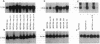Abstract
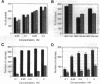
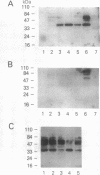
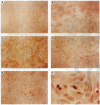
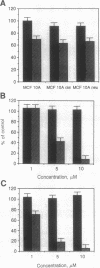
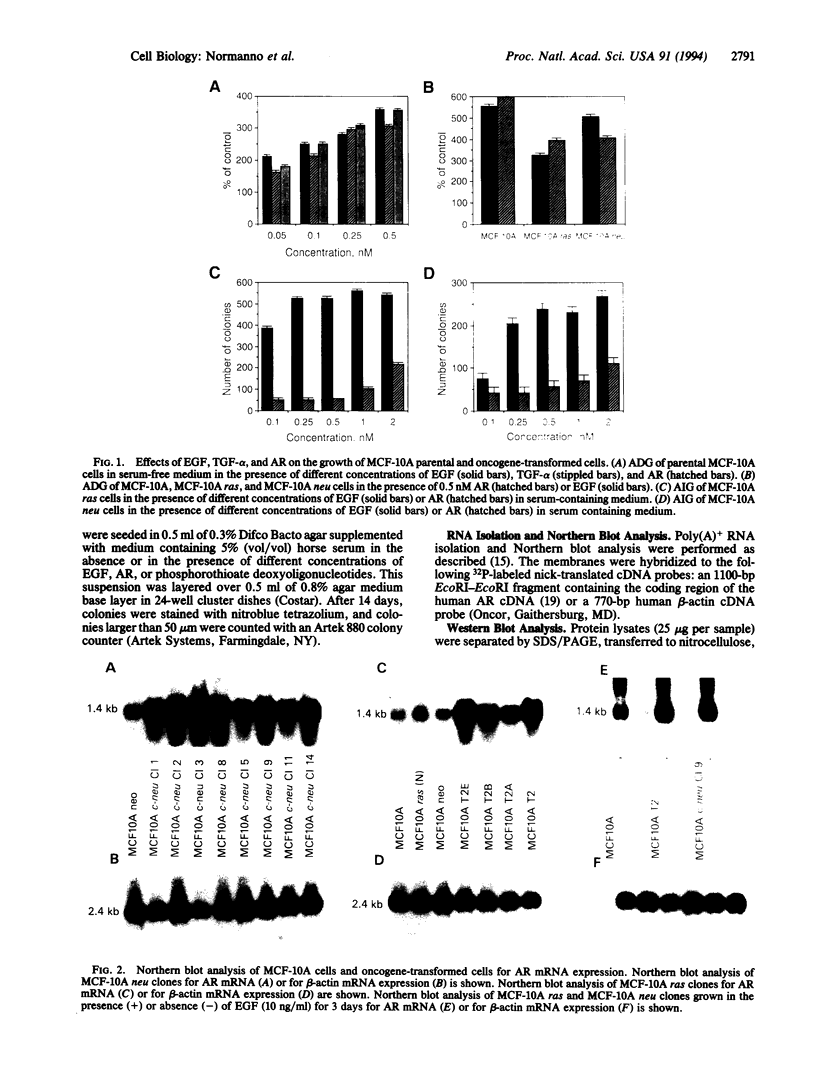
Amphiregulin (AR), a member of the epidermal growth factor (EGF) family, was found to be as potent as EGF in stimulating the anchorage-dependent growth (ADG) of immortalized, nontransformed human mammary epithelial MCF-10A cells. MCF-10A cells transformed by either an activated human c-Ha-ras protooncogene (MCF-10A ras) or by overexpression of a nonactivated rat c-neu gene (MCF-10A neu) exhibited a 35% reduction in the response to AR in ADG when compared to MCF-10A cells, but AR was still as potent as EGF in these transformants. Exogenous AR exhibited only 15-20% of the activity of EGF in stimulating the anchorage-independent growth, a response that is normally dependent upon exogenous EGF, of the oncogene-transformed MCF-10A cells. MCF-10A cells express low levels of a 1.4-kb AR mRNA transcript, while MCF-10A ras and MCF-10A neu cells display a 15- to 30-fold increase in the levels of AR mRNA and endogenous AR protein as determined by Western blot analysis. Exogenous EGF was found to induced both the AR mRNA and protein in the MCF-10A parental and transformed cells. A 20-mer phosphorothioate antisense deoxyoligonucleotide complementary to the 5' sequence of AR mRNA was able to significantly reduce the levels of endogenous AR protein and to inhibit the EGF-stimulated ADG and anchorage-independent growth of MCF-10A ras and MCF-10A neu cells. These data suggest that AR may function as an EGF-dependent autocrine growth factor in mammary epithelial cells that have been transformed by either a point-mutated c-Ha-ras or c-neu.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bates S. E., Davidson N. E., Valverius E. M., Freter C. E., Dickson R. B., Tam J. P., Kudlow J. E., Lippman M. E., Salomon D. S. Expression of transforming growth factor alpha and its messenger ribonucleic acid in human breast cancer: its regulation by estrogen and its possible functional significance. Mol Endocrinol. 1988 Jun;2(6):543–555. doi: 10.1210/mend-2-6-543. [DOI] [PubMed] [Google Scholar]
- Ciardiello F., Kim N., McGeady M. L., Liscia D. S., Saeki T., Bianco C., Salomon D. S. Expression of transforming growth factor alpha (TGF alpha) in breast cancer. Ann Oncol. 1991 Mar;2(3):169–182. doi: 10.1093/oxfordjournals.annonc.a057897. [DOI] [PubMed] [Google Scholar]
- Ciardiello F., Kim N., Saeki T., Dono R., Persico M. G., Plowman G. D., Garrigues J., Radke S., Todaro G. J., Salomon D. S. Differential expression of epidermal growth factor-related proteins in human colorectal tumors. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7792–7796. doi: 10.1073/pnas.88.17.7792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciardiello F., McGeady M. L., Kim N., Basolo F., Hynes N., Langton B. C., Yokozaki H., Saeki T., Elliott J. W., Masui H. Transforming growth factor-alpha expression is enhanced in human mammary epithelial cells transformed by an activated c-Ha-ras protooncogene but not by the c-neu protooncogene, and overexpression of the transforming growth factor-alpha complementary DNA leads to transformation. Cell Growth Differ. 1990 Sep;1(9):407–420. [PubMed] [Google Scholar]
