point), each to the nearest 0·01 mm. The size of the capsule in mosses is probably a good indicator of relative spore number (Glime 2007a).
Statistics
Due to the inherent censoring of the leaf damage data, Tobit regression models (Tobin, 1958) were used to test for treatment effects. Generalized linear models with binomial errors (logistic regression; Kutner et al., 2004) were used to determine whether the drying regime and/or sporophyte phenophase affected the likelihood of sporophyte survival. Sporophyte fitness parameters were examined using ANOVA. Multiple comparisons with Tukey contrasts were used to compare effects across drying treatments. Box–Cox transformations (Box and Cox, 1964) of seta length data were necessary to make the data more normally distributed prior to analysis. The effects of both drying regime and sporophyte phenophase were examined simultaneously in the models. In only one case (see section Leaf damage, below) was the interaction term significant; for the rest it was removed from the final model. The sporophyte development times (Table 1) were analysed using Cox proportional hazards regression models (Therneau and Grambsch, 2000). All post-embryonic phases were pooled in most analyses excepting those where different parts of the post-embryonic timeline were specifically examined. All analyses were performed using R version 3.1.0 (R Core Development Team, 2014).
RESULTS
Water content
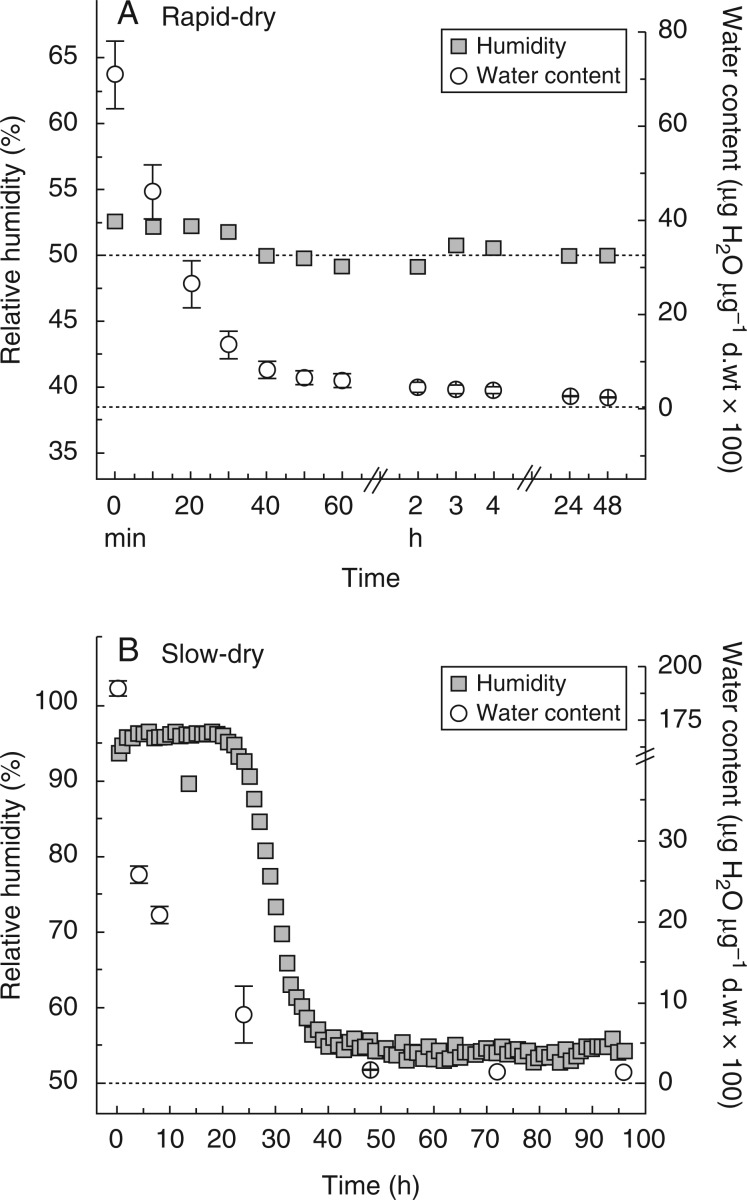
Water content decreased rapidly during the first 40 min in the rapid drying treatment (Fig. 1A), reaching very close to equilibration in 2 h. In the slow drying treatment, water content declined much more slowly, reaching equilibrium with 50 % RH after ∼35 h (Fig. 1B). The higher initial water content of slowly dried shoots was caused by the presence of external free water on the unblotted plants. When soil was removed, water content on a dry weight basis was 12–13 % (Fig. 1 caption).
Fig. 1.
Water content and relative humidity (RH) experienced by shoots (vegetative shoot + rhizoids with soil) and sporophytes (sporophyte at seta elongation phenophase + shoot + rhizoids with soil) of Aloina ambigua during an experimental drying event (mean ± s.e., n = 3 for each; s.e. values were too small to show on graph for RH; upper dotted line is 50 % RH, lower dotted line is 0·0 % water content). (A) Rapid drying. (B) Slow drying. Trials for RH and water content were independent tests because the occupation of space within the Petri dish by an iButton is significant and may affect water loss. Water content = [shoot mass equilibrated at 50 % RH – d.wt]/d.wt, where d.wt is dry weight. Water content for vegetative shoots with rhizoids removed and equilibrated at 50 % RH = 0·118 ± 0·006 µg H2O µg d.wt–1 (n = 6). Water content for sporophytes at early seta elongation phase with rhizoids removed and equilibrated at 50 % RH = 0·130 ± 0·011 µg H2O µg d.wt–1 (n = 6).
