Abstract
Background
Autosomal dominant polycystic kidney disease (ADPKD) results in kidney cyst development and enlargement, resulting in chronic kidney disease (CKD) leading to renal failure. This study sought to determine if ADPKD patients in the early stages of CKD contribute to a sizable economic burden for the US health care system.
Methods
This was a retrospective, matched cohort study, reviewing medical resource utilization (MRU) and costs for adults in a US private-payer claims database with a diagnosis code of ADPKD (ICD-9-CM 753.13). ADPKD patients were matched by age grouping (0–17, 18–34, 35–44, 45–54, 55–64, and 65+ years) and sex to controls to understand the burden of ADPKD. Descriptive statistics on 6-month MRU and costs were assessed by CKD stages, dialysis use, or previous renal transplant.
Results
The analysis included ADPKD patients in CKD stages 1–5 (n=316 to n=860), dialysis (n=586), and post-transplant (n=615). Mean ages did not differ across CKD stages (range 43–56 years). Men were the majority in the later stages but the minority in the early stages. The proportion of patients with at least one hospitalization increased with CKD stage, (12% to >40% CKD stage 2 to stage 5, dialysis or post-transplant). The majority had at least one hospital outpatient visit and at least one pharmacy claim. Total 6-month per-patient costs were greater among ADPKD patients than in age-matched and sex-matched healthy non-ADPKD controls (P<0.001 for all comparisons).
Conclusion
ADPKD patients with normal kidney function are associated with a significant economic burden to the health care system relative to the general population. Any treatments that delay progression to later stages of CKD may provide potential health care cost offsets.
Keywords: autosomal dominant polycystic kidney disease, medical resource utilization, chronic kidney disease
Introduction
Autosomal dominant polycystic kidney disease (ADPKD) is the most common inherited kidney disease and the fourth leading cause of end-stage renal disease (ESRD) in the USA. ADPKD is characterized by progressive cyst development and growth which contributes to loss of kidney function. The prevalence of ADPKD estimates range from one in 400 to one in 4,000 diagnosed patients.1
ADPKD patients experience renal-related complications and failure due to cyst development.2 Appearance, timing, and severity of signs and symptoms correlate with the number, distribution, and growth rate of these cysts.3 ADPKD accounts for approximately 10% of all ESRD cases.4 Most symptoms and renal complications occur before an ADPKD diagnosis is made and decades prior to loss of kidney function.5 In 2010, approximately 2.3% of incident ESRD patients had ADPKD with increasing incidence and a median age for ESRD onset of 54 years.6
Currently available therapies focus on limiting the morbidity and mortality associated with ADPKD by only treating a limited number of complications that contribute to ESRD (eg, cyst infections, hypertension, and modifiable cardiovascular risk factors) rather than targeting the inhibition of cyst formation which contributes to the loss of kidney function.1 While restricting dietary protein intake, controlling blood pressure, and administration of renin–angiotensin system inhibitors may delay the onset of ESRD, they have not been shown to prevent its onset.7–9
Reliable information regarding ADKPD-specific medical costs is rare. One study showed that medical and pharmacy costs of combined ADPKD and polycystic kidney disease in a privately insured population correlated inversely with kidney function.10 Although informative, the inclusion of both ADPKD and the broader diagnosis of polycystic kidney disease, based on the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnosis code for polycystic kidney disease (753.12), potentially resulted in including patients without ADPKD. The study relied on charge data rather than actual payer reimbursement, and it did not determine the relative costs associated with dialysis or transplant. Most importantly, the economic impact of ADPKD was not assessed relative to medical costs for healthy age-matched and sex-matched controls.
There is a perception that the disease burden of ADPKD during the early stages of the disease, when kidney function is still classified as normal (glomerular filtration rate [GFR] ≥60 mL/min/1.73 m2), does not place a sizeable burden on medical resource utilization (MRU). The hypothesis at the start of this research was that ADPKD patients, even early in disease, would pose a sizeable burden to the US health care system. While we investigated the economic burden of ADPKD across all stages of kidney disease, dialysis, and transplantation, the emphasis in this study was the economic burden of ADPKD when kidney function was still considered normal. We evaluated MRU and costs associated with ADPKD from a payer perspective, characterized MRU and costs among clinically relevant subgroups, and examined the cost of disease progression.
Materials and methods
Study design and data sources
This retrospective, longitudinal cohort study was based on health insurance claims data from a US private-payer data source using the most recent 5-year period (July 1, 2005 to June 30, 2010). The Truven Health MarketScan® Commercial Claims and Encounters Database (Truven Health Analytics, Inc., Ann Arbor, MI, USA) including inpatient, outpatient, and outpatient prescription drug claims for over 170 million privately insured individuals in the USA annually from approximately 90 large employers and health plans, with insurance provided under various fee-for-service and capitated health plans. This was supplemented by data from the MarketScan Lab Database, which contains community-based diagnostic test results integrated with claims data from health plan contributors for a subset of their covered lives.
Study population
The ADPKD study population comprised patients within the database who had:
at least one index medical claim carrying an ICD-9-CM code of ADPKD (753.13)
a medical claim within 6 months of the index medical claim for ADPKD carrying a diagnosis code indicating chronic kidney disease (CKD) stage (585.1–585.6; note that CKD stage 5 and ESRD patients were combined into a single group), dialysis, previous renal transplant (V42.0), or a creatinine laboratory test result
≥6 months of pre-index eligibility and 6 months of post-index eligibility (ie, the observational period).