- Clair T., Miller W. R., Cho-Chung Y. S. Prognostic significance of the expression of a ras protein with a molecular weight of 21,000 by human breast cancer. Cancer Res. 1987 Oct 15;47(20):5290–5293. [PubMed] [Google Scholar]
- Cook P. W., Mattox P. A., Keeble W. W., Pittelkow M. R., Plowman G. D., Shoyab M., Adelman J. P., Shipley G. D. A heparin sulfate-regulated human keratinocyte autocrine factor is similar or identical to amphiregulin. Mol Cell Biol. 1991 May;11(5):2547–2557. doi: 10.1128/mcb.11.5.2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dati C., Muraca R., Tazartes O., Antoniotti S., Perroteau I., Giai M., Cortese P., Sismondi P., Saglio G., De Bortoli M. c-erbB-2 and ras expression levels in breast cancer are correlated and show a co-operative association with unfavorable clinical outcome. Int J Cancer. 1991 Apr 1;47(6):833–838. doi: 10.1002/ijc.2910470607. [DOI] [PubMed] [Google Scholar]
- Decker S. J. Epidermal growth factor and transforming growth factor-alpha induce differential processing of the epidermal growth factor receptor. Biochem Biophys Res Commun. 1990 Jan 30;166(2):615–621. doi: 10.1016/0006-291x(90)90853-f. [DOI] [PubMed] [Google Scholar]
- Derynck R. Transforming growth factor alpha. Cell. 1988 Aug 26;54(5):593–595. doi: 10.1016/s0092-8674(88)80001-1. [DOI] [PubMed] [Google Scholar]
- Ebner R., Derynck R. Epidermal growth factor and transforming growth factor-alpha: differential intracellular routing and processing of ligand-receptor complexes. Cell Regul. 1991 Aug;2(8):599–612. doi: 10.1091/mbc.2.8.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson G. R., Kannan B., Shoyab M., Stromberg K. Amphiregulin induces tyrosine phosphorylation of the epidermal growth factor receptor and p185erbB2. Evidence that amphiregulin acts exclusively through the epidermal growth factor receptor at the surface of human epithelial cells. J Biol Chem. 1993 Feb 5;268(4):2924–2931. [PubMed] [Google Scholar]
- Johnson G. R., Saeki T., Gordon A. W., Shoyab M., Salomon D. S., Stromberg K. Autocrine action of amphiregulin in a colon carcinoma cell line and immunocytochemical localization of amphiregulin in human colon. J Cell Biol. 1992 Aug;118(3):741–751. doi: 10.1083/jcb.118.3.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura H. Schwannoma-derived growth factor must be transported into the nucleus to exert its mitogenic activity. Proc Natl Acad Sci U S A. 1993 Mar 15;90(6):2165–2169. doi: 10.1073/pnas.90.6.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klagsbrun M., Baird A. A dual receptor system is required for basic fibroblast growth factor activity. Cell. 1991 Oct 18;67(2):229–231. doi: 10.1016/0092-8674(91)90173-v. [DOI] [PubMed] [Google Scholar]
- LeJeune S., Leek R., Horak E., Plowman G., Greenall M., Harris A. L. Amphiregulin, epidermal growth factor receptor, and estrogen receptor expression in human primary breast cancer. Cancer Res. 1993 Aug 1;53(15):3597–3602. [PubMed] [Google Scholar]
- Li S., Plowman G. D., Buckley S. D., Shipley G. D. Heparin inhibition of autonomous growth implicates amphiregulin as an autocrine growth factor for normal human mammary epithelial cells. J Cell Physiol. 1992 Oct;153(1):103–111. doi: 10.1002/jcp.1041530114. [DOI] [PubMed] [Google Scholar]