Leaf damage
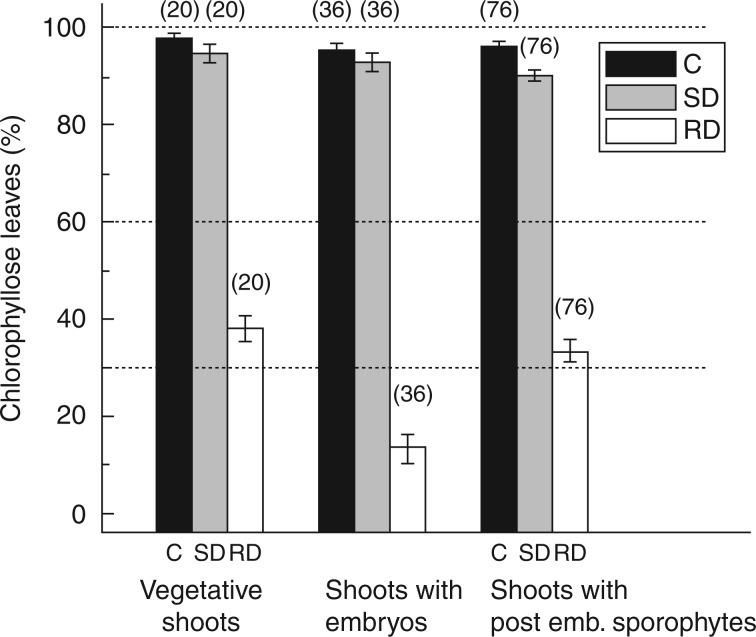
Drying regime had a significant effect on leaf damage, with both undried controls (z = 19·069, P < 0·001) and slowly dried plants (z = 18·696, P < 0·001) showing less damage than rapidly dried plants. There was no significant difference between the undried controls and the slowly dried plants (z = 1·035, P = 0·74), each of which exhibited minimal damage. Sporophyte phenophase also had a significant effect on leaf damage. The embryonic plants showed significantly more damage than both the vegetative plants (z = 5·523, P < 0·001) and the post-embryonic plants (z = 6·159, P < 0·001; including up to 6 d post-embryonic). However, there was an interaction between the two factors such that the difference between the leaf damage shown by plants of differing sporophyte phenophases was only seen in the plants that were rapidly dried (z = –3·027, P = 0·002; Figs 2A–C and 3).
Fig. 3.
Leaf damage as the percentage of chlorophyllose leaves (visual estimate) following desiccation treatments in Aloina ambigua. Shoots were assessed 7 d following rehydration from the desiccation event. C, control undried shoots; SD, shoots dried slowly over a 30-h period; RD, shoots dried rapidly in <1 h. Data are mean ± s.e., with n values in parentheses.
Fig. 2.
Images of the moss Aloina ambigua at least 7 d following rehydration from the desiccation treatment. (A) Typical control shoot (undried, transplanted). (B) Typical slowly dried shoot. (C) Typical rapidly dried shoot. Sporophyte phenophases: (D) early embryo; (E) late embryo; (F) early seta elongation; (G) late seta elongation showing two sporophytes; (H) early capsule expansion (pre-meiosis, showing rupture of the calyptral tissue); (I) meiosis, arrow at discoloured operculum; (J) postmeiosis; (K) abortive embryonic sporophyte (at arrow) following rapid drying.
Survival of sporophytes
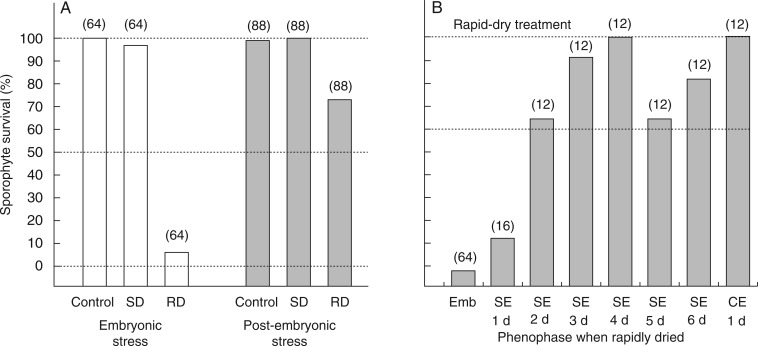
Drying regime had a significant effect on sporophyte survival, with both undried controls (z = 5·248, P < 0·001) and slowly dried (z = 7·127, P < 0·001) plants showing higher survival than rapidly dried plants. Very few (8 %) embryonic sporophytes survived rapid drying (5 of 64 embryos survived to meiosis), yet 97 % survived slow drying. There was no significant difference between the survival of undried controls and the slowly dried plants (z = 0·567, P = 0·91), with the transplantation of undried (control) and slowly dried sporophytes resulting in nearly 100 % survival of both embryonic and post-embryonic phenophases. Sporophyte phenophase also had a significant effect on sporophyte survival, with the post-embryonic sporophytes significantly more likely to survive (z = 6·413, P < 0·001). However, because of the effect of drying regime, this difference was almost entirely due to the survival rates of rapidly dried plants (Fig. 4A). As development proceeded from the first day of seta elongation to capsule expansion (15 d on average), the likelihood of sporophyte survival increased significantly (z = 3·319, P < 0·001). A day-by-day analysis of post-embryonic sporophyte survival up to day 6 (from the beginning of seta elongation) and including the first day of capsule expansion (pre-meiosis) revealed that survival dramatically improved between the first and second days of post-embryonic seta elongation, increasing from 19 % to 67 % (Fig. 4B).
Fig. 4.
Survival of embryonic and post-embryonic sporophytes (the complex of maternal shoot and attached sporophyte) of Aloina ambigua. (A) Plants exposed to slow desiccation (SD) or rapid desiccation (RD) and undried controls (Control). (B) Plants exposed to rapid desiccation at phenophases from embryo (Emb), 1–6 days following the start of seta elongation (SE) and at the onset of capsule expansion (CE). The n-values are in parentheses.
