Abstract
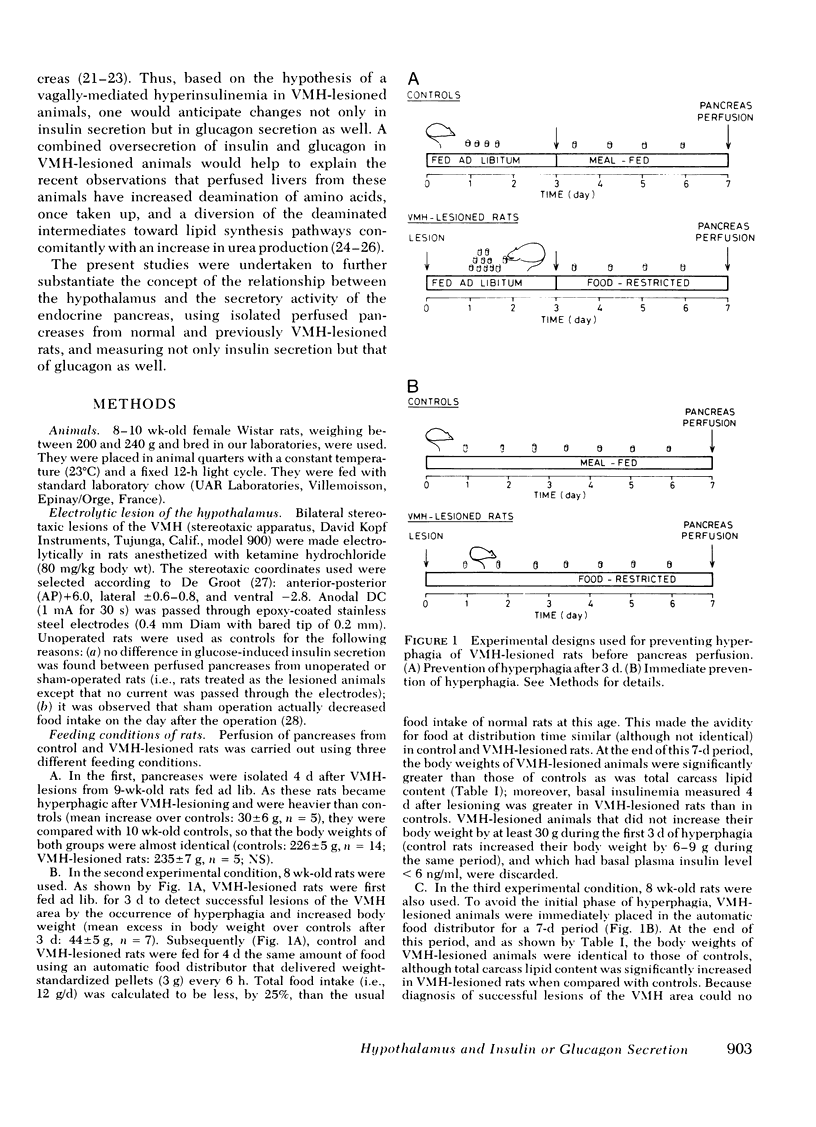
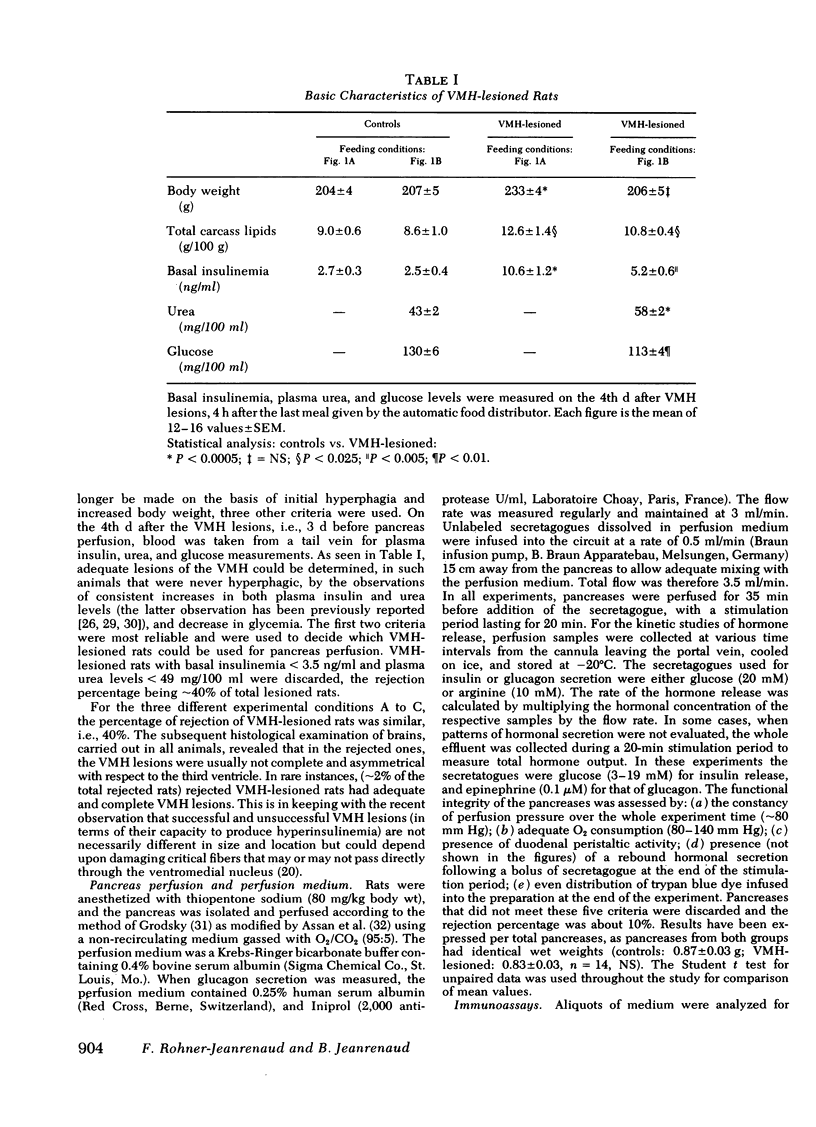
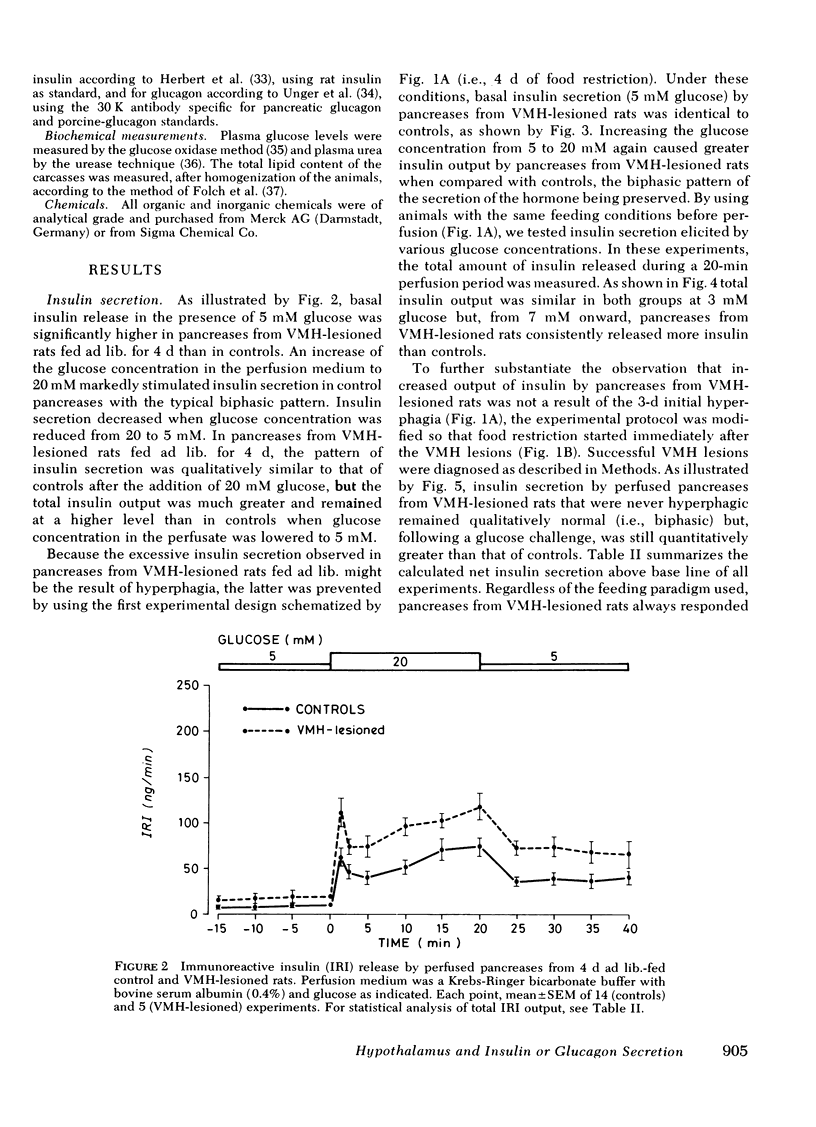
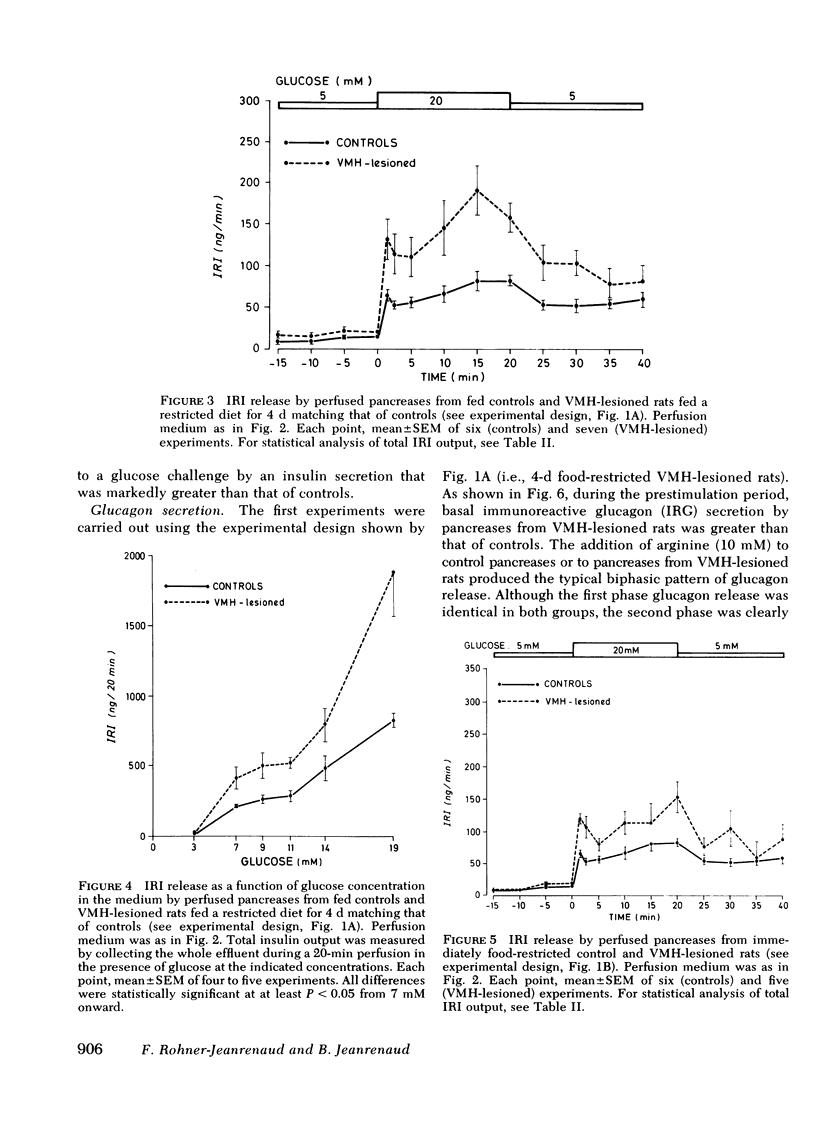
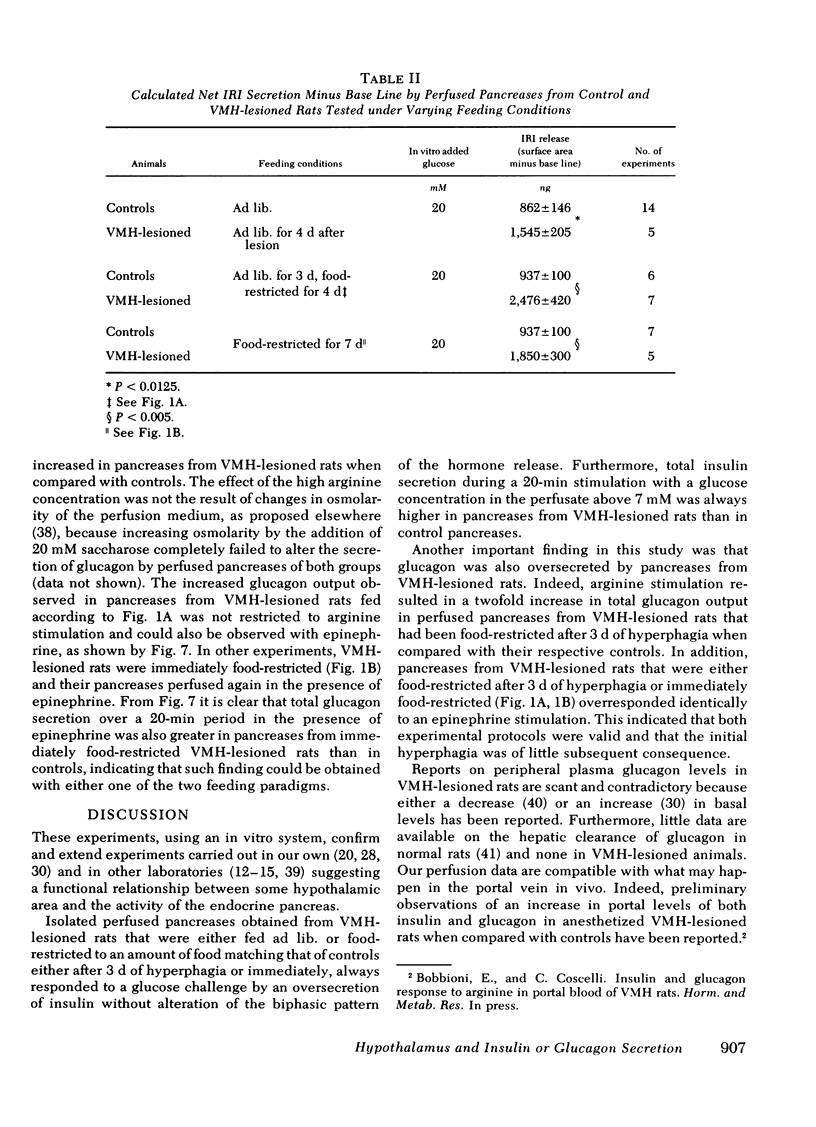
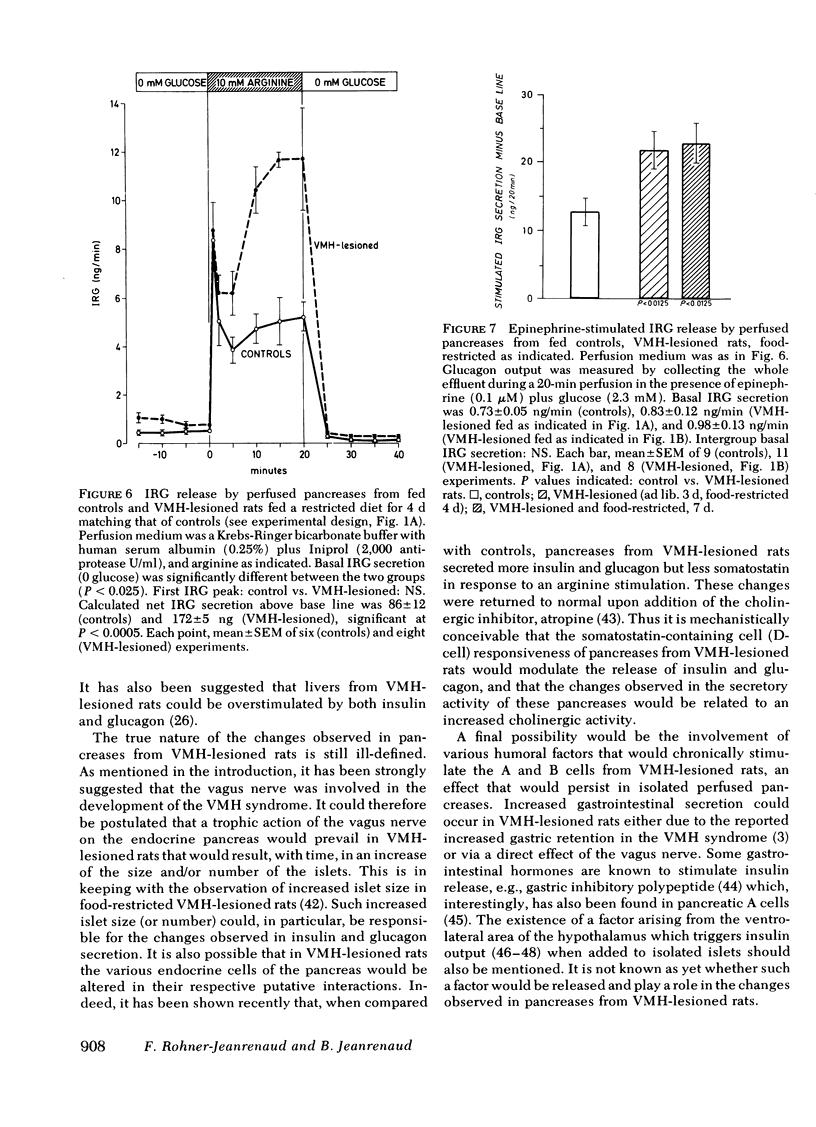
The existence of a relationship between the ventromedial hypothalamic area (VMH) and the activity of the endocrine pancreas has been shown previously. This relationship has been further tested and extended in the present study, using isolated perfused pancreases from rats previously lesioned (4-7 d) in the VMH. It was found that in isolated pancreases obtained from rats fed ad lib. for 4 d after VMH lesions (i.e., that were hyperphagic), the typical biphasic pattern of insulin secretion was observed following glucose stimulation (20 mM) and that the total insulin output was much greater than that of controls. The increased insulin output was not a result of hyperphagia because similar results were obtained using pancreases obtained from VMH-lesioned rats in which a food restriction matching exactly that of control rats was started either immediately of 3 d after the lesions. Pancreases from such food-restricted VMH-lesioned rats oversecreted insulin, when compared with controls fed the same amount, from 7 mM of glucose concentration in perfusion medium onwards. After the addition of arginine (10 mM), the total output of glucagon by