Abstract
Exposure to environmental contaminants, such as organochlorine insecticides during critical periods of neurodevelopment has been shown to be a major contributor to several neuropsychological deficits seen in children, adolescence, and adults. Although the neurobehavioral outcomes resulting from exposure to these compounds are known the neurotransmitter circuitry and molecular targets that mediate these endpoints have not been identified. Given the importance of the frontal cortex in facilitating numerous neuropsychological processes, our current study sought to investigate the effects of developmental exposure to the organochlorine insecticide, endosulfan, on the expression of specific proteins associated with neurotransmission in the frontal cortex. Utilizing in vitro models we were able to show endosulfan reduces cell viability in IMR-32 neuroblastoma cells in addition to reducing synaptic puncta and neurite outgrowth in primary cultured neurons isolated from the frontal cortex of mice. Elaborating these findings to an in vivo model we found that developmental exposure of female mice to endosulfan during gestation and lactation elicited significant alterations to the GABAergic (GAT1, vGAT, GABAA receptor), glutamatergic (vGlut and GluN2B receptor), and dopaminergic (DAT, TH, VMAT2, and D2 receptor) neurotransmitter systems in the frontal cortex of male offspring. These findings identify damage to critical neurotransmitter circuits and proteins in the frontal cortex, which may underlie the neurobehavioral deficits observed following developmental exposure to endosulfan and other organochlorine insecticides.
Keywords: Cognition, Dopamine, GABA, Glutamate, Memory, Schizophrenia
INTRODUCTION
Increasing evidence suggests that exposure to environmental chemicals during critical periods of brain development can have detrimental effects on normal neuropsychological development (Bellinger, 2013; Grandjean and Landrigan, 2014). Chlorinated pesticides have been used extensively to mitigate the detrimental health effects of insects to the human population as well as food crops in agricultural settings. In general, these compounds are extremely lipophilic and resistant to degradation, which allows them to accumulate and persist in the environment for several years following their use. In the last decade epidemiological and laboratory findings have identified chlorinated pesticides, including DDT, dieldrin, heptachlor, and endosulfan, as potential risk factors for neurobehavioral deficits following exposure during critical periods of neurodevelopment (Caudle et al., 2005; Eskenazi et al., 2008; Puertas et al., 2010; Ribas-Fito et al., 2007; Richardson et al., 2006; Richardson et al., 2008; Roberts et al., 2007; Sioen et al., 2013).
While most organochlorine pesticides have seen their manufacture and use reduced or discontinued, the impact on human health by endosulfan specifically has just recently been recognized, resulting in proposed use reduction and potential elimination within the next several years. However, like other organochlorines, endosulfan is extremely resistant to degradation and breakdown exhibiting a half-life of up to 6 years, which allows it to remain in the environment, leading to repeated or continual exposure to the human population. As a result, endosulfan has been demonstrated to accumulate in significant levels in human tissue, including fat, liver, kidney, and brain. In addition, high levels of endosulfan have been recorded in the cord blood and breast milk of pregnant women (Jimenez Torres et al., 2006; Moreno Frias et al., 2004). These findings raise particular concern for exposure during fetal development, both during gestation as well as postnatally, and the effect that this exposure may have on development of the nervous system.
Neurodevelopment is composed of multiple critical processes that occur in utero and continue throughout infancy, childhood, and adolescence, which must be precisely accomplished in order to ensure the proper maturation, connection, and function of neural circuits (Rice and Barone, 2000). A key mediator of neurodevelopment is the neurotransmitter GABA, which acts upon all neuronal types via the GABA receptors located on adjacent neurons to facilitate various aspects of neurogenesis, neuronal migration and synaptic formation (Akerman and Cline, 2007; Ben-Ari, 2002; Represa and Ben-Ari, 2005). The functional importance of the GABAergic system in the context of developmental exposure to endosulfan is of interest given that the premier target of endosulfan is inhibition of the GABAA receptors (Casida, 1993; Kamijima and Casida, 2000), resulting in an alteration in GABAergic signaling in the central nervous system. Reports have shown that exposure to GABAA antagonists cause an alteration in neuronal outgrowth and migration in the cortex resulting in a disordered cortical architecture similar to that found in human patients (Behar et al., 1998; Gleeson and Walsh, 2000; Liu et al., 1997).
The frontal cortex, which is extensively innervated by the GABAergic, glutamatergic and dopaminergic neurotransmitter systems, has been implicated in mediating many cognitive functions often found to be altered in multiple neurobehavioral disorders, including cognitive deficits, autism spectrum disorder (ASD), and schizophrenia (DeLorey et al., 2008; Fatemi et al., 2010; Gaspar et al., 2009; Lewis and Sweet, 2009; Mohler, 2007; Zahr et al., 2008). Indeed, it appears that deficits in cognitive processes arise from disruptions in proteins critical for normal functioning of these neurotransmitter systems (Bragina et al., 2008; Dzirasa et al., 2009; Homayoun and Moghaddam, 2007; Simons et al., 2013). Although a few studies have identified modifications to levels of neurotransmitters, such as GABA, glutamate, and several monoamines in the frontal cortex of animals developmentally exposed to endosulfan (Cabaleiro et al., 2008; Lakshmana and Raju, 1994), our understanding of the cellular and molecular processes that may underlie these alterations and contribute to subsequent neurobehavioral deficits are largely unknown. Thus, our current study sought to investigate the potential neuronal targets in the frontal cortex of animals developmentally exposed to endosulfan. Using in vitro and in vivo models we evaluated the neurotoxic potential of endosulfan on a neuroblastoma cell line in addition to primary cultured neurons isolated from the frontal cortex of postnatal mice. These data then helped to inform our assessment of specific markers of the GABA, glutamate, and dopamine circuits in the frontal cortex of mice exposed to endosulfan throughout gestation and lactation. This information will serve to elaborate our understanding of the neurological targets and alterations following endosulfan exposure and will provide insight into the potential neuropathological endpoints responsible for subsequent neurobehavioral indices.
MATERIALS AND METHODS
Chemicals and reagents
α-Endosulfan was purchased from Accustandard (New Haven, CT). Hibernate A and Hibernate A- Calcium were purchased from BrainBits (Springfield, IL). B27, DNase1, and Neurobasal A were purchased from Life Technologies (Carlsbad, CA). Papain was obtained from Sigma (St. Louis, MO). Dispase II was purchased from Roche (Nutley, NJ). The BCA protein assay kit was obtained from Pierce (Rockford, IL). Aphidicolin was purchased from A.G. Scientific (San Diego, CA). Monoclonal anti-rat dopamine transporter (DAT) and polyclonal anti-rabbit tyrosine hydroxylase (TH) were purchased from EMD Millipore (Billerica, MA). Rabbit anti-D2 receptor and monoclonal anti-mouse actin antibodies were purchased from Sigma (St. Louis, MO). Rabbit anti-GABA transporter 1 (GAT1), rabbit anti-vesicular GABA transporter (vGAT), mouse anti-GABAA 2α receptor subunit, rabbit anti-vesicular glutamate transporter (vGlut) were purchased from Synaptic Systems (Germany), rabbit anti-synapsin and mouse anti-GluN2B receptor subunit were purchased from BD Transduction (San Jose, CA), mouse anti-microtubule associated protein 2 (MAP2) antibodies was purchased from Abcam (San Francisco, CA). Polyclonal anti-rabbit vesicular monoamine transporter 2 (VMAT2) antibodies were generated by Covance to the C-terminal sequence in mouse (CTQNNVQPYPVGDDEESESD). Secondary antibodies conjugated to horseradish peroxidase were obtained from Jackson Immunoresearch Laboratories (West Grove, PA). Secondary antibodies conjugated to fluorescent tags were obtained from Life Technologies (Grand Island, NY). SuperSignal West Dura Extended duration substrate and stripping buffer were obtained from Pierce. 3,3′ Diaminobenzidine (DAB) was purchased from Sigma (St. Louis, MO).
