Abstract
Delayed recall at the primacy position (first few items on a list) has been shown to predict cognitive decline in cognitively intact elderly participants, with poorer delayed primacy performance associated with more pronounced generalized cognitive decline during follow-up. We have previously suggested that this association is due to delayed primacy performance indexing memory consolidation, which in turn is thought to depend upon hippocampal function. Here, we test the hypothesis that hippocampal size is associated with delayed primacy performance in cognitively intact elderly individuals.
Data were analyzed from a group (N=81) of cognitively intact participants, aged 60 or above. Serial position performance was measured with the Buschke selective reminding test (BSRT). Hippocampal size was automatically measured via MRI, and unbiased voxel-based analyses were also conducted to explore further regional specificity of memory performance. We conducted regression analyses of hippocampus volumes on serial position performance; other predictors included age, family history of Alzheimer's disease (AD), APOE ε4 status, education, and total intracranial volume.
Our results collectively suggest that there is a preferential association between hippocampal volume and delayed primacy performance. These findings are consistent with the hypothesis that delayed primacy consolidation is associated with hippocampal size, and shed light on the relationship between delayed primacy performance and generalized cognitive decline in cognitively intact individuals, suggesting that delayed primacy consolidation may serve as a sensitive marker of hippocampal health in these individuals.
Keywords: Serial Position, Primacy, Hippocampus, Structural MRI
Introduction
A decline in episodic memory performance is an early key symptom in Alzheimer's disease (AD) and is considered critical for the prediction of the disease (e.g., e.g., McKhann et al., 2011; Sperling et al., 2011), especially when memory is tested after a delay (Gomar, Bobes-Bascaran, Conejero-Goldberg, Davies & Goldberg, 2011). Recently, Bruno, Reiss, Petkova, Sidtis and Pomara (2013) have shown that a detailed analysis of serial position performance in delayed recall tests is more sensitive to the prediction of subsequent cognitive decline in healthy elderly subjects compared to total delayed recall performance. Serial position refers to the pattern in free recall whereby early-list items (primacy) and late-list items (recency) are remembered better than items learned in the middle (Murdock, 1962; Glanzer, 1972). Bruno et al. (2013), using a verbal memory task, tested a group of cognitively intact individuals over a span of up to seven years and showed that delayed recall at the primacy position was a better predictor of generalized cognitive decline than total memory performance, or performance anywhere else on the list (e.g., recency). Poorer delayed primacy recall was associated with greater subsequent decline.
Bruno et al. (2013) argued that the predictive advantage of delayed primacy performance over the other memory indices, including immediate primacy performance, was due to its reliance upon memory consolidation (McGaugh, 2000). Primacy effects are typically explained as a consequence of increased opportunities for rehearsal of early list items as compared to items learned later (Rundus, 1971; Tan & Ward, 2000). More rehearsal is expected to lead to better encoding of the information and, consequently, stronger memories, although alternative interpretations have also been put forward (e.g., Brown, Neath & Chater, 2007). If the value of primacy performance in predicting cognitive decline were due to its ability to index effective use of rehearsal strategies, then little predictive difference would be expected between immediate and delayed primacy. However, since Bruno et al. (2013) isolated delayed primacy performance as the best predictor of subsequent generalized cognitive ability, it is arguable that a process of consolidation, requiring time and structural changes to stabilize memory traces and render them more resistant to interference, is required.
Consolidation is thought to depend upon hippocampal function (Wixted, 2004; Wixted & Cai, 2013) and Bruno et al. (2013) have suggested that the assessment of delayed primacy performance could work as a proxy measure for hippocampal integrity. Associations between the hippocampus and primacy have been reported in the literature, albeit with mixed methods and results. Hermann et al. (1996) conducted a study to examine serial position performance in participants who underwent anterior temporal lobectomy, including resection of the hippocampus. Hermann et al. (1996) measured memory over five learning trials with the California Verbal Learning Test (CVLT), and observed a drop in primacy performance (first four words), which they interpreted as a loss of consolidation ability, only in those participants who underwent resection of the left hippocampus when this was not sclerotic prior to the surgery. In other words, only the removal of a relatively healthy left hippocampus caused a drop in primacy recall performance in the participants. In contrast, Albuquerque, Loureiro and Martins (2008), also using the CVLT, reported that only participants with focal frontal lesions, but not participants with mesotemporal lesions, showed reduced primacy performance.
In a study using functional magnetic resonance imaging (fMRI), Strange, Otten, Josephs, Rugg and Dolan (2002) tested 14 healthy participants, aged 19 to 32. Participants were asked to learn a set of 12 words and then free recall the words; this procedure was repeated over 30 consecutive trials. Strange et al. (2002) found that retrieval of early list items (first two items were classed as primacy) was associated with activation of the right anterior hippocampus, the posterior fusiform, and parahippocampal areas (bilaterally), but that these areas were not engaged with retrieval of later words. In contrast, Talmi, Grady, Goshen-Gottstein and Moscovitch (2005), who also tested young adults (n=10) with fMRI, found that primacy items were associated with left hippocampal activation in a series of recognition memory tasks.
Despite some inconsistencies, there is evidence that hippocampal integrity is associated with successful retrieval of primacy items. Therefore, following Bruno et al. (2013), we hypothesize that hippocampal size should predict the likelihood of successful retrieval of primacy words in a delayed memory task in a group of cognitively intact elderly volunteers. Moreover, considering the prominence of delayed performance, we hypothesize that hippocampal volume should be a better predictor of primacy performance after a delay as compared to primacy performance immediately after study (consolidation hypothesis). Finally, we also hypothesize (primacy specificity hypothesis) that there should be a special relationship between the hippocampus and delayed primacy performance, but not between the former and delayed recall performance for items learned afterwards (non-primacy).
To test our hypotheses, we examined the relationship between hippocampal gray matter volume and delayed primacy performance in two groups of participants who were cognitively intact and at least 60 years of age. The first group comprised 54 individuals, originally enrolled as controls for a study on Major Depressive Disorder (MDD), whereas the second group consisted of 28 volunteers recruited for a study on the effects of benzodiazepines on cognition (all tested prior to drug/placebo administration). These two groups were merged for the purpose of the present study. To estimate the specificity of the relationship between delayed primacy performance and hippocampal size, we employed a number of relevant control variables in our analyses, and also explored separately the potential impact on memory of potential indicators of AD-related pathology (see Methods), such as APOE ε4 status (e.g., Corder et al., 1993).
Memory performance was measured with the Buschke Selective Reminding Test (BSRT; Buschke & Fuld, 1974). The BSRT (see Procedure), despite some minor differences, is analogous to the test used in Bruno et al. (2013; i.e., Rey Auditory Verbal Learning Test). Primacy was defined as the first four words on the study list, and the delayed task occurred roughly 15-20 minutes after the end of the learning trials. The study prediction was that larger hippocampal volumes would be associated with more primacy words retrieved in the delayed task.
