Abstract
Heart failure (HF) may be accompanied by considerable alterations of left ventricular (LV) volume, depending on the particular phenotype. Two major types of HF have been identified, although heterogeneity within each category may be considerable. All variants of HF show substantially elevated LV filling pressures, which tend to induce changes in LV size and shape. Yet, one type of HF is characterized by near-normal values for LV end-diastolic volume (EDV) and even a smaller end-systolic volume (ESV) than in matched groups of persons without cardiac disease. Furthermore, accumulating evidence indicates that, both in terms of shape and size, in men and women, the heart reacts differently to adaptive stimuli as well as to certain pharmacological interventions. Adjustments of ESV and EDV such as in HF patients are associated with (reverse) remodeling mechanisms. Therefore, it is logical to analyze HF subtypes in a graphical representation that relates ESV to EDV. Following this route, one may expect that the two major phenotypes of HF are identified as distinct entities localized in different areas of the LV volume domain. The precise coordinates of this position imply unique characteristics in terms of the actual operating point for LV volume regulation. Evidently, ejection fraction (EF; equal to 1 minus the ratio of ESV and EDV) carries little information within the LV volume representation. Thus far, classification of HF is based on information regarding EF combined with EDV. Our analysis shows that ESV in the two HF groups follows different patterns in dependency of EDV. This observation suggests that a superior HF classification system should primarily be founded on information embodied by ESV.
Keywords: heart failure, ventricular volume regulation, ejection fraction
Introduction
Analytical frameworks offer researchers not only techniques and paradigmatic modes of understanding disease, but also specific ways of constructing analogies and developing classifications.
quoted from Amsterdamska and Hiddinga1
Studying the geometry of the left ventricle (LV) is a central issue for cardiovascular physiologists since the classic observations on the pulmonary circulation in the 16th and 17th centuries described by Miguel Servetus (1511–1553)2 from Spain, followed by Robert Fludd (1574–1637)3 and William Harvey (1578–1657),4 both from England.5 Owing to instructions formulated by Calvin, poor Miguel died young because he was, together with his book, burnt at the stake in Champel (near Geneva). Robert found time to expand his ideas about blood circulation by working on a comprehensive system to describe the whole of creation,6 besides attempts to develop a perpetuum mobile. William, after all, became famous, thanks to Robert who encouraged him to get the concept on pulmonary circulation published, notwithstanding Miguel’s fate. It is not impossible that on a rainy afternoon, William discovered Miguel’s book in the library at the time when he resided in Padua. Much later, the Irish laryngologist Sir Robert H. Woods (1865–1938) emphasized the significance of ventricular geometry in the analysis of cardiac function, when in 1892, he applied Laplace’s law to the heart.7 A particular shape as well as a matching (or optimal) cavity size are important for the proper functioning of the LV.8–14 The two requirements assure efficient pumping, ie, the desired performance (in terms of pressure level and cardiac output (CO), both at rest and during exercise) at minimal energetic cost in relation to myocardial oxygen consumption.15
The healthy LV resembles an oblique ellipsoid (much like a pear, but rather flat on one side – reflecting the interventric-ular septum), implying that a standard fiber shortening of only 15% permits up to 70% of forward pumping relative to the LV filling volume. This high level of efficiency is achieved by the helical course of the muscle fibers, also termed geodesic curves arrangement16 or helical ventricular myocardial band17,18 in combination with the LV torsion, which becomes manifest during contraction. In addition, we observe that a naturally occurring vortex movement of the blood within the LV cavity results in an optimal transport direction. Facing the fact that both inlet (mitral valves) and outlet (aortic valves) for the LV are located in the same geometrical plane, the bloodstream has to reverse direction, and this is accomplished by the creation of a forceful inlet jet initiating rotation of the blood near the apex, thus resembling a whirlpool. Obviously, depending on the type and extent of aberrations relative to the natural shape and size, these two processes (ie, torsion and vortex) become disturbed to a mild or more severe degree.
In contrast to the healthy human LV with an ellipsoid shape, the pathologically dilated LV tends to become more spherical, thus attaining a better volume-to-surface ratio. Volume overload and pressure overload are pronounced causes of alterations with respect to size and shape. Aortic stenosis and chronic hypertension impose pressure overload on the LV and induce concentric hypertrophy without appreciable dilatation. LV wall thickness may increase by 25–75%, thereby doubling or tripling LV mass. Chronic aortic or mitral regurgitation and dilated cardiomyopathy (DCM) impose a volume overload with LV chamber dilatation, with or without hypertrophy. This leads to a more globoid heart with increased base-apex and short-axis dimensions. Hypertrophy decreases myocardial compliance and, as a consequence, impairs diastolic filling.19 Impaired diastolic reserve results in a dobutamine stress-induced increase in LV end-diastolic pressure (EDP) of patients with HF with preserved ejection fraction (HFpEF), thus giving rise to exercise intolerance with breathlessness.20
Description of dynamic geometrical information on the LV comprises time-dependent details on both size and shape during the cardiac cycle. When considering the volume domain for the characteristics of LV-operating conditions, it is obvious that aspects of the shape cannot be neglected. Ideally, a three-dimensional graph should be employed to simultaneously depict variations in intraventricular volume and characteristics of shape as a function of time.
For practical purposes, the apex to base length of the LV has been divided into three portions, namely, basal (corresponding to the mitral leaflets and tendinous cords), mid-ventricular (mitral papillary muscles), and apical levels. Each level is then further divided into segments, thus forming the basis for regional wall motion analysis.19 Although in the past most authors have concluded that major axis shortening contributes minimally to volume displacement during ejection, the exact contribution and the geometry of major axis shortening have varied substantially.21 The degree of non-sphericity has, for example, been calculated and expressed as eccentricity,21,22 but unfortunately, this approach was not adopted as a routine measure when evaluating the LV function. With advancing age, a change in LV geometry is also observed.19 Recently, the importance of long axis (shortening) has regained interest, particularly with respect to heart failure (HF).23–25 Indeed, it would be informative to include such a measure of LV shape into the LV volume domain representation. The present contribution primarily concerns the LV, but mostly the considerations mentioned equally apply to the right ventricle (RV).
Twisting of heart muscle fibers has been described as a helical arrangement with geodesic properties.16 An application of advanced techniques such as magnetic resonance imaging or speckle-tracking echocardiography allows for the accurate determination of both apical and basal rotations.26 The latter two are in opposite directions during ventricular contraction, leading to LV torsional deformation during systole. Twist can be defined as the net difference of the two rotation angles. Torsion is the net result of counterclockwise rotation of the base with respect to the clockwise rotation of the apex along the LV long axis.27 Normal torsion is a component of systolic function and contributes to an energy-efficient ejection. The subsequent LV untwisting is a key determinant of diastolic function.27 However, the isovolumic relaxation time constant does not correlate with the untwisting rate in HFpEF patients.27 LV torsion can be calculated as twist divided by LV size.26 Also, children and young adult patients with acute myocarditis and normal values for ejection fraction (EF) having LV systolic and diastolic dysfunction (DD) on echocardiography were studied. Tissue velocity, deformation, and twist parameters (increased twist rate and earlier time to peak twist) showed potential to improve the detection of these patients.28 Twist during contraction contributes to exercise capacity,29 and delayed untwist significantly limits exercise capacity in patients with non-obstructive hypertrophic cardiomyopathy.29 The effect of DD on the timing of LV diastolic longitudinal and circumferential expansions and their load dependence has recently been described. The intraventricular pressure difference (IVPD; as a measure of the strength of LV diastolic suction, with a value of 2.2 mmHg as the lower limit of normal) from the left atrium (LA) to the LV apex is obtained using color M-mode Doppler data to integrate the one-dimensional incompressible Euler equation. In DD, the normal dependence of longitudinal strain rate echocardiography (SRE) on IVPD is reduced, while the relationship of circumferential SRE to IVPD is unchanged. Normally, the LV expands symmetrically during early diastole, and both longitudinal and circumferential expansions are related to the IVPD. With DD, early diastolic longitudinal LV expansion is delayed, occurring after the IVPD and LV filling, resulting in their relative independence from the IVPD. In contrast, with DD, circumferential SRE and mitral inflow are not delayed, while their normal relation to the IVPD is unchanged.30 Also, it has been found that global LV longitudinal strain is closely associated with increased neurohormonal activation after acute myocardial infarction (MI) in patients with both HF phenotypes.31
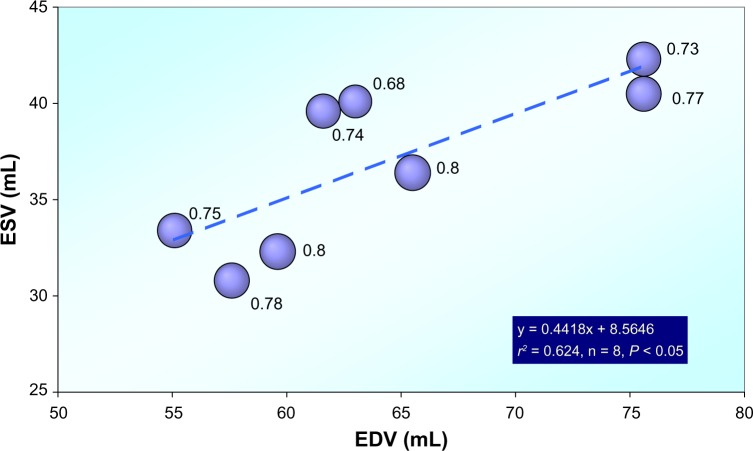
Rankin et al found for instrumented dogs in the conscious state, a shortening of the minor axis diameter, lengthening of the major axis diameter, and slight thickening or thinning of the wall during isovolumic contraction (isovolumic ellipticalization pattern, see Fig. 1). During caval vein occlusion, the contraction pattern changed from isovolumic ellipticalization to isovolumic sphericalization as the end-diastolic volume (EDV) decreased.21 Thus, the exact pattern of left ventricular contraction was found to be a function of LV volume.22 The geometry of the LV was represented as a three-dimensional ellipsoidal shell. Left ventricular eccentricity was found to be a linear function of ventricular volume during both diastole and ejection. However, the relationship was not the same for diastole and ejection, and during diastole, the LV was more spherical at large volumes and more elliptical at small volumes than during ejection. The rearrangements in geometry observed during isovolumic contraction appear to be transitional stages from the diastolic to the ejection-phase relationship. Thus, during isovolumic contraction, the LV becomes more elliptical at large volumes and more spherical at small volumes. These relationships are not altered significantly by increased afterload or inotropic interventions. Nowadays, applying statistically derived fiber models, even personalized cardiac geometry comes within reach.32
Figure 1.
Dynamic geometry of the left ventricle as studied in eight chronically instrumented conscious dogs. ESV versus EDV.
Note: Bubble size indicates eccentricity at the end of systole. Data derived from Rankin et al.21
Hypertrophy
Alterations in cardiac structure and function have been recognized as markers of increased risk for cardiovascular events. LV (static) geometry can be defined on the basis of LV mass and relative wall thickness (RWT).14 Chronic LV pressure overload states, as seen in long-standing systemic arterial hypertension or LV outflow tract obstruction, are well tolerated for many years. In such pathophysiologic circumstances, an increase in the ratio of LV wall thickness to chamber radius mitigates but does not necessarily fully normalize the after-load (forces against which the myocardium shortens during systole). Although beneficial, this adaptive response, known as concentric hypertrophy, may also cause a reduction in LV chamber distensibility and, in some instances, an increase in the elastic stiffness of the myocardium. With extended passage of time, the increased afterload imposed by hypertension or LV outflow obstruction outstrips all salutary adaptive mechanisms, preload reserve becomes exhausted despite LV chamber dilation, and basal contractility becomes mismatched to the level of afterload, provoking a lower extent and speed of shortening of the LV chamber. Concomitant elevation of both the LV EDP and LA means pressure gives rise to pulmonary capillary and alveolar congestion. Accompanying reduced fractional shortening is associated with an inadequate CO during exercise and sometimes even at rest. The patient with a pressure overload condition is then exhibiting the clinical syndrome of congestive HF (CHF).33
Ventricular Remodeling in Pressure Overload States
Though the traditional biomechanical construct of the pathophysiology of pressure overload hypertrophy (POH) has validity, it fails today to capture fully the much broader adaptations (genetic, molecular, and neurohumoral) that are known to accompany the development of ventricular dilation, and other clinical manifestations of HF, in diseases associated with POH. These latter changes have come to be known as cardiac remodeling, a term that, at first reception, connotes gross pathoanatomic change,34 but which, by extended definition, encompasses a host of alterations in homeostatic mechanisms (endocrine, autocrine, and paracrine). Under normal conditions, these mechanisms control vascular tone, blood volume, basal contractile state, and apoptosis, as well as the architectural integrity and organization of myocardial sarcomeres. These homeostatic mechanisms, like concentric hypertrophy, can be considered not only protective and reparative but also detrimental. With sustained pressure overload, experimental and clinical evidence shows that tissue heterogeneity develops with an imbalance struck between the muscular and non-muscular components of the myocardium. Scarring replaces necrotic myocytes, and a reactive fibrosis is observed in the interstitium and perivascular areas. Hypertrophy and hyperplasia of vascular smooth cells lead to medial thickening and loss of coronary arteriolar vasodilator reserve. Associated with these changes, the LV dilates, myocardial contractility may be either normal or diminished, diastolic chamber stiffness is variably normal or decreased, and myocardial stiffness may be variably normal or increased. What seems indisputable is that in humans, an augmented incidence of CHF, arrhythmias, and coronary events occurs.33
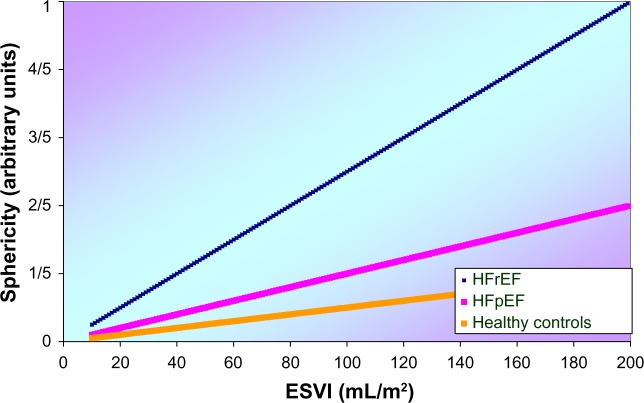
Cardiac remodeling involves molecular, cellular, and interstitial changes that manifest clinically as changes in size, shape, and function of the heart after injury or stress stimulation. Although the expression cardiac remodeling initially referred to changes following MI, it is clear that similar processes transpire following other types of injury such as with pressure overload, inflammatory disease (myocarditis), idiopathic DCM, and volume overload. While the etiologies of these diseases differ, they share molecular, biochemical, and cellular events to collectively change the shape of the myocar-dium.35 The net result of these modifications at the ultrastruc-tural level is expressed by typical macroscopic findings, per diagnostic group visible as characteristic properties in terms of size and shape (Fig. 2).
