Abstract
Autism spectrum disorders (ASD) are characterized by social impairments and restricted/stereotyped behaviors and currently affect an estimated 1 in 68 children aged 8 years old. While there has been substantial recent focus on ASD in research, both the biological pathology and, perhaps consequently, a fully effective treatment have yet to be realized. What has remained throughout is the hypothesis that ASD has neurobiological underpinnings and the observation that both the phenotypic expression and likely the underlying etiology is highly heterogeneous. Given the neurodevelopmental basis of ASD, a biologically based marker (biomarker) could prove useful not only for diagnostic and prognostic purposes, but also for stratification and response indices for pharmaceutical development. In this review, we examine the current state of the field for MEG-related biomarkers in ASD. We describe several potential biomarkers (middle latency delays [M50/M100], mismatch negativity latency, gamma-band oscillatory activity), and investigate their relation to symptomology, core domains of dysfunction (e.g., language impairment), and putative biological underpinnings.
Keywords: ASD, biomarker, MEG, signature, translational, latency delay, Gamma
Introduction
Autism spectrum disorders (ASD) are defined by restricted/stereotyped behaviors and social impairments [1] and have a massive impact on both the individuals with ASD and society itself. Currently, an estimated 1 in 68 children aged 8 years old suffer from an ASD [2]. Neither the biological pathology nor, perhaps consequently, a fully effective treatment have yet to be realized for ASD, even after substantial focus on ASD in research. In fact, the list of possible pathogenic mechanisms has dramatically increased, including multiple genetic mutations, combinations thereof and gene-environment interactions [3], manifesting through a variety of mechanisms, including imbalance of excitation and inhibition [4] as well as hypo/hyper-connectivity [5]. What seems to be of consensus is that ASD have neurobiological underpinnings and, moreover, phenotypic expression, and likely the underlying etiology is highly heterogeneous. As such, a biologically based marker (biomarker), rooted in one or more of the various etiologies, could prove useful for both diagnostic and prognostic purposes. Furthermore, a valuable role of such a biomarker lies in subject/patient stratification in both clinical trials (e.g., by population enrichment through biologically based inclusion criteria) and subsequently matching/selecting treatment to patient. In such a way, the heterogeneous ASD population would be biologically divided — thus made at least somewhat less heterogeneous — into good candidates for a particular treatment (a sub-population that would be targeted from inclusion) versus a less favorable sub-population. Additionally, it would provide biologically targeted objective indices of drug target engagement and activity for dose finding and as early signals of possible efficacy. Particularly since recruitment would be targeted toward that subset of the heterogeneous autism spectrum that exhibit this particular endophenotype, it should be expected to respond (perhaps, normalize) if indeed it is biologically tuned to the mode of action of the drug being evaluated. Exploring the biological basis of ASD in the pursuit of such biomarkers will also likely continue to identify substrates and neurobiological mechanisms underpinning the symptoms of ASD, informing our neurobiological understanding of the disorder and the basis of its comorbidities (e.g., attention deficit hyperactivity disorder and seizure disorders, both associated with neurotransmitter imbalance) as well as providing targets for further development of interventions.
In general, for a biomarker to be effective, it must be sensitive and specific to the disorder/dysfunctional domain, scale with severity, be simple to implement, and demonstrate robustness. An additional opportunity emerges in use of the biomarker to bridge the gap between clinical and pre-clinical environments, essentially providing a degree of validity to pre-clinical models that a biologically relevant phenomenon or trait is, in fact, being recapitulated [6]. As the label itself implies, a “bio” marker owes a plausible biological mechanism (or, at least, hypothesis), commonly rooted in both clinical and pre-clinical findings. Several emerging biomarkers have been proposed for adoption in ASD, ranging from blood- based assays [7], magnetic resonance imaging (MRI) [8], positron emission tomography (PET) imaging [9] and electrophysiology, and electroencephalography (EEG) or magnetoencephalography (MEG) [6,10-12]. In addition, other markers not as firmly rooted in biology have been also suggested for adoption in ASD, such as behavioral and eye-tracking performance [13]. This review focuses on electrophysiological biomarkers, as they satisfy the above measurement requirements and offer plausible biological hypotheses for their basis. Specifically, while both EEG and MEG methodologies have been used to probe ASD, in this review, we will focus on MEG.
Current Electrophysiological Signatures of ASD: Non-Invasive, Precise, and Sensitive to Neuronal Mass Firing
Of the various classes of candidate biomarkers, electrophysiological signatures of ASD hold particular promise in the study and treatment of ASD because of the non-invasive nature of recordings for a population traditionally challenged by imaging procedure compliance demands (Table 1). Passive paradigms, along with recording of early, obligate responses offer strategies to defend against confounding influences of attention, performance, and movement. Over the last several decades, researchers have explored numerous electrophysiological signatures and have found varying relationships of these measures to ASD symptom severity and specificity [14]. While this association of observational findings and symptomatology and the non-invasive nature of recordings are not unique to electrophysiological methods (e.g., other imaging methods such as fMRI/MRI/PET show such profiles [15,16]), electrophysiology has the important distinction of being exquisitely sensitive to the temporal dimension, and, thus, unlike MRI, fMRI, and PET, electrophysiological methodologies allow investigations and characterization of the timing, synchrony, and connectivity of actual neural activity rather than simply spatial identification of secondary associations (e.g., hemodynamic response through neurovascular coupling). While exhibiting sensitivity to similar neuronal electrical activity as EEG, MEG extends the capabilities of EEG with a more precise spatial resolution than its electrical measuring cousin. In contrast to EEG, MEG’s resolution is in the range of millimeters [17], similar to the range of fMRI. In addition, MEG is relatively immune (via the use of spatial filtering-based source localization) from contaminating EMG artifact in high frequency bands arising from scalp and facial muscle [18] as well as microsaccadic eye movements [19]. Signatures derived from electrophysiological measures, and especially MEG, technically offer the potential of increased sensitivity as well as specificity for neurophysiological abnormalities that may underlie symptom domains of ASD and thus present opportunities for evaluation as potential biomarkers.
Table 1. Electrophysiological signatures of ASD and their potential for biomarker use.
| Biomarker | Simple to Implement | Sensitive | Specific | Scale | Responsive to Treatment | Biological Basis | Predictive |
| M50/100 Latency Delay | √ | √ | √ | √ | √ (pre-clinical) | √ (hypothesized) | untested |
| MMF/N | √ | √ | to RDoC domain but not clinical label | √ | untested | less clear | untested |
| Gamma Band Dysfunction | √ | √ | to RDoC domains, but not clinical label | √ | √ | √ | √ |
M50 and M100 Latency Delays: Predictive with a Plausible Biological Basis
One of the most replicable electrophysiologic findings is the latency delay of middle-latency M50/M100 responses in ASD as compared to typically developing children. First demonstrated by Gage and colleagues, right hemisphere M100 latencies exhibit both altered maturation [20] and reduced representational dynamic range [21]. These findings went against some concurrent findings that resolved no latency prolongation of the M100 response [22], though this study has exclusively low functioning subjects with ASD for the experimental group, and methodological differences such as choice of stimuli (1000Hz/1200Hz standard/deviant) may account for the lack of findings. Furthermore, the M100 response was defined as the “first power maximum following the 50-msec latency,” which has been shown to be either the M50 or M100, depending on the age — and potentially, diagnostic status — of the subject [23]. While it is posited that both M50 and M100 are delayed in ASD, to detect M50 latency prolongation, larger cohort sizes have been needed [24]; importantly, distinction should be made between responses that are “late M50s” versus “early or typical M100s” as potential overlap in latency range can obscure group findings; topographic representations of surface magnetic fields can readily aid this resolution. In addition to this potential misattribution, M100 response latencies, even in response to simple sinusoidal tones, are not stationary. Middle latency-evoked potentials can differ depending on stimulus properties such as frequency [25], intensity [26], and other features [27]. As such, it is important to standardize all stimulus properties and delivery methodology. For instance, we the authors always conduct experiments with stimuli at 45 dB above sensation level, well above the plateau of intensity effects of the stimuli on latency. Also, during each session, multiple (200, 300, 500, and 1000Hz tones) stimuli are used to both fully characterize responses and allow comparison to other institutions that use different frequencies. Direct comparisons of M100 latencies recorded at different sites occurred in a recent multi-center collaboration [28]. Here, great care was undertaken to standardize all aspects of the stimuli presentation, although a persistent up-to-5 ms delay continued to exist between sites, which may either reflect technical precision limits or imperfect subject matching between sites or a combination thereof.