IMR-32 neuroblastoma cells
Given that endosulfan has been shown to target and inhibit GABAA receptors, the IMR-32 neuroblastoma cell line was chosen to investigate the neurotoxic potential of endosulfan due to their documented expression of GABAA receptors and response to other GABAergic compounds, in vitro (Anderson et al., 1993; Noble et al., 1993; Sapp and Yeh, 2000). Cells were cultured in DMEM F12 media supplemented with 100 units/ml penicillin, 100 units/ml streptomycin, and 10% fetal bovine serum. Cells were cultured at 37°C in a humidified atmosphere with 5% CO2 and propagated according to the protocol provided by ATCC. When cells were confluent, they were passaged to 40,000 cells per well in 96-well plates at 100 μl for treatment with endosulfan. Cell death was assessed using the WST-1 Cell Proliferation assay. Following treatment for 24 hrs with 25, 50, 100, 200, 300, 400 μM of endosulfan dissolved in DMSO, 10 μl/well of Cell Proliferation Reagent WST-1 was added to cells and incubated for 3 hrs at 37°C and 5% CO2. Cytotoxicity was then measured by enzymatic cleavage of the tetrazolium salt WST-1 to a water-soluble formazan dye detected by spectral absorbance. Viable cells form more formazan than less viable cells. Spectral absorbance was measured at 450 nanometers on an Epoch BioTek microplate spectrophotometer and analyzed using Gen5 software (2.0) and GraphPad software.
Primary culture of cortical neurons
Primary cortical cultures were generated as previously described (Bradner et al., 2013a), with modifications. Briefly, neuron cultures from postnatal mice (postnatal day 1–3) PND 1–3 were isolated from the frontal cortex, which included the medial aspect of the frontal cortex but did not include tissue from the striatum or limbic system. Mouse brains were dissected in ice cold Hibernate A supplemented with B27. Following isolation of the relevant region and the removal of meninges, tissue pieces were chemically treated with a dissociation solution containing Papain (1 mg/ml), Dispase II (1.2 units/ml), and DNase 1 (1 μl/ml) dissolved in Hibernate A- Calcium for 20 min at 37°C and gently agitated every 5 mins. Tissue was then rinsed in plating media containing Neurobasal-A, 10% heat inactivated fetal bovine serum, pen-strep, and mechanically dissociated using gentle trituration. Cells were plated on poly-d-lysine pre-coated 96 well plates at 40,000 cells per well. Plating media was removed and immediately switched to Neurobasal-A based culture media containing B27, 1% L-glutamine, 1% penicillin-streptomycin, and aphidicolin (1 μg/ml) after 2 hrs, in vitro. Primary cultures were treated 2 hrs after plating with increasing concentrations of endosulfan (1–5 μM) dissolved in DMSO and further diluted in cell culture media. For all control and endosulfan treatment experiments the final concentration of DMSO was <0.05% and no toxicity was observed at this percentage. Neurons were continually treated with endosulfan or control for 9 days, with fresh endosulfan and media added daily. Following treatment, cultures were fixed in 4% PFA for 20 mins and incubated overnight in mouse anti-MAP2 and rabbit anti-synapsin at 4°C. The following day, cultures were incubated with fluorescent secondary antibodies, goat anti-mouse 572 and goat anti-rabbit 488 for 1 hr at room temperature. After staining with DAPI, cells were rinsed and stored in PBS. Images of treated cultures were obtained using the 10x objective on an Array Scan VTI HCS (Cellomics; Pittsburgh, PA). Twenty-five contiguous fields were taken per well, DAPI+ nuclei were counted, MAP2+ cell bodies were identified, and neurite length and synaptic puncta were recorded for all fields containing at least one MAP2+ cell body. Objects were identified and measured using the neuronal profiling bioapplication from Thermo Scientific. Image analysis workflow was identical to that determined by Harrill et al., (2011). In brief, cell bodies were selected or rejected based upon predefined pixel intensity and morphological parameters. Neurites emerging from selected cell bodies were individually identified and traced while synapsin positive puncta were identified based upon signal intensity. Only synapsin positive puncta that were: 1) within the boundaries of the neurite or 2) within 1 μM of the traced neurite were quantified. Synapsin positive puncta that did not adhere to these parameters where not included in analysis. Statistical significance between the control and treatment groups for neuron count, and neurite length, and synaptic puncta were determined using GraphPad analysis software.
Animals and treatment
Eight week old female and male C57BL/6J mice purchased from Jackson Laboratory (Bar Harbor, ME, USA) were used for developmental studies. Mice were maintained on a 12:12 light/dark cycle. Food and water were available ad libitum. All procedures were conducted in accordance with the Guide for Care and Use of Laboratory Animals (National Institutes of Health) and were previously approved by the Institutional Animal Care and Use Committee at Emory University.
Female mice were randomly assigned to treatment groups and were orally dosed with 1 mg/kg endosulfan dissolved in corn oil vehicle and mixed with peanut butter every 2 days for 2 weeks prior to introducing male mice for breeding. Control mice received an equivalent amount of corn oil vehicle in peanut butter. Mice were monitored to ensure total consumption of the treatment dose, which generally occurred within 10 mins. Oral exposure was to mimic the most likely route of exposure to endosulfan in the current human population, which is through ingestion of contaminated food. The chosen dosage is 7.36-fold less than the acute oral LD50 in mice no observable adverse effects level (NOAEL) (Smith, 1991). Peanut butter was chosen as the method of exposure to reduce stress to the dam during gestation. Stress from repeated injections via oral gavage during gestation has been shown to alter GABAA subunit development (Liu et al., 1997). Dosing continued on the same schedule throughout gestation and lactation and dams were allowed to give birth and litters were culled to 6–8 pups/litter on PND 1, to ensure standardized nutritional availability. On PND 21 pups were separated by litter and by sex until approximately 12 weeks of age when male offspring were sampled. Each litter was considered as an individual unit of analysis (N=6–8).
Immunoblot analysis
Western blots were used to quantify the amount of several targets, including GAD67, GAT1, vGAT, GABAA 2α receptor subunit, vGlut, GluN2B receptor subunit, DAT, TH, VMAT2, and dopamine D2 receptor in samples of frontal cortex isolated from treated and control mice. Analysis was performed as previously described (Bradner et al., 2013b). Briefly, samples were homogenized, subjected to polyacrylamide gel electrophoresis and electrophoretically transferred to polyvinylidene difluoride membranes. Nonspecific sites were blocked in 7.5% nonfat dry milk in Tris-buffered saline and then membranes incubated overnight in primary antibody. Antibody binding was detected using a horseradish peroxidase secondary antibody (1:10,000) and enhanced chemiluminescence. The luminescence signal was captured on an Alpha Innotech Fluorochem imaging system and stored as a digital image. Membranes were stripped for 15 mins at room temperature with Pierce Stripping Buffer and sequentially reprobed with additional antibodies, including actin. Actin blots were used to ensure equal protein loading across samples.
Immunohistochemistry
Tissue staining was performed as described previously (Bradner et al., 2013b; Caudle et al., 2006). Briefly, whole brains were immersion fixed in 4% paraformaldehyde and serially sectioned at 40 μm. Sections were incubated with rabbit anti-GAT1, rabbit anti-vGAT, mouse anti-GABAA 2α receptor subunit, and rabbit anti-vGlut overnight and then incubated in a biotinylated secondary antibody for 1 hr at room temperature. Visualization was performed using DAB for 3 mins at room temperature. After DAB, tissue was mounted on slides, dehydrated, and coverslipped.
Statistical analysis
All analysis was performed on raw data for each treatment group by one-way ANOVA or Students t-test. Post hoc analysis was performed using Tukey’s post hoc test. Significance is reported at the p < 0.05 level.
RESULTS
Effects of endosulfan on IMR-32 cells
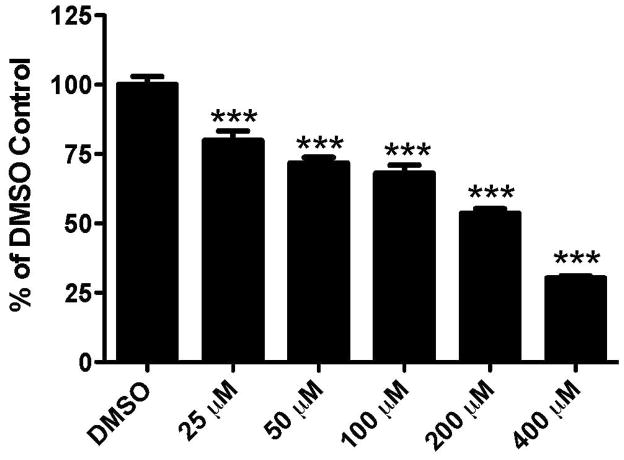
We first assessed the general cytotoxicity of endosulfan on the IMR-32 neuroblastoma cell line. This cell line was chosen as it has been shown to express GABAergic receptors (Anderson et al., 1993; Noble et al., 1993; Sapp and Yeh, 2000). Given that endosulfan has been demonstrated to be a GABAA receptor antagonist, these cells serve as an effective in vitro model to evaluate endosulfan toxicity on neuronal cells. Exposure to increasing concentrations of endosulfan for 24 hrs caused a reduction in cell viability with 25 μM causing a 20% reduction in viability while 400 μM resulted in a 70% decrease (Figure 1). These findings provided a generalized assessment of the neurotoxicity of endosulfan on a neurally-derived cell line, further supporting a potential role for endosulfan in neurotoxicity in the mammalian brain.