Clinically relevant patient subgroups were identified: a hypertensive subgroup (patients with at least one medical claim carrying a hypertension ICD-9-CM code during the pre-index or post-index period) high risk for renal progression subgroup (hypertension diagnosed before age 35 years, hematuria occurring before age 30 years, or presence of albuminuria or one of the following: CKD stage 2 by age 30 years, CKD stage 3 by age 50 years, and CKD stage 4/5, ESRD, or transplant by age 55 years).
MRU and costs for ADPKD patients overall and by subgroup were classified by disease stage group according to CKD stage, dialysis status, and post-transplant status using ICD-9-CM codes on medical claims and for patients with laboratory results by estimated GFR. In addition, if patients had a serum creatinine result available allowing for calculation of estimated GFR, CKD stage was determined based on estimated GFR using the CKD-EPI equation (without adjustment for race). If patients progressed through different CKD stages during the observation period, the MRU and cost analyses of each observed CKD stage were included.
A random sample of a sex-matched and age-matched cohort of healthy patients without polycystic kidney disease or ADPKD provided baseline MRU and cost data for comparison in order to determine the costs of care at all CKD stages of ADPKD for an otherwise healthy population. Therefore, patients with CKD or pre-existing or concomitant comorbidities were not used as matching criteria.
Study endpoints
Six-month MRU and associated costs for ADPKD patients were the main study endpoints. All-cause MRU in the hospital inpatient, hospital outpatient, physician office, and ESRD facility settings during the 6-month study period was summarized using medical claims with ICD-9-CM codes, Current Procedural Terminology codes, and/or Healthcare Common Procedure Coding System codes derived from medical and pharmacy claims. Outpatient prescription utilization was measured using National Drug Codes on outpatient pharmacy claims; inpatient pharmacy medication utilization data were unavailable.
Costs associated with all-cause MRU were derived from medical and pharmacy claims paid by the insurer (including outpatient prescription costs), summarized over a 6-month period, and adjusted to the most recent available data (2010) by the Consumer Price Index. Probabilities of transitioning between disease stages, remaining in the same estimated GFR/CKD/dialysis or transplant status category, transitioning to subsequent stages, and dying in the hospital setting over a 6–12-month period were measured.
Analysis methods
Descriptive statistics were summarized. Categorical variables are presented as the count and percentage of patients in each category, and chi-square tests were used to compare differences across all groups. Continuous variables were summarized using the mean, standard deviation, median, and range, as appropriate. Kruskal–Wallis tests were used to compare costs across groups, and Student’s t-tests were used to test all other continuous variables using SAS® version 9.1.3 software (SAS Institute Inc., Cary, NC, USA).
Results
Demographic and clinical characteristics
Age and sex for the matched-control cohort for each disease stage were similar to that of ADPKD patients (Table 1). Diabetes, coronary artery disease, heart failure, hypertension, and neoplasms (malignant and benign) were more common among ADPKD patients across disease stages relative to the matched controls, except for diabetes in ADPKD stage 1.
Table 1.
Demographic and clinical characteristics of ADPKD and non-ADPKD matched controla population
| Group | Age Mean (SD) |
Age ≥65 years n (%) |
Female n (%) |
High risk for early progression n (%) |
Diabetes, n (%) |
CAD, n (%) |
Heart failure n (%) |
Hypertension n (%) |
Neoplasms n (%) |
|---|---|---|---|---|---|---|---|---|---|
| ADPKD stage 1 CKD (n=316) |
43.4 (16.0) | 18 (5.7) | 195 (61.7) | 133 (42.1) | 16 (5.1) | 28 (8.9) | 12 (3.8) | 198 (62.7) | 78 (24.7) |
| Non-ADPKD matched controls (n=1,580) |
43.2 (16.7) | 111 (7.0) | 975 (61.7) | N/A | 102 (6.5) | 62 (3.9) | 15 (0.9) | 243 (15.4) | 151 (9.6) |
| ADPKD stage 2 CKD (n=404) |
46.7 (12.2) | 17 (4.2) | 220 (54.5) | 219 (54.2) | 40 (9.9) | 56 (13.9) | 11 (2.7) | 301 (74.5) | 108 (26.7) |
| Non-ADPKD matched controls (n=2,020) |
46.60 (13.8) | 133 (6.6) | 1,100 (54.5) | N/A | 123 (6.1) | 71 (3.5) | 19 (0.9) | 294 (14.6) | 199 (9.9) |
| ADPKD stage 3 CKD (n=860) |
52.5 (12.5) | 104 (12.1) | 397 (46.2) | 508 (59.1) | 132 (15.3) | 156 (18.1) | 53 (6.2) | 682 (79.3) | 212 (24.7) |
| Non-ADPKD matched controls (n=4,300) |
52.42 (13.3) | 610 (14.2) | 1,985 (46.2) | N/A | 349 (8.1) | 248 (5.8) | 72 (1.7) | 854 (19.9) | 514 (12.0) |