Methods
Subjects
Participants in the first group were recruited via advertisements in local newspapers and flyers, or from the Memory Education and Research Initiative (MERI) program at the Nathan Kline Institute for Psychiatric Research; participants' recruitment was part of a study on late-life MDD (Bruno et al., 2012; Bruno, Nierenberg, Ritchie, Lutz & Pomara, 2012; Pillai et al., 2012; Pomara et al., 2012). All participants provided formal consent prior to testing and received compensation for up to $450.00 for their time and efforts. A total of 133 participants were recruited for the study. In order to maintain a cognitively intact sample without major indication of cerebrovascular disease, we excluded participants who presented MRI evidence of confluent deep or periventricular white matter hyperintensities, defined as one or more hyperintense lesions measuring at least 10 mm in any direction on the FLAIR scan (see MRI Acquisition below), or had a Mini-Mental State Examination (MMSE) score below 28. Excluding participants with MDD left us with a total of 54.
For the second group, a total of 76 participants were recruited originally through advertisement. All participants formally and in writing consented prior to testing and were paid $200 for their time and efforts. Participants' recruitment was for a study on the combined effects of Lorazepam and APOE variants on cognition (Pomara, Willoughby, Wesnes, Greenblatt & Sidtis, 2005; Pomara, Facelle, Roth, Willoughby, Greenblatt & Sidtis, 2006), but all data analysed here was taken from baseline performance on week 1 (i.e., prior to drug/placebo administration). Participants did not show any cognitive impairment, and were free of significant neurological or medical illnesses, as determined by laboratory tests and medical examination; they were not currently using any psychotropic medication, as determined by a urine toxicology exam; and did not meet the DSM-IV criteria for a psychiatric disorder after evaluation. Participants also had an MMSE score of 28 or higher and a Clinical Dementia Rating of 0. A total of 28 participants received an MRI scan of the head and are included in the present analysis. Table 1 reports the population demographics split by cohort. Both studies received ethical approval by the institutional review boards of the Nathan Kline Institute for Psychiatric Research and the New York University School of Medicine, and were conducted at these institutions.
Table 1.
Study demographics by cohort: Number of subjects (i.e., N); Age in years (mean and standard deviation, and range); MMSE score (with standard deviation); Gender (proportion of females); Years of Education (with standard deviation); Family history of AD (FHAD, number of cases); and APOE ε4 status (number of cases). T-tests and Fisher's exact tests were used to test for significant differences across groups; p values are reported on the far right column.
| Cohort 1 | Cohort 2 | p value | |
|---|---|---|---|
| N | 53 | 28 | |
| Age | 67.68 (5.93) 60-82 |
64.46 (3.78) 60-73 |
0.004 |
| MMSE | 29.68 (0.51) | 29.39 (0.79) | 0.089 |
| Gender (females) | 31 (59%) | 17 (61%) | 1.000 |
| Education (years) | 16.45 (2.54) | 15.93 (2.40) | 0.371 |
| FHAD (yes) | 13 (25%) | 11 (39%) | 0.204 |
| APOE ε4 (yes) | 10 (19%) | 13 (46%) | 0.018 |
MRI Acquisition
The MRI acquisition was performed on a 1.5 T Siemens Vision system (Erlangen, Germany) at the Nathan Kline Institute. All images were acquired using a sagittal magnetization prepared rapid gradient-echo sequence [MPRAGE; repetition time (TR)/echo time (TE)=11.4/11.9 ms, 1 excitation, (NEX), matrix=256 × 256, FOV=307 mm, 1.2mm3 isotropic voxel, 172 slices, no gap]. For evaluation of white matter hyperintensities, we used a fluid attenuated inversion recovery sequence [FLAIR; TR/TE=9000/119 ms, inversion time=2400 ms, NEX=1, matrix 256 × 256, FOV=240 mm, slice thickness=4 mm, 1 mm gap].
MRI preprocessing and analysis
MRI data processing followed procedures described previously (Grothe, Ewers, Krause, Heinsen & Teipel, 2014; Teipel, Heinsen, Amaro, Grinberg, Krause, & Grothe, 2014) and illustrated in Figure 1. Briefly, MPRAGE images were segmented into gray matter, white matter, and cerebrospinal fluid partitions and high-dimensionally registered to Montreal Neurological Institute (MM) standard space, using a segmentation routine without reliance on tissue priors and the diffeomorphic DARTEL warping algorithm (Ashburner, 2007), respectively, both implemented in the VBM8-toolbox. Warping parameters were applied to individual gray matter maps and voxel values were modulated to account for the volumetric differences introduced by the high-dimensional warps, such that the total amount of gray matter volume present before warping was preserved.
Figure 1.
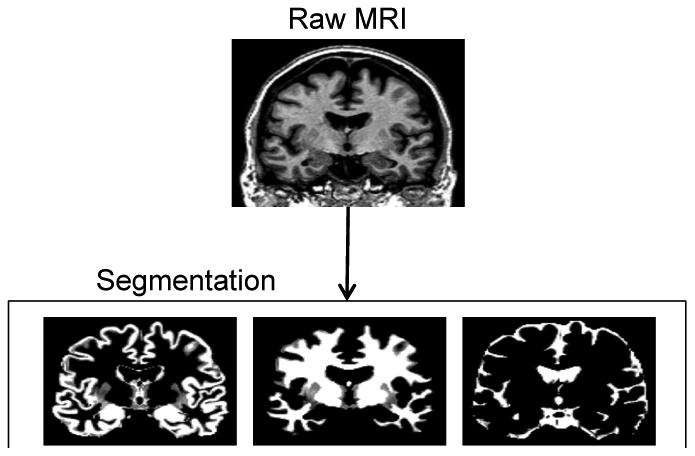
Illustration of the automated volumetry approach used to extract hippocampal volumes for each subject.
Individual gray matter volumes of the hippocampus were extracted automatically from the warped gray matter segments by summing up the modulated voxel values within a predefined hippocampus mask in template space. This mask was obtained by manual delineation of the hippocampus in the MNI standard space template used for high-dimensional image normalization in the VBM8 toolbox. Tracing of the hippocampus outlines followed recently developed international consensus criteria for manual hippocampus segmentation on MRI (Boccardi et al., 2013; http://www.hippocampal-protocol.net/SOPs/index.php) and was performed by a certified tracer (MJG) using MultiTracer 1.0 software (http://www.loni.usc.edu/Software/MultiTracer). Figure 2 illustrates the hippocampal regions of interest (ROIs). The total intracranial volume (TIV) was used in the statistical model to account for differences in head size (see below), and was calculated as the sum of the total segmented gray matter, white matter and cerebrospinal fluid volumes in native space. For complementary voxel-wise analyses, warped gray matter maps were smoothed with an isotropic smoothing kernel of 8 mm full-width at half maximum.