Delays in the M100 latency were recapitulated later in a larger cohort and survived co-varying for age and language ability [29]. Generally, these delays are of the order of ~10ms (Figure 1) and thus, in themselves, quite demanding of measurement precision. M50 latency delays were also observed in a similarly large-sized sample [24]. While M50 responses are larger in amplitude as well as later in children than adults [23], both the M50 and M100 latency delays seen in ASD are ~10 percent of the typical latency (M50 [24], M100 [29]). Thus, M50 delays (~5ms) are even more demanding to resolve, so less statistical power may be needed to resolve M100 delays (given similar measurement precision), explaining the predominance of focus on this later component. In both cases, large sample studies are necessary to avoid misrepresenting the broad distribution of responses across the spectrum. Nonetheless, M50 latency delays in ASD have also been replicated in later analyses [30]. Maturational changes in both M100 (especially right hemisphere) and M50 (bilaterally resolved) suggest that although there is a pattern of latency shortening with increasing age — in fact, at a rate similar to that of typical development — a persistent shift or prolongation of latency exists in ASD compared to controls at any given age, thereby defining an atypical developmental trajectory, despite potentially similar rate [20,24].
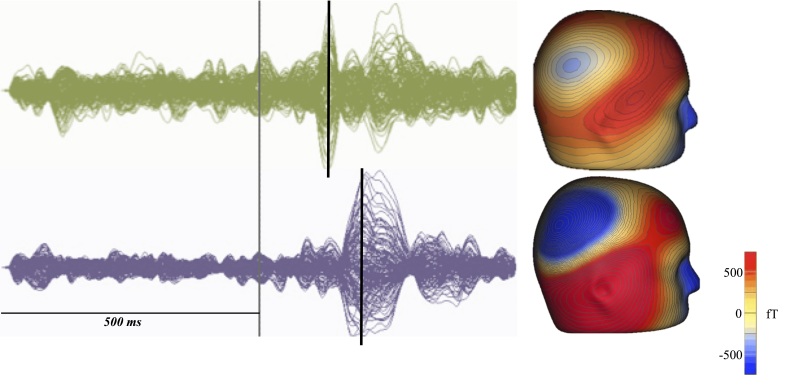
Figure 1.
Delayed M100 response latency in ASD, when compared to age matched typically developing controls. ASD (bottom) demonstrate a delay of the M100 response compared to typically developing controls (top) as detected by MEG. At left, ERP of typically developing (green) and children with ASD (purple), with stimulus marked in gray and M100 denoted by black bar. At right, 3D topographic representations of the M100 responses demonstrate that both populations M100 arise from the region of auditory cortex (top: TD; bottom: ASD).
Confounding these results are the observations that M100 responses are detectable less often in young children with ASD and comorbid language impairment than typically developing children [30], potentially skewing the distribution of samples in whom responses are indeed obtained. Another yet unresolved aspect of M50/M100 latency delays are their association with ultimate clinical language impairment. M50 latency and, to a lesser extent, M100 latencies are reported to predict oral language ability [31], though this has failed to be replicated in all cohorts [29]. Recent evidence suggests an association of M50/M100 latencies with language ability is better seen with more specific intermediate-level language phenotyping (e.g., phonological processing using the CTOPP), although this has not been fully evaluated. Interestingly, M100 delays seem specific to ASD and are not evident in specific language impairment (SLI) [32]. Thus, currently, it appears that the M50/M100 delays may indicate atypical auditory processing and may indeed underlie clinical language impairments, but a direct and striking correlational mapping between simple M50/M100 latency and ultimate high-level behavioral language performance is not present.
Beyond a diagnostic and potentially prognostic role, M50/M100 latency delays also show the translational promise of signaling treatment efficacy in pre-clinical models. Prenatal valproic acid insult in rodents can recapitulate key aspects of ASD in the offspring, including several auditory related alterations such as auditory cortex (N1) latency delays [6]. In this rodent model, behavioral training can recover otherwise delayed auditory P1 (analogous to M50) latencies [33]. Similar changes have been found in children with ASD, with behavioral training recovering N1 (EEG equivalent of M100) latencies in children with ASD, though care should be taken due to the extremely small number of participants [34]. Partial support for this finding arises from studies on other sensory modalities, where recovery of typical event-related potential (ERP) latencies through the Early Start Denver Model (a high intensity, applied behavioral analysis-based intervention focusing on social and emotion domains shown to be effective in a randomized clinical trial [35]) was concurrent with behavioral improvements, though in a larger sample cohort [36]. Interestingly, recent pre-clinical work now suggests a link between N1 latency and sociability in several studies [37,38], though similar associations of M100 and behavioral measures of ASD have yet to be thoroughly studied clinically (Table 2).
Table 2. Summary of MEG based M50/M100 findings in ASD, and their relation to structural and behavioral measures.
| Study | M50 | M100 | Correlate to Age | Correlate to IQ | Correlate to Language Impairment | Correlate to White Matter Microstructure |
| Gage et al., 2003 [21] | Not Reported | Reduced dynamic range of M100 latency to different frequencies in the right hemisphere | Within-subject-normalized M100 latencies were not modulated by age (ANOVA) | Not Tested | Not Tested | Not Tested |
| Gage et al., 2003 [20] | Not Tested | M100 latency is prolonged in ASD | Altered maturation of M100 in right hemisphere | Not Tested | Not Tested | Not Tested |
| Tecchio et al., 2003 [22] | Not Tested | No M100 differences exhibited between TD and ASD | M100 decreased as a function of age (only 1 hemisphere reported) | Not Tested | Not Tested | Not Tested |
| Oram Cardy et al., 2004 [23] | No differences exhibited between TD and ASD | No differences exhibited between TD and ASD | Both TD and ASD children have delayed M50/M100 latencies compare to healthy adults | Not Tested | Not Tested | Not Tested |
| Roberts et al., 2010 [29] | No resolvable difference in latency or strength | Delayed M100 in right hemisphere of ASD | Age correlated to M100 latency in TD only | No relationship between cognitive ability and M100 latency | No relationship between language ability and M100 latency | Not Tested |
| Roberts et al., 2013 [24] | 10% delayed M50 when averaged across hemisphere | Not Reported | ASD and TD both show maturation, with no difference in slope of fit, but with different intercepts | No relationship between cognitive ability and M100 latency | No relationship between language ability and M100 latency | FA and M50 latency negatively correlate in TD only |
| Edgar et al., 2013 [62] | Not Tested | 10% delay in ASD in right hemisphere | Age predicts M100 latency | Not Tested | Not Tested | Not Tested |
| Edgar et al., 2014 [30] | Delayed M50 for both ASD+/-LI (Language Impairment) | M100 detected less often in ASD +LI than TD in younger children (6-10yrs old). In older subject (11-15 years old) groups, ASD-LI has more responses than ASD+LI. M100 delayed in ASD | Age predicts M50/100 latency | No relationship between cognitive ability and M100 latency | No relationship between language ability and M100 latency | Not Tested |
| Oram Cardy et al., 2008 [31] | Not Reported | Not Reported | Left hemisphere M50/M100 predicted age | No relationship between cognitive ability and M100 latency | Right hemisphere M50 and to less extent M100 predicts language ability, especially true for receptive language | Not Tested |
| Roberts et al., 2012 [32] | Not Tested | M100 not delayed in SLI | Not Tested | No relationship between cognitive ability and M100 latency | Not Tested | Not Tested |
Finally, in fulfillment of a biomarker’s plausible biological basis, early event-related potential/field (ERP/ERF) latency delays could, in principle, arise from multiple mechanisms. Currently, the best experimentally supported hypotheses are either white matter alterations leading to poor signal conduction [24,39-41] or synaptic dysfunction [42-46] leading to poor signal transduction. Additional mechanisms and combinations can, of course, also be considered. White matter alterations not directly visible to electrophysiologic techniques can be probed using diffusion-sensitive MRI called diffusion tensor imaging (DTI). Such methods also afford a quantitative description of properties of the white matter microstructure, often represented in the terms “mean diffusivity (MD)” and “fractional anisotropy (FA).” These are then used to draw inferences about white matter developmental changes. While DTI indices such as FA are sometimes interpreted as measures of microstructural “integrity,” it should be borne in mind that there are several underlying metrics and their combination in summary measures such as FA can obscure findings. For example, although the thalamocortical white matter projections of children with ASD exhibit similar mean FA microstructural metrics to typically developing controls, the age-FA relationship evident in typical development [39] was not present in ASD. When compared directly to the M50 response latency, increased FA correlated with decreased M50 latency, suggesting a biophysical hypothesis linking white matter conduction velocity and cortical response timing; such a relationship was not observed in ASD [24]. However, a more in-depth investigation in Roberts et al. [24] showed that white matter maturation in ASD might still be occurring (as resolved in changing mean diffusivity or the eigenvalue components thereof), despite being unresolved in FA, but importantly through an atypical mechanism driven by atypical maturational trajectories of axial and radial diffusivity components [24].
