Abstract
Objective
The sparse existing research on ipsilesional neglect supports an association of this disorder with damage to the right frontal and subcortical brain networks. It is believed that dysfunction in these networks may result in primarily “aiming”, motor-intentional spatial errors. The purpose of this study was to confirm whether frontal-subcortical circuits are indeed commonly affected in ipsilesional neglect and to determine the relative presence of “aiming”, motor-intentional versus “where”, perceptual-attentional spatial errors in these individuals.
Methods
We identified 12 participants with ipsilesional neglect based on a computerized line bisection task and used the line bisection data to quantify participants' perceptual-attentional and motor-intentional errors. We were able to discriminate between these two biases using the algebraic solutions for two separate equations, one for “aiming” and one for “where” biases. Lesion mapping was conducted for all participants using MRICroN® software; lesion checklist and overlap analysis were created from these images.
Results
A greater percentage of participants with ipsilesional neglect had frontal/subcortical damage (83%) compared to the expected percentage (27%) observed in published patient samples with contralesional neglect. We observed the greatest area of lesion overlap in frontal lobe white matter pathways. Nevertheless, participants with ipsilesional neglect made primarily “where” rather than “aiming” spatial errors.
Conclusion
Our data confirms previous research suggesting that ipsilesional neglect may result from lesions to the right frontal-subcortical networks. Furthermore, in our group, ipsilesional neglect was also strongly associated with primarily “where”, perceptual-attentional bias, and less so with “aiming” motor-intentional spatial bias.
Keywords: ipsilesional neglect, spatial neglect, attention, stroke, right brain
Spatial neglect is a disorder demonstrated by patients as a failure to report, respond to or orient towards stimuli, causing functional disability (Heilman & Valenstein, 1979; Barrett & Burkholder, 2006). Neglect usually results from and is most severe following right hemisphere damage (reviewed in Barrett et al., 2006) and most commonly impairs processes, or responses to, stimuli in contralesional, left space. However, spatial neglect is a complex and heterogeneous disorder (e.g., Coslett, 1997). Although contralesional neglect occurs more frequently, cases of ipsilesional or right-sided neglect after right stroke have also been described (e.g., Kwon & Heilman, 1991; Robertson, Halligan, Bergego, Homberg, Pizzamiglio, Weber & Wilson, 1994; Beschin, Basso & Della Sala, 2000). However, the literature on ipsilesional neglect is scarce and much less is known about this relatively rare disorder than its widely studied counterpart, contralesional neglect. The few cases that have been reported with lesion localization data show that frontal-subcortial lesions are more common in patients with ipsilesional, relative to contralesional neglect (Kim, Na, Kim, Adair, Lee & Heilman, 1999; Na, Adair, Choi, Seo, Kang & Heilman, 2000).
Motor-Intentional and Perceptual-Attentional Neglect
Stroke patients with spatial neglect may make different types of spatial performance errors. Performance deficits may occur because of pathological perceptual-attentional awareness of stimuli in one side of space (“where” spatial function), or may occur because of deficits affecting motor-intentional movement preparation (“aiming” spatial function) errors (Barrett, 2014); Buxbaum et al., 2004; Na, Adair, Williamson, Schwartz, Haws & Heilman, 1998; Bisiach, 1990; Heilman, 2004). One method for fractionating these specific components of spatial performance employs a variation of the line bisection task. Na and colleagues (1998) used a video apparatus to dissociate “aiming” versus “where” spatial errors. The video image was manipulated so that there were two conditions: In the Natural condition the right side of the line to be bisected appeared on the right side of the screen and vice versa. However, in the Reversed condition, the video image was rotated 180 degrees, so that the right side of the line to be bisected appeared on the left side of the screen (see Chen et al., 2011 for a detailed description and picture of the task). Rightward movements of the hand appear leftward and vice versa. In the Natural condition, patients with contralesional neglect typically make right-sided line bisection errors. In the Reversed condition, however, stroke patients can demonstrate two different patterns of performance. In some individuals, a failure to move leftward (directional hypokinesia) results in persistent rightward errors: the right-left reversal of visual feedback has no effect on the primary direction of performance errors. Other individuals, however, have a primary perceptual-attentional unawareness of the left side of the line, or fail to represent the left side of the line internally as they represent the right side of the line (Adair and Barrett, 2008). In these subjects, right-left reversal of the line to be bisected results in the patient making leftward, rather than rightward, errors in the workspace.
Using the line bisection method of Na et al. (1999), Kim et al (1999) found that among a small sample of five patients with ipsilesional neglect some exhibited primarily “where” spatial bias (n =2), and others primarily “aiming” spatial bias (n =3). Thus, it is not clear whether ipsilesional neglect is preferential associated with one of these forms of spatial bias.
Furthermore, while Na et al. (1998) used their paradigm to categorize participants as having a primary motor-intentional vs. a primary perceptual-attentional bias, many neglect patients have a combination of both biases (Barrett & Burkholder, 2006; Goedert, Chen, Boston, Foundas & Barrett, 2014). Recognizing this, we followed the method of Barrett and Burkholder (2006) for quantifying the amount of motor-intentional “aiming” and perceptual-attentional “where” biases, calculating both for every participant. The quantification of both biases can be achieved by algebraically solving the following equations based on the participant's error in the natural and reversed viewing conditions:
| (Eq. 1) |
| (Eq. 2) |
When a participant performs line bisections under the natural viewing condition, both “aiming” and “where” bias are considered as contributing to the spatial bias- the visual input and the movement itself are directionally congruent (an additive effect of “aiming” and “where” biases). When a participant performs line bisections under the reverse viewing condition, the visual input (or “where” component) becomes mirrored imaged, or reversed in direction, while the motor movement (or “aiming” component) remains unchanged. In this case, “aiming” is subtracted from “where” biases to represent their different valences. Were ipsilesional neglect to result from a compensatory strategy, as has been suggested previously (Robertson et al., 1994), then participants with ipsilesional neglect should show greater “aiming” than “where” bias when it is quantified in this manner.