Figure 2.
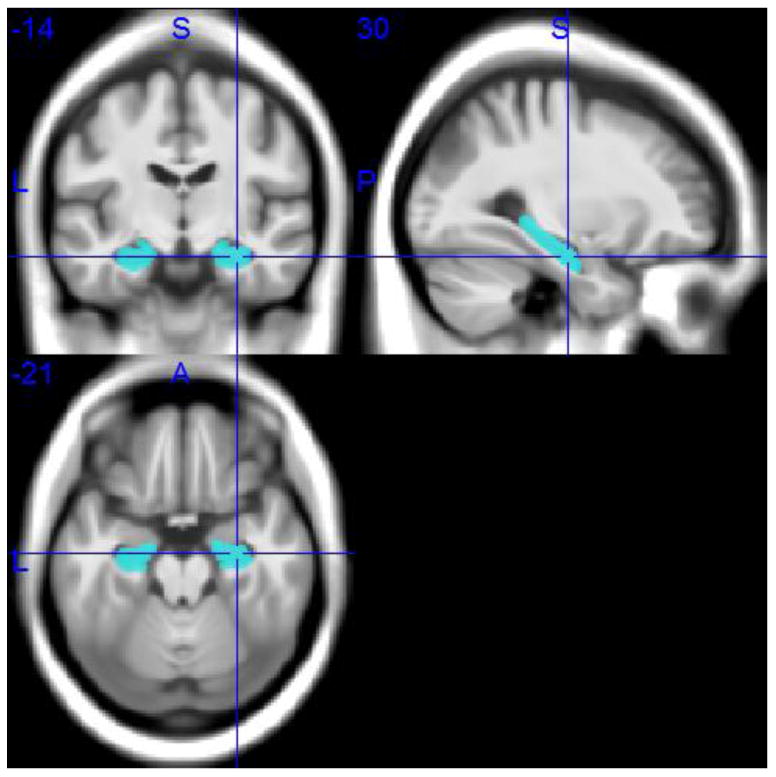
Hippocampal ROIs in MNI space.
Procedure
The first cohort was examined at the Nathan Kline Institute for Psychiatric Research and at the Clinical and Translational Science Institute, New York University Langone Medical Center, over three visits on three successive weeks. On the first visit, after providing informed consent, participants were administered a general medical intake questionnaire to obtain family and medical history information; after this, vital signs were measured, blood was drawn for APOE genotyping, and the MMSE score was obtained. On a second visit, participants received an MRI scan of the head. Comprehensive neuropsychological assessment, including the BSRT, took place on a third visit. The BSRT comprises a list of 16 unrelated nouns, which are presented orally to participants at a rate of 2 seconds each. Participants are asked to recall as many words as possible and to indicate when no more words can be recalled. Two trials are the focus of our analysis: in the first trial (immediate recall), participants are asked to free recall as many words as possible immediately after presentation of the study list; in contrast, in the delayed trial, participants are asked to free recall after a 15-20 minutes gap from initial learning and testing. More information on the study procedures is also available in Pomara et al. (2012). The second cohort was examined at the Nathan Kline Institute for Psychiatric Research. Diagnostic evaluations took place one week before testing proper began. All relevant testing for the present study was conducted in a single session beginning approximately at 9 AM under non-fasting conditions, after obtaining vital signs. A comprehensive neuropsychological assessment, including the BSRT, was administered to evaluate cognitive performance.
Study Design and Analysis
To test our hypothesis that hippocampal size predicts delayed primacy performance (dP), we carried out multiple linear regression analyses. In order to assess the specificity of hippocampus volume for dP vs. other types of memory performance, analog regression models were calculated separately for delayed non-primacy performance (dNP)1, and immediate primacy performance (iP) as outcome variables. Primacy performance was defined as the number of recalled items from the first four on the study list, whereas non-primacy performance was defined as all words recalled minus primacy words. Immediate performance refers to the first BSRT trial, and delayed performance refers to the delayed BSRT trial. To allow for a direct comparison, we employed proportions for both primacy and non-primacy in the analyses, dividing the total number of correctly recalled items by four and 12, respectively. Once one participant was removed due to missing data, thus leading to a total N of 81, all outcome variables were normally distributed based on assessment of skewness and kurtosis (absolute z-value < 3.29; Kim, 2013).
The main predictor was hippocampal gray matter volume (expressed in mm3), and we tested three hypothesis families (Rutherford, 2012; p. 72): 1) whether hippocampal volume predicted dP, 2) whether the relationship between hippocampal volume and dP was greater than the relationship between hippocampal volume and iP (i.e., the consolidation hypothesis), and 3) whether the relationship between hippocampal volume and dP was greater than the relationship between hippocampal volume and dNP (i.e., the primacy specificity hypothesis). For this reason, to correct for testing three hypothesis families, we lowered the α level from 0.05 to 0.017 following Sidak's adjustment.
We employed a three-model testing procedure. Model 1 included all control variables: Cohort (group 1 or 2); age; sex; the MMSE score as a measure of general cognitive ability; years of education as a proxy of cognitive reserve (e.g., Bruno, Brown, Kapucu, Marmar & Pomara, 2014); and TIV, measured in cm3, to control for head size. Model 2 included the predictor, hippocampal gray matter volume. Finally, Model 3 included two potential AD markers: family history of AD (FHAD), which has been shown to be a strong risk factor for AD (e.g., Berti et al., 2011; Silverman, Ciresi, Smith, Marin & Schnaider-Beeri, 2005); and APOE ε4 status. As a criterion for multicollinearity, we set the variance inflation factor (VIF) to 4. To evaluate directly differences in the magnitude of the association between hippocampal size and different types of memory performance, we compared the partial correlation coefficients (controlled for the Model 1 variables) for dP with those for dNP and iP using Steiger's Z test (Steiger, 1980; conventionally, the threshold for significance is set to |1.96| in 2-tailed tests).
In order to assess the regional specificity of the different types of memory performance, i.e. whether the memory tests are specific for hippocampal volume or show similar associations with gray matter volume in other parts of the brain, we further conducted regionally unbiased voxel-based regression analyses. Thus, dP, dNP, and iP scores were used as predictor variables in separate voxel-wise regression models on warped gray matter maps, while controlling for the Model 1 variables. Results were assessed at a statistical threshold of p< 0.001, uncorrected, and a cluster extension threshold of 50 continuous voxels. Although this choice of statistical thresholding reduces control over type 1 errors when compared to the use of more conservative multiple comparison correction procedures, it also lowers the risk for type 2 errors, which is critical in the context of these complementary analyses addressing the regional specificity of the observed memory-hippocampus associations.
