Abstract
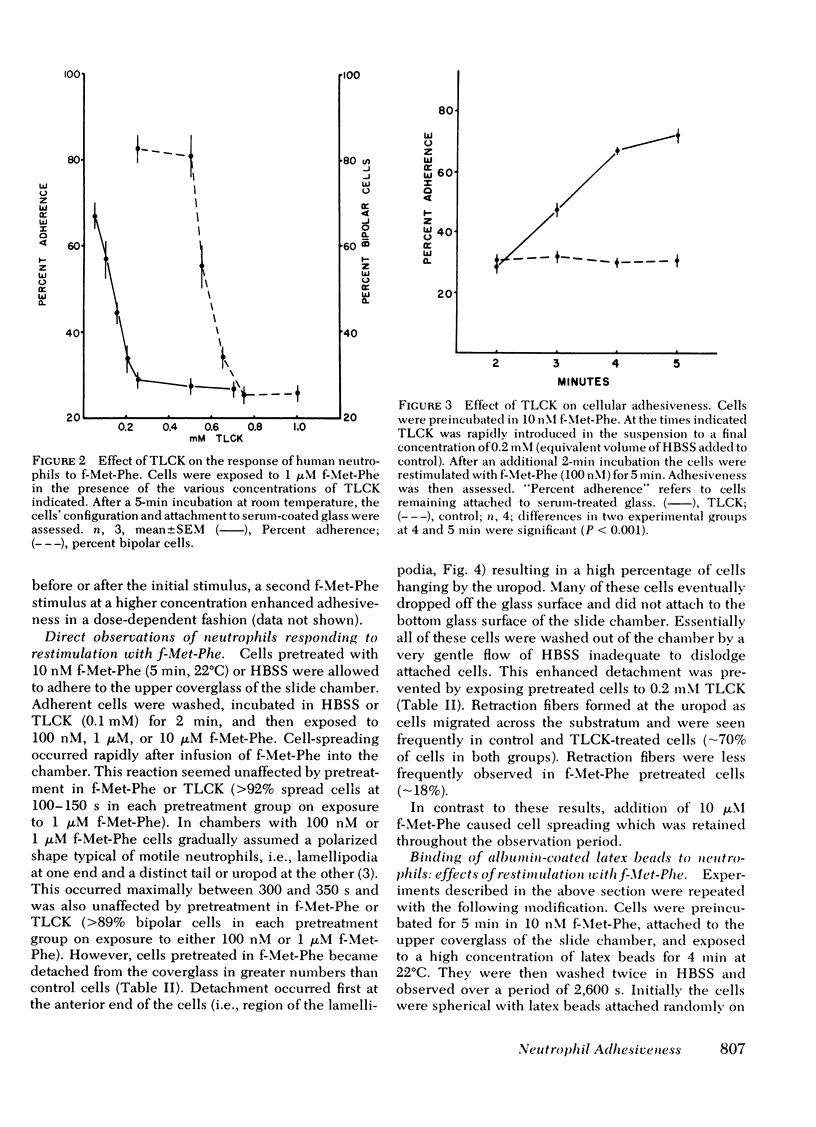
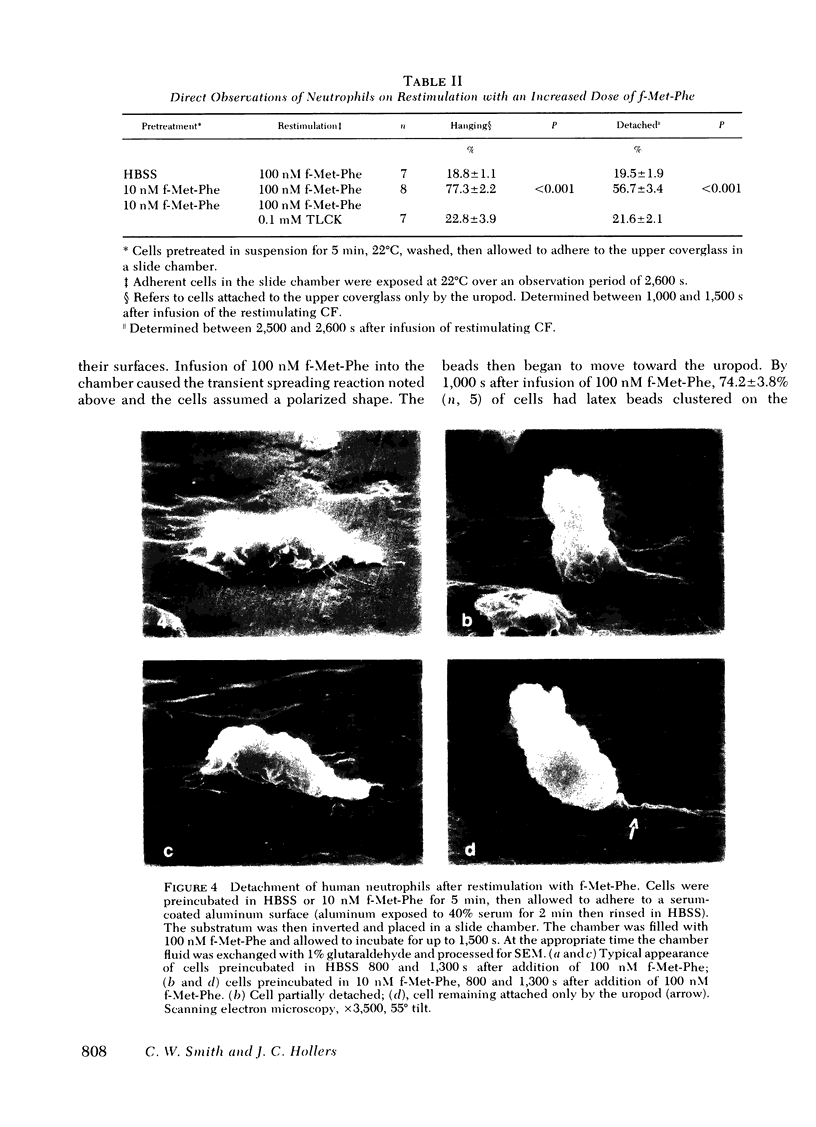
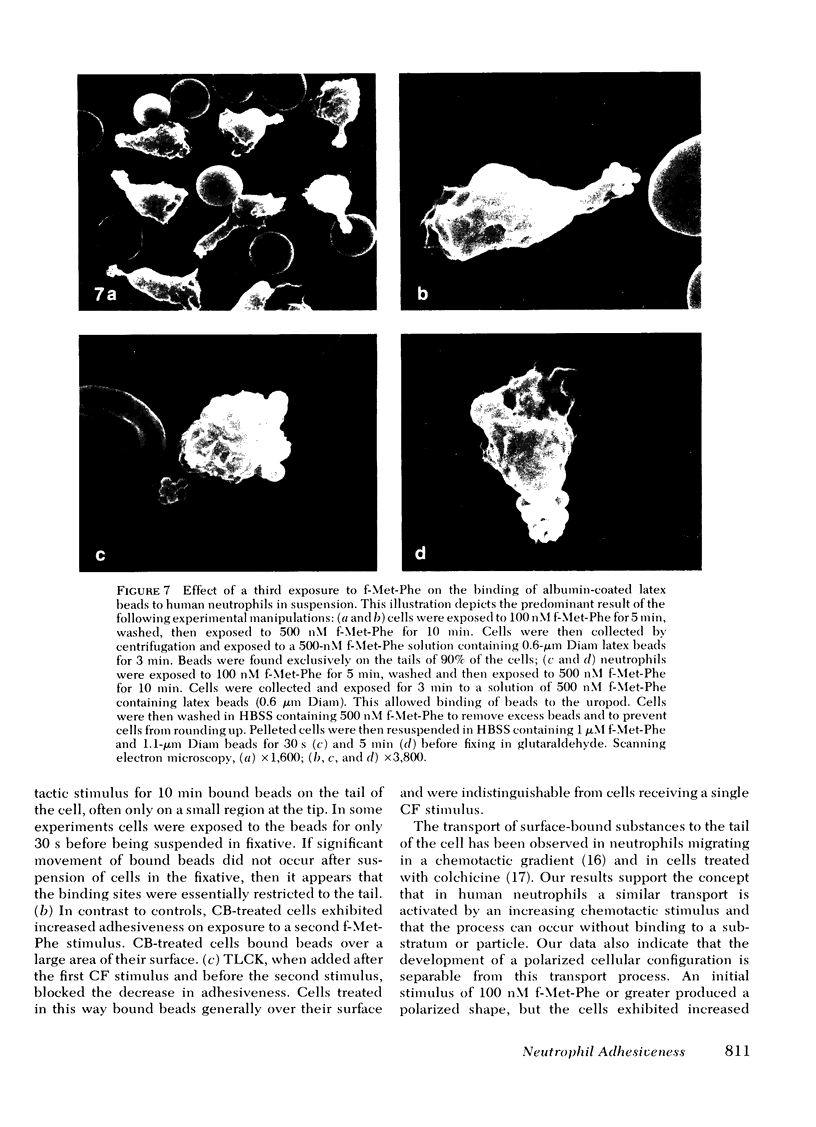
Human peripheral blood neutrophils obtained from healthy adults were examined in vitro. We assessed the effects of sequential stepwise increases in the concentration of the chemotactic dipeptide N-formyl-l-methionyl-l-phenylalanine (f-Met-Phe) on neutrophil attachment to serum-coated glass, detachment from serum-coated glass and the distribution on the cell surface of binding sites for albumin-coated latex beads. The initial exposure to f-Met-Phe resulted in increased adhesiveness and binding of latex beads in a random pattern over the cell surface. The second exposure to f-Met-Phe resulted in decreased adherence, detachment of neutrophils from serum-coated glass, and movement of binding sites for latex beads to the uropod. Enhanced adhesiveness and redistribution of binding sites were blocked by 0.1 mM N-α-p-tosyl-l-lysine chloromethyl ketone, a concentration that did not reduce the change in cellular shape caused by f-Met-Phe. Cytochalasin B (5 μg/ml) blocked the redistribution of binding sites as well as the change in shape. The third exposure to f-Met-Phe was given along with the latex beads. The stimulus was stopped after 2 min by fixing cells in suspension with glutaraldehyde. If the third exposure was at a concentration higher than the second, the beads were bound in the region of the lamellipodia in 70% of the cells. If lower, binding to the lamellipodia was found in a significantly smaller proportion of cells (13%). The results support the concept that neutrophils develop a polarized distribution of f-Met-Phe-induced adhesion sites in response to increasing concentrations of f-Met-Phe, and these sites flow from the region of the lamellipodia to the uropod.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allan R. B., Wilkinson P. C. A visual analysis of chemotactic and chemokinetic locomotion of human neutrophil leucocytes. Use of a new chemotaxis assay with Candida albicans as gradient source. Exp Cell Res. 1978 Jan;111(1):191–203. doi: 10.1016/0014-4827(78)90249-5. [DOI] [PubMed] [Google Scholar]