Neuroanatomical Correlates of Ipsilesional Neglect
Although contralesional neglect is traditionally associated with posterior cortical lesions located in the parietal lobe (Vallar & Perani, 1986), the temporal-parietal-occipital (TPO) junction (Leibovitch, Black, Caldwell, Ebert, Ehrlich & Szalai, 1998) and the superior temporal gyrus (STG;Karnath, Himmelbach & Rorden, 2002; Karnath, Berger, Kuker & Rorden, 2004; Karnath, Rennig, Johannsen & Rorden, 2011), there is less information about the anatomical correlates of ipsilesional neglect. Studies of patients with ipsilesional neglect associated this syndrome with right hemisphere lesions including the frontal lobe (Na, Adair, Choi, Seo, Kang & Heilman, 2000; Kim et al, 1999; Robertson et al, 1994), the dorsolateral frontal lobe (Kwon & Heilman, 1991), the anterior cerebral artery territory including the high mesial frontal cortex (Drago et al., 2006), the temporal lobe (Na, Adair, Choi, Seo, Kang & Heilman, 2000; Robertson et al, 1994), parietal and occipital lobe (Robertson et al, 1994), insula (Na et al, 2000), the middle cerebral artery territory (Schwartz, Barrett, Kim & Heilman, 1999), the thalamus and caudate (Barrett, Peterlin & Heilman, 2003) and the basal ganglia (Na et al, 2000; Kim et al, 1999). Lesion data reported in these papers support the frontal lobe (36%) and subcortical structures (50%) as being most the most commonly lesioned sites in ipsilesional neglect (Refer to Table 1).
Table 1.
Published Ipsilesional Neglect Lesion Data
| Study | N of ipsilesional neglect | Frontal | Parietal | Temporal | Occipital | Insula | Subcortical |
|---|---|---|---|---|---|---|---|
| Kwon & Heilman, 1991 | 1 | 1 | 0 | 0 | 0 | 0 | 0 |
| Schwartz et al, 1999 | 1 | 1 | 1 | 1 | 1 | 0 | 0 |
| Barrett at al, 2003 | 1 | 0 | 0 | 0 | 0 | 0 | 1 |
| Drago et al, 2006 | 1 | 1 | 0 | 0 | 0 | 0 | 0 |
| Robertson et al, 1994 | 8 | 3 | 5 | 3 | 1 | 0 | 1 |
| Kim et al, 1999 | 5 | 1 | 0 | 0 | 0 | 0 | 5 |
| Na et al, 2000 | 5 | 1 | 0 | 1 | 0 | 1 | 4 |
| Totals | 22 | 8 | 6 | 5 | 2 | 1 | 11 |
| % lesion from all cases | 36% | 27% | 22% | 9% | 4% | 50% |
Notes. Subcortical refers to the thalamus, caudate, basal ganglia, internal capsule, corona radiate and centrum semiovale.
Current Study
In this study, we examined the anatomical and behavioral correlates of ipsilesional neglect in a retrospective analysis of patients selected from a cohort of consecutive post-stroke individuals recruited in a study of spatial neglect recovery. We tested two hypotheses: 1) ipsilesional neglect will be associated with a primary “aiming” spatial bias, and 2) ipsilesional neglect will be associated with damage to the frontal lobe and subcortical structures. Study participants underwent evaluation of “where” and “aiming” spatial bias with a line bisection task, and we also compared the location of their brain lesions with past reports of brain lesion locations associated with contralateral neglect.
Methods
Archival Dataset
Participants were selected from a dataset (N = 132) reflecting a consecutive sample (December 2, 2008 to June 15, 2011) of right-hemisphere stroke patients with suspected spatial neglect, enrolled from an inpatient rehabilitation hospital five to ten days post-stroke. Participants in this dataset had unilateral right hemisphere brain damage, with no history of dementia, uncontrolled glaucoma, or previous head trauma with loss of consciousness (data on subsets of the patients with contralesional neglect in this dataset have previously been published; Chen et al., 2012; Goedert et al., 2012, 2013). All 132 patients were evaluated with the Mini-Mental Status Examination (MMSE; Folstein, Folstein, & McHugh, 1975), Behavioural Inattention Test (BIT; Wilson, Cockburn & Halligan, 1987), Catherine Bergego Scale (CBS; Azouvi, Olivier, Montety, Samuel, Louis-Dreyfus & Tesio, 2003) and Barthel Index (Mahoney & Barthel, 1965). The BIT, a paper-and-pencil neglect assessment, consists of a line bisection test, three cancellation test (lines, stars, and letters), and three drawing tests (figures, shapes, and representational drawing). The CBS is a functional assessment of neglect and was used to evaluate the participant's abilities in functional activities, specific for the left side of space, and was performed by an occupational therapist blind to the purpose of the study using the Kessler Foundation-Neglect Assessment Process (Chen et al., 2012). Study participants also underwent spatial performance testing to detect and quantify “where” and “aiming” spatial bias (Chen et al., 2011).
Participant Selection Criteria
Using the existing data from these 132 patients, we identified participants with ipsilesional neglect. Participants were categorized as having ipsilesional neglect if they demonstrated abnormal leftward error in the natural viewing condition of the computerized line bisection task. Cut off values for defining abnormal leftward error were based on Chen et al (2011), which assessed age-related and sex-specific differences in the spatial bias of healthy participants (N = 44) completing a line bisection task. Table 2 depicts the means and standard deviations of the age- and sex-specific healthy groups from Chen et al. (2011). Here, patients were categorized as having ipsilesional neglect if their line bisection error fell more than two standard deviations outside the lower cutoff (leftward error) for the appropriate age- and gender-based healthy group. We assigned each sex/ age group its own cutoff score, because Chen et al. (2011) demonstrated a difference in normal line bisection performance with age and gender.
Table 2.
Mean and SD of Line Bisections in Healthy Individuals in Chen et al. (2011)
| Young (22–56) | Old (57–93) | |||
|---|---|---|---|---|
| Sex | M (SD, n) | Cutoff | M (SD, n) | Cutoff |
| Males | −.91 (3.03, 12) | −6.97 | 2.53 (2.88, 10) | −3.18 |
| Females | −3.06 (2.71, 10) | −8.48 | −4.15 (6.09, 12) | −16.37 |
Notes. M = mean, SD = standard deviation. Negative numbers denote a leftward deviation.
Participants
We identified 14 participants (10 male, 4 female) with ipsilesional neglect. Two participants from this group were dropped from further analysis because on brain scan review, one participant had bilateral strokes, and one participant did not score below a conventional BIT cutoff (≥129) for neglect. The remaining twelve participants were distributed as follows: older women (n = 2); older men (n = 7); young women (n = 2); young men (n = 1). Compared to the archival contralesional dataset, this group of twelve ipsilesional participants tested at a similar time post-stroke [M=23.62, SD=21.33 days for contralesional; M=18.25, SD=7.34 days for ipsilesional, t(36.01) = 1.85, p = .072]. There was also a similar rate of hemianopia among the ipsilesional (n = 1) and contralesional participants (n = 12; p = 1.00 for the Fisher's exact test comparing the hemianopia rates). Table 3 shows additional demographic and clinical data for the twelve participants classified as having ipsilesional neglect.
Table 3.