| ADPKD stage 4 CKD (n=611) |
54.9 (12.5) | 102 (16.7) | 296 (48.4) | 358 (58.6) | 108 (17.7) | 124 (20.3) | 54 (8.8) | 505 (82.7) | 158 (25.9) |
| Non-ADPKD matched controls (n=3,055) |
54.8 (13.2) | 587 (19.2) | 1,480 (48.4) | N/A | 279 (9.1) | 212 (6.9) | 64 (2.1) | 648 (21.2) | 395 (12.9) |
| ADPKD stage 5 CKD (n=842) |
55.2 (11.5) | 141 (16.7) | 378 (44.9) | 504 (59.9) | 151 (17.9) | 220 (26.1) | 104 (12.4) | 734 (87.2) | 259 (30.8) |
| Non-ADPKD matched controls (n=4,210) |
55.4 (12.4) | 806 (19.1) | 1,890 (44.9) | N/A | 407 (9.7) | 291 (6.9) | 78 (1.9) | 937 (22.3) | 521 (12.4) |
| ADPKD dialysis (n=586) |
55.8 (11.4) | 104 (17.7) | 250 (42.7) | 329 (56.1) | 102 (17.4) | 162 (27.6) | 89 (15.2) | 524 (89.4) | 184 (31.4) |
| Non-ADPKD matched controls (n=2,930) |
56.0 (12.3) | 592 (20.2) | 1,250 (42.7) | N/A | 307 (10.5) | 200 (6.8) | 49 (1.7) | 668 (22.8) | 363 (12.4) |
| ADPKD post- transplant (n=615) |
53.3 (10.0) | 54 (8.8) | 267 (43.4) | 381 (62.0) | 117 (19.0) | 133 (21.6) | 50 (8.1) | 505 (82.1) | 215 (35.0) |
| Non-ADPKD matched controls (n=3,075) |
53.8 (11.4) | 360 (11.7) | 1,335 (43.4) | N/A | 297 (9.7) | 147 (4.8) | 33 (1.1) | 614 (20.0) | 392 (12.7) |
Notes:
ADPKD patients were grouped by CKD stage and matched by age and sex to a control population without ADPKD. Less than 1.0% of all non-ADPKD matched control patients had a diagnosis for CKD, procedure for dialysis, or an indicator for post-transplant.
Abbreviations: ADPKD, autosomal dominant polycystic kidney disease; CAD, coronary artery disease; CKD, chronic kidney disease; N/A, not available; SD, standard deviation.
The average ages of the ADPKD population ranged from the early 40s in patients with stage 1 disease to the mid-50s in patients with stage 5 disease, on dialysis, or post-transplant. Most patients were aged 36–55 years. While women comprised the majority of stage 1 and 2 patients, men comprised the majority in every remaining CKD subgroup. High risk of progression increased in frequency as CKD severity increased, ranging from 42% in stage 1 to 62% among post-transplant patients. ADPKD patients were at least twice as likely to have a diagnosis of malignant or benign neoplasm as compared with controls.
Six-month medical resource use
The proportion of patients experiencing at least one hospitalization during the observation period increased with increasing CKD stage (Table 2). Per patient, the mean number of hospitalizations over 6 months averaged between 0.2 (CKD stage 1) and 0.7 (post-transplant). Among hospitalized patients, the average length of stay ranged from 5.7 days (CKD stage 1) to 7.7 days (CKD stage 3), with similar lengths of stay among dialysis patients (8.7 days) and post-transplant patients (8.6 days).
Table 2.
Demographic and clinical characteristics of ADPKD study population by CKD disease stage
| Setting of care | CKD Stage 1 (n=316) |
CKD Stage 2 (n=404) |
CKD Stage 3 (n=860) |
CKD Stage 4 (n=611) |
CKD Stage 5 (n=842) |
Dialysis (n=586) |
Post-transplant (n=615) |
P-value |
|---|---|---|---|---|---|---|---|---|
| Hospitalizations | ||||||||
| n (%) | 51 (16.1) | 49 (12.1) | 150 (17.4) | 141 (23.1) | 360 (42.8) | 274 (46.8) | 252 (41.0) | <0.0001 |
| Mean (SD) | 0.22 (0.56) | 0.18 (0.59) | 0.19 (0.57) | 0.24 (0.66) | 0.31 (0.72) | 0.59 (0.84) | 0.70 (0.97) | <0.0001 |
| Total length of stay, mean (SD) (days)a | 5.69 (7.17) | 7.22 (11.39) | 7.66 (12.09) | 6.17 (10.55) | 7.55 (8.95) | 8.71 (11.43) | 8.56 (13.67) | 0.3253 |
| Hospital outpatient visits | ||||||||
| n (%) | 218 (69.0) | 284 (70.3) | 668 (77.7) | 504 (82.5) | 779 (92.5) | 556 (94.9) | 575 (93.5) | <0.0001 |
| Mean (SD) | 3.05 (5.23) | 2.60 (4.07) | 3.50 (4.73) | 5.39 (6.89) | 11.87 (16.59) | 13.82 (19.51) | 10.10 (12.22) | <0.0001 |
| Outpatient physician office visits | ||||||||
| n (%) | 308 (97.5) | 391 (96.8) | 835 (97.1) | 601 (98.4) | 793 (94.2) | 540 (92.2) | 574 (93.3) | <0.0001 |
| Mean (SD) | 7.03 (6.19) | 6.68 (6.04) | 7.48 (6.84) | 8.87 (6.71) | 9.16 (8.44) | 8.45 (10.18) | 8.97 (8.32) | <0.0001 |
| Outpatient prescription claims | ||||||||
| n (%) | 281 (88.9) | 379 (93.8) | 824 (95.8) | 581 (95.1) | 803 (95.4) | 553 (94.4) | 579 (94.1) | 0.0003 |
| Mean (SD) | 12.22 (12.08) | 14.68 (13.98) | 18.03 (14.82) | 19.50 (14.35) | 23.31 (16.29) | 23.09 (16.48) | 27.76 (18.60) | <0.0001 |
| ESRD facility | ||||||||
| Visited an ESRD facility, n (%) | 15 (4.7) | 2 (0.5) | 15 (1.7) | 39 (6.4) | 292 (34.7) | 321 (54.8) | 26 (4.2) | <0.0001 |
| Mean (SD) ESRD visits (n) | 1.21 (8.32) | 0.19 (3.43) | 0.46 (6.44) | 1.48 (8.38) | 10.38 (26.07) | 21.22 (38.97) | 1.14 (9.18) | <0.0001 |
Note:
Mean number of hospitalizations and mean lengths of hospitalization stay were calculated only among patients with at least one visit.