Results
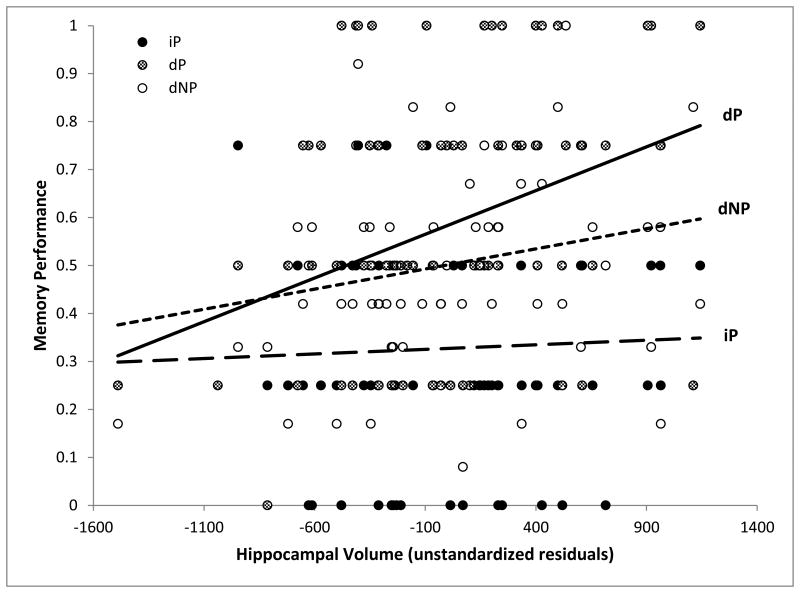
Table 2 reports the memory performance scores across all the participants. No issues of multicollinearity were observed (VIF ≤ 2.462). When examining dP, Model 2 [F(7,73)=3.270, p=.004] provided a better fit than Model 1 [F(6,74)=1.664, p=.142], and Model 3 [F(9,71)=2.486, p=.016]. Adding hippocampal volume (Model 2) significantly increased variance explained [F(1,73)=11.488, p=.001, ΔR2 =.120], and, as expected, the size of the hippocampus was positively associated with dP performance [β=.462, partial R=.369]. Hippocampal volume remained a significant predictor in Model 3 [p=.002, β=.463, partial R=.359]. No other variable, including the Model 3 predictors, came close in this analysis to a significant level (p's ≥ .110). When the same analysis was carried out on iP, all models [Model 1, F(6,74)=5.014, p<.001; Model 2, F(7,73)=4.276, p=.001; Model 3, F(9,71)=3.427, p=.001] fit the data well, but hippocampal volume was not a significant predictor [F(1,73)=0.179, p=.673, ΔR2 =.002, β=.056, partial R=.049]. Model 3 predictors did not yield significant correlations (p's ≥ .275), nor were there any other associations of interest. Finally, hippocampal gray matter volume was positively correlated with dNP, although not significantly so [F(1,73)=4.296, p=.042, ΔR2=.040; Model 2 estimates: β=266, partial R=236; Model 3 estimates: p=.069, β=.247, partial R=.214], and all models fit the data [Model 1, F(6,74)=5.022, p<.001; Model 2, F(7,73)=5.110, p<.001; Model 3, F(9,71)=3.919, p<.001]. Only two predictors, in any of the models, reached the significance level of the test: sex [Model 1: p=.010, β=.337, partial R=.296; Model 2: p=.007, β=.340, partial R=.306], indicating better memory for females; and the MMSE score [Model 1: p=.015, β=.254, partial R=.278; Model 2: p=.008, β=.276, partial R=.306]. To evaluate the relative magnitude of the associations between hippocampal size and memory, we compared iP (partial R = .049) to dP (partial R = .369), and dP to dNP (partial R = .236). The comparison between iP and dP produced a Z value of 2.31 [p=.020], whereas the comparison between dP and dNP yielded a Z value of 1.03 [p=.305]. The relationship between memory performance and hippocampal gray matter volume is illustrated in Figure 3.
Table 2.
Memory performance, dP, dNP and iP (expressed as mean proportion and SD), by cohort. T-tests were used as a test of difference; significance values are reported on the far right column.
| Cohort 1 | Cohort 2 | p value | |
|---|---|---|---|
| dP | 0.64 (0.25) | 0.48 (0.27) | 0.013 |
| dNP | 0.53 (0.21) | 0.45 (0.23) | 0.100 |
| iP | 0.40 (0.23) | 0.19 (0.16) | <0.001 |
Figure 3.
Scatterplot of the relationship between hippocampal gray matter volume (unstandardized residuals obtained by controlling for Model 1 variables) on the X-axis, and memory performance (expressed as a proportion) on the Y-axis. dP = delayed primacy performance; dNP = delayed non-primacy performance; iP = immediate primacy performance.
Results of the unbiased voxel-based regression analyses across the whole brain are summarized in Figure 4. dP showed associations with gray matter volume in a bilateral medial temporal lobe cluster, corresponding to the entorhinal cortex and the hippocampal head (Figure 4, top row). Additional clusters corresponded to the bilateral superior temporal gyrus and the left posterior middle and inferior temporal gyri. dNP also showed associations with gray matter volume in a medial temporal lobe cluster, albeit restricted to the left hemisphere and mainly corresponding to the amygdala and the hippocampal head (Figure 4, middle row). Further effects for the association with dNP were seen in the bilateral superior temporal gyrus, the left temporal pole, and also in several frontal lobe clusters, including bilateral middle frontal gyrus as well as left inferior frontal gyrus and frontal operculum. In contrast to the delayed recall scores, iP showed associations with gray matter volumes in the bilateral orbitofrontal cortex and the basal ganglia (putamen), but not in the hippocampus or surrounding medial temporal lobe structures (Figure 4, bottom row). Table 3 reports the coordinates for significant clusters in the voxel-wise analyses.
Figure 4.
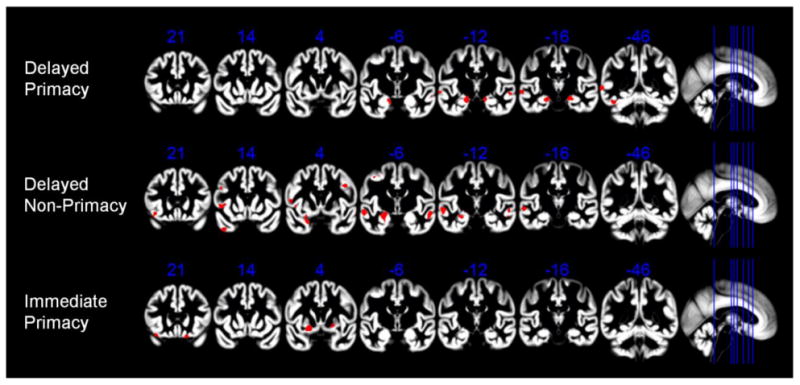
Voxel-based analyses of the associations between different types of memory performance and regional gray matter volumes. Effects of delayed primacy, delayed non-primacy, and immediate primacy performance on regional gray matter volume are shown on representative coronal sections through the MNI space template that was used for high-dimensional spatial normalization. Statistical threshold was set to p < 0.001, uncorrected, with a minimum cluster extension threshold of 50 continuous voxels. Numbers in blue indicate y-coordinates of MNI space. Effects corresponding to the hippocampus and surrounding structures of the medial temporal lobe are seen on coronal slices with MNI y-coordinates -6, -12, and -16.