Demographic and clinical data of twelve participants with ipsilesional neglect
| Sex | Age | Edu. | MMSE | BIT | Barthel | CBS | Where | Aiming | |
|---|---|---|---|---|---|---|---|---|---|
| S1 | F | 30 | 14 | 30 | 128 | 90 | 2 | −35.35 | −1.63 |
| S2 | M | 74 | 11 | 22 | 114 | 65 | 17 | −1.67 | −2.51 |
| S3 | M | 66 | 12 | 25 | 26 | 5 | 23 | −0.83 | −9.98 |
| S4 | M | 53 | 16 | 29 | 67 | 10 | 5 | −11.79 | −3.44 |
| S5 | F | 59 | 12 | 21 | 65 | 35 | 23 | −14.00 | −2.35 |
| S6 | F | 41 | 18+ | 26 | 67 | 30 | - | −43.44 | −4.57 |
| S7 | M | 67 | 9 | 21 | 58 | 15 | 20 | −14.46 | 3.08 |
| S8 | M | 68 | 8 | 13 | 104 | 10 | 21 | −10.73 | 6.00 |
| S9 | M | 76 | 12 | 23 | 59 | 0 | 27 | −25.90 | −13.79 |
| S11 | F | 76 | 8 | 17 | 101 | 20 | - | −10.00 | −15.92 |
|
| |||||||||
| Mean | - | 61 | 12 | 22.7 | 78.9 | 28 | 17.25 | −16.82 | −4.51 |
| SD | - | 15.48 | 3.30 | 5.19 | 31.39 | 28.79 | 8.01 | 13.99 | 6.93 |
|
| |||||||||
| S10 | M | 76 | 18 | 29 | 129 | 60 | - | −1.55 | −4.77 |
| S12 | M | 78 | 12 | 16 | 93 | 30 | 5 | −11.22 | −5.18 |
|
| |||||||||
| Mean | - | 77 | 15 | 22.5 | 111 | 60 | - | −6.38 | −4.97 |
| SD | - | 1.14 | 4.24 | 9.19 | 25.46 | 21.21 | - | 6.84 | .29 |
Notes. F= female, M=male; Edu.= Education in years; Where and Aiming error in mm (analysis performed on Z-scores); S1–S9 & S11 are individuals whose brain scans were classified as demonstrating frontal lobe/subcortical damage; S10 and S12 are individuals without classified frontal lobe/subcortical damage on brain imaging.
Procedure
Quantification of “Where” and “Aiming” Bias
All participants sat centrally in front a computer screen, which was 60cm away. The room was dimmed, and the experimenter sat out of the participant's view. The participants used their right hand to move a computer mouse that was located under a shelf on the desk in front of them. The shelf blocked participants' view of their hand movements. In order to monitor what their hand was doing they had to look at the computer screen. After a series of 8 practice trials, the participant bisected 32 horizontal lines, 16 in the natural condition and 16 in the reverse condition. Each line was presented at a visual angel of 23.537 degrees and appeared in black in the center of a white screen. The starting location of the cursor alternated each trial, with half of the trials starting in the upper right-hand and half starting in the upper left-hand corner of the computer screen. In the Natural condition, the cursor on the screen moved in the same direction as the hand movement. For example, rightward movement of the hand moved the cursor to the right. In the Reversed condition, the cursor on the screen moved in the opposite direction as the hand movement. For example, rightward movement of the hand moved the leftward. Errors were recorded as deviation (mm) from the true center of the line, with rightward errors coded as positive and leftward errors coded as negative.
In order to separate “where” and “aiming” errors we used an equation which has been previously described by Barrett & Burkholder (2006). The algebraically solution to Equations 1 and 2 allows us to separately quantify “where” and “aiming” bias (see also Fortis, Goedert & Barrett, 2011):
| (Eq. 3) |
| (Eq. 4) |
A “where” bias and “aiming” bias score was calculated for each participant using the above equations.
Lesion Mapping
Clinical brain scans were used to map lesion location in each study participant. Lesions were mapped from T1-weighted and FLAIR images (with DWI when available) to a standard template that matched the clinical image (Karnath et al.,2004; www.mricro.com). Patients who only had a clinical CT scan (4 of 12) were not excluded. The lesion analysis method consisted of generating individual maps of the full-extent of the lesion visualized on standardized axial templates. A trained experimenter mapped all of the patients' scans by manually locating the lesion based on standard landmarks such as gyri, sulci, gray matter and white matter boundaries, or vascular territories (Barta, Dhingra, Royall & Schwartz, 1997; Ono, Kubik & Abernathey, 1990; Talairach & Tournoux, 1988). A computer-guided cursor was used to draw the full-extent of the lesion including orthogonal views to assist with the accurate delineation of lesion borders. Each lesion map was realigned into stereotaxic Montreal Neurological Institute (MNI) space to overlay them on standard brain templates. In order to improve the lesion-mapping method, the following procedures were followed: an experienced examiner mapped all of the lesions, and these lesion maps were reviewed by a 2nd rater following procedures previously described to generate the final lesion maps (Cola, Daniels, Corey, Lemen, Romero, & Foundas, 2010; Daniels & Foundas, 1999; Foundas, Daniels, Vasterling, 1998; Hanna-Pladdy et al., 2001; Naeser & Hayward, 1978; Damasio & Damasio, 1989).
Two approaches to lesion localization were used including: (1) anatomical checklist, and (2) areas of overlap. The anatomical checklist (see Table 4) was used to identify cortical (frontal, temporal, parietal, occipital, and insula) and subcortical (gray and white matter) lesion locations. Subcortical lesion locations included the thalamus, caudate/putamen, globus pallidus, subthalamus, internal/external/extreme capsule, and the periventricular white matter. Region of interest maps were generated for subsequent analysis in MRIcro® (Rorden and Brett, 2000) (to compute group overlap and for group comparison) including an examination of areas of overlap. The VLBM analysis was performed with the MRIcroN® nonparametric mapping software (Rorden et al. 2007). Lesion volume (cm3) was also calculated for each subject from the lesion map.
Table 4.