Abbreviations: ADPKD, autosomal dominant polycystic kidney disease; CKD, chronic kidney disease; ESRD, end-stage renal disease; SD, standard deviation.
Most ADPKD patients had at least one hospital outpatient visit, occurring in more than 90% of CKD stage 5, dialysis, and post-transplant patients. Per patient, the average number of hospital outpatient visits increased with increasing CKD stage, ranging from 2.6 in CKD stage 2 to 13.8 among dialysis patients. Over 90% of patients had at least one physician office visit over 6 months, ranging from an average of 6.7 visits in CKD stage 2 to 9.2 in CKD stage 5. Similarly, high proportions of patients across subgroups had at least one outpatient pharmacy claim, with the 6-month mean number per patient ranging from 12.2 in CKD stage 1 (equivalent to two prescriptions per patient, assuming a 30-day prescription) to 23.3 in CKD stage 5.
Very few patients in CKD stages 1 through 4 and very few post-transplant patients had an ESRD facility visit over the observation period, although some were associated with acute kidney injury according to ICD-9-CM codes. However, many lacked explanatory codes. Nearly 35% of CKD stage 5 patients (10.4 visits per patient) and nearly 55% of dialysis patients (21.2 charges per patient) reported at least one ESRD facility charge over 6 months.
Direct medical costs
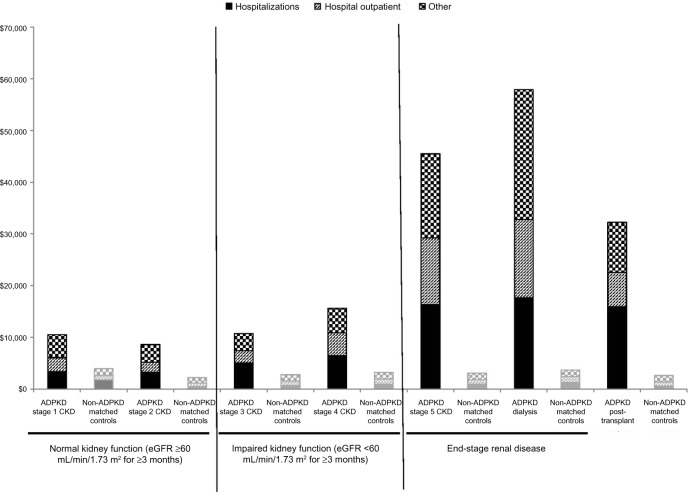
Six-month all-cause direct medical costs varied widely by disease stage and were far greater among CKD stage 5 and dialysis patients than in any other subgroup (Figure 1). Cost drivers were hospitalizations (the most common diagnosis codes used were related to hypertension, cyst complications, anemia, and dialysis-related complications) and hospital outpatient visits, which together accounted for more than half the total costs in each group. Other key cost drivers differed by CKD stage: prescription drug costs for patients with CKD stages 1–4 (over 10% of total costs) and post-transplant patients (over 20%) and ESRD facility visits for CKD stage 5 patients (23%) and dialysis patients (32%).
Figure 1.
All-cause medical, outpatient pharmacy, and total costs for patients with ADPKD compared with non-ADPKD matched-controla patients during the 6-month observation period (presented by CKD disease stage, in 2010 US dollars). aADPKD patients were grouped by CKD stage and matched by age and sex to a control population without ADPKD. Less than 1.0% of all non-ADPKD matched control patients had a diagnosis for CKD, procedure for dialysis, or an indicator for post-transplant. Plot illustrates the contrast in costs between ADPKD patients and matched controls. Additionally, increasing costs are observed with worsening CKD stage.
Abbreviations: ADPKD, autosomal dominant polycystic kidney disease; CKD, chronic kidney disease; ESRD, end-stage renal disease; eGFR, estimated glomerular filtration rate.
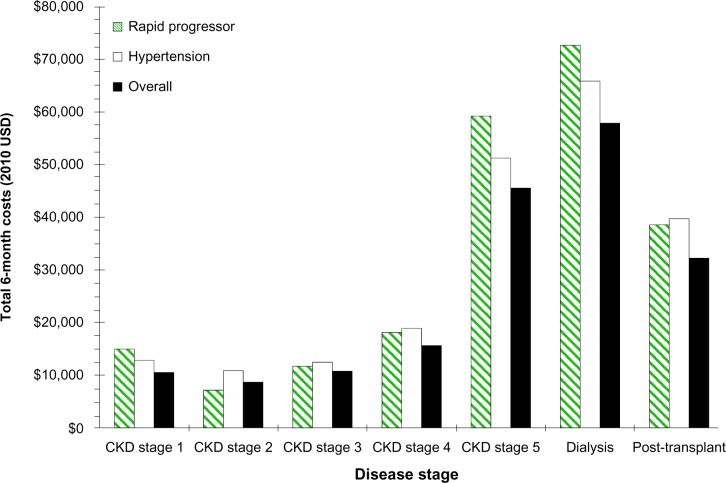
All-cause direct 6-month medical costs, by patient subgroup, are shown in Figure 2. High-risk for renal disease progression and early-onset hypertensive patients incurred higher costs than the overall sample for each CKD stage except CKD stage 2. Costs were higher among those at risk for progression in CKD stage 1 and 5 and the dialysis groups and among those with hypertension in CKD stage 2.