- Berlin R. D., Oliver J. M. Analogous ultrastructure and surface properties during capping and phagocytosis in leukocytes. J Cell Biol. 1978 Jun;77(3):789–804. doi: 10.1083/jcb.77.3.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beukers H., Deierkauf F. A., Blom C. P., Deierkauf M., Riemersma J. C. Effects of albumin on the phagocytosis of polystyrene spherules by rabbit polymorphonuclear leucocytes. J Cell Physiol. 1978 Oct;97(1):29–36. doi: 10.1002/jcp.1040970105. [DOI] [PubMed] [Google Scholar]
- Fehr J., Dahinden C. Modulating influence of chemotactic factor-induced cell adhesiveness on granulocyte function. J Clin Invest. 1979 Jul;64(1):8–16. doi: 10.1172/JCI109466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetzl E. J., Austen K. F. Active site chemotactic factors and the regulation of the human neutrophil chemotactic response. Antibiot Chemother (1971) 1974;19:218–232. doi: 10.1159/000395433. [DOI] [PubMed] [Google Scholar]
- Hatch G. E., Gardner D. E., Menzel D. B. Chemiluminescence of phagocytic cells caused by N-formylmethionyl peptides. J Exp Med. 1978 Jan 1;147(1):182–195. doi: 10.1084/jem.147.1.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson P. M., Zanolari B., Schwartzman N. A., Hong S. R. Intracellular control of human neutrophil secretion. I. C5a-induced stimulus-specific desensitization and the effects of cytochalasin B. J Immunol. 1978 Sep;121(3):851–855. [PubMed] [Google Scholar]
- Lichtman M. A., Weed R. I. Alteration of the cell periphery during granulocyte maturation: relationship to cell function. Blood. 1972 Mar;39(3):301–316. [PubMed] [Google Scholar]
