Abstract
The human vaginal microbiota plays an important role in the maintenance of a woman's health, as well as of her partner's and newborns’. When this predominantly Lactobacillus community is disrupted, decreased in abundance and replaced by different anaerobes, bacterial vaginosis (BV) may occur. BV is associated with ascending infections and obstetrical complications, such as chorioamnionitis and preterm delivery, as well as with urinary tract infections and sexually transmitted infections. In BV the overgrowth of anaerobes produces noxious substances like polyamines and other compounds that trigger the release of pro-inflammatory cytokines interleukin (IL)-1 β and IL-8. BV can profoundly affect, with different mechanisms, all the phases of a woman's life in relation to reproduction, before pregnancy, during fertilization, through and at the end of pregnancy. BV can directly affect fertility, since an ascending dissemination of the involved species may lead to tubal factor infertility. Moreover, the increased risk of acquiring sexually transmitted diseases contributes to damage to reproductive health. Exogenous strains of lactobacilli have been suggested as a means of re-establishing a normal healthy vaginal flora. Carefully selected probiotic strains can eliminate BV and also exert an antiviral effect, thus reducing viral load and preventing foetal and neonatal infection. The administration of beneficial microorganisms (probiotics) can aid recovery from infection and restore and maintain a healthy vaginal ecosystem, thus improving female health also in relation to reproductive health.
Keywords: Bacterial vaginosis, lactobacilli, probiotics, reproductive health, vaginal microbiota
Introduction
In healthy women of child-bearing age the protective mucosa in the vagina is populated with microflora typically dominated by lactobacilli, and their dominance over pathogenic anaerobes is positively associated with vaginal health. The majority of healthy women evidence only Lactobacillus in the vagina. The function of lactobacilli is to maintain an environment that limits the growth of pathogenic microorganisms1.
Bacterial vaginosis (BV) represents the most common vaginal syndrome affecting fertile, premenopausal and pregnant women, with an incidence rate ranging from 20 to 50 per cent2. The prevalence varies with age, ethnicity, education and poverty. BV is not caused by one specific pathogenic microorganism, but rather by an imbalance of vaginal microbiota. In BV lactobacilli are replaced by Gardnerella vaginalis, Atopobium vaginae, Prevotella, Veillonella, Megasphaera and other anaerobic microorganisms difficult to culture3. In BV the overgrowth of anaerobes produces noxious substances like polyamines and other compounds that trigger the release of pro-inflammatory cytokines interleukin (IL)-1 β and IL-84. BV is frequently disregarded since the symptoms are often absent or insignificant; however, the clinical consequences can be important. Alterations in vaginal microbiology have been associated with many pathological conditions including late miscarriage and premature birth5. Preterm birth is a major obstetric problem and a leading cause of neonatal morbidity and mortality. Therefore, the bacterial biota of the human vagina can have a profound impact not only on the health of women but also on that of their neonates.
A change in the vaginal microbiota has been also associated with pelvic inflammatory disease (PID) and cervicitis and is a risk factor for urinary tract infection and for the acquisition of sexually transmitted disease (STD)6,7. An increasing body of data now indicates that abnormal vaginal flora lacking lactobacilli facilitates the acquisition of infections by parasites like Trichomonas vaginalis and bacteria such as Neisseria gonorrhoeae and Chlamydia trachomatis8. All these infections can cause damage to reproductive health and infertility. Moreover, several longitudinal observational studies have demonstrated that the absence of vaginal lactobacilli, as assessed by culture or Gram stain, is an independent risk factor for viral sexually transmitted infections (STIs) such as HIV, papillomavirus and herpes simplex virus infections7,9. BV may predispose to the acquisition of STIs upon exposure because the local cytokine production associated with BV may facilitate the acquisition of infections. BV can profoundly affect, with different mechanisms, all the phases of a woman's life in relation to reproduction, before pregnancy, during fertilization, through and at the end of pregnancy (Fig. 1). The bacterial biota of the human vagina can influence neonatal health by increasing the possibility of maternal-foetal and maternal-neonatal transmission of infections.
