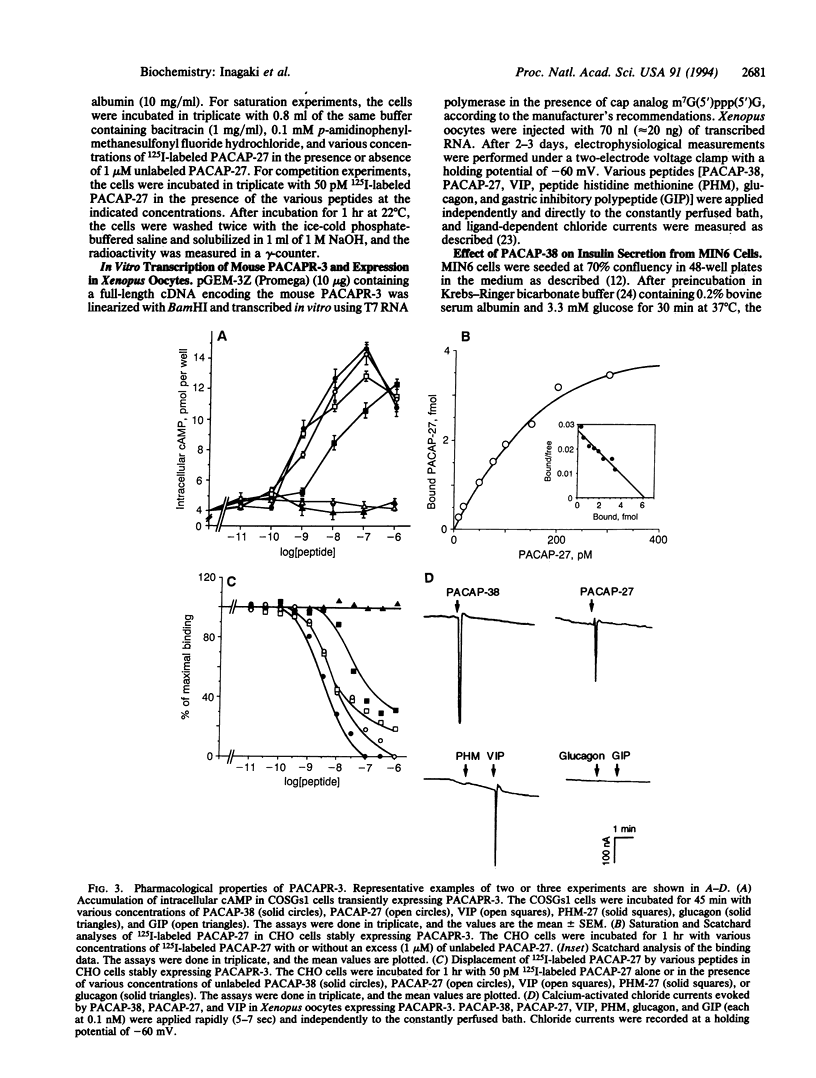
Abstract
Pituitary adenylate cyclase-activating polypeptide (PACAP) is a neuropeptide belonging to the vasoactive intestinal polypeptide/glucagon/secretin family. It is widely distributed in the body, and a variety of biological actions have been reported. PACAP exerts its biological effects by binding to specific receptors that are coupled to GTP-binding proteins. Recent studies have shown that there is a family of PACAP receptors (PACAPRs), and two members of this family have been identified. We report here the cloning, functional expression, and tissue distribution of a third PACAPR subtype, designated PACAPR-3. The cDNA encoding PACAPR-3 has been isolated from a mouse insulin-secreting beta-cell line MIN6 cDNA library. Mouse PACAPR-3 is a protein of 437 amino acids that has 50% and 51% identity with rat PACAP type I and type II receptors, respectively. Expression of recombinant mouse PACAPR-3 in mammalian cells shows that it binds to vasoactive intestinal polypeptide as well as PACAP-38 and -27, with a slightly higher affinity for PACAP-38, and is positively coupled to adenylate cyclase. The expression of PACAPR-3 in Xenopus oocytes indicates that calcium-activated chloride currents are evoked by PACAP and vasoactive intestinal polypeptide, suggesting that PACAPR-3 can also be coupled to phospholipase C. RNA blot analysis studies reveal that PACAPR-3 mRNA is expressed at high levels in MIN6, at moderate levels in pancreatic islets and other insulin-secreting cell lines, HIT-T15 and RINm5F, as well as in the lung, brain, stomach, and colon, and at low levels in the heart. Furthermore, insulin secretion from MIN6 cells is significantly stimulated by PACAP-38. These results suggest that the diverse biological effects of PACAP are mediated by a family of structurally related proteins and that PACAPR-3 participates in the regulation of insulin secretion.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arimura A. Pituitary adenylate cyclase activating polypeptide (PACAP): discovery and current status of research. Regul Pept. 1992 Feb 18;37(3):287–303. [PubMed] [Google Scholar]
- Arimura A., Somogyvári-Vigh A., Miyata A., Mizuno K., Coy D. H., Kitada C. Tissue distribution of PACAP as determined by RIA: highly abundant in the rat brain and testes. Endocrinology. 1991 Nov;129(5):2787–2789. doi: 10.1210/endo-129-5-2787. [DOI] [PubMed] [Google Scholar]
- Blondel O., Takeda J., Janssen H., Seino S., Bell G. I. Sequence and functional characterization of a third inositol trisphosphate receptor subtype, IP3R-3, expressed in pancreatic islets, kidney, gastrointestinal tract, and other tissues. J Biol Chem. 1993 May 25;268(15):11356–11363. [PubMed] [Google Scholar]
- Dohlman H. G., Thorner J., Caron M. G., Lefkowitz R. J. Model systems for the study of seven-transmembrane-segment receptors. Annu Rev Biochem. 1991;60:653–688. doi: 10.1146/annurev.bi.60.070191.003253. [DOI] [PubMed] [Google Scholar]
- Fridolf T., Sundler F., Ahrén B. Pituitary adenylate cyclase-activating polypeptide (PACAP): occurrence in rodent pancreas and effects on insulin and glucagon secretion in the mouse. Cell Tissue Res. 1992 Aug;269(2):275–279. doi: 10.1007/BF00319618. [DOI] [PubMed] [Google Scholar]
