Abstract
There have been significant advances in the understanding of the biology and treatment of non-small cell lung cancer (NSCLC) over the past few years. A number of molecularly targeted agents are in the clinic or in development for patients with advanced NSCLC (Table 1). We are beginning to understand the mechanisms of acquired resistance following exposure to tyrosine kinase inhibitors in patients with oncogene addicted NSCLC. The advent of next generation sequencing has enabled to study comprehensively genomic alterations in lung cancer. Finally, early results from immune checkpoint inhibitors are very encouraging. This review summarizes recent advances in the area of cancer genomics, targeted therapies and immunotherapy.
Keywords: NSCLC, TARGETED THERAPIES, IMMUNOTHERAPY
1. MOLECULAR GENETICS OF HUMAN LUNG CANCER
Lung cancer has traditionally been classified by histologic subtype and immunohistochemical characteristics. However, this classification has been complicated by the recognition that several clinically actionable somatic genetic alterations can be identified in the distinct histologic subtype of lung cancer and that some of these alterations can be found in more than one histology. Through comprehensive genomic analysis it is known that all lung cancers carry high rates of somatic mutation, high levels of inter- and intra-chromosomal rearrangement and copy number alterations as compared to other tumor types.1 Exploitation of these genomic aberrations has become an attractive and efficacious treatment strategy and has underscored the need for multiplexed genetic testing as part of the routine care of patients with lung cancer. To stratify patients into clinically relevant subgroups, the combination of histomorphological, immunohistochemical and genetic analysis is now employed for routinely for patients with newly diagnosed lung cancer and is the standard of care in newly diagnosed adenocarcinoma in which Epidermal Growth Factor Receptor (EGFR) mutation and Anaplastic Lymphoma Kinase (ALK) rearrangement testing have been incorporated into standard treatment algorithms. Additionally many institutions are now routinely testing for alterations such as ROS, RET, BRAF, and HER2 which have shown initial promise in tailored cancer treatment.
1.1 Lung Adenocarcinoma
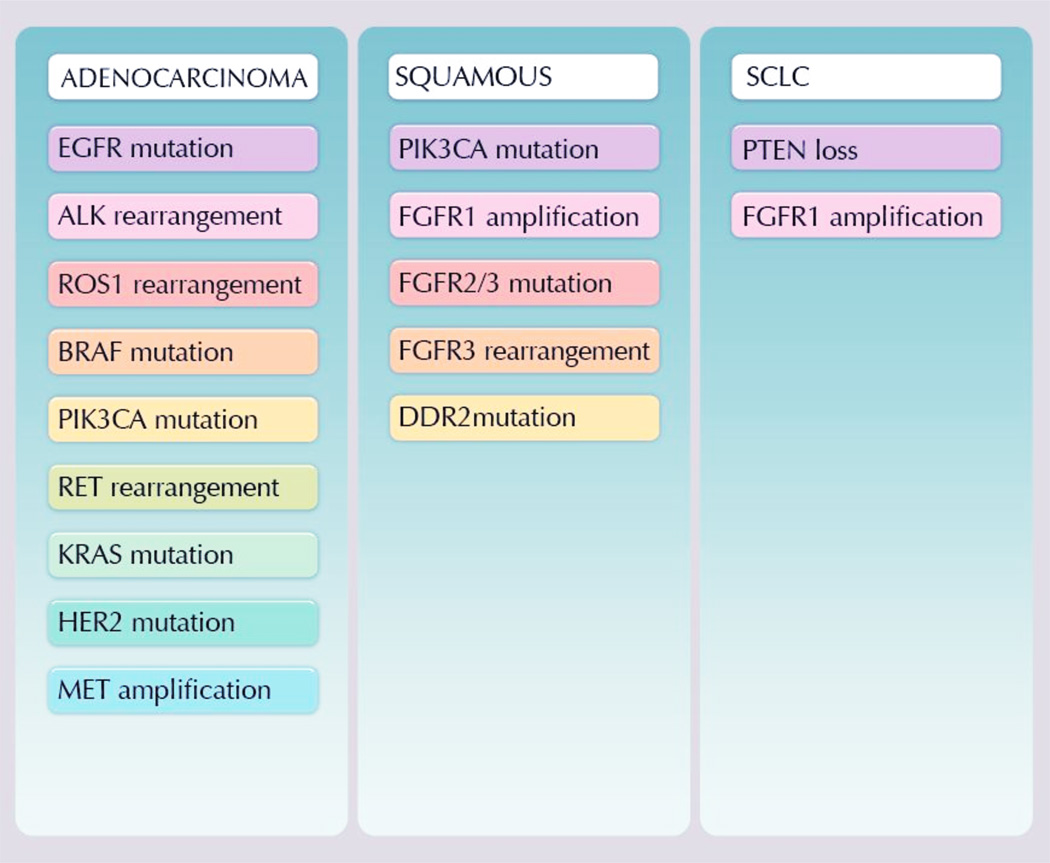
Lung adenocarcinoma is one of the best genetically characterized human epithelial malignancies and recent discoveries of targetable driver mutations have highlighted the impressive cadre of molecular alterations present in this disease. The identification of oncogenic activation of particular tyrosine kinases in some patients with advanced NSCLC most notably mutations in EGFR2–4 or rearrangements of the ALK gene5, has led to a paradigm shift and the development of specific molecular treatments for patients. These clinical successes have revolutionized the field and stimulated the investigation into additional, potential targetable, generation aberrations across all lung cancer histologies (Figure 1).
Figure 1.
Potential targetable oncogenes by histology subtype
For patients with lung adenocarcinoma the impact of genetic testing has led to changes in the standard diagnostic algorithms with recommendations from the International Association for the Study of Lung Cancer (IASLC) and National comprehensive Cancer Network (NCCN) that newly diagnosed patients with advanced disease be tested for EGFR mutation and ALK fusion testing. Additionally many institutions are now routinely testing for alterations in genes such as ROS, RET, MET, BRAF, and HER2 which have shown initial promise in tailored cancer treatment.
The need to perform detailed molecular testing of lung cancers began with the correlation of EGFR mutations and sensitivity to gefitinib and erlotinib in lung adenocarcinoma, typically in patients with modest tobacco exposure EGFR tyrosine kinase inhibitors (TKIs) are now the established first line therapy in patients with NSCLC known to have activating mutations in EGFR.6, 7 The majority of these tumors initially respond to EGFR TKIs, but subsequently develop resistance to therapy, with a median time to progression of 9 months.8 Recent work has demonstrated the value of additional molecular testing at the time of acquired resistance in EGFR TKI-responsive patients, as nearly half of patients with disease progression will carry a secondary EGFR mutation, such as T790M, which can now be successfully targeted with third generation EGFR TKIs such as AZD9291 and CO-1686.8, 9 Additional mechanisms of resistance to EGFR inhibitors have been defined in re-biopsy cohorts many of which are associated with a potential for response to other targeted agents or with response to other chemotherapies (small cell transformation).8
Receptor tyrosine kinase gene rearrangements, such as ALK, ROS and RET, are identified in 1–8% of lung adenocarcinomas.10 Patients’ whose tumors harbor ALK fusion, as well as ROS1 rearrangements demonstrate a response to crizotinib and other TKIs.11, 12 However, similar to their EGFR counterparts, these patients ultimately recur. This has led to molecular characterization of mechanisms of acquired resistance and the clinical use of ALK and ROS inhibitors with expanded mechanisms of action such as LDK378.
Interestingly, some of the most frequent genomic alterations in adenocarcinoma, such as mutations in TP53, KRAS, and STK11, have proven difficult to target and therapeutically exploit.13, 14 The mitogen activation pathway (MAPK) is often implicated in the development of lung adenocarcinoma however little success has been garnered therapeutically. The most common mechanism for MAPK activation is through substitutions mutations in 12th, 13th and 61st amino of KRAS. Activating KRAS mutations are observed in approximately 20 to 25% of lung adenocarcinomas in the United States and are generally associated with a history of smoking. The presence of a KRAS mutation appears to have at most a limited effect on overall survival (OS) in patients with early stage NSCLC, although some data have suggested that it was associated with inferior prognosis. Efforts to identify specific RAS inhibitors for KRAS-mutated lung cancer have proven challenging with the current focus of targeted therapeutics for patients with KRAS-mutated lung cancer is against downstream effectors of activated KRAS such as MEK1/MEK2, PI3K and AKT. Recent Phase II data examining combination use of selumetenib, an inhibitor of MEK1/MEK2 and docetaxel has been shown to have promising activity KRAS-mutant patient population.15 Additional work on downstream effectors in the KRAS mutant pathway is crucial and currently several clinical trials employing the inhibition of PI3KCA, MEK and PTEN are in progress.
1.2 Squamous Cell Lung Cancer
Genotyping alone has improved survival in patients who harbor a targetable mutation such as EGFR mutation and EML4-ALK fusions. However, there has been limited advancement in targeted approaches in squamous cell carcinoma until recently. Recent genomic profiling in squamous cell carcinoma has highlighted a number of new molecular targets including the FGFR family kinases. Fibroblast growth factor receptors are cell surface tyrosine kinase receptors that mediates cell survival and proliferation. Gene amplification of FGFR1 has been detected in 7 to 25% of squamous tumors, and extensive profiling has identified low-frequency activating mutations and copy number alterations in all of the FGF receptors.16–18 Small molecule inhibitors of FGFR1 are in clinical development and a case report of a NSCLC patient with tumor regression in response to the FGFR small molecule tyrosine kinase inhibitor BGJ398 has been presented.19 Additionally, a number of different mutations have been identified in FGFR2 and FGFR3, including a recurrent fusion of FGFR3 and TACC3, which provides much needed insight into the oncogenic pathways operating in SCC and makes a strong case for applying FGFR inhibitors in this disease.20–22 Targetable alterations in lung squamous cell carcinomas also include members of the PI3K pathway, DDR2 and potentially the NRF2/KEAP1/CUL3 antioxidant response pathway. In contrast to lung adenocarcinomas, there does not appear to be a substantial cohort of non-smokers with squamous cell lung cancer and the disease appears to be more genomically homogeneous.