Fig. 1.
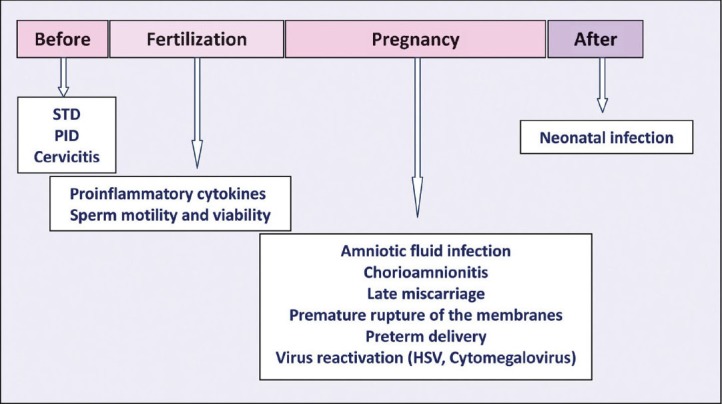
Bacterial vaginosis in relation to reproductive health. STD, sexually transmitted disease; PID, pelvic inflammatory disease.
BV and fertility
Infectious agents can impair various important human functions, including reproduction. Bacteria, fungi, viruses and parasites are able to interfere with the reproductive function in both sexes. In female, C. trachomatis and N. gonorrhoeae are certainly involved in cervical, tubal, and peritoneal damage, while Herpes simplex cervicitis is less dangerous10.
BV can directly affect fertility, since an ascending dissemination of the involved species may lead to tubal factor infertility. Moreover, the increased risk of acquiring STDs contributes to damage to reproductive health. STIs (like chlamidiosis) may be present without symptoms or with symptoms that are mild and transient, but these may have severe long-term consequences such as infertility and ectopic pregnancy11. C. trachomatis is a major cause of tubal obstructions, lacerations and ectopic pregnancy. STDs can also result in acute pelvic inflammatory disease (PID) which in turn can lead to sterility10.
Treatment for BV in non pregnant women involves oral or local administration of metronidazole or clindamycin and varies in efficacy from 48 to 85 per cent for absence of infection three or more weeks after treatment5. The long-term cure rate is low, BV recurs in up to 40 per cent of women within three months after initiation of antibiotic therapy and in up to 50 per cent of women after six months12. There are several unpleasant side effects and disadvantages associated with these therapies, including superinfections with pathogenic microorganisms and susceptibility of lactobacilli to clindamycin13,14. More importantly, some vaginal pathogens, particularly G. vaginalis and anaerobic bacteria, have shown increasing drug resistance15.
The use of probiotics to re-establish a physiological microbial flora of the female urogenital tract has long been under consideration and is now supported by some clinical evidence. The rationale for the use of probiotics in women is based on the genitourinary regulatory role played by the vaginal health microbiota and the need for restoration of this microbial ecosystem after insult16. Lactobacilli are the organisms commonly used as probiotics.
For probiotic applications to the urogenital tract to be successful, it is critical that strains are carefully selected. Lactobacillus strains (Lactobacillus brevis CD2, L. salivarius FV2 and L. plantarum FV9), present in Florisia vaginal tablets - a probiotic product for vaginal use - have been selected for properties relating to mucosal colonization, i.e. their ability to adhere at high levels to human epithelial cells and to temporarily colonize the human vagina, for the production of antimicrobial compounds effective towards BV-related microorganisms and for the capacity to inhibit pathogen binding to the cell membrane17,18. Florisia vaginal tablets have been assessed for effectiveness in the treatment of symptomatic bacterial vaginosis in a double blind, placebo-controlled clinical trial. Treatment with the probiotic preparation was 61 per cent effective in eliminating BV and 50 per cent effective in restoring ‘normal vaginal flora’ as determined by Gram stain three weeks after therapy19. It must be emphasized that the therapy, although not typically pharmacological but entirely probiotic, achieved a cure rate in the lower range of typical pharmacological therapies.