As such, DTI supports the notion of ongoing, but atypical, maturation of the thalamocortical white matter, perhaps accounting for M50/M100 maturational trajectories showing age-related latency shortening, but nonetheless persistent delays with respect to typically developing controls. Further insight to the biological underpinnings of early ERP-related latency delays in ASD comes from subjects with 16p11.2 abnormalities. These subjects are at greater risk for ASD (15 percent of 16p11.2 alterations also demonstrating ASD [47]). A multi-site collaboration recently observed M100 latency delays in subjects with 16p11.2 deletions. Such delays were again observed in conjunction with white matter abnormalities [48], though the exact interpretation of such microstructure alterations is unclear, due to increases in the size of several anatomic structures and white matter in general. Interestingly, pre-clinical work with rodent models that recapitulates key aspects of ASD suggest that M100 latency delays are actually due to delays in anterior auditory field (AAF) rather than primary auditory cortex responses [46], spatial scales that are unresolvable by MEG [17]. This last finding may suggest that synaptic dysfunction may partially account for prolonged latencies, due to improper integration in auditory cortex.
Mismatch Negativity/Field (MMN/F): Predicts Language Impairment and ASD, though Less-Direct Biological Underpinning
Similar to the M50/M100 latency delays in response to simple auditory presentation of sinusoidal tones seen in ASD, children with ASD can also exhibit a latency delay of the auditory magnetic mismatch field [49]. The magnetic mismatch field (MMF) and its EEG counterpart, mismatch negativity (MMN), refer to the resultant ERP derived from subtracting a standard or frequent stimulus’ ERP from that of a rare or novel stimulus [50] and essentially representing the “difference wave” as an index of “change detection.” To perform such an experiment, a stream of standard stimuli are presented, with irregular interruption by the deviant stimuli (e.g., /a//a//a//a//u//a/). Responses for the standard stimuli and deviant stimuli are separately averaged into ERPs, and then the standard stimulus ERP is subtracted from the deviant stimulus ERP. The MMF/N measure is sensitive to changing stimulus parameters, for example, a phoneme category boundary being crossed between two stimuli [50]. This signature exhibits face validity, since change detection is critical for the accurate processing of speech, and, moreover, language impairment is a commonly observed feature of ASD [51]. The MMF/N latency deficit is not, however, dependent on the use of language stimuli (i.e., vowels); delays are also observed to sinusoidal tones [49], implicating an auditory processing sensitivity. Furthermore, the MMF delay [38] is in addition to any intrinsic ASD-related delay in the earlier M100 component.
Analogous alterations to MMF latencies have been replicated using different paradigms [52], though the exact nature of the alteration is not stationary; for example, the MMF is sometimes missing in ASD populations [22]. Similar results have been exhibited via EEG, though they are not always consistent [53]. For instance, Gomot and colleagues have found shortened MMF latencies using EEG, although it is not clear if the difference is due to the use of higher frequency stimuli than aforementioned reported studies with findings of prolongation of latencies or small sample sizes [54,55]. Others have also found no differences in MMF latency when using multiple stimuli types, but in small sample populations [56].
Interestingly, from the view of a biomarker, MMF latency delays scales within the ASD cohort with clinical measures of language ability and is a sensitive and specific predictor of language impairment [10]. When compared to another MEG signature (e.g., M100 latency delay) or a structural imaging marker, MMF timing alterations have a better accuracy for both predicting both ASD and also language impairment within ASD [57]. The specificity of MMF to ASD-related language impairment is in question, though, as selective MMF delays also exist in children who demonstrate language impairment but are not on the autism spectrum [32].
When considering MMN/F alterations, it is necessary to keep in mind results from a similar paradigm: rapid temporal processing. This paradigm consists of two rapidly presented tones (e.g., 150ms apart), and studies have demonstrated that the earlier components of the second tone are less likely to be exhibited by language-impaired children regardless of ASD diagnosis as compared to children with no language perturbations (typically developing and ASD) [58]. The specificity of this may also be called into question with dyslexic adults demonstrating weaker amplitude responses [59], although its sensitivity for impaired language function is supported. As such, it may be that both rapid temporal processing measures and MMF/N are best considered markers of language domains and not specific for clinical labels.
What remains unknown is the biological basis for the MMF/N and how this might be related to the biological perturbations frequently exhibited by those with ASD. Addressing this indirectly, however, there is emerging evidence from DTI studies that white matter microstructure of the left hemisphere arcuate fasciculus (a key part of the language pathway) also exhibits atypicalities in ASD and indeed as a function of language impairment. Visual comparison of quantitative associations between MMF latency and clinical language assessment on the one hand and white matter microstructural metrics versus clinical language assessment on the other are strikingly analogous (Figure 2). A direct hypothesized mechanism for the biological coupling of the white matter and cortical measures is not yet available, in contrast to the M50/M100 and thalamocortical white matter association discussed above.
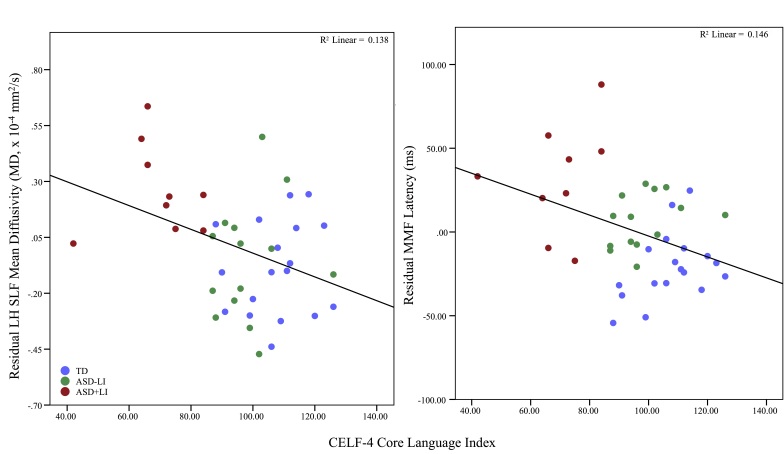
Figure 2.
Mismatch fields and microstructure of arcuate fasciculus demonstrate qualitatively similar relationships to language ability. Age corrected mean diffusivity of the arcuate fasciculus (left) and age corrected mismatch field latency latency (right) both show negative correlations to language ability as indexed via the CELF-4 core language index. No hypothesis currently directly links the latency of mismatch fields to the white matter microstructure of the arcuate fasciculus, in contrast to the M50/100 relationship to thalamocortical white matter microstructure. Nonetheless, a clear analogy is offered.
Gamma-Band Oscillatory Activity: Pervasive, Replicable, Predictive with a Specific Putative Biological Basis
A recent topic of increased study for ASD is Gamma-band oscillatory activity (Gamma [30-80 Hz]). While not specific to ASD [60], Gamma dysfunction (“oscillopathy”) may relate to certain core domains of symptomology, with the ultimately impacted system/domain dependent on the time of critical dysfunction or region involved [61]. Importantly, auditory response-related Gamma has been repeatedly demonstrated to be altered in ASD [6,62-66]. Evoked (phase-locked) Gamma power for both transient responses to simple sinusoidal tones and driven by 40Hz auditory steady state stimuli is reduced in ASD [65,66]. Concomitant with such phase-locked deficits, increased induced (non-phase locked activity) activity [64] has been observed, though this does not consistently achieve statistical significance [6]. Interestingly, during spoken word recognition, a reverse profile of Gamma dysfunction occurs compared to simpler stimuli; evoked Gamma is increased, and total (both phase and non-phase locked) Gamma power, which is driven by induced power changes, responses are diminished [63]. As such, the exact Gamma dysfunction exhibited seems dependent on stimulus type.
For both types of stimuli (simple/complex), relationships to symptomatology and/or core functional domains can be found. The total Gamma power elicited by the spoken word recognition task was positively correlated to figurative language abilities in controls, with qualitatively the opposite association exhibited in ASD. Separately, the transient and steady state Gamma responses that result from simpler stimuli both correlate to more basic functionality such as communication and symptom severity rating of ASD [62,65]. The relationship of Gamma in response to simpler stimuli and more basic functioning has an intuitive basis, since this Gamma dysfunction is in a lower level of sensory processing.