1.3 Small Cell Lung Cancer
With the development of targeted agents, the treatment paradigm of NSCLC continues to evolve however the discovery of a clinically actionable mutation in small cell lung cancer (SCLC) remains elusive. SCLC remains an exceptionally aggressive malignancy with limited treatment options in the relapsed/refractory setting. SCLC has a high mutation rate, likely secondary to tobacco carcinogen exposure in this patient population. This high rate of mutation makes the identification of pathologically relevant driver mutations difficult. Genomic sequencing has confirmed a high prevalence of difficult to target TP53 and RB1 inactivation mutations. Next generation sequencing has been applied to the SCLC genome in hopes of identifying new therapeutic targets and recent work has showcased the identification of significantly mutated genes in SCLC cell lines. These aberrations include genetic alterations affecting histone modifying enzymes CREBPP, EP300, and LFF as well as PTEN mutations, FGFR1 and SOX2 amplification. Recent work on SOX2 demonstrates the prevalence of SOX2 amplification in SCLC cell lines with expression of SOX2 strongly correlated with increased gene copy number and clinical stage leading the authors to postulate if SOX2 is a genuine SCLC driver mutation.23, 24
2. EPIGENETIC THERAPY IN THE TREATMENT OF LUNG CANCER
The contributions of epigenetic dysregulation to carcinogenesis through aberrant DNA methylation and altered chromatin configuration continue to be expanded.25 The cancer epigenome contains a variety of tumor-specific alterations which define subgroups of disease, alter transcriptional patterns, and may reflect therapeutic sensitivities.26–28
Recent genomic sequencing efforts have further underscored the inextricable connections between genetic and epigenetic mechanisms of carcinogenesis, including alternative mechanisms leading to loss of individual tumor suppressor gene function, as well as cooperation of defects affecting key signaling pathways. A signature example in lung cancer is the CDKN2A locus, encoding both the p16 tumor suppressor (a key regulator of cell-cycle progression from G1 to S-phase) as well as ARF (which can sequester MDM2, leading to stabilization of the tumor suppressor p53). Comprehensive genomic analysis of squamous cell lung cancers by the Cancer Genome Atlas (TCGA) Research Network has demonstrated CDKN2A loss of function in the large majority of cases, the most common mechanisms being homozygous deletion in 29%, site-specific promoter hypermethylation leading to gene silencing in 21%, and missense or truncating mutation in 18%.27
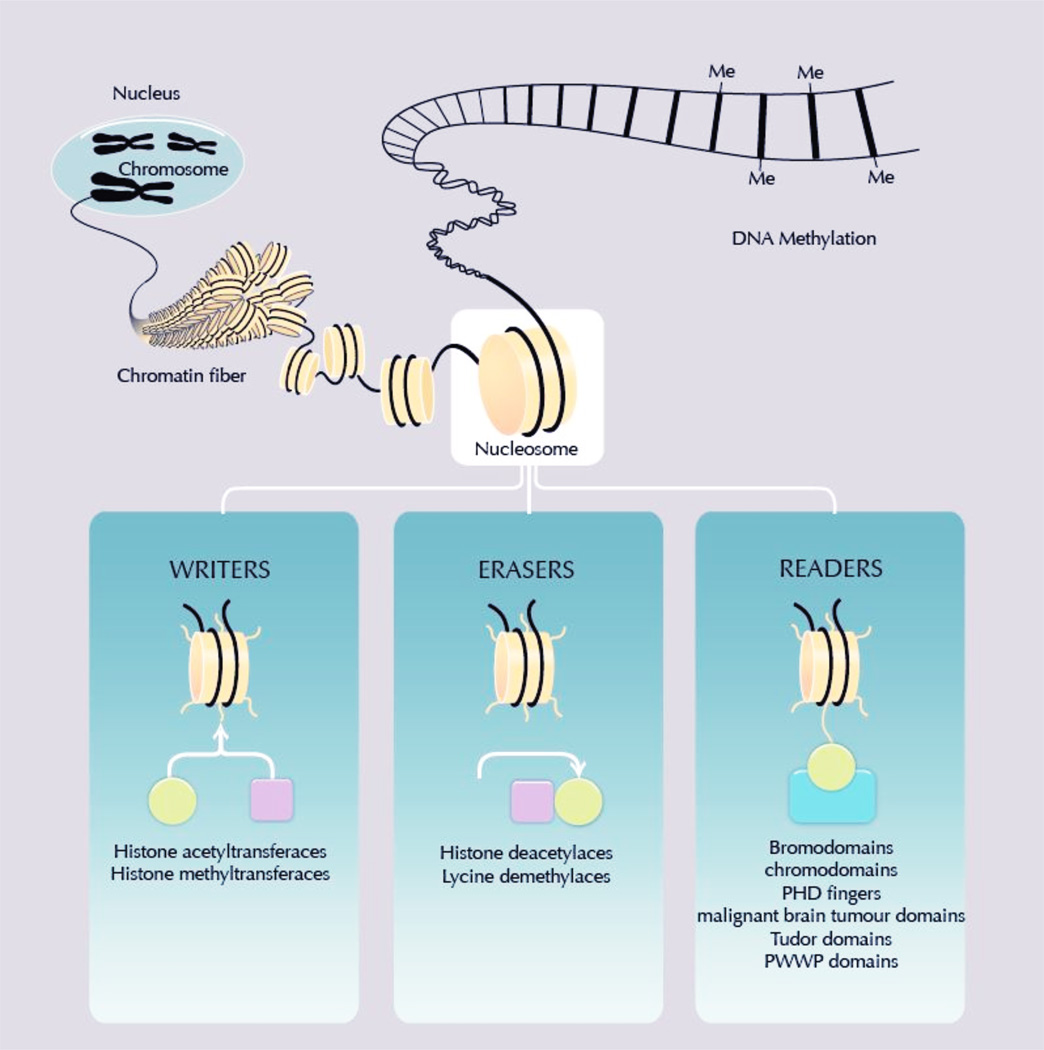
The TCGA has also demonstrated, across tumor types, high rates of genetic alterations in key epigenetic regulators. These include members of the mixed-lineage leukemia (MLL) gene family of histone methyltransferases, mutated in 20 and 18 percent of squamous and non-squamous lung cancers, respectively.29 Chromatin modifying enzymes which add and remove histone modifications, termed “writers” and “erasers”, affect the state of chromatin compaction of DNA making it more or less accessible for the transcription of genes which play various roles in a cancer cell’s transformation (Figure 2). Beyond the vast changes in DNA methylation which characterize cancer, these enzymes contribute to an altered landscape of transcriptional regulation not only in the hematologic malignancies for which epigenetic therapy has found early success, but also in a broad range of solid tumors including lung cancers.25, 29 Mutations of the isocitrate dehydrogenase genes, found across many tumor types, most notably gliomas and sarcomas but also including a small percentage of lung cancers, result in a CpG island methylator phenotype (CIMP) with therapeutic implications.29, 30 Multiple CIMP-like states have been described, and the molecular abnormalities responsible for these phenotypes are beginning to be defined; to date these include alterations in genes affecting the metabolism of methylated cytosines as well as mutations in DNA methyltransferases.30, 31
Figure 2.
Chromatin modifying enzymes
Historically, identification and demonstration of re-expression of individual tumor suppressor genes has provided the rationale for the clinical development of DNA hypomethylating agents and histone deacetylase inhibitors in various hematologic malignancies.32 In fact, of course, hundreds of genes are affected by these therapies. Many of the described “hallmarks of cancer,” broad programs of normal cellular functions subverted during carcinogenesis, are altered by epigenetic reprogramming.33 Epigenetically directed therapies have the potential to concurrently affect multiple relevant pathways critical to cancer proliferation, survival, and metastatic capacity. Site-specific hypermethylation of promoters for genes controlling stem cell maturation has been implicated as a mechanism contributing to replicative immortality and clonogenic potential in cancer.34, 35 Promoter demethylation by DNA methyltransferase inhibitors such as azacitidine can markedly reduce replicative capacity in cancer cell ines, associated with reversal of a program of cancer-specific changes in methylation.36 Mutations in epigenetic regulators such as the polycomb-repressive complex protein BMI-1 and the histone methyltransferase EZH2 can enhance clonogenic potential of cancer cells.35 Additionally, genetic alterations of chromatin regulatory genes with established roles in proliferation, inhibition of apoptosis and senescence, and promotion of genomic instability, have been described.35
In addition to affecting key elements of carcinogenesis, epigenetic therapy may have a role in the treatment of acquired resistance to mutationally targeted therapy. For example, inhibition of the histone demethylase KDM5A has been shown to preferentially eliminate clonogenic survivors to EGFR tyrosine kinase therapy in EGFR-mutant NSCLC cell lines.37
While epigenetic therapy regimens employing DNA hypomethylating agents or histone deacetylase inhibitors have become standard-of-care therapies in myelodysplastic syndrome and peripheral T-cell lymphoma, these treatments have only begun to be clinically investigated in lung cancer or other solid tumors. Combinatorial epigenetic therapy consisting of the DNA hypomethylating agent azacitidine and the histone deacetylase inhibitor entinostat has been shown to result in rare objective responses in lung cancer.38 Data from this initial study suggest that combinatorial epigenetic therapy may prime lung cancers for improved responses to subsequent therapy, notably including immunotherapy. Preclinical models suggest that multiple pathways down-regulated in tumors as a mechanism of immune escape and evasion may be re-expressed in response to epigenetic therapy and may augment the effectiveness of PD-1 immune checkpoint blockade.39 Thus epigenetic therapy may prime tumors to respond to immunotherapeutic strategies by overcoming tumor mechanisms including increased antigen presentation, up-regulation of PD-L1 expression and augmentation of interferon and cytokine signaling within the tumor.40
In addition to next generation hypomethylating agents and histone deacetylase inhibitors, there are a host of novel epigenetic therapies targeting chromatin modifying enzymes now being translated into clinical testing in lung cancer and other solid tumors. EZH2 inhibitors are currently in early phase clinical development, and may be of relevance in the treatment of lung cancers including small cell lung cancers, in which EZH2 appears to be frequently overexpressed. A DOT1L inhibitor is being developed for acute leukemias defined by alterations in MLL, a gene family of histone methyltransferases also commonly mutated or otherwise genetically altered in lung cancer. Beyond targeting chromatin modifying enzymes that write or erase histone marks, bromodomain and extra-terminal (BET) histone binding proteins possess what may be termed a “reading” function focused on histone acetylation; trials of inhibitors of the protein-protein interaction have been initiated in small cell lung cancer.41–43 The evolving knowledge of the prevalence and array of identifiable defects in chromatin regulators and DNA-methylation phenotypes suggests a large number of potential targets and strategies for epigenetic therapy beyond those which have formed the basis of much clinical investigation of epigenetic therapies to date. Thoracic oncologists can expect an expanding portfolio of novel epigenetically targeted agents with potential for clinical application to lung cancer over the next several years.
3. EGFR MUTATION-POSITIVE NSCLC
The approach toward lung cancer therapeutics has undergone a major paradigm shift in the last ten years. The impetus to move toward larger and more frequent biopsies and perform upfront genotyping at the time of diagnosis came in large part with the recognition that between 10 and 20% of US lung cancer patients had tumors carrying an EGFR mutation, a biomarker of oncogene addiction that correlates strongly with response to EGFR TKIs.44 There are several subtypes of EGFR mutations, but the two most frequent, L858R and del19, comprise 90% of the cases and are also the most tightly associated with robust response to therapy.45 In this review, L858R and del19 mutations will be referred to collectively as “common mutations”. We will review the current treatment recommendations for EGFR-mutant patients and the pivotal studies that shape the basis for the recommendations.
3.1 Advanced Stage Disease: First-Line
When EGFR mutations were first discovered, several single arm phase 2 studies were quickly performed confirming that patients with advanced lung cancer and common EGFR mutations did very well with first-line gefitinib and erlotinib therapy, with response rates of 60–75% and median progression-free survival (PFS) of approximately 9–10 months.46–48 While these results were two-to-three fold better than what was achieved with the current standard-of-care platinum doublet chemotherapy regimens, there was still some skepticism about whether a randomized trial would favor an EGFR TKI or not, because EGFR-mutant patients seemed to do better on chemotherapy than EGFR wild-type patients. However, this debate was settled when the IPASS study was published.
IPASS was a large randomized trial of about 1200 patients done in Asia, where EGFR mutations are 2–3 times more common than in Western countries.6 All the subjects were non-smokers with adenocarcinoma, both of which are clinical features that have been associated with increased incidence of EGFR mutations. Patients did NOT have to have an EGFR mutation to enter the trial, but among the subset that had tissue available for EGFR mutation testing, about 60% were positive for common EGFR mutations. The IPASS design compared 1st-line gefitinib to 1st-line chemotherapy with carboplatin and paclitaxel for up to 6 cycles with a primary end-point of PFS. The results showed that in the overall intention-to-treat population gefitinib had an improved PFS compared to chemotherapy with a hazard ratio (HR) of 0.74 and a 95% confidence interval (CI) 0.65–0.85 (Table 2). However, when examining the subset of patients with tissue available for genotyping, it became clear that the overall positive results for gefitinib were exclusively due to the contribution from the EGFR-mutant cohort of patients, who had an even more impressive PFS benefit from first-line gefitinib, HR= 0.48 (95% CI0.36, 0.46). Conversely, the EGFR wild-type patients showed that front-line EGFR TKI was a harmful strategy for them with HR = 2.85 (95% CI 2.1, 4.0). In addition to a PFS benefit, patients with EGFR mutations treated with gefitinib had an improved quality of life compared to those treated with chemotherapy.49 Hence, practice changed significantly with the IPASS publication in two major ways: 1) the importance of early genotyping was appreciated and giving 1st-line EGFR TKIs to patients with EGFR mutations became an accepted therapeutic strategy and 2) because the wild-type patients did so poorly with 1st-line gefitinib in lieu of chemotherapy, it became obvious that if one didn’t know the mutation status for a patient, then EGFR TKIs should not be given, at least in the 1st-line setting.
Table 2.