Figure 1.
Exposure of IMR-32 neuroblastoma cells to endosulfan results in a dose-dependent reduction in cell viability. Cells were exposed to increasing concentrations of endosulfan for 24 hrs and then assessed with the WST-1 Cell Proliferation Assay. Columns represent the percent change from DMSO control for each concentration of endosulfan. Data represent the mean ± SEM of 8–12 experimental replicates per treatment group performed across 3 separate experiments. ***Values significantly different from the DMSO control (p < 0.001).
Effects of endosulfan on primary cultured cortical neurons
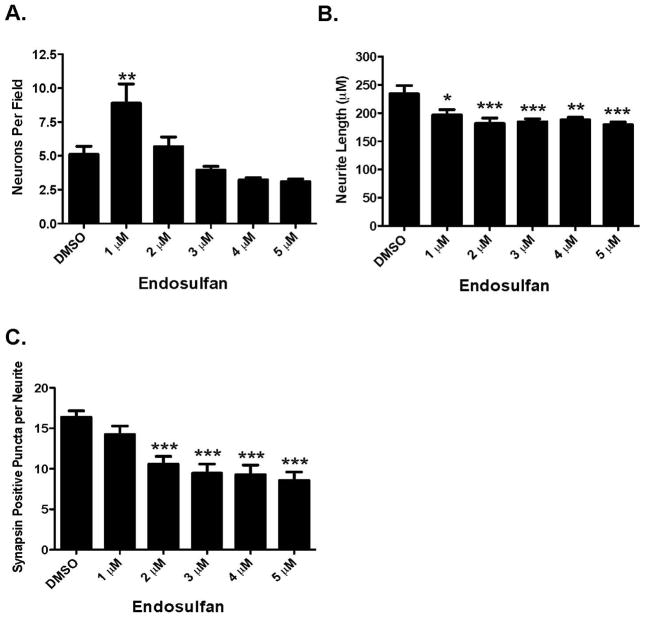
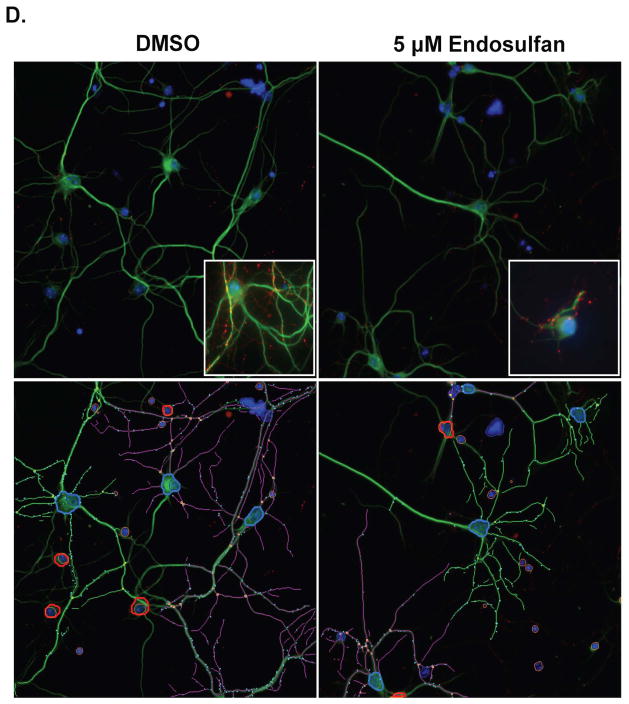
Given these results, we next sought to elaborate the complexity of our exposure paradigm by examining the effects of endosulfan exposure on primary cultured neurons isolated from the frontal cortex. This region is extensively innervated by the GABAergic, glutamatergic, and dopaminergic neurotransmitter systems and disruption of neural circuitry within this region has been implicated in several neurological disorders. Exposure of neurons to increasing concentrations of endosulfan for 9 days resulted in dose-dependent alterations to neuron number, neurite length, and synapsin positive puncta. Initial exposure to 1 μM endosulfan caused a 47% increase in the number of neuronal cells present in the culture. In contrast, subsequent exposure to 5 μM of endosulfan resulted in a 36% reduction in the number of neurons in our cultures (Figure 2A). However, these higher concentrations of endosulfan did not elicit a significant effect on neuron number in our cultures. Using this same treatment paradigm we also simultaneously assessed the effect of endosulfan treatment on the outgrowth of neurites from the cell body. Exposure to endosulfan caused a significant reduction in neurite length ranging from 16% with 1 μM to 24% with 5 μM (Figure 2B and D), suggesting that exposure to endosulfan may cause moderate damage to the neuronal processes in the frontal cortex prior to affecting the neuronal cell body. Evaluation of alterations to synapsin positive puncta in neurons exposed to endosulfan demonstrated a more robust reduction beginning with a 12.5% decrease with 1 μM of endosulfan up to a 50% reduction with 5 μM (Figure 2C and D). These decreases in synaptic puncta suggest that this endpoint may be especially sensitive to the effects of endosulfan exposure.
Figure 2.
Exposure of cortical primary cultures to endosulfan causes a loss of cortical neurons as well as reductions in neurite outgrowth and synapse formation. (A) Treatment of cortical cultures caused a significant reduction in the number of MAP2+ neurons, compared with DMSO control. (B) Assessment of neurite length in these neurons demonstrated a greater loss of neurite outgrowth compared with DMSO control. (C) Evaluation of synapse formation also showed a substantial reduction, compared with DMSO control. Columns represent the percent change from DMSO control for each concentration of endosulfan. (D) Representative images of primary cultured cortical neurons treated with DMSO or 5 μM endosulfan for 9 days and immunolabeled with MAP2 (green), synapsin (red), and Hoechst nuclear counterstain (blue). Insets represent higher magnification images of cortical cultures treated with DMSO or endosulfan. Images below are traces of the same images to highlight our use of the automated quantification of neuron number, neurite length, and the number of synapsin positve puncta in our DMSO and endosulfan treated cultures. Blue outline denotes a cell body that was selected for analysis. In contrast, red outline denotes cell bodies that did not meet our analysis parameters and were not selected or included for analysis. Green and purple traces represent neurites assigned to different cell bodies. Cyan dots denote synapsin positive puncta that were adherent to our parameters for analysis. Data represent the mean ± SEM of 17 experimental replicates per treatment group performed across 3 separate experiments. *Values for treatments significantly different from DMSO control (p < 0.05). **Values for treatments significantly different from DMSO control (p < 0.01). ***Values significantly different from DMSO control (p < 0.001).
Effects of developmental exposure to endosulfan on the frontal cortex, in vivo
Next, we generated an in vivo developmental exposure model in order to evaluate the neurological effects of endosulfan on the developing nervous system in the frontal cortex of male offspring. Dams treated with endosulfan or control did not display any overt signs of toxicity and there were no adverse effects on pup growth rate or other general health endpoints (data not shown). Additionally, treatment with endosulfan did not influence sex ratios of the litters or numbers of pups per litter (data not shown).