pancreases from food-restricted VMH-lesioned rats was twice that of controls. Qualitatively, the arginine-induced glucagon secretion by pancreases from food-restricted VMH-lesioned rats retained its biphasic pattern. Similarly, epinephrine (0.1 μM) elicited a greater glucagon release by pancreases from food-restricted VMH-lesioned rats when compared with controls. These data further support the concept of a link (as yet undefined) between the hypothalamus and the endocrine pancreas, as lesions of the VMH area resulted in abnormal secretion not only of insulin, but of glucagon as well.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Assan R., Attali J. R., Ballerio G., Boillot J., Girard J. R. Glucagon secretion induced by natural and artificial amino acids in the perfused rat pancreas. Diabetes. 1977 Apr;26(4):300–307. doi: 10.2337/diab.26.4.300. [DOI] [PubMed] [Google Scholar]
- Assimacopoulos-Jeannet F., Jeanrenaud B. The hormonal and metabolic basis of experimental obesity. Clin Endocrinol Metab. 1976 Jul;5(2):337–365. doi: 10.1016/s0300-595x(76)80025-4. [DOI] [PubMed] [Google Scholar]
- BOGDANOVE E. M., SPIRTOS B. N., HALMI N. S. Further observations on pituitary structure and function in rats bearing hypothalamic lesions. Endocrinology. 1955 Sep;57(3):302–315. doi: 10.1210/endo-57-3-302. [DOI] [PubMed] [Google Scholar]