- Fukumoto H., Seino S., Imura H., Seino Y., Eddy R. L., Fukushima Y., Byers M. G., Shows T. B., Bell G. I. Sequence, tissue distribution, and chromosomal localization of mRNA encoding a human glucose transporter-like protein. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5434–5438. doi: 10.1073/pnas.85.15.5434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto H., Ishihara T., Shigemoto R., Mori K., Nagata S. Molecular cloning and tissue distribution of a receptor for pituitary adenylate cyclase-activating polypeptide. Neuron. 1993 Aug;11(2):333–342. doi: 10.1016/0896-6273(93)90188-w. [DOI] [PubMed] [Google Scholar]
- Hosoya M., Onda H., Ogi K., Masuda Y., Miyamoto Y., Ohtaki T., Okazaki H., Arimura A., Fujino M. Molecular cloning and functional expression of rat cDNAs encoding the receptor for pituitary adenylate cyclase activating polypeptide (PACAP). Biochem Biophys Res Commun. 1993 Jul 15;194(1):133–143. doi: 10.1006/bbrc.1993.1795. [DOI] [PubMed] [Google Scholar]
- Inagaki N., Maekawa T., Sudo T., Ishii S., Seino Y., Imura H. c-Jun represses the human insulin promoter activity that depends on multiple cAMP response elements. Proc Natl Acad Sci U S A. 1992 Feb 1;89(3):1045–1049. doi: 10.1073/pnas.89.3.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki N., Yasuda K., Inoue G., Okamoto Y., Yano H., Someya Y., Ohmoto Y., Deguchi K., Imagawa K., Imura H. Glucose as regulator of glucose transport activity and glucose-transporter mRNA in hamster beta-cell line. Diabetes. 1992 May;41(5):592–597. doi: 10.2337/diab.41.5.592. [DOI] [PubMed] [Google Scholar]
- Ishihara T., Nakamura S., Kaziro Y., Takahashi T., Takahashi K., Nagata S. Molecular cloning and expression of a cDNA encoding the secretin receptor. EMBO J. 1991 Jul;10(7):1635–1641. doi: 10.1002/j.1460-2075.1991.tb07686.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara T., Shigemoto R., Mori K., Takahashi K., Nagata S. Functional expression and tissue distribution of a novel receptor for vasoactive intestinal polypeptide. Neuron. 1992 Apr;8(4):811–819. doi: 10.1016/0896-6273(92)90101-i. [DOI] [PubMed] [Google Scholar]
- Jelinek L. J., Lok S., Rosenberg G. B., Smith R. A., Grant F. J., Biggs S., Bensch P. A., Kuijper J. L., Sheppard P. O., Sprecher C. A. Expression cloning and signaling properties of the rat glucagon receptor. Science. 1993 Mar 12;259(5101):1614–1616. doi: 10.1126/science.8384375. [DOI] [PubMed] [Google Scholar]
- Jüppner H., Abou-Samra A. B., Freeman M., Kong X. F., Schipani E., Richards J., Kolakowski L. F., Jr, Hock J., Potts J. T., Jr, Kronenberg H. M. A G protein-linked receptor for parathyroid hormone and parathyroid hormone-related peptide. Science. 1991 Nov 15;254(5034):1024–1026. doi: 10.1126/science.1658941. [DOI] [PubMed] [Google Scholar]
- Kawai K., Ohse C., Watanabe Y., Suzuki S., Yamashita K., Ohashi S. Pituitary adenylate cyclase activating polypeptide stimulates insulin release from the isolated perfused rat pancreas. Life Sci. 1992;50(4):257–261. doi: 10.1016/0024-3205(92)90332-j. [DOI] [PubMed] [Google Scholar]
- Kimura C., Ohkubo S., Ogi K., Hosoya M., Itoh Y., Onda H., Miyata A., Jiang L., Dahl R. R., Stibbs H. H. A novel peptide which stimulates adenylate cyclase: molecular cloning and characterization of the ovine and human cDNAs. Biochem Biophys Res Commun. 1990 Jan 15;166(1):81–89. doi: 10.1016/0006-291x(90)91914-e. [DOI] [PubMed] [Google Scholar]
- Lin C., Lin S. C., Chang C. P., Rosenfeld M. G. Pit-1-dependent expression of the receptor for growth hormone releasing factor mediates pituitary cell growth. Nature. 1992 Dec 24;360(6406):765–768. doi: 10.1038/360765a0. [DOI] [PubMed] [Google Scholar]