Gamma deficits are not specific to the auditory system; replicable high frequency oscillatory alterations have been found elsewhere (e.g., visual system [67,68], and at rest [69-71]), with some finding correlations of these Gamma perturbations to symptom severity [71]. Similar to the alterations seen at rest, pre-stimulus measures of Gamma are increased in ASD (sometimes considered evidence of a “noisy” brain), with elevated pre-stimulus Gamma corresponding to language abilities in ASD [62]. Together, such findings demonstrate an emerging coherent picture of the pervasiveness and importance of Gamma oscillopathy with regard to symptomatology and core domain dysfunction in ASD and its potential suitability as a biomarker. Moreover, in fulfillment of this status, Gamma functioning is also predictive of later outcome; infants’ frontal region Gamma not only correlates to current [72] but also future [73] cognitive and language abilities, both of which are core domains associated with ASD. Also during infancy, Gamma metrics are discriminative for risk status (low versus high) for ASD; two separate studies have demonstrated differential Gamma profiles in low risk (no sibling with ASD) versus high risk (at least one sibling with ASD) for autism [74,75]. Furthermore, after Tierney et al. removed all subjects that finally met criteria for ASD, difference still remained significant, suggesting that Gamma may be an endophenotype of ASD [59]. This is supported by work by Rojas and colleagues, where two studies have identified Gamma dysfunction in first-degree relatives of ASD [64,65]. While the identification of Gamma dysfunction in high-risk infants or first-degree relative of people with ASD may paradoxically undermine the use of Gamma as a diagnostic biomarker (since it is also altered in “unaffected”/undiagnosed relatives), a broad autism phenotype (BAP) has long been associated within first-degree relatives of people with ASD [76], suggesting a possible biological sensitivity that exceeds clinical resolution and categorization.
Lastly, Gamma-related metrics also hold the potential for measuring treatment efficacy, not only for synaptically targeted pharmaceuticals but also for behavioral interventions, as a reversal of Gamma perturbations coincided and correlated with effective intervention using Program for the Education and Enrichment of Relational Skills (PEERs) [77], a behavioral intervention utilizing parental assistance for the teaching of social functioning to teens with ASD, shown to be effective in a randomized controlled study [78].
Biological Evidence for Human Oscillopathies
Gamma is posited to depend on numerous constituents, including potassium channel subtypes, spike conductance trajectories, and a strong role for both glutamatergic and γ-Aminobutyric acid (GABA)-ergic signaling [79]. There are two widely accepted models for the mechanism of Gamma generation: the I-I model and E-I model. In both models, inhibitory feedback onto specific cells (interneurons and pyramidal cells, respectively, for each model) cause a temporary quiescence in population firing [79]. Therefore, Gamma is thought to arise from local circuit interactions that depend heavily on GABAergic/glutamatergic receptor-based kinetics [79]. Supporting this putative GABA-Gamma interaction are findings that relative cortical GABA levels correlate to Gamma in motor and visual cortex [80,81], and initial work by our lab and others suggest this is true for auditory cortex as well. Such emerging correlations are not without controversy, with at least one other laboratory showing no correlation between relative cortical GABA and Gamma [82]. The possibility of regional variability in this coupling also cannot be excluded in accounting for varying observations in the literature, along with the usual suspects of measurement precision and sample size.
With this link between GABA and the mechanistic underpinning of Gamma, and the clear Gamma alterations in ASD, it is not surprising that the balance of excitation and inhibition has been posed as a pathogenic mechanism for ASD [4]. In addition, there has been substantial data from human studies to suggest the existence of such an imbalance [83]. The high comorbidity of seizure disorders such as epilepsy in ASD also points to hyper-excitability and an imbalance in GABA and glutamate [84]. Indeed, the high co-occurrence of seizure disorders in ASD and the known glutamatergic/gabaergic basis of some, if not all, seizures may have led in considerable part to Rubenstein and Merzenich’s proposed model of E/I imbalance as a basis for ASD [4]. With respect to glutamate-related alterations, a GluR6 receptor subunit has been tied to ASD inheritance [85-87]. Subjects with ASD can also demonstrate increased expression of glutamate transmission-related messenger RNA (mRNA) and proteins, for example, Excitatory Amino Acid Transporters (EAAT) and α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) isotypes [88]. Additionally, Magnetic Resonance Spectroscopy (MRS) has demonstrated possible increased glutamate in the cortex of ASD individuals [89], although this last finding is not unambiguously interpreted because of the role of glutamate in other intracellular processes [90]. For inhibition, the 15q11 locus, which encodes many GABA signaling-related proteins, is deleted in as much as 3 percent of the ASD population [91]. Also, both GABA production enzymes (glutamic acid decarboxylase [GAD] 65 and GAD 67 and receptors [GABAA and GABAB]) have been found to be decreased in postmortem studies of subjects with ASD [92-94]. This is supported by several studies in which subjects with ASD exhibited decreased cortical GABA concentrations [42-44]. As such, Gamma activity in general, and oscillopathies in particular, seem not only supported with a biological mechanism, but this mechanism appears to be altered in ASD, enhancing the optimism of Gamma-based measures as candidate biomarkers.
Mouse Model Verification of the Biological Mechanisms of Intermediate Phenotypes: Clinical and Basic Research Informing Each Other
Recent work has shown that mouse models that recapitulate key aspects of ASD, whether due to prenatal environmental insult [6], pharmacological treatment [95], or genetic manipulation [37,96,97], demonstrate analogous perturbations to their auditory electrophysiological responses with both delayed early/middle latencies and aberrant oscillatory profile. Such alterations can be related to the synaptic/circuitry alterations exhibited by these mice. In mice treated prenatally with valproic acid, Gamma deficits are correlated to neuroligin-3 (NLGN3), a protein integral in the building and maintenance of synapses [6]. This same prenatal treatment also causes a hyper-connectivity and hyper-excitability within circuit functioning [98]. Lastly, when studied in the aforementioned mouse models, altered GABAergic cell expression is found. Mice treated prenatally with valproic acid display reductions in Parvalbumin containing (PV+) interneuron cells counts in [99], cells thought to be crucial in Gamma activity [100]. Such PV+ modulation is also observed in another animal model exhibiting stimulus-related Gamma activity deficits, with down-regulated PV+ cell count and protein expression in NR1neo-/- mice [97]. Interestingly, the NR1 neo-/- mouse model’s hippocampal pyramidal cells also exhibit intrinsic hyper-excitability, further demonstrating altered E/I balance [97]. These alterations to synaptic transmission and circuit parameters could lead not only to alterations to oscillatory activity, but also delays in ERP components. Figure 3 draws some analogies between human studies of ASD and various preclinical models probing aspects of ASD neurobiology.
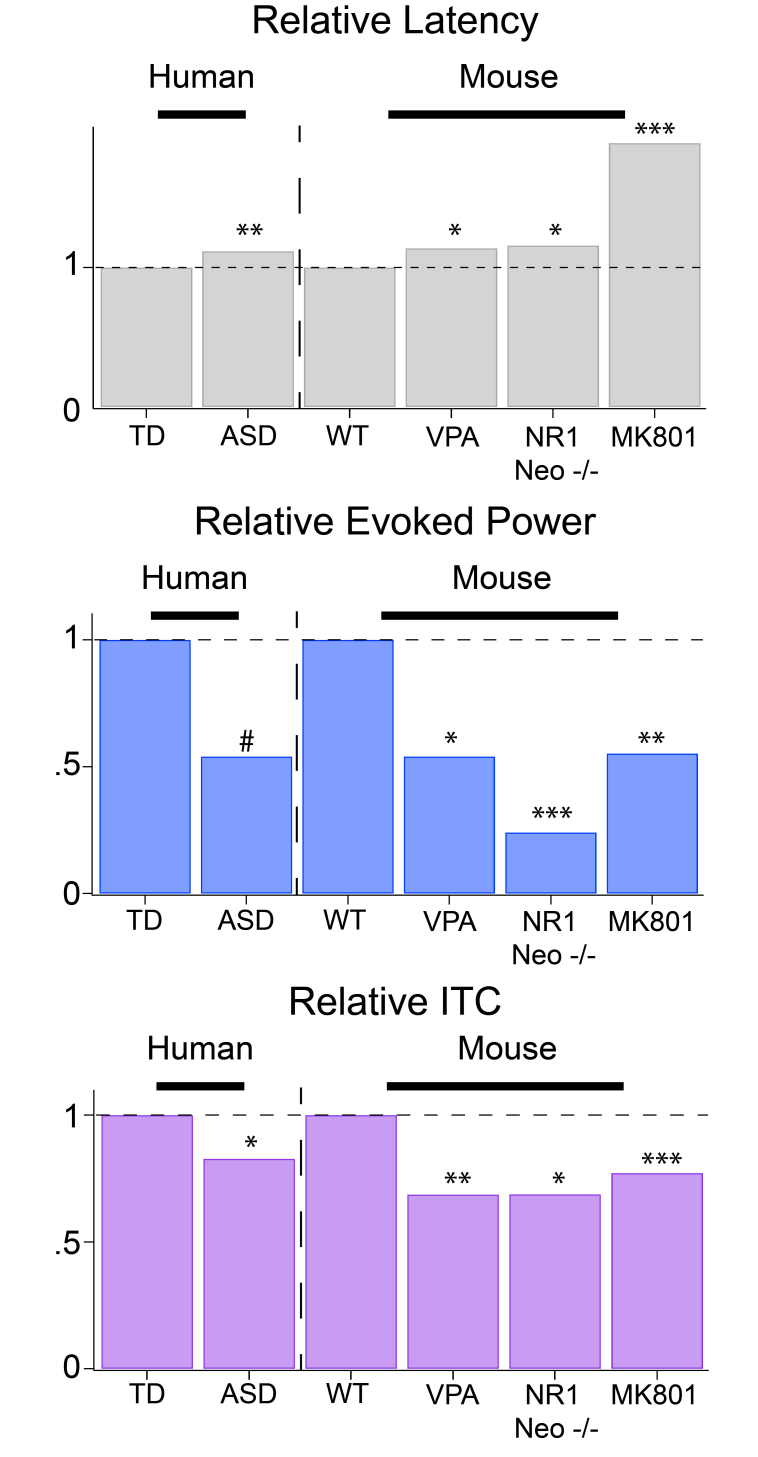
Figure 3.
