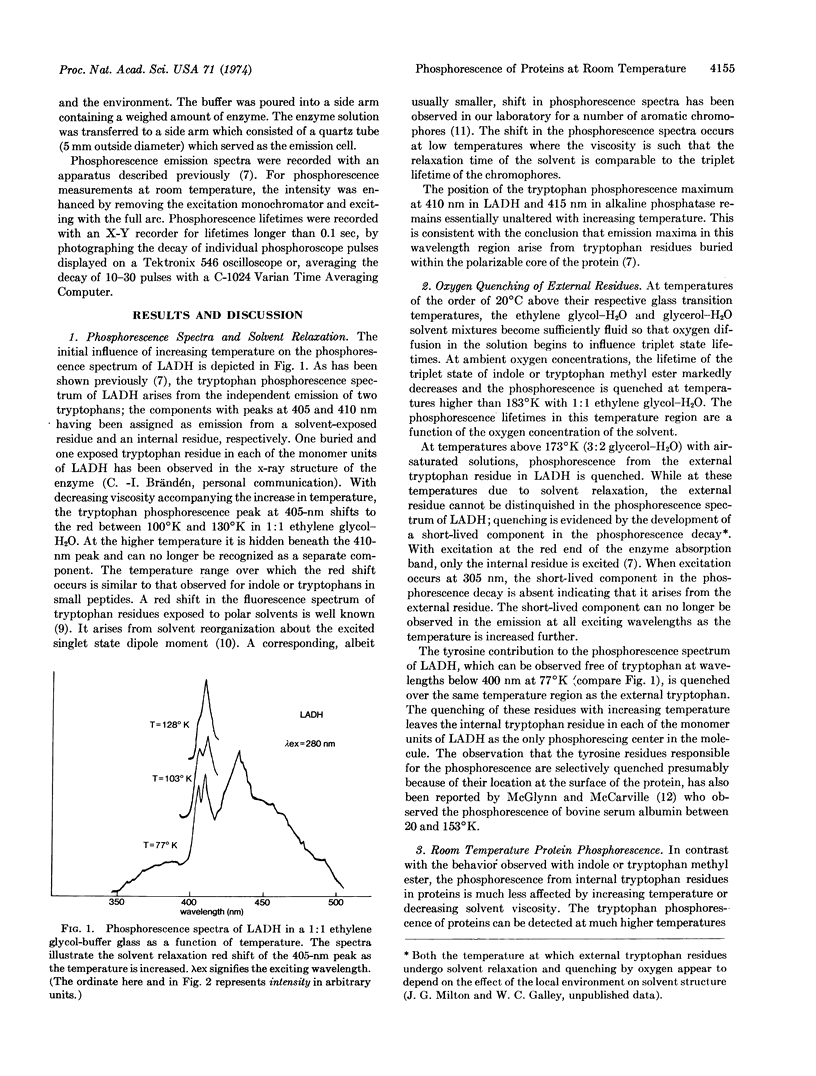
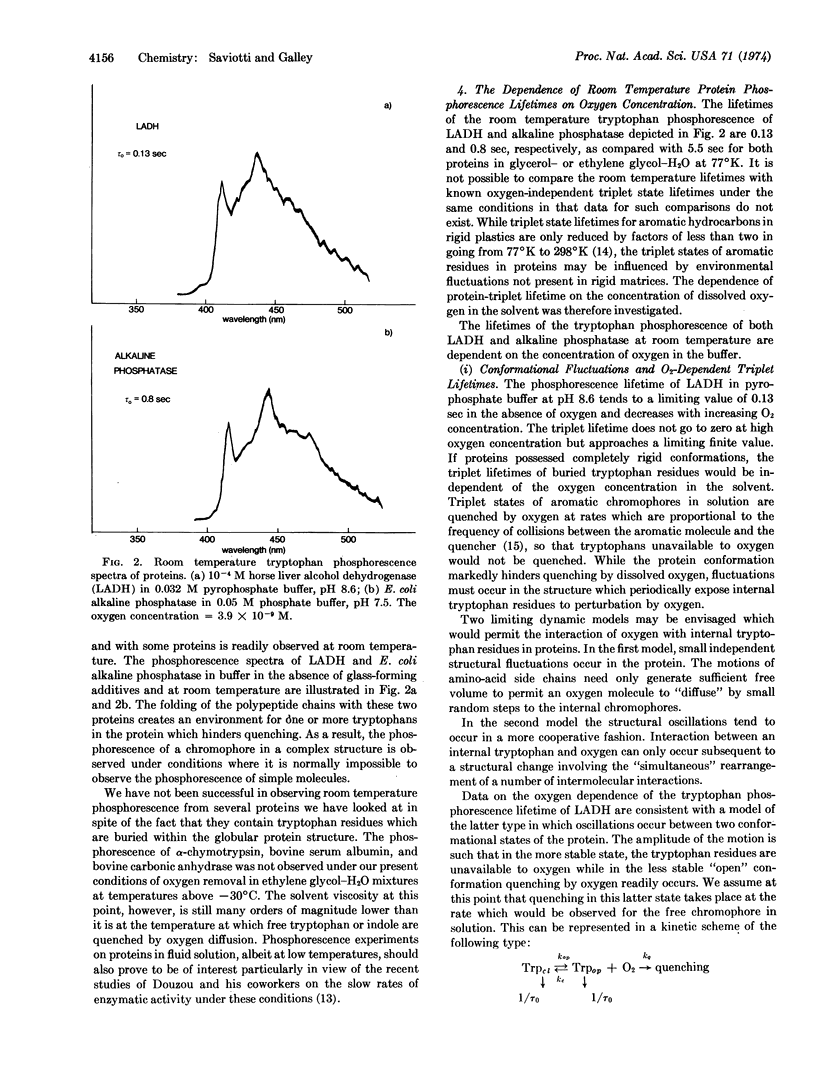
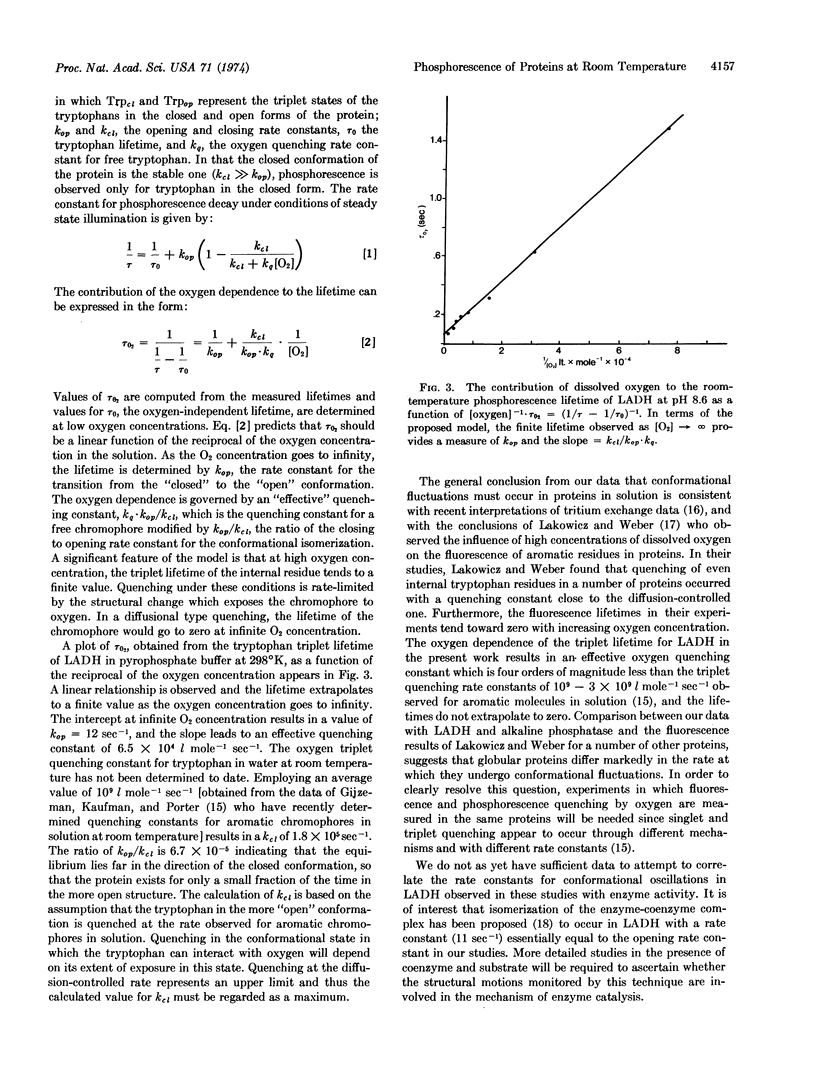
Abstract
While the phosphorescence of aromatic chromophores in solution is normally quenched through diffusion of dissolved oxygen and other solvent-mediated processes, the phosphorescence of some proteins in solution is observed at room temperature. The tryptophan phosphorescence arises from residues which are hindered from interaction with oxygen by the folding of the polypeptide chains. Measurements of the phosphorescence lifetime of horse liver alcohol dehydrogenase (alcohol: NAD+ oxidoreductase, EC 1.1.1.1) as a function of oxygen concentration indicate that internal tryptophan residues are periodically exposed to oxygen. This permits the calculation of rate constants for conformational oscillations in the enzyme. The present article illustrates the feasibility of employing phosphorescence in the study of proteins in solution in general and the utility of such experiments in probing the dynamic aspects of protein structure.