Summary of Randomized Trials Examining Genotype-Customized First-Line EGFR TKI Therapy
| Study | Treatment | N | Response Rate |
Median PFS, mo |
HR for PFS (95% CI) |
Median OS, mo |
HR for OS (95% CI) |
|---|---|---|---|---|---|---|---|
| IPASS 6, 54 | Gefitinib | 132 | 71% | 9.6 | 0.48 (0.36, 0.64) | 21.6 | 1.00 (0.76, 1.33) |
| Carbo/Pac | 129 | 47% | 6.3 | 21.96 | |||
| WJTOG 34057* | Gefitinib | 86 | 62% | 9.2 | 0.49 (0.35, 0.71) | NR | NR |
| Cis/doce | 86 | 31% | 6.3 | NR | |||
| NEJ 0028 | Gefitinib | 114 | 74% | 10.4 | 0.36 (0.25, 0.51) | 30.5 | No ratio provided P=0.31 |
| Carbo/pac | 114 | 31% | 5.5 | 23.6 | |||
| OPTIMAL*50 | Erlotinib | 83 | 83% | 13.1 | 0.16 (0.10, 0.26) | NR | NR |
| Carbo/gem | 82 | 36% | 4.6 | NR | |||
| EURTAC*51 | Erlotinib | 86 | 58% | 9.7 | 0.37 (0.25, 0.54) | 19.3 | 1.04 (0.65, 1.68) |
| Carbo or cis/gem or doce | 87 | 15% | 5.2 | 19.5 | |||
| Lux-Lung 352 | Afatinib | 230 | 69% | 11.1 | 0.58 (0.43, 0.78) | 28.2 | 0.88 (no CI provided) |
| Cis/pem | 115 | 44% | 6.9 | 28.2 | |||
| Lux-Lung 3: Afatinib arm, common mutations only* | 203 | 75% | 13.6 | 0.47 (0.34, 0.65) | 31.5 | 0.78 (0.58, 1.06) | |
| Lux-Lung 3: Cis/pem arm, common mutations only* | 104 | 43% | 6.9 | 28.6 | |||
| Lux-Lung 3: Afatinib arm, exon 19 del only56 | 112 | NR | NR | 0.28 (0.18, 0.44) | 33.3 | 0.54 (0.36, 0.79) | |
| Lux-Lung 3: Cis/pem arm, exon 19 del only | 57 | NR | NR | 21.1 | |||
| Lux-Lung 653 | Afatinib | 242 | 74% | 11.0 | 0.28 (0.20, 0.39) | 23.1 | 0.93 (no CI provided) |
| Cis/gem | 122 | 31% | 5.6 | 23.5 | |||
| Lux-Lung 6: Afatinib arm, common mutations only* | 216 | NR | 11.0 | 0·25 (0.18, 0.35) | 23.6 | 0.83 (0.62, 1.09) | |
| Lux-Lung 6: Cis/gem arm, common mutations only* | 108 | NR | 5.6 | 23.5 | |||
| Lux-Lung 6: Afatinib arm, exon 19 del only56 | 124 | NR | NR | 0·20 (0·13 0·33) | 31.4 | 0.64 (0.44, 0.94) | |
| Lux-Lung 6: Cis/gem arm, exon 19 del only | 62 | NR | NR | 18.4 | |||
Only L858R and del 19 mutants were included in this study; NR = no mature data reported
After IPASS, several other randomized trials were completed in rapid succession, each confirming similar benefits from first-line gefitinib or erlotinib, primarily for patients with common EGFR mutations (Table 2).7, 50–53 The EURTAC study was especially important from this collection of studies as it was the first such trial performed in a Western population.52 In EURTAC, Spanish and Italian EGFR mutant patients (common mutations only) treated with first-line erlotinib had improved PFS compared to investigator choice chemotherapy (either cisplatin/docetaxel or cisplatin/gemcitabine), HR = 0.37 (95% CI 0.25−0.54). None of the studies examining first-line gefitinib or erlotinib have demonstrated a survival advantage for the genotype-directed therapy, presumably because EGFR mutants have a very robust response rate and PFS when EGFR TKIs are given in the 2nd or later-line setting, thus allowing them to “catch up” to the benefit achieved with first-line therapy.
More recently, two randomized studies were completed with the 2nd-generation EGFR TKI afatinib as first-line therapy for EGFR mutation-positive patients.54, 55 In contrast to the 1st-generation drugs erlotinib and gefitinib, 2nd-generation EGFR TKIs are “irreversibly binding” meaning that instead of ATP-competitive binding at the receptor, the drug forms a direct chemical covalent bond with the EGFR receptor. In addition, afatinib binds all the ErbB receptors, not just EGFR. Lux-lung 3 was a global study comparing afatinib to cisplatin/pemetrexed and Lux-lung 6 was performed in China only, comparing afatinib to cisplatin/gemcitabine. Similar to the prior studies, the afatinib trials showed a superior PFS, response rate, and quality of life for genotype-directed treatment, particularly among the 90% of trial participants with common EGFR mutations. As a result, afatinb was FDA approved in 2013 as first-line therapy for patients with L858R and deletion 19 EGFR mutations. At the 2014 ASCO meeting, we also learned that among exon 19 deletion mutants, first-line afatinib appears to improve OS compared to first-line chemotherapy.56 Although these results were from a post-hoc subgroup analysis, the survival benefit was large (approximately one year), and was replicated in both Lux-lung 3 and Lux-lung 6 with highly significant p-values (Table 2). The L858R patients did not have a survival advantage with afatinib, similar to results from other studies with gefitinib and erlotinib.
Because of this collection of research, the current standard approach in the US is to test all patients with newly diagnosed advanced adenocarcinoma for EGFR mutations and, if positive for a common mutation, to treat with either afatinib or erlotinib.57 If patients are symptomatic from their cancer and cannot wait for the results of mutation testing to return, chemotherapy should be started as EGFR TKIs should only be given in the first-line setting to patients known to have an EGFR mutation. There are uncommon mutations that are still considered sensitizing to EGFR TKIs such as L861Q, G719X and S768I. However, it is important to note that the exon 20 insertion/deletion mutations are typically not sensitive to erlotinib, gefitinib and afatinib.58
3.2 Advanced Stage Disease: Special considerations for EGFR mutant patients
There are several considerations in the management of EGFR mutant patients that are unique compared to historical approaches for treating lung cancer: 1) When to start EGFR TKIs if chemotherapy was given prior to mutation test result availability, 2) Should EGFR TKIs be continued beyond progression and, 3) How to work-up EGFR mutation positive lung cancer with acquired resistance to the first EGFR TKI. There are no definitive randomized trials that give us direction about these issues, but clinical experience is now large and consensus recommendations are emerging. For patients that were unable to wait for EGFR mutation test results prior to starting first line chemotherapy, it is always difficult to know when and how to start an EGFR TKI after the mutation is discovered. Options range from beginning the TKI immediately after the test results returns to not until the patient progresses and 2nd-line therapy is indicated. One popular approach is to complete 4–6 cycles of the first-line chemotherapy (assuming the patient is tolerating therapy and is not progressing through it) and then switch to the EGFR TKI, similar to a maintenance approach, however no clinical trials have addressed this specific sitution.58
A more common question is under what circumstances to continue an EGFR TKI when the patient is progressing on therapy. The discussion arises because it has been observed that even when EGFR mutants are radiographically progressing through an EGFR therapy, removal of that therapy can hasten a clinically-significant flare in the disease in up to 25% of cases, leading to hospitalization and/or death in approximately one week in the initial publication.59 The disease flare is thought to be due to a mix of clones within the tumor, some of which are still sensitive to the EGFR TKI and remain under control even while other clones are growing. Removal of the suppressive TKI can allow many more cells to divide compared to keeping the suppressive TKI on board.
EGFR mutants have two distinctive patterns of progression not historically distinguished in lung cancer treatment paradigms: 1) progression in only one site while the rest of the disease remains stable, and 2) very slow and indolent progression in multiple anatomic locations. There is mounting evidence suggesting that if progression is only in one location then local treatment (surgery or radiation) followed by continued EGFR TKI therapy can yield good outcomes.60, 61 In one study using this approach, EGFR mutant patients had controlled disease for a median of 6 months after the locally-directed therapy before further progression was noted.60 In addition, clinical experience is accumulating supporting the notion that patients who are having slow and indolent progression while on an EGFR TKI can achieve significant additional time on therapy after meeting RECIST criteria for progression.62, 63 One single institution experience documented that 88% of EGFR patients received ongoing EGFR TKI beyond RECIST-defined progression and the median time until a change in therapy was necessary from that point was 10 months.63
Once a patient is progressing sufficient to demand a change in systemic therapy, there is an additional question of whether one should stop the EGFR TKI and switch to chemotherapy or continue the EGFR TKI along with adding chemotherapy. Again, the observation of a clinically-significant flare in disease if the EGFR TKI is stopped has fueled interest in this question. Prospective randomized studies are in process which will provide further guidance but retrospective studies suggest that response rates may be higher if the EGFR TKI is continued while chemotherapy is added.64
Considering a biopsy at the time of progression on the initial EGFR TKI is an emerging standard for EGFR mutants.56 Initially this was a maneuver primarily done for research purposes, in order to gain a better understanding of the range of molecular mechanisms of acquired resistance and to consider customizing clinical trial options for patients. It then became appreciated that a small portion of patients would have a transformation from adenocarcinoma harboring an EGFR mutation to small cell lung cancer with the same EGFR mutation as an escape mechanism from their EGFR TKI.65, 66 This transformation, although rare, facilitated broader clinical interest in repeat biopsies because the biopsy might indicate a new therapeutic direction. In the current era, data is rapidly accumulating that 3rd-generation EGFR TKIs may have high activity among those with acquired resistance by virtue of the T790M mutation in exon 20, the single mutation that accounts for 50–65% of acquired resistance, see below.67, 68 This provides yet an additional and compelling clinical indication for biopsy at the time of acquired resistance.
3.3 Treatment of Acquired Resistance
Even though initial therapy with an EGFR TKI is quite effective, acquired resistance still develops after 10–15 months. When the 2nd-generation EGFR TKIs were developed, there was great hope that these would be highly effective for patients with acquired resistance because laboratory studies showed these compounds had a high level of activity against T790M in vitro.69 Unfortunately, in clinical trials all three 2nd-generation EGFR TKIs tested (neratinib, afatinib and dacomitinib) have had disappointing results with response rates in the single digits.70–72 The explanation behind the discordant pre-clinical and clinical results is thought to be that the 2nd-generation drugs have a high degree of wild-type EGFR potency so dose escalation is limited by rash, diarrhea, and other side effects resulting from wild-type EGFR inhibition. Hence, in patients it appears difficult to achieve drug concentrations sufficient to inhibit T790M.
The first successful clinical trial for EGFR acquired resistance was a phase I trial examining the combination of afatinib and cetuximab.73 This trial expanded when activity was observed to ultimately include 93 patients. The response rate was 32%, significantly higher than the 7% observed with single agent afatinib. However, the toxicity of this regimen is not insignificant, with 18% of patients having grade 3 acneiform rash (rash that limits ADLs and covers >30% of the body surface area). Interestingly, the pre-clinical evidence for this combination suggested that T790M mutants would preferentially benefit,74 however the clinical observation has been that response is roughly equal regardless of T790M status.
A new class of 3rd-generation EGFR TKIs have recently entered clinical study.67, 68 These differ from the prior generations of EGRF TKIs because while they have potent inhibition of both activating EGFR mutations and T790M, wild-type inhibition is close to zero, allowing dose escalation to concentrations that can effectively overcome acquired resistance. Two compounds have had mature results presented in abstract form thus far, CO-1686 and AZD9291. Both have demonstrated response rates of about 60% among those with biopsy-proven T790M. Mature PFS is not yet available but responses appear to be durable for at least 6 months in most patients in the preliminary data. As suspected, rash and diarrhea are extremely uncommon during therapy with 3rd-generation EGFR TKIs. CO-1686 causes hyperglycemia, which is typically controlled with oral medications.
3.4 Early Stage Disease
Whenever there is a successful strategy for treating advanced stage disease, such as EGFR TKIs for patients with EGFR mutations, there is interest in moving the therapy from late stage disease to early stage disease with the hope of increasing cure rates. Studies are just beginning that will look at incorporation of EGFR TKIs into multi-modality therapy for stage III disease. However, two studies have been completed offering preliminary data about adjuvant erlotinib. The SELECT study was a single-arm multi-center study of 2 years of adjuvant erlotinib for patients with common EGFR mutations.75 One hundred patients were treated (stage I n=45, stage II n=27, stage III n=28) and the primary end-point of 2-year disease free survival was 89% (by stage: I - 96%, II - 78%, IIIA - 91%), which was significantly improved compared to the pre-defined historical control 2-year disease free survival for EGFR mutants followed with observation alone. In addition, after a median duration of follow-up of over 3 years, of the 29 patients that recurred, only 4 recurred while one erlotinib and 25 recurred after erlotinib was completed, raising speculation that duration of therapy may be important. The RADIANT study was a randomized study of 2 years of adjuvant erlotinib vs. placebo that enrolled a broader population of lung cancer patients among which 16% harbored EGFR mutations.76 Though the overall study was negative, the subgroup analysis of EGFR mutants suggested that erlotinib provided a disease-free survival advantage with HR = 0.61 (95% CI 0.38, 0.98) but no OS advantage in this preliminary study. A more definitive prospective randomized trial including only EGFR mutants and powered to examine OS is set to begin this year.