Lesion locations of the 12 participants identified with Ipsilesional Neglect
| Subject | Image | FL | PL | SSM | TL | OL | TPO | Insula | Subcortical |
|---|---|---|---|---|---|---|---|---|---|
| S1 | MRI | 0 | 0 | 0 | X | X | 0 | 0 | X |
| S2* | MRI | 0 | 0 | 0 | 0 | 0 | 0 | 0 | X |
| S3* | CT | X | X | 0 | X | 0 | X | X | X |
| S4* | CT | X | 0 | 0 | 0 | 0 | 0 | 0 | X |
| S5* | MRI | 0 | 0 | 0 | 0 | 0 | 0 | 0 | X |
| S6 | MRI | X | X | 0 | 0 | 0 | X | 0 | X |
| S7 | MRI | 0 | 0 | 0 | 0 | 0 | 0 | 0 | X |
| S8* | MRI | X | 0 | X | X | 0 | 0 | X | X |
| S9* | CT | X | 0 | X | 0 | 0 | 0 | X | X |
| S10 | MRI | 0 | 0 | X | 0 | 0 | 0 | 0 | 0 |
| S11* | CT | X | X | X | X | 0 | 0 | X | X |
| S12 | MRI | 0 | 0 | 0 | 0 | X | 0 | 0 | 0 |
| Total | 6 | 3 | 4 | 4 | 2 | 2 | 4 | 10 | |
| % | 50% | 25% | 33% | 33% | 17% | 17% | 33% | 83% |
Notes. Above, “x” indicates that the region was lesioned; values of 0 indicate that no lesion was detected. An* indicates damage to anterior white matter.
Results
Lesion Analysis
Table 4 summarizes lesion locations identified in our subjects. Consistent with our hypothesis ten of the twelve participants (83%) with ipsilesional neglect had damage to right frontal-subcortical regions.
We conducted a χ2 goodness of fit analysis to determine whether participants with ipsilesional neglect had a greater incidence of right frontal-subcortical damage, relative to the incidence typically observed in stroke patients with contralesional neglect. We identified five anatomical studies on patients in the acute stage of stroke recovery from contralesional neglect (Leibovitch et al, 1998; Karnath, Himmelbach & Rorden, 2002; Mort et al, 2003; Karnath, Renning, Johannsen & Rorden, 2011; Chen, Goedert, Shah, Foundas & Barrett, 2012). Using the weighted average of these five studies may not be the best comparison to our ipsilesional neglect sample because of differences in the exclusion criteria. Thus, we completed a Chi-square goodness-of- fit analysis excluding the two studies conducted by Karnath and colleagues (Karnath, Himmelbach & Rorden, 2002; Karnath, Renning, Johannsen & Rorden, 2011), because unlike our participant sample and those of the Leibovitch et al. (1988), Mort et al. (2003), and Chen et al. (2012) studies, the Karnath studies excluded individuals with visual field deficits. The weighted average proportion of contralesional patients with frontal or subcortical damage from these three studies was 0.3471. The expected values derived from the weighted average of these three selected studies are shown in Table 5. The results of the Chi-square analysis indicated that a greater proportion of participants with ipsilesional neglect in the current sample had frontal or subcortical damage, compared to expected proportions observed in published patient samples with contralesional neglect (χ2 (1,N=12) =12.55, p<.001).
Table 5.
Observed and Expected Values Derived from the Select Weighted Average
| Observed | Expected | |
|---|---|---|
| Frontal Lobe or Subcortical Damage | 10 | 4.16 |
| Other Damage | 2 | 7.84 |
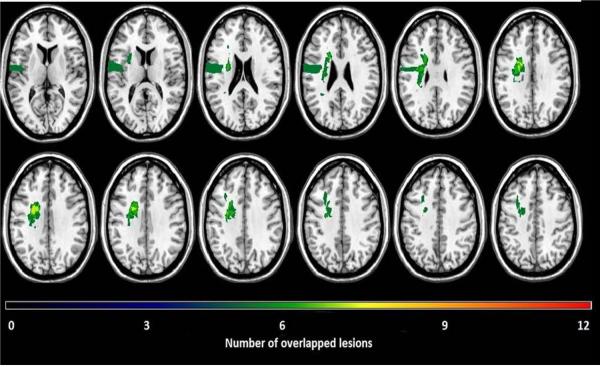
Lesion overlap analysis of the twelve participants in the study (Figure 1) indicates the brain regions most commonly affected in this sample of ipsilesional neglect participants. Lesion overlap analysis depicts the greatest areas of overlap in frontal lobe white matter; this region had a maximum overlap for 8 out of the 12 participants. The results from the lesion overlap are also in support of our hypothesis, implicating damage to the frontal lobe as an important anatomical correlate of ipsilesional neglect.
Figure 1.
Lesion overlap of the 12 participants identified with ipsilesional neglect. Each lesion was plotted onto a normal template brain using MRIcroN® software (Rorden, Karnath,&Bonilha, 2007). Colors denoting increasing numbers of participants having a lesion in a specific region, from “black” (n=1) to “red” (n=12).
“Where” and “Aiming” Spatial Bias
In order to evaluate whether participants with ipsilesional neglect had a greater extent of “aiming” relative to “where” spatial bias, we performed a Wilcoxon signed-rank test to compare the extent of “where” and” aiming” deficits. We used this nonparametric statistical analysis because of the small sample and skewed distribution of the “where” and “aiming” spatial bias fractions. Because “where” errors are typically greater in magnitude than are “aiming” errors, we transformed all the raw scores into Z-scores using the means and standard deviations for healthy participants' from Chen et al. (2011). This allowed us to compare “where” versus “aiming” spatial bias without being confounded by the general tendency of a greater “where” bias that is observed even among neurologically unimpaired individuals. The results from the analysis showed that there was a statistically significant difference between “where” (Mean= − 5.83, SD= 5.05) and “aiming” (Mean= − 2.04, SD= 3.75) errors (Z= −1.96, p=.050)1. Contrary to our hypothesis, as a group individuals with ipsilesional neglect demonstrated a greater “where”, perceptual-attentional spatial bias than “aiming”, motor-intentional bias.
Discussion
Ipsilesional (left-sided) neglect is a rare disorder following right brain injury. In this study we assessed a cohort of twelve patients with this syndrome in order to examine anatomical correlates and to determine the nature of the spatial processing bias (i.e., primarily “where” or “aiming” bias). There were two main results: first, the predominant lesion sites were subcortical structures including the thalamus, caudate/basal ganglia and adjacent white matter (i.e., internal capsule), and portions of the frontal cortex with extension into the periventricular white matter. The greatest area of lesion overlap was in the frontal white matter with a maximum overlap in 7 of 12 subjects (58%). Ten of twelve patients (83%) in our sample had lesions in either frontal cortex or subcortical regions. The second main finding was that the extent of “where” feedback-dependent errors was greater than the extent of “aiming” errors in this sample of ipsilesional neglect participants. .