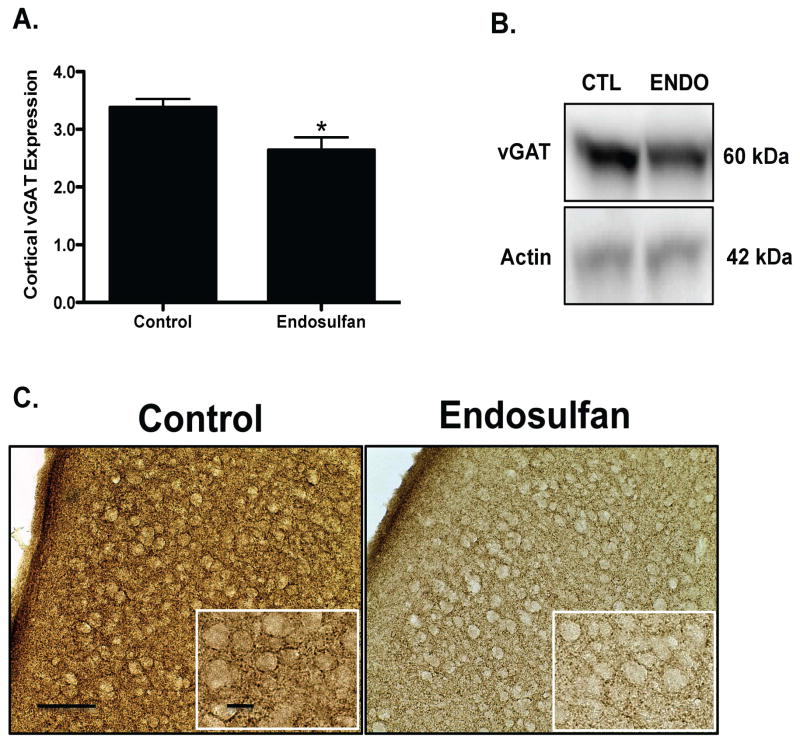
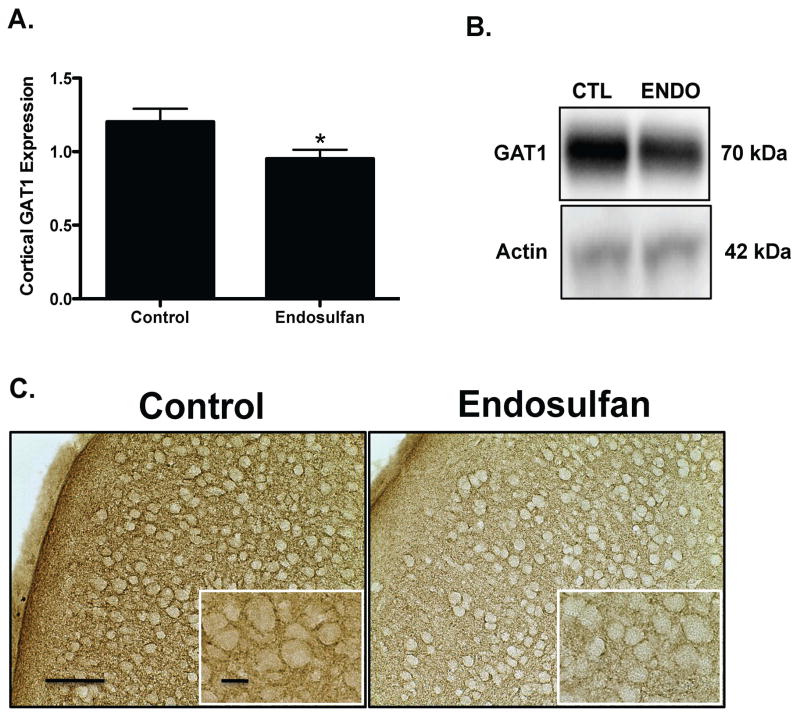
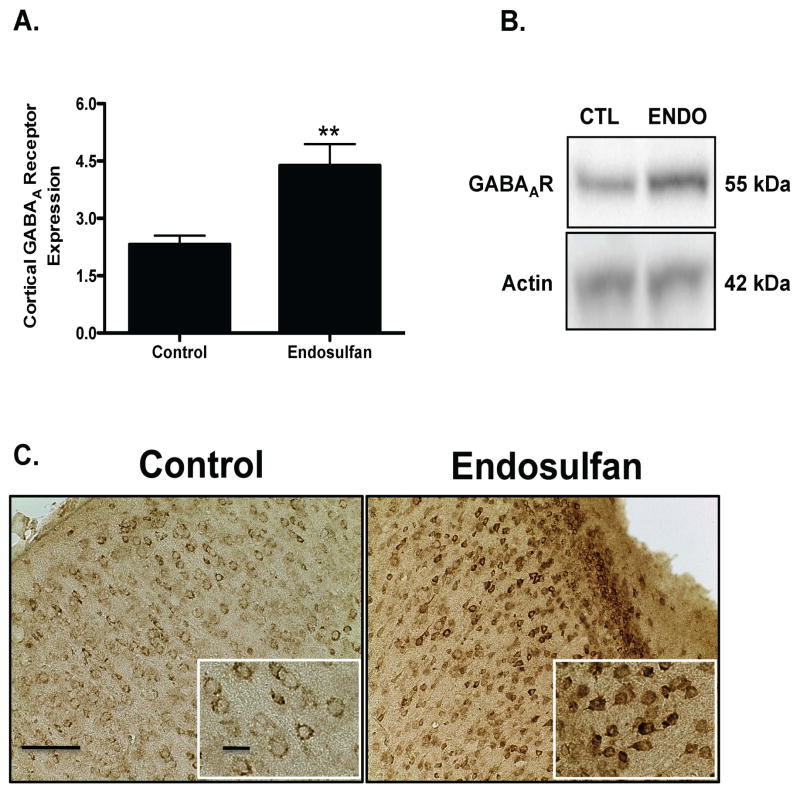
Assessment of alterations to several markers of the GABAergic neurotransmitter system in the frontal cortex demonstrated a 23% reduction in protein expression of vGAT in the offspring, compared to control treated as assessed by immunoblotting and immunohistochemical methods (Figure 3A). Similarly, the plasmalemmal GABA transporter, GAT1, showed a 21% decrease in protein expression relative to control (Figure 4A). In contrast, protein expression of the GABAA 2α receptor subunit was increased by 91% in endosulfan exposed offspring, compared to control (Figure 5A). No change was seen in GAD67 expression in the frontal cortex (data not shown). These findings indicate that exposure to endosulfan during neurodevelopment causes significant alterations in the expression of proteins that are critical to the function of the GABA neurotransmitter system in the frontal cortex.
Figure 3.
Developmental exposure of male offspring to endosulfan significantly reduces the expression of vGAT in the frontal cortex. Female mice were administered either 0 (control) or 1 mg/kg endosulfan throughout gestation and lactation. Expression of vGAT in the frontal cortex was determined by immunoblot (A) in male offspring. Representative immunoblot (B) and immunohistochemical (C) images for vGAT are included. Data represent mean ± SEM (6–8 animals each from a different litter per treatment group). *Values for animals that are significantly different from controls (p < 0.05). Scale Bars: 100 μm and 50 μM, respectively.
Figure 4.
Developmental exposure of male offspring to endosulfan significantly reduces the expression of GAT1 in the frontal cortex. Female mice were administered either 0 (control) or 1 mg/kg endosulfan throughout gestation and lactation. Expression of GAT1 in the frontal cortex was determined by immunoblot (A) in male offspring. Representative immunoblot (B) and immunohistochemical (C) images for GAT1 are included. Data represent mean ± SEM (6–8 animals each from a different litter per treatment group). *Values for animals that are significantly different from controls (p < 0.05). Scale Bars: 100 μm and 50 μM, respectively.
Figure 5.
Developmental exposure of male offspring to endosulfan significantly increases the expression of GABAA 2α receptor subunit in the frontal cortex. Female mice were administered either 0 (control) or 1 mg/kg endosulfan throughout gestation and lactation. Expression of GABAA 2α receptor subunit in the frontal cortex was determined by immunoblot (A) in male offspring. Representative immunoblot (B) and immunohistochemical (C) images for GABAA 2α receptor subunit are included. Data represent mean ± SEM (6–8 animals each from a different litter per treatment group). **Values for animals that are significantly different from controls (p < 0.01). Scale Bars: 100 μm and 50 μM, respectively.