The efficacy of vaginal probiotic treatment on the recurrence rate of BV has also been evaluated20,21. Lactobacillus supplementation (EcoVag®Vaginal capsules containing L. gasseri Lba EB01-DSM 14869 and L. rhamnosus Lbp PB01-DSM 14870) after clindamycin treatment did not improve the efficacy of BV therapy during the first month of treatment, but it significantly reduced the recurrence rate of BV at 6 months from initiation of treatment20. Moreover, probiotic prophylaxis with vaginal capsules containing L. rhamnosus, L. acidophilus, and Streptococcus thermophilus (Probaclac Vaginal) resulted in lower recurrence rates for BV in women with a history of recurrent BV21.
BV and fertilization
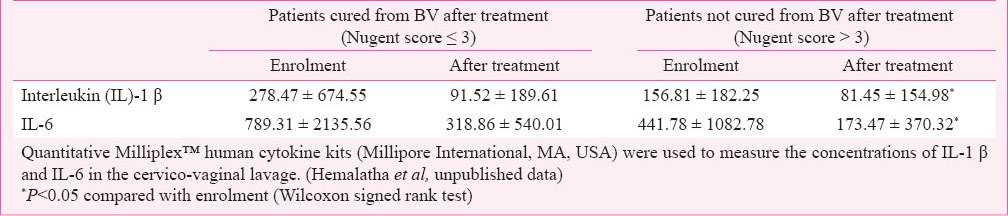
BV could indirectly affect the fertilization. Spandorfer et al22 investigated abnormal vaginal flora and vaginal pro-inflammatory cytokines in women with idiopathic infertility undergoing in vitro fertilization. A correlation between bacterial vaginosis, elevated IL-1 β and IL-8, and idiopathic infertility was demonstrated. A recent study conducted in India evaluated the effectiveness of probiotic lactobacilli (Florisia vaginal tablets) on vaginal health and pro-inflammatory cytokines23. As shown in Table I, vaginal cytokines were significantly reduced in women cured from BV as well as in women that resulted not cured from BV (NCW) after probiotic treatment. The beneficial effect of lactobacilli treatment on local pro-inflammatory cytokines observed in the presence of a still altered vaginal microbiota suggests that probiotic administration could reduce the consequences of the host response to microbial products even in the absence of a substantial restoration of a healthy vaginal microbiota.
Table I.
Effect of probiotic vaginal tablets on cytokine concentration
Recently it has been demonstrated that the lipopolysaccharide (LPS) receptor, Toll like receptor 4 (TLR4), and peptidoglycan receptor, TLR2, are expressed in human sperm24. The authors demonstrated that after recognizing bacterial endotoxins, the activated TLRs reduce sperm motility, induce sperm apoptosis and significantly impair the potential for fertilization.
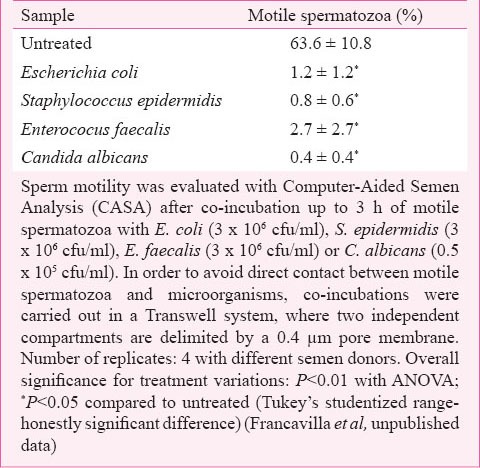
Many microorganisms seem to be involved in male reproductive failure in various ways, and to different degrees of statistical association. Infections of the male genito-urinary tract may cause a microbial colonization of the semen that may affect sperm motility and viability. However, the presence of an altered microbiota in the female genital tract may also affect the fertilization potential. It has been demonstrated that genital discharges of asymptomatic infertile women are characterized by the presence of Gardnerella vaginalis, Enterobacteriaceae or Enterococci and Streptococcus agalactiae25. Studies from our group indicated that products released by Escherichia coli, Enterococcus faecalis, Staphylococcus epidermidis and Candida albicans, common opportunistic microorganisms of the female genital tract, strongly reduced sperm motility (Table II). It is possible to hypothesize that bacterial cell wall components are responsible for the detrimental effect exerted by Gram positive and Gram negative bacteria on sperm motility. The negative effect caused by C. albicans suggests that other mechanisms could be also involved.