Time course of sporophyte development
The interval from the early embryo stage (1 mm long) to the postmeiotic capsule stage was ∼40 d in culture. Sporophyte development time was analysed for those plants that achieved postmeiosis and were dried prior to capsule expansion (rapidly dried plants were excluded). The total time from seta elongation to postmeiosis was used as the metric of development speed. The sporophyte development times of the slowly dried plants were not different from those of the undried controls (z = 1·243, P = 0·214). However, sporophytes that were treated (dried or controls) in the embryonic state took significantly longer to complete development (z = 2·029, P = 0·042) than those treated during the seta elongation phase.
Sporophyte fitness
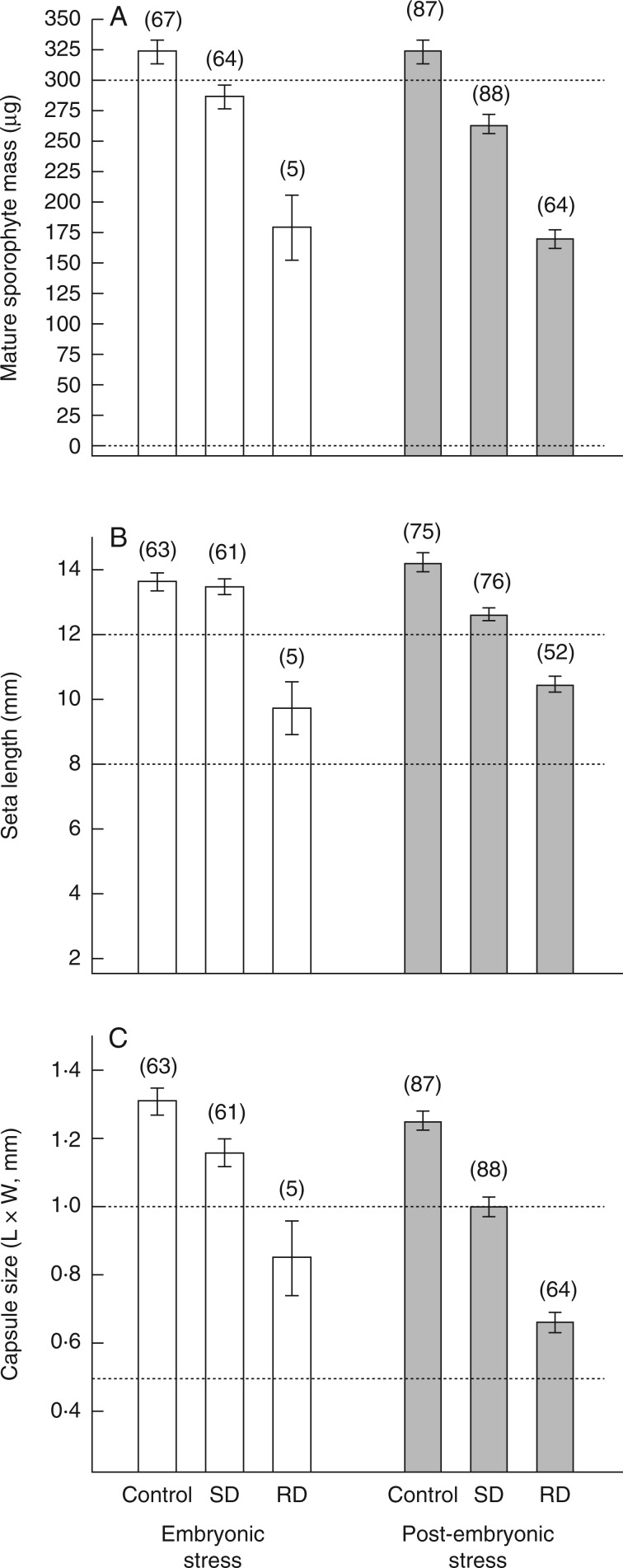
Sporophyte fitness was assessed using three measures of postmeiotic sporophyte size: total mass, seta length and capsule (theca) size. Initial sporophyte phenophase did not have a significant effect on final sporophyte biomass (t = –1·241, P = 0·215). However, the effects of drying regime were significant, with both slowly dried (z = –4·08, P < 0·001) and rapidly dried (z = –12·52, P < 0·001) plants producing sporophytes having a lower final biomass than undried controls. The two treatments also differed from each other with respect to final sporophyte mass, with rapidly dried plants producing a lower mass than slowly dried plants (z = –10·04, P < 0·001; Fig. 5A). Initial sporophyte phenophase did not have a significant effect on final seta length (z = 0·296, P = 0·767). However, the effects of drying regime were significant, with both slowly dried (z = 4·024, P < 0·001) and rapidly dried (z = 11·643, P < 0·001) plants producing sporophytes with a shorter final seta length than undried controls. The two treatments also differed from each other with respect to final seta length, rapidly dried plants producing shorter setae than slowly dried plants (z = 8·552, P < 0·001; Fig. 5B). Unlike the other two measures, final capsule size was significantly affected by initial sporophyte phenophase (z = –3·375, P = 0·003), with plants dried during the post-embryonic phenophase producing smaller capsules. The effects of drying regime were also significant, with both slowly dried (z = 5·986, P < 0·001) and rapidly dried (z = 13·797, P < 0·001) plants producing smaller capsules than undried controls. The two treatments also differed from each other with respect to capsule size, with rapidly dried plants producing smaller capsules than slowly dried plants (z = 9·044, P < 0·001; Fig. 5C). Although not assessed across treatments, capsules reaching postmeiosis (treated and controls) contained viable spores (from a limited test of several capsules).
Fig. 5.
Final sporophyte measures of fitness in cultures of Aloina ambigua where individual shoot + sporophyte complexes were exposed to slowly dried (SD), rapidly dried (RD) and undried conditions (Control) at two initial phenophases (embryonic and post-embryonic) and allowed to mature following transplantation and rehydration. (A) Sporophyte mass. (B) Seta length. (C) Capsule (theca) area (length × width). Data are mean ± s.e., with n values in parentheses.