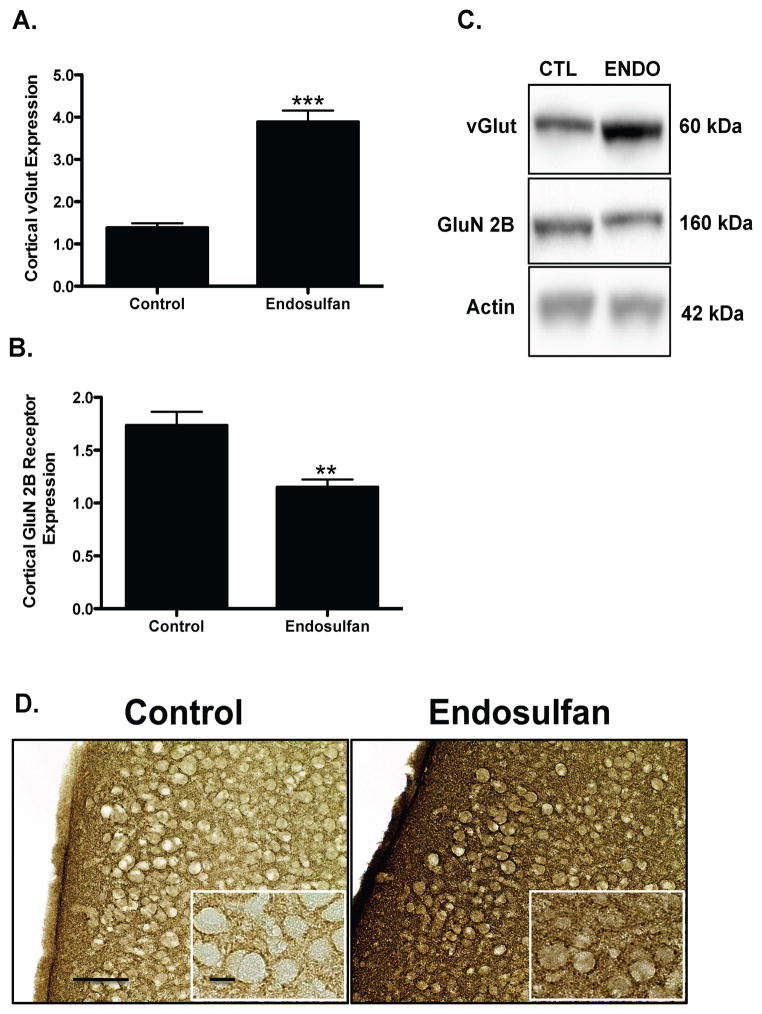
Evaluation of the glutamatergic neurotransmitter system revealed similar alterations to protein expression, with endosulfan exposure eliciting a 178% increase in expression of the vGlut in the frontal cortex of offspring (Figure 6A). In contrast, the postsynaptic glutamate receptor, GluN2B receptor was significantly decreased by 32%, compared with control offspring (Figure 6B). These results suggest that developmental exposure to endosulfan results in changes in proteins involved in glutamatergic neurotransmission, as seen by elevations in vGlut and reductions in GluN2B receptor subunit.
Figure 6.
Developmental exposure of male offspring to endosulfan significantly increases the expression of vGlut and reduces expression of the GluN2B receptor in the frontal cortex. Female mice were administered either 0 (control) or 1 mg/kg endosulfan throughout gestation and lactation. Expression of vGlut in the frontal cortex was determined by immunoblot (A) in male offspring. Representative immunoblot (C) and immunohistochemical (D) images for vGlut are included. Conversely, expression of the GluN2B receptor were reduced in the frontal cortex of male offspring (B). Data represent mean ± SEM (6–8 animals each from a different litter per treatment group). **Values for animals that are significantly different from controls (p < 0.01). ***Values for animals that are significantly different from controls (p < 0.001). Scale Bars: 100 μm and 50 μM, respectively.
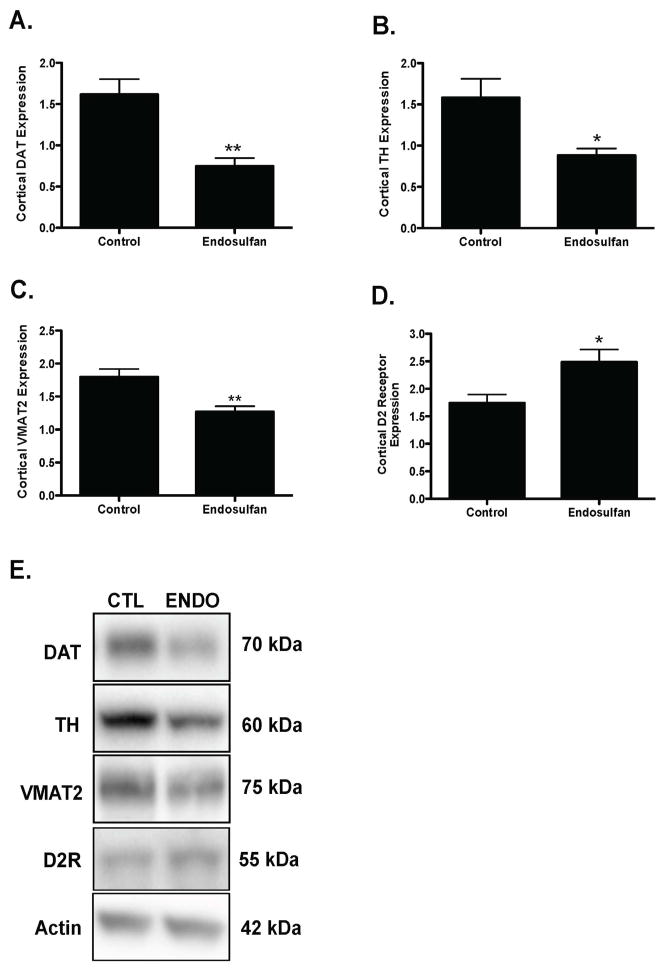
Finally, investigation of the effects of developmental endosulfan exposure on the dopaminergic neurotransmitter system in the frontal cortex demonstrated a 53% reduction in expression of the plasma membrane dopamine transporter, DAT, compared with control treated animals (Figure 7A). Similarly, the expression of the rate-limiting enzyme in dopamine synthesis, TH was also reduced by 44% in the endosulfan-exposed offspring (Figure 7B), with a concomitant 39% reduction in expression of VMAT2 (Figure 7C). In contrast to the reductions seen in DAT, VMAT2, and TH, expression of the dopaminergic D2 receptor was increased 90% in the endosulfan treated animals (Figure 7D). These data demonstrate a significant alteration in both presynaptic and postsynaptic dopaminergic proteins that are important in mediating dopaminergic neurotransmission.
Figure 7.
Developmental exposure of male offspring to endosulfan significantly reduces the expression of DAT, TH, VMAT2, and D2 receptor in the frontal cortex. Female mice were administered either 0 (control) or 1 mg/kg endosulfan throughout gestation and lactation. Expression of DAT (A), TH (B), VMAT2 (C), D2 receptor (D) in the frontal cortex was determined by immunoblot in male offspring. Representative immunoblot (E) images for DAT, TH, VMAT2, and D2 receptor are included. Data represent mean ± SEM (6–8 animals each from a different litter per treatment group). *Values for animals that are significantly different from controls (p < 0.05). **Values for animals that are significantly different from controls (p < 0.01).
DISCUSSION
Recent epidemiological studies have demonstrated exposure to organochlorine compounds, including pesticides during critical periods of neurodevelopment, can significantly contribute to several cognitive and psychomotor deficits in children (Eskenazi et al., 2008; Puertas et al., 2010; Ribas-Fito et al., 2007; Roberts et al., 2007; Sioen et al., 2013). However, further identification of specific compounds contributing to these neurological alterations and subsequent elucidation of their underlying mechanisms remains to be established. In this study we first utilized in vitro models to establish the neurotoxic potential of endosulfan in both the IMR-32 neuroblastoma cell line as well as cortical neurons isolated from postnatal mouse brain. These findings were then elaborated to an in vivo model of developmental exposure to endosulfan. Data collected from each of these models demonstrates that exposure to endosulfan during critical periods of neurodevelopment causes a significant alteration to proteins involved in maintaining normal neurotransmitter signaling in the GABAergic, glutamatergic, and dopaminergic neurotransmitter systems that innervate the frontal cortex. These findings further suggest that these neurotransmitter circuits are especially vulnerable to exposure to endosulfan during development and these detriments may contribute to many of the cognitive and psychological deficits observed in children developmentally exposed to pesticides.
In order to gain an understanding of the general neurotoxicity of endosulfan on neuronal cells we first evaluated its impact on cell viability using the IMR-32 human neuroblastoma cell line that expresses measurable and functional levels of GABAA receptor (Anderson et al., 1993; Noble et al., 1993; Sapp and Yeh, 2000). Having this model was important as endosulfan specifically targets and inhibits this receptor, thus providing us with a unique in vitro model that more appropriately mirrors the biological processes of mammalian neurons being affected by endosulfan. Indeed, as we observed a significant dose-dependent reduction in cell viability in IMR-32 cells following exposure to endosulfan, this loss was greatly diminished at similar concentrations of endosulfan in SK-N-SH cells, another human neuroblastoma cell line (data not shown) that does not appear to contain relevant levels of the GABAA receptor (Starosta-Rubinstein et al., 1987). While this cell line has not been previously used to evaluate the neurotoxic potential of endosulfan, it has been used to investigate other insecticides that may potentially interact with the GABAA receptors, such as pyrethroid and organophosphorous insecticides (Cova et al., 1995; Fakata et al., 1998).