Table II.
Effect of common opportunistic microorganisms of the female genital tract on sperm motility
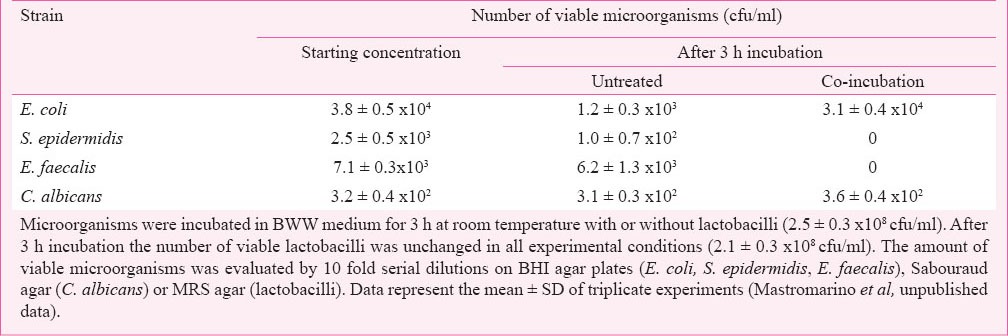
In the presence of a healthy vaginal microbiota, opportunistic microorganisms are in very low numbers. The absence of lactobacilli and their by-product lactic acid triggers an increase of vaginal pH, thereby disrupting physiological mechanisms that might inactivate or contain the pathogens16,26. Moreover, lactobacilli are able to produce other bacteriostatic and bactericidal compounds (H2O2, bacteriocins) capable of killing bacteria. A commercially available lactobacilli mixture (Florisia CD Pharma India Pvt. Ltd. India) showed a bactericidal effect on common opportunistic microorganisms of the female genital tract. As shown in Table III, E. faecalis and S. epidermidis were inactivated after 3 h co-incubation with physiological concentrations of lactobacilli whereas E. coli and C. albicans were unaffected. The inhibition was exerted without any effect on pH values suggesting that properly selected lactobacilli may be of prophylactic value in preventing various urogenital disorders in women.
Table III.
Bactericidal effect of Florisia probiotic lactobacilli on common opportunistic microorganisms of the female genital tract
A study by Barbonetti et al27 evaluated the effect of Florisia probiotic lactobacilli on sperm motility and viability. The rationale for this study was based on the increased concentration of IL-1 β in vaginal environment of BV-affected women that could trigger the generation of reactive oxygen species (ROS). ROS-induced lipid peroxidation, affecting membrane integrity and flexibility, represents a major factor in the aetiology of sperm dysfunction, because it impairs both tail motion and sperm-oocyte fusion. In this study, fluorophore-loaded sperm samples were preincubated with the probiotic mix in a Transwell system in which two independent compartments are delimited by a 0.4-μm pore membrane. Motile sperm suspension and viable lactobacilli were placed on the lower and the upper compartments of the chamber, respectively. After 30 min of incubation, sperm lipid peroxidation was induced by exposure to ferrous sulphate added to the lower compartment and lipid peroxidation was evaluated by flow cytometry. Pretreatment with lactobacilli prevented sperm lipid peroxidation induced by ferrous sulphate and the detrimental effect exerted on both cell motility and viability.