Figure 2.
A schematic model for the left ventricle in terms of ellipsoid shape and sphericity for three different conditions, ie, control, HFpEF, and HF with reduced ejection fraction (HFrEF).
Relative Pathogenic Roles of Myocardial Contractility and Cardiac Remodeling
The response of the heart to pressure overload is hypertrophy. The initial adaptive hypertrophic response of the myocardium is characterized by structural and molecular changes, such as wall thickening, and increases in cardiomyocyte size and contractile protein content without cellular proliferation. In later stages, LV hypertrophy becomes maladaptive. This maladaptive remodeling process is characterized by LV dilation and impaired function. It is a major risk factor for the development of CHF and a cause for ischemia, arrhythmia, and sudden death.36 It is within the broader construct of the natural history of POH that the question has been posed: “How important is reduced contractility itself, as opposed to other effects of cardiac remodeling, in the manifestations of CHF?” This question was studied in a rat model of POH created by suprarenal artery banding. Approximately one-half of the POH animals studied developed manifestations of CHF (POH-F group) with lung edema, significant LV chamber dilation, and a non-significant increase in wall thickness compared to control rats. The other one-half showed concentric myocardial hypertrophy in response to the pressure overload but no evidence of LV chamber dilation or an increase in their lung weight to body weight ratio (POH-NF group). Compared to controls, both POH groups showed reduction in myocardial contractility when assessed by ex vivo end-systolic stress–strain relations; by contrast, the in vivo systolic myocardial end-systolic myocardial stiffness analysis failed to detect a depression of myocardial contractility. The in vivo stress-shortening relations suggested a small and equivalent decrement in the two POH groups compared to controls. Despite this essential equivalency of myocardial contractility, whether normal or depressed, only the POH-F group manifested ventricular dilation, wall thinning, and lung edema, leading the investigators to conclude that CHF was dependent primarily upon the processes of remodeling. It is important to recognize that CHF could not be attributed solely and categorically to one pathogenetic mechanism as opposed to the other. Although there is inconsistency between the in vivo and ex vivo systolic stiffness analyses, some reduction in contractility appeared to be operative in both groups. This depression may be time dependent and is witnessed only if the offending disease process has been operative for a sufficient period of time. Nevertheless, because of the important influence of intrinsic ventricular size (or chamber remodeling based upon increased sarcomeres laid down in series) on the pressure–diameter or pressure–volume relation, around the year 1985, some investigators in the field often analyzed myocardial contractility by an end-systolic force-normalized dimension relation. Myocardial stiffness, calculated from the stiffness constant of the midwall stress–strain relation, is particularly appealing for overcoming the confounding effects of chamber size. The use of this construct would seem particularly important in an experimental or clinical investigation where chamber remodeling is occurring. Thus, the finding that end-systolic myocardial stiffness was equivalent in both POH groups, despite significant dilation only in those showing CHF, appears supportable.33
A recent study reported significant sex differences in LV remodeling because of pressure overload. Women develop more concentric hypertrophy with smaller ventricular diameter, greater RWT, and a better systolic function, while men demonstrate more pronounced chamber dilation. Compared to men in this study, the women had a significantly (P < 0.001) smaller LV end-diastolic diameter, less (P < 0.004) LV mass, and larger (P < 0.036) RWT. Remarkably, EF was not different.36 These sex differences are partly expected to be the outcome of differences in myocardial collagen mRNA expression and architecture between males and females. However, the molecular mechanisms underlying the sexually dimorphic response of the heart to pressure overload, possibly leading to HF, are not completely understood. Moreover, inflammation is expected to contribute to sex differences in LV remodeling. These authors also found a significant increase in activation of inflammatory signaling in female mice lacking estrogen receptor β.37 However, little is known about the regulation of the inflammatory response in human LV remodeling. Thus, in pressure overload, when comparing men to women, distinct molecular processes are found that regulate remodeling. Maladaptive LV remodeling occurs more frequently in men and is associated with greater activation of profibrotic and inflammatory markers.36 Recognition of the detrimental role of neurohu-moral activation in HF has led to neurohumoral antagonism, leading to reduced morbidity and mortality. Yet, the persisting disability and death rates remain unacceptably high. The search for novel strategies requires understanding of basic cardiac physiology at levels ranging from the molecular to the systemic in order to identify new targets for the treatment of HF. This approach includes analysis of diastolic ventricular interaction and cardiac energetics. Emerging therapeutic approaches (for example, biventricular pacing in HF) have proved successful, and identification of novel therapy modes (for example, per-hexiline as an energy augmentation agent) is on the horizon.38
Adjusting Ventricular Size and Shape
LV size and muscle mass variation may induce deviations of shape (as they occur in certain cardiopathological conditions) but usually at the expense of efficiency. Pathologic cardiac remodeling can be dramatic and rapid. Myosin heavy-chain synthesis increases by as much as 35% within hours after exposure to an elevated afterload. Enforced expression of an activated transgenic Akt1 gene triggers an increase in cardiac mass by 60% in just two weeks. Concentric remodeling is an increase in RWT but with normal cardiac mass. Concentric hypertrophy is an increase in RWT and cardiac mass with little or no change in chamber volume. Eccentric hypertrophy is an increase in cardiac mass with increased chamber volume.35,39
The highly endurance-trained athlete’s heart represents the most extreme form of cardiac adaptation to physical stress, but its circulatory alterations largely remain obscure. Increased myocardial blood transition time enables higher oxygen extraction levels with a lower myocardial blood flow and a higher vascular resistance. These physiological adaptations to exercise training occur independently of the level of oxygen consumption and together with training-induced bradycardia may serve as mechanisms to increase functional reserve of the human heart.40
Dilation and hypertrophy are common modes of adaptation in the failing heart. Many pathological changes are accompanied by a tendency of the LV to remodel the shape and assume a more spherical form, thus decreasing efficiency. Variations in cavity shape are important but difficult to measure. Alterations in size can rather easily be determined by measuring LV end-systolic volume (ESV) and EDV.11 Clearly, it is not very informative to consider only the ratio of ESV and EDV. Yet, in clinical practice, this route is often followed by calculating the index EF as (1 − ESV/EDV) × 100%.
Modification of Size and Shape by Therapeutic Intervention
Various techniques have been developed to modify size and shape of the LV in cardiac patients with serious mechanical abnormalities of their ventricular wall. In particular, for HF patients, a spectrum of surgical interventions aiming at LV volume reduction has been developed, partly from a physiologic perspective.41 Details on surgical approaches are covered by various publications. Reduce or reshape has been a central issue.13 LV reconstruction with respect to LV aneurysm and reshaping techniques for patients with end-systolic volume index (ESVI) >60 mL/m2 has been described.42 Surgical ventricular reconstruction (SVR) involves resection of scar, septal exclusion, cavity reduction by endoventricular patch, and complete coronary grafting.43 Recently, the persistence of volume reduction employing a technique designed to be used on beating hearts without ventriculotomy or cardiopulmonary bypass was demonstrated. The extent of volume reduction was consistent with results of conventional SVR in experienced centers. The 11 months follow-up outcomes validate the further development of technical iterations, leading to a clinical study employing a closed chest endovascular platform.44 Medical and device therapies that reduce HF-related morbidity and mortality also lead to decreased LV volume and mass, and return to a more normal elliptical shape of the LV. These reversal manifestations are because of changes in myocyte size, structure, and organization, which have been referred to collectively as reverse remodeling.45 Moreover, there are subsets of patients whose hearts have undergone reverse remodeling either spontaneously or after medical or device therapies and whose clinical course is associated with freedom from future HF events. This phenomenon has been referred to as myocardial recovery. Despite the frequent interchangeable use of the terms myocardial recovery and reverse remodeling to describe the reversal of various aspects of the HF phenotype after medical and device therapy, the literature suggests that there are important differences between these two phenomena and that myocardial recovery and reverse remodeling are not synonymous.45 Furthermore, it is known that cardiac resynchronization therapy may reduce ESV up to 30% (with a moderate amelioration of EF), especially in patients referred to as super-responders.46
Heart Failure
HF may occur at any level of EF, not just at reduced EF. DD is apparent in all patients with HF, regardless of EF.47 However, diagnosing DD is difficult (particularly when relying on echocardiographic images) and not well defined; some of the patients in clinical trials may not have had HF, while only about 65% of patients in one substudy had objective evidence of DD.48
HFpEF indicates that the EDV is appropriate for the stroke volume (SV), and HF with reduced ejection fraction (HFrEF) means that the EDV is enlarged relative to SV (ie, the LV is dilated). Most therapies proven to be effective in HFrEF (such as angiotensin converting enzyme (ACE)-inhibitors, angiotensin receptor blockers, beta-blockers (BB), and cardiac resynchronization) reverse LV dilation. However, these therapies fail to be effective in HFpEF.47
Remarkably, it is reported that in an HFpEF group, even smaller ESVs occur than in a matched group of persons without cardiac disease.49 Also, EF is even significantly (67 vs 62%, P < 0.02) higher in the HFpEF group than in controls with non-cardiac causes of breathlessness who had significantly (P < 0.01) lower systolic and diastolic blood pressures.20 Similar findings have been noted elsewhere.50
It has been suggested that diastolic and systolic HF form a continuum rather than two different entities, despite the fact that understanding the mechanisms involved in HFpEF remains a complex task.48 Classification and the exact mechanism of HFpEF are still debated.51 The pathophysiology of HFpEF is complex, but the molecular aspects of HFpEF as well as the profoundly disturbed hemodynamics with particular focus on exercise hemodynamic abnormalities have been studied, indicating that increased vascular and LV stiffness, inadequate LV relaxation (related to fibrosis and changed titin isoform expression), atrial dysfunction, rising blood pressure during exercise, chronotropic incompetence, and comorbidities (such as diabetes, obesity, anemia, and kidney problems) all contribute.52 Titin is a large cytoskeletal protein involved in the resting stiffness of the myocardium. A shift toward the stiffer N2B isoform was shown in the HFpEF group, in contrast to an N2BA isoform, in the HFrEF group.53 Later, it was found that protein kinase G-dependent phosphorylation of the N2B was involved. Also, endothelial dysfunction plays a certain key role in DD.
Pressure loading in a canine model of LV dysfunction with pEF and rEF resulted in similar degrees of LV dilatation, increased filling pressures, and increased index of myocardial performance.54 Also in dogs, Mathieu et al investigated the physiopathology of HFpEF in a model of healed MI induced by coronary ligation while using echocardiography, levels of neurohormones, and conductance catheter measurements two months after a coronary artery ligation, compared to controls. Healed MI was associated with preserved echocardiographic EF (57%), increased NT-proBNP, decreased aldosterone, unchanged norepinephrine, marked decrease of end-systolic elastance (2.1 vs 6.1 mmHg/mL, P < 0.001), and coupling index (0.6 vs 1.4, P < 0.001), but preserved LV capacitance (70 vs 61 mL at 20 mmHg, P = NS) and stiffness constant. From their canine model of healed MI, it may be concluded that HF is essentially characterized by an altered contractility with ventricular-arterial uncoupling despite vascular compensation, rather than by abnormal diastolic function.55
The two phenotypes not only differ in terms of their volume regulation56 but also dissimilar outcome of BB therapy in both HF phenotypes has been observed. Hamdani et al investigated whether BB have distinct myocardial effects in HFpEF and HFrEF. Myocardial structure, cardiomyocyte function, and myocardial protein composition were compared in HFpEF and HFrEF patients without or with BB. Patients without coronary artery disease were divided into those with and without BB for both phenotypes. Using LV endomyocardial biopsies, they assessed collagen volume fraction (CVF) and cardiomyocyte diameter (MyD) by histomorphometry, phosphorylation of myofilament proteins by ProQ-Diamond phosphostained 1D-gels, and expression of beta-adrenergic signaling and calcium handling proteins by western immunoblotting. Cardiomyocytes were also isolated from the biopsies to measure active force (Factive), resting force (Fpassive), and calcium sensitivity (pCa50). Myocardial effects of BB therapy were either shared by HFpEF and HFrEF, unique to HFpEF or unique to HFrEF. Higher Factive, higher pCa50, lower phosphorylation of troponin I and myosin-binding protein C, and lower beta-2 adrenergic receptor expression were shared. Higher Fpassive, lower CVF, lower MyD, and lower expression of stimulatory G protein were unique to HFpEF, and lower expression of inhibitory G protein was unique to HFrEF.57
In an editorial,12 focus was on restoring the normal elliptical shape of the LV in cases of spherical dilation, with preference to analyze ESVI in combination with percent asynergy, rather than the single index EF. Furthermore, it was noted that the term remodeling is used extensively in current research. In the dictionary edited by Segen,58 we find the following description for the lemma remodeling: The progressive LV dilation that follows an MI, a finding that has prognostic import; remodeling can be quantified by measuring the ESV and EDV, along with a reference to a publication.59 As an earlier source, we found Pfeffer et al describing that captopril attenuates LV remodeling (dilation) and deterioration in performance in rats with chronic MI.34
It is anticipated that HFpEF will emerge as the predominant form of HF throughout the world in the near future because of two factors: (1) greater availability of therapeutic interventions that limit myocardial damage (particularly in the setting of an acute MI) should reduce the incidence of HFrEF and (2) since diastolic properties of the heart that occur with aging are clearly involved in HFpEF, this phenotype will become more common as the mean age of the population increases.60
The healthy LV displays a uniform contraction, more precisely, a somewhat asymmetrical 3D-contraction against the rather fixed structures of the interventricular septum. The contraction process normally starts near the apex and moves up toward the base, and is accompanied by a torsion that shortens the long axis by about 10%. Dyssynchrony constitutes an important disturbance of the process, leading to depressed cardiac performance. The combination of muscle fiber shortening, rotation of the apex in the direction of the base, and the squeezing action because of the progressing activation pattern normally result in a forward pumping efficiency of about 70%. This means that the amount of blood effectively pumped into the aorta (a quantity termed SV) comprises more than two-thirds of the filling volume (EDV) in humans. This ratio is duly reflected by the term EF. Indeed, this fraction is nothing more than a dimensionless ratio, implying that ventricles of totally different shapes and/or sizes may have identical values for EF. This notion underscores the intrinsic ambiguity of EF as an indicator of cardiac function. Taking EF as a starting point, one may wonder what impact regarding discriminating power remains in, for example, HF patients. Clearly, wall motion abnormalities deserve special attention because they may occur in an LV with near-normal shape and size. Apart from ESV, such local wall motion deviations (or percent asynergy) need consideration.12
Starling’s law and end-systolic volume
With a few exceptions,61 the insights offered by a representation of ESV versus EDV have not been studied in detail in the literature. Of particular interest is the implicit role of EF in this graphical representation. Already in 1981 we published a paper62 showing that the significant (inverse) correlation between EF and ESV becomes blurred in patients chronically using BB. When submitting this material, it was not yet clear whether the volume regulation graph (VRG) constitutes the cornerstone for the analysis of ventricular dynamics. In 1998, we demonstrated63 that the VRG (at that time still called the alternative starling curve – abbreviated as ASC) offers a unifying framework to visually interpret ventricular function, both for LV and RV. Related clinical studies concerned estimation of myocardial oxygen consumption (MVO2) on the basis of the VRG,64 effects of aging in dogs with cardiac disease,65 and diabetes mellitus66 in humans in connection with the ASC, as well as investigations in growing foals67 and two types of HF patients.56 Along the theoretical line, we clarified the role of ASC versus classical Starling curve63 and the mathematical connection between ASC determinants and the systolic elastance concept.66,68 Finally, we dismantled the presumed pivotal role of EF, developed a Monte Carlo simulation model for HF phenotype transitions,69 and formulated the dependence of LV-arterial coupling on the characteristics of the ASC.70 Recently, we replaced the term ASC by VRG because the former unintendedly seems to suggest that Starling’s law is of minor importance, while the latter abbreviation more appropriately focuses on the central issue regarding regulation of LV volume.