- Logan A. Intracrine regulation at the nucleus--a further mechanism of growth factor activity? J Endocrinol. 1990 Jun;125(3):339–343. doi: 10.1677/joe.0.1250339. [DOI] [PubMed] [Google Scholar]
- Modrell B., McDonald V. L., Shoyab M. The interaction of amphiregulin with nuclei and putative nuclear localization sequence binding proteins. Growth Factors. 1992;7(4):305–314. doi: 10.3109/08977199209046413. [DOI] [PubMed] [Google Scholar]
- Murphy P. R., Sato Y., Knee R. S. Phosphorothioate antisense oligonucleotides against basic fibroblast growth factor inhibit anchorage-dependent and anchorage-independent growth of a malignant glioblastoma cell line. Mol Endocrinol. 1992 Jun;6(6):877–884. doi: 10.1210/mend.6.6.1323055. [DOI] [PubMed] [Google Scholar]
- Plowman G. D., Green J. M., McDonald V. L., Neubauer M. G., Disteche C. M., Todaro G. J., Shoyab M. The amphiregulin gene encodes a novel epidermal growth factor-related protein with tumor-inhibitory activity. Mol Cell Biol. 1990 May;10(5):1969–1981. doi: 10.1128/mcb.10.5.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Querzoli P., Marchetti E., Bagni A., Marzola A., Fabris G., Nenci I. Expression of p21 ras gene products in breast cancer relates to histological types and to receptor and nodal status. Breast Cancer Res Treat. 1988 Sep;12(1):23–30. doi: 10.1007/BF01805736. [DOI] [PubMed] [Google Scholar]
- Saeki T., Cristiano A., Lynch M. J., Brattain M., Kim N., Normanno N., Kenney N., Ciardiello F., Salomon D. S. Regulation by estrogen through the 5'-flanking region of the transforming growth factor alpha gene. Mol Endocrinol. 1991 Dec;5(12):1955–1963. doi: 10.1210/mend-5-12-1955. [DOI] [PubMed] [Google Scholar]
- Saeki T., Stromberg K., Qi C. F., Gullick W. J., Tahara E., Normanno N., Ciardiello F., Kenney N., Johnson G. R., Salomon D. S. Differential immunohistochemical detection of amphiregulin and cripto in human normal colon and colorectal tumors. Cancer Res. 1992 Jun 15;52(12):3467–3473. [PubMed] [Google Scholar]
- Salomon D. S., Ciardiello F., Valverius E. M., Kim N. The role of ras gene expression and transforming growth factor alpha production in the etiology and progression of rodent and human breast cancer. Cancer Treat Res. 1991;53:107–157. doi: 10.1007/978-1-4615-3940-7_6. [DOI] [PubMed] [Google Scholar]
- Sherman L., Stocker K. M., Morrison R., Ciment G. Basic fibroblast growth factor (bFGF) acts intracellularly to cause the transdifferentiation of avian neural crest-derived Schwann cell precursors into melanocytes. Development. 1993 Aug;118(4):1313–1326. doi: 10.1242/dev.118.4.1313. [DOI] [PubMed] [Google Scholar]
- Shoyab M., Plowman G. D., McDonald V. L., Bradley J. G., Todaro G. J. Structure and function of human amphiregulin: a member of the epidermal growth factor family. Science. 1989 Feb 24;243(4894 Pt 1):1074–1076. doi: 10.1126/science.2466334. [DOI] [PubMed] [Google Scholar]
- Soule H. D., Maloney T. M., Wolman S. R., Peterson W. D., Jr, Brenz R., McGrath C. M., Russo J., Pauley R. J., Jones R. F., Brooks S. C. Isolation and characterization of a spontaneously immortalized human breast epithelial cell line, MCF-10. Cancer Res. 1990 Sep 15;50(18):6075–6086. [PubMed] [Google Scholar]