- Lin H. Y., Harris T. L., Flannery M. S., Aruffo A., Kaji E. H., Gorn A., Kolakowski L. F., Jr, Lodish H. F., Goldring S. R. Expression cloning of an adenylate cyclase-coupled calcitonin receptor. Science. 1991 Nov 15;254(5034):1022–1024. doi: 10.1126/science.1658940. [DOI] [PubMed] [Google Scholar]
- Lutz E. M., Sheward W. J., West K. M., Morrow J. A., Fink G., Harmar A. J. The VIP2 receptor: molecular characterisation of a cDNA encoding a novel receptor for vasoactive intestinal peptide. FEBS Lett. 1993 Nov 8;334(1):3–8. doi: 10.1016/0014-5793(93)81668-p. [DOI] [PubMed] [Google Scholar]
- Mayo K. E. Molecular cloning and expression of a pituitary-specific receptor for growth hormone-releasing hormone. Mol Endocrinol. 1992 Oct;6(10):1734–1744. doi: 10.1210/mend.6.10.1333056. [DOI] [PubMed] [Google Scholar]
- Miyata A., Arimura A., Dahl R. R., Minamino N., Uehara A., Jiang L., Culler M. D., Coy D. H. Isolation of a novel 38 residue-hypothalamic polypeptide which stimulates adenylate cyclase in pituitary cells. Biochem Biophys Res Commun. 1989 Oct 16;164(1):567–574. doi: 10.1016/0006-291x(89)91757-9. [DOI] [PubMed] [Google Scholar]
- Miyazaki J., Araki K., Yamato E., Ikegami H., Asano T., Shibasaki Y., Oka Y., Yamamura K. Establishment of a pancreatic beta cell line that retains glucose-inducible insulin secretion: special reference to expression of glucose transporter isoforms. Endocrinology. 1990 Jul;127(1):126–132. doi: 10.1210/endo-127-1-126. [DOI] [PubMed] [Google Scholar]
- Morrow J. A., Lutz E. M., West K. M., Fink G., Harmar A. J. Molecular cloning and expression of a cDNA encoding a receptor for pituitary adenylate cyclase activating polypeptide (PACAP). FEBS Lett. 1993 Aug 23;329(1-2):99–105. doi: 10.1016/0014-5793(93)80202-6. [DOI] [PubMed] [Google Scholar]
- Pisegna J. R., Wank S. A. Molecular cloning and functional expression of the pituitary adenylate cyclase-activating polypeptide type I receptor. Proc Natl Acad Sci U S A. 1993 Jul 1;90(13):6345–6349. doi: 10.1073/pnas.90.13.6345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seino S., Chen L., Seino M., Blondel O., Takeda J., Johnson J. H., Bell G. I. Cloning of the alpha 1 subunit of a voltage-dependent calcium channel expressed in pancreatic beta cells. Proc Natl Acad Sci U S A. 1992 Jan 15;89(2):584–588. doi: 10.1073/pnas.89.2.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spengler D., Waeber C., Pantaloni C., Holsboer F., Bockaert J., Seeburg P. H., Journot L. Differential signal transduction by five splice variants of the PACAP receptor. Nature. 1993 Sep 9;365(6442):170–175. doi: 10.1038/365170a0. [DOI] [PubMed] [Google Scholar]
- Thorens B. Expression cloning of the pancreatic beta cell receptor for the gluco-incretin hormone glucagon-like peptide 1. Proc Natl Acad Sci U S A. 1992 Sep 15;89(18):8641–8645. doi: 10.1073/pnas.89.18.8641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada Y., Post S. R., Wang K., Tager H. S., Bell G. I., Seino S. Cloning and functional characterization of a family of human and mouse somatostatin receptors expressed in brain, gastrointestinal tract, and kidney. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):251–255. doi: 10.1073/pnas.89.1.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota Y., Sasai Y., Tanaka K., Fujiwara T., Tsuchida K., Shigemoto R., Kakizuka A., Ohkubo H., Nakanishi S. Molecular characterization of a functional cDNA for rat substance P receptor. J Biol Chem. 1989 Oct 25;264(30):17649–17652. [PubMed] [Google Scholar]