Extending our findings in the IMR-32 cells to a more sophisticated in vitro system we found that cortical neurons from postnatal mouse pups were also vulnerable to the neurotoxic effects of endosulfan, demonstrating a reduction in neuronal viability as well as neurite outgrowth and synapse formation. These findings are well aligned with previous studies that utilized brainstem neurons to examine the role of GABA as a trophic factor in neuronal development. Using dieldrin, another organochlorine insecticide similar to endosulfan, investigators were able to demonstrate the importance of GABAergic signaling in neuronal development and subsequent detriment to this process when these signals were altered, such as the case following blockade of the GABAA receptors (Liu et al., 1997). Thus, we hypothesize that the alterations to cortical neurons following exposure to endosulfan are mediated by this disruption in GABAergic signaling. In contrast to these findings, we initially demonstrate a significant increase in the number of neurons following treatment with 1 μM endosulfan for 9 days. Although the precise mechanisms responsible for this effect are unclear, it would appear that exposure to endosulfan at this concentration is attenuating neuronal loss, rather than increasing neuronal proliferation, as cultured neurons are post-mitotic and incapable of dividing. One possible mechanism by which this effect is mediated is through the activation of the estrogen receptor by endosulfan. Endosulfan, in addition to other organochlorine compounds, has routinely been shown to interact with the estrogen receptor and elicit estrogenic effects. Given the importance of estrogen in promoting various aspects of neuronal development, including neuronal survival (Garcia-Segura et al., 2001; Micevych and Dominguez, 2009), exposure to low levels of endosulfan could be inhibiting those general cell death processes through the estrogen receptor.
Interestingly, our sensitive in vitro exposure platform was able to detect alterations to neurite outgrowth and the number of synapsin positive puncta that occurred prior to any measurable loss of cortical neurons in culture. While we can not rule out pruning or destabilization of newly formed synapses as a contributor to our reduction in synapsin positive puncta, our results suggest that development and establishment of putative synapses are uniquely vulnerable to the effects of endosulfan, relative to the neuronal cell body. These alterations were not observed in cortical cultures that were treated with endosulfan at a single time point of 10 DIV, further supporting the importance of exposure to endosulfan during critical periods of neuronal growth (data not shown). Similar alterations in neurite outgrowth have also been seen in the presence of other organochlorine insecticides and compounds that antagonize the GABAA receptors, including dieldrin and bicuculline, implicating the importance of proper GABAergic signaling in neuronal morphology and growth (Liu et al., 1997). Furthermore, we have seen similar results in the frontal cortex following exposure to the flame retardant compounds, DE-71 (Bradner et al., 2013a), suggesting that neuronal outgrowth and number of synapsin positive puncta are susceptible to different neurotoxic agents and should be considered as a sensitive marker of neurotoxicity. These findings were instrumental in fine-tuning our in vivo assessment of developmental exposure to endosulfan by directing our focus towards investigating alterations in specific synaptic markers involved in mediating neuronal signaling in the frontal cortex.
As the frontal cortex is densely innervated by various neuronal populations, we focused our investigation on the GABAergic, glutamatergic, and dopaminergic circuits as these are involved in various aspects of normal functioning in the frontal cortex (Floresco and Magyar, 2006; Lewis and Moghaddam, 2006). The most striking finding from our developmental study is the extensive alteration to specific synaptic proteins in each of these neurotransmitter systems that are crucial for maintaining proper neuronal signaling in these circuits, suggesting that the synapse may be critically vulnerable to developmental exposure to endosulfan.
Changes in GABAergic neurons showed significant reductions in the presynaptic proteins, vGAT, which is involved in packing GABA into synaptic vesicles, and GAT1, which resides on the plasma membrane and removes GABA from the synapse, terminating its effects at the receptors. In contrast, assessment of the postsynaptic GABAA 2α receptor showed a significant elevation in expression. Although we did not evaluate levels of GABA in the frontal cortex of offspring in our study, a previous study observed significant alterations in GABA in the prefrontal cortex of male offspring whose mothers had been exposed to .61 or 6.12 mg/kg/day of endosulfan during gestation and lactation (Cabaleiro et al., 2008). These findings are interesting as the lowest concentration aligns well with our exposure paradigm of 1 mg/kg/every other day, suggesting that disruption of vGAT and GAT1 may be involved in alterations to GABA levels in the frontal cortex. This hypothesis is further supported with studies that generated vGAT or GAT1 knockout mice and found an elevation in GABA levels in the cortex of knockout animals and demonstrating an alteration in the function of the GABA receptors (Bragina et al., 2008; Saito et al., 2010). Thus, a potential loss of GABA in the synapse could explain the elevated expression of the GABAA 2α receptor subuit, indicating a compensatory increase to compensate for a reduction in GABA signaling. Indeed, trafficking of GABAA receptors to and from the plasma membrane is highly dependent upon their activation. For example, under circumstances of increased activation, as in the case of a GABAA receptor agonist, like ethanol, GABAA receptors will be internalized, reducing their availability on the membrane (Jacob et al., 2008). In contrast, an alteration in GABAergic signaling that causes a reduction in stimulation could result in trafficking of GABAA receptors to the membrane. Similar alterations in GABAergic proteins and signaling in the frontal cortex have routinely been identified in neurological disorders, including schizophrenia and autism spectrum disorder (Lewis and Sweet, 2009).
In addition to the GABAergic neurotransmitter circuit, alterations in the glutamatergic pathway were also observed in the frontal cortex of mice exposed to endosulfan. In contrast to the changes seen with GABA, we observed a substantial elevation in the expression of the vGlut as well as a significant reduction in the expression of the GluN2B receptor subunit. Although previous work has shown an increase in glutamate levels in the prefrontal cortex of rats developmentally exposed to endosulfan, glutamatergic synaptic proteins that may underlie this elevation were not investigated (Cabaleiro et al., 2008). Our findings align closely with other studies showing a substantial elevation in glutamate release following overexpression of vGlut in drosophila, as well as the ability of NMDA receptors to internalize from the plasma membrane under conditions of activation (Daniels et al., 2011; Lau and Zukin, 2007). Similar to the GABAergic circuit, alterations in glutamatergic signaling have also been suggested to underlie the neurological dysfunction seen in neurological disorders. Studies of patients with schizophrenia have found that they exhibit a generalized hypofunctioning of the glutamatergic circuit that appears to be mediated by the NMDA receptors (Gonzalez-Burgos and Lewis, 2012).
Finally, the frontal cortex receives extensive dopaminergic projections that arise from the mesocortical dopamine system and serve to modulate several aspects of behavior. In our study, developmental exposure to endosulfan caused significant alterations to specific dopaminergic proteins that are critically involved in mediating normal dopamine signaling in the frontal cortex. Thus, reductions in DAT, VMAT2, and TH were seen while an elevation in the D2 dopamine receptor was also observed. To our knowledge, our findings represent the first evidence that developmental exposure can affect the expression of specific proteins in the dopaminergic system. Although previous work has evaluated the effects of developmental as well as adult exposure to endosulfan on the dopamine system, only one study has focused on alterations to this circuit in the frontal cortex, finding a lack of effect on levels of dopamine in this region (Cabaleiro et al., 2008). However, there were significant alterations in ratios of the dopamine metabolites to levels of dopamine, suggesting a disruption in dopamine handling, which could be mediated by changes in DAT and VMAT2. As mentioned above, the dopaminergic projections to the frontal cortex play an imperative role in various behavioral outputs and alterations to this circuit have been implicated in neurological dysfunction including alterations in working memory, ADHD, and schizophrenia (Akil et al., 1999; Chudasama and Robbins, 2006; Floresco and Magyar, 2006; Seamans and Yang, 2004).
While many of the alterations observed in the GABAergic, glutamatergic, and dopaminergic neurotransmitter systems can be explained as compensatory responses, it cannot be ruled out that these changes in expression are simply due to alterations incurred as a result of disruption to normal development and signaling of the GABAergic neurotransmitter system caused by endosulfan. Indeed, the importance of GABA as a trophic factor during early stages of neurodevelopment has been well documented and alteration in GABAergic signaling has been shown to have significant influence on numerous neurodevelopmental processes, including neuronal proliferation, migration, and synapse formation (Akerman and Cline, 2007; Ben-Ari, 2002; Represa and Ben-Ari, 2005). While the exact mechanisms responsible for mediating these disruptions are still being investigated, several studies have shown interruption of the GABAergic signal at various points, such as blockade of calcium homeostasis or sodium influx with tetrodotoxin can facilitate decrements in neuronal circuitry. Furthermore, previous work has shown developmental exposure to other organochlorine compounds, including dieldrin and heptachlor, elicit changes to transcription factors responsible for mediating expression of dopaminergic proteins. Investigation of these changes as potential contributors to the changes we observed in our study should be further evaluated. Thus, as endosulfan specifically antagonizes GABAergic signaling via blockade of GABAA receptors, our findings implicate endosulfan as a significant neurodevelopmental disruptor, capable of impacting multiple aspects of the neuronal circuitry in the frontal cortex.