Theoretical Background
ESV and EDV are related during a cardiac cycle. A linear equation is assumed for the VRG:
| (1) |
with Pearson’s coefficient of correlation r, where α (mL) is the volume axis intercept and β (dimensionless) the slope of the regression line. Besides EDV, the VRG may include further determinants Fi (independent of end-diastolic volume index (EDVI) if their cross-products vanish) with corresponding weight factors Wi. For each i, both Fi and Wi are to be identified using multiple regression analysis. Thus, equation (1) can be generalized to yield
| (2) |
with new α′ (mL) as the volume axis intercept and β′ (dimensionless) as the slope. Σ is the sum sign, and i represents a running number. Fi can be continuous variables, such as age, heart rate (HR), and LV filling pressure, as well as dichotomous covariates such as gender and administration of particular medication. The VRG permits easy introduction of third and higher dimensions, including (dichotomous) covariables by employing a stratification procedure. Subsequently, the crude model can be reduced by selectively omitting elements contained in ΣWiFi.
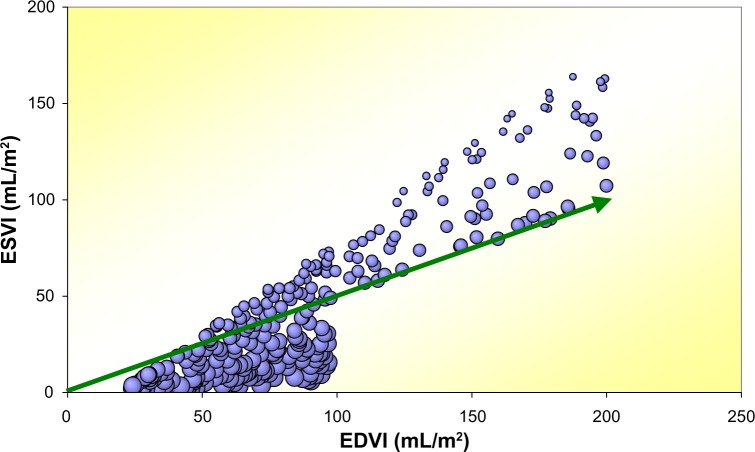
This approach allows for a more personalized description that includes age, sex, adherence to pharmaceutical agents, etc. Figure 3 illustrates the two common HF phenotypes based on Monte Carlo-generated surrogate patients. The green arrow represents the linear dividing line as suggested in various guidelines.71 Bubble size reflects the pertinent value of EF, and obviously, decreases as ESVI becomes larger at identical EDVI values.
Figure 3.
Monte Carlo generated HF data for simulation of HFpEF and HFrEF patients with EF cutoff at 50%, n = 287.
Note: Bubble size corresponds with the pertaining magnitude of EF for each surrogate patient. The green arrow is the 50% iso-EF line.
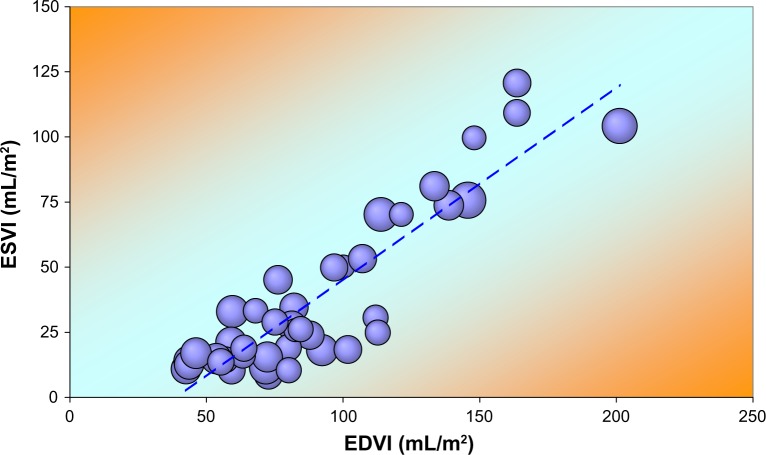
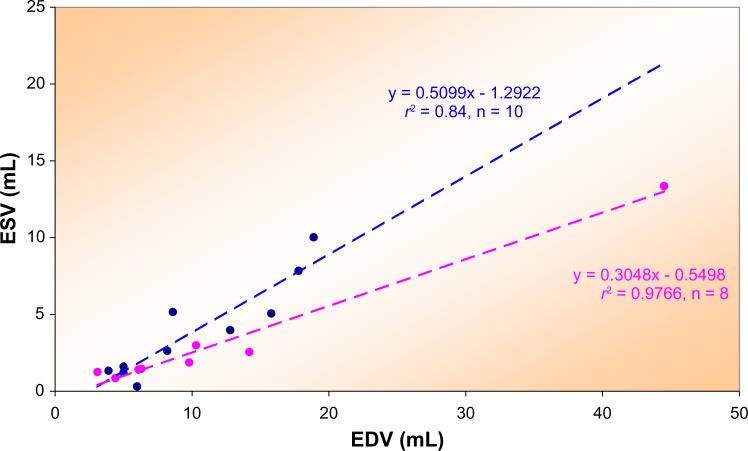
As an example of a distinct subgroup, we show (Fig. 4) the VRG for men 50 years and older, not using BB and with EDP <17 mmHg. Bubble size reflects HR (range 47–114 bpm). Linear regression yields:
Figure 4.
Volume regulation graph illustrating ESVI versus EDVI for 42 older men not using BB and without HF.
Note: Bubble size reflects HR. The broken line results from linear regression analysis (see text).
Various codeterminants of the VRG are briefly discussed:
– Age: advancing age is the most important non-modifiable prognostic factor for long-term prognosis, whereas LV function assessed clinically or measured as either EF or ESV is the most important modifiable factor.72 Compared to the situation at birth, the human heart’s mass increases by about 13 times when reaching the age of 24 years, and the ratio of heart mass to body mass decreases in the time span from 0.76 to 0.45%.16 The growth process has been studied echocardiographically in foals.67 In the normal elderly heart, LV geometry is altered with a more sigmoid septum, while the LV outflow tract changes shape and direction, simulating hypertrophic cardiomyopathy.19 Aging is also associated with increased LV torsion secondary to reduced rotational deformation delay and increased peak basal rotation. LV torsion and strain patterns in patients with HFpEF are similar to age-related changes, apart from circumferential strain, which is enhanced in patients with HFpEF. Both LV torsion and untwist rate are significantly correlated with EDVI and ESVI.27 Although the underlying causes of HFpEF have yet to be fully delineated, changes in the diastolic properties of the heart that occur with aging are clearly involved.60,73,74 Age is also the dominant risk factor for cardiovascular diseases. Understanding the coupling between the LV and arterial system provides important mechanistic insights into the complex cardiovascular system and its changes with aging in the absence and presence of disease. Arterial-ventricular coupling (k) can be expressed as Emax/Ea, where Emax is the LV end-systolic elastance and Ea is the effective arterial elastance, which is a measure of the net arterial load exerted on the LV. Age-associated alterations in arterial structure and function, including diameter, wall thickness, wall stiffness, and endothelial dysfunction, contribute to a gradual increase in resting Ea with age. Remarkably, there is a corresponding increase in resting Emax with age, because of alterations in LV remodeling (loss in myocyte number, increased collagen) and function. These age-adaptations at rest likely occur, at least, in response to the age-associated increase in Ea. Optimal coupling at rest is also maintained when aging is accompanied by the presence of hypertension and obesity. In contrast, in HF patients (either with rEF or pEF), k at rest is impaired. Thus, although increased stiffness of the arteries itself has important physiological and clinical relevance, such changes also have major implications for the heart.74–76 Finally, age variance of LV diameters was studied in dogs with cardiac disease.65
– Gender: Gender differences in electrophysiological gene expression in failing and non-failing human hearts have been reported.77 Gender effects on LV volume regulation in patients with beta-adrenergic blockade have been detected.78 Another study reported significant sex differences in LV remodeling because of pressure overload. Women develop more concentric hypertrophy with smaller ventricular diameter, greater RWT, and a better systolic function, while men demonstrate more pronounced chamber dilation.36 HF in women differs in many aspects from that of men. Contrasts in origin, diagnostic yield, prognosis, and possibly response to treatment have been outlined. Some of these differences may have a pathophysiological basis.79
– HR: Sinus cycle length is longer in men than women.80 This difference appears to be associated with a gender difference in exercise capacity rather than intrinsic gender-related properties of the sinus node or differences in autonomic tone. In addition, exercise-induced bradycardia is mediated by autonomic as well as non-autonomic factors in both genders.80 In the LV volume domain, we detected a parallel leftward shift of the ESV– EDV regression line for the LV in pigs as well as for the RV in horses after atropine administration,81 implying a significant change in α in equation (1).
– Presence of atrial fibrillation: The presence of atrial fibrillation and effect on the VRG has been described.82
– Presence of diabetes mellitus: The presence of diabetes mellitus has been analyzed using the VRG.66
– Thyroid dysfunction: Using radionuclide ventriculography, LV volume was studied in 18 patients with hyperthyroidism before and after treatment. Under untreated conditions, we found significant correlations between triiodothyronine (T3) level and EF, Emax, and k. However, these associations disappeared with return to the euthyroid state.83
– LV filling pressure and diastolic stiffness: Differences were detected when comparing women and men.84
– Medication: BB has been shown to alter the VRG78 and EF versus ESV relationship.62
– Positive inotropic intervention: In 107 acute MI patients, LV volume was measured by 2D echocardiography during baseline, during low-dose (10 μg/kg·minute i.v.) dobutamine infusion, and after three months. The three resulting VRGs were almost identical, but ESV decreased (P < 0.0001) during dobutamine compared to baseline and repeat measurements.85
Factors that Determine EF
Four hallmark papers have pointed out the complex nature of the index EF.86–89 Because EF is regarded a crucial metric in the evaluation of cardiac patients and considered the key to classification in cases of HF, we derive an expression that relates EF to ESV (Figs. 5 and 10). As a starting point, we employ the volume domain description as expressed by equation (1), now without inclusion of the term ΣWiFi. This leads to a reformulation of EF in terms of the key parameters EDVave, r2, α, and β, where EDVave is the average value of EDV for the group under consideration.56 See Supplementary File.
Figure 5.
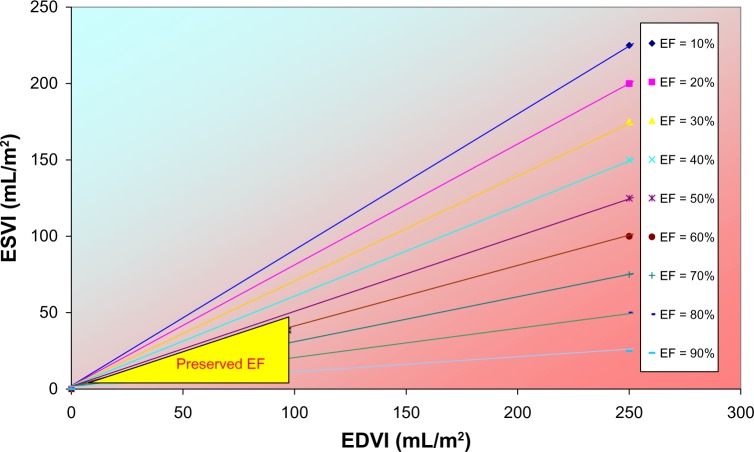
Volume domain as defined by ESVI and EDVI.
Note: The yellow triangle identifies the region reserved for patients with HF and preserved EF on the basis of current guidelines.71 The colored lines refer to trajectories with fixed values for EF, ranging from 10 to 90%. By mentioning a particular value of EF, we only know on which line we can find a patient, not the precise location as described by the ESVI and EDVI coordinates.
Figure 10.
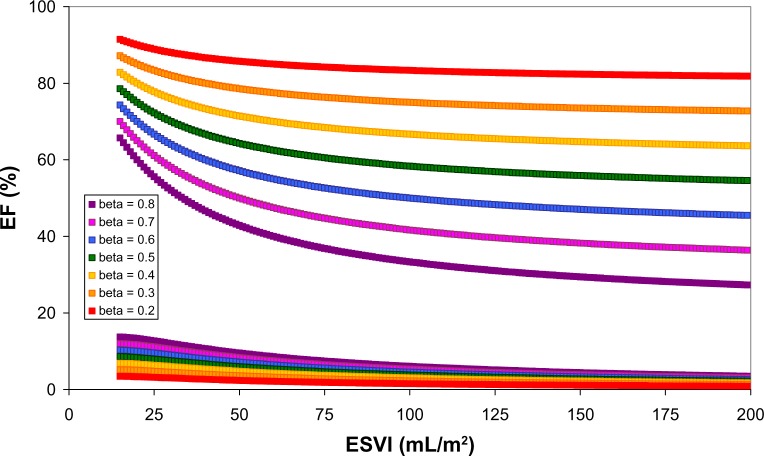
Application of the volume regulation graph (VRG) concept aiming at theoretically quantifying consequences of variations of the two VRG determinants (see equation 1) on EF as a function of the ESVI. Upper tracings: anticipated effects on (absolute value of) EF as induced by stepwise variations of the VRG-associated slope (each curve at a fixed value for β, ranging from 0.2 to 0.8) with constant α = 20 mL/m2. For comparison in a recent study (n = 1459), we found β = 0.65 and α = −24.80 mL/m2.78 The lower portion illustrates for the same increments of β the differences of EF when α increases from −20 to −10 mL/m2. Calculations are based on equation (3). The slope β as well as the intercept α may vary for distinct patients groups and, therefore, induce β-dependent changes for EF as documented in this paper. The impact of variation of α in this example is small compared to the contribution related to β. Note that a lower value for β is generally associated with higher EF values (upper portion) as well as the differences (lower part) induced by α at identical readings of ESVI. By quantitatively predicting the gain in terms of EF in dependence of an induced change for β, this figure illustrates the possible application of personalized therapy.