Previous investigators have suggested that spatial processing in “where” and “aiming” spatial networks may be neuroanatomically distinct. A primary “where” spatial bias may be associated with posterior cortical lesions, while “aiming” spatial bias might be associated with anterior cortical and subcortical brain injury (Na et al., 1998; Barrett et al., 1999; Barrett et al., 2001; Vossel, Eschenbeck, Weiss & Fink, 2010). The frontal lobe may be strongly associated with motor-intentional “aiming” neglect because of its association with exploration and motor preparation in three-dimensional space (Passingham, 1995). Also, frontal systems may inhibit subcortical or parietal regions stimulating approach behaviors, and thus frontal cortical damage may cause a pathologic release of asymmetric approach motor behaviors (Denny-Brown and Chambers, 1958; Drago et al., 2006).
As suggested by Drago et al. (2006), frontal lobe damage may be common in in ipsilesional neglect if contralesional bias is explained by a deficit in avoidance behavior (Kwon & Heilman, 1991). If this is true, individuals with ipsilesional neglect may actually be approaching the contralateral portion of a stimulus, rather than neglecting the ipsilateral side. This alternative may explain why ipsilesional neglect is frequently task-dependent; it may be more frequent when acting on stimuli with attentionally-salient characteristics. Although why anterior white matter damage should be associated with ipsilesional neglect is not yet clear, white matter disconnections are associated with contralesional neglect symptoms (Doricchi, Theibaut de Schotten, Tomaiuolo & Bartolomeo, 2008; He, Snyder, Vincent, Epstein & Shulman, 2007; Karnath, Rorden & Ticini, 2009; Urbanski et al., 2011).
Our results showed that ipsilesional neglect was not strongly associated with an “aiming” spatial bias; rather, it was more strongly associated with “where”, feedback-dependent spatial bias. Although the reasons for this pattern of association are not yet known, stroke patients with “where” spatial bias may make errors consistent with degraded internal left spatial representations (abnormal left-sided imagery or visual working memory; Heilman et al., 2012 & Adair and Barrett, 2008). When an internal representation of the left side of space is degraded disproportionately to perceptual-attentional left spatial awareness, the novelty of perceived left-sided stimuli may actually be enhanced, since these stimuli do not match an internal expectation. Whether individuals with a primary “where” spatial bias and ipsilesional neglect demonstrate a primary “where” representational bias, and whether their leftward errors demonstrate a response to enhanced novelty in that spatial region, should be evaluated in future studies.
It has been suggested that ipsilesional neglect may result from a compensation strategy that patients with contralesional neglect acquire after learning that they systematically ignore the left side of space (Robertson et al., 1994). By this argument, patients make more movements towards the left to compensate for their attentional deficit. If this were true, however, we would have expected to see a greater extent of the aiming bias than the where bias in these ipsilesional participants. However, we observed the opposite. Furthermore, the idea that ipsilesional neglect is compensatory suggests that it may emerge later post-stroke. When comparing the time post-stroke of our ipsilesional sample to that of our archival contralesional sample, we failed to find any significant differences. Indeed, the data trended in the opposite direction, with the ipsilesional participants testing slightly closer to their stroke date than the contralesional patients. Thus, our data are not consistent with the idea that ipsilesional neglect results from a compensatory strategy.
One difficulty with group studies examining the characteristics of patients with ipsilesional neglect is inconsistent identification of the ipsilesional neglect syndrome. A criterion validated by demonstrating association with functional disability would be most desirable, as proposed for contralesional neglect (Barrett and Burkholder, 2006), but such a criterion is not yet available. Thus, different studies of ipsilesional neglect included participants with very different characteristics. We chose to use leftward line bisection errors exceeding that in an age-sex matched sample, but others used different methods. Other studies on ipsilesional neglect used 95% confidence intervals for line bisection errors of control subjects (Kim et al, 1999) or displaying ipsilesional (right sided) neglect on one out of three different types of neglect assessments (Robertson et al, 1994). We chose not to identify ipsilesional participants based on any other type of neglect assessment besides line bisection because those individuals who display symptoms of ipsilesional neglect on a cancellation task for example, maybe be different from those who display symptoms of ipsilesional neglect on line bisection tasks. By only using one criterion we hoped to reduce individual differences. We chose not to use a 95% confidence intervals because it is not an appropriate criterion by which to define the behavior of individuals as normal/abnormal: the confidence interval refers to the range of likely values for the population mean in a distribution of sample means, and its value depends on the size of the sample from which it is computed. It does not provide quantitative information about the likely distribution of individual scores in the population. Since the confidence interval underestimates the variability of individual scores, defining individual scores as abnormal on the basis of confidence intervals leads to more individuals being classified as abnormal. Thus, we constructed an interval based on standard deviations around the mean because we were categorizing individual scores. Since there is not yet a standardized method of identifying stroke survivors with ipsilesional neglect, it is possible that if we altered our inclusion criteria we would have gotten a very different participant sample with very different results.
Similar to the potential task-dependency for defining ipsilesional neglect, it is possible that the quantitative values we derived for the magnitude of “where” and “aiming” biases from the reversed line bisection task may change if derived from a different task. Researchers have employed different kinds of tasks to disentangle perceptual-attentional from motor-intentional deficits (e.g., pulley system, Bisiach, Geminiani, Berti, & Rusconi, 1990; perceptual and motor versions of the Landmark task, Toraldo, McIntosh, Dijkerman, & Milner, 2004). Previously, it has been observed that classification of participants as having a primary perceptual-attentional vs. primary motor-intentional bias may change with the task (Harvey, Kramer-McCaffery, Dow, Murphy & Gilchrist, 2002). While we acknowledge the potential for task-dependency, we suggest that it may be a greater problem for binary classification of participants' biases as either perceptual-attentional or motor-intentional than it is for simultaneously quantifying both forms of bias. While different tasks may possess different demands that alter the magnitude of individuals' “where” and “aiming” deficits, Landmark and traditional line bisection also recruit common spatial-attentional mechanisms and common neural regions (Çiçek, Deouell, & Knight, 2009). The quantitative measures used here have been validated, with perceptual manipulations selectively altering “where” and not “aiming” biases, and conversely, motor manipulations selectively altering “aiming” but not “where” (Garza, Eslinger, & Barrett, 2008). Additionally, these measures respond in a qualitatively similar way to experimental manipulations and neglect treatment as do the perceptual and manual versions of the Landmark task (e.g., compare Fortis, Chen, Goedert & Barret, 2011 and Striemer & Danckert, 2010).
Conclusion
Our data indicated that in a consecutive cohort of stroke patients suspected to have spatial neglect, 9% made potentially pathologic spatial errors suggestive of ipsilesional spatial neglect. As a group, the twelve subjects in our sample with ipsilesional neglect had a greater likelihood of damage to the frontal lobe or subcortical regions than did prior published contralesional neglect groups, with frontal white matter the area of greatest lesion overlap in our sample. We also identified an unexpected association of ipsilesional neglect with “where” spatial bias. Clinical implications of these results are not yet clear, but future study is warranted to determine whether ipsilesional neglect may be associated with different functional deficits than contralesional neglect, and whether this syndrome may also require different and specific rehabilitative treatments (Schwartz et al., 1998).