- Berthoud H. R., Jeanrenaud B. Acute hyperinsulinemia and its reversal by vagotomy after lesions of the ventromedial hypothalamus in anesthetized rats. Endocrinology. 1979 Jul;105(1):146–151. doi: 10.1210/endo-105-1-146. [DOI] [PubMed] [Google Scholar]
- Bloom S. R., Edwards A. V., Vaughan N. J. The role of the autonomic innervation in the control of glucagon release during hypoglycaemia in the calf. J Physiol. 1974 Feb;236(3):611–623. doi: 10.1113/jphysiol.1974.sp010455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray G. A., Gallagher T. F., Jr Manifestations of hypothalamic obesity in man: a comprehensive investigation of eight patients and a reveiw of the literature. Medicine (Baltimore) 1975 Jul;54(4):301–330. doi: 10.1097/00005792-197507000-00002. [DOI] [PubMed] [Google Scholar]
- Chikamori K., Masuda K., Izumi H., Isaka K., Tezuka U. Effect of vagotomy on hyperinsulinemia in obese rats with hypothalamic lesions. Endocrinol Jpn. 1977 Jun;24(3):251–258. doi: 10.1507/endocrj1954.24.251. [DOI] [PubMed] [Google Scholar]
- Creutzfeldt W., Ebert R., Willms B., Frerichs H., Brown J. C. Gastric inhibitory polypeptide (GIP) and insulin in obesity: increased response to stimulation and defective feedback control of serum levels. Diabetologia. 1978 Jan 14;14(1):15–24. doi: 10.1007/BF00429703. [DOI] [PubMed] [Google Scholar]