- Nelson R. D., McCormack R. T., Fiegel V. D., Herron M., Simmons R. L., Quie P. G. Chemotactic deactivation of human neutrophils: possible relationship to stimulation of oxidative metabolism. Infect Immun. 1979 Feb;23(2):282–286. doi: 10.1128/iai.23.2.282-286.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson R. D., McCormack R. T., Fiegel V. D., Simmons R. L. Chemotactic deactivation of human neutrophils: evidence for nonspecific and specific components. Infect Immun. 1978 Nov;22(2):441–444. doi: 10.1128/iai.22.2.441-444.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan G. B., Borysenko J. Z., Karnovsky M. J. Factors affecting the redistribution of surface-bound concanavalin A on human polymorphonuclear leukocytes. J Cell Biol. 1974 Aug;62(2):351–365. doi: 10.1083/jcb.62.2.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Showell H. J., Williams D., Becker E. L., Naccache P. H., Sha'afi R. Desensitization and deactivation of the secretory responsiveness of rabbit neutrophils induced by the chemotactic peptide, formyl-methionyl-leucyl-phenylalanine. J Reticuloendothel Soc. 1979 Feb;25(2):139–150. [PubMed] [Google Scholar]
- Smith C. W., Hollers J. C., Patrick R. A., Hassett C. Motility and adhesiveness in human neutrophils. Effects of chemotactic factors. J Clin Invest. 1979 Feb;63(2):221–229. doi: 10.1172/JCI109293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward P. A., Becker E. L. The deactivation of rabbit neutrophils by chemotactic factor and the nature of the activatable esterase. J Exp Med. 1968 Apr 1;127(4):693–709. doi: 10.1084/jem.127.4.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigmond S. H. Ability of polymorphonuclear leukocytes to orient in gradients of chemotactic factors. J Cell Biol. 1977 Nov;75(2 Pt 1):606–616. doi: 10.1083/jcb.75.2.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigmond S. H. Chemotaxis by polymorphonuclear leukocytes. J Cell Biol. 1978 May;77(2):269–287. doi: 10.1083/jcb.77.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]