Volume Domain Representation and Systolic Elastance Concept
Systolic elastance Emax is defined by the ratio of end-systolic pressure (ESP) and (ESV-Vo). Vo is the volume axis intercept. Increasing discussion has emerged about the interpretation of Vo, especially once it became clear that the relationship essentially shows a nonlinear behavior. However, a Legendre transformation method was devised to extend the end-systolic P–V relationships to the nonlinear realm.90
The analytical expression for the VRG incorporates ESV, EDV, α, and β. EF depends on ESV and EDV. Consequently, the VRG concept and the elastance model are interrelated. The connection between Emax and EF in HF has been extensively described.91 We investigated the position of Vo within the LV volume domain by using published data.92 Results are illustrated in Figure 6, suggesting a correspondence between ESVI and Vo as previously reported.68,93 In conscious, autonomically intact dogs, the use of stepwise, steady-state afterload variations to obtain ESP–ESV data points was employed to construct the systolic elastance curve.94 Unfortunately, incorrect estimates for Emax were found. In the case of afterload reduction, Emax(TRUE) is underestimated by an average of 16.3%, and in the case of afterload increase, Emax(TRUE) is overestimated by an average of 37.1%.94 Determination of Emax and Vo requires successive measurements in the same subject, which is not always feasible. Therefore, various alternative routes have been explored, aiming at the determination via a single-beat measurement.95–98 Clearly, EF as well as k can be expressed in terms of α and β. Coupling in HF during rest and exercise, including effect of age and gender, has been extensively described.74,75
Figure 6.
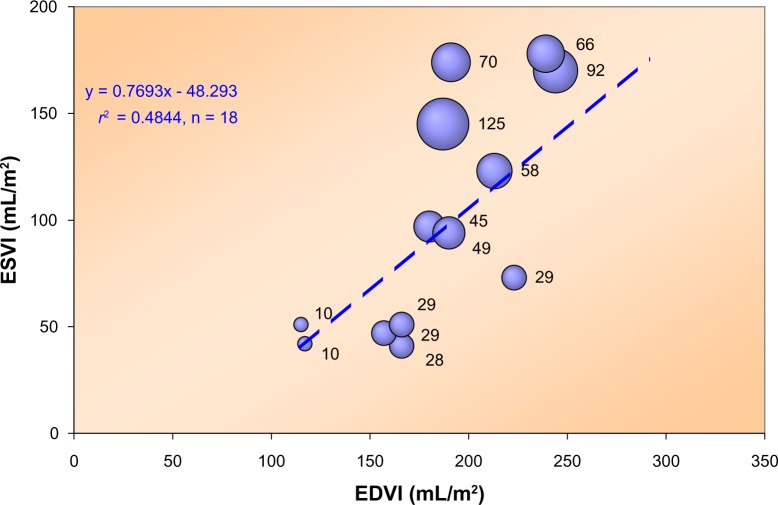
Relationship between ESVI and EDVI for 18 patients with varying degrees of LV dysfunction, described by Grossman et al.92
Note: The bubble size reflects the value of the volume intercept (expressed as mL/m2 with actual number indicated next to each bubble) of the end-systolic pressure–volume relationship. Clearly, this intercept tends to increase for larger values of ESVI. As a matter of fact, we found an excellent linear relationship (r2 = 0.62 with P < 0.001) between ESVI (control values) and the intercept. Pressure refers to the dicrotic notch level.
Importance of ESV
From a historical perspective, ESV(I) is important because it is the sole determinant of the strict definition of the Starling curve, ie, variation of EDV(I), while afterload and contractility remain constant.99 Even a new law of the heart was formulated in a publication on the relationship between ESV and ventricular mechanical impulse.100 A year before, this specific central issue was highlighted by considering not the filling properties (as described by Frank and Starling) but rather the regulation of the degree of emptying of the LV by the force of ventricular contraction.101 Combining filling volume (EDV) and residual volume (ESV) results in the VRG. This approach honors both those who sympathize with ESV and the Starling adepts.76
The relationship between ESV and EDV has been documented on the basis of numerous data sets.61,65,99 Since both ESV and EDV are primary determinants of LV function, one may wonder if one of the two is sufficient to globally assess LV performance. This idea seems attractive when considering distinct patient groups. Indeed, if all ESV–EDV data pairs for patients with an identical diagnosis belong to the same cluster as characterized by the regression line, then information on one variable would suffice. As a matter of fact, several studies have already documented that the single use of ESV(I) provides a valuable criterion, eg, for separating survivors.102 In these studies, a multivariate analysis with log-rank test and the Cox proportional hazards model showed that ESV (χ2 = 82.9) had greater predictive value for survival than EDV (χ2 = 59.0) or EF (χ2 = 46.6) in 605 male patients with coronary artery disease, whereas stepwise analysis showed that once the relationship between survival and ESV had been fitted, there was no additional significant predictive information in either EDV or EF. These authors produced a graphical representation of ESV versus EF showing two significantly different regression lines for the two groups. Using data published on children with cardiac disease,103 we were able to calculate (see Fig. 7) similar regression lines for survivors versus non-survivors in this age group.56
Figure 7.
A plot of ESV versus EDV for pediatric patients with heart disease as described by Suda et al.103
Note: Regression lines for two groups of children with cardiac disease differ (P < 0.012), as did the regression lines for EF versus ESV. The blue symbols refer to those who survived, while the purple symbols pertain to the non-survivors. Similar results were reported by White et al.102 in 605 adult patients when analyzing EF versus ESV.
Interestingly, a proposal was advanced implying that clinical trials in coronary thrombosis can record as end points either death or an index of LV function (EF or ESV), which can be used as a surrogate for long-term mortality.104 During administration of 25 mg BB i.v. in patients with cardiomyopathy, ESV was significantly (P < 0.0005) increased by 21%.105 Next, the importance of ESV as a predictor of postoperative LV performance in volume overload from valvular regurgitation was documented.106 Furthermore, the crucial role of ESV (rather than of afterload as reflected by ESP) was emphasized within the framework of the mechanics of LV relaxation.107 Also, certain ESV limits as a consistent indicator of variations in inotropic state were defined.95
Extending the work by Norris, LV function was advanced as an end point.108 The ESV criterion was employed to define the relation between LV dilatation and global and regional cardiac dysfunction, and to identify early predictors of enlargement and chronic HF in patients after MI.109 In addition, ESVI is considered the major predictor of survival after coronary artery bypass graft surgery in patients with impaired LV function.110 Furthermore, it was found that either EF or ESV is the most important modifiable factor for the prognosis of acute MI patients.72 Another group reported that LV ESVI in patients with ischemic cardiomyopathy predicts postoperative ventricular function.111 LV ESVI (with cutoff at 40 mL/m2) early into reperfusion therapy for MI was found to strongly predict adverse outcomes, including early and late mortality, thus, establishing the role of very early LV dilatation on outcome in MI and the usefulness when identifying high-risk patients.112 Preoperative ESVI predicts the development of postoperative CHF and the actuarial survival rate in patients with ischemic cardiomyopathy.113 Invoking the concept that nature is simple, it was reasoned that fiber angle (not EF) is important, that shape (not EF) defines prognosis, that MVO2 falls if the LV becomes more spherical, and that in CHF, the disease must be treated – not the symptom.12 Direction and magnitude of changes in LV cavity size and mass over time were studied in 59 patients to investigate if these changes were related to those in exercise performance in patients with chronic HF. At baseline, the group as a whole had moderate to severe LV dysfunction with an end-systolic diameter (ESD) of 60 ± 11 mm and LV mass of 500 ± 200 g. Changes in LV size and mass per year were significantly and best related to ESD with r = −0.56.114 The increase in ESV after exercise independently predicts mortality in patients with coronary heart disease.115 It was suggested to develop individualized clinical strategies based upon a consideration of the important role that LV remodeling plays in the pathogenesis of HF and to begin incorporating measurements of LV volume and mass into the clinical decision-making process.11 Recently, for LV reconstruction surgery (LV aneurysm/reshaping techniques), a break point at ESVI >60 mL/m2 was proposed.42
Cutoff Values for EF
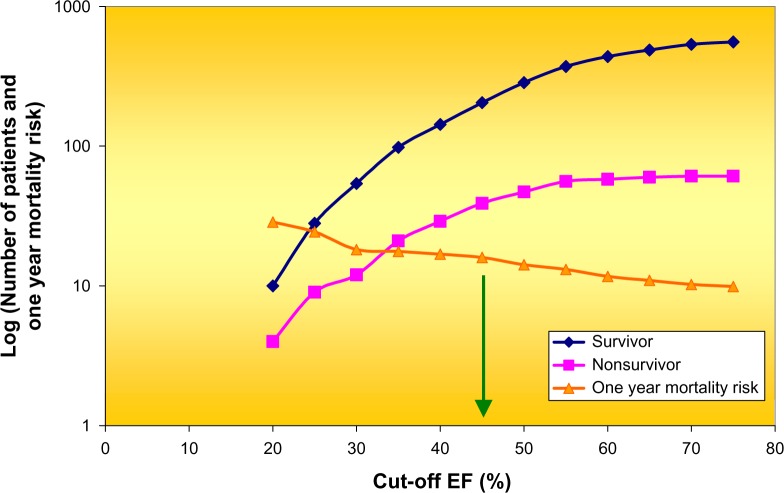
Even before the HFpEF era, we see broad attention for the possible discriminatory power of EF. Prevalence of EF-classes exhibits a non-Gaussian distribution, as illustrated in Figure 8. This example shows that at an upper break point value of 40% for EF in combination with at least 10 ventricular premature complexes (VPCs), the relative risk of sudden death for up to two years was 11 times that of patients without these conditions.116
Figure 8.
Cumulative distribution of survivors (n = 569) and non-survivors (n = 63) of acute MI plus the one-year mortality in dependence of break point selected for EF. An EF of less than 45% (see green arrow) best defined a high-risk group. Note the logarithmic scale along the ordinate. Data derived from Ahnve et al.116
Thus, cutoff values selected at 40 and 45% have not been uncommon and demonstrated to be of relevance in various clinical settings. Based on this tradition, it is not surprising to discover that current investigations on HF again center around EF cutoff values between 35 and 55%71 or at 45%.117 In a Japanese study, the EF distribution in 1692 patients (n = 985 for HFrEF and n = 429 for HFpEF) included a borderline/gray zone with 40% < EF < 50%, and 278 patients were therefore not classified.118 Also, HFpEF is variously defined as >40–45%.119 Elsewhere, patients were categorized in three groups: those with an EF of <40% (HFrEF), those with an EF of 40–50% (HF with borderline EF), and those with an EF of >50% (HFpEF).120 Two groups were studied in detail: those with an EF of <40% and those with an EF of >50%. The presenting history and clinical examination findings were similar for both groups, but HFpEF patients were more likely to be older and female and to have a history of hypertension and atrial fibrillation.120 While coronary heart disease was clearly associated with a lower EF, overlapping characteristics between HFrEF and HFpEF were observed in the middle of the LV EF spectrum, whose range was termed the intermediate EF group with EF 35–55%.121 Elsewhere, a survey of various papers with EF cutoff between 40 and 55% has been presented.51 Borderline HF patients (in the zone of the ESV– EDV plane where EF ranges between 40 and 55%, see Fig. 5) are all located within a region, which in many other studies is conveniently neglected and benevolently referred to as a gray zone. Unfortunately, this zone includes the clinically interesting patients because they concern exactly the group where one may expect the highest frequency of transitions between HFpEF and HFrEF.69
The classification of HFpEF is still debated, while biomarkers of myocyte stress, inflammation, extracellular matrix remodeling, GDF-15, cystatin C, resistin, and galectin-3 are under investigation.51 The crucial question now is whether a boundary value selected at 50% is just an intuitively induced best bet or a rigorous estimate supported by sound reasoning. Machine learning techniques and advanced statistical approaches may shed another light on how to best classify HF patients. A linear divider such as a single breaking point for EF may be too simple. Indeed, there is already evidence that the lower limit for EF in elderly women with HFpEF is much higher.122
HF: Definitions, Criteria, Mechanisms, and Criticism
Definitions
European society of cardiology (ESC) guidelines 2012
HF can be defined as an abnormality of cardiac structure or function leading to failure of the heart to deliver oxygen at a rate commensurate with the requirements of the metabolizing tissues, despite normal filling pressures (or only at the expense of increased filling pressures). HF is defined as a syndrome in which patients have typical symptoms and signs resulting from an abnormality of cardiac structure or function. The diagnosis of HF can be difficult. Many of the symptoms of HF are non-discriminating and, therefore, of limited diagnostic value. Many of the signs of HF result from sodium and water retention and resolve quickly with diuretic therapy, ie, may be absent in patients receiving such treatment. Demonstration of an underlying cardiac cause is therefore central to the diagnosis of HF. This is usually myocardial disease causing systolic ventricular dysfunction. However, abnormalities of ventricular diastolic function or of the valves, pericardium, endocardium, heart rhythm, and conduction can also cause HF, and more than one abnormality can be present.71
Fukuta and Little
Fukuta and Little define HF as the pathological state in which the heart is unable to pump blood at a rate required by the metabolizing tissues or can do so only with an elevated filling pressure. HF in adults most frequently results from the inability of the LV to fill (diastolic performance) and/or eject (systolic performance) blood. The severity of HF and its prognosis are more closely related to the degree of diastolic filling abnormalities than the EF. This underscores the importance of understanding the mechanisms of diastolic abnormalities in HF.123
Mann
Mann states that HF is a clinical syndrome caused by dysregulated calcium handling and abnormal cardiac pumping capacity.124 Upregulation of microRNA-25 impairs calcium handling leading to pump dysfunction, and targeting microRNA-25 using antisense oligonucleotides reverses pump dysfunction and improves survival in mice with HF.125 More generally, microRNAs are being applied in diagnosis and treatment of cardiovascular disease because in recent years, they have emerged as master regulators of gene expression.126 The insight that decreases in LV volume and mass occur secondary to the recovery of the myocardium at the cellular and molecular levels have engendered a wider appreciation of the importance of LV remodeling as a mechanism for worsening HF.11 Despite recent insights into the recognition of the importance of LV reverse remodeling in HF, many clinicians do not consider simple measurements of LV structure (ie, LV volume) in their routine clinical decision-making process.11 Instead, they often rely on EF when making decisions about medical and surgical treatment options. There are probably multiple reasons of why the use of LV volumes has not gained wider acceptance in day-to-day clinical management of HF patients. Clinicians remain extremely comfortable using EF (referring to LV function) to assess HF patients.11 Importantly, LV volumes predict outcome more reliably than does the EF.11,102 Moreover, knowledge regarding LV volumes is extremely useful in optimizing patient selection for surgical and device therapies.11 Based on the foregoing arguments, we suggest that it is time to begin developing individualized clinical strategies based upon a consideration of the important role that LV remodeling plays in the pathogenesis of HF and that we incorporate measurements of LV volume and mass into the decision-making process.11
Various criteria for diagnosing HF
An annotated historical survey is presented elsewhere.127 Therefore, we present only a few classification systems as used for diagnosing HF:
First, we consider the traditional criteria formulated by Forrester et al.128: elevated filling pressure and cardiac index (CI) is <2.2 L/minute m2. The value is almost 19% lower than the level that these investigators considered to be normal (2.7 L/minute m2). We applied these criteria to our HF patient groups described before.56 Figure 9 illustrates the four groups analyzed, namely, HFpEF (complying with Forrester criteria or not) and HFrEF (either in accordance with Forrester criteria or violating them). It can be derived that there is only partial congruence between both approaches, although it is evident that the regression lines for HFpEF differ from those for HFrEF.