Figure 2.
All-cause total costs of patients with ADPKD by disease stage and by subgroup (high risk for early progression, hypertensive, and overall). Plot illustrates the increasing costs observed with worsening CKD stage and impact of subgroup on costs.
Abbreviations: ADPKD, autosomal dominant polycystic kidney disease; CKD, chronic kidney disease.
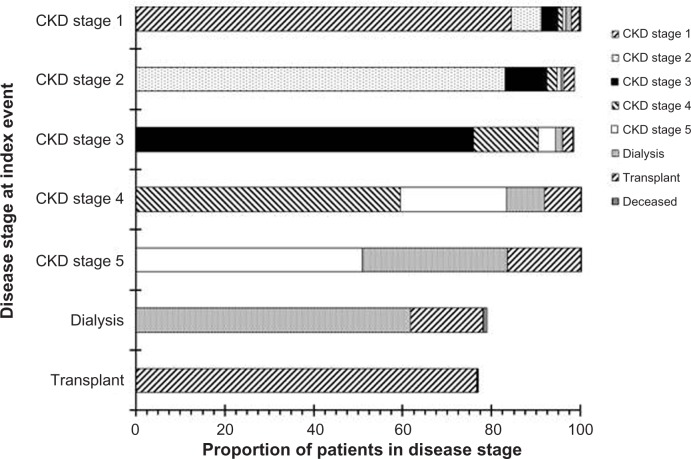
Disease progression probabilities
Six-month disease progression probabilities are shown in Figure 3. Among CKD stage 1 patients, 7% advanced to CKD stage 2, 4% to CKD stage 3, and <6% to other CKD stages. Among CKD stage 2 patients, >9% advanced to CKD stage 3, and >2% transitioned to the post-transplant state. Among CKD stage 3 patients, 14% advanced to CKD stage 4, >4% to CKD stage 5, and >3% to the post-transplant state. Among CKD stage 4 patients, 23% progressed to CKD stage 5, 9% transitioned into dialysis, and 7% moved into the post-transplant state. Among CKD stage 5 patients, 55% did not progress, 31% transitioned into dialysis, and 14% moved into the post-transplant state.
Figure 3.
Probability of disease progression by baseline disease stage.
Notes: “Deceased” refers to in-hospital mortality. Only “forward” progression (eg, from stage 1 to stage 2) probabilities are displayed. As such, 19% of dialysis patients were classified as stage 5 after 6 months, and 21% of post-transplant patients were classified as CKD stage 1–5 (with 11% classified as CKD stage 2 or 3) after 6 months.
Abbreviation: CKD, chronic kidney disease.
MRU and medical costs in matched controls versus ADPKD patients
Proportionately, across CKD stages, 4–6 times as many ADPKD patients with CKD stages 1–2 were hospitalized compared with their age-matched and sex-matched controls (Table 3). Mean costs for hospitalizations were substantially higher in ADPKD patients than in their matched controls, ranging from two to eight times higher for CKD stages 1 and 2 compared with matched controls. Mean hospital outpatient visit costs for CKD stages 1–2 ADPKD patients were approximately 3–4 times that of their controls, and differences between groups increased with the more severe disease stages. Compared with their matched controls, mean total costs for CKD stages 1 and 2 were three to four times greater and for CKD stages 3, 4, and 5, and were 4–19 times greater for dialysis patients.
Table 3.
Analysis of 6-month MRU and costs between ADPKD and non-ADPKD matched-controla patients
| Group | Hospitalizations
|
Hospital outpatient visits
|
Total
|
|||||
|---|---|---|---|---|---|---|---|---|
| n (%) | Mean costs, US$ (SD) | Median costs, US$ (range) | n (%) | Mean costs, US$ (SD) | Median costs, US$ (range) | Mean costs, US$ (SD) | Median costs, US$ (range) | |
| ADPKD stage 1 CKD (n=316) | 51 (16.1) | 3,351 (12,447) | 0 (0–79,636) | 218 (69.0) | 2,687 (9,606) | 287 (0–128,277) | 10,509 (25,186) | 2,186 (0–229,209) |
| Non-ADPKD matched controls (n=1,580) | 53 (3.4) | 1,499 (17,087) | 0 (0–444,330) | 418 (26.5) | 663 (3,138) | 0 (0–59,941) | 3,315 (19,255) | 416 (0–445,268) |
| ADPKD stage 2 CKD (n=404) | 49 (12.1) | 3,253 (19,435) | 0 (0–253,856) | 284 (70.3) | 1,903 (4,348) | 321 (0–40,424) | 8,673 (28,056) | 2,314 (0–324,609) |
| Non-ADPKD matched controls (n=2,020) | 39 (1.9) | 372 (3,687) | 0 (0–82,913) | 505 (25.0) | 583 (2,407) | 0 (0–43,822) | 1,870 (5,999) | 433 (0–130,738) |
| ADPKD stage 3 CKD (n=860) | 150 (17.4) | 5,014 (27,228) | 0 (0–601,435) | 668 (77.7) | 2,411 (6,957) | 453 (0–111,601) | 10,754 (30,929) | 3,109 (0–620,783) |
| Non-ADPKD matched controls (n=4,300) | 130 (3.0) | 650 (6,616) | 0 (0–220,286) | 1,142 (26.6) | 668 (2,916) | 0 (0–67,910) | 2,452 (8,935) | 514 (0–242,513) |
| ADPKD stage 4 CKD (n=611) | 141 (23.1) | 6,482 (21,090) | 0 (0–179,428) | 504 (82.5) | 4,391 (11,264) | 750 (0–172,441) | 15,617 (30,257) | 4,534 (0–229,002) |
| Non-ADPKD matched controls (n=3,055) | 123 (4.0) | 824 (8,492) | 0 (0–242,173) | 829 (27.1) | 727 (3,545) | 0 (0–79,480) | 2,752 (10,855) | 584 (0–243,217) |
| ADPKD stage 5 CKD (n=842) | 360 (42.8) | 16,350 (41,186) | 2 (0–509,341) | 779 (92.5) | 12,925 (31,342) | 3,155 (0–341,299) | 45,528 (65,340) | 15,876 (0–535,396) |
| Non-ADPKD matched controls (n=4,210) | 140 (3.3) | 679 (9,746) | 0 (0–444,330) | 1,156 (27.5) | 675 (3,255) | 0 (0–79,480) | 2,509 (11,779) | 550 (0–445,268) |
| ADPKD dialysis (n=586) | 274 (46.8) | 17,667 (51,648) | 518 (0–573,117) | 556 (94.9) | 15,136 (30,143) | 3,958 (0–305,464) | 57,877 (78,968) | 25,994 (35–630,791) |
