Abstract
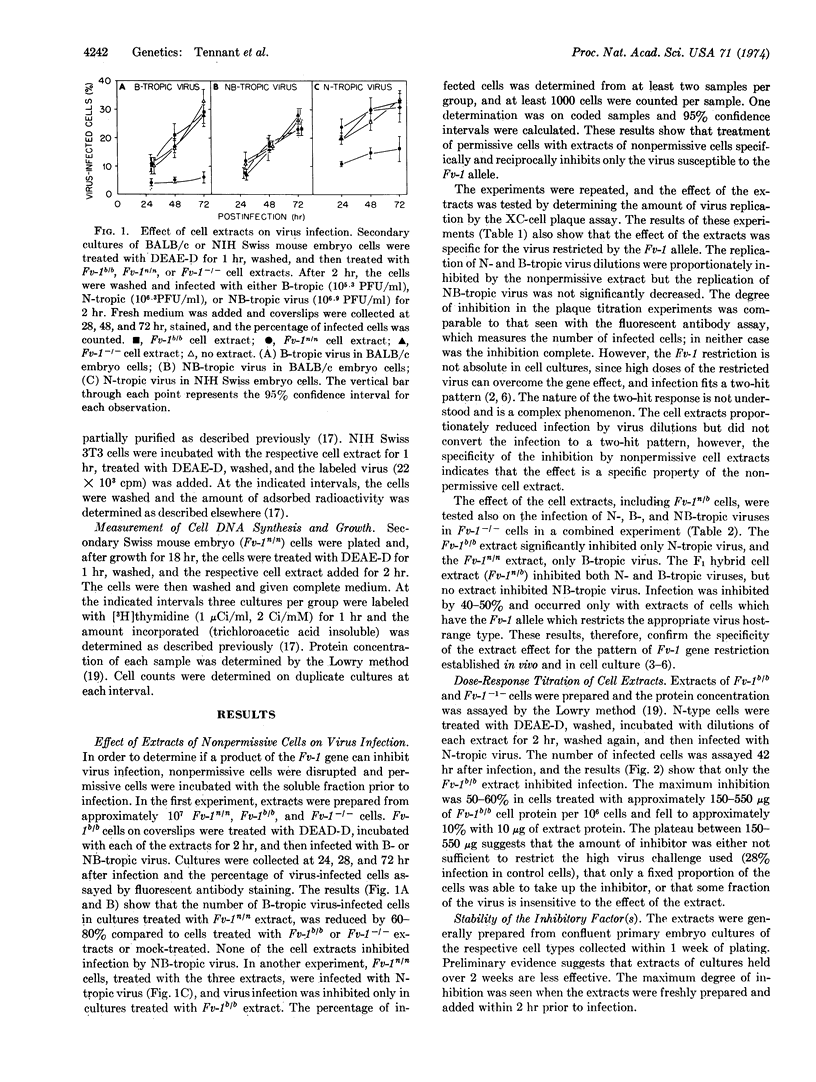
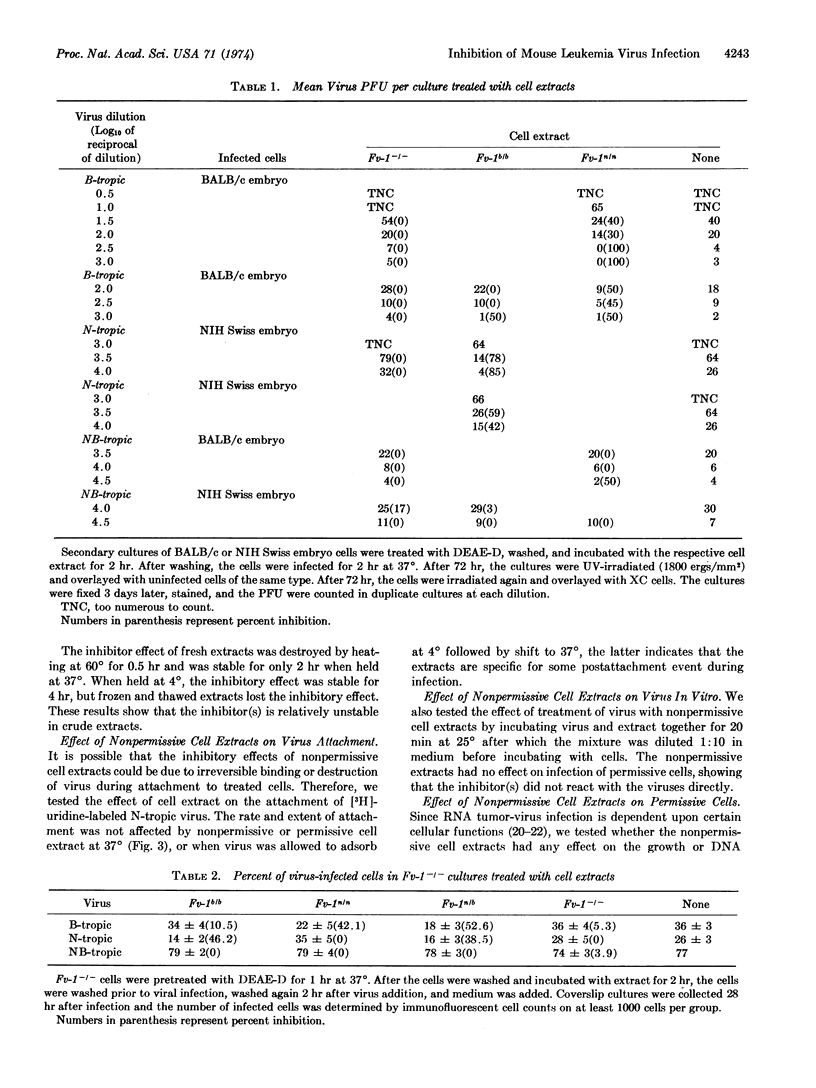
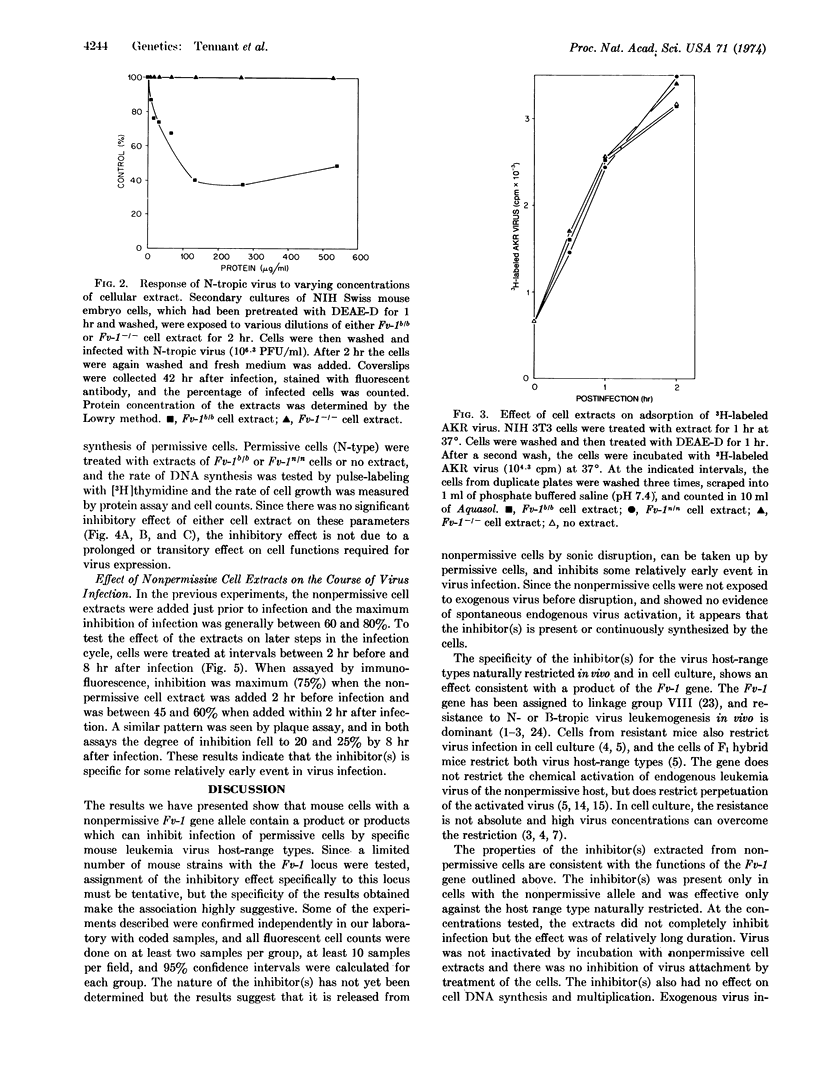
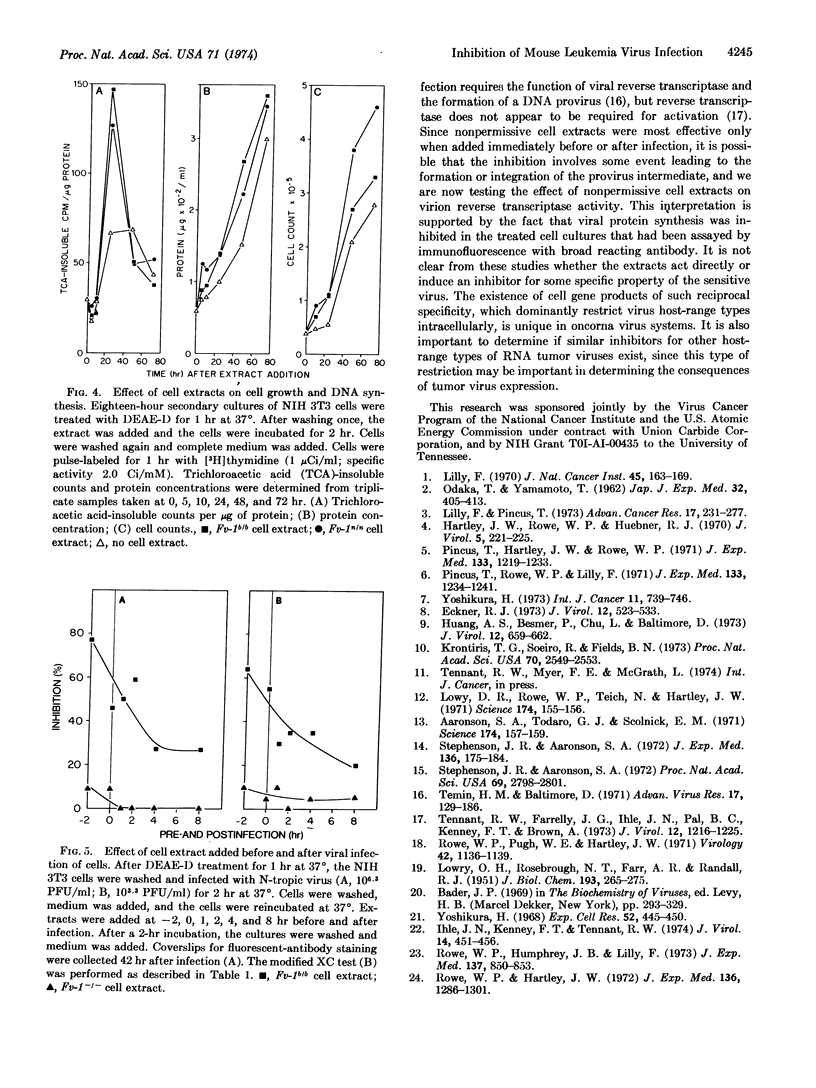
Soluble extracts of mouse cells with Fv-1n or Fv-1b gene alleles specifically and reciprocally inhibit infection of B- or N-tropic mouse leukemia viruses in permissive cell cultures. NB-tropic virus infection was not inhibited by either cell extract. Extracts from Fv-1- cells did not inhibit infection by the three virus host-range types, but N- or B-tropic virus infection of Fv-1- cells was inhibited by extracts of the nonpermissive cells, and Fv-1nb cell extracts inhibited both viruses. The maximum degree of inhibition was 50-80% as determined by immunofluorescent or plaque assays, with extracts containing up to 500 μg/ml of nonpermissive cell protein. The inhibitor(s) is relatively unstable since activity is lost after 2 hr at 37° or 30 min at 56°. The inhibitor(s) was most effective if added 2 hr before or within 2 hr after infection, did not react with the virus directly, inhibit virus attachment, or inhibit the normal cell functions tested. These results indicate that nonpermissive mouse cells contain a product, possibly determined by the Fv-1 gene, which inhibits some early postpenetration event(s) in leukemia virus infection.