DISCUSSION
The major finding of this study was foreshadowed by Richard T. T. Forman nearly 50 years ago. Forman (1965) formulated a phenological classification for mosses, and reasoned that the growth of the embryo should be separated from the extension of the seta, ‘since the ecological tolerances of the two stages are likely to be different’. For the moss A. ambigua, the embryonic sporophyte and the vegetative shoot survive desiccation by adopting the strategy of IDT. This mode of DT requires a slow period of desiccation in order for development to resume (sporophyte) or to exhibit minimal shoot damage (gametophyte; Hájek and Vicherová, 2014). However, once the embryonic phenophase gives way to the post-embryonic phenophase of seta elongation, the strategy of DT transitions during the first 48 h of seta elongation to one of predominantly CDT with respect to sporophyte survival; thus, our first two hypotheses (a consistent DT strategy across sporophyte phenophases and between shoots and sporophytes) were not sustained. These post-embryonic (and pre-meiotic) sporophytes can (mostly, ∼70 %) tolerate drying at a rapid rate (<60 min) to equilibration at 50 % RH. The transition to CDT was expected to occur at some point of development, perhaps at or after meiosis, as inferred from the strong DT exhibited by the spores of bryophytes (Glime, 2007b). The finding that the transition to CDT occurs relatively early and abruptly during sporophyte development was surprising and may be a fairly common pattern (noted on a pilot basis for three additional species of Pottiaceae; L. R. Stark, pers. observ.). It represents the second case (the first case for a bryophyte) of a single plant structure (the sporophyte) exhibiting a transition from one mode of DT (IDT) to the alternative mode of DT (CDT; Testo and Watkins, 2012, with sporophytes of the fern Asplenium transitioning from IDT to desiccation-sensitive). Among animals, where DT is less frequent than among plants, variation in DT exists between the larval and adult phases of the fly Polypedilum vanderplanki (Watanabe et al., 2002).
The third hypothesis, that desiccation stress slows the developmental progression of the sporophyte and reduces sexual fitness by leading to the production of smaller sporophytes (with fewer spores) compared with unstressed sporophytes in Aloina, was mostly sustained. Desiccated sporophytes were smaller in total biomass at maturity, had shorter setae (with the exception of slowly dried embryonic sporophytes) and had smaller spore-bearing capsules than undried sporophytes. Further, rapidly dried sporophytes were much smaller in all respects than slowly dried sporophytes. However, the developmental timeline of the sporophyte was relatively unaffected by desiccation, with the time courses nearly identical. The rapidly dried shoots with attached embryonic sporophytes suffered significantly more damage than rapidly dried shoots with attached post-embryonic sporophytes, indicating vulnerability of the entire shoot–sporophyte complex during early sporophyte development.
Formulation of a new hypothesis: the continuum hypothesis of ecological DT in bryophytes
Abel (1956) drew attention to the variability of DT based on the developmental stages of mosses and the gradient of responses to desiccation in the leaves of mosses, clearly noticing the existence of both constitutive and inducible ecological strategies. In a 1995 retrospect on DT in plants, Bewley indicated that ‘it is unlikely that there is a single strategy for desiccation tolerance in the many tolerant species and tissues within the plant kingdom but, rather, many variations on a theme, involving both protection and repair mechanisms’. In 2006, Pressel et al. noted that ecological strategies of DT in bryophytes probably exist within two endpoints, IDT and CDT: induced and constitutive desiccation-tolerance are not sharply separated, and there is evidence that some desiccation-tolerance may be induced to a greater or less degree in most species (Werner et al., 1991; Oliver et al., 1998; Beckett, 2001). Furthermore, studies of Polytrichum led Proctor et al. (2007b) to note the lack of a sharp boundary between the DT mechanisms of bryophytes and vascular plants. The sporophyte survival and fitness data presented here lend credence to these suggestions. First, although sporophyte survival following rapid drying increased from 8 % to >70 % during the transition from embryonic to post-embryonic sporophytes, constitutive protection remained partial through seta elongation with respect to survival, provided ≥2 d had passed from the termination of the embryonic phenophase. Once the capsule begins to expand (pre-meiosis), DT appears to be entirely constitutive. Second, the sporophytes that survived after exposure to rapid drying were as a group markedly smaller in total biomass, seta length and capsule volume than undried or slowly dried sporophytes. The significant differences in final measures of sporophyte size between post-embryonic sporophytes that were slowly dried versus those that were rapidly dried indicate that these maturing sporophytes employ an inducible strategy (in part) of DT. Thus, we have embryonic sporophytes of Aloina employing a very strong IDT strategy of DT (only able to survive a desiccation event in large numbers when desiccation occurs slowly), whereas post-embryonic sporophytes employ a weaker strategy of IDT, which, when taking survival into consideration, can be considered a moderate CDT strategy.
Given (1) recent data indicating that all mosses, including some species of Sphagnum (not a true moss), are likely to be DT to some degree, including even aquatic mosses and Physcomitrella (Proctor et al., 2007a; Cruz de Carvalho et al., 2011, 2012; Greenwood and Stark, 2014; Hájek and Vicherová, 2014), (2) the probability that most mechanistic studies of bryophyte DT failed to adequately deharden plants prior to testing, thereby distorting the prominence of constitutive processes over inducible ones (Stark et al., 2014), and (3) the fact that the field of bryophyte ecological DT is presently without a unifying hypothesis to frame future research (unlike the mechanistic paradigm of bryophyte DT, which states that DT is conferred through a combination of constitutive protection coupled with rehydration-induced repair processes; Proctor et al., 2007a), we would like to elevate the suggestion of Pressel et al. (2006) and Wood (2007) to a hypothesis that complements and validates the early work of Abel (1956), and call it the ‘continuum hypothesis of ecological DT in bryophytes’. This hypothesis states that ecological DT in mosses (and probably also liverworts) exists along a gradient from a strictly inducible strategy to a predominantly constitutive strategy, with absolute CDT and IDT probably unobtainable. It predicts that life-history phases of bryophytes may exhibit different strategies of DT, even within the same species (e.g. protonema, juvenile shoots, adult shoots, embryos, post-embryonic sporophytes, antheridia, sperm and spores). It also predicts that complete constitutive protection can be approached but not reached; thus, ecological DT can be viewed along an inducibility gradient (weakly to strongly inducible; we note that the pre-meiotic capsules tested here appeared to be entirely CDT, but we considered only one response variable—maturation through meiosis—and when other responses can be measured this assessment may change). Most ecological strategies of DT are expected to occur along this gradient, with the more constitutive plants or structures (e.g. shoots of Syntrichia and Grimmia species) expected to employ at least in small measure an inducible component of DT. Testing this hypothesis requires the use of either dehardened (deacclimated) field material or laboratory-grown cultures and a method of applying rapid drying to such plants or structures. This hypothesis has a historical parallel among the DT seed literature: attempts to classify seed DT as either orthodox or recalcitrant (Pammenter and Berjak, 1999) proved difficult, prompting the suggestion that (quoting from Walters et al., 2002) ‘in reality, a continuum exists among seed species, based on desiccation response’.