4. HER 2/3 POSTIVE NSCLC
4.1 The role of HER2 and HER3 in NSCLC
HER2 and HER3 (also known as ERBB2 and ERBB3, respectively) are members of the HER/ERBB receptor tyrosine kinase family, which also includes EGFR and HER4. Although these receptors all mediate cell proliferation and survival through downstream MAPK and PI3K pathways, they vary in regards to the ability to bind ligand and the presence of an active tyrosine kinase domain. For example, HER2 has no known high-affinity ligand and therefore utilizes homo- or hetero-dimerization for activation, and HER3 has no tyrosine kinase activity and relies on heterodimerization to induce downstream signaling. The most powerful signaling heterodimer is that of HER2 and HER3, which can function as an oncogenic unit.77
Oncogenic HER2 kinase domain mutations were first reported in NSCLC in 2004.78 Since that time, several studies have found the rate of kinase domain HER2 mutations in NSCLC to be approximately 2–4%.79–81 These mutations are most commonly in-frame insertions in exon 20 with duplication of amino acids YVMA at codon 775; infrequently, insertions in other codons or point mutations can be found that lead to constitutive activation of downstream pathways resulting in cell growth and survival. More recently, extracellular domain mutations were detected in HER2 and found to be oncogenic, including a S310F mutation in exon 8 detected in 1 of 188 lung adenocarcinomas,82 a S310Y mutation in 1 of 63 squamous cell lung cancers,83 and 1 S310F and 1 S310Y mutation in 258 lung adenocarcinomas sequenced by the Cancer Genome Atlas Network. Across these studies, the frequency of extracellular domain mutations appears to be <1%.
In contrast to HER2, there have been no reports of mutations in the HER3 gene. However, HER3 has been implicated as an escape mechanism for drugs that inhibit signaling through EGFR and HER2.84, 85 Attempts at therapeutically targeting both HER2 and HER3 are ongoing.
4.2 Clinical features of patients with HER2-mutated NSCLC
Patients with HER2-mutant NSCLC have distinct clinicopathologic characteristics, similar to those whose tumors harbor EGFR mutations. In the largest reported study to date of 65 patients with HER2-mutant NSCLC, the median age of diagnosis was 60.4 years (range 31–86), 69% were female, 52% were never-smokers, and all tumors were adenocarcinomas.81 Although HER2 mutations are relatively rare in lung cancer, the rate of detection can be enriched by testing never-smoker patients with adenocarcinoma or adenosquamous histology without an EGFR mutation, in which case the frequency is approximately 14%.79 HER2 mutations are mutually exclusive with point mutations in EGFR, KRAS, BRAF, NRAS, PIK3CA, MEK1 and AKT, as well as rearrangements in ALK.80
4.3 Preclinical and clinical data for therapeutics targeting HER2 and HER3
Both small molecular inhibitors and monoclonal antibodies targeting HER2 are under investigation. Currently there is limited data for patients treated on prospective clinical trials, however preclinical studies and retrospective data from patients treated with off-label, commercially available agents show promise in targeting HER2 in those with HER2-mutant NSCLC. Below are several compounds under investigation.
4.4 Trastuzumab
In contrast to breast cancer, HER2 overexpression or amplification does not predict for benefit from trastuzumab in lung cancer. However, the presence of a HER2 mutation may be a predictive biomarker for response to trastuzumab in NSCLC. In a retrospective study of 16 patients with HER2-mutant NSCLC, a total of 22 anti-HER2 treatments were assessed.81 Of the patients who received trastuzumab-based regimens (trastuzumab combined with carboplatin, paclitaxel, carboplatin/paclitaxel, vinorelbine, or docetaxel), the response rate was 60% (9 out of 15 regimens tested) and disease control rate was 100%. One patient received trastuzumab alone and had a partial response (PR).
4.5 Afatinib
Afatinib is an irreversible small molecular inhibitor of EGFR and HER2 that is approved for use in the first-line setting for patients with EGFR-mutant NSCLC. In lung cancer cell lines harboring a HER2 insertion mutation in the tyrosine kinase domain, afatinib was effective at inhibiting survival, whereas erlotinib was not.86 Interestingly, afatinib was also effective at inhibiting survival in cell lines transformed with the HER2 extracellular domain mutation.82 The clinical activity of afatinib in HER2-mutant NSCLC has been evaluated in the same retrospective study discussed above.81 Three patients who had progressed after receiving trastuzumab-based therapy were treated with afatinib, which resulted in 100% disease control rate (1 PR and 2 stable disease [SD]). In the only prospective study with afatinib in this population, 5 patients with NSCLC harboring a HER2 kinase domain mutation were treated with afatinib, followed by the option to add weekly paclitaxel at 80mg/m2 to afatinib at progression.87 Of the 3 patients evaluable for response (2 patients withdrew early due to toxicity), 2 had a partial response to afatinib alone and 1 had stable disease with afatinib and a PR once paclitaxel was added.
4.6 Dacomitinib
Dacomitinib is an irreversible pan-HER tyrosine kinase inhibitor. A Phase II study in patients that included patients with NSCLC and HER2 amplification or mutation with any number of prior lines of therapy treated patients with dacomitinib 45mg or 30mg with the option to escalate to 45mg once daily.88 Of the 16 evaluable patients in the HER2 cohort, there were 2 with a partial response, both of whom had a HER2 mutation. Final results from this study are pending.
4.7 Neratinib
Preclinical mouse models of HER2-mutant lung cancer have demonstrated that HER2 inhibition plus mTOR inhibition results in significant tumor shrinkage over either alone.89 Based on this and other preclinical data, the combination of neratinib, an irreversible pan-HER small molecule inhibitor, and temsirolimus, an mTOR inhibitor, was studied in a Phase I trial including patients with multiple tumor types.90 Six patients out of the 60 on the trial had HER2-mutant NSCLC. Among them, two had a PR (one of whom had prior trastuzumab) and the remainder had stable disease.
4.8 MM-111
MM-111 is a bispecific fully human antibody targeting HER2 and HER3. In preclinical studies of HER2-overexpressing cancer cells, MM111 inhibits cell proliferation, particularly when used in combination with other HER2 inhibitors such as trastuzumab.91 A Phase I trial in multiple tumor types with HER2 positivity is testing MM-111 combined with various HER2-targeted agents and chemotherapeutics to determine the maximally tolerated dose, safety and efficacy. This drug has not yet been tested in patients with HER2-mutant NSCLC.
4.9 MEHD 7945 A
In contrast to the previously discussed compounds, MEHD 7945 A does not target HER2, but instead is a dual-action human IgG1 monoclonal antibody that targets EGFR and HER3. In cell lines and xenograft models of tumors resistant to the EGFR inhibitors cetuximab or erlotinib, MEHD 7945 A was able to overcome resistance and inhibit tumor growth.92 Clinically, the safety and activity of MEHD 7945 A was studied in a Phase I trial in multiple tumor types.93 Nine patients with NSCLC were included, of which 2 had stable disease as their best response. The final report from this study is pending.
4.10 MM-121
Unique compared to the other drugs discussed here, MM-121 is a monoclonal antibody that only targets HER3. It is being developed in combination with other targeted agents or chemotherapeutics, which is not surprising given the lack of known alterations in HER3 in human cancers. Specifically, MM-121 is being tested in combination with erlotinib, with early signals of clinical benefit in patients with EGFR-mutant NSCLC and erlotinib resistance.94
5. ALK-POSITIVE NSCLC
Chromosomal rearrangements of ALK are present in 3–7% of NSCLC. The resulting ALK fusions, such as EML4-ALK, function as potent oncogenic drivers and lead to a state of oncogene addiction. In the clinic, this phenomenon underlies the marked responsiveness of ALK-positive tumors to small molecule ALK tyrosine kinase inhibition. Crizotinib, a multitargeted tyrosine kinase inhibitor (TKI) of ALK, ROS1 and cMET, was the first ALK inhibitor tested in the clinic and helped to establish ALK as a therapeutic target in NSCLC.12 To date, nine other ALK inhibitors have now entered clinical development, with promising early results in both crizotinib-naïve and crizotinib-resistant disease. Here we review the latest data on crizotinib and select next-generation ALK inhibitors in TKI-naïve patients with ALK-positive NSCLC.
5.1 Crizotinib
Phase 1 and 2 studies have shown that crizotinib is highly active in patients with advanced, ALK-positive NSCLC. In the phase 1 trial (PROFILE 1001), the objective response rate among 143 evaluable patients was 61%, and median PFS was 9.7 months.95 Updated results from the phase 2 study of crizotinib (PROFILE 1005) were recently reported in the US FDA label. Among 765 patients with advanced, ALK-positive NSCLC, the objective response rate was 48% and median duration of response was 11 months;96 the follow-up of these phase 2 patients was too short to evaluate PFS. Based on the response rates observed in the phase 1 and 2 studies, along with its favorable side effect profile, crizotinib was granted accelerated approval by the FDA in August 2011 for patients with advanced, ALK-positive NSCLC. This approval occurred almost exactly 4 years after the first report of ALK rearrangements in NSCLC.97
Recently, the results of the first prospective, randomized phase 3 trial comparing crizotinib with standard chemotherapy in advanced, ALK-positive NSCLC (PROFILE 1007) were reported.11 In this study, 347 ALK-positive patients who had failed one prior platinum-based chemotherapy regimen were randomized 1:1 to receive either crizotinib as their second-line therapy, or pemetrexed or docetaxel chemotherapy. Compared with standard single-agent chemotherapy, treatment with crizotinib resulted in a significantly longer progression-free survival and a tripling of the objective response rate. The median progression-free survival with crizotinib was 7.7 months by independent radiology review, compared to 3.0 months with chemotherapy. Consistent with previous single-arm studies, the response rate with crizotinib was 65%, as opposed to 20% with chemotherapy, thus confirming the significant antitumor activity of crizotinib in advanced ALK-positive NSCLC.
In this study, crizotinib was more active than either pemetrexed or docetaxel chemotherapy in ALK-positive NSCLC.11 Consistent with previous studies in unselected patients with advanced NSCLC, the efficacy of second-line docetaxel in ALK-positive NSCLC was minimal, with a median progression free survival of 2.6 months and an objective response rate of 6.9%.98 In contrast, pemetrexed showed greater activity than expected based on previous second-line studies.99 Median progression free survival was 4.2 months in ALK-positive patients, as compared with 3.5 months in unselected NSCLC patients with adenocarcinoma histology. The response rate to pemetrexed was also higher in this study at 29.3%, as compared with 12.8% in the general population of lung adenocarcinomas.100 While these findings suggest that patients with ALK-positive NSCLC may be more responsive than average to pemetrexed-based chemotherapy, the benefit of pemetrexed appears to be less than that originally suggested by small retrospective studies,101, 102 and importantly, significantly less than that with crizotinib.
In a prespecified interim analysis, OS was found to be similar between the crizotinib and chemotherapy arms with a median OS of 20.3 and 22.8 months, respectively.11 This analysis was immature with a total of 96 deaths (40% of the required events) and censoring of over 70% of patients in either treatment arm. In addition, the analysis was likely confounded by the high crossover rate of patients in the chemotherapy group. Nearly 90% of patients who were treated with chemotherapy and had disease progression crossed over to receive crizotinib. This issue has similarly complicated the analysis of OS in multiple randomized phase 3 studies of EGFR tyrosine kinase inhibitors in advanced EGFR mutant NSCLC. In these studies where the crossover rate from chemotherapy to targeted therapy ranged from 64% to 95%, no difference in OS was demonstrated despite substantial improvements in progression-free survival with the targeted therapy.
Several important issues regarding the role of crizotinib in ALK-positive NSCLC remain to be addressed. In many countries, crizotinib is an approved therapy for patients with advanced, ALK-positive NSCLC with no requirement for prior treatment. As a result, crizotinib can be prescribed as first-line therapy. While the first-line use of EGFR tyrosine kinase inhibitors in advanced EGFR-mutant NSCLC has been established in multiple randomized phase 3 studies, there is limited data on the use of crizotinib in the first-line setting. In the original phase 1 study, there were 24 patients who received crizotinib as their first systemic therapy.95 In this small cohort, the objective response rate was 64% and median progression-free survival was 18.3 months, suggesting that first-line crizotinib may be at least equivalent if not more effective than crizotinib in the second-line setting and beyond. A randomized phase 3 trial comparing crizotinib with platinum/pemetrexed chemotherapy in newly diagnosed, advanced ALK-positive NSCLC (ClinicalTrials.gov number, NCT01154140) has recently completed enrollment, and results may be reported at ASCO 2014. A similar phase 3 trial in Asia comparing first-line crizotinib to platinum/pemetrexed chemotherapy in ALK-positive NSCLC patients (ClinicalTrials.gov number NCT01639001) is ongoing.
5.2 Next-generation ALK Inhibitors: Alectinib and Ceritinib
Alectinib (RO5424802) is a highly potent and selective TKI targeting ALK, but not ROS1 or cMET.103 Alectinib was first evaluated in a phase 1/2 study in Japan and enrolled a total of 70 Japanese patients with advanced, ALK-positive NSCLC who were crizotinib-naïve.104 In contrast to the PROFILE 1001/5/7 studies which used ALK FISH only, patients were identified as ALK-positive using ALK immunohistochemistry (IHC), followed by ALK FISH for confirmation. In the phase 2 portion of the study, the ORR with alectinib dosed at 300 mg twice daily was remarkably high at 94%. With a median follow-up of only 7.6 months, median PFS is not yet known, but durable responses exceeding 12 months have been reported.