Table 3.
Coordinates for significant clusters in the voxel-wise analyses by iP, dP and dNP.
| Cluster size | Peak t-values | MNI coordinates | |||
|---|---|---|---|---|---|
| x | y | z | |||
| iP | 104 | 4.48 | 16 | 22 | -27 |
| 282 | 3.84 | 20 | 8 | -14 | |
| 65 | 3.78 | -36 | 23 | -24 | |
| 67 | 3.68 | 20 | 4 | -6 | |
|
| |||||
| dP | 133 | 4.28 | -66 | -45 | 1 |
| 299 | 3.99 | -18 | -15 | -18 | |
| 97 | 3.98 | -63 | -16 | -6 | |
| 168 | 3.81 | 21 | -18 | -17 | |
| 66 | 3.80 | 60 | -10 | -9 | |
| 65 | 3.70 | -44 | -48 | -24 | |
|
| |||||
| 334 | 4.44 | -57 | -9 | -9 | |
| dNP | 367 | 4.15 | -50 | 9 | 4 |
| 4.01 | -44 | 11 | -3 | ||
| 3.31 | -38 | 15 | 3 | ||
| 53 | 4.10 | -33 | -81 | 10 | |
| 120 | 4.05 | 44 | 6 | 34 | |
| 102 | 4.02 | -40 | 14 | -44 | |
| 159 | 3.92 | -40 | -1 | 48 | |
| 3.58 | -30 | -3 | 51 | ||
| 93 | 3.89 | -40 | 9 | 22 | |
| 3.52 | -45 | 14 | 30 | ||
| 552 | 3.79 | -21 | -4 | -15 | |
| 3.66 | -20 | 5 | -30 | ||
| 230 | 3.77 | 60 | -4 | -14 | |
| 3.49 | 54 | -4 | -24 | ||
| 3.43 | 56 | -12 | -20 | ||
| 64 | 3.54 | -38 | 23 | -14 | |
| 3.40 | -40 | 23 | -21 | ||
Discussion
Consistent with the work of other researchers (e.g., Egli, Beck, Berres, Foldi, Monsch & Sollberger, 2014; Howieson et al., 2011; La Rue, Hermann, Jones, Johnson, Ashtana & Sager, 2008; Martin et al., 2013), Bruno et al. (2013) showed that primacy performance in delayed trials is a predictor of generalized cognitive decline from a baseline of intact cognition; the authors suggested that delayed primacy performance was reliant on intact consolidation and, therefore, could act as an indirect measure of hippocampal health. In the present study, we set out to test this hypothesis by analyzing data from a group of cognitively intact elderly participants who underwent an MRI scan of the head, and took part in a neuropsychological test battery that included memory testing (BSRT). Our findings, over two sets of analyses, are largely consistent with Bruno et al.'s (2013) suggestion. We observed that hippocampal gray matter volumes were associated with performance from the primacy region (first four words) in a delayed free recall task, but not with performance in the immediate recall trials, and that larger volumes were correlated with better recall. In addition, we found the association between hippocampal size and non-primacy delayed recall performance to be weaker, although the direct comparison of correlation coefficients between delayed primacy and non-primacy performance did not yield a significant difference.
The lack of statistical difference between primacy and non-primacy coefficients raises the issue of the degree of specificity of the association between hippocampal volume and delayed primacy performance as compared to delayed non-primacy performance. However, a series of complementary regionally unbiased voxel-based analyses reported significant associations between delayed primacy performance and the bilateral hippocampus, in addition to clusters in the superior temporal gyrus, and the left posterior middle and inferior temporal gyri. In contrast, delayed non-primacy performance showed a left lateralized effect in the Amygdala/Hippocampus as well as several temporal and also frontal clusters. Therefore, qualitatively, these unbiased voxel-based analyses appear to be consistent with a somewhat higher regional selectivity of primacy for the hippocampus as compared to non-primacy. Thus, taken together, our findings largely support the notion that the hippocampus plays an important role in delayed primacy recall and suggest that testing for this type of performance could be used as a resource for prediction of hippocampal integrity in cognitively healthy, older populations.
A key question that emerges from our results pertains to the specificity of the delayed primacy relationship with the hippocampus and neighboring areas, as opposed to memory for items elsewhere on the study list. This is indeed an important question that cannot be answered exhaustively by this study. However, a few considerations are possible. First of all, it has been highlighted how, despite its importance for the formation of long-lasting memories, the medial-temporal lobe, which includes the hippocampal formation, becomes progressively less involved in the maintenance of information in memory over time (e.g., Wixted & Cai, 2013). Hence, despite our emphasis here on retrieval processes, it is possible that the hippocampus may be primarily involved within the process of encoding new information and, thus, with the formation of memories that are effectively preserved, rather than with its extraction from storage. In this respect, it is worth noting that primacy effects are typically found to depend upon increased rehearsal opportunities for early-list items as opposed to items that are learned later (Rundus, 1971; Tan & Ward, 2000; although see Sederberg et al., 2006, for an account that incorporates both rehearsal and focused attention), which would benefit and strengthen the encoding process. Importantly, there is some evidence, both in clinical (Brown, Delia Sala, Foster & Vousden, 2007) and non-clinical (Davachi & Wagner, 2002) studies, that hippocampal regions are involved in the rehearsal process. Therefore, we can tentatively propose that the specificity of the relationship between delayed primacy performance and the hippocampus is dependent upon the role the hippocampus plays (perhaps in conjunction with the dorsolateral prefrontal cortex; see Innocenti et al., 2012) in facilitating rehearsal at learning, which in turn would lead to enhanced consolidation of primacy items. Further studies, however, are needed to clarify these points.
The relationship between hippocampal volume and episodic memory function in older individuals is somewhat complex with extreme variability noted and limited evidence of a positive correlation between size and performance (Van Petten, 2004). Our study may tentatively offer an explanation for these inconsistent findings. If the hippocampus is primarily involved with the retrieval of early list items, as our results appear to indicate, then it may be that studies using total list memory performance only find associations between hippocampal size and memory when, incidentally, primacy and non-primacy outputs are highly correlated. For instance, let us assume that Participant A free recalls all primacy items (e.g., 4) and all non-primacy items (e.g., 12) for a total of 16 items; Participant B free recalls 0 primacy items, but all non-primacy items for a total of 12; and finally Participant C free recalls all primacy items, but 0 non-primacy items for a total of 4. Based on our findings, Participant C would be expected to have a larger hippocampus than Participant B due to better primacy performance, despite a lower total score; moreover, Participant A would be expected to have roughly a similar-sized hippocampus to Participant C despite a much higher total score. In this example, examining total performance without considering serial position would lead to rejecting, erroneously, the hypothesis that hippocampal volume and episodic memory are positively correlated. This simple example illustrates how shifting the focus from total performance onto primacy performance, and particularly delayed primacy, may help clarify the relationship between hippocampal volume and episodic memory ability (also see Bruno et al., In Press, for a similar argument).