Keywords: conformational fluctuations, liver alcohol dehydrogenase
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLAND D. E. Eugenol lignin: its chemical properties and significance. Biochem J. 1961 Oct;81:23–28. doi: 10.1042/bj0810023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brändén C. I., Eklund H., Nordström B., Boiwe T., Söderlund G., Zeppezauer E., Ohlsson I., Akeson A. Structure of liver alcohol dehydrogenase at 2.9-angstrom resolution. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2439–2442. doi: 10.1073/pnas.70.8.2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debey P., Balny C., Douzou P. Enzyme assay in microsomes below zero degrees. Proc Natl Acad Sci U S A. 1973 Sep;70(9):2633–2636. doi: 10.1073/pnas.70.9.2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galley W. C. On the triplet states of polynucleotide--acridine complexes. I. Triplet energy delocalization in the 9-aminoacridine-DNA complex. Biopolymers. 1968;6(9):1279–1296. doi: 10.1002/bip.1968.360060905. [DOI] [PubMed] [Google Scholar]
- Galley W. C., Purkey R. M. Role of heterogeneity of the solvation site in electronic spectra in solution. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1116–1121. doi: 10.1073/pnas.67.3.1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galley W. C., Stryer L. Triplet-singlet energy transfer in proteins. Biochemistry. 1969 May;8(5):1831–1838. doi: 10.1021/bi00833a008. [DOI] [PubMed] [Google Scholar]
- Galley W. C., Stryer L. Triplet-triplet energy transfer in proteins as a criterion of proximity. Proc Natl Acad Sci U S A. 1968 May;60(1):108–114. doi: 10.1073/pnas.60.1.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISENBERG I., LESLIE R. B., BAIRD S. L., Jr, ROSENBLUTH R., BERSOHN R. DELAYED FLUORESCENCE IN DNA-ACRIDINE DYE COMPLEXES. Proc Natl Acad Sci U S A. 1964 Aug;52:379–387. doi: 10.1073/pnas.52.2.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jörnvall H. Horse liver alcohol dehydrogenase. The primary structure of the protein chain of the ethanol-active isoenzyme. Eur J Biochem. 1970 Sep;16(1):25–40. doi: 10.1111/j.1432-1033.1970.tb01049.x. [DOI] [PubMed] [Google Scholar]
- Lakowicz J. R., Weber G. Quenching of protein fluorescence by oxygen. Detection of structural fluctuations in proteins on the nanosecond time scale. Biochemistry. 1973 Oct 9;12(21):4171–4179. doi: 10.1021/bi00745a021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarville M. E., McGlynn S. P. Delayed luminescence of organic mixed crystals. IX. Amino acids and proteins. Photochem Photobiol. 1969 Sep;10(3):171–181. doi: 10.1111/j.1751-1097.1969.tb05677.x. [DOI] [PubMed] [Google Scholar]
- Purkey R. M., Galley W. C. Phosphorescence studies of environmental heterogeneity for tryptophyl residues in proteins. Biochemistry. 1970 Sep 1;9(18):3569–3575. doi: 10.1021/bi00820a010. [DOI] [PubMed] [Google Scholar]
- Shore J. D., Gutfreund H. Transients in the reactions of liver alcohol dehydrogenase. Biochemistry. 1970 Nov 24;9(24):4655–4659. doi: 10.1021/bi00826a006. [DOI] [PubMed] [Google Scholar]
- TEALE F. W. The ultraviolet fluorescence of proteins in neutral solution. Biochem J. 1960 Aug;76:381–388. doi: 10.1042/bj0760381. [DOI] [PMC free article] [PubMed] [Google Scholar]