Similarly, the next-generation ALK inhibitor ceritinib (LDK378) has also demonstrated high response rates in crizotinib-naïve ALK-positive NSCLC. In preclinical studies, ceritinib is also more potent and selective than crizotinib, targeting ALK and ROS1 but not cMET.105 In a global phase 1 study, ceritinib was highly active in patients with advanced, ALK-positive NSCLC.106 Among 34 patients who had not received an ALK inhibitor and who received ceritinib at doses of 400 mg or higher, the ORR was 62%, and median PFS was 10.4 months. Of note, in contrast to the phase 1 study of alectinib, this study included both Asian and Caucasian patients. In addition, the ceritinib study required only ALK FISH testing to demonstrate ALK rearrangement, as opposed to both ALK IHC and ALK FISH. Thus, these two factors, ethnicity and diagnostic testing, could explain the differences in efficacy seen between alectinib and ceritinib in the TKI-naïve ALK-positive population.
6. ROS1, RET AND NTRK1 POSITIVE NSCLC
6.1 Biology
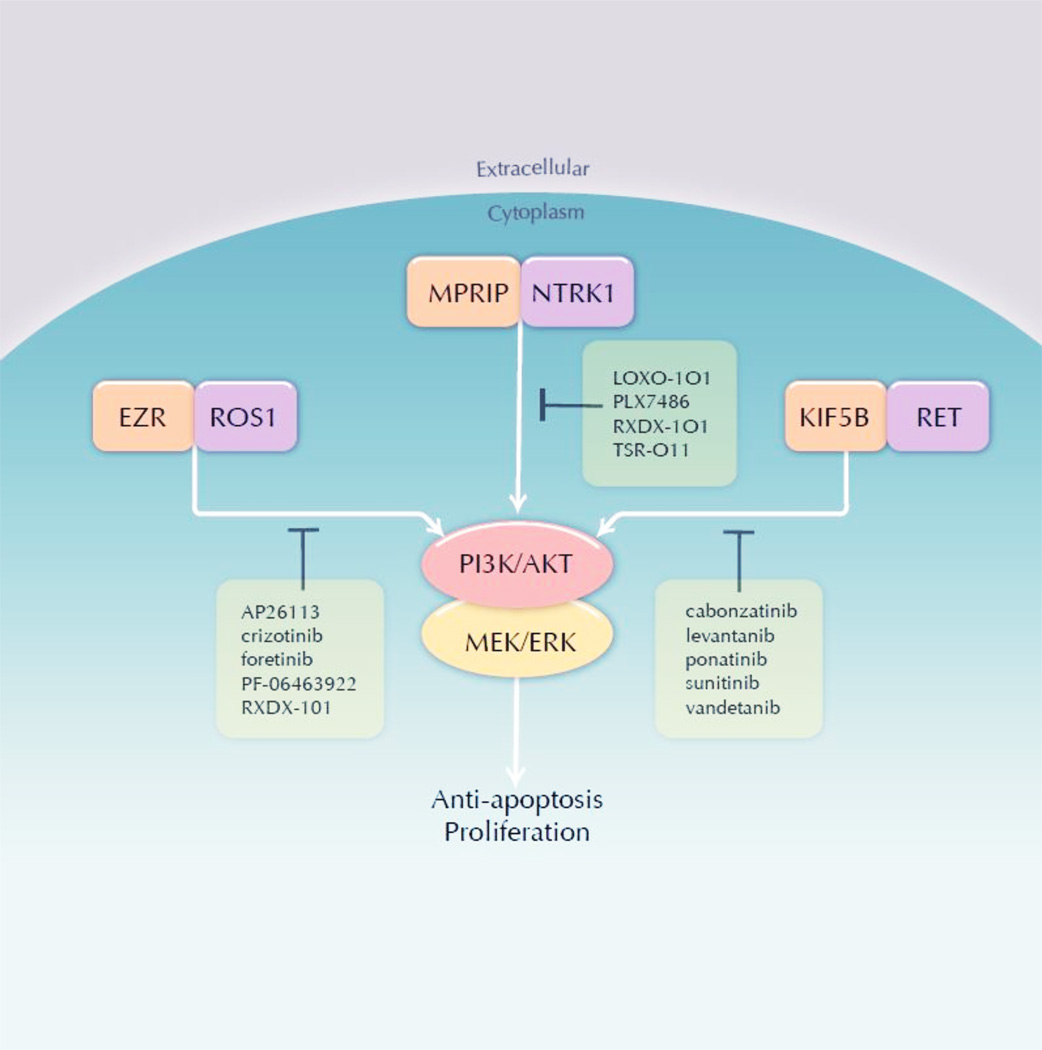
Gene fusions result from large-scale inter- or intra- rearrangements or chromosomal deletions that join pieces of two disparate genes and result in chimeric mRNA transcripts and proteins. The gene fusions described here contain sequences from the 5’ region of an unrelated partner gene and the 3’ region of genes encoding receptor tyrosine kinases (RTKs): ROS1, RET, and NTRK1. These gene fusions always have an intact kinase domain encoded by the 3’ gene region, but contain varying 5’ sequences from other genes. These partner genes typically provide 2 critical components: a promoter that allows sufficient transcription of the novel gene and sequences that encode oligomerization domains. ROS1, RET, TRKA (encoded by the NTRK1 gene) are not highly expressed in most lung adenocarcinomas, but their upstream partner joins a promoter that drives sufficient expression in the tumor cell.108–110 The typical mode of activation for these RTKs cannot occur because they lack the extracellular domains harboring the ligand-binding domain; however, the oligomerization domain, for example coiled-coil domains encoded by the partners KIF5B, CCDC6, EZR, TPM3 and MPRIP facilitate dimerization of the fusion protein.97, 111, 112 It is currently unknown whether different fusion partners, which can target the fusion proteins to different cellular compartments, induce differential tumor behavior, including drug sensitivity.113 Activation of the kinase domain initiates a downstream signaling cascade that ultimately activates MAPK and AKT signaling, which leads to cellular proliferation among other tumorigenic properties.112, 114, 115 This dominant signaling role makes targeted inhibition of these oncogenes an attractive therapeutic strategy (Figure 3).
Figure 3.
ROS1, NTRK1 and RET inhibitors.
6.2 Incidence
Multiple studies have investigated the incidence of the oncogenic fusions using a variety of techniques, including fluorescence in situ hybridization (FISH), IHC, next generation sequencing (NGS) of RNA and DNA, and polymerase chain reaction (PCR); however, an approved companion diagnostic is not yet available for these oncogenes.108, 111, 112, 114, 116–118 Typically, these oncogene fusions do not overlap with other dominant oncogenes, but unbiased studies have demonstrated overlap of ROS1 fusions and EGFR, KRAS, and BRAF mutations, similar to dual oncogenes observed in ALK positive cases.117–122 The incidence of ROS1, RET, and NTRK1 gene fusions appears to be in the range of 1–3%, although in studies using enriched cohorts (i.e., negative for other oncogenes) the reported incidence is higher.108, 111, 112, 114, 116–118 Although associations have been drawn with age, sex, and smoking history, there is no reliable clinical selection for oncogenic fusions and thus these factors should not be used as criteria for selection of patients to undergo testing.111, 112, 114, 116, 123, 124 Although these gene fusions are widely associated with adenocarcinoma histology, this is not an ideal selection criteria as squamous cell and other histologies have been associated with ROS1, RET, and NTRK1 fusions.108, 116, 117, 125, 126
6.3 ROS1, RET and NTRK in other disease types
Although these oncogenic fusions occur infrequently in lung cancer, interest in these targets is bolstered by their occurrence in other malignancies. ROS1 has been detected in gastric cancer, colorectal cancer, Spitzoid neoplasms and numerous others.113, 119, 127, 128 RET fusions have long been identified in papillary thyroid cancer but have also been identified in CMML and others.128–130 An oncogenic NTRK1 fusion was first detected in a colorectal cancer specimen, later found to be prevalent in papillary thyroid cancers, and now identified in multiple other tumor types.128, 129, 131–133 Many TRKA inhibitors have activity against two homologous RTKs, TRKB (NTKR2) and TRKC (NTRK3). These genes are also involved in gene fusions across multiple cancer types, perhaps broadening the appeal of these pan-TRK inhibitors.134–137
Inhibitors of ROS1, RET and TRK
6.4 ROS1
Crizotinib (Pfizer) has been approved for use in ALK+ NSCLC. ROS1 has high homology to ALK and many ALK inhibitors also display ROS1 inhibition.113 An expanded phase I trial is the first trial to report clinical outcomes of ROS1+ NSCLC patients treated with crizotinib (NCT00585195). The most recent update of 35 patients demonstrated an objective response rate (ORR) of 60% and a 6-month PFS rate of 76%, very similar to studies of the same drug in ALK+ NSCLC patients.95, 138 Foretinib (XL-880, GlaxoSmithKline) is a multi-kinase inhibitor with activity against ROS1 (as well as RET, MET, AXL and other kinases) that has a planned ROS1 cohort in an upcoming clinical trial (NCT01068587).139 Ceritinib (LDK378, Novartis) is a potent second generation ALK inhibitor that displays weaker ROS1 inhibition;105 however, this drug is not currently in clinical trials enrolling ROS1+ NSCLC patients. AP26113 (Ariad) is currently in clinical trials for ALK+ NSCLC and also has activity against ROS1, but is currently not yet enrolling ROS1+ NSCLC patients (NCT01449461). PF-06463922 (Pfizer) is a next generation ALK/ROS1 inhibitor that is currently enrolling crizotinib-naïve or TKI-resistant ROS1 patients (NCT01970865).140
6.5 RET
Multiple RET inhibitors are undergoing clinical trials in RET+ NSCLC patients and many of these drugs are multi-kinase inhibitors. A clinical trial of cabozantinib (XL184, Exelixis), a RET inhibitor (in addition to MET and VEGFR2) is currently accruing RET+ NSCLC patients (NCT01639508). Early results from this trial demonstrated confirmed partial responses (PR) in 2 patients and prolonged stable disease (31 weeks) in a third patient demonstrating early clinical activity of this RET inhibitor in RET gene fusion positive patients.141 A phase II clinical trial of vandetanib (AstraZeneca), a dual RET and EGFR inhibitor, in RET+ NSCLC is currently accruing patients (NCT01823068). A patient treated off-protocol with vandetanib 300mg once daily showed a clinical response.142 An additional patient treated with off-protocol vandetanib showed prolonged stable disease 6 months on drug.143 Lenvantinib (E7080, Eisai) is multi-kinase inhibitor (VEGFR1–3, FGFR1–3, SCFR, and PDGFR) with activity against RET and is currently enrolling patients in a phase II clinical trial (NCT01877083).144 Clinical trials of ponatinib (AP24534, Ariad), a multikinase inhibitor with RET activity, in RET+ NSCLC are planned (NCT01935336).145, 146 Sunitinib (Pfizer) is another multikinase inhibitor currently in a phase 2 clinical trial of never smokers with lung adenocarcinoma and has a secondary endpoint to evaluate benefit in patient with RET gene fusions (NCT01829217).
6.6 TRK
LOXO-101 (Loxo) is a selective pan-TRK inhibitor (TRKA, TRKB, and TRKC) that is planned to shortly enter first in man phase I clinical trials. RXDX-101 (Ignyta) is a pan-TRK inhibitor that also has ALK/ROS1 activity with reported CNS penetration and is currently in phase I clinical trials. TSR-011 (Tesaro) is an ALK inhibitor with ~10 selectivity over the TRK family of RTKs and is currently in a phase I clinical trial (NCT0204848).147 PLX7486 (Plexxikon), a pan-TRK inhibitor with additional activity against Fms, is currently in clinical trials as a single agent and in combination with chemotherapy in patients with solid tumors (NCT01804530). This study will also evaluate cancer-related pain as TRKA signaling can modulate pain: Mutations in the NTRK1 gene are the cause of the autosomal recessive syndrome of congenital insensitivity to pain with anhydrosis (CIPA).148 A major focus of next generation ALK inhibitors has been to improve CNS penetration to more effectively treat the brain metastases that occur frequently in patients demonstrating disease progression on crizotinib;149 however, CNS penetration may not be a desired effect of pan-TRK inhibitors. Inhibition of TRKB has been linked to ataxia and other serious neurologic side effects, mimicking the phenotype of the mutant stargazer (stg) mice, which demonstrate ataxia and lack brain-derived neurotrophic factor (BDNF), the TRKB cognate ligand.150, 151
6.7 Resistance
Resistance mechanisms to cognate inhibitors of ROS1 are similar to mechanisms of drug resistance observed for tumors bearing ALK fusions or EGFR mutations. The first described mechanism of resistance was a patient with a ROS1 kinase domain mutation.152 This mutation, G2032R, is analogous to the ALK G1202R and adjacent to the D1203N and S1206Y mutation located at the solvent front; all of these mutations induce resistance to crizotinib.153–155 Preclinical data suggests that foretinib and PF-06463922 can inhibit ROS1 G2032R and that AP26113 can overcome the predicted ROS1 gatekeeper mutation, L2026M.139, 140, 156 Resistance mechanisms to RET inhibitors have yet to be described in NSCLC patients; however, ponatinib has demonstrated activity against oncogenic RET carrying substitutions at the predicted gatekeeper residue, V804.145, 146 TRKA harbors a bulky tyrosine residue at the conserved gatekeeper position perhaps making this position a less likely site of mutation to decrease inhibitor binding. Bypass signaling has also recently been described in a ROS1+ cell line model of drug resistance. The lung adenocarcinoma cell line with SLC34A2-ROS1, HCC78, with in vitro induced resistance to a ROS1 kinase inhibitor, switched oncogene dependence away from ROS1 to EGFR.157 This mechanism of resistant suggests the need for combination strategies to prevent or overcome resistance.