Keywords: gene action, cell culture, virus host range, restriction mechanism
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson S. A., Todaro G. J., Scolnick E. M. Induction of murine C-type viruses from clonal lines of virus-free BALB-3T3 cells. Science. 1971 Oct 8;174(4005):157–159. doi: 10.1126/science.174.4005.157. [DOI] [PubMed] [Google Scholar]
- Eckner R. J. Helper-dependent properties of Friend spleen focus-forming virus: effect of the Fv-1 gene on the late stages in virus synthesis. J Virol. 1973 Sep;12(3):523–533. doi: 10.1128/jvi.12.3.523-533.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley J. W., Rowe W. P., Huebner R. J. Host-range restrictions of murine leukemia viruses in mouse embryo cell cultures. J Virol. 1970 Feb;5(2):221–225. doi: 10.1128/jvi.5.2.221-225.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang A. S., Besmer P., Chu L., Baltimore D. Growth of pseudotypes of vesicular stomatitis virus with N-tropic murine leukemia virus coats in cells resistant to N-tropic viruses. J Virol. 1973 Sep;12(3):659–662. doi: 10.1128/jvi.12.3.659-662.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihle J. N., Kenney F. T., Tennant R. W. Evidence for a stable intermediate in leukemia virus activation in AKR mouse embryo cells. J Virol. 1974 Sep;14(3):451–456. doi: 10.1128/jvi.14.3.451-456.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krontiris T. G., Soeiro R., Fields B. N. Host restriction of Friend leukemia virus. Role of the viral outer coat. Proc Natl Acad Sci U S A. 1973 Sep;70(9):2549–2553. doi: 10.1073/pnas.70.9.2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lilly F. Fv-2: identification and location of a second gene governing the spleen focus response to Friend leukemia virus in mice. J Natl Cancer Inst. 1970 Jul;45(1):163–169. [PubMed] [Google Scholar]
- Lowy D. R., Rowe W. P., Teich N., Hartley J. W. Murine leukemia virus: high-frequency activation in vitro by 5-iododeoxyuridine and 5-bromodeoxyuridine. Science. 1971 Oct 8;174(4005):155–156. doi: 10.1126/science.174.4005.155. [DOI] [PubMed] [Google Scholar]
- ODAKA T., EDAKA T., YAMAMOTTO T. Inheritance of susceptibility to Friend mouse leukemia virus. Jpn J Exp Med. 1962 Oct;32:405–413. [PubMed] [Google Scholar]
- Pincus T., Hartley J. W., Rowe W. P. A major genetic locus affecting resistance to infection with murine leukemia viruses. I. Tissue culture studies of naturally occurring viruses. J Exp Med. 1971 Jun 1;133(6):1219–1233. doi: 10.1084/jem.133.6.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pincus T., Rowe W. P., Lilly F. A major genetic locus affecting resistance to infection with murine leukemia viruses. II. Apparent identity to a major locus described for resistance to friend murine leukemia virus. J Exp Med. 1971 Jun 1;133(6):1234–1241. doi: 10.1084/jem.133.6.1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe W. P., Hartley J. W. Studies of genetic transmission of murine leukemia virus by AKR mice. II. Crosses with Fv-1 b strains of mice. J Exp Med. 1972 Nov 1;136(5):1286–1301. doi: 10.1084/jem.136.5.1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe W. P., Humphrey J. B., Lilly F. A major genetic locus affecting resistance to infection with murine leukemia viruses. 3. Assignment of the Fv-1 locus to linkage group 8 of the mouse. J Exp Med. 1973 Mar 1;137(3):850–853. doi: 10.1084/jem.137.3.850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe W. P., Pugh W. E., Hartley J. W. Plaque assay techniques for murine leukemia viruses. Virology. 1970 Dec;42(4):1136–1139. doi: 10.1016/0042-6822(70)90362-4. [DOI] [PubMed] [Google Scholar]
- Stephenson J. R., Aaronson S. A. A genetic locus for inducibility of C-type in BALB-c cells: the effect of a nonlinked regulatory gene on detection of virus after chemical activation. Proc Natl Acad Sci U S A. 1972 Oct;69(10):2798–2801. doi: 10.1073/pnas.69.10.2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson J. R., Aaronson S. A. Genetic factors influencing C-type RNA virus induction. J Exp Med. 1972 Jul 1;136(1):175–184. doi: 10.1084/jem.136.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temin H. M., Baltimore D. RNA-directed DNA synthesis and RNA tumor viruses. Adv Virus Res. 1972;17:129–186. doi: 10.1016/s0065-3527(08)60749-6. [DOI] [PubMed] [Google Scholar]
- Tennant R. W., Farrelly J. G., Ihle J. N., Pal B. C., Kenney F. T., Brown A. Effects of polyadenylic acids on functions of murine RNA tumor viruses. J Virol. 1973 Dec;12(6):1216–1225. doi: 10.1128/jvi.12.6.1216-1225.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikura H. Requirement of cellular DNA synthesis for the growth of Friend leukemia virus. Exp Cell Res. 1968 Oct;52(2):445–450. doi: 10.1016/0014-4827(68)90486-2. [DOI] [PubMed] [Google Scholar]
- Yoshikura H. Ultraviolet inactivation of murine leukemia virus and its assay in permissive and non-permissive cells. Int J Cancer. 1973 May;11(3):739–746. doi: 10.1002/ijc.2910110325. [DOI] [PubMed] [Google Scholar]