| Non-ADPKD matched controls (n=2,930) | 107 (3.7) | 984 (11,964) | 0 (0–444,330) | 817 (27.9) | 767 (3,832) | 0 (0–79,480) | 2,925 (14,143) | 552 (0–445,268) |
| ADPKD post-transplant (n=615) | 252 (41.0) | 15,938 (42,376) | 0 (0–491,270) | 575 (93.5) | 6,614 (16,056) | 1,855 (0–180,184) | 32,234 (52,051) | 12,460 (0–500,055) |
| Non-ADPKD matched controls (n=3,075) | 85 (2.8) | 456 (4,067) | 0 (0–120,056) | 811 (26.4) | 649 (2,481) | 0 (0–45,874) | 2,245 (7,009) | 518 (0–134,012) |
Notes:
ADPKD patients were grouped by CKD stage and were matched by age and sex to controls without ADPKD. Less than 1.0 % of all non-ADPKD matched control patients had a diagnosis for CKD, procedure for dialysis, or an indicator for post-transplant.
Abbreviations: ADPKD, autosomal dominant polycystic kidney disease; CKD, chronic kidney disease; MRU, medical resource use; SD, standard deviation.
Discussion
The results of this study show that higher rates of MRU and costs (in all stages of kidney disease) were associated with ADPKD patients, and transitions between CKD disease stages increased with each progressive disease stage. While the disease burden of CKD is well characterized, the disease burden of ADPKD is less well understood. This analysis of commercial claims data provides real-world estimates of MRU and costs associated with ADPKD from a private US payer perspective. Specifically, this study evaluated costs from a payer perspective, assessing reimbursements rather than charges, compared cost and utilization relative to an age-matched and sex-matched healthy control cohort, evaluated economic outcomes in key patient subgroups, and studied transitions among CKD disease states.
This study showed the relative burden of ADPKD patients compared with age-matched and sex-matched healthy controls without ADPKD and that significant disease burden due to ADPKD is not only associated with patients in later stages of kidney disease, but also with patients early in the course of their disease when kidney function is still characterized as normal.
As CKD progresses, the proportion of patients at high risk for early progression of ADPKD increases. As expected, ADPKD patients with later CKD stages had higher MRU and more inpatient hospitalizations, hospital outpatient visits, physician office visits, prescription drug claims, and visits to ESRD facilities than did patients with the earlier stages of CKD.
Costs increased substantially as patients progressed from CKD stage 4 to 5 and then to ESRD, with costs among dialysis patients greatly exceeding that of post-transplant patients. Hospitalizations and hospital outpatient visits were key cost drivers (>50% of total costs) in all groups. ESRD facility costs were substantial among dialysis patients and those with CKD stage 5, while prescription drugs were relatively more important cost drivers among post-transplant and CKD stage 1–4 patients. ESRD facility costs in earlier CKD stages represent the potential consequences of acute kidney injury events, such as pyelonephritis and nephrolithiasis, which are related to the presence of ADPKD. These cost increases and cost drivers are consistent with the data reported by Lentine et al.10
Although our study sample represents those patients who actively sought health care, our analysis shows that a sizeable proportion of ADPKD patients seek care early, when signs and symptoms may not have been present. Considering that the database does not include many Medicare patients and that most of the ADPKD patients sampled in this study were found to be at high risk for progression to ESRD, these patients may have a more aggressive form of ADPKD than the general ADPKD population. However, given the hereditary nature of ADPKD and the informed nature of this patient group, at-risk individuals may have been actively seeking preventative health care before signs and symptoms developed and uncovered early-onset hypertension, albuminuria, or other risk factors for progression to ESRD. There were more women in the early CKD stages than in the later disease stages. This group could represent a subgroup of ADPKD patients who seek or require medical care at earlier CKD stages, ie, women with ADPKD who become pregnant and receive health care and are at increased risk for the development of hypertension during pregnancy.11 Even among ADPKD patients with an earlier CKD stage, hospitalizations and hospitalization costs were greater than in their healthy age-matched and sex-matched controls. Similar results were found for hospital outpatient costs.