7. BRAF MUTANT NSCLC
BRAF, an oncogene encoding a RAS-regulated kinase that promotes cell growth, has generated recent interest in oncology.158, 159 The majority of BRAF mutations promote kinase activation, enhancing the kinase’s ability to directly phosphorylate MEK.159 The BRAF exon 15 mutation in which glutamine is substituted for a valine at residue 600 (V600E) destabilizes the inactive kinase conformation, leading to continual downstream phosphorylation in the MAPK signaling cascade. BRAF mutations are found in approximately 50% of melanomas, and treatment for metastatic melanoma using selectively targeted BRAF V600E inhibitors has elicited high response rates (RR).160 Yet, colorectal cancers harboring the same BRAF mutation rarely respond to BRAF inhibitor monotherapy.161 Clinical investigation targeting specific BRAF mutations in NSCLC is ongoing.
7.1 Prevalence of BRAF Mutations in NSCLC
In 2002, two studies identified BRAF mutations in 1.6–3% of NSCLC.159, 162 Based on these findings and improved genotyping techniques, a 2011 US study examined tissue from 697 patients with lung adenocarcinoma of which BRAF mutations in codons V600, D594 and G469 occurred in 3% of NSCLC cases.163 An analysis looking at the gene more broadly conducted in Italy in 2011 identified BRAF mutations in 4.9% of patient cases.164 In contrast to melanoma where 90% of BRAF mutations are V600E, approximately half of the BRAF mutations in the general NSCLC population are non-V600E.165 A comprehensive genomic study for squamous cell lung cancer identified BRAF mutations in 4% of cases, all of which were non-V600E.27 The V600E mutation has been associated with a more destructive tumor, with a poor prognosis (significantly shorter DFS and OS).164 V600E mutations have been reported as significantly more common in females than males (8.6% vs. 0.9%) and were less strongly associated with cigarette smoking.164 BRAF mutations in an Asian population were detected at a lower frequency (1.3%).166
7.2 Clinical data with BRAF inhibitors
Vemurafenib, a V600E BRAF inhibitor used in melanoma, has been associated with anti-tumor activity in NSCLC.167 Dabrafenib has been more rigorously evaluated. In an interim analysis of a single arm trial, the overall RR for single agent dabrafenib was 54%, and it was generally well-tolerated.168 As a result, the FDA granted breakthrough status to dabrafenib for V600E mutation-positive NSCLC in January 2014. During a clinical trial of the Src family tyrosine kinase inhibitor dasatinib for advanced NSCLC, a profound antitumor effect was seen in one patient, and that patient was subsequently found to have a kinase-inactivating non-V600E BRAF mutation, Y472CBRAF.169 When studying dasatinib in NSCLC cell lines with an endogenous inactivating BRAF mutation, the cell lines experienced senescence, which was reversed with transfection of active BRAF.169
7.3 Selected ongoing trials with BRAF inhibitors
Currently, a phase II, non-randomized, open-label study of dabrafenib as a monotherapy and in combination with trametinib, a mitogen-activated protein kinase inhibitor, is recruiting stage IV NSCLC participants with BRAF V600E mutations (NCT01336634). A study evaluating dasatinib in subjects with advanced cancers harboring a DDR2 mutation or an inactivating BRAF mutation is currently enrolling (NCT01514864). A phase II, open-label second-line study of GSK1120212, which is closed to enrollment, compared trametinib with docetaxel in stage IV NSCLC with a mutation in KRAS, NRAS, BRAF, or MEK1 gene. (Clinicaltrials.gov No.: NCT01362296).
8. KRAS MUTANT NSCLC
8.1 Biology and Nomenclature
In lung cancer, KRAS (chromosome 12p12.1) is the principal member of the Ras family (which also includes HRAS [11p15.5] and NRAS [1p13.1]) involved in tumorigenesis. The HRAS and KRAS genes were initially identified from studies of two cancer-causing viruses, the Harvey sarcoma virus and the Kirsten sarcoma virus. These viruses were originally discovered in rats by Jennifer Harvey and Werner Kirsten, hence the name Rat sarcoma (Ras).170 NRAS is so named for its initial identification in human neuroblastoma cells. All RAS proteins undergo complex, multi-step post-translational modification including farnesylation, gerangylgerangylation, and palmitoylation.
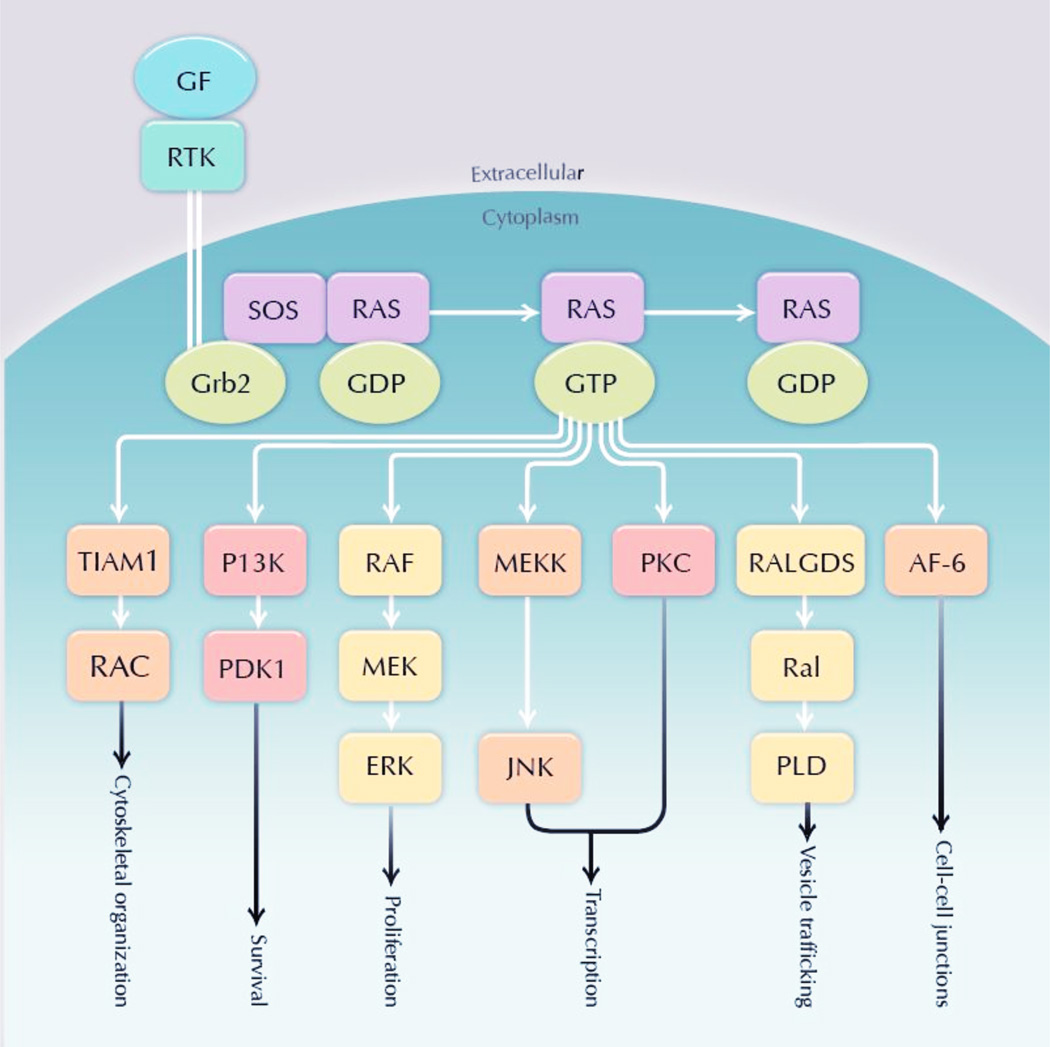
KRAS activation begins with stimulation of various upstream receptors, most EGFR in lung cancer. Adaptor proteins interact with the intracellular domain of EGFR and recruit guanine nucleotide exchange factors that interact with RAS to promote the exchange of guanosine diphosphate (GDP) for guanosine triphosphate (GTP). With binding of GTP, activated KRAS phosphorylates downstream signaling cascade proteins in until GTP is converted to GDP through a GTPase activity instrinsic to the Ras family enzymes. The end effect is that KRAS kinase and signaling capacity is higher when the enzyme is bound to GTP instead of GDP. Key downstream effectors include the RAF/mitogen-activated protein kinase kinase (MEK)/extracellular signal-regulated kinase (ERK) cascade (controlling cellular proliferation), PI3K/AKT/mammalian target of rapamycin (mTOR) cascade (controlling survival), and pathways affecting tumor invasion and vesicle trafficking (Figure 4).
Figure 4.
KRAS Mutations in NSCLC
8.2 Role in tumorigenesis
KRAS acquires tumorigenic properties when mutations arise that decrease its intrinsic GTPase activity. The resulting RAS proteins are locked in the GTP-bound conformation independent of upstream signals. This causes marked up-regulation of RAS kinase activity and downstream growth and mitotic signaling. Overall, RAS mutations occur in approximately 30% of all human cancers, with KRAS mutations the most common and best characterized.171 KRAS mutations result in single amino acid substitutions primarily at residues G12, G13, or Q61. In addition to lung cancer, KRAS mutations occur in 70–90% of pancreas cancer, 30–40% of colorectal cancer, 30% of biliary tract cancer, 20% of melanoma, 15% of endometrial cancer, and 15% of ovarian cancer.172
In lung cancer, KRAS mutations occur commonly at codon 12 (within exon 2) (>80%), occasionally at codon 13, and rarely at codon 61. Approximately 80% of codon 12 mutations are guanine/thymidine (G/T) (purine for pyrimidine) nucleotide transversions,173 which are considered the characteristic mutation related to tobacco smoke exposure. KRAS mutations in lung tumors from never smokers are typically G/A (purine for purine) transversions. The two most common mutations in NSCLC, G12C (approximately40% of cases) and G12V (~20%), arise from G/T transversions.174 Other principal mutations include G12D (17%), G12A (7%), and G12S (5%).175
8.3 Clinical significance
KRAS mutations occur in approximately 20–30% of NSCLC.176, 177 KRAS mutations occur predominantly in adenocarcinoma histology, have been reported rarely in squamous cell carcinoma, but have not been observed in small cell lung cancer.178, 179 In contrast to EGFR mutations and ALK and ROS1 mutations, KRAS mutations are associated with smoking.180 Among lifetime non-smokers with lung cancer, KRAS mutations occur only in 2–6% of cases.173, 181 KRAS mutations are mutually exclusive of EGFR, ALK, and ROS1 aberrations.
The prognostic role of KRAS mutations is not clear. In a meta-analysis of 24 studies incorporating various disease stages, treatments, and KRAS mutation detection methods, KRAS mutations were associated with worse survival (HR 1.35; 95% CI, 1.16–1.56).182 However, in a pooled analysis of 1,543 patients with resected early-stage NSCLC (of whom 300 had KRAS mutations); there was no difference in OS between KRAS mutant and KRAS wild type cases.173 No significant benefit from adjuvant chemotherapy was noted for wild-type cases or codon 12 mutations; among the 24 codon 13 mutation cases, adjuvant chemotherapy was deleterious (HR 5.78; 95% CI, 2.06–16.2).
In advanced NSCLC, KRAS mutations predict resistance to EGFR tyrosine kinase inhibitors.181 However, the mutual exclusivity of KRAS and EGFR mutations and the strong association between the latter and sensitivity to EGFR TKIs limit the clinical utility of KRAS mutations as a selection biomarker in current clinical practice. In contrast to colorectal cancer, in NSCLC KRAS mutations are not clearly associated with resistance to the anti-EGFR monoclonal antibody cetuximab.183
8.4 Treatment of KRAS mutant NSCLC
At the present time, there are no targeted therapies clinically available for NSCLC patients with KRAS mutations. High affinity binding to the GTP substrate has hindered the development of therapeutic agents that inhibit KRAS directly. In late 2013, initial reports of KRAS G12C inhibitors that bind to an allosteric site specific to the mutant molecule were published,184, 185 but such drugs are likely years away from clinical use.