An obvious limitation of our study was that the sample was made up of two separate cohorts, which, despite similarities, had been recruited at different times for different purposes. Although we tried to overcome this issue by controlling for cohort in the statistical analysis, it would still have been preferable to obtain and analyze data from a single, homogeneous group. For these reasons, further investigations are recommended, including studies investigating clinical populations of interest (e.g., individuals with mild cognitive impairment).
AD is a devastating illness whose cause or causes are currently unknown, and for which there currently is no cure. Nonetheless, early, pre-clinical, intervention has been proposed as a viable solution to delay the onset of the disease and reduce prevalence (Emery, 2011; but see Ames, 2011). The identification of subjects at elevated risk of conversion to AD has benefited a great deal from research into the genetic correlates of AD (e.g., the APOE ε4 allele; Blennow, DeLeon & Zetterberg, 2006; Corder et al., 1993). Another avenue for early detection that has received considerable attention has been the study of disease biomarkers, including plasma (e.g., Yaffe et al., 2011) and cerebrospinal fluid (e.g., Pomara et al., 2012) levels of Aβ and tau proteins (e.g., Blennow & Zetterberg, 2013; Osorio et al., 2013). A third area for research is the study of brain structure. Tondelli, Wilcock, Nichelli, De Jager, Jenkinson and Zamboni (2012), for example, have shown that volumetric analysis of MRI data can be used to predict conversion to AD up to ten years prior to the disease onset: participants with preclinical AD show reductions in volume in the right medial temporal lobe, which includes the hippocampus, and the posterior cingulate/precuneus (see also Apostolova et al., 2010; Achterberg et al., 2013; den Heijer, Geerlings, Hoebeek, Hofman, Koudstaal & Breteler, 2006). Similar results on the predictive value of MRI-based hippocampal volume measurements have been shown in familial AD cohorts, such as the DIAN study (Bateman et al., 2012). These findings have prompted, somehow controversially (e.g., Le Couteur, Doust, Creasey & Brayne, 2013), the suggestion that hippocampal size could be screened in the general population to promote early identification and preventive interventions (Ferrarini et al., 2014).
Despite their promise and proven effectiveness, genetic, biomarker and MRI testing also present some limitations, with the main one being cost and availability. These issues become especially important when considering developing countries, where, according to the World Alzheimer Report 2009 by Alzheimer's Disease International, it is estimated that a large proportion of the world increase in AD prevalence will be observed. Therefore, it is vital that research produces less costly alternatives that can either act as a surrogate for early identification of AD risk, or, at least, aid the recognition of cases that would warrant further examination. In this respect, future lines of inquiry should explore whether our findings, corroborating the role of the hippocampus in delayed primacy effects, have clinical utility by investigating whether delayed primacy performance can be used as a predictor of conversion to AD from a cognitively healthy baseline.
Highlights.
The study sample constituted cognitively intact adults aged 60 or higher.
Delayed free recall for primacy words was associated with hippocampal volume.
This association was weaker for delayed non-primacy performance.
Acknowledgments
We would like to thank Marina Boccardi for expert advice and quality check of the traced hippocampus outlines following the EADC-ADNI harmonized hippocampus protocol, as well as Luigi Antelmi for helping with format conversion of the traced hippocampus mask. We would also like to acknowledge the help provided by Chelsea Reichert. The results of Study 2 were presented as a poster at the 2014 meeting of the Alzheimer's Association International Conference, Copenhagen, Denmark.
Funding: These studies were funded in part by NIMH grants (R01 MH-080405 & R01 MH-056994) to NP.
Footnotes
Given the emphasis on delayed primacy recall, and in consideration of the fact that recency effects are largely reduced after a delay (e.g., Glanzer & Cunitz, 1966), we have opted to compare primacy to non-primacy performance rather than employing the typical three sections of the serial position: primacy, middle and recency.
Conflict of Interest: No conflict of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Achterberg HC, van der Lijn F, den Heijer T, Vernooij MW, Ikram MA, Messen WJ, de Bruijne M. Hippocampal shape is predictive for the development of dementia in a normal, elderly population. Human brain mapping. 2014;35(5):2359–2371. doi: 10.1002/hbm.22333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert M, Moss MB, tanzi R, Jones K. Preclinical prediction of AD using neuropsychological tests. Journal of the International Neuropsychological Society. 2001;7:631–639. doi: 10.1017/s1355617701755105. [DOI] [PubMed] [Google Scholar]
- Albuquerque L, Loureiro C, Martins IP. Effect of lesion site on serial position during list learning: A study with the CVLT. International Journal of Neuroscience. 2008;118(7):917–933. doi: 10.1080/00207450701591081. [DOI] [PubMed] [Google Scholar]
- Ames D. Negative argument for debate with VO Emery for J Neural Transmission. Journal of Neural Transmission. 2011;118(9):1379–1381. doi: 10.1007/s00702-011-0678-6. [DOI] [PubMed] [Google Scholar]
- Apostolova LG, Mosconi L, Thompson PM, Green AE, Hwang KS, Ramirez A, Mistur R, Tsui WH, de Leon MJ. Subregional hippocampal atrophy predicts Alzheimer's dementia in the cognitively normal. Neurobiology of aging. 2010;31(7):1077–1088. doi: 10.1016/j.neurobiolaging.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38(1):95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Bateman RJ, Xiong C, Benzinger TL, Fagan AM, Goate A, Fox NC, Morris JC. Clinical and biomarker changes in dominantly inherited Alzheimer's disease. New England Journal of Medicine. 2012;367(9):795–804. doi: 10.1056/NEJMoa1202753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berti V, Mosconi L, Glodzik L, Li Y, Murray J, De Santi S, De Leon MJ. Structural brain changes in normal individuals with a maternal history of Alzheimer's. Neurobiology of aging. 2011;32(12):2325–e17. doi: 10.1016/j.neurobiolaging.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blennow K, DeLeon MJ, Zetterberg H. Alzheimer's disease. Lancet. 2006;368:387–403. doi: 10.1016/S0140-6736(06)69113-7. [DOI] [PubMed] [Google Scholar]