Acknowledgments
The authors would like to thank Peii Chen, PhD for providing helpful feedback in the preparation of this manuscript.
Footnotes
Using all five samples of contralesional patients yielded similar results: The weighted average proportion of contralesional patients exhibiting frontal-subcortical lesions across all five studies was .273, resulting in an expected value of 3.28 for frontal-subcortical damage and of 8.72 for other damage. The ipsilesional sample reported here differed from these expected values (χ2 (1, N=12) =18.95, p<.001).
Although our main analysis was on the quantitative measures comparing the magnitude of the Where and Aiming biases in this sample, we also performed a post-hoc analysis in which we categorized participants as having either a primary where (n = 8) or primary aiming bias (n = 4). Separate lesion overlaps for these two groups did not reveal any substantive differences in the lesion patterns.
References
- Adair JC, Barrett AM. Spatial neglect clinical and neuroscience review: a wealth of information on the poverty of attention. Annals of the NY Academy of Science. 2008;1142:21–43. doi: 10.1196/annals.1444.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azouvi P, Olivier S, Montety G, Samuel C, Louis-Dreyfus A, Tesio L. Behavioral assessment of unilateral neglect: Study of psychometric properties of the Catherine Bergego Scale. Archives of Physical Medicine and Rehabilitation. 2003;84(1):51–57. doi: 10.1053/apmr.2003.50062. doi: 10.1053/apmr.2003.50062. [DOI] [PubMed] [Google Scholar]
- Barrett AM. Perceptual-attentional “where” and motor-intentional “aiming” spatial systems. In: Coslett HB, Chatterjee A, editors. The Roots of Cognitive Neuroscience: Behavioral Neurology and Neuropsychology. Oxford University Press; New York: 2014. [Google Scholar]
- Barrett AM, Buxbaum LJ, Coslett HB, Edwards E, Heilman KM, Hillis AE, Milberg WP, Robertson IH. Cognitive rehabilitation interventions for neglect and related disorders: Moving from bench to bedside in stroke patients. Journal of Cognitive Neuroscience. 2006;18:1223–1236. doi: 10.1162/jocn.2006.18.7.1223. doi: 10.1162/jocn.2006.18.7.1223. [DOI] [PubMed] [Google Scholar]
- Barrett AM, Burkholder S. Monocular patching in subjects with right-hemisphere stroke affects perceptual-attentional bias. Journal of Rehabilitation Research and Development. 2006;43:337–346. doi: 10.1682/jrrd.2005.01.0015. doi: 10.1682/JRRD.2005.01.0015. [DOI] [PubMed] [Google Scholar]
- Barrett AM, Peterlin L, Heilman KM. Ipsilateral neglect versus hemianopic compensation. Neurology. 2003;61:120–123. doi: 10.1212/01.wnl.0000072331.56477.12. doi: 10.1212/01.WNL.0000072331.56477.12. [DOI] [PubMed] [Google Scholar]
- Barrett AM, Crucian GP, Beversdorf DQ, Heilman KM. Monocular Patching May Worsen Sensory-Attentional Neglect: A Case Report. Archives of Physical Medicine and Rehabilitation. 2001;82:516–518. doi: 10.1053/apmr.2001.21973. [DOI] [PubMed] [Google Scholar]
- Barrett AM, Schwartz RL, Crucian GP, Heilman KM. Adverse Effect of Dopamine Agonist Therapy in a Patient With Motor-Intentional Neglect. Archives of Physical Medicine and Rehabilitation. 1999;80:600–603. doi: 10.1016/s0003-9993(99)90205-8. [DOI] [PubMed] [Google Scholar]
- Barta PE, Dhingra L, Royall R, Schwartz E. Improving stereological estimates for the volume of structures identified in three-dimensional arrays of spatial data. Journal of Neuroscience Methods. 1997;75:111–118. doi: 10.1016/s0165-0270(97)00049-6. doi.org/10.1016/S0165-0270(97)00049-6. [DOI] [PubMed] [Google Scholar]
- Beschin N, Basso A, Della Sala S. Perceiving left and imagining right: dissociations in neglect. Cortex. 2000;36:401–414. doi: 10.1016/s0010-9452(08)70849-9. doi: 10.1016/S0010-9452(08)70849-9. [DOI] [PubMed] [Google Scholar]
- Bisiach E, Geminiani G, Berti A, Rusconi ML. Perceptual and premotor factors of unilateral neglect. Neurology. 1990;40(8):1278. doi: 10.1212/wnl.40.8.1278. doi:10.1212/WNL.40.8.1278. [DOI] [PubMed] [Google Scholar]
- Buxbaum LJ, Ferraro MK, Veramonti T, Farne A, Whyte J, Ladavas E, Franssinetti F, Coslett HB. Hemispatial Neglect: Subtypes, neuroanatomy, and disability. Neurology. 2004;62(5):749–756. doi: 10.1212/01.wnl.0000113730.73031.f4. doi: 10.1212/01.WNL.0000113730.73031.F4. [DOI] [PubMed] [Google Scholar]
- Chen P, Goedert KM, Murray E, Kelly K, Ahmeti S, Barrett AM. Spatial Bias and Right hemisphere function sex-specific changes with age. Journal of the International Neuropsychological Society. 2011;17:455–462. doi: 10.1017/S135561771100004X. doi: 10.1017/S13556 1771100004X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P, Geodert KM, Shah P, Foundas AL, Barrett AM. Integrity of medial temporal networks in spatial neglect predicts better recovery with prism adaptation treatment. Brain Imaging & Behavior. 2012 doi: 10.1007/s11682-012-9200-5. Epub ahead of print. doi: 10.1007/s11682-012-9200-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicek M, Deouell LY, Knight RT. Brain activity during landmark and line bisection tasks. Frontiers in Human Neuroscience. 2009;3:7. doi: 10.3389/neuro.09.007.2009. doi:10.3389/neuro.09.007.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cola MG, Daniels SK, Corey DM, Lemen LC, Romero M, Foundas AL. Relevance of subcortical stroke in dysphagia. Stroke. 2010;41:482–486. doi: 10.1161/STROKEAHA.109.566133. doi: 10.1161/STROKEAHA.109.566133. [DOI] [PubMed] [Google Scholar]