Pre-clinical work mirrors that of clinical research for multiple biomarkers. M100 latency (top) delays seen in humans are mirrored in several mouse models derived from different mechanisms .The same mice also exhibit decreases in evoked GAMMA responses (middle) and GAMMA inter-trial coherence (ITC) (bottom). (VPA, prenatal insult; NR1,Neo-/-, genetic indult; MK801, pharmacological challenge). Adapted from Port et al. [61]. ***P < 0.001, **P < 0.01, *P < 0.05, #P < 0.1
Such pre-clinical work has also informed ongoing human studies. Several mouse models exhibit negative correlations between sociability and baseline Gamma-band activity [38,97]. This prompted work that showed similar results in children with ASD [62]. A putative link between baseline Gamma and sociability was demonstrated using optogenetics by Yizhar and colleagues, who demonstrated increased baseline Gamma-band activity due to increased excitatory drive, which coincided with a decrease in sociability; additionally, such a deficit could be reversed by recovering E/I balance [101]. Separately, others have demonstrated that pharmacological reversal of Gamma signal to noise deficits in a genetic insult model of ASD with GABA-B agonist treatment reversed behavior impairments [97]. Such recovery of symptomology coinciding with reversal of Gamma abnormalities is relevant and reflected in humans; for example, as mentioned above, Gamma-band function is partially recovered with PEERS intervention [77].
Conclusion and Outlook
MEG-based biomarkers display great potential for use in ASD. Not only are biomarkers presented here grounded in biology, each also have substantial support from the literature for their use. These MEG biomarkers may, in fact, be deemed more suitable for ASD than more invasive assays (i.e., blood-based), since the subject is only mildly inconvenienced. Moreover, compared to other imaging techniques, MEG is favorable due to the use of non-claustrophobia/anxiety inducing setups for which MRI-based techniques suffer due to the requirement of a magnetic bores and loud machine noise and minimal sensory contact as opposed to applying EEG head caps.
Although the current state of electrophysiological biomarkers in ASD is promising, there are still unresolved issues regarding the sensitivity and specificity of each candidate signature. Therefore, at the moment, the greatest promise may be toward a multivariate combination of such biomarkers [57]. The unresolved concerns for clinical specificity of such biomarkers may, in fact, reflect the nature of biological dysfunction and offer opportunities to investigate comorbidities and potentially redefine clinical categories. What these biomarkers may actually detect are core domain alterations and not ASD per se. As such, this would explain the occurrence of similar abnormalities in other disorders (e.g., Gamma alterations in bipolar [102] and schizophrenia [103]). This is not unprecedented; the new focus from the National Institute of Mental Health on Research Domain Criteria encourages testing the relationship between biomarkers and specific cognitive and sensory domains even if the biomarkers are not selective for traditionally defined disorders. While further study is needed to perfect these biomarkers to the point of clinical use, promise is nevertheless exhibited even in these early stages. Indeed, this approach may be more clinically relevant in disorders as heterogeneous as ASD as roles extend beyond diagnosis and prognosis toward stratification (sub-population definition) and monitoring of treatment response. For instance, if a biomarker could stratify subjects with ASD based on certain biological alterations (i.e., poor synaptic function as revealed by Gamma alterations versus poor signal transmission as reveled by white matter alterations), new sub-types of ASD (those with/without Gamma and/or white matter alterations) could be defined. Then treatments targeted to those alterations could be implemented and monitored for efficacy.
Acknowledgments
This work was supported in part by grants (TR) from NIH R01DC008871 and P30HD026979 and the Nancy Lurie Marks Family Foundation and a pre-doctoral fellowship for RGP from the Autism Science Foundation. Dr Roberts is grateful to the Oberkircher family for the Oberkircher Family Endowed Chair in Pediatric Radiology at CHOP.
Abbreviations
- ASD
autism spectrum disorders
- biomarker
biologically based marker
- EEG
electroencephalography
- MEG
magnetoencephalography
- MRI
magnetic resonance imaging
- fMRI
functional magnetic resonance imaging
- PET
positron emission tomography
- ERP
event-related potential
- ERF
event-related field
- EMG
electromyography
- DTI
diffusion tensor imaging
- FA
fractional anisotropy
- MMF
magnetic mismatch field
- MMN
mismatch negativity
- Gamma
30-80 Hz oscillatory activity
- GABA
γ-Aminobutyric acid
- mRNA
messenger RNA
- EAAT
excitatory amino acid transporters
- AMPA
α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- GAD65/67
glutamic acid decarboxylase
- MRS
magnetic resonance spectroscopy
- NLGN3
neuroligin-3
- PV
parvalbumin
- PEERS
Program for the Education and Enrichment of Relational Skills
Conflict of interest
TPLR currently is a consultant for Prism Clinical Imaging and has given talks on behalf of Siemens Medical Solutions and Elekta. He has received research grant support in the past from Seaside Therapeutics. All other authors are free of conflicts of interest.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th edition. Washington, DC: American Psychiatric Publishing; 2013. [Google Scholar]
- Developmental Disabilities Monitoring Network Surveillance Year 2010 Principal Investigators; Centers for Disease Control and Prevention (CDC) Prevalence of autism spectrum disorder among children aged 8 years — autism and developmental disabilities monitoring network, 11 sites, United States, 2010. MMWR Surveill Summ. 2014;63(2):1–21. [PubMed] [Google Scholar]
- Pardo CA, Eberhart CG. The neurobiology of autism. Brain Pathol. 2007;17(4):434–447. doi: 10.1111/j.1750-3639.2007.00102.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubenstein JLR, Merzenich MM. Model of autism: increased ratio of excitation/inhibition in key neural systems. Genes Brain Behav. 2003;2(5):255–267. doi: 10.1034/j.1601-183x.2003.00037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belmonte MK, Allen G, Beckel-Mitchener A, Boulanger LM, Carper RA, Webb SJ. Autism and abnormal development of brain connectivity. J Neurosci. 2004;24(42):9228–9231. doi: 10.1523/JNEUROSCI.3340-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandal MJ, Edgar JC, Ehrlichman RS, Mehta M, Roberts TPL, Siegel SJ. Validating γ oscillations and delayed auditory responses as translational biomarkers of autism. Biol Psychiatry. 2010;68(12):1100–1106. doi: 10.1016/j.biopsych.2010.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson GM, Horne WC, Chatterjee D, Cohen DJ. The hyperserotonemia of autism. Ann NY Acad Sci. 1990;600:331-40; discussion 341-. doi: 10.1111/j.1749-6632.1990.tb16893.x. [DOI] [PubMed] [Google Scholar]