- Blennow K, Zetterberg H. The application of cerebrospinal fluid biomarkers in early diagnosis of Alzheimer disease. Medical Clinics of North America. 2013;97(3):369–376. doi: 10.1016/j.mcna.2012.12.012. [DOI] [PubMed] [Google Scholar]
- Boccardi M, Bocchetta M, Ganzola R, Robitaille N, Redolfi A, Duchesne S, Frisoni GB. Operationalizing protocol differences for EADC-ADNI manual hippocampal segmentation. Alzheimer's & Dementia. 2013 doi: 10.1016/j.jalz.2013.03.001. [DOI] [PubMed] [Google Scholar]
- Brown GD, Delia Sala S, Foster JK, Vousden JI. Amnesia, rehearsal, and temporal distinctiveness models of recall. Psychonomic Bulletin & Review. 2007;14(2):256–260. doi: 10.3758/bf03194061. [DOI] [PubMed] [Google Scholar]
- Brown GD, Neath I, Chater N. A temporal ratio model of memory. Psychological review. 2007;114(3):539. doi: 10.1037/0033-295X.114.3.539. [DOI] [PubMed] [Google Scholar]
- Bruno D, Brown AD, Kapucu A, Marmar CR, Pomara N. Cognitive Reserve and Emotional Stimuli in Older Individuals: Level of Education Moderates the Age-Related Positivity Effect. Experimental aging research. 2014;40(2):208–223. doi: 10.1080/0361073X.2014.882212. [DOI] [PubMed] [Google Scholar]
- Bruno D, Grothe MJ, Nierenberg J, Teipel SJ, Zetterberg H, Blennow K, Pomara N. The relationship between CSF tau markers, hippocampal volume and delayed primacy performance in cognitively intact elderly individuals. Alzheimer's & Dementia: Diagnosis, Assessment and Disease Monitoring. doi: 10.1016/j.dadm.2014.11.002. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno D, Nierenberg J, Ritchie JC, Lutz MW, Pomara N. CSF Cortisol concentrations in healthy elderly are affected by both APOE and TOMM40. Psychoneuroendocrinology. 2012;37:366–371. doi: 10.1016/j.psyneuen.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno D, Pomara N, Nierenberg J, Ritchie JC, Lutz MW, Zetterberg H, Blennow K. Levels of cerebrospinal fluid neurofilament light protein in healthy elderly vary as a function of TOMM40 variants. Experimental Gerontology. 2012;47:347–352. doi: 10.1016/j.exger.2011.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno D, Reiss RT, Petkova E, Sidtis JJ, Pomara N. Decreased Recall of Primacy Words Predicts Cognitive Decline. Archives of clinical neuropsychology. 2013;28(2):95–103. doi: 10.1093/arclin/acs116. [DOI] [PubMed] [Google Scholar]
- Buschke H, Fuld PA. Evaluating storage, retention, and retrieval in disordered memory and learning. Neurology. 1974;24(11):1019–1019. doi: 10.1212/wnl.24.11.1019. [DOI] [PubMed] [Google Scholar]
- Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small G, Pericak-Vance MA. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science. 1993;261(5123):921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- Davachi L, Wagner AD. Hippocampal contributions to episodic encoding: insights from relational and item-based learning. Journal of Neurophysiology. 2002;88(2):982–990. doi: 10.1152/jn.2002.88.2.982. [DOI] [PubMed] [Google Scholar]
- den Heijer T, Geerlings MI, Hoebeek FE, Hofman A, Koudstaal PJ, Breteler MM. Use of hippocampal and amygdalar volumes on magnetic resonance imaging to predict dementia in cognitively intact elderly people. Archives of general psychiatry. 2006;63(1):57–62. doi: 10.1001/archpsyc.63.1.57. [DOI] [PubMed] [Google Scholar]
- Egli SC, Beck IR, Berres M, Foldi NS, Monsch AU, Sollberger M. Serial position effects are sensitive predictors of conversion from MCI to Alzheimer's disease dementia. Alzheimer's & Dementia. 2014 doi: 10.1016/j.jalz.2013.09.012. [DOI] [PubMed] [Google Scholar]
- Emery VOB. Alzheimer disease: are we intervening too late? Journal of Neural Transmission. 2011;118(9):1361–1378. doi: 10.1007/s00702-011-0663-0. [DOI] [PubMed] [Google Scholar]
- Ferrarini L, van Lew B, Reiber JH, Gandin C, Galluzzo L, Scafato E, Pievani M. Hippocampal atrophy in people with memory deficits: results from the population-based IPREA study. International Psychogeriatrics. 2014;26(07):1067–1081. doi: 10.1017/S1041610213002627. [DOI] [PubMed] [Google Scholar]
- Glanzer M. Storage mechanisms in recall. In: Bower GH, editor. The psychology of learning and motivation. New York: Academic Press; 1972. pp. 129–153. [Google Scholar]
- Glanzer M, Cunitz AR. Two storage mechanisms in free recall. Journal of Verbal Learning and Verbal Behavior. 1966;5:351–360. [Google Scholar]
- Gomar JJ, Bobes-Bascaran MT, Conejero-Goldberg C, Davies P, Goldberg TE. Utility of combinations of biomarkers, cognitive markers, and risk factors to predict conversion from mild cognitive impairment to Alzheimer disease in patients in the Alzheimer's disease neuroimaging initiative. Archives of General Psychiatry. 2011;68:961–969. doi: 10.1001/archgenpsychiatry.2011.96. [DOI] [PubMed] [Google Scholar]
- Grothe MJ, Ewers M, Krause B, Heinsen H, Teipel SJ. Basal forebrain atrophy and cortical amyloid deposition in nondemented elderly subjects. Alzheimer's & Dementia. 2014 doi: 10.1016/j.jalz.2013.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann BP, Seidenberg M, Wyler A, Davies K, Christeson J, Moran M, Stroup E. The effects of human hippocampal resection on the serial position curve. Cortex. 1996;32:323–334. doi: 10.1016/s0010-9452(96)80054-2. [DOI] [PubMed] [Google Scholar]
- Howieson DB, Mattek N, Seeyle AM, Dodge HH, Wasserman D, Zitzelberger T, Jeffrey K. Journal of Clinical and Experimental Neuropsychology. 2011;33:292–299. doi: 10.1080/13803395.2010.516742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innocenti I, Cappa SF, Feurra M, Giovannelli F, Santarnecchi E, Bianco G, Cincotta M, Rossi S. TMS interference with primacy and recency mechanisms reveals bimodal episodic encoding in the human brain. Journal of cognitive neuroscience. 2013;25(1):109–116. doi: 10.1162/jocn_a_00304. [DOI] [PubMed] [Google Scholar]