Next, we survey the Framingham CHF criteria, which require validation of two major or one major plus two minor criteria. Majors are paroxysmal nocturnal dyspnea, orthopnea, elevated jugular venous pressure, pulmonary rales, third heart sound, cardiomegaly or pulmonary edema on chest radiograph, and weight loss more than 4.5 kg within five days in response to HF therapy. Minor criteria include peripheral edema, night cough, dyspnea on exertion, hepatomegaly, pleural effusion, and HR above 120 bpm.129
The ESC presented in 2012 the latest guidelines, along with a list of the New York Heart Association (NYHA) functional classification dating back to 1943.71 HF is defined, clinically, as a syndrome in which patients have typical symptoms (eg, breathlessness and fatigue) and signs (eg, ankle swelling, elevated jugular venous pressure, pulmonary crackles, and displaced apex beat) resulting from an abnormality of cardiac structure or function. Demonstration of an underlying cause is essential as the precise pathology determines the specific treatment. Patients with HF because of LV dysfunction are categorized as HFrEF (or systolic HF) versus HFpEF (or diastolic HF). The ESC committee members emphasize that the main terminology used to describe HF is historical and is based on measurement of LV EF. As a consequence, the treatment schedules as provided indicate EF cut-offs at 45, 40 and 35% (cf. individual iso-EF lines in the LV volume domain shown in Fig. 5) to recommend angiotensin-converting-enzyme inhibitor (ACEI), BB, mineralocorticoid receptor antagonist therapy, or implantation of cardioverter defibrillator.71 In patients with reduced contraction and emptying of the LV (ie, systolic dysfunction), SV is maintained by an increase in EDV (because the LV dilates), ie, the heart ejects a smaller fraction of a larger filling volume. The more severe the systolic dysfunction, the more the EF is reduced from normal and, generally, the greater the EDV and ESV. The EF is considered important in HF, not only because of its prognostic importance (the lower the EF, the poorer the survival) but also because most clinical trials selected patients based upon EF, usually measured using a radionuclide technique or echocardiography.71 However, stating that nowadays EF is important because in the past we created a framework based on EF may entail the risk that we harvest our own initial conditions. The situation somewhat resembles the post hoc ergo propter hoc sophism, which has been described elsewhere.130 Furthermore, the ESC committee continues, the major trials in patients with HFrEF, mainly enrolled patients with an EF ≤35%, and it is only in these patients that effective therapies have been demonstrated to date.
Discordant ventricular failure versus concordant failure: Forrester’s classification system does not take into account the role of blood volume in the pulmonary bed and RV function, nor does it encompass a possible role for central venous pressure (CVP) as an independent index for circulatory evaluation.127 The latter researchers have developed a computerized cardiovascular model for analyzing hemodynamic characteristics and volume movement in simulated acute HF. They show that by simultaneously integrating LV with RV function, the model provides a better understanding of how pulmonary capillary wedge pressure (PCWP) and CVP interact in HF. Additionally, they propose a physiologic HF classification system on the basis of these findings. Basically, they developed a lumped-parameter cardiovascular model and analyzed forms of HF in which the RV and LV failed disproportionately (discordant ventricular failure) versus equally (concordant failure). Acute discordant pump failure was characterized by a passive volume movement, with fluid accumulation and pressure elevation in the circuit upstream of the failed pump. In biventricular failure, less volume was mobilized. These findings negate the prevalent teaching that pulmonary congestion in LV failure results primarily from the backing up of elevated LV filling pressure. They also reveal a limitation of the Forrester 1976 classification, namely, that PCWP and CI are not independent indices of the circulatory system.127
Figure 9.
Volume regulation graph for HF patients (n = 63) described elsewhere,56 and excluding those using BB. They are now subdivided into four groups not only on the basis of reduced (r) or preserved (p) EF but also by applying the classical Forrester criteria. The Forrester compliant group with HFpEF is too small (n = 5) to find a significant regression line. The other three groups yield significant regression equations. Note that the slope of the regression line for the HFpEF subgroup is significantly lower than those for both HFrEF lines.
Criticism to established criteria for HF
One study demonstrated that when invasive pressure measurements are not included, only 5% of their outpatients with HFpEF fulfilled the ESC definition, thus illustrating the weakness of echocardiographic estimates of DD.131 Another investigation indicated that only 0.8% (sic) of all their HF patients fulfill the ESC guidelines.132
The current paradigm regarding classification of HF assumes a linear divider, namely, EF at a cutoff level according to the liberal preference of the individual investigator. However, there is no rationale to support linearity. It is very well conceivable that the cutoff value is not a fixed number but rather varies with the size of the heart. Such a nonlinear discriminator would, in particular, be more appropriate when dilated hearts are involved. Machine learning techniques may yield other guidelines because they do not assume a linear discriminator but indeed create nonlinear (hyper)planes to distinguish groups. Adhering to a nonlinear framework also necessitates a reevaluation of the present simple concept of transition from one phenotype to another, just by crossing a straight dividing line. Moreover, the dividing line does not necessarily follow the EF trajectory. In fact, there are strong indications that the line best separating both groups runs perpendicular to the one proposed in the current paradigm. Additionally, the specific dividing criterion may depend on gender122 and medication.78,84 This observation is supported by the finding that HF in women differs in many aspects from that of men. Contrasts in origin, diagnostic yield, prognosis, and possibly response to treatment have been outlined. Some of these differences may have a pathophysiological basis.79
A third phenotype of HF, namely, with recovered EF, has been proposed as a separate entity.133,134 It remains to be seen if this effect is solely induced by medication78,84 and if the increase in EF is crucial for the borderline HF group. In addition, 25% of patients with DCM with the recent onset of HF (<6 months) have a relatively benign clinical course with a spontaneous improvement in symptoms. DCM patients who undergo unassisted resolution of symptoms show that this process is accompanied by recovery of LV function.135 Yet another distinct subset of HFpEF has been described, featuring pathophysiology similar to HFrEF but with eccentric hypertrophy.136
Dilated LA with dysfunction has been reported in HFpEF patients.114,137 While emphasizing the feasibility of measuring LA volume and the need to include this information during the diagnostic process, this characteristic has even been advanced as a criterion.138
Possible mechanisms for high EF in HF
It is hypothesized that a greater than normal EF in HFpEF translates into a more forceful contraction, while storing elastic energy (during systole) in the myocardial tissue.50 When systole terminates, the contractile elements detach and the stored energy is utilized for rapid relengthening of the contractile elements of the myocardium, before mitral valve opening. The abrupt expansion of the LV decreases LV pressure to a minimum, thereby increasing the pressure gradient between the LA and LV. This pressure gradient accounts for an efficient early LV filling. Using the energy stored during systole in favor of early LV filling is referred to as elastic recoil (also termed diastolic suction). The greater the tension difference between ESV and LV slack volume (occurring during the isovolumic relaxation period when volume remains constant but tension changes), the greater the energy stored in the myocardium, with subsequent release as elastic recoil.50
Considering augmented systolic wall thickening, yet another mechanism for preserved EF was proposed.23 The mathematical model predicts that the normal EF in patients with HF may be explained by the presence of LV hypertrophy. The resulting amplified radial thickening in the setting of reduced long-axis shortening explains the preservation of EF. Thus, the reduced SV in the precompensated state rather than DD may be the cause of HFpEF.
What is EF?
Numerically, the value of EF depends on prevailing ESV(I), α, β, r2, and average EDV(I) (see Supplementary File). However, EF does not directly embody information on the factor time. Yet, time in terms of HR plays a crucial role in circulatory dynamics.139 The clinically and physiologically relevant measure of CO is calculated by multiplying the differential (EDV–ESV) by HR. CI is defined as CO normalized for body surface area (BSA).
In a large population, we found no association between EF and HR78:
Even in the absence of BB, we found no clear relationship:
Yet, the metric CI has been important, eg, as a criterion in the Forrester model.128 Traditional HF classification systems include the Forrester hemodynamic subsets, which use two indices: PCWP and CI. As a modification, it was hypothesized that changes in PCWP and CVP, and in the phenotypes of HF, might be better evaluated by cardiovascular modeling. Therefore, a lumped-parameter cardiovascular model was developed to analyze forms of HF in which the right LV failed disproportionately (discordant ventricular failure) versus equally (concordant failure).127 Note that neither of these two approaches invokes EF as a determinant.
Over the years, a sort of cult has been created around the index EF, where every external factor became evaluated against EF. For example, pretended beneficial influences of sauna bathing in the rehabilitation of hypertensive patients with ischemic heart disease following aortocoronary venous bypass operation has been expressed in terms of changes in EF, regardless of LV volume.140
Which elements determine EF in a population? For an individual, it is obviously two volume determinations, namely, at the end of systole and end of diastole. However, when ana-lyzing characteristics of a particular group of patients (be it selected on the basis of diagnosis, medication, intervention, gender, or age), the situation appears more complex. To describe the dynamics of a group, we require additional information as embodied by EDVIave, γ, and δ. The latter two depend on the features of the pertaining VRG, ie, α, β, and r. What we gain by following this route is the power to predict data pairs for any patient defined by the same group-related properties. Also, it becomes possible to predict the outcome of intervention and the likelihood of transition to another phenotype (as is the case for HF and BB) in dependence of gender differences. EF has also been analyzed in other species. EF in conscious dogs was 29.6% in normals versus 18.8% in HFrEF canine patients.141 Elsewhere, pEF was reportedly 57%.55
Problems arise when an index does not do what it should do, namely, reflect the generally adopted interpretation. This statement also applies to EF. In the prevailing paradigm, healthy LVs exhibit high values for EF (ie, above 60–70%), and one certainly would not expect a high value for EF in a failing heart. However, with the arrival of the new name, HF with preserved EF syndrome, we are confronted with a contradictio in terminis. This is nothing different from stating a syndrome where the value of EF has nothing to do with the common connotation. Even worse, it is precisely the opposite of what one always ascertains. It is as strange as talking about hayfever, actually being a condition with anything but fever. Besides, EF does not correlate very well with any performance indicator proposed thus far. It remains to be specified if the reason for the discordance stems from imperfections intrinsic to the new candidates or simply originates from the inadequacy of EF. Indeed, the ESC warns: EF is not an index of contractility as it depends on volumes, preload, afterload, HR, and valvular function, and is not the same as SV. SV may be maintained by LV dilation in a patient with HFrEF, whereas it may be reduced in patients with HFpEF and concentric LV hypertrophy. EF may also be preserved (and SV reduced) in patients with significant mitral regurgitation. Thus, EF must be interpreted in its clinical context.71
Since EF is not a universal indicator of LV performance, it has been suggested that EF rather reflects k. Indeed with the inclusion of several assumptions, it is possible to derive a relationship between EF and k. Additionally, it has been demonstrated that the regression coefficients of the VRG are related68 to k and to the intercept Vo of the Emax relationship:
Elsewhere, we have shown that k = [(1 − β)ESV − α]/β(ESV − Vo).56
Furthermore, a further reduction applies yielding EF = 1 −(1/(k + 1)) if Vo vanishes. Therefore, a simple connection exists between EF and the slope β, as well as the intercept α. The value for k index was found to exhibit mismatch in HFpEF patients74,75,119,142–144 as well as during dobutamine stress test in HFpEF.20
What is the Effect of an Intervention on the VRG?
We have seen that in the VRG representation, we can express the slope β = (ESVI − α)/EDVI, ie, for small α, the slope ≈ (100 − EF)/100. However, in practice, α cannot be neglected.
Now we can add a correction factor to equation (1) that modifies the slope from control state to a new operating line induced by intervention or by superimposing the effect of gender differences.
General expression: slope β = (ESVI − α)/EDVI − φ * θ
where φ is the Kronecker delta145 and θ is the impact (ie, strength of the effect) because of a particular intervention.
As an example: θ(BB) ≈ 0.2 and θ(female) ≈ 0.1.
Additionally, θ(HFrEF) ≈ −0.2 and θ(HFpEF) ≈ 0.2, both compared to non-HF patients with BB.
Figure 10 illustrates anticipated effects on (absolute value of) EF as induced by stepwise variations of the VRG-associated slope (each curve at a fixed value for β, ranging from 0.2 to 0.8) with constant α = 20 mL/m2. For comparison, in a recent study (n = 1459) we found β = 0.65 and α = −24.80 mL/m2.78 The lower portion illustrates for the same increments of β the differences of EF when α increases from −20 to −10 mL/m2. Calculations are based on equation (3). The slope β as well as the intercept α may vary for distinct patients groups and, therefore, induce β-dependent changes for EF as documented in this paper. The impact of variation of α in this example is small compared to the contribution related to β. Note that a lower value for β is generally associated with higher EF values (upper portion) as well as the differences (lower part) induced by α at identical readings of ESVI. This figure is an example of possible application of personalized therapy.
Discussion
Alongside the routine application of the index known as EF during half of a century, we notice a permanent striving to discover an even better indicator to judge the performance of the healthy and diseased heart. The continuing enthusiasm to search for an alternative holy grail induces the feeling that sooner or later, EF will be unmasked as a surrogate with the risk of dethroning the hailed favorite. After exploring what is known and unknown about HFpEF, one group of authors proposed to arrive at a new classification system independent of EF. Our investigations support this notion and suggest ESV as the next candidate.