- Ingalhalikar M, Parker D, Bloy L, Roberts TPL, Verma R. Diffusion based abnormality markers of pathology: toward learned diagnostic prediction of ASD. Neuroimage. 2011;57(3):918–927. doi: 10.1016/j.neuroimage.2011.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chugani DC, Muzik O, Behen M, Rothermel R, Janisse JJ, Lee J. et al. Developmental changes in brain serotonin synthesis capacity in autistic and nonautistic children. Ann Neurol. 1999;45(3):287–295. doi: 10.1002/1531-8249(199903)45:3<287::aid-ana3>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Roberts TPL, Cannon KM, Tavabi K, Blaskey L, Khan SY, Monroe JF. et al. Auditory magnetic mismatch field latency: a biomarker for language impairment in autism. Biol Psychiatry. 2011;70(3):263–269. doi: 10.1016/j.biopsych.2011.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dani VS, Chang Q, Maffei A, Turrigiano GG, Jaenisch R, Nelson SB. Reduced cortical activity due to a shift in the balance between excitation and inhibition in a mouse model of Rett syndrome. Proc Natl Acad Sci USA. 2005;102(35):12560–12565. doi: 10.1073/pnas.0506071102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas DC, Wilson LB. γ-band abnormalities as markers of autism spectrum disorders. Biomark Med. 2014;8(3):353–368. doi: 10.2217/bmm.14.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice K, Moriuchi JM, Jones W, Klin A. Parsing heterogeneity in autism spectrum disorders: visual scanning of dynamic social scenes in school-aged children. J Am Acad Child Adolesc Psychiatry. 2012;51(3):238–248. doi: 10.1016/j.jaac.2011.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts TPL, Schmidt GL, Egeth M, Blaskey L, Rey MM, Edgar JC. et al. Electrophysiological signatures: magnetoencephalographic studies of the neural correlates of language impairment in autism spectrum disorders. Int J Psychophysiol. 2008;68(2):149–160. doi: 10.1016/j.ijpsycho.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ecker C, Suckling J, Deoni SC, Lombardo MV, Bullmore ET, Baron-Cohen S. et al. Brain anatomy and its relationship to behavior in adults with autism spectrum disorder: a multicenter magnetic resonance imaging study. Arch Gen Psychiatry. 2012;69(2):195–209. doi: 10.1001/archgenpsychiatry.2011.1251. [DOI] [PubMed] [Google Scholar]
- Lange N, Dubray MB, Lee JE, Froimowitz MP, Froehlich A, Adluru N. et al. Atypical diffusion tensor hemispheric asymmetry in autism. Autism Res. 2010;3(6):350–358. doi: 10.1002/aur.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leahy RM, Mosher JC, Spencer ME, Huang MX, Lewine JD. A study of dipole localization accuracy for MEG and EEG using a human skull phantom. Electroencephalogr Clin Neurophysiol. 1998;107(2):159–173. doi: 10.1016/s0013-4694(98)00057-1. [DOI] [PubMed] [Google Scholar]
- Muthukumaraswamy SD. High-frequency brain activity and muscle artifacts in MEG/EEG: a review and recommendations. Front Hum Neurosci. 2013;7:138. doi: 10.3389/fnhum.2013.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuval-Greenberg S, Tomer O, Keren AS, Nelken I, Deouell LY. Transient induced gamma-band response in EEG as a manifestation of miniature saccades. Neuron. 2008;58(3):429–441. doi: 10.1016/j.neuron.2008.03.027. [DOI] [PubMed] [Google Scholar]
- Gage NM, Siegel B, Roberts TPL. Cortical auditory system maturational abnormalities in children with autism disorder: an MEG investigation. Brain Res Dev Brain Res. 2003;144(2):201–209. doi: 10.1016/s0165-3806(03)00172-x. [DOI] [PubMed] [Google Scholar]
- Gage NM, Siegel B, Callen M, Roberts TPL. Cortical sound processing in children with autism disorder: an MEG investigation. Neuroreport. 2003;14(16):2047–2051. doi: 10.1097/00001756-200311140-00008. [DOI] [PubMed] [Google Scholar]
- Tecchio F, Benassi F, Zappasodi F, Gialloreti LE, Palermo M, Seri S. et al. Auditory sensory processing in autism: a magnetoencephalographic study. Biol Psychiatry. 2003;54(6):647–654. doi: 10.1016/s0006-3223(03)00295-6. [DOI] [PubMed] [Google Scholar]
- Oram Cardy JE, Ferrari P, Flagg EJ, Roberts W, Roberts TPL. Prominence of M50 auditory evoked response over M100 in childhood and autism. Neuroreport. 2004;15(12):1867–1870. doi: 10.1097/00001756-200408260-00006. [DOI] [PubMed] [Google Scholar]
- Roberts TPL, Lanza MR, Dell J, Qasmieh S, Hines K, Blaskey L. et al. Maturational differences in thalamocortical white matter microstructure and auditory evoked response latencies in autism spectrum disorders. Brain Res. 2013;1537:79–85. doi: 10.1016/j.brainres.2013.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts TP, Poeppel D. Latency of auditory evoked M100 as a function of tone frequency. Neuroreport. 1996;7(6):1138–1140. doi: 10.1097/00001756-199604260-00007. [DOI] [PubMed] [Google Scholar]
- Stufflebeam SM, Poeppel D, Rowley HA, Roberts TP. Peri-threshold encoding of stimulus frequency and intensity in the M100 latency. Neuroreport. 1998;9(1):91–94. doi: 10.1097/00001756-199801050-00018. [DOI] [PubMed] [Google Scholar]
- Roberts TP, Ferrari P, Stufflebeam SM, Poeppel D. Latency of the auditory evoked neuromagnetic field components: stimulus dependence and insights toward perception. J Clin Neurophysiol. 2000;17(2):114–129. doi: 10.1097/00004691-200003000-00002. [DOI] [PubMed] [Google Scholar]
- Jenkins J, Chow V, Blaskey L, Kuschner E, Qasmieh S, Gaetz L. et al. Auditory Evoked M100 Response Latency Is Delayed in Children with 16p11.2 Deletion but not 16p11.2 Duplication. Cereb Cortex. 2014 doi: 10.1093/cercor/bhv008. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts TPL, Khan SY, Rey M, Monroe JF, Cannon K, Blaskey L. et al. MEG detection of delayed auditory evoked responses in autism spectrum disorders: towards an imaging biomarker for autism. Autism Res. 2010;3(1):8–18. doi: 10.1002/aur.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar JC, Lanza MR, Daina AB, Monroe JF, Khan SY, Blaskey L. et al. Missing and delayed auditory responses in young and older children with autism spectrum disorders. Front Hum Neurosci. 2014;8:417. doi: 10.3389/fnhum.2014.00417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oram Cardy JE, Flagg EJ, Roberts W, Roberts TPL. Auditory evoked fields predict language ability and impairment in children. Int J Psychophysiol. 2008;68(2):170–175. doi: 10.1016/j.ijpsycho.2007.10.015. [DOI] [PubMed] [Google Scholar]
- Roberts TPL, Heiken K, Kahn SY, Qasmieh S, Blaskey L, Solot C. et al. Delayed magnetic mismatch negativity field, but not auditory M100 response, in specific language impairment. Neuroreport. 2012;23(8):463–468. doi: 10.1097/WNR.0b013e32835202b6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engineer CT, Centanni TM, Im KW, Kilgard MP. Speech sound discrimination training improves auditory cortex responses in a rat model of autism. Front Syst Neurosci. 2014;8:1–10. doi: 10.3389/fnsys.2014.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo NM, Hornickel J, Nicol T, Zecker S, Kraus N. Biological changes in auditory function following training in children with autism spectrum disorders. Behav Brain Funct. 2010;6:60. doi: 10.1186/1744-9081-6-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson G, Rogers S, Munson J, Smith M, Winter J, Greenson J. et al. Randomized, controlled trial of an intervention for toddlers with autism: the Early Start Denver Model. Pediatrics. 2010;125(1):e17–e23. doi: 10.1542/peds.2009-0958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson G, Jones EJH, Merkle K, Venema K, Lowy R, Faja S. et al. Early behavioral intervention is associated with normalized brain activity in young children with autism. J Am Acad Child Adolesc Psychiatry. 2012;51(11):1150–1159. doi: 10.1016/j.jaac.2012.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders JA, Tatard-Leitman VM, Suh J, Billingslea EN, Roberts TP, Siegel SJ. Knockout of NMDA receptors in parvalbumin interneurons recreates autism-like phenotypes. Autism Res. 2013;6(2):69–77. doi: 10.1002/aur.1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billingslea EN, Tatard-Leitman VM, Anguiano J, Jutzeler CR, Suh J, Saunders JA. et al. Parvalbumin cell ablation of NMDA-R1 causes increased resting network excitability with associated social and self-care deficits. Neuropsychopharmacology. 2014;39(7):1603–1613. doi: 10.1038/npp.2014.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts TPL, Khan SY, Blaskey L, Dell J, Levy SE, Zarnow DM. et al. Developmental correlation of diffusion anisotropy with auditory-evoked response. Neuroreport. 2009;20(18):1586–1591. doi: 10.1097/WNR.0b013e3283306854. [DOI] [PubMed] [Google Scholar]
- Stufflebeam SM, Witzel T, Mikulski S, Hämäläinen MS, Temereanca S, Barton JJS. et al. A non-invasive method to relate the timing of neural activity to white matter microstructural integrity. Neuroimage. 2008;42(2):710–716. doi: 10.1016/j.neuroimage.2008.04.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dockstader C, Gaetz W, Rockel C, Mabbott DJ. White matter maturation in visual and motor areas predicts the latency of visual activation in children. Hum Brain Mapp. 2012;33(1):179–191. doi: 10.1002/hbm.21203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada M, Taki MM, Nose A, Kubo H, Mori K, Nishitani H. et al. Non-invasive evaluation of the GABAergic/glutamatergic system in autistic patients observed by MEGA-editing proton MR spectroscopy using a clinical 3 tesla instrument. J Autism Dev Disord. 2011;41(4):447–454. doi: 10.1007/s10803-010-1065-0. [DOI] [PubMed] [Google Scholar]