An often-overlooked yet thorough analysis of DT in mosses was carried out by Abel (1956) and merits mention here. He assessed detached leaves of 61 species of mosses by exposing leaves to RHs ranging from 0 % to 100 % with and without a predesiccation treatment for 24 h at 96 % RH, assessing damage by cell viability as determined visually. Although it is uncertain whether Abel intentionally deacclimated the leaves prior to the test beyond a few days, and certainly the behaviour of detached leaves may deviate from that of whole shoots, his results are consistent with the continuum hypothesis proposed here: 17 species exhibited CDT (using exposure to 50 % RH directly), 30 species exhibited IDT (tolerating desiccation after a pretreatment/partial drying treatment) and the remaining species exhibited extreme sensitivity to desiccation even on exposure to 80 % RH. Finding a significant number of species to be desiccation-sensitive may derive from the relatively short time used (24 h) for the pretreatments; some species may require a more protracted pre-exposure to partially dry conditions (e.g. Physcomitrella) in order to induce DT.
Inducible desiccation tolerance in shoots
The shoots of A. ambigua exhibit a fairly clear inducible strategy of DT (IDT), with rapidly dried shoots incurring roughly twice the leaf damage as undried controls or slowly dried shoots, and similar damage being incurred between vegetative and post-embryonic sporophytic shoots (shoots bearing sporophytes). We used a leaf damage assay that corresponds well with chlorophyll fluorescence assays (Stark et al., 2013, 2014). This mode of vegetative DT, considered to be uncommon in bryophytes (Toldi et al., 2009), is reported here for a third arid-land species of bryophyte, which along with Pterygoneurum lamellatum and Crossidium crassinerve (Stark et al., 2014) brings the number of known IDT bryophyte species to 16 (summarized in Stark et al., 2013) if we add here the protonema of Ditrichum plumbicola, as inferred from a rapid drying treatment (Rowntree et al., 2007). This number is expected to increase as studies of DT using dehardened shoots are performed. Consistent with the continuum hypothesis of ecological DT in mosses presented above, shoots of A. ambigua retained some constitutive protection, with the shoot apex and portions of leaves retaining chlorophyll after rapid drying and the shoot being capable of regeneration.
Shoots bearing embryos that were subjected to rapid drying suffered significantly more damage than either vegetative shoots or shoots bearing post-embryonic sporophytes that were also subjected to rapid drying. In many cases all of the leaves surrounding the embryo were bleached. This result may derive from differences in maternal partitioning of resources between the two sporophyte phenophases, creating increased vulnerability to shoots bearing an embryo.
Self-compatibility and duration of sporophyte development
Self-compatibility in bryophytes is known to occur in 21 species, while self-incompatibility has been detected in three species, including Aloina bifrons (Stark and Brinda, 2013). By cloning a single line of A. ambigua here and allowing it to self-fertilize in culture, the sexual condition (rhizautoicy) was confirmed and self-compatibility demonstrated. From a subcultured single plant to embryonic sporophyte took ∼4–5 months. The duration of sporophyte development in culture took ∼40 d, as follows: from detectable embryos to seta elongation, 13 d; from seta elongation to capsule expansion, 15 d; from capsule expansion to meiosis, 9 d; and from meiosis to postmeiosis, 4 d. This compares with 60–65 d from seta elongation to postmeiosis in cultures of Funaria hygrometrica (Garner and Paolillo, 1973) and ∼35 d in Phascum cuspidatum (Hughes and Wiggin, 1969).
Evolution of CDT in sporophytes
While the proximate mechanism for conferring partial constitutive DT in post-embryonic sporophytes may be a by-product of the transfer of sucrose from the maternal shoot to the sporophyte, the ultimate evolutionary factor may reside in the variation in desiccation intensity experienced by the sporophyte as it elongates. We propose that the factor promoting the transition from a predominantly IDT sporophyte to a partially CDT sporophyte involves increased environmental desiccation pressure as the sporophyte elongates away from the maternal shoot. As the seta extends in length (in mosses with exserted capsules), drying pressures on distal setal tissues are bound to be greater than on embryonic tissues protected by often specialized and elongated perichaetial leaves at the apex of the maternal shoot. Sporophytes may be subject to faster rates of desiccation as the seta elongates too fast for an inducible system of DT to confer protection, thus selecting for a partially constitutive strategy of DT. This hypothesis is supported by the recent finding of a specialized cuticle on the calyptra of F. hygrometrica that is postulated to reduce water loss during maturation of the sporophyte and confers desiccation protection that reduces sporophyte damage, enhances survival, reduces peristome developmental malfunctions and increases spore output (Budke et al., 2012, 2013). Furthermore, in F. hygrometrica the cuticle on the young post-embryonic sporophytes is less well developed than on older sporophytes, prompting Budke et al. (2012) to hypothesize that protection of early post-embryonic sporophytes against desiccation is provided by the maternal calyptra.