The same group evaluated the protective effects of vaginal probiotic lactobacilli on the sperm damage induced by different microorganisms, frequently present in the vaginal environment. Using the Transwell system, they demonstrated that sperm co-incubation for 1 h with Lactobacillus strains (L. brevis CD2, L. salivarius FV2 and L. plantarum FV9), present in Florisia vaginal tablets eliminated the negative impact exerted by microorganisms on sperm motility and viability (Fig. 2). These preliminary in vitro results indicate the potential for vaginal probiotic lactobacilli to improve the fertilization potential of the female host in the presence of vaginal disorders.
Fig. 2.
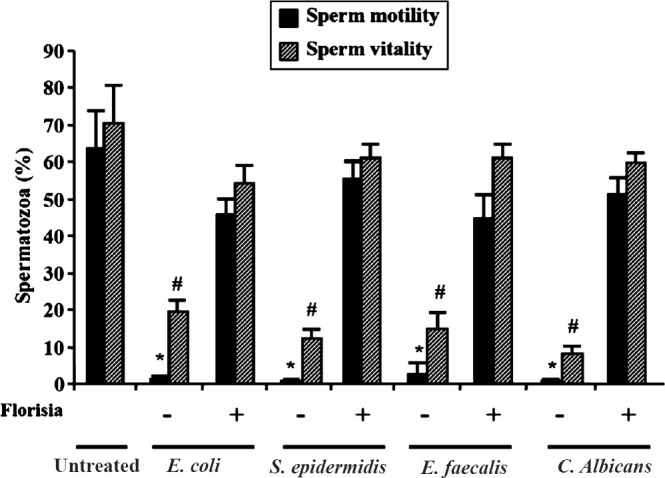
Effect of lactobacilli on sperm functions. Sperm motility was evaluated with Computer-Aided Semen Analysis (CASA) and sperm vitality was evaluated under light microscope by the eosin technique, after coincubation of motile spermatozoa with E.coli (3 × 106 cfu/ml), S. epidermidis (3 × 106 cfu/ml), E. faecalis (3 × 106 cfu/ml) or C. albicans (0.5 × 105 cfu/ml. To avoid direct contact between motile spermatozoa and microorganisms, co-incubations were carried out in a Transwell system, where two independent compartments are delimited by a 0.4 μm pore membrane. Number of replicates: 4 with different semen donors. Overall significance for treatment variations: P<0.01 with ANOVA; * and #P<0.05 vs untreated and vs samples with Florisia (Tukey's studentized range-honestly significant difference). Francavilla et al., unpublished data.
BV and pregnancy
Case-control and cohort studies have shown that BV in pregnant women is associated with several adverse health outcomes. Besides obstetrical complications, unrecognized chlamydial and gonococcal infections and virus reactivation during pregnancy can determine maternal-foetal or maternal-infant transmission. The Centers for Disease Control have recommended that only patients at high risk for preterm delivery specifically only those with a previous history of a spontaneous preterm birth should be treated with antibiotics if they are found to have bacterial vaginosis5,28.
Metronidazole is the drug of choice and multiple studies and meta-analyses have not demonstrated a consistent association between metronidazole use during pregnancy and teratogenic or mutagenic effects in infants. However, metronidazole is a pregnancy category B drug since animal studies have revealed no evidence of harm to the foetus, but no adequate, well-controlled studies among pregnant women have been conducted.
Probiotic use to treat BV during pregnancy could represent an effective alternative option to antibiotics. Several recent studies have indicated that exposure to probiotics during pregnancy is safe. In 2009, a meta-analysis and systematic review of randomized controlled trials of probiotic use in more than 1500 pregnant women did not report an increase in adverse foetal outcomes29. There was no increase in the incidence of miscarriages or malformations and no significant difference in birth weight, gestational age, or the incidence of cesarean section. Similar results were obtained from randomized control trials on more than 2000 pregnant women published following the above meta-analysis30. Oral administration of multistrain probiotics (VSL#3) to improve pregnancy outcomes has recently been tested in a pilot study, with some success in terms of modulating immune parameters and the vaginal microbiota31. The use of selected probiotic strains can eliminate BV and also exert an antiviral effect, thus reducing viral load and preventing foetal and neonatal infection.