Acknowledgments
FUNDING SOURCES
This work was supported by National Institutes of Health grants [R00ES017477 and P01ES016731 to W.M.C.]
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interests with the material presented in this manuscript.
References
- Akerman CJ, Cline HT. Refining the roles of GABAergic signaling during neural circuit formation. Trends Neurosci. 2007;30(8):382–389. doi: 10.1016/j.tins.2007.06.002. [DOI] [PubMed] [Google Scholar]
- Akil M, Pierri JN, Whitehead RE, Edgar CL, Mohila C, Sampson AR, Lewis DA. Lamina-specific alterations in the dopamine innervation of the prefrontal cortex in schizophrenic subjects. Am J Psychiatry. 1999;156(10):1580–1589. doi: 10.1176/ajp.156.10.1580. [DOI] [PubMed] [Google Scholar]
- Anderson SM, De Souza RJ, Cross AJ. The human neuroblastoma cell line, IMR-32 possesses a GABAA receptor lacking the benzodiazepine modulatory site. Neuropharmacology. 1993;32(5):455–460. doi: 10.1016/0028-3908(93)90169-4. [DOI] [PubMed] [Google Scholar]
- Behar TN, Schaffner AE, Scott CA, O’Connell C, Barker JL. Differential response of cortical plate and ventricular zone cells to GABA as a migration stimulus. J Neurosci. 1998;18(16):6378–6387. doi: 10.1523/JNEUROSCI.18-16-06378.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellinger DC. Prenatal Exposures to Environmental Chemicals and Children’s Neurodevelopment: An Update. Safety and health at work. 2013;4(1):1–11. doi: 10.5491/SHAW.2013.4.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ari Y. Excitatory actions of gaba during development: the nature of the nurture. Nat Rev Neurosci. 2002;3(9):728–739. doi: 10.1038/nrn920. [DOI] [PubMed] [Google Scholar]
- Bradner JM, Suragh TA, Caudle WM. Alterations to the circuitry of the frontal cortex following exposure to the polybrominated diphenyl ether mixture, DE-71. Toxicology. 2013a;312:48–55. doi: 10.1016/j.tox.2013.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradner JM, Suragh TA, Wilson WW, Lazo CR, Stout KA, Kim HM, Wang MZ, Walker DI, Pennell KD, Richardson JR, Miller GW, Caudle WM. Exposure to the polybrominated diphenyl ether mixture DE-71 damages the nigrostriatal dopamine system: role of dopamine handling in neurotoxicity. Exp Neurol. 2013b;241:138–147. doi: 10.1016/j.expneurol.2012.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bragina L, Marchionni I, Omrani A, Cozzi A, Pellegrini-Giampietro DE, Cherubini E, Conti F. GAT-1 regulates both tonic and phasic GABA(A) receptor-mediated inhibition in the cerebral cortex. J Neurochem. 2008;105(5):1781–1793. doi: 10.1111/j.1471-4159.2008.05273.x. [DOI] [PubMed] [Google Scholar]
- Cabaleiro T, Caride A, Romero A, Lafuente A. Effects of in utero and lactational exposure to endosulfan in prefrontal cortex of male rats. Toxicol Lett. 2008;176(1):58–67. doi: 10.1016/j.toxlet.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Casida JE. Insecticide action at the GABA-gated chloride channel: recognition, progress, and prospects. Archives of insect biochemistry and physiology. 1993;22(1–2):13–23. doi: 10.1002/arch.940220104. [DOI] [PubMed] [Google Scholar]
- Caudle WM, Richardson JR, Delea KC, Guillot TS, Wang M, Pennell KD, Miller GW. Polychlorinated biphenyl-induced reduction of dopamine transporter expression as a precursor to Parkinson’s disease-associated dopamine toxicity. Toxicol Sci. 2006;92(2):490–499. doi: 10.1093/toxsci/kfl018. [DOI] [PubMed] [Google Scholar]
- Caudle WM, Richardson JR, Wang M, Miller GW. Perinatal heptachlor exposure increases expression of presynaptic dopaminergic markers in mouse striatum. Neurotoxicology. 2005;26(4):721–728. doi: 10.1016/j.neuro.2004.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chudasama Y, Robbins TW. Functions of frontostriatal systems in cognition: comparative neuropsychopharmacological studies in rats, monkeys and humans. Biological psychology. 2006;73(1):19–38. doi: 10.1016/j.biopsycho.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Cova D, Perego R, Nebuloni C, Fontana G, Molinari GP. In vitro cytotoxicity of fenthion and related metabolites in human neuroblastoma cell lines. Chemosphere. 1995;30(9):1709–1715. doi: 10.1016/0045-6535(95)00056-e. [DOI] [PubMed] [Google Scholar]
- Daniels RW, Miller BR, DiAntonio A. Increased vesicular glutamate transporter expression causes excitotoxic neurodegeneration. Neurobiol Dis. 2011;41(2):415–420. doi: 10.1016/j.nbd.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLorey TM, Sahbaie P, Hashemi E, Homanics GE, Clark JD. Gabrb3 gene deficient mice exhibit impaired social and exploratory behaviors, deficits in non-selective attention and hypoplasia of cerebellar vermal lobules: a potential model of autism spectrum disorder. Behav Brain Res. 2008;187(2):207–220. doi: 10.1016/j.bbr.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzirasa K, Ramsey AJ, Takahashi DY, Stapleton J, Potes JM, Williams JK, Gainetdinov RR, Sameshima K, Caron MG, Nicolelis MA. Hyperdopaminergia and NMDA receptor hypofunction disrupt neural phase signaling. J Neurosci. 2009;29(25):8215–8224. doi: 10.1523/JNEUROSCI.1773-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskenazi B, Rosas LG, Marks AR, Bradman A, Harley K, Holland N, Johnson C, Fenster L, Barr DB. Pesticide toxicity and the developing brain. Basic Clin Pharmacol Toxicol. 2008;102(2):228–236. doi: 10.1111/j.1742-7843.2007.00171.x. [DOI] [PubMed] [Google Scholar]
- Fakata KL, Swanson SA, Vorce RL, Stemmer PM. Pyrethroid insecticides as phosphatase inhibitors. Biochem Pharmacol. 1998;55(12):2017–2022. doi: 10.1016/s0006-2952(98)00076-8. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Reutiman TJ, Folsom TD, Rooney RJ, Patel DH, Thuras PD. mRNA and protein levels for GABAAalpha4, alpha5, beta1 and GABABR1 receptors are altered in brains from subjects with autism. J Autism Dev Disord. 2010;40(6):743–750. doi: 10.1007/s10803-009-0924-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, Magyar O. Mesocortical dopamine modulation of executive functions: beyond working memory. Psychopharmacology (Berl) 2006;188(4):567–585. doi: 10.1007/s00213-006-0404-5. [DOI] [PubMed] [Google Scholar]
- Garcia-Segura LM, Azcoitia I, DonCarlos LL. Neuroprotection by estradiol. Prog Neurobiol. 2001;63(1):29–60. doi: 10.1016/s0301-0082(00)00025-3. [DOI] [PubMed] [Google Scholar]
- Gaspar PA, Bustamante ML, Silva H, Aboitiz F. Molecular mechanisms underlying glutamatergic dysfunction in schizophrenia: therapeutic implications. J Neurochem. 2009;111(4):891–900. doi: 10.1111/j.1471-4159.2009.06325.x. [DOI] [PubMed] [Google Scholar]
- Gleeson JG, Walsh CA. Neuronal migration disorders: from genetic diseases to developmental mechanisms. Trends Neurosci. 2000;23(8):352–359. doi: 10.1016/s0166-2236(00)01607-6. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Burgos G, Lewis DA. NMDA receptor hypofunction, parvalbumin-positive neurons, and cortical gamma oscillations in schizophrenia. Schizophr Bull. 2012;38(5):950–957. doi: 10.1093/schbul/sbs010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandjean P, Landrigan PJ. Neurobehavioural effects of developmental toxicity. Lancet Neurol. 2014;13(3):330–338. doi: 10.1016/S1474-4422(13)70278-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrill JA, Robinette BL, Mundy WR. Use of high content image analysis to detect chemical-induced changes in synaptogenesis in vitro. Toxicol In Vitro. 2011;25(1):368–387. doi: 10.1016/j.tiv.2010.10.011. [DOI] [PubMed] [Google Scholar]