- Rojas DC, Singel D, Steinmetz S, Hepburn S, Brown MS. Decreased left perisylvian GABA concentration in children with autism and unaffected siblings. Neuroimage. 2014;86:28–34. doi: 10.1016/j.neuroimage.2013.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaetz W, Bloy L, Wang DJ, Port RG, Blaskey L, Levy SE. et al. GABA estimation in the brains of children on the autism spectrum: measurement precision and regional cortical variation. Neuroimage. 2014;86:1–9. doi: 10.1016/j.neuroimage.2013.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee A, García-Oscos F, Roychowdhury S, Galindo LC, Hall S, Kilgard MP. et al. Impairment of cortical GABAergic synaptic transmission in an environmental rat model of autism. Int J Neuropsychopharmacol. 2013;16(6):1309–1318. doi: 10.1017/S1461145712001216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engineer CT, Centanni TM, Im KW, Borland MS, Moreno NA, Carraway RS. et al. Degraded auditory processing in a rat model of autism limits the speech representation in non-primary auditory cortex. Dev Neurobiol. 2014;74(10):972–986. doi: 10.1002/dneu.22175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zufferey F, Sherr EH, Sherr ND, Hanson E, Maillard AM, Hippolyte L. et al. A 600 kb deletion syndrome at 16p11.2 leads to energy imbalance and neuropsychiatric disorders. J Med Genet. 2012;49(10):660–668. doi: 10.1136/jmedgenet-2012-101203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen JP, Chang YS, Pojman NJ, Bukshpun P, Wakahiro MLJ, Marco EJ. et al. Aberrant white matter microstructure in children with 16p11.2 deletions. J Neurosci. 2014;34(18):6214–6223. doi: 10.1523/JNEUROSCI.4495-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oram Cardy JE, Flagg EJ, Roberts W, Roberts TPL. Delayed mismatch field for speech and non-speech sounds in children with autism. Neuroreport. 2005;16(5):521–525. doi: 10.1097/00001756-200504040-00021. [DOI] [PubMed] [Google Scholar]
- Näätänen R. The perception of speech sounds by the human brain as reflected by the mismatch negativity (MMN) and its magnetic equivalent (MMNm) Psychophysiology. 2001;38(1):1–21. doi: 10.1017/s0048577201000208. [DOI] [PubMed] [Google Scholar]
- Tager-Flusberg H, Joseph R, Folstein S. Current directions in research on autism. Ment Retard Dev Disabil Res Rev. 2001;7(1):21–29. doi: 10.1002/1098-2779(200102)7:1<21::AID-MRDD1004>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Kasai K, Hashimoto O, Kawakubo Y, Yumoto M, Kamio S, Itoh K. et al. Delayed automatic detection of change in speech sounds in adults with autism: a magnetoencephalographic study. Clin Neurophysiol. 2005;116(7):1655–1664. doi: 10.1016/j.clinph.2005.03.007. [DOI] [PubMed] [Google Scholar]
- O’Connor K. Auditory processing in autism spectrum disorder: a review. Neurosci Biobehav Rev. 2012;36(2):836–854. doi: 10.1016/j.neubiorev.2011.11.008. [DOI] [PubMed] [Google Scholar]
- Gomot M, Giard M-H, Adrien J-L, Barthelemy C, Bruneau N. Hypersensitivity to acoustic change in children with autism: Electrophysiological evidence of left frontal cortex dysfunctioning. Psychophysiology. 2002;39(5):577–584. doi: 10.1017.S0048577202394058. [DOI] [PubMed] [Google Scholar]
- Gomot M, Blanc R, Clery H, Roux S, Barthelemy C, Bruneau N. Candidate electrophysiological endophenotypes of hyper-reactivity to change in autism. J Autism Dev Disord. 2011;41(6):705–714. doi: 10.1007/s10803-010-1091-y. [DOI] [PubMed] [Google Scholar]
- Ceponiene R, Lepistö T, Shestakova A, Vanhala R, Alku P, Näätänen R. et al. Speech-sound-selective auditory impairment in children with autism: they can perceive but do not attend. Proc Natl Acad Sci USA. 2003;100(9):5567–5572. doi: 10.1073/pnas.0835631100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingalhalikar M, Parker WA, Bloy L, Roberts TPL, Verma R. Creating multimodal predictors using missing data: Classifying and subtyping autism spectrum disorder. J Neurosci Methods. 2014;235:1–9. doi: 10.1016/j.jneumeth.2014.06.030. [DOI] [PubMed] [Google Scholar]
- Oram Cardy JE, Flagg EJ, Roberts W, Brian J, Roberts TPL. Magnetoencephalography identifies rapid temporal processing deficit in autism and language impairment. Neuroreport. 2005;16(4):329–332. doi: 10.1097/00001756-200503150-00005. [DOI] [PubMed] [Google Scholar]
- Nagarajan S, Mahncke H, Salz T, Tallal P, Roberts T, Merzenich MM. Cortical auditory signal processing in poor readers. Proc Natl Acad Sci USA. 1999;96(11):6483–6488. doi: 10.1073/pnas.96.11.6483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann CS, Demiralp T. Human EEG gamma oscillations in neuropsychiatric disorders. Clin Neurophysiol. 2005;116(12):2719–2733. doi: 10.1016/j.clinph.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Port RG, Gandal MJ, Roberts TPL, Siegel SJ, Carlson GC. Convergence of circuit dysfunction in ASD: a common bridge between diverse genetic and environmental risk factors and common clinical electrophysiology. Front Cell Neurosci. 2014;8:1–14. doi: 10.3389/fncel.2014.00414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar JC, Khan SY, Blaskey L, Chow VY, Rey M, Gaetz W. et al. Neuromagnetic Oscillations Predict Evoked-Response Latency Delays and Core Language Deficits in Autism Spectrum Disorders. J Autism Dev Disord. 2013 Aug 21; doi: 10.1007/s10803-013-1904-x. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden KL, Hepburn S, Winterrowd E, Schmidt GL, Rojas DC. Abnormalities in gamma-band responses to language stimuli in first-degree relatives of children with autism spectrum disorder: an MEG study. BMC Psychiatry. 2012;12(1):213. doi: 10.1186/1471-244X-12-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas DC, Maharajh K, Teale P, Rogers SJ. Reduced neural synchronization of gamma-band MEG oscillations in first-degree relatives of children with autism. BMC Psychiatry. 2008;8:66. doi: 10.1186/1471-244X-8-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas DC, Teale P, Maharajh K, Kronberg E, Youngpeter K, Wilson LB. et al. Transient and steady-state auditory gamma-band responses in first-degree relatives of people with autism spectrum disorder. Mol Autism. 2011;2(1):11. doi: 10.1186/2040-2392-2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson TW, Rojas DC, Reite ML, Teale PD, Rogers SJ. Children and adolescents with autism exhibit reduced MEG steady-state gamma responses. Biol Psychiatry. 2007;22(3):192–197. doi: 10.1016/j.biopsych.2006.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grice SJ, Spratling MW, Karmiloff-Smith A, Halit H, Csibra G, de Haan M. et al. Disordered visual processing and oscillatory brain activity in autism and Williams syndrome. Neuroreport. 2001;12(12):2697–2700. doi: 10.1097/00001756-200108280-00021. [DOI] [PubMed] [Google Scholar]
- Sun L, Grützner C, Bölte S, Wibral M, Tozman T, Schlitt S. et al. Impaired gamma-band activity during perceptual organization in adults with autism spectrum disorders: evidence for dysfunctional network activity in frontal-posterior cortices. J Neurosci. 2012;32(28):9563–9573. doi: 10.1523/JNEUROSCI.1073-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornew L, Roberts TPL, Blaskey L, Edgar JC. Resting-state oscillatory activity in autism spectrum disorders. J Autism Dev Disord. 2012;42(9):1884–1894. doi: 10.1007/s10803-011-1431-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orekhova EV, Stroganova TA, Nygren G, Tsetlin MM, Posikera IN, Gillberg C. et al. Excess of high frequency electroencephalogram oscillations in boys with autism. Biol Psychiatry. 2007;62(9):1022–1029. doi: 10.1016/j.biopsych.2006.12.029. [DOI] [PubMed] [Google Scholar]