Almost traditionally, various painstaking attempts have been undertaken to challenge EF as the pretended gold standard for the evaluation of LV function. Amidst them are various candidates invariably accompanied by the illustrious epithet maximum, eg, maximum circumferential shortening velocity (Vmax), maximum rate of ventricular pressure development (dP/dt)max, and maximal elastance of the ventricle (Emax) occurring at the end of systole. Recently, twist and apical rotation have been shown to correlate well with dP/dt, and are proposed as a new index of cardiac performance.26
SUMOylation is a posttranslational modification, in which small ubiquitin-related modifier (SUMO) proteins are covalently conjugated to target proteins via a series of enzymatic reactions, and they may be implicated in human cardiovascular disorders.146 How do ultrastructural components such as titin,53 CVF, and MyD, phosphorylation of myofila-mentary proteins and expression of beta-adrenergic signaling and calcium handling proteins57, SUMOylation146, and genetics of electrical phenomena77 translate into macroscopic determinants such as ESV and EF? Although etiologies of cardiac diseases may differ, they share molecular, biochemical, and cellular events to collectively change the shape of the myocardium.35
Despite overwhelming but (alas) transient interest in successive alternatives, it became again and again clear that EF (owing to its simplicity, and because of the universal belief of the community) survived in the clinic as a convenient index.11,56,71 Interestingly, after an initial period of glory, every new candidate became attacked by quibbling cardiophysiologists or similar meddlers pointing out frustrating shortcomings such as afterload dependence or nonlinearity.147–149 Possibly, clinicians felt somewhat disturbed by those diverging controversies and itching limitations that turbulence readily invites them to peacefully adhere to EF.
How to solve this inconvenient situation in the long term? Why not venture in the opposite direction and search for the minimum, rather than the earlier attempts in the direction of a maximum (cf. Vmax, (dP/dt)max, and Emax)? An interesting candidate may be end-contraction volume (a sort of Vmin during the cardiac cycle, to apply a formulation in the language of the proposed paradigm). In this case, a logical and fascinating route emerges with end-systolic volume (ESV) as a building stone. Various publications have already highlighted the clinical relevance of considering ESV (eg, when timing valve replacement in aortic stenosis and in volume-reduction procedures for the dilated ventricle). Although rarely identified as such, ESV not only comprises the fundamental determinant of the latest favorite Emax but also features as the major component of favorite EF.56,62
Thus, this contribution reviews the relevance of EF in the evaluation of HF patients. The mere fact that the numerical value of EF is determined by a cocktail of cardiovascular parameters is truly troubling.56,70 The conclusion of my analysis is promising: the way to heaven looks to be paved by stones designed around ESV. Insights can be enhanced by considering the LV volume domain, particularly by analyzing the relationship between ESV and EDV for various clinically relevant entities.
Conclusions
Thus far, classification of HF is based on information regarding EDV combined with the ratio of ESV and EDV. Our analysis in the LV volume domain shows that in the two major HF subgroups, ESV follows different patterns in dependence of EDV. This observation clarifies that a superior classification system regarding HF is primarily founded on information embodied by ESV. Future research should concentrate on machine learning techniques and advanced statistical approaches to establish solid guidelines to distinguish various HF phenotypes. The clinical relevance of EF requires reevaluation, in particular, in view of reported gender differences.
Supplementary Data
Supplementary File. Analytical description of the relationship between EF and ESV.
Acknowledgments
The author appreciates the stimulating and constructive discussions he had during more than three decades with his con-frères Prof. John K.-J. Li and Prof. J. Yasha Kresh. Prof. Guy R. Heyndrickx kindly made the patient data available. Among numerous other original ideas, Dr. Neal Handly asked attention for SUMOylation, and he was day and night on duty to exchange ideas and polish my thoughts with a rapidity and efficacy as if they all were emergency cases.
Footnotes
Author Contributions
Conceived and designed the experiments: PK. Analyzed the data: PK. Wrote the first draft of the manuscript: PK. Contributed to the writing of the manuscript: PK. Agreed with manuscript results and conclusions: PK. Jointly developed the structure and arguments for the paper: PK. Made critical revisions and approved the final version: PK. The author reviewed and approved the final manuscript.
ACADEMIC EDITOR: Thomas E. Vanhecke, Editor in Chief
FUNDING: Author discloses no funding sources.
COMPETING INTERESTS: Author discloses no potential conflicts of interest.
Paper subject to independent expert blind peer review by minimum of two reviewers. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
REFERENCES
- 1.Amsterdamska O, Hiddinga A. The analyzed body. In: Cooter E, Pickstone J, editors. Companion to Medicine in the Twentieth Century. London: Routledge; 2003. p. 430. (Chap. 27) [Google Scholar]
- 2.Servetus M. Christianismi Restitutio. Vienne, France: Baltasar Arnoullet; 1553. [Google Scholar]
- 3.Fludd R. Utriusque Cosmi, Maioris scilicet et Minoris, metaphysica, physica, atque technica Historia. Frankfurt: Theodor de Bry; pp. 1617–21. [Google Scholar]
- 4.Harvey W. Exercitatio anatomica de motu cordis et sanguinis in animalibus. Frankfurt: W Fitzer; p. 1628. [PubMed] [Google Scholar]
- 5.Clendening L. Source Book of Medical History. 2nd ed. New York: Dover Publications Inc; 1960. [Google Scholar]
- 6.Huisman T. The Finger of God; Anatomical Practice in 17th Century Leiden. Leiden: Primavera Press; 2009. [Google Scholar]
- 7.Woods RH. A few applications of a physical theorem to membranes in the human body in a state of tension. J Anat Physiol. 1892;26:362–70. [PMC free article] [PubMed] [Google Scholar]
- 8.Burton AC. Importance of shape and size of the heart. Am Heart J. 1957;54:801–809. doi: 10.1016/0002-8703(57)90186-2. [DOI] [PubMed] [Google Scholar]
- 9.Sandler H, Alderman E. Determination of left ventricular size and shape. Circ Res. 1974;34:1–8. doi: 10.1161/01.res.40.4.1. [DOI] [PubMed] [Google Scholar]
- 10.Ford LE. Heart size (editorial) Circ Res. 1976;39:297–303. doi: 10.1161/01.res.39.3.297. [DOI] [PubMed] [Google Scholar]
- 11.Mann DL, Bogaev R, Buckberg GD. Cardiac remodelling and myocardial recovery: lost in translation? Eur J Heart Fail. 2010;12(8):789–96. doi: 10.1093/eurjhf/hfq113. [DOI] [PubMed] [Google Scholar]
- 12.Buckberg GD. Congestive heart failure: treat the disease, not the symptom – return to normalcy. J Thorac Cardiovasc Surg. 2001;121:628–37. doi: 10.1067/mtc.2001.114075. [DOI] [PubMed] [Google Scholar]
- 13.Menicanti LA. Reduce or reshape, this is the question! Eur J Cardiothorac Surg. 2014 doi: 10.1093/ejcta/ezu254. [DOI] [PubMed] [Google Scholar]
- 14.Shah AM, Pfeffer MA. Left ventricular size, mass, and shape. JACC Heart Failure. 2014;2:523–5. doi: 10.1016/j.jchf.2014.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoffman JI, Buckberg GD. The myocardial oxygen supply: demand index revisited. J Am Heart Assoc. 2014;3(1):e000285. doi: 10.1161/JAHA.113.000285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thompson DW. On Growth and Form. New York: Dover; 1917. 1992. (Republication of the 1942 Edition). [Google Scholar]
- 17.Buckberg GD, Hoffman JI, Coghlan HC, Nanda NC. Ventricular structure-function relations in health and disease: part I. The normal heart. Eur J Cardiothorac Surg. 2014 doi: 10.1093/ejcts/ezu278. [DOI] [PubMed] [Google Scholar]
- 18.Buckberg GD, Hoffman JI, Coghlan HC, Nanda NC. Ventricular structure-function relations in health and disease: part II. Clinical considerations. Eur J Cardiothorac Surg. 2014 doi: 10.1093/ejcts/ezu279. [DOI] [PubMed] [Google Scholar]
- 19.Murphy JG, Lloyd MA, editors. Mayo Clinic Cardiology. 3rd ed. Rochester MN: Mayo Clinic Scientific Press; 2007. [Google Scholar]
- 20.Chattopadhyay S, Alamgir MF, Nikitin NP, Rigby AS, Clark AL, Cleland JG. Lack of diastolic reserve in patients with heart failure and normal ejection fraction. Circ Heart Fail. 2010;3(1):35–43. doi: 10.1161/CIRCHEARTFAILURE.108.824888. [DOI] [PubMed] [Google Scholar]
- 21.Rankin JS, McHale PA, Arentzen CE, Ling D, Greenfield JC, Jr, Anderson RW. The three-dimensional dynamic geometry of the left ventricle in the conscious dog. Circ Res. 1976;39(3):304–13. doi: 10.1161/01.res.39.3.304. [DOI] [PubMed] [Google Scholar]
- 22.Olsen CO, Rankin JS, Arentzen CE, Ring WS, McHale PA, Anderson RW. The deformational characteristics of the left ventricle in the conscious dog. Circ Res. 1981;49(4):843–55. doi: 10.1161/01.res.49.4.843. [DOI] [PubMed] [Google Scholar]
- 23.MacIver DH, Townsend M. A novel mechanism of heart failure with normal ejection fraction. Heart. 2008;94(4):446–9. doi: 10.1136/hrt.2006.114082. [DOI] [PubMed] [Google Scholar]
- 24.Yip G, Wang M, Zhang Y, Fung JWH, Ho PY, Sanderson JE. Left ventricular long axis function in diastolic heart failure is reduced in both diastole and systole: time for a redefinition? Heart. 2002;87:121–5. doi: 10.1136/heart.87.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yip GW, Zhang Y, Tan PY, et al. Left ventricular long-axis changes in early diastole and systole: impact of systolic function on diastole. Clin Sci (Lond) 2002;102(5):515–22. [PubMed] [Google Scholar]
- 26.Song JK. How does the left ventricle work? Ventricular rotation as a new index of cardiac performance. Korean Circ J. 2009;39:347–51. doi: 10.4070/kcj.2009.39.9.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Phan TT, Shivu GN, Abozguia K, Gnanadevan M, Ahmed I, Frenneaux M. Left ventricular torsion and strain patterns in heart failure with normal ejection fraction are similar to age-related changes. Eur J Echocardiogr. 2009;10(6):793–800. doi: 10.1093/ejechocard/jep072. [DOI] [PubMed] [Google Scholar]
- 28.Khoo NS, Smallhorn JF, Atallah J, Kaneko S, Mackie AS, Paterson I. Altered left ventricular tissue velocities, deformation and twist in children and young adults with acute myocarditis and normal ejection fraction. J Am Soc Echocardiogr. 2012;25(3):294–303. doi: 10.1016/j.echo.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 29.Abozguia K, Nallur-Shivu G, Phan TT, et al. Left ventricular strain and untwist in hypertrophic cardiomyopathy: relation to exercise capacity. Am Heart J. 2010;159(5):825–32. doi: 10.1016/j.ahj.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iwano H, Pu M, Upadhya B, Meyers B, Vlachos P, Little WC. Delay of left ventricular longitudinal expansion with diastolic dysfunction: impact on load dependence of e’ and longitudinal strain rate. Physiol Rep. 2014;2(7):pii: e12082. doi: 10.14814/phy2.12082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ersbøll M, Valeur N, Mogensen UM, et al. Global left ventricular longitudinal strain is closely associated with increased neurohormonal activation after acute myocardial infarction in patients with both reduced and preserved ejection fraction: a two-dimensional speckle tracking study. Eur J Heart Fail. 2012;14(10):1121–9. doi: 10.1093/eurjhf/hfs107. [DOI] [PubMed] [Google Scholar]
- 32.Lekadir K, Pashaei A, Hoogendoorn C, Pereanez M, Alba X, Frangi AF. Effect of statistically derived fiber models on the estimation of cardiac electrical activation. IEEE Trans BME. 2014;61:2740–8. doi: 10.1109/TBME.2014.2327025. [DOI] [PubMed] [Google Scholar]
- 33.Peterson KL. Pressure overload hypertrophy and congestive heart failure; where is the “Achilles’ heel”? J Am Coll Cardiol. 2002;39(4):672–5. doi: 10.1016/s0735-1097(01)01790-9. [DOI] [PubMed] [Google Scholar]
- 34.Pfeffer JM, Pfeffer MA, Braunwald E. Influence of chronic captopril therapy on the infarcted left ventricle of the rat. Circ Res. 1985;57:84–95. doi: 10.1161/01.res.57.1.84. [DOI] [PubMed] [Google Scholar]
- 35.Kehat I, Molkentin JD. Molecular pathways underlying cardiac remodeling during pathophysiological stimulation. Circulation. 2010;122:2727–35. doi: 10.1161/CIRCULATIONAHA.110.942268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kararigas G, Dworatzek E, Petrov G, et al. Sex-dependent regulation of fibrosis and inflammation in human left ventricular remodelling under pressure overload. Europ J Heart Fail. 2014;16:1160–7. doi: 10.1002/ejhf.171. [DOI] [PubMed] [Google Scholar]
- 37.Kararigas G, Fliegner D, Gustafsson JA, Regitz-Zagrosek V. Role of the estrogen/estrogen-receptor-beta axis in the genomic response to pressure overload-induced hypertrophy. Physiol Genomics. 2011;43:438–46. doi: 10.1152/physiolgenomics.00199.2010. [DOI] [PubMed] [Google Scholar]
- 38.Ashrafian H, Williams L, Frenneaux MP. The pathophysiology of heart failure: a tale of two old paradigms revisited. Clin Med. 2008;8(2):192–7. doi: 10.7861/clinmedicine.8-2-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hill JA, Olson EN. Cardiac plasticity. N Engl J Med. 2008;358:1370–80. doi: 10.1056/NEJMra072139. [DOI] [PubMed] [Google Scholar]