We propose that the proximate mechanism for CDT in post-embryonic sporophytes involves the one-way transfer of nutrients, including most prominently sucrose, from the shoot to the developing sporophyte, which is well documented in mosses (e.g. Chevallier et al., 1977). This flow of sucrose may infuse the developing sporophyte shortly after the termination of the embryonic phenophase. Most of the transfer occurs during capsule expansion (Proctor, 1977), although in species with long setae the movement of sucrose through setal tissues may begin earlier than capsule expansion and confer CDT quickly in non-photosynthetic setal tissues. Because the foot is not well established during the embryonic phenophase, embryos are not expected to have levels of sucrose that would allow survival of rapid drying. However, once the seta elongation phenophase (and all phenophases subsequent to seta elongation) is reached, sucrose levels imported from the gametophyte (Renault et al., 1992) are expected to be present in the sporophyte, and therefore the sporophyte is expected to better tolerate rapid drying events. The sporophyte–gametophyte junction consists of a placental region where abundant small vacuoles may be present in the embryonic sporophyte, and these vacuoles are postulated to protect the rapidly growing sporophytes from water stress in Acaulon muticum (Rushing and Anderson, 1996); wall ingrowths are characteristic of the sporophyte–gametophyte junction and facilitate the flow of solutes and nutrients into the sporophyte (Yip and Rushing, 1999).
It is possible that a slower rate of drying may allow proteins and sucrose to interact in a more protective manner (Wolkers et al., 2001). In two bryophyte species, slow-drying treatment induces the production of either endogenous ABA and/or dehydrin production (Werner et al., 1991; Hellwege et al., 1994), with exogenously applied ABA known to elevate sucrose levels and provide protection from rapid drying events in several species (e.g. Pence, 1998; Oldenhof et al., 2006). Exogenously applied ABA ‘triggers the expression of numerous genes in Physcomitrella, including those of several homologs to stress proteins that accumulate in other plants … included among these are two dehydrin-like proteins’ (Koster et al., 2010). Thus, ABA is likely to be produced during a slow drying event (noting that it has yet to be found in Physcomitrella patens or Syntrichia ruralis) and is integral to the IDT strategy of DT in both bryophytes and DT vascular plants.
ACKNOWLEDGEMENTS
We thank Joshua Greenwood, Melvin Oliver and Brent Mishler for conceptual input to this experiment; Michael Proctor for helpful translations from the early German work in the field; funding and a permit from the US National Park Service (Great Basin Cooperative Ecosystem Studies Unit, Cooperative Agreement Number H8R07060001), allowing collections within the Grand Canyon–Parashant National Monument; Gabriela Benito, Matthew Glen, Wendie Welch and Elisha Rhodes for media, nutrient solution, water preparation and contributing ideas assisting this research; R. Riker for assistance with the colour plate; Natalie Schimbrowski for translating critical German passages of Abel (1956); and the comments of two anonymous referees that strengthened the paper. L.R.S. was supported by a UNLV faculty sabbatical leave during part of this project.
LITERATURE CITED
- Abel WO. 1956. Die Austrocknungsresistenz der Laubmoose. Sitzungsberichte. Österreichische Akademie der Wissenschaften. Mathematisch-naturwissenschaftliche Klasse, Abteilung. I, 165: 619–707. [Google Scholar]
- Alpert P, Oliver MJ. 2002. Drying without dying. In: Black M, Pritchard HW. eds. Desiccation and survival in plants: drying without dying. Wallingford: CABI Publishing, 3–43. [Google Scholar]
- Barker DH, Stark LR, Zimpfer JF, McLetchie ND, Smith 2005. Evidence of drought-induced stress on biotic crust moss in the Mojave Desert. Plant, Cell and Environment 28: 939–947. [Google Scholar]
- Beckett RP. 2001. ABA-induced tolerance to ion leakage during rehydration following desiccation in the moss Atrichum androgynum. Plant Growth Regulation 35: 131–135. [Google Scholar]
- Bewley JD. 1995. Physiological aspects of desiccation tolerance—a retrospect. International Journal of Plant Sciences 156: 393–403. [Google Scholar]
- Bopp M, Werner O. 1993. Abscisic acid and desiccation tolerance in mosses. Botanica Acta 106: 103–106. [Google Scholar]
- Box GEP, Cox DR. 1964. An analysis of transformations (with discussion). Journal of the Royal Statistical Society B 26: 211–252. [Google Scholar]
- Budke JM, Goffinet B, Jones CS. 2012. The cuticle on the gametophyte calyptra matures before the sporophyte cuticle in the moss Funaria hygrometrica (Funariaceae). American Journal of Botany 99: 14–22. [DOI] [PubMed] [Google Scholar]
- Budke JM, Goffinet B, Jones CS. 2013. Dehydration protection provided by a maternal cuticle improves offspring fitness in the moss Funaria hygrometrica. Annals of Botany 111: 781–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaghan TV, Collins NJ, Callaghan CH. 1978. Photosynthesis, growth and reproduction of Hylocomium splendens and Polytrichum commune in Swedish Lapland. Oikos 31: 73–88. [Google Scholar]
- Chevallier D, Nurit F, Pesey H. 1977. Orthophosphate absorption by the sporophyte of Funaria hygrometrica during maturation. Annals of Botany 41: 527–531. [Google Scholar]
- Cruz de Carvalho R, Branquinho C, Marques da Dilva J. 2011. Physiological consequences of desiccation in the aquatic bryophyte Fontinalis antipyretica. Planta 234: 195–205. [DOI] [PubMed] [Google Scholar]