Vaginal lactobacilli are shown to inhibit Herpes simplex type 2 (HSV-2) replication in culture cells32. It was demonstrated that highly adhesive Lactobacillus strains inhibited HSV-2 infection when present during virus adsorption and all the tested strains significantly reduced virus yield when present during HSV-2 multiplication. A soluble factor(s) released from lactobacilli was responsible for the inhibition. In a subsequent study the mechanisms of L. brevis antiviral activity towards HSV-2 was evaluated33. Bacterial extract from L. brevis cells and L. brevis cell wall strongly inhibited HSV-2 replication. The inhibitory activity was resistant to high temperature and protease digestion and appeared to be associated with compounds with a molecular weight higher than 10,000 kDa. S-layer removal from L. brevis cells strongly reduced the antiviral activity of both bacterial extract and cell wall fragments. It was concluded that the inhibitory activity was due to a heat-resistant non protein cell surface bacterial component.
Conclusions
Indigenous microorganisms can help the host overcome infection, maintain vaginal health and prevent infections of the reproductive tract. The administration of beneficial microorganisms (probiotics) can also aid recovery from infection and restore and maintain a healthy vaginal ecosystem, thus improving female health also in relation to reproductive health.
References
- 1.Rönnqvist PD, Forsgren-Brusk UB, Grahn-Håkansson EE. Lactobacilli in the female genital tract in relation to other genital microbes and vaginal pH. Acta Obstet Gynecol Scand. 2006;85:726–35. doi: 10.1080/00016340600578357. [DOI] [PubMed] [Google Scholar]
- 2.Allsworth JE, Peipert JF. Prevalence of bacterial vaginosis: 2001-2004 National Health and Nutrition Examination Survey data. Obstet Gynecol. 2007;109:114–20. doi: 10.1097/01.AOG.0000247627.84791.91. [DOI] [PubMed] [Google Scholar]
- 3.Fredricks DN, Fiedler TL, Marrazzo JM. Molecular identification of bacteria associated with bacterial vaginosis. N Engl J Med. 2005;353:1899–911. doi: 10.1056/NEJMoa043802. [DOI] [PubMed] [Google Scholar]
- 4.Romero R, Chaiworapongsa T, Kuivaniemi H, Tromp G. Bacterial vaginosis, the inflammatory response and the risk of preterm birth: a role for genetic epidemiology in the prevention of preterm birth. Am J Obstet Gynecol. 2004;190:1509–19. doi: 10.1016/j.ajog.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 5.Koumans EH, Markowitz LE, Hogan V CDC BV Working Group. Indications for therapy and treatment recommendations for bacterial vaginosis in non-pregnant and pregnant women: a synthesis of data. Clin infect Dis. 2002;35(Suppl 2):S152–72. doi: 10.1086/342103. [DOI] [PubMed] [Google Scholar]
- 6.Harmanli OH, Cheng GY, Nyirjesy P, Chatwani A, Gaughan JP. Urinary tract infections in women with bacterial vaginosis. Obstet Gynecol. 2000;95:710–2. doi: 10.1016/s0029-7844(99)00632-8. [DOI] [PubMed] [Google Scholar]
- 7.Martin HL, Richardson BA, Nyange PM, Lavreys L, Hillier SL, Chohan B, et al. Vaginal lactobacilli, microbial flora, and risk of human immunodeficiency virus type 1 and sexually transmitted disease acquisition. J Infect Dis. 1999;180:1863–8. doi: 10.1086/315127. [DOI] [PubMed] [Google Scholar]