- Coslett HB. Neglect in vision and visual imagery: A double dissociation. Brain. 1997;120:1163–1171. doi: 10.1093/brain/120.7.1163. doi: 10.1093/brain/120.7.1163. [DOI] [PubMed] [Google Scholar]
- Damasio H, Damasio AR. Lesion analysis in neuropsychology. Oxford University Press; New York: 1989. pp. 38–39. [Google Scholar]
- Daniels SK, Foundas AL. Association of lesion localization with risk of aspiration in acute stroke patients. Journal of Neuroimaging. 1999;9:91–98. doi: 10.1111/jon19999291. [DOI] [PubMed] [Google Scholar]
- Denny-Brown Derek, Chambers RA. The parietal lobe and behavior. Association for Research in Nervous & Mental Disease. 1958;36:35–117. [PubMed] [Google Scholar]
- Doricchi F, Thiebaut de Schotten T, Tomaiuolo F, Bartolomeo P. White matter (dis)connections and gray matter (dys)functions in visual neglect: Gaining insights into the brain networks of spatial awareness. Cortex. 2008;44:983–995. doi: 10.1016/j.cortex.2008.03.006. doi: 0.1016/j.cortex.2008.03.006. [DOI] [PubMed] [Google Scholar]
- Drago V, Jeong Y, Crucian GP, Fitzgerald DB, Finney GR, Mizuno T, Pisani F, Heilman KM. Ipsilesional Attentional-Approach Neglect or Crossover Effect. Neurocase. 2006;12(4):206–211. doi: 10.1080/13554790600598758. doi: 10.1080/13554790600598758. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-Mental State”: A Practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Fortis P, Chen P, Goedert KM, Barrett AM. Effects of prism adaptation on motor-intentional spatial bias in neglect. Neuroreport. 2011;22(14):700–705. doi: 10.1097/WNR.0b013e32834a3e20. doi:10.1097/WNR.0b013e32834a3e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortis P, Goedert KM, Barrett AM. Prism adaptation differently affects motor-intentional and perceptual-attentional biases in healthy individuals. Neuropsychologia. 2011;49(9):2718–2727. doi: 10.1016/j.neuropsychologia.2011.05.020. doi: 10.1016/j.neuropsychologia.2011.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foundas AL, Daniels S, Vasterling J. Anomia: Case studies with lesion localization. Neurocase. 1998;4:35–43. doi:10.1080/13554799808410605. [Google Scholar]
- Garza JP, Eslinger PJ, Barrett AM. Perceptual-attentional and motor-intentional bias in near and far space. Brain and Cognition. 2008;68(1):9–14. doi: 10.1016/j.bandc.2008.02.006. doi:10.1016/j.bandc.2008.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goedert KM, Chen P, Botticello A, Masmela JR, Adler U, Barrett AM. Psychometric Evaluation of Neglect Assessment Reveals Motor-Exploratory Predictor of Functional Disability in Acute-Stage Spatial Neglect. Archives of Physical Medicine and Rehabilitation. 2012;93(1):137–142. doi: 10.1016/j.apmr.2011.06.036. doi: 10.1016/j.apmr.2011.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goedert KM, Chen P, Boston RC, Barrett AM. Presence of motor-intentional deficits predicts functional improvement of spatial neglect with prism adaptation. Neurorehabilitation and Neural Repair. 2014;28:483–492. doi: 10.1177/1545968313516872. doi: 10.1177/1545968313516872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna-Pladdy B, Daniels SK, Fieselman MA, Thompson K, Vasterling JJ, Heilman KM, Foundas AL. Praxis lateralization: Errors in right and left hemisphere damage. Cortex. 2001;37:219–230. doi: 10.1016/s0010-9452(08)70569-0. doi.org/10.1016/S0010-9452(08)70569-0. [DOI] [PubMed] [Google Scholar]
- Harvey M, Kramer-McCaffery T, Dow L, Murphy PJ, Gilchrist ID. Categorization of 'perceptual' and 'premotor' neglect patients across different tasks: Is there strong evidence for a dichotomy? Neuropsychologia. 2002;40(8):1387–1395. doi: 10.1016/s0028-3932(01)00202-0. doi:S0028393201002020. [DOI] [PubMed] [Google Scholar]
- He BJ, Snyder AZ, Vincent JL, Epstein A, Shulman AG, Corbetta M. Breakdown of Functional Connectivity in Frontoparietal Networks Underlies Behavioral Deficits in Spatial Neglect. Neuron. 2007;53:905–918. doi: 10.1016/j.neuron.2007.02.013. doi: 10.1016/j.neuron.2007.02.013. [DOI] [PubMed] [Google Scholar]
- Heilman KM. Intentional Neglect. Frontiers in Bioscience. 2004;9:694–705. doi: 10.2741/1261. [DOI] [PubMed] [Google Scholar]
- Heilman KM, Bowers D, Valensein E, Watson RT. Hemispace and hemisptial neglect. Advanced in Psychology. 1987;45:115–150. [Google Scholar]
- Heilman KM, Valenstein E. Mechanisms underlying hemispatial neglect. Annals of Neurology. 1979;5:166–170. doi: 10.1002/ana.410050210. doi: 10.1002/ana.410050210. [DOI] [PubMed] [Google Scholar]
- Heilman KM, Valenstein E, Watson RT. The what and how of neglect. Neuropsychological Rehabilitation. 1994;4(2):133–139. [Google Scholar]
- Heilman KM, Watson RT, Valenstein E. Neglect and related disorders. In: Heilman KM, Valenstein E, editors. Clinical Neuropsychology. 5th ed. Oxford University; New York: 2012. pp. 296–348. [Google Scholar]
- Karnath H, Berger MF, Kuker W, Rorden C. The anatomy of spatial neglect based on voxelwise statistical analysis: A study of 140 patients. Cerebral Cortex. 2004;23:1164–1172. doi: 10.1093/cercor/bhh076. doi: 10.1093/cercor/bhh076. [DOI] [PubMed] [Google Scholar]
- Karnath H, Hillemlbach M, Rorden C. The subcortical anatomy of human spatial neglect: putamen, caudate nucleus and pulvinar. Brain. 2002;125:350–360. doi: 10.1093/brain/awf032. doi: 10.1093/brain/awf032. [DOI] [PubMed] [Google Scholar]