- 40.Heinonen I, Kudomi N, Kemppainen J, et al. Myocardial blood flow and its transit time, oxygen utilization, and efficiency of highly endurance-trained human heart. Basic Res Cardiol. 2014;109:413. doi: 10.1007/s00395-014-0413-1. [DOI] [PubMed] [Google Scholar]
- 41.Artrip JH, Oz MC, Burkhoff D. Left ventricular volume reduction surgery for heart failure; a physiologic perspective. J Thorac Cardiovasc Surg. 2001;122:775–82. doi: 10.1067/mtc.2001.116208. [DOI] [PubMed] [Google Scholar]
- 42.Castelvecchio S, Menicanti L. Left ventricular reconstruction: update to left ventricular aneurysm/reshaping techniques. Multimed Man Cardiothorac Surg. 2013;2013:mmt002. doi: 10.1093/mmcts/mmt002. [DOI] [PubMed] [Google Scholar]
- 43.Di Donato M, Sabatier M, Dor V, RESTORE Group Surgical ventricular restoration in patients with postinfarction coronary artery disease: effectiveness on spontaneous and inducible ventricular tachycardia. Semin Thorac Cardiovasc Surg. 2001;13(4):480–5. doi: 10.1053/stcs.2001.30137. [DOI] [PubMed] [Google Scholar]
- 44.Wechsler AS, Sadowski J, Kapelak B, et al. Durability of epicardial ventricular restoration without ventriculotomy. Eur J Cardiothorac Surg. 2013;44(3):e189–92. doi: 10.1093/ejcts/ezt292. [discussion e192] [DOI] [PubMed] [Google Scholar]
- 45.Mann DL, Barger PM, Burkhoff D. Myocardial recovery and the failing heart: myth, magic, or molecular target? J Am Coll Cardiol. 2012;60(24):2465–72. doi: 10.1016/j.jacc.2012.06.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van der Heijden AC, Höke U, Thijssen J, et al. Super-responders to cardiac resynchronization therapy remain at risk for ventricular arrhythmias and benefit from defibrillator treatment. Eur J Heart Fail. 2014;16(10):1104–11. doi: 10.1002/ejhf.152. [DOI] [PubMed] [Google Scholar]
- 47.Iwano H, Little WC. Heart failure: what does ejection fraction have to do with it? J Cardiol. 2013;62(1):1–3. doi: 10.1016/j.jjcc.2013.02.017. [DOI] [PubMed] [Google Scholar]
- 48.Brunner-La Rocca HP. Do we understand why the heart fails? Eur Heart J. 2008;20:698–700. doi: 10.1093/eurheartj/ehn031. [DOI] [PubMed] [Google Scholar]
- 49.Prasad A, Hastings JL, Shibata S, et al. Characterization of static and dynamic left ventricular diastolic function in patients with heart failure with a preserved ejection fraction. Circ Heart Fail. 2010;3(5):617–26. doi: 10.1161/CIRCHEARTFAILURE.109.867044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dori G, Rudman M, Lichtenstein O, Schliamser JE. Ejection fraction in patients with heart failure and preserved ejection fraction is greater than that in controls – a mechanism facilitating left ventricular filling and maximizing cardiac output. Med Hypotheses. 2012;79(3):384–7. doi: 10.1016/j.mehy.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 51.Cheng JM, Akkerhuis KM, Battes LC, et al. Biomarkers of heart failure with normal ejection fraction: a systematic review. Eur J Heart Fail. 2013;15(12):1350–62. doi: 10.1093/eurjhf/hft106. [DOI] [PubMed] [Google Scholar]
- 52.Phan TT, Shivu GN, Abozguia K, Sanderson JE, Frenneaux M. The pathophysiology of heart failure with preserved ejection fraction: from molecular mechanisms to exercise haemodynamics. Int J Cardiol. 2012;158(3):337–43. doi: 10.1016/j.ijcard.2011.06.113. [DOI] [PubMed] [Google Scholar]
- 53.Van Heerebeek L, Borbely A, Niessen HW, et al. Myocardial structure and function differ in systolic and diastolic heart failure. Circulation. 2006;113:1966–73. doi: 10.1161/CIRCULATIONAHA.105.587519. [DOI] [PubMed] [Google Scholar]
- 54.Lavine SJ, Conetta DA. Comparison of the effect of pressure loading on left ventricular size, systolic and diastolic function in canines with left ventricular dysfunction with preserved and reduced ejection fraction. Cardiovasc Ultrasound. 2008;6:57. doi: 10.1186/1476-7120-6-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mathieu M, El Oumeiri B, Touihri K, et al. Ventricular-arterial uncoupling in heart failure with preserved ejection fraction after myocardial infarction in dogs – invasive versus echocardiographic evaluation. BMC Cardiovasc Disord. 2010;10:32. doi: 10.1186/1471-2261-10-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kerkhof PLM, Kresh JY, Li JK-J, Heyndrickx GR. Left ventricular volume regulation in heart failure with preserved ejection fraction. Physiol Rep. 2013;1:e00007. doi: 10.1002/phy2.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hamdani N, Paulus WJ, van Heerebeek L, et al. Distinct myocardial effects of beta-blocker therapy in heart failure with normal and reduced left ventricular ejection fraction. Eur Heart J. 2009;30(15):1863–72. doi: 10.1093/eurheartj/ehp189. [DOI] [PubMed] [Google Scholar]
- 58.Segen JC. Current Med Talk, a Dictionary of Medical Terms, Slang and Jargon. Stamford, CT: Appleton and Lange; 1995. [Google Scholar]
- 59.Pfeffer MA, Braunwald E, Moyé LA, et al. Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction. Results of the survival and ventricular enlargement trial. The SAVE Investigators. N Engl J Med. 1992;327(10):669–77. doi: 10.1056/NEJM199209033271001. [DOI] [PubMed] [Google Scholar]
- 60.Greenberg B. Heart failure preserved ejection fraction with coronary artery disease; Time for a new classification? J Am Coll Cardiol. 2014;63(25_PA):2828–30. doi: 10.1016/j.jacc.2014.03.033. [DOI] [PubMed] [Google Scholar]
- 61.Kerkhof PLM. Annotations regarding the starling curve and rationale for an alternative. Comm Theor Biol. 2000;5:437–53. [Google Scholar]
- 62.Kerkhof PLM, Baan J, Buis B, Arntzenius AC. Relations between ejection fraction and ventricular volume, and their alteration by chronic beta-blockade. Br Heart J. 1981;46:17–22. doi: 10.1136/hrt.46.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Beringer JY, Kerkhof PLM. A unifying representation of ventricular volumetric indexes. IEEE Trans BME. 1998;45:365–71. doi: 10.1109/10.661161. [DOI] [PubMed] [Google Scholar]
- 64.Kerkhof PLM, Weber MF, Vanoverschelde J-L J, Cheng CP. Neural modulation of myocardial oxygen consumption: experiments and model study based on the alternative Starling curve description; 17th Annual International Conference of the IEEE-EMBS; Montreal. 1995. [Google Scholar]
- 65.Kerkhof PLM, Roos A, ter Haar G, Kocsis S, Pijnenburg HL, Stokhof AA. Age variance of left ventricular diameters in dogs with cardiac disease. J Gerontol Biol Sci. 1998;53:B25–31. doi: 10.1093/gerona/53a.1.b25. [DOI] [PubMed] [Google Scholar]
- 66.Kerkhof PLM. Does diabetes mellitus interfere with cardiac volume regulation and affect the interpretation of ventricular performance?; XIII World Congress of Cardiology; Rio de Janeiro, JACC. 1998. [Google Scholar]
- 67.van Baren PA, Knopjes S, Voorhout G, Kerkhof PLM. Development of left ventricular size in foals as a function of growth. J Mol Cell Cardiol. 1999;31:A107. [Google Scholar]
- 68.Kerkhof PLM, Li JK-J. Identification of left ventricular volume regulation on the basis of the elastance concept; 24th Annual International Conference of the IEEE EMBS; Houston. 2002. [Google Scholar]
- 69.Kerkhof PLM, Li JK-J, Kresh JY, Heyndrickx GR. Cardiac volume regulation: transition among two phenotypes in heart failure. Faseb J. 2014;28:1073–2. [Google Scholar]
- 70.Kerkhof PLM, Li JK, Heyndrickx GR. Effective arterial elastance and arterial compliance in heart failure patients with preserved ejection fraction. Conf Proc IEEE Eng Med Biol Soc. 2013;2013:691–4. doi: 10.1109/EMBC.2013.6609594. [DOI] [PubMed] [Google Scholar]
- 71.McMurray JJ, Adamopoulos S, Anker SD, et al. ESC Committee for Prac-tice Guidelines ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2012;33(14):1787–847. doi: 10.1093/eurheartj/ehs104. [DOI] [PubMed] [Google Scholar]
- 72.Woo KS, White HD. Factors affecting outcome after recovery from myocardial infarction. Annu Rev Med. 1994;45:325–39. doi: 10.1146/annurev.med.45.1.325. [DOI] [PubMed] [Google Scholar]
- 73.Shim CY. Arterial-cardiac interaction: the concept and implications. J Cardio-vasc Ultrasound. 2011;19:62–6. doi: 10.4250/jcu.2011.19.2.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chantler PD, Lakatta EG. Arterial-ventricular coupling with aging and disease. Front Physiol. 2012;3:90. doi: 10.3389/fphys.2012.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chantler PD, Lakatta EG, Najjar SS. Arterial-ventricular coupling: mechanistic insights into cardiovascular performance at rest and during exercise. J Appl Physiol. 2008;105(4):1342–51. doi: 10.1152/japplphysiol.90600.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chantler PD, Melenovsky V, Schulman SP, et al. Use of the Frank-Starling mechanism during exercise is linked to exercise-induced changes in arterial load. Am J Physiol Heart Circ Physiol. 2012;302(1):H349–58. doi: 10.1152/ajpheart.00147.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ambrosi CM, Yamada KA, Nerbonne JM, Efimov IR. Gender differences in electrophysiological gene expression in failing and non-failing human hearts. PLoS One. 2013;8(1):e54635. doi: 10.1371/journal.pone.0054635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kerkhof PLM, Handly N, Heyndrickx GR. Gender effects on left ventricular volume regulation in patients with beta-adrenergic blockade. 2015. [submitted] [Google Scholar]
- 79.Petrie MC, Dawson NF, Murdoch DR, Davie AP, McMurray JJ. Failure of women’s hearts. Circulation. 1999;99(17):2334–41. doi: 10.1161/01.cir.99.17.2334. [DOI] [PubMed] [Google Scholar]
- 80.Burke JH, Goldberger JJ, Ehlert FA, Kruse JT, Parker MA, Kadish AH. Gender differences in heart rate before and after autonomic blockade: evidence against an intrinsic gender effect. Am J Med. 1996;100(5):537–43. doi: 10.1016/s0002-9343(96)00018-6. [DOI] [PubMed] [Google Scholar]
- 81.Kerkhof PLM, Li JK-J, Kresh JY. An analytical expression for the regulation of ventricular volume in the normal and diseased heart. Cardiovasc Eng. 2002;2:37–48. [Google Scholar]
- 82.Kerkhof PLM. Is regulation of left ventricular dimension affected by the presence of atrial fibrillation? J Mol Cell Cardiol. 1998;30:A71. [Google Scholar]
- 83.Kerkhof PLM, Tielens ET, Berghout A. Hyperthyroid state and left ventricular volume regulation in human patients. FASEB J. 2001;15:A1137. [Google Scholar]
- 84.Kerkhof PLM, Li JK-J, Kresh JY, Heyndrickx GR. Left ventricular diastolic elastance is higher in women compared to men, and elevated with beta-blockade. Faseb J. 2015;29:799–5. [Google Scholar]
- 85.Kerkhof PLM, Nijland F, Kamp O. Effect of low dose dobutamine on left ventricular volume regulation in patients with myocardial infarction related to wall motion abnormalities. FASEB J. 2002;16:A1. [Google Scholar]
- 86.Hugenholtz PG, Wagner HR. Assessment of myocardial function in congenital heart disease. In: Adams FH, Swan HJC, Hall VE, editors. Pathophysiology of Congenital Heart Diseases. Berkeley: University of California Press; 1970. pp. 201–30. [PubMed] [Google Scholar]
- 87.Robotham JL, Takata M, Berman M, Harasawa Y. Ejection fraction revisited. Anesthesiology. 1991;74:172–83. doi: 10.1097/00000542-199101000-00026. [DOI] [PubMed] [Google Scholar]
- 88.Marmor A, Jain D, Zaret B. Beyond ejection fraction. J Nucl Cardiol. 1994;1:477–86. doi: 10.1007/BF02961601. [DOI] [PubMed] [Google Scholar]
- 89.Manisty CH, Francis DP. Ejection fraction: a measure of desperation? Heart. 2008;94:400–1. doi: 10.1136/hrt.2007.118976. [DOI] [PubMed] [Google Scholar]
- 90.Kerkhof PLM, Huisman GH. Application of Legendre transformation to the end-systolic behavior of the ventricle; VIth International Conference Cardiovascular System Dynamics Society; Oxford, UK. 1982. [Google Scholar]
- 91.Shoucri RM. ESPVR, ejection fraction and heart failure. Cardiovasc Eng. 2010;10:207–12. doi: 10.1007/s10558-010-9105-0. [DOI] [PubMed] [Google Scholar]
- 92.Grossman W, Braunwald E, Mann T, McLaurin LP, Green LH. Contractile state of the left ventricle in man as evaluated from end-systolic pressure–volume relations. Circulation. 1977;56(5):845–52. doi: 10.1161/01.cir.56.5.845. [DOI] [PubMed] [Google Scholar]
- 93.Kerkhof PLM, Li JK-J. Effective end/systolic volume of the left ventricle and its contribution to cardiac function indexes; 25th Annual International Conference of the IEEE EMBS; Cancun. 2003. [Google Scholar]
- 94.Crottogini AJ, Willshaw P, Barra JG, Pichel RH. Left ventricular end-systolic elastance is incorrectly estimated by the use of stepwise afterload variations in conscious, unsedated, autonomically intact dogs. Circulation. 1994;90(3):1431–40. doi: 10.1161/01.cir.90.3.1431. [DOI] [PubMed] [Google Scholar]
- 95.Crottogini AJ, Willshaw P, Barra JG, Armentano R, Cabrera Fischer EI, Pichel RH. Inconsistency of the slope and the volume intercept of the end-systolic pressure–volume relationship as individual indexes of inotropic state in conscious dogs: presentation of an index combining both variables. Circulation. 1987;76(5):1115–26. doi: 10.1161/01.cir.76.5.1115. [DOI] [PubMed] [Google Scholar]
- 96.Takeuchi M, Igarashi Y, Tomimoto S, et al. Single-beat estimation of the slope of the end-systolic pressure–volume relation in the human left ventricle. Circulation. 1991;83(1):202–12. doi: 10.1161/01.cir.83.1.202. [DOI] [PubMed] [Google Scholar]