- 8.Wiesenfeld H, Hillier S, Krohn MA, Landers DV, Sweet RL. Bacterial vaginosis is a strong predictor of Neisseria gonorrhoeae and Chlamydia trachomatis infection. Clin Infect Dis. 2003;36:663–8. doi: 10.1086/367658. [DOI] [PubMed] [Google Scholar]
- 9.Cherpes TL, Meyn LA, Krohn MA, Lurie JG, Hillier SL. Association between acquisition of herpes simplex virus type 2 in women and bacterial vaginosis. Clin Infect Dis. 2003;37:319–25. doi: 10.1086/375819. [DOI] [PubMed] [Google Scholar]
- 10.Pellati D, Mylonakis I, Bertoloni G, Fiore C, Andrisani A, Ambrosini G, et al. Genital tract infections and infertility. Eur J Obstet Gynecol Reprod Biol. 2008;140:3–11. doi: 10.1016/j.ejogrb.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 11.Workowski KA, Berman SM. Centers for Disease Control and Prevention Sexually Transmitted Disease Treatment Guidelines. Clin Infect Dis. 2011;53(Suppl 3):S59–63. doi: 10.1093/cid/cir694. [DOI] [PubMed] [Google Scholar]
- 12.Bradshaw CS, Morton AN, Hocking J, Garland SM, Morris MB, Moss LM, et al. High recurrence rates of bacterial vaginosis over the course of 12 months after oral metronidazole therapy and factors associated with recurrence. J Infect Dis. 2006;193:1478–86. doi: 10.1086/503780. [DOI] [PubMed] [Google Scholar]
- 13.Sobel JD, Ferris D, Schwebke J, Nyirjesy P, Wiesenfeld HC, Peipert J, et al. Suppressive antibacterial therapy with 0.75% metronidazole vaginal gel to prevent recurrent bacterial vaginosis. Am J Obstet Gynecol. 2006;194:1283–9. doi: 10.1016/j.ajog.2005.11.041. [DOI] [PubMed] [Google Scholar]
- 14.Bayer AS, Chow AW, Concepcion N, Guze LB. Susceptibility of 40 lactobacilli to six antimicrobial agents with broad Gram-positive anaerobic spectra. Antimicrob Agents Chemother. 1978;14:720–2. doi: 10.1128/aac.14.5.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beigi RH, Austin MN, Meyn LA, Krohn MA, Hillier SL. Antimicrobial resistance associated with the treatment of bacterial vaginosis. Am J Obstet Gynecol. 2004;191:1124–9. doi: 10.1016/j.ajog.2004.05.033. [DOI] [PubMed] [Google Scholar]
- 16.Mastromarino P, Vitali B, Mosca L. Bacterial vaginosis: a review on clinical trials with probiotics. New Microbiol. 2013;36:229–38. [PubMed] [Google Scholar]
- 17.Maggi L, Mastromarino P, Macchia S, Brigidi P, Pirovano F, Matteuzzi D. Technological and biological evaluation of tablets containing different strains of lactobacilli for vaginal administration. Eur J Pharm Biopharm. 2000;50:389–95. doi: 10.1016/s0939-6411(00)00121-1. [DOI] [PubMed] [Google Scholar]
- 18.Mastromarino P, Brigidi P, Macchia S, Maggi L, Pirovano F, Trinchieri V, et al. Characterization and selection of vaginal Lactobacillus containing strains for the preparation of vaginal tablets. J Appl Microbiol. 2002;93:884–93. doi: 10.1046/j.1365-2672.2002.01759.x. [DOI] [PubMed] [Google Scholar]
- 19.Mastromarino P, Macchia S, Meggiorini L, Trinchieri V, Mosca L, Perluigi M, et al. Effectiveness of Lactobacillus containing vaginal tablets in the treatment of symptomatic bacterial vaginosis. Clin Microbiol Infect. 2009;15:67–74. doi: 10.1111/j.1469-0691.2008.02112.x. [DOI] [PubMed] [Google Scholar]
- 20.Larsson PG, Stray-Pedersen B, Ryttig KR, Larsen S. Human lactobacilli as supplementation of clindamycin to patients with bacterial vaginosis reduce the recurrence rate; a 6-month, double-blind, randomized, placebo-controlled study. BMC Womens Health. 2008;8:3. doi: 10.1186/1472-6874-8-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ya W, Reifer C, Miller LE. Efficacy of vaginal probiotic capsules for recurrent bacterial vaginosis: a double-blind, randomized, placebo-controlled study. Am J Obstet Gynecol. 2010;203:120.e1–6. doi: 10.1016/j.ajog.2010.05.023. [DOI] [PubMed] [Google Scholar]