- Cruz de Carvalho R, Catalá M, Marques da Silva J, Branquinho C, Barreno E. 2012. The impact of dehydration rate on the production and cellular location of reactive oxygen species in an aquatic moss. Annals of Botany 110: 1007–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgadillo C. 2007. Aloina. In: Flora North America Editorial Committee. eds. Flora North America, Volume 27, Bryophytes: mosses, part 1. New York: Oxford University Press, 614–617. [Google Scholar]
- Forman RTT. 1965. A system of studying moss phenology. Bryologist 68: 289–300. [Google Scholar]
- Gallego MT, Cano MJ, Ros RM, Guerra J. 1999. The genus Aloina (Pottiaceae, Musci) in the Mediterranean region and neighbouring areas. Nova Hedwigia 69: 173–194. [Google Scholar]
- Garner D, Paolillo DJ., Jr 1973. A time-course of sporophyte development in Funaria hygrometrica Hedw. Bryologist 76: 356–360. [Google Scholar]
- Glime JM. 2007a. Ecophysiology of development: sporophyte. In: Bryophyte ecology, Volume 1. Physiological ecology . Ebook sponsored by Michigan Technological University and the International Association of Bryologists, Chapter 5-9. http://www.bryoecol.mtu.edu/ (25 September 2013). [Google Scholar]
- Glime JM. 2007b. Ecophysiology of development: spore germination. In: Bryophyte Ecology, Volume 1. Physiological ecology . Ebook sponsored by Michigan Technological University and the International Association of Bryologists, Chapter 5-2. http://www.bryoecol.mtu.edu/ (25 September 2013). [Google Scholar]
- Greenwood JL, Stark LR. 2014. Rate of drying determines extent of desiccation tolerance in Physcomitrella patens. Functional Plant Biology 41: 460–467. [DOI] [PubMed] [Google Scholar]
- Hájek T, Vicherová E. 2014. Desiccation tolerance of Sphagnum revisited: a puzzle resolved. Plant Biology 16: 765–773. [DOI] [PubMed] [Google Scholar]
- Hellwege EM, Dietz K, Volk OH, Hartung W. 1994. Abscisic acid and the induction of desiccation tolerance in the extremely xerophilic liverwort Exormotheca holstii. Planta 194: 525–531. [Google Scholar]
- Hoagland DR, Arnon DI. 1938. The water-culture method for growing plants without soil. California Agricultural Experiment Station Circular 347: 1–39. [Google Scholar]
- Horsley K, Stark LR, McLetchie DN. 2011. Does the silver moss Bryum argenteum exhibit sex-specific patterns in vegetative growth rate, asexual fitness or prezygotic reproductive investment? Annals of Botany 107: 897–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes JG, Wiggin AJA. 1969. Light intensity and sexual reproduction in Phascum cuspidatum Hedw. Transactions of the British Bryological Society 5: 823–826. [Google Scholar]
- Koster KL, Balsamo RA, Espinoza C, Oliver MJ. 2010. Desiccation sensitivity and tolerance in the moss Physcomitrella patens: assessing limits and damage. Plant Growth Regulation 62: 293–302. [Google Scholar]
- Kutner MH, Nachtsheim CJ, Neter J, Li W. 2004. Applied linear statistical models , 5th edn New York: McGraw-Hill/Irwin. [Google Scholar]
- Longton RE. 1988. The biology of polar bryophytes and lichens. Cambridge: Cambridge University Press. [Google Scholar]
- Longton RE, Greene SW. 1969. Relationship between sex distribution and sporophyte production in Pleurozium schreberi (Brid.) Mitt. Annals of Botany 33: 107–126. [Google Scholar]
- McLetchie DN, Stark LR. 2006. Sporophyte and gametophyte generations differ in responses to thermotolerance in the moss Microbryum. Annals of Botany 97: 505–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris DH, Shevock JR. 2004. Contributions toward a bryoflora of California: II. A key to the mosses. Madroño 51: 133–269. [Google Scholar]
- Oldenhof H, Wolkers WF, Bowman JL, Tablin F, Crowe JH. 2006. Freezing and desiccation tolerance in the moss Physcomitrella patens: an in situ Fourier transform infrared spectroscopic study. Biochimica et Biophysica Acta 1760: 1226–1234. [DOI] [PubMed] [Google Scholar]
- Oliver MJ, Wood AJ, O’Mahony P. 1998. ‘To dryness and beyond’—preparation for the dried state and rehydration in vegetative desiccation-tolerant plants. Plant Growth Regulation 24: 193–201. [Google Scholar]
- Oliver MJ, Velten J, Wood AJ. 2000. Bryophytes as experimental models for the study of environmental stress tolerance: Tortula ruralis and desiccation-tolerance in mosses. Plant Ecology 151: 73–84. [Google Scholar]
- Pammenter NW, Berjak P. 1999. A review of recalcitrant seed physiology in relation to desiccation-tolerance mechanisms. Seed Science Research 9: 13–37. [Google Scholar]
- Pence VC. 1998. Cryopreservation of bryophytes: the effects of ABA and encapsulation dehydration. Bryologist 101: 278–281. [Google Scholar]
- Pressel S, Duckett JG. 2010. Cytological insights into the desiccation biology of a model system: moss protonemata. New Phytologist 185: 944–963. [DOI] [PubMed] [Google Scholar]
- Pressel S, Ligrone R, Duckett JG. 2006. Effects of de- and rehydration on food-conducting cells in the moss Polytrichum formosum: a cytological study. Annals of Botany 98: 67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proctor MCF. 1977. Evidence on the carbon nutrition of moss sporophytes from 14CO2 uptake and the subsequent movement of labelled assimilate. Journal of Bryology 9: 375–386. [Google Scholar]
- Proctor MCF, Pence VC. 2002. Vegetative tissues: bryophytes, vascular ‘resurrection plants' and vegetative propagules. In: Pritchard H, Black M. eds. Desiccation and plant survival. Wallingford: CABI Publishing, 207–237. [Google Scholar]
- Proctor MCF, Oliver MJ, Wood AJ, et al. 2007a. Desiccation-tolerance in bryophytes: a review. Bryologist 110: 595–621. [Google Scholar]