- Maxwell CR, Villalobos ME, Schultz RT, Herpertz-Dahlmann B, Konrad K, Kohls G. Atypical Laterality of Resting Gamma Oscillations in Autism Spectrum Disorders. J Autism Dev Disord. 2013 doi: 10.1007/s10803-013-1842-7. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benasich AA, Gou Z, Choudhury N, Harris KD. Early cognitive and language skills are linked to resting frontal gamma power across the first 3 years. Behav Brain Res. 2008;195(2):215–222. doi: 10.1016/j.bbr.2008.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gou Z, Choudhury N, Benasich AA. Resting frontal gamma power at 16, 24 and 36 months predicts individual differences in language and cognition at 4 and 5 years. Behav Brain Res. 2011;220(2):263–270. doi: 10.1016/j.bbr.2011.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tierney AL, Gabard-Durnam L, Vogel-Farley V, Tager-Flusberg H, Nelson CA. Developmental trajectories of resting EEG power: an endophenotype of autism spectrum disorder. PLoS One. 2012;7(6):e39127. doi: 10.1371/journal.pone.0039127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsabbagh M, Volein A, Csibra G, Holmboe K, Garwood H, Tucker L. et al. Neural correlates of eye gaze processing in the infant broader autism phenotype. Biol Psychiatry. 2009;65(1):31–38. doi: 10.1016/j.biopsych.2008.09.034. [DOI] [PubMed] [Google Scholar]
- Piven J, Palmer P, Landa R, Santangelo S, Jacobi D, Childress D. Personality and Language Characteristics in Parents From Multiple-Incidence Autism Families. Am J Med Genet. 1997;74(4):398–411. [PubMed] [Google Scholar]
- Van Hecke AV, Stevens S, Carson AM, Karst JS, Dolan B, Schohl K. et al. Measuring the Plasticity of Social Approach: A Randomized Controlled Trial of the Effects of the PEERS Intervention on EEG Asymmetry in Adolescents with Autism Spectrum Disorders. J Autism Dev Disord. 2013 June 28; doi: 10.1007/s10803-013-1883-y. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Laugeson EA, Frankel F, Mogil C, Dillon AR. Parent-assisted social skills training to improve friendships in teens with autism spectrum disorders. J Autism Dev Disord. 2009;39(4):596–606. doi: 10.1007/s10803-008-0664-5. [DOI] [PubMed] [Google Scholar]
- Buzsáki G, Wang X-J. Mechanisms of gamma oscillations. Annu Rev Neurosci. 2012;35:203–225. doi: 10.1146/annurev-neuro-062111-150444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaetz W, Edgar JC, Wang DJ, Roberts TPL. Relating MEG measured motor cortical oscillations to resting γ-aminobutyric acid (GABA) concentration. Neuroimage. 2011;55(2):616–621. doi: 10.1016/j.neuroimage.2010.12.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthukumaraswamy SD, Edden RAE, Jones DK, Swettenham JB, Singh KD. Resting GABA concentration predicts peak gamma frequency and fMRI amplitude in response to visual stimulation in humans. Proc Natl Acad Sci USA. 2009;106(20):8356–8361. doi: 10.1073/pnas.0900728106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousijn H, Haegens S, Wallis G, Near J, Stokes MG, Harrison PJ. et al. Resting GABA and glutamate concentrations do not predict visual gamma frequency or amplitude. Proc Natl Acad Sci USA. 2014;111(25):9301–9306. doi: 10.1073/pnas.1321072111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coghlan S, Horder J, Inkster B, Mendez MA, Murphy DG, Nutt DJ. GABA system dysfunction in autism and related disorders: from synapse to symptoms. Neurosci Biobehav Rev. 2012;36(9):2044–2055. doi: 10.1016/j.neubiorev.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks-Kayal A. Epilepsy and autism spectrum disorders: are there common developmental mechanisms? Brain Dev. 2010;32(9):731–738. doi: 10.1016/j.braindev.2010.04.010. [DOI] [PubMed] [Google Scholar]
- Jamain S, Betancur C, Quach H, Philippe A, Fellous M, Giros B. et al. Linkage and association of the glutamate receptor 6 gene with autism. Mol Psychiatry. 2002;7(3):302–310. doi: 10.1038/sj.mp.4000979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuang M, Liu J, Jia MX, Yang JZ, Wu SP, Gong XH. et al. Family-based association study between autism and glutamate receptor 6 gene in Chinese Han trios. Am J Med Genet B Neuropsychiatr Genet. 2004;131B(1):48–50. doi: 10.1002/ajmg.b.30025. [DOI] [PubMed] [Google Scholar]
- Strutz-Seebohm N, Korniychuk G, Schwarz R, Baltaev R, Ureche ON, Mack AF. et al. Functional significance of the kainate receptor GluR6(M836I) mutation that is linked to autism. Cell Physiol Biochem. 2006;18(4-5):287–294. doi: 10.1159/000097675. [DOI] [PubMed] [Google Scholar]
- Purcell AE, Jeon OH, Zimmerman AW, Blue ME, Pevsner J. Postmortem brain abnormalities of the glutamate neurotransmitter system in autism. Neurology. 2001;57(9):1618–1628. doi: 10.1212/wnl.57.9.1618. [DOI] [PubMed] [Google Scholar]
- Brown MS, Singel D, Hepburn S, Rojas DC. Increased glutamate concentration in the auditory cortex of persons with autism and first-degree relatives: a (1)H-MRS study. Autism Res. 2013;6(1):1–10. doi: 10.1002/aur.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertz L. The Glutamate-Glutamine (GABA) Cycle: Importance of Late Postnatal Development and Potential Reciprocal Interactions between Biosynthesis and Degradation. Front Endocrinol (Lausanne) 2013;4:59. doi: 10.3389/fendo.2013.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meguro-Horike M, Yasui DH, Powell W, Schroeder DI, Oshimura M, Lasalle JM. et al. Neuron-specific impairment of inter-chromosomal pairing and transcription in a novel model of human 15q-duplication syndrome. Hum Mol Genet. 2011;20(19):3798–3810. doi: 10.1093/hmg/ddr298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatemi SH, Halt AR, Stary JM, Kanodia R, Schulz SC, Realmuto GR. Glutamic acid decarboxylase 65 and 67 kDa proteins are reduced in autistic parietal and cerebellar cortices. Biol Psychiatry. 2002;52(8):805–810. doi: 10.1016/s0006-3223(02)01430-0. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Reutiman TJ, Folsom TD, Thuras PD. GABA(A) receptor downregulation in brains of subjects with autism. J Autism Dev Disord. 2009;39(2):223–230. doi: 10.1007/s10803-008-0646-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatemi SH, Folsom TD, Reutiman TJ, Thuras PD. Expression of GABA(B) receptors is altered in brains of subjects with autism. Cerebellum. 2009;8(1):64–69. doi: 10.1007/s12311-008-0075-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders JA, Gandal MJ, Roberts TP, Siegel SJ. NMDA antagonist MK801 recreates auditory electrophysiology disruption present in autism and other neurodevelopmental disorders. Behav Brain Res. 2012;234(2):233–237. doi: 10.1016/j.bbr.2012.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandal MJ, Anderson RL, Billingslea EN, Carlson GC, Roberts TPL, Siegel SJ. Mice with reduced NMDA receptor expression: more consistent with autism than schizophrenia? Genes Brain Behav. 2012;11(6):740–750. doi: 10.1111/j.1601-183X.2012.00816.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandal MJ, Sisti J, Klook K, Ortinski PI, Leitman V, Liang Y. et al. GABAB-mediated rescue of altered excitatory-inhibitory balance, gamma synchrony and behavioral deficits following constitutive NMDAR-hypofunction. Transl Psychiatry. 2012;2(7):e142. doi: 10.1038/tp.2012.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaldi T, Silberberg G, Markram H. Hyperconnectivity of local neocortical microcircuitry induced by prenatal exposure to valproic acid. Cereb Cortex. 2008;18(4):763–770. doi: 10.1093/cercor/bhm117. [DOI] [PubMed] [Google Scholar]
- Gogolla N, Leblanc JJ, Quast KB, Südhof TC, Fagiolini M, Hensch TK. Common circuit defect of excitatory-inhibitory balance in mouse models of autism. J Neurodev Disord. 2009;1(2):172–181. doi: 10.1007/s11689-009-9023-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardin JA, Carlén M, Meletis K, Knoblich U, Zhang F, Deisseroth K. et al. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature. 2009;459(7247):663–667. doi: 10.1038/nature08002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yizhar O, Fenno LE, Prigge M, Schneider F, Davidson TJ, O’Shea DJ. et al. Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature. 2011;477(7363):171–178. doi: 10.1038/nature10360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maharajh K, Abrams D, Rojas DC, Teale P, Reite ML. Auditory steady state and transient gamma band activity in bipolar disorder. Int Congr Ser. 2007;1300:707–710. [Google Scholar]
- Kwon JS, O’Donnell BF, Wallenstein GV, Greene RW, Hirayasu Y, Nestor PG. et al. Gamma frequency-range abnormalities to auditory stimulation in schizophrenia. Arch Gen Psychiatry. 1999;56(11):1001–1005. doi: 10.1001/archpsyc.56.11.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]