- 22.Spandorfer SD, Neuer A, Giraldo PC, Rosenwaks Z, Witkin SS. Relationship of abnormal vaginal flora, proinflammatory cytokines and idiopathic infertility in women undergoing IVF. J Reprod Med. 2001;46:806–10. [PubMed] [Google Scholar]
- 23.Hemalatha R, Mastromarino P, Ramalaxmi BA, Balakrishna NV, Sesikeran B. Effectiveness of vaginal tablets containing lactobacilli versus pH tablets on vaginal health and inflammatory cytokines: a randomized, double-blind study. Eur J Clin Microbiol Infect Dis. 2012;31:3097–105. doi: 10.1007/s10096-012-1671-1. [DOI] [PubMed] [Google Scholar]
- 24.Fujita Y, Mihara T, Okazaki T, Shitanaka M, Kushino R, Ikeda C, et al. Toll-like receptors (TLR) 2 and 4 on human sperm recognize bacterial endotoxins and mediate apoptosis. Hum Reprod. 2011;26:2799–806. doi: 10.1093/humrep/der234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Casari E, Ferrario A, Morenghi E, Montanelli A. Gardnerella, Trichomonas vaginalis, Candida, Chlamydia trachomatis, Mycoplasma hominis and Ureaplasma urealyticum in the genital discharge of symptomatic fertile and asymptomatic infertile women. New Microbiol. 2010;33:69–76. [PubMed] [Google Scholar]
- 26.Nardis C, Mosca L, Mastromarino P. Vaginal microbiota and viral sexually transmitted diseases. Ann Ig. 2013;25:443–56. doi: 10.7416/ai.2013.1946. [DOI] [PubMed] [Google Scholar]
- 27.Barbonetti A, Cinque B, Vassallo MR, Mineo S, Francavilla S, Cifone MG, et al. Effect of vaginal probiotic lactobacilli on in vitro-induced sperm lipid peroxidation and its impact on sperm motility and viability. Fertil Steril. 2011;95:2485–8. doi: 10.1016/j.fertnstert.2011.03.066. [DOI] [PubMed] [Google Scholar]
- 28.Sexually transmitted diseases treatment guidelines 2002. Centers for Disease Control and Prevention. MMWR Recomm Rep. 2002;51:1–78. [PubMed] [Google Scholar]
- 29.Dugoua JJ, Machado M, Zhu X, Chen X, Koren G, Einarson TR. Probiotic safety in pregnancy: a systematic review and meta-analysis of randomized controlled trials of Lactobacillus, bifidobacterium, and Saccharomyces spp. J Obstet Gynaecol Can. 2009;31:542–52. doi: 10.1016/S1701-2163(16)34218-9. [DOI] [PubMed] [Google Scholar]
- 30.Elias J, Bozzo P, Einarson A. Are probiotics safe for use during pregnancy and lactation? Can Fam Physician. 2011;57:299–301. [PMC free article] [PubMed] [Google Scholar]
- 31.Vitali B, Cruciani F, Baldassarre ME, Capursi T, Spisni E, Valerii MC, et al. Dietary supplementation with probiotics during late pregnancy: outcome on vaginal microbiota and cytokine secretion. BMC Microbiol. 2012;12:236. doi: 10.1186/1471-2180-12-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Conti C, Malacrino C, Mastromarino P. Inhibition of herpes simplex virus type 2 by vaginal lactobacilli. J Physiol Pharmacol. 2009;60(Suppl 6):19–26. [PubMed] [Google Scholar]
- 33.Mastromarino P, Cacciotti F, Masci A, Mosca L. Antiviral activity of Lactobacillus brevis towards herpes simplex virus type 2: role of cell wall associated components. Anaerobe. 2011;17:334–6. doi: 10.1016/j.anaerobe.2011.04.022. [DOI] [PubMed] [Google Scholar]