- Ernest M. J., Chen C. L., Feigelson P. Induction of tyrosine aminotransferase synthesis in isolated liver cell suspensions. Absolute dependence of induction on glucocorticoids and glucagon or cyclic AMP. J Biol Chem. 1977 Oct 10;252(19):6783–6791. [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Frohman L. A., Bernardis L. L. Growth hormone and insulin levels in weanling rats with ventromedial hypothalamic lesions. Endocrinology. 1968 Jun;82(6):1125–1132. doi: 10.1210/endo-82-6-1125. [DOI] [PubMed] [Google Scholar]
- Frohman L. A., Bernardis L. L., Schnatz J. D., Burek L. Plasma insulin and triglyceride levels after hypothalamic lesions in weanling rats. Am J Physiol. 1969 Jun;216(6):1496–1501. doi: 10.1152/ajplegacy.1969.216.6.1496. [DOI] [PubMed] [Google Scholar]
- Frohman L. A., Ezdinli E. Z., Javid R. Effect of vagotomy and vagal stimulation on insulin secretion. Diabetes. 1967 Jul;16(7):443–448. doi: 10.2337/diab.16.7.443. [DOI] [PubMed] [Google Scholar]
- Grodsky G. M., Bennett L. L., Smith D. F., Schmid F. G. Effect of pulse administration of glucose or glucagon on insulin secretion in vitro. Metabolism. 1967 Mar;16(3):222–233. doi: 10.1016/0026-0495(67)90171-0. [DOI] [PubMed] [Google Scholar]
- Hahn H. J., Ziegler M. Investigations on isolated islets of langerhans in vitro. 16.Modification of the glucose-dependent inhibition of glucagon secretion. Biochim Biophys Acta. 1977 Oct 25;499(3):362–372. doi: 10.1016/0304-4165(77)90067-8. [DOI] [PubMed] [Google Scholar]