As hospitalizations and hospital outpatient visits were the largest contributors to total costs among early-stage CKD patients (>50% of costs for stage 1–2), total costs for these patients were much greater than for their matched controls. Additionally, a high proportion of ADPKD patients had a benign or malignant neoplasm, with more than twice as many early-stage ADPKD patients affected compared with matched controls. Of these early-stage patients, 69% had benign neoplasms; among those with malignant neoplasms, most common was skin cancer, which increased in frequency with worsening kidney function and again in post-transplant patients. ADPKD patients also had higher rates of diabetes, coronary artery disease, heart failure, and hypertension than their matched controls. While it is not surprising that ADPKD patients with decreasing kidney function tended to be associated with increased MRU and costs compared with their matched controls, it is worthwhile to note that ADPKD patients in the early stages of their disease are associated with substantial increases in MRU and costs.
In this study, the rate of disease progression increased with each successive CKD stage. ADPKD patients with early-stage CKD were less likely to progress to advanced disease, while those with later-stage CKD were more likely to progress to more advanced disease. These observations are consistent with the CRISP (Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease) cohort, wherein once GFR began to decline, progression became unavoidable.12 Given that MRU and costs only increase as patients progress into worsening kidney function, it is in the best interests of patients, providers, and payers that continued research focuses on early treatment to delay progression of ADPKD.
Limitations
The current study had several limitations. Index events were determined using the first observed inpatient or outpatient claim with an indicator of CKD stage or status or a laboratory result for a creatinine test within 6 months of a medical claim for ADPKD. This possibly caused inflated estimates of MRU and cost, particularly in early-stage CKD, as we might expect increased diagnostics and repeated physician visits in the period immediately following the index event. Our study population had elevated MRU and costs in the month of the index event, but consistent per-month MRU and costs were observed for up to 3 years thereafter. Therefore, while we may have slightly inflated estimates of initial resource use and costs, we believe the effect was minimal.
Dialysis patients were found to have 21.2 ESRD facility charges in a 6-month time period, consistent with expected weekly billing practices but likely not reflective of actual facility visit numbers.
As expected, more ADPKD patients had diabetes, heart failure, coronary artery disease, and/or neoplasms than did matched controls. The proportions of matched controls with these diseases were similar to their reported prevalence in the general population,13–15 suggesting that the control population was reflective of a general population.
The typical limitations of research using a medical and pharmacy claims database apply to this study. Only patients who actively sought health care or had access to or concerns about a medical diagnosis and who had full commercial insurance were represented in the MarketScan data. As such, our sample accurately represents the payer perspective, but may not be generalizable to the broader ADPKD population, particularly patients with earlier stages of ADPKD. Given that those who do not present to a medical provider are not captured in this study, background percentages may be underreported. However, the increased resource use and background proportions among early-stage ADPKD patients when compared with controls remained relevant and important. In addition, the validity of this analysis is dependent on the accuracy of coding in the claims; coding errors or concerns about pre-existing conditions may mean that our sample missed some ADPKD patients actively seeking care and may have misclassified patients into incorrect CKD stages. Given concerns about preexisting conditions, ADPKD is not always coded or explicitly diagnosed, although it should be noted that the analysis by Lentine et al10 found no clear differences in demographic characteristics, estimated GFR, or charges between patients with a diagnosis of ADPKD (ie, ICD-9-CM 753.13) and patients with a diagnosis of polycystic kidney disease (ie, ICD-9-CM 753.12, ADPKD not specified by diagnosis code).
This study relied mostly upon CKD disease stages defined by ICD-9-CM diagnosis codes to distinguish disease severity among ADPKD patients because standard ADPKD-specific classification criteria have not yet been established. In reality, the stage and severity of ADPKD are informally determined not only by associated kidney function, but also by age, cyst burden, and symptoms. When available, estimated GFR values were used to determine kidney function, but data for laboratory results were not available for most study patients. Using stages of measured or estimated GFR to determine disease severity or stage in ADPKD is not necessarily the best measure because kidney function may appear normal for years while compensatory renal mechanisms mask the decline in functioning kidney tissue. However, this underscores the relevance and importance of our finding that ADPKD patients who appear to still have normal kidney function are associated with increased MRU and costs. Recognition of early markers of disease progression may be valuable to identify high-risk ADPKD patients for treatment with more intensive interventions.
While private payer reimbursements are valid measures more of cost than of provider charges, they tend to be higher than Medicare reimbursement and are not perfect proxies for payer costs. Further, because we reported costs by different settings of care and we noted the proportion of total costs for each setting, we chose to report means rather than medians when comparing costs between patients with ADPKD and their matched controls. It is important to understand that cost data are almost always skewed to non-normal distributions, and that the mean will be sensitive to any outliers. However, while median costs were lower than mean costs for both ADPKD patients and matched controls, the relative difference in median costs was actually greater, and statistical comparisons using the Kruskal–Wallis test showed all comparisons to be statistically significant. The full societal burden associated with ADPKD is likely understated and should be explored further, because indirect costs, such as productivity effects, lost wages, and caregiver support were not measured in our study. Finally, the retrospective nature of claims analysis does not allow outcomes to be interpreted in terms of causal relationships.