Therapeutic strategies against KRAS mutant cancers that have been investigated clinically include inhibition of post-translational modification, inhibition of effector pathways, and synthetic lethality.
8.5 Post-translational modification
To date, this strategy has had little clinical efficacy. Farnesyl transferase inhibitors (FTIs) have failed to inhibit KRAS due to alternative prenylation by geranylgeranyl transferase. (GGTase).186 Combined FTI and GGTase inhibitor therapy has been associated with excessive toxicity.187
8.6 Effector pathway inhibition
Several clinical trials have evaluated MEK inhibition alone or in combination with other therapies for KRAS mutant lung cancer. In a phase 2 clinical trial of docetaxel ± the MEK inhibitor selumetinib (AZD6244; AstraZeneca) for previously treated advanced KRAS mutant NSCLC, selumetinib was associated with improved PFS (5.3 v 2.1 mos; 80% CI, 0.42–0.79; P=0.14) and a trend toward improved OS (9.4 v 5.2 mos; 80% CI, 0.56–1.4; P=0.21).15 Another phase 2 trial randomizing patients to selumetinib alone or in combination with erlotinib has completed enrollment (NCT01229150). Other MEK inhibitors under study specifically in KRAS mutant NSCLC include MEK162 (Novartis) combined with erlotinib (NCT01859026) and trametinib (GSK1120212, GlaxoSmithKline) monotherapy (NCT01362296).
A possible benefit of BRAF inhibition in KRAS mutant NSCLC was suggested in the BATTLE (Biomarker-integrated Approaches of Targeted Therapy for Lung Cancer Elimination) trial. In that study, 11/14 (79%) patients with KRAS/BRAF mutations had disease control at 8 weeks with sorafenib.188 However, in preclinical models, BRAF inhibitors appear ineffective against RAS mutant cells, paradoxically potentiating RAF/MeK/ERK signaling.189 This phenomenon, which has been attributed to CRAF activation, is evident clinically in the development of KRAS mutant cutaneous squamous cell carcinomas in melanoma patients treated with BRAF inhibitors.190, 191
A number of recent and ongoing clinical trials have focused on the PI3K/AKT/mTOR signaling cascade. Specific agents under investigation in KRAS mutant NSCLC include the mTOR inhibitor ridaforolimus (IPI-504; Infinity Pharmaceuticals) (NCT00818675), the mTOR inhibitor everolimus in combination with the HSP90 inhibitor retaspimycin (NCT01427946), everolimus in combination with trametinib (NCT00955773), and the dual PI3K-mTOR inhibitor BEZ235 (Novartis) in combination with MEK162 (Novartis) (NCT01337765, NCT01363232).
The RHOA-focal adhesion kinase (FAK) axis has emerged as a critical mediator of RAS signal transduction. In transgenic and orthotopic mouse models of KRAS mutant lung adenocarcinoma, FAK inhibition resulted in inhibition of tumor growth and prolongation of survival,192 leading to an ongoing multicenter phase 2 trial of the FAK inhibitor defactenib (VS-6063; Verastem) in previously treated advanced KRAS mutant NSCLC (NCT01951690).
In a randomized phase 2 clinical of erlotinib ± the c-MET inhibitor tivantinib (ARQ-197; ArQule), an exploratory analysis revealed that the small cohort with KRAS mutations achieved a PFS HR of 0.18 (95% CI, 0.05–0.70).193 This benefit was hypothesized to be related to a putative feedback loop through which EGFR acts as a downstream mediator of KRAS signaling, interactions between hepatocyte growth factor (the MET ligand) and KRAS, or non-MET mediated pathways. A subsequent randomized phase 2 clinical trial of erlotinib + ARQ-197 versus single-agent chemotherapy in previously treated advanced KRAS mutant NSCLC (NCT0139578) has completed accrual.
8.7 Synthetic lethality
With synthetic lethality, KRAS-mutant cancer cells are selectively killed via inhibition of a second protein. In KRAS mutant cell lines, RNAi-based synthetic lethal screens have identified several potential targets. A number of these, including CDK4, STK33, TBK1, and PLK1, encode protein kinases and may therefore be amenable to small molecule inhibition.194
9. NSCLC WITH PI3K PATHWAY ALTERATIONS
Phosphatidylinositol 3-kinase (PI3K) signaling plays important roles in metabolism, growth, survival, and motility. The Class IA PI3Ks are most clearly associated with human cancer and are activated by growth factor stimulation through receptor tyrosine kinases. Class IA PI3Ks are composed of a regulatory subunit and catalytic subunit. The regulatory subunit, p85, is encoded by PIK3R1, PIK3R2, and PIK3R3, while the catalytic subunit has three isoforms, p110α, p110β, p110δ, encoded by PIK3CA, PIK3CB, and PIK3D. Binding of p85 to phosphotyrosine residues on receptor tyrosine kinases releases the inhibition of p110 by p85 and causes localization of PI3K to the plasma membrane, where it can phosphorylate PIP2 (phosphatidylinositol 4,5-bisphosphate) to produce PIP3 which in turn propagates intracellular signaling via AKT and PDK1 and other PIP3 dependent signaling pathways. Phosphatase and tensin homology deleted on chromosome 10 (PTEN) dephosphorylates PIP3 to PIP2, thus inhibiting PI3K-dependent signaling and acting as a tumor suppressor. PI3K can also be activated by RAS or by G-protein coupled receptors which bind directly to the catalytic subunit. PI3K-AKT signaling regulates multiple downstream pathways including the bcl2 family members, forkhead transcription factors, MDM2/p53, mTORC1/2, and NFKB pathways, to promote cell survival and inhibit apoptosis.195–197
9.1 Genetic Alterations in PI3K pathway
Genetic alterations of elements in the PI3K pathway have been described in lung cancer as well as other tumor types. PIK3CA encodes the gene for the p110α isoform of the catalytic subunit ofPI3K. Both copy number gains and mutations in PIK3CA have been identified in lung cancer. PIK3CA copy number gains occur in approximately 20% of lung cancers, with higher frequency in squamous cell carcinomas.198–200 Somatic mutations in PIK3CA have also been described and promote activation of the PI3K signaling pathway.201 Mutations in PIK3CA are clustered in two hotspot regions in exons 9 and 20 encoding the helical and kinase domains of the protein, respectively. These mutations lead to increased lipid kinase activity and constitutive PI3K-AKT signaling.201 The mechanism of action is different based on mutation type; for example, the helical domain mutants E545K and E542K interfere with the inhibitory interaction between the regulatory subunit p85 and the catalytic unit p110α, while the kinase domain mutant H1047R is located near the activation loop and leads to constitutive signaling through the kinase.195 PIK3CA mutations have been reported in 1–5% of NSCLC cell lines and tumors.198, 202 Kawano et al202 found PIK3CA mutations in 6.5 % of lung squamous cell carcinomas, and less often in lung adenocarcinomas (1.5%). PIK3CA mutations often do not exist in isolation, and coexistence with other mutations, such as KRAS, NRAS, BRAF, and EGFR, is common.203–205 A study among patients with lung adenocarcinoma in the U.S. reported 70% of cases with PIK3CA mutation had a coexisting driver mutation, with the most frequent partner being KRAS.205
The tumor suppressor gene PTEN encodes a lipid phosphatase that negatively regulates the PI3K/AKT pathway, and loss of PTEN leads to constitutive PI3K-AKT signaling. Somatic PTEN deletions and mutations, and inactivation of PTEN by epigenetic mechanisms such as methylation or microRNA silencing, are seen in multiple cancers.206 PTEN mutations occur in approximately 5% of lung cancers and are significantly associated with squamous cell rather than adenocarcinoma histology (10.2% v 1.7%).207 Reduction or loss of PTEN expression has been reported in up to 70% of non-small cell lung cancer, both adenocarcinoma and squamous cell.208
Other mutations in elements of the PI3K pathway have also been reported. For example, a somatic mutation in AKT, E17K, constitutively activates the protein kinase.209 The AKT1 E17K mutation was found in 5.5% (2/36) squamous cell lung cancers, but not (0/53) in lung adenocarcinoma.210 PIK3R1 mutations causing truncations or in-frame deletions have also been reported and are thought to relieve the inhibitory effect of p85 on p110, thereby activating PI3K signaling.
9.2 Drug Development
There are multiple PI3K inhibitors in development, with specificity ranging from pan-PI3K inhibitors to isoform-selective PI3K inhibitors and dual PI3K/MTOR inhibitors. As a class, common adverse events have been hyperglycemia (which is thought to be due to the role PI3K plays in the insulin signaling pathway), maculopapular rash, and gastrointestinal issues such as nausea, vomiting, dyspepsia, diarrhea, and stomatitis. In addition to the phase 1 studies of PI3K inhibitors that enrolled all tumor types, there are many ongoing trials with a focus on lung cancer, both as monotherapy and in combination with other agents. There have been multiple Phase 1/1b studies combining various PI3K inhibitors with MEK inhibitors which have enrolled expansion cohorts of patients with KRAS mutated lung cancer; efficacy results from these trials are awaited. In non-molecularly selected lung cancer populations, currently ongoing trials include GDC0941 in combination with carboplatin, paclitaxel, with or without bevacizumab, and BKM120 in combination with docetaxel or carboplatin/pemetrexed. BKM120 is also being tested singly and in combination with EGFR inhibitors in molecularly selected cohorts. GDC0032 is also being tested in combination with chemotherapy agents including docetaxel and paclitaxel.
Preclinical data have suggested that cancers harboring activating mutations in PIK3CA may be among the most sensitive to single-agent PI3K pathway inhibitors.195 In general, the clinically observed activity of PI3K inhibitors as monotherapy has been modest, and it is not entirely clear how well molecular alterations in PI3K pathway correlate with antitumor effect.211 In one institution’s cumulative phase 1 experience, patients with PI3K mutations who were enrolled in phase 1 trials with PI3K/AKT/MTOR inhibitors had a higher partial response rate than wildtype PI3K patients on their best phase 1 therapy.203 However, the response rates reported (18% for PI3K mutated patients versus 8% for wildtype, with H1047R mutations faring best with a 38% PR rate),203 still leave much room for improvement and are not comparable to the response rates achieved with the landmark targeted therapies used for EGFR or ALK inhibition. The frequent coexistence of other driver mutations may mean that single agent PI3K inhibition may not be sufficient if the coexisting driver is not effectively targeted as well. In addition, signaling feedback loops may be activated that promote growth via alternative pathways; for example mTORC1 inhibition leads to activation of PI3K pathway via a feedback loop, limiting single agent mTORC1 efficacy.195 Finally, it remains unclear whether the drugs in development thus far are achieving an adequate therapeutic window; observed pathway inhibition in various trials has ranged from 30–90%, and it is possible that at the dosing levels achieved there may not be sufficient pathway inhibition to have an antitumor effect.211
10. MET POSITIVE NSCLC
The MET/hepatocyte growth factor (HGF) pathway has been identified as a potential therapeutic target in multiple solid tumors, including non-small cell lung cancer.212–214 The MET gene on chromosome 7q21–31 encodes the HGF receptor (HGFR), which is a single-chain heterodimer consisting of a 50-kDa extracellular α-chain and a 140-kDa transmembrane β-chain. Binding of the HGF ligand leads to dimerization of the receptor and phosphorylation of the intracellular tyrosine kinase (TK) domain.215 This results in activation of downstream signaling pathways, such as PI3K-AKT and RAS-MAP-kinase, which are involved in cell survival and apoptosis, cell proliferation and differentiation, cytoskeletal function, angiogenesis, as well as other cellular functions.216, 217 There is also crosstalk between MET and other receptor tyrosine kinases, including EGFR/ERBB family of receptors, which can result in HGF-independent activation of the MET pathway.212, 213, 218 Ligand-mediated MET is tightly controlled through recruitment of CBL (E3 ubiquitin ligase), which binds to the regulatory site of the juxtamembrane domain of HGFR and leads to ubiquination of HGFR into clathrin-coated vesicles, with ultimate degradation.219
Aberrant signaling of the MET pathway can occur through overexpression of HGF or HGFR, decreased degradation of HGFR, MET amplification or MET mutations.212, 213 In NSCLC, the most common mechanism for aberrant MET signaling is overexpression of HGF and HGFR. HGFR overexpression is associated with poor prognosis and has been reported to occur in up to 61% of NSCLC,220, 221 including 25 – 67% of patients with adenocarcinoma of the lung.220 The prevalence of de Novo MET amplification is low (≤ 5%)11–15, but is also associated with poor prognosis.221 Importantly, MET amplification has been identified as a mechanism for acquired resistance to EGFR tyrosine kinase inhibition in a subset (5–20%) of patients with activating EGFR mutations through ERBB3-dependent activation of the PI3K pathway.85, 222, 223 It is also seen that amplification can be de novo without resistance. Both somatic and germline MET mutations have been identified in multiple solid tumors.212 In NSCLC, mutations in the extracellular semaphorin domain (exon 2) and intracellular juxtamembrane domain (exon 14–15, including exon skipping), which can affect ligand binding and receptor downregulation, respectively, have been described.220, 224, 225 In a recently reported series that included 106 patients with NSCLC who underwent MET mutational analysis, approximately 4% were found to be MET-mutation positive (exon 14–15).226
Both in-vitro and in-vivo preclinical models have established the utility of MET pathway inhibitors to suppress HGF-dependent and -independent MET phosphorylation and activation of downstream pathways, resulting in inhibition of both tumor growth and metastasis.212 Dual inhibition of EGFR and MET in in-vivo tumor xenograft models has been shown to be additive and potentially synergistic in NSCLC, including in tumors with acquired resistance to EGFR TKIs.227, 228 Recently we have also shown that MET can synergize with its family member RON.229 MET targeting strategies have included inhibitors of the HGF-HGFR binding, including HGF antagonists, HGFR inhibitors and decoy MET, as well as small molecule inhibitors of the intracellular tyrosine kinase domain.212–214 The preclinical experience has led to clinical testing of both single-agent and combination strategies to inhibit the MET pathway in NSCLC.