- Proctor MCF, Ligrone R, Duckett JG. 2007b. Desiccation tolerance in the moss Polytrichum formosum: physiological and fine-structural changes during desiccation and recovery. Annals of Botany 99: 75–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Development Core Team. 2014. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing. http://www.R-project.org/. [Google Scholar]
- Renault S, Bonnemain JL, Faye L, Gaudillere JP. 1992. Physiological aspects of sugar exchange between the gametophyte and the sporophyte of Polytrichum formosum. Plant Physiology 100: 1815–1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowntree JK, Duckett JG, Mortimer CL, Ramsay MM, Pressel S. 2007. Formation of specialized propagules resistant to desiccation and cryopreservation in the threatened moss Ditrichum plumbicola (Ditrichales, Bryopsida). Annals of Botany 100: 483–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushing AE, Anderson WB. 1996. The sporophyte-gametophyte junction in the moss Acaulon muticum (Pottiaceae): early stages of development. American Journal of Botany 83: 1274–1281. [Google Scholar]
- Schonbeck MW, Bewley JD. 1981. Responses of the moss Tortula ruralis to desiccation treatments. I. Effects of minimum water content and rates of dehydration and rehydration. Canadian Journal of Botany 59: 2698–2706. [Google Scholar]
- Shaw AJ, Goffinet B. 2000. Bryophyte biology. Cambridge: Cambridge University Press. [Google Scholar]
- Stark LR. 2001. Widespread sporophyte abortion following summer rains in Mojave Desert populations of Grimmia orbicularis. Bryologist 104: 115–125. [Google Scholar]
- Stark LR. 2002. New frontiers in bryology: phenology and its repercussions on the reproductive ecology of mosses. Bryologist 105: 204–218. [Google Scholar]
- Stark LR. 2005. Phenology of patch hydration, patch temperature and sexual reproductive output over a four-year period in the desert moss Crossidium crassinerve. Journal of Bryology 27: 231–240. [Google Scholar]
- Stark LR, Brinda JC. 2013. An experimental demonstration of rhizautoicy, self-incompatibility, and reproductive investment in Aloina bifrons (Pottiaceae). Bryologist 116: 43–52. [Google Scholar]
- Stark LR, Stephenson AG. 1983. Reproductive biology in Entodon cladorrhizans (Bryopsida, Entodontaceae). II. Resource-limited reproduction and sporophyte abortion. Systematic Botany 8: 389–394. [Google Scholar]
- Stark LR, Mishler BD, McLetchie DN. 2000. The cost of realized sexual reproduction: assessing patterns of reproductive allocation and sporophyte abortion in a desert moss. American Journal of Botany 87: 1599–1608. [PubMed] [Google Scholar]
- Stark LR, Oliver MJ, Mishler BD, McLetchie DN. 2007. Generational differences in response to desiccation stress in the desert moss Tortula inermis. Annals of Botany 99: 53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark LR, Brinda JC, McLetchie DN. 2011. Effects of increased summer precipitation and N deposition on Mojave Desert populations of the biological crust moss Syntrichia caninervis. Journal of Arid Environments 75: 457–463. [Google Scholar]
- Stark LR, Greenwood JL, Brinda JC, Oliver MJ. 2013. The desert moss Pterygoneurum lamellatum exhibits inducible desiccation tolerance: effects of rate of drying on shoot damage and regeneration. American Journal of Botany 100: 1522–1531. [DOI] [PubMed] [Google Scholar]
- Stark LR, Greenwood JL, Brinda JC, Oliver MJ. 2014. Physiological history may mask the inherent inducible desiccation tolerance strategy of the desert moss Crossidium crassinerve. Plant Biology 16: 935–946. [DOI] [PubMed] [Google Scholar]
- Testo WL, Watkins JE. 2012. Influence of plant size on the ecophysiology of the epiphytic fern Asplenium auritum (Aspleniaceae) from Costa Rica. American Journal of Botany 99: 1840–1846. [DOI] [PubMed] [Google Scholar]
- Therneau TM, Grambsch PM. 2000. Modeling survival data: extending the Cox model. New York: Springer. [Google Scholar]
- Tobin J. 1958. Estimation of relationships for limited dependent variables. Econometrica 26: 24–36. [Google Scholar]
- Toldi O, Tuba Z, Scott P. 2009. Vegetative desiccation tolerance: is it a goldmine for bioengineering crops? Plant Science 176: 187–199. [Google Scholar]
- Walters C, Farrant JM, Pammenter NW, Berjak P. 2002. Desiccation stress and damage. In: Black M, Pritchard HW. eds. Desiccation and survival in plants: drying without dying. New York: CABI Publishing, 263–291. [Google Scholar]
- Watanabe M, Kikawada T, Minagawa N, Yukuhiro Fokuda T. 2002. Mechanism allowing an insect to survive complete dehydration and extreme temperatures. Journal of Experimental Biology 205: 2799–2802. [DOI] [PubMed] [Google Scholar]
- Watkins JE, Jr, Mack MC, Sinclair TR, Mulkey SS. 2007. Ecological and evolutionary consequences of desiccation tolerance in tropical fern gametophytes. New Phytologist 176: 708–717. [DOI] [PubMed] [Google Scholar]
- Werner O, Ros Espín RM, Bopp M, Atzorn R. 1991. Abscisic-acid-induced drought tolerance in Funaria hygrometrica Hedw. Planta 186: 99–103. [DOI] [PubMed] [Google Scholar]
- Wolkers WF, McCready S, Brandt WF, Lindsey GG, Hoekstra FA. 2001. Isolation and characterization of a D-7 LEA protein from pollen that stabilizes glasses in vitro. Biochimica et Biophysica Acta 1544: 196–206. [DOI] [PubMed] [Google Scholar]
- Wood AJ. 2007. The nature and distribution of vegetative desiccation tolerance in hornworts, liverworts and mosses. Bryologist 110: 163–177. [Google Scholar]
- Yip KL, Rushing AE. 1999. An ultrastructural and developmental study of the sporophyte-gametophyte junction in Ephemerum cohaerens. Bryologist 102: 179–195. [Google Scholar]