Conclusion
These results indicate that early-stage ADPKD patients with normal kidney function are associated with a sizable economic burden to the health care system relative to the general population. Nevertheless, ADPKD is far costlier at later stages, which is a concern given that disease progression rates increase as CKD worsens. Therefore, potential cost offsets could be achieved by early intervention to delay progression of ADPKD disease.
Acknowledgments
The authors thank Helen M Wilfehrt (Covance Inc.), for editorial support in preparation of this manuscript.
Footnotes
Disclosure
Otsuka Pharmaceutical Development and Commercialization, Inc. (Otsuka), sponsored this research. Covance Inc. received funding for this work. The authors include employees from Otsuka and Covance Inc., based on their roles in study design, analysis and interpretation of data, writing and revising the manuscript, and the decision to submit the manuscript for publication. TK and CS are employees of Covance Inc. which received funding for this research. HK and DO are employees of Otsuka. AC and RDP serve as investigators and steering committee members for several Otsuka clinical trials. RDP also serves as a consultant for Sanofi and Vertex, and AC serves as a consultant for Sanofi and Pfizer Pharmaceuticals.
References
- 1.Torres VE, Harris PC, Pirson Y. Autosomal dominant polycystic kidney disease. Lancet. 2007;369:1287–1301. doi: 10.1016/S0140-6736(07)60601-1. [DOI] [PubMed] [Google Scholar]
- 2.Zhou J, Pei Y. Autosomal dominant polycystic kidney disease. In: Mount DB, Pollack MR, editors. Molecular and Genetic Basis of Renal Disease: A Companion to Brenner and Rector’s The Kidney. Philadelphia, PA, USA: Saunders Elsevier; 2008. [Google Scholar]
- 3.Grantham JJ, Chapman AB, Torres VE. Volume progression in autosomal dominant polycystic kidney disease: the major factor determining clinical outcomes. Clin J Am Soc Nephrol. 2006;1:148–157. doi: 10.2215/CJN.00330705. [DOI] [PubMed] [Google Scholar]
- 4.Gabow PA. Autosomal dominant polycystic kidney disease. Am J Kidney Dis. 1993;22:511–512. doi: 10.1016/s0272-6386(12)80921-8. [DOI] [PubMed] [Google Scholar]
- 5.Taylor M, Johnson AM, Tison M, Fain P, Schrier RW. Earlier diagnosis of autosomal dominant polycystic kidney disease: importance of family history and implications for cardiovascular and renal complications. Am J Kidney Dis. 2005;46:415–423. doi: 10.1053/j.ajkd.2005.05.029. [DOI] [PubMed] [Google Scholar]
- 6.US Renal Data System . USRDS 2012 annual data report: atlas of chronic kidney disease and end-stage renal disease in the United States. Bethesda, MD, USA: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2012. [Accessed December 4, 2014]. Available from: http://www.ajkd.org/issue/S0272-6386%2812%29X0003-9. [Google Scholar]
- 7.Schrier R, McFann K, Johnson A, et al. Cardiac and renal effects of standard versus rigorous blood pressure control in autosomal-dominant polycystic kidney disease: results of a seven-year prospective randomized study. J Am Soc Nephrol. 2002;13:1733–1739. doi: 10.1097/01.asn.0000018407.60002.b9. [DOI] [PubMed] [Google Scholar]
- 8.Maschio G, Alberti D, Janin G, et al. Effect of the angiotensin-converting-enzyme inhibitor benazepril on the progression of chronic renal insufficiency. The Angiotensin-Converting-Enzyme Inhibition in Progressive Renal Insufficiency Study Group. N Engl J Med. 1996;334:939–945. doi: 10.1056/NEJM199604113341502. [DOI] [PubMed] [Google Scholar]
- 9.Klahr S, Breyer JA, Beck GJ, et al. Dietary protein restriction, blood pressure control, and the progression of polycystic kidney disease. Modification of Diet in Renal Disease Study Group. J Am Soc Nephrol. 1995;5:2037–2047. doi: 10.1681/ASN.V5122037. [DOI] [PubMed] [Google Scholar]
- 10.Lentine KL, Xiao H, Machnicki G, Gheorghian A, Schnitzler MA. Renal function and healthcare costs in patients with polycystic kidney disease. Clin J Am Soc Nephrol. 2010;5:1471–1479. doi: 10.2215/CJN.00780110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chapman AB, Johnson AM, Gabow PA. Pregnancy outcome and its relationship to progression of renal failure in autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 1994;5:1178–1185. doi: 10.1681/ASN.V551178. [DOI] [PubMed] [Google Scholar]
- 12.Rule AD, Torres VE, Chapman AB, et al. CRISP Consortium Comparison of methods for determining renal function decline in early autosomal dominant polycystic kidney disease: The Consortium of Radiologic Imaging Studies of Polycystic Kidney Disease Cohort. J Am Soc Nephrol. 2006;17:854–862. doi: 10.1681/ASN.2005070697. [DOI] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention Prevalence of Coronary Heart Disease – United States, 2006–2010. MMWR Morb Mortal Wkly Rep. 2011;60:1377–1411. [PubMed] [Google Scholar]
- 14.Go AS, Mozaffarian D, Roger VL, et al. American Heart Association Statistics Committee and Stroke Statistics Committee Heart disease and stroke statistics – 2013 update: a report from the American Heart Association. Circulation. 2013;127:e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schiller JS, Lucas JW, Ward BW, Peregoy JA. Summary health statistics for US adults: National Health Interview Survey, 2010. Vital Health Stat 10. 2012;(252):1–207. [PubMed] [Google Scholar]