- Homayoun H, Moghaddam B. NMDA receptor hypofunction produces opposite effects on prefrontal cortex interneurons and pyramidal neurons. J Neurosci. 2007;27(43):11496–11500. doi: 10.1523/JNEUROSCI.2213-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob TC, Moss SJ, Jurd R. GABA(A) receptor trafficking and its role in the dynamic modulation of neuronal inhibition. Nat Rev Neurosci. 2008;9(5):331–343. doi: 10.1038/nrn2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez Torres M, Campoy Folgoso C, Canabate Reche F, Rivas Velasco A, Cerrillo Garcia I, Mariscal Arcas M, Olea-Serrano F. Organochlorine pesticides in serum and adipose tissue of pregnant women in Southern Spain giving birth by cesarean section. Sci Total Environ. 2006;372(1):32–38. doi: 10.1016/j.scitotenv.2006.07.009. [DOI] [PubMed] [Google Scholar]
- Kamijima M, Casida JE. Regional modification of [(3)H]Ethynylbicycloorthobenzoate binding in mouse brain GABA(A) receptor by endosulfan, fipronil, and avermectin B(1a) Toxicol Appl Pharmacol. 2000;163(2):188–194. doi: 10.1006/taap.1999.8865. [DOI] [PubMed] [Google Scholar]
- Lakshmana MK, Raju TR. Endosulfan induces small but significant changes in the levels of noradrenaline, dopamine and serotonin in the developing rat brain and deficits in the operant learning performance. Toxicology. 1994;91(2):139–150. doi: 10.1016/0300-483x(94)90140-6. [DOI] [PubMed] [Google Scholar]
- Lau CG, Zukin RS. NMDA receptor trafficking in synaptic plasticity and neuropsychiatric disorders. Nat Rev Neurosci. 2007;8(6):413–426. doi: 10.1038/nrn2153. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Moghaddam B. Cognitive dysfunction in schizophrenia: convergence of gamma-aminobutyric acid and glutamate alterations. Arch Neurol. 2006;63(10):1372–1376. doi: 10.1001/archneur.63.10.1372. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Sweet RA. Schizophrenia from a neural circuitry perspective: advancing toward rational pharmacological therapies. J Clin Invest. 2009;119(4):706–716. doi: 10.1172/JCI37335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Morrow AL, Devaud L, Grayson DR, Lauder JM. GABAA receptors mediate trophic effects of GABA on embryonic brainstem monoamine neurons in vitro. J Neurosci. 1997;17(7):2420–2428. doi: 10.1523/JNEUROSCI.17-07-02420.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micevych P, Dominguez R. Membrane estradiol signaling in the brain. Front Neuroendocrinol. 2009;30(3):315–327. doi: 10.1016/j.yfrne.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohler H. Molecular regulation of cognitive functions and developmental plasticity: impact of GABAA receptors. J Neurochem. 2007;102(1):1–12. doi: 10.1111/j.1471-4159.2007.04454.x. [DOI] [PubMed] [Google Scholar]
- Moreno Frias M, Jimenez Torres M, Garrido Frenich A, Martinez Vidal JL, Olea-Serrano F, Olea N. Determination of organochlorine compounds in human biological samples by GC-MS/MS. Biomedical chromatography: BMC. 2004;18(2):102–111. doi: 10.1002/bmc.300. [DOI] [PubMed] [Google Scholar]
- Noble PJ, Anderson SM, De Souza RJ, Cross AJ, Stephenson FA. Identification of the GABAA receptor alpha 3 subunit in the IMR-32 neuroblastoma cell line. J Neurochem. 1993;61(2):752–755. doi: 10.1111/j.1471-4159.1993.tb02182.x. [DOI] [PubMed] [Google Scholar]
- Puertas R, Lopez-Espinosa MJ, Cruz F, Ramos R, Freire C, Perez-Garcia M, Abril A, Julvez J, Salvatierra M, Campoy C, Olea N. Prenatal exposure to mirex impairs neurodevelopment at age of 4 years. Neurotoxicology. 2010;31(1):154–160. doi: 10.1016/j.neuro.2009.09.009. [DOI] [PubMed] [Google Scholar]
- Represa A, Ben-Ari Y. Trophic actions of GABA on neuronal development. Trends Neurosci. 2005;28(6):278–283. doi: 10.1016/j.tins.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Ribas-Fito N, Torrent M, Carrizo D, Julvez J, Grimalt JO, Sunyer J. Exposure to hexachlorobenzene during pregnancy and children’s social behavior at 4 years of age. Environ Health Perspect. 2007;115(3):447–450. doi: 10.1289/ehp.9314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice D, Barone S., Jr Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ Health Perspect. 2000;108(Suppl 3):511–533. doi: 10.1289/ehp.00108s3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson JR, Caudle WM, Wang M, Dean ED, Pennell KD, Miller GW. Developmental exposure to the pesticide dieldrin alters the dopamine system and increases neurotoxicity in an animal model of Parkinson’s disease. Faseb J. 2006;20(10):1695–1697. doi: 10.1096/fj.06-5864fje. [DOI] [PubMed] [Google Scholar]
- Richardson JR, Caudle WM, Wang MZ, Dean ED, Pennell KD, Miller GW. Developmental heptachlor exposure increases susceptibility of dopamine neurons to N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)in a gender-specific manner. Neurotoxicology. 2008;29(5):855–863. doi: 10.1016/j.neuro.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts EM, English PB, Grether JK, Windham GC, Somberg L, Wolff C. Maternal residence near agricultural pesticide applications and autism spectrum disorders among children in the California Central Valley. Environ Health Perspect. 2007;115(10):1482–1489. doi: 10.1289/ehp.10168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito K, Kakizaki T, Hayashi R, Nishimaru H, Furukawa T, Nakazato Y, Takamori S, Ebihara S, Uematsu M, Mishina M, Miyazaki J, Yokoyama M, Konishi S, Inoue K, Fukuda A, Fukumoto M, Nakamura K, Obata K, Yanagawa Y. The physiological roles of vesicular GABA transporter during embryonic development: a study using knockout mice. Mol Brain. 2010;3:40. doi: 10.1186/1756-6606-3-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapp DW, Yeh HH. Heterogeneity of GABA(A) receptor-mediated responses in the human IMR-32 neuroblastoma cell line. J Neurosci Res. 2000;60(4):504–510. doi: 10.1002/(SICI)1097-4547(20000515)60:4<504::AID-JNR9>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Seamans JK, Yang CR. The principal features and mechanisms of dopamine modulation in the prefrontal cortex. Prog Neurobiol. 2004;74(1):1–58. doi: 10.1016/j.pneurobio.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Simons CJ, van Winkel R Group. Intermediate phenotype analysis of patients, unaffected siblings, and healthy controls identifies VMAT2 as a candidate gene for psychotic disorder and neurocognition. Schizophr Bull. 2013;39(4):848–856. doi: 10.1093/schbul/sbs067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sioen I, Den Hond E, Nelen V, Van de Mieroop E, Croes K, Van Larebeke N, Nawrot TS, Schoeters G. Prenatal exposure to environmental contaminants and behavioural problems at age 7–8years. Environ Int. 2013;59:225–231. doi: 10.1016/j.envint.2013.06.014. [DOI] [PubMed] [Google Scholar]
- Smith AG. Chlorinated Hydrocarbon Insecticides. In: Hayes WJ, Laws ER, editors. Pesticide Toxicology. New York: Academic Press Inc; 1991. [Google Scholar]
- Starosta-Rubinstein S, Ciliax BJ, Penney JB, McKeever P, Young AB. Imaging of a glioma using peripheral benzodiazepine receptor ligands. Proc Natl Acad Sci U S A. 1987;84(3):891–895. doi: 10.1073/pnas.84.3.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahr NM, Mayer D, Pfefferbaum A, Sullivan EV. Low striatal glutamate levels underlie cognitive decline in the elderly: evidence from in vivo molecular spectroscopy. Cereb Cortex. 2008;18(10):2241–2250. doi: 10.1093/cercor/bhm250. [DOI] [PMC free article] [PubMed] [Google Scholar]