- Kim HY. Statistical notes for clinical researchers: assessing normal distribution (2) using skewness and kurtosis. Restorative dentistry & endodontics. 2013;38(1):52–54. doi: 10.5395/rde.2013.38.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Rue A, Hermann B, Jones JJ, Johnson S, Ashtana S, Sager MA. Effect of parental family history of Alzheimer's disease on serial position profiles. Alzheimer's and Dementia. 2008;4:285–290. doi: 10.1016/j.jalz.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Couteur DG, Doust J, Creasey H, Brayne C. Political drive to screen for pre-dementia: not evidence based and ignores the harms of diagnosis. BMJ: British Medical Journal. 2013:347. doi: 10.1136/bmj.f5125. [DOI] [PubMed] [Google Scholar]
- Martin ME, Sasson Y, Crivelli L, Roldán Gerschovich E, Campos JA, Calcagno ML, Allegri RF. Relevance of the serial position effect in the differential diagnosis of mild cognitive impairment, Alzheimer-type dementia, and normal ageing. Neurología (English Edition) 2013;28(4):219–225. doi: 10.1016/j.nrl.2012.04.013. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. Memory: a century of consolidation. Science. 2000;287:248–251. doi: 10.1126/science.287.5451.248. [DOI] [PubMed] [Google Scholar]
- McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Jr, Kawas CH, Phelps CH. The diagnosis of dementia due to Alzheimer's disease: Recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimer's & Dementia. 2011;7(3):263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdock BB. The serial position effect of free recall. Journal of Experimental Psychology. 1962;64:482–488. [Google Scholar]
- Osorio RS, Pirraglia E, Gumb T, Mantua J, Ayappa I, Williams S, de Leon MJ. Imaging and Cerebrospinal Fluid Biomarkers in the Search for Alzheimer's Disease Mechanisms. Neurodegenerative Diseases. 2013;13(2-3):163–165. doi: 10.1159/000355063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillai A, Bruno D, Sarreal AS, Hernando RT, Saint-Louis LA, Nierenberg J, Ginsberg SD, Pomara N, Mehta PD, Zetterberg H, Blennow K, Buckley PF. Plasma BDNF levels vary in relation to body weight in females. PLoS One. 2012;7:e39358. doi: 10.1371/journal.pone.0039358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomara N, Bruno D, Sarreal A, Hernando R, Nierenberg JJ, Petkova E, Sidtis JJ, Mehta PD, Wisniewski TM, Pratico D, Zetterberg H, Blennow K. Lower CSF amyloid beta peptides and higher F2-isoprostanes in cognitively intact elderly individuals with major depressive disorder. The American Journal of Psychiatry. 2012;169:523–530. doi: 10.1176/appi.ajp.2011.11081153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomara N, Facelle TM, Roth AE, Willoughby LM, Greenblatt DJ, Sidtis JJ. Dose-dependent retrograde facilitation of verbal memory in healthy elderly after acute oral lorazepam administration. Psychopharmacology. 2006;185(4):487–494. doi: 10.1007/s00213-006-0336-0. [DOI] [PubMed] [Google Scholar]
- Pomara N, Shao B, Wisniewski T, Mehta PD. Decreases in Plasma Aβ1-40 Levels with Aging in Non-Demented Elderly with ApoE-ε4 Allele. Neurochemical research. 1998;23(12):1563–1566. doi: 10.1023/a:1020936222286. [DOI] [PubMed] [Google Scholar]
- Pomara N, Willoughby L, Wesnes K, Greenblatt DJ, Sidtis JJ. Apolipoprotein E ε4 allele and lorazepam effects on memory in high-functioning older adults. Archives of general psychiatry. 2005;62(2):209–216. doi: 10.1001/archpsyc.62.2.209. [DOI] [PubMed] [Google Scholar]
- Rundus D. Analysis of rehearsal processes in free recall. Journal of Experimental Psychology. 1971;89:63–77. [Google Scholar]
- Rutherford A. ANOVA and ANCOVA: a GLM approach. John Wiley & Sons; 2012. [Google Scholar]
- Sederberg PB, Gauthier LV, Terushkin V, Miller JF, Barnathan JA, Kahana MJ. Oscillatory correlates of the primacy effect in episodic memory. Neurolmage. 2006;32:1422–1431. doi: 10.1016/j.neuroimage.2006.04.223. [DOI] [PubMed] [Google Scholar]
- Silverman JM, Ciresi G, Smith CJ, Marin DB, Schnaider-Beeri M. ariability of familial risk of Alzheimer disease across the late life span. Archives of General Psychiatry. 2005;62:565–573. doi: 10.1001/archpsyc.62.5.565. [DOI] [PubMed] [Google Scholar]
- Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, Phelps CH. Toward defining the preclinical stages of Alzheimer's disease: Recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimer's & Dementia. 2011;7(3):280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiger JH. Tests for comparing elements of a correlation matrix. Psychological Bulletin. 1980;87:245–251. [Google Scholar]
- Strange BA, Otten LJ, Josephs O, Rugg MD, Dolan RJ. Dissociable human perirhinal, hippocampal, and parahippocampal roles during verbal encoding. The Journal of Neuroscience. 2002;22(2):523–528. doi: 10.1523/JNEUROSCI.22-02-00523.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talmi D, Grady CL, Goshen-Gottstein Y, Moscovitch M. Neuroimaging the Serial Position Curve A Test of Single-Store Versus Dual-Store Models. Psychological Science. 2005;16(9):716–723. doi: 10.1111/j.1467-9280.2005.01601.x. [DOI] [PubMed] [Google Scholar]
- Teipel S, Heinsen H, Amaro E, Jr, Grinberg LT, Krause B, Grothe M, Alzheimer's Disease Neuroimaging Initiative Cholinergic basal forebrain atrophy predicts amyloid burden in Alzheimer's disease. Neurobiol Aging. 2014 Mar;35(3):482–91. doi: 10.1016/j.neurobiolaging.2013.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan L, Ward G. A recency-based account of the primacy effect in free recall. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2000;26(6):1589. doi: 10.1037//0278-7393.26.6.1589. [DOI] [PubMed] [Google Scholar]
- Tondelli M, Wilcock GK, Nichelli P, De Jager CA, Jenkinson M, Zamboni G. Structural MRI changes detectable up to ten years before clinical Alzheimer's disease. Neurobiology of aging. 2012;33(4):825–e25. doi: 10.1016/j.neurobiolaging.2011.05.018. [DOI] [PubMed] [Google Scholar]
- Van Petten C. Relationship between hippocampal volume and memory ability in healthy individuals across the lifespan: review and meta-analysis. Neuropsychologia. 2004;42(10):1394–1413. doi: 10.1016/j.neuropsychologia.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Wixted JT. The psychology and neuroscience of forgetting. Annual Review of Psychology. 2004;55:235–269. doi: 10.1146/annurev.psych.55.090902.141555. [DOI] [PubMed] [Google Scholar]
- Wixted J, Cai DJ. Memory Consolidation. The Oxford Handbook of Cognitive Neuroscience, Volume 1: Core Topics. 2013;1:436. [Google Scholar]