- Karnath H, Rennig J, Johannsen L, Rorden C. The anatomy underlying acute versus chronic spatial neglect: a longitudinal study. Brain. 2011;134:903–912. doi: 10.1093/brain/awq355. doi: 10.1093/brain/awq355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnath H, Rorden C, Ticini LF. Damage to white matter fiber tracts in acute spatial neglect. Cerebral Cortex. 2009;19:2331–2337. doi: 10.1093/cercor/bhn250. doi: 10.1093/cerebr/bbn250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M, Na DL, Kim GM, Adair JC, Lee KH, Heilman KM. Ipsilesional neglect: behavioural and anatomical features. Journal of Neurosurgery Psychiatry. 1999;67:35–38. doi: 10.1136/jnnp.67.1.35. doi: 10.1136/jnnp.67.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon S, Heilman KM. Ipsilateral neglect in a patient following unilateral frontal lesion. Neurology. 1991;41:2001–2004. doi: 10.1212/wnl.41.12.2001. [DOI] [PubMed] [Google Scholar]
- Leibovitch FS, Black SE, Caldwell CB, Ebert PL, Ehrlich LE, Szalai JP. Brain-behavior correlation in hemispatial neglect using CT and SPECT. Neurology. 1998;50:901–908. doi: 10.1212/wnl.50.4.901. [DOI] [PubMed] [Google Scholar]
- Mahoney FI, Barthel D. “Functional evaluation: the Barthel Index.”. Maryland State Medical Journal. 1965;14:56–61. [PubMed] [Google Scholar]
- Mesulam MM. A cortical network for directed attention and unilateral neglect. Annals of Neurology. 1981;10:309–325. doi: 10.1002/ana.410100402. [DOI] [PubMed] [Google Scholar]
- Mort DJ, Malhorta P, Mannan SK, Rorden C, Pambakian A, Kennard C, Husain M. The anatomy of visual neglect. Brain. 2003;126:1986–1977. doi: 10.1093/brain/awg200. doi: 10.1093/brain/awg200. [DOI] [PubMed] [Google Scholar]
- Na DL, Adair JC, Williamson DJG, Schwartz RL, Haws B, Heilman KM. Dissociation of sensory-attentional from motor-intentional neglect. Journal of Neurology Neurosurgery Psychiatry. 1998;64:331–338. doi: 10.1136/jnnp.64.3.331. doi: 10.1136/jnnp.64.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Na DL, Adair JC, Choi SH, Seo DW, Kang Y, Heilman KM. Ipsilesional versus contralesional neglect depends on attentional demands. Cortex. 2000;36:455–467. doi: 10.1016/s0010-9452(08)70532-x. doi: 10.1016/S0010-9452(08)70532-X. [DOI] [PubMed] [Google Scholar]
- Naeser MA, Hayward RW. Lesion localization in aphasia with cranial computerized topography and the Boston Diagnostic Aphasia Exam. Neurology. 1978;28:545–551. doi: 10.1212/wnl.28.6.545. doi: 10.1212/WNL.28.6.545. [DOI] [PubMed] [Google Scholar]
- Nys GMS, van Zandvoort MJE, van der Worp HB, Kappelle LJ, de Hann EHF. Neuropsychological and neuroanatomical correlates of perseverative responses in subacute stroke. Brain. 2006;129:2148–2157. doi: 10.1093/brain/awl199. doi: 10.1093/brain/awl199. [DOI] [PubMed] [Google Scholar]
- Ono M, Kubik S, Abernathey CD. Atlas of the Cerebral Sulci. Georg Thieme Verlag Stuttgart; New York: 1990. [Google Scholar]
- Passingham R. The frontal lobes and voluntary action. Oxford University Press; New York: 1995. [Google Scholar]
- Robertson IH, Halligan PW, Bergego C, Homberg V, Pizzamiglio L, Weber E, Wilson BA. Right neglect following right hemisphere damage? Cortex. 1994;30:199–213. doi: 10.1016/s0010-9452(13)80193-1. [DOI] [PubMed] [Google Scholar]
- Rorden C, Brett M. Stereotaxic display of brain lesions. Behavioral Neurology. 2000;12:191–200. doi: 10.1155/2000/421719. [DOI] [PubMed] [Google Scholar]
- Rorden C, Karnath HO, Bonilha L. Improving lesion symptom mapping. Journal of Cognitive Neuroscience. 2007;19(7):1081–1088. doi: 10.1162/jocn.2007.19.7.1081. [DOI] [PubMed] [Google Scholar]
- Schwartz RL, Barrett AM, Kim M, Heilman KM. Ipsilesional intentional neglect and the effect of cueing. Neurology. 1999;53:2017–2022. doi: 10.1212/wnl.53.9.2017. [DOI] [PubMed] [Google Scholar]
- Striemer CL, Danckert J. Dissociating perceptual and motor effects of prism adaptation in neglect. Neuroreport. 2010;21(6):436–441. doi: 10.1097/WNR.0b013e328338592f. doi:10.1097/WNR.0b013e328338592f. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain. 3-Dimesnsional Proportional System: An Approach to Cerebral Imaging. Thieme Medical Publishers; New York: 1988. [Google Scholar]
- Toraldo A, McIntosh RD, Dijkerman HC, Milner AD. A revised method for analysing neglect using the landmark task. Cortex: A Journal Devoted to the Study of the Nervous System and Behavior. 2004;40(3):415–431. doi: 10.1016/s0010-9452(08)70136-9. doi:10.1016/S0010-9452(08)70136-9. [DOI] [PubMed] [Google Scholar]
- Vallar G, Perani D. The anatomy of unilateral neglect after right-hemisphere stroke lesions. A clinical/CT scan correlation study in man. Neuropsychologia. 1986;24(5):609–622. doi: 10.1016/0028-3932(86)90001-1. doi: 10.1016/0028-3932(86)90001-1. [DOI] [PubMed] [Google Scholar]
- Urbanski M, Thiebaut de Schotten M, Rodrigo S, Oppenheim C, Touzé E, Méder JF, Moreau K, Loeper-Jeny C, Dubois B, Bartolomeo P. DTI-MR tractography of white matter damage in stroke patients with neglect. Experimental Brain Research. 2011;208(4):491–505. doi: 10.1007/s00221-010-2496-8. doi: 10.1007/s00221-010-2496-8. [DOI] [PubMed] [Google Scholar]
- Verdon V, Schwartz S, Lovbald KO, Hauert CA, Vuilleumier P. Neuroanatomy of hemispatial neglect and its functional components: a study using voxel-based lesion mapping. Brain. 2010;133:880–894. doi: 10.1093/brain/awp305. doi: 10.1093/brain/awp305. [DOI] [PubMed] [Google Scholar]
- Vossel S, Eschenbeck P, Weiss PH, Fink GR. The neural basis of perceptual bias and response bias in the Landmark task. Neuropsychologia. 2010;48:3949–3954. doi: 10.1016/j.neuropsychologia.2010.09.022. doi: 10.1016/j.neuropsychologia.2010.09.022. [DOI] [PubMed] [Google Scholar]
- Wilson BA, Cockburn J, Halligan PW. Behavioural Inattention Test. Thames Valley Test Co; Titchfield: 1987. 1987. [Google Scholar]