- Hales C. N., Kennedy G. C. Plasma glucose, non-esterified fatty acid and insulin concentrations in hypothalamic-hyperphagic rats. Biochem J. 1964 Mar;90(3):620–624. doi: 10.1042/bj0900620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han P. W. Hypothalamic obesity in rats without hyperphagia. Trans N Y Acad Sci. 1967 Dec;30(2):229–243. doi: 10.1111/j.2164-0947.1967.tb02460.x. [DOI] [PubMed] [Google Scholar]
- Han P. W., Yu Y. K., Chow S. L. Enlarged pancreatic islets of tube-fed hypophysectomized rats bearing hypothalamic lesions. Am J Physiol. 1970 Mar;218(3):769–771. doi: 10.1152/ajplegacy.1970.218.3.769. [DOI] [PubMed] [Google Scholar]
- Herbert V., Lau K. S., Gottlieb C. W., Bleicher S. J. Coated charcoal immunoassay of insulin. J Clin Endocrinol Metab. 1965 Oct;25(10):1375–1384. doi: 10.1210/jcem-25-10-1375. [DOI] [PubMed] [Google Scholar]
- Hill D. E., Mayes S., DiBattista D., Lockhart-Ewart R., Martin J. M. Hypothalamic regulation of insulin release in rhesus monkeys. Diabetes. 1977 Aug;26(8):726–731. doi: 10.2337/diab.26.8.726. [DOI] [PubMed] [Google Scholar]
- Hinman D. J., Griffith D. R. Effects of ventromedial hypothalamic lesions on thyroid secretion rate in rats. Horm Metab Res. 1973 Jan;5(1):48–50. doi: 10.1055/s-0028-1093982. [DOI] [PubMed] [Google Scholar]