- 97.Lascano EC, Negroni JA, Barra JG, Crottogini AJ, Pichel RH. Single-beat evaluation of left ventricular inotropic state in conscious dogs. Am J Physiol. 1989;256(1 pt 2):H56–65. doi: 10.1152/ajpheart.1989.256.1.H56. [DOI] [PubMed] [Google Scholar]
- 98.Hayashi K, Shigemi K, Shishido T, Sugimachi M, Sunagawa K. Single-beat estimation of ventricular end-systolic elastance-effective arterial elastance as an index of ventricular mechanoenergetic performance. Anesthesiology. 2000;92(6):1769–76. doi: 10.1097/00000542-200006000-00037. [DOI] [PubMed] [Google Scholar]
- 99.Kerkhof PLM. Importance of end-systolic volume for the evaluation of cardiac pump performance. In: EI Chazov, Smirnov VN, Oganov RG., editors. Cardiology, An International Perspective. New York: Plenum Press; 1984. pp. 1339–52. [Google Scholar]
- 100.Duomarco JL, Esponda AC. On the relationship between end systolic volume and ventricular mechanical impulse – a new law of the heart. Acta Physiol Latinoam. 1958;8:164–75. [Google Scholar]
- 101.Holt JP. Regulation of the degree of emptying of the left ventricle by the force of ventricular contraction. Circ Res. 1957;5(3):281–7. doi: 10.1161/01.res.5.3.281. [DOI] [PubMed] [Google Scholar]
- 102.White HD, Norris RM, Brown MA, Brandt PW, Whitlock RM, Wild CJ. Left ventricular end-systolic volume as the major determinant of survival after recovery from myocardial infarction. Circulation. 1987;76(1):44–51. doi: 10.1161/01.cir.76.1.44. [DOI] [PubMed] [Google Scholar]
- 103.Suda K, Kohl T, Kovalchin JP, Silverman NH. Echocardiographic predictors of poor outcome in infants with hypertrophic cardiomyopathy. Am J Cardiol. 1997;80:595–600. doi: 10.1016/s0002-9149(97)00428-1. [DOI] [PubMed] [Google Scholar]
- 104.Norris RM, White HD. Therapeutic trials in coronary thrombosis should measure left ventricular function as primary end-point of treatment. Lancet. 1988;1(8577):104–6. doi: 10.1016/s0140-6736(88)90295-4. [DOI] [PubMed] [Google Scholar]
- 105.Ikram H, Chan W, Bennett SI, Bones PJ. Haemodynamic effects of acute beta-adrenergic receptor blockade in congestive cardiomyopathy. Br Heart J. 1979;42(3):311–5. doi: 10.1136/hrt.42.3.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Borow KM, Green LH, Mann T, et al. End-systolic volume as a predictor of postoperative left ventricular performance in volume overload from valvular regurgitation. Am J Med. 1980;68(5):655–63. doi: 10.1016/0002-9343(80)90251-x. [DOI] [PubMed] [Google Scholar]
- 107.Chemla D, Coirault C, Hébert J-L, Lecarpentier Y. Mechanics of relaxation of the human heart. News Physiol Sci. 2000;15:78–83. doi: 10.1152/physiologyonline.2000.15.2.78. [DOI] [PubMed] [Google Scholar]
- 108.White HD, Norris RM. Left-ventricular function as an end-point. Lancet. 1989;2(8664):676. doi: 10.1016/s0140-6736(89)90916-1. [DOI] [PubMed] [Google Scholar]
- 109.Gaudron P, Eilles C, Kugler I, Ertl G. Progressive left ventricular dysfunction and remodeling after myocardial infarction. Potential mechanisms and early predictors. Circulation. 1993;87(3):755–63. doi: 10.1161/01.cir.87.3.755. [DOI] [PubMed] [Google Scholar]
- 110.Hamer AW, Takayama M, Abraham KA, et al. End-systolic volume and long-term survival after coronary artery bypass graft surgery in patients with impaired left ventricular function. Circulation. 1994;90(6):2899–904. doi: 10.1161/01.cir.90.6.2899. [DOI] [PubMed] [Google Scholar]
- 111.Yamaguchi A, Ino T, Adachi H, Mizuhara A, Murata S, Kamio H. Left ventricular end-systolic volume index in patients with ischemic cardiomyopathy predicts postoperative ventricular function. Ann Thorac Surg. 1995;60:1059–62. doi: 10.1016/0003-4975(95)00488-7. [DOI] [PubMed] [Google Scholar]
- 112.Migrino RQ, Young JB, Ellis SG, et al. End-systolic volume index at 90 to 180 minutes into reperfusion therapy for acute myocardial infarction is a strong predictor of early and late mortality. The global utilization of streptokinase and t-PA for occluded coronary arteries (GUSTO)-I angiographic investigators. Circulation. 1997;96(1):116–21. doi: 10.1161/01.cir.96.1.116. [DOI] [PubMed] [Google Scholar]
- 113.Yamaguchi A, Ino T, Adachi H, et al. Left ventricular volume predicts postoperative course in patients with ischemic cardiomyopathy. Ann Thorac Surg. 1998;65(2):434–8. doi: 10.1016/s0003-4975(97)01155-7. [DOI] [PubMed] [Google Scholar]
- 114.Florea VG, Henein MY, Anker SD, Francis DP, Gibson DG, Coats AJ. Relation of changes over time in ventricular size and function to those in exercise capacity in patients with chronic heart failure. Am Heart J. 2000;139(5):913–7. doi: 10.1016/s0002-8703(00)90025-5. [DOI] [PubMed] [Google Scholar]
- 115.Turakhia MP, McManus DD, Whooley MA, Schiller NB. Increase in end-systolic volume after exercise independently predicts mortality in patients with coronary heart disease: data from the heart and soul study. Eur Heart J. 2009;30:2478–84. doi: 10.1093/eurheartj/ehp270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ahnve S, Gilpin E, Henning H, Curtis G, Collins D, Ross J., Jr Limitations and advantages of the ejection fraction for defining high risk after acute myocardial infarction. Am J Cardiol. 1986;58(10):872–8. doi: 10.1016/s0002-9149(86)80002-9. [Erratum in Am J Cardiol 198 Mar 1; 59(6):A12] [DOI] [PubMed] [Google Scholar]
- 117.Norman HS, Oujiri J, Larue SJ, Chapman CB, Margulies KB, Sweitzer NK. Decreased cardiac functional reserve in heart failure with preserved systolic function. J Card Fail. 2011;17:301–8. doi: 10.1016/j.cardfail.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tsutsui H, Tsuchihashi-Makaya M. Clinical characteristics and outcomes of heart failure with preserved ejection fraction: lessons from epidemiological studies. J Cardiol. 2010;55:13–22. doi: 10.1016/j.jjcc.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 119.Frenneaux M, Williams L. Ventricular-arterial and ventricular-ventricular interactions and their relevance to diastolic filling. Prog Cardiovasc Dis. 2007;49(4):252–62. doi: 10.1016/j.pcad.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 120.Bhatia RS, Tu JV, Lee DS, et al. Outcome of heart failure with preserved ejection fraction in a population-based study. N Engl J Med. 2006;355(3):260–9. doi: 10.1056/NEJMoa051530. [DOI] [PubMed] [Google Scholar]
- 121.Ho JE, Gona P, Pencina MJ, et al. Discriminating clinical features of heart failure with preserved vs. reduced ejection fraction in the community. Eur Heart J. 2012;33(14):1734–41. doi: 10.1093/eurheartj/ehs070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kaila K, Haykowsky MJ, Thompson RB, Paterson DI. Heart failure with preserved ejection fraction in the elderly: scope of the problem. Heart Fail Rev. 2012;17(4–5):555–62. doi: 10.1007/s10741-011-9273-z. [DOI] [PubMed] [Google Scholar]
- 123.Fukuta H, Little WC. The cardiac cycle and the physiologic basis of left ventricular contraction, ejection, relaxation, and filling. Heart Fail Clin. 2008;4(1):1–11. doi: 10.1016/j.hfc.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mann DL. Getting pumped about heart failure. Cell Metab. 2014;19(6):896–7. doi: 10.1016/j.cmet.2014.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wahlquist C, Jeong D, Rojas-Muñoz A, et al. Inhibition of miR-25 improves cardiac contractility in the failing heart. Nature. 2014;508(7497):531–5. doi: 10.1038/nature13073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wronska A, Kurkowska-Jastrzebska I, Santulli G. Application of micro-RNAs in diagnosis and treatment of cardiovascular disease. Acta Physiol (Oxf) 2015;213:60–83. doi: 10.1111/apha.12416. [DOI] [PubMed] [Google Scholar]
- 127.Ma TS, Bozkurt B, Paniagua D, Kar B, Ramasubbu K, Rothe CF. Central venous pressure and pulmonary capillary wedge pressure; Fresh clinical perspectives from a new model of discordant and concordant heart failure. Tex Heart Inst J. 2011;38:627–38. [PMC free article] [PubMed] [Google Scholar]
- 128.Forrester JS, Diamond G, Chatterjee K, Swan HJ. Medical therapy of acute myocardial infarction by application of hemodynamic subsets (first of two parts) N Engl J Med. 1976;295(24):1356–62. doi: 10.1056/NEJM197612092952406. [DOI] [PubMed] [Google Scholar]
- 129.Redfield MM, Jacobsen SJ, Burnett JC, Jr, Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA. 2003;289(2):194–202. doi: 10.1001/jama.289.2.194. [DOI] [PubMed] [Google Scholar]
- 130.Skrabanek P, McCormick J. Follies and Fallacies in Medicine. 2nd ed. Whithorn: The Tarragon Press; 1992. [Google Scholar]
- 131.Penicka M, Bartunek J, Trakalova H, et al. Heart failure with preserved ejection fraction in outpatients with unexplained dyspnea: a pressure–volume loop analysis. J Am Coll Cardiol. 2010;55:1701–10. doi: 10.1016/j.jacc.2009.11.076. [DOI] [PubMed] [Google Scholar]
- 132.Patel HC, Hayward C, di Mario C, Cowie MR, Lyon AR, Rosen SD. Heart failure with preserved ejection fraction: the impact of stricter definitions. Eur J Heart Fail. 2014;16(7):767–71. doi: 10.1002/ejhf.106. [DOI] [PubMed] [Google Scholar]
- 133.Punnoose LR, Givertz MM, Lewis EF, Pratibhu P, Stevenson LW, Desai AS. Heart failure with recovered ejection fraction: a distinct clinical entity. J Card Fail. 2011;17(7):527–32. doi: 10.1016/j.cardfail.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 134.de Groote P, Fertin M, Duva Pentiah A, Goéminne C, Lamblin N, Bauters C. Long-term functional and clinical follow-up of patients with heart failure with recovered left ventricular ejection fraction after β-blocker therapy. Circ Heart Fail. 2014;7:434–9. doi: 10.1161/CIRCHEARTFAILURE.113.000813. [DOI] [PubMed] [Google Scholar]
- 135.Givertz MM, Mann DL. Epidemiology and natural history of recovery of left ventricular function in recent onset dilated cardiomyopathies. Curr Heart Fail Rep. 2013;10(4):321–30. doi: 10.1007/s11897-013-0157-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Katz DH, Beussink L, Sauer AJ, Freed BH, Burke MA, Shah SJ. Prevalence, clinical characteristics, and outcomes associated with eccentric versus concentric left ventricular hypertrophy in heart failure with preserved ejection fraction. Am J Cardiol. 2013;112:1158–64. doi: 10.1016/j.amjcard.2013.05.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Welles CC, Ku IA, Kwan DM, Whooley MA, Schiller NB, Turakhia MP. Left atrial function predicts heart failure hospitalization in subjects with preserved ejection fraction and coronary heart disease: longitudinal data from the heart and soul study. J Am Coll Cardiol. 2012;59(7):673–80. doi: 10.1016/j.jacc.2011.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Mottram PM, Harper RW, Peverill RE. Heart failure with normal ejection fraction: the diagnostic importance of left atrial volume. J Am Coll Cardiol. 2011;57(13):1499. doi: 10.1016/j.jacc.2010.10.046. [author replies 1499–500] [DOI] [PubMed] [Google Scholar]
- 139.Rose WC, Schwaber JS. Analysis of heart rate-based control of arterial blood pressure. Am J Physiol. 1996;271:H812–22. doi: 10.1152/ajpheart.1996.271.2.H812. [DOI] [PubMed] [Google Scholar]
- 140.Winterfeld HJ, Siewert H, Strangfeld D, et al. Use of walking and sauna therapy in the rehabilitation of hypertensive patients with ischemic heart disease following aortocoronary venous bypass operation with special reference to hemody-namics. Z Kardiol. 1988;77:190–3. [in German] [PubMed] [Google Scholar]
- 141.Masutani S, Cheng HJ, Hyttilä-Hopponen M, et al. Orally available levo-simendan dose-related positive inotropic and lusitropic effect in conscious chronically instrumented normal and heart failure dogs. J Pharmacol Exp Ther. 2008;325(1):236–47. doi: 10.1124/jpet.107.134940. [DOI] [PubMed] [Google Scholar]
- 142.Sasayama S, Asanoi H. Coupling between the heart and arterial system in heart failure. Am J Med. 1991;90(5B):14S–8. doi: 10.1016/0002-9343(91)90267-2. [DOI] [PubMed] [Google Scholar]
- 143.Fox JM, Maurer MS. Ventriculovascular coupling in systolic and diastolic heart failure. Curr Heart Fail Rep. 2005;2(4):204–11. doi: 10.1007/BF02696651. [DOI] [PubMed] [Google Scholar]
- 144.Borlaug BA, Kass DA. Ventricular-vascular interaction in heart failure. Heart Fail Clin. 2008;4(1):23–36. doi: 10.1016/j.hfc.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Feynman RP, Leighton RB, Sands M. The Feynman Lectures on Physics. Part II. 2nd ed. Reading, MA: Addison-Wesley Publishing Company; 1966. pp. 31–6. [Google Scholar]
- 146.Beketaev I, Wang J. Sumoylation in gene regulation and cardiac disease: potential for drug discovery. Adv Genomics Genet. 2014;4:185–92. [Google Scholar]
- 147.van der Velde ET, Burkhoff D, Steendijk P, Karsdon J, Sagawa K, Baan J. Non-linearity and load sensitivity of end-systolic pressure–volume relation of canine left ventricle in vivo. Circulation. 1991;83(1):315–27. doi: 10.1161/01.cir.83.1.315. [DOI] [PubMed] [Google Scholar]
- 148.Aghajani E, Muller S, Kjorstad KE, et al. The pressure–volume loop revisited: is the search for a cardiac contractility index a futile cycle? Shock. 2006;25(4):370–6. doi: 10.1097/01.shk.0000209521.20496.7a. [DOI] [PubMed] [Google Scholar]
- 149.Van den Berg A, Flameng W, Herijgers P. Parameters of ventricular contractility in mice: influence of load and sensitivity to changes in inotropic state. Pflügers Arch. 2008;455(6):987–94. doi: 10.1007/s00424-007-0362-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary File. Analytical description of the relationship between EF and ESV.