- Hustvedt B. E., Lovo A., Reichl D. The effect of ventromedial hypothalamic lesions on metabolism and insulin secretion in rats on a controlled feeding regimen. Nutr Metab. 1976;20(4):264–271. doi: 10.1159/000175708. [DOI] [PubMed] [Google Scholar]
- Idahl L. A., Martin J. M. Stimulation of insulin release by a ventro-lateral hypothalamic factor. J Endocrinol. 1971 Nov;51(3):601–602. doi: 10.1677/joe.0.0510601. [DOI] [PubMed] [Google Scholar]
- Inoue S., Bray G. A., Mullen Y. S. Effect of transplantation of pancreas on development of hypothalamic obesity. Nature. 1977 Apr 21;266(5604):742–744. doi: 10.1038/266742a0. [DOI] [PubMed] [Google Scholar]
- Inoue S., Bray G. A. The effects of subdiaphragmatic vagotomy in rats with ventromedial hypothalamic obesity. Endocrinology. 1977 Jan;100(1):108–114. doi: 10.1210/endo-100-1-108. [DOI] [PubMed] [Google Scholar]
- Jaspan J. B., Huen A. H., Morley C. G., Moossa A. R., Rubenstein A. H. The role of the liver in glucagon metabolism. J Clin Invest. 1977 Aug;60(2):421–428. doi: 10.1172/JCI108791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneto A., Kosaka K., Nakao K. Effects of stimulation of the vagus nerve on insulin secretion. Endocrinology. 1967 Mar;80(3):530–536. doi: 10.1210/endo-80-3-530. [DOI] [PubMed] [Google Scholar]
- Lockhart-Ewart R. B., Mok C., Martin J. M. Neuroendocrine control of insulin secretion. Diabetes. 1976 Feb;25(2):96–100. doi: 10.2337/diab.25.2.96. [DOI] [PubMed] [Google Scholar]
- MARSHALL N. B., BARRNETT R. J., MAYER J. Hypothalamic lesions in goldthioglucose injected mice. Proc Soc Exp Biol Med. 1955 Oct;90(1):240–244. doi: 10.3181/00379727-90-21995. [DOI] [PubMed] [Google Scholar]
- Rohner F., Dufour A. C., Karakash C., Le Marchand Y., Ruf K. B., Jeanrenaud B. Immediate effect of lesion of the ventromedial hypothalamic area upon glucose-induced insulin secretion in anaesthetized rats. Diabetologia. 1977 May;13(3):239–242. doi: 10.1007/BF01219706. [DOI] [PubMed] [Google Scholar]
- Smith P. H., Merchant F. W., Johnson D. G., Fujimoto W. Y., Williams R. H. Immunocytochemical localization of a gastric imhibitory polypeptide-like material within A-cells of the endocrine pancreas. Am J Anat. 1977 Aug;149(4):585–590. doi: 10.1002/aja.1001490410. [DOI] [PubMed] [Google Scholar]
- Snodgrass P. J., Lin R. C., Müller W. A., Aoki T. T. Induction of urea cycle enzymes of rat liver by glucagon. J Biol Chem. 1978 Apr 25;253(8):2748–2753. [PubMed] [Google Scholar]
- Unger R. H., Aguilar-Parada E., Müller W. A., Eisentraut A. M. Studies of pancreatic alpha cell function in normal and diabetic subjects. J Clin Invest. 1970 Apr;49(4):837–848. doi: 10.1172/JCI106297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- York D. A., Bray G. A. Dependence of hypothalamic obesity on insulin, the pituitary and the adrenal gland. Endocrinology. 1972 Apr;90(4):885–894. doi: 10.1210/endo-90-4-885. [DOI] [PubMed] [Google Scholar]