10.1 Monoclonal Antibodies Targeting HGF and HGFR
AMG 102 (rilotumumab) and AV 299 (ficlatuzumab) are monoclonal antibodies targeting HGF. Rilotumumab is a fully humanized monoclonal antibody that has been shown to improve the activity of chemotherapy in pre-clinical and clinical testing in tumors that overexpress MET.230, 231 In phase I testing as a single agent, rilotumumab was well tolerated with most common treatment-related adverse events including fatigue (13%), constipation (8%), and nausea (8%).232 A phase I/II trial is currently ongoing evaluating rilotumumab with erlotinib in previously treated patients with NSCLC (NCT01233687). Ficlatuzumab is a human anti-HGF IgG1 monoclonal antibody.233 In phase I testing, ficlatuzumab was well tolerated with no additional safety signals identified when combined with an EGFR TKI.234 In a randomized phase II trial comparing gefitinib to gefitinib plus ficlatuzumab in never or former light smokers with previously untreated adenocarcinoma of the lung, there was no significant difference in response rate (40% versus 43%) or progression free survival (4.7 versus 5.6 months) between the two groups (gefitinib versus gefitinib + ficlatuzumab, respectively).235 Interestingly, in subgroup analysis, patients with activating mutations in the EGFR gene and low MET expression appeared to gain the most benefit from the combination (overall response rate 70 versus 44% and median PFS 11.0 versus 5.5 months).235
MetMab (Onartuzumab) and LY-2875358 are monoclonal antibodies directed against the MET receptor. MetMab is a humanized, monovalent monoclonal antibody that inhibits HGF/MET binding without inducing MET dimerization.236, 237 In contrast, LY-2875358 is a bivalent MET receptor antibody that can inhibit both HGF-mediated signaling by binding to the MET receptor, as well as HGF-independent activation of the MET pathway by inducing internalization and degradation of MET.238 LY-2875358 has confirmed antitumor activity in in vivo and in vitro models.239, 240 A phase I trial as a single agent and in combination with erlotinib has been reported with no dose limiting toxicities, serious or grade III adverse events.241 Currently, LY-2875358 is being evaluated in 2 phase II trials, including a randomized phase II with erlotinib versus erlotinib alone in patients with advanced-stage EGFR-mutated NSCLC (NCT01897480), as well as a single-agent or combined with erlotinib in patients with MET diagnostic positive NSCLC that has progressed on erlotinib.
Onartuzumab has been evaluated in a randomized phase II trial in patients with recurrent NSCLC in combination with erlotinib versus erlotinib alone.242 There was no significant difference in the primary PFS endpoint in the intent to treat population (HR), 1.09, p = 0.69). However, in the pre-specified MET-positive population (defined by a score of 2–3+ by immunohistochemistry (≥ 50% of cells with strong or moderate or higher staining using CONFIRM SP44 anti-MET monoclonal antibody, Ventana)), the combination arm was associated with improved PFS (HR, 0.53; p = 0.04) and OS (HR, 0.37; p = 0.002).242 A phase III trial evaluating this combination versus erlotinib alone in MET-positive patients with advanced-stage NSCLC who have received prior chemotherapy is ongoing (MetLung; NCT01456325).
10.2 MET Tyrosine Kinase Inhibitors
Targeting the MET tyrosine kinase has the potential to inhibit both HGF-dependent and independent signaling through the MET pathway. There are a number of MET tyrosine kinase inhibitors currently undergoing testing in early phase clinical trials.213 Although crizotinib is FDA-approved for ALK-translocated NSCLC, it also has in vitro activity against MET. In a case report, a patient with advanced-stage MET-amplified (MET/CEP7 ratio > 5.0) and ALK-negative NSCLC was reported achieved a rapid and durable response after treatment with crizotinib.243
Cabozantinib (XL184) is an ATP competitive inhibitor of MET, VEGFR and RET with documented phase II activity in an unselected pre-treated cohort of 60 patients with advanced NSCLC (overall response rate 10%; disease control rate 40%).244 The combination of cabozantinib with erlotinib was also shown to be active in a phase IB trial of patients with previously treated NSCLC, the majority of who had received prior erlotinib.245 In this trial, 2 of 53 patients had confirmed MET gene copy number gain and both achieved tumor shrinkage with the combination.
Based on a promising randomized phase II trial, the phase III MARQUEE trial was initiated to test the combination of tivantinib (ARQ 197), a non-ATP competitive tyrosine kinase inhibitor of MET, with erlotinib in previously treated patients with advanced NSCLC. This trial was stopped at the interim analysis because the primary OS endpoint was not met. Recent in vitro studies demonstrated that tivantinib is a cytotoxic drug affecting microtubule dynamics with activity in cell lines independent of MET activity. It is feasible that tivantinib is a weak MET inhibitor and has differential activity in different tumors.246–248
11. FGFR POSITIVE NSCLC
Dysregulation of FGFR family signaling has been described in a broad range of cancers, including lung, breast, prostate, myeloma, sarcoma, bladder, and endometrial cancers, among others.249–254 Amplification, translocation, and point mutations involving FGFR family members have all been described across the various tumor types, and each of these genetic alterations occurs in lung cancer.
The FGF/FGFR family consists of 18 FGF ligands which bind to four homologous FGFR receptor tyrosine kinases (FGFR 1, 2, 3, and 4). A typical FGFR is composed of an extracellular domain with three immunoglobulin (Ig)-like domains, a transmembrane domain, and a split tyrosine kinase domain. Binding of FGF ligand to FGFRs induces receptor dimerization, which leads to transphosphorylation of a tyrosine in the activation loop of the tyrosine kinase domain. Activation leads to downstream signaling via the PI3K/AKT and RAS/MAPK pathways which are central to growth, survival migration and angiogenesis.249–254
11.1 Amplification
Amplification at 8p12 was observed in multiple studies of squamous cell lung cancer,16, 18, 255 and FGFR1 has been identified as a potential candidate gene in this region. Weiss et al identified focal amplifications in FGFR1 corresponding to the 8p12 amplification in a study of 155 primary squamous cell lung cancer specimens, which they validated in an independent set of 153 squamous cell lung cancers.18 Similarly, Dutt et al reported FGFR1 amplification in approximately 20% of squamous cell lung cancers, and rarely in adenocarcinoma (3%). Inhibition of FGFR1 in amplified cell lines and in mouse models with FGFR1 amplified engrafted tumors showed growth inhibition and induced apoptosis.16
It remains unclear whether FGFR1 amplification is a prognostic marker in lung cancer. Weiss reported that FGFR1 amplification (copy number > 9 by FISH) had a trend toward worse survival compared to patients who lacked FGFR1 amplification (copy number = 2 by FISH). Multiple studies have investigated the potential prognostic role of FGFR1 among patients with squamous cell lung cancer; some have reported no effect of FGFR1 amplification on survival,256, 257 while others have reported inferior survival with FGFR1 amplification,18, 258, 259 and one reported potential improved survival.260 Comparison across studies is limited by the heterogeneity in definitions of amplification, and there is not yet a defined standard in the field.
11.2 Fusions
In addition to FGFR1 amplification, fusions involving FGFR3 have recently been reported in lung cancer.20–22 Fusions involving FGFR3 have been reported in other cancers including glioblastoma and bladder cancer.261 Kim et al performed whole exome sequencing of lung squamous cell cancers from Korean patients and identified an in-frame fusion of FGFR3 with TACC3.20 Overall two of 148 Korean lung squamous cell cancers had this fusion; probing the TCGA dataset revealed another four of 178 samples with the FGFR3-TACC3 fusion.20 Majewski et al used kinome-centred RNA sequencing on 95 lung cancer samples and identified two squamous cell lung cancers with FGFR3-TACC3 fusions.21 FGFR3 fusions resulted in overexpression of fusion proteins and enhanced proliferation of cells, as well as activation of downstream MAPK-ERK pathways.22 Studies in bladder cancer and glioblastoma have invoked various hypotheses for the transforming capacity of FGFR3-TACC3, including constitutive activation and signaling via downstream MAPK pathway,262 localization to the mitotic spindle, causing chromosomal missegregation and aneuploidy,263 or loss of a 3’UTR miR-99a binding site resulting in enhanced expression of the fusion transcripts.264 Importantly, multiple studies have shown sensitivity of FGFR3 fusion cell lines and xenograft models to FGFR inhibitors.22, 262, 263
11.3 Point mutations
Point mutations in FGFR have also been identified as potentially oncogenic, in particular mutations in FGFR2 and FGFR3. Analysis of whole exome data from TCGA identified five FGFR2 and six FGFR3 mutations from 178 tumor/normal pairs.265 The observed mutations fell within both the extracellular and kinase domains of FGFR2 and FGFR3, and included previously identified mutations in other tumor types as well as novel mutations. Some of these mutations were transforming in anchorage-independent growth assays and xenograft assays. In particular, extracellular domain mutations W290C and S320C in FGFR2 and S249C in FGFR3, as well as kinase domain mutations K660E and K660N in FGFR2, significantly increased colony formation in anchorage-independent growth assays as compared to wild type (in contrast, FGFR2 E471Q and T787K, and FGFR3 S435C and K717M were not transforming). The transforming ability of the specific FGFR2 and FGFR3 mutations was inhibited by multiple of the small molecule FGFR inhibitors currently in clinical development.265
11.4 Drugs
Many FGFR inhibitors are in development and have multitargeted activity and inhibit other kinases in addition to FGFR, most notably VEGFR, PDGFR, FLT3, RET, KIT, among others.261 Selective FGFR inhibitors are also in development and preliminary results have been reported for some of these trials. A phase 1 study of BGJ398 is enrolling patients with advanced solid malignancies with FGFR1 or FGFR2 amplification or FGFR3 mutation. A preliminary report in 2012 reported 26 patients having been treated, including 10 with FGFR1 amplified breast cancer and 3 with FGFR1 amplified lung squamous cell cancer. The most frequent adverse events included diarrhea, fatigue, nausea, and hyperphosphatemia, with dose limiting toxicities of grade 3 elevations in transaminases and grade 2 corneal events. Hyperphosphatemia may be a class effect due to blockade of FGF23 signaling but seems controllable with phosphate binders and diuretics. One patient with lung cancer and FGFR1 amplification (FGFR1/CEP8 ratio of 2.6 by FISH) had a confirmed partial response.266 A phase 1 study of AZD4547, with selection for FGFR1 and FGFR2 amplification in the later phases of the study has completed accrual, and final results are pending. In a preliminary report, dose limiting toxicities included hyperphosphatemia, renal failure, mucositis, and increased transaminases. A preliminary report in 2013 reported on 21 patients with FGFR1 or FGFR2 amplified tumors on study. One patient with FGFR1 amplified lung squamous cancer had a partial response,267 with another patient with FGFR1 amplified lung squamous cancer having a prolonged period of stable disease. While some are selecting for specific FGFR family alterations, others are more inclusive and enroll specific tumor types without molecular characterization required a priori. Class specific effects of the selective FGFR inhibitors are thought to include hyperphosphatemia and tissue calcification due to FGF23 blockade; while this is a class-specific adverse event, increases in FGF23, phosphate, and vitamin D levels may also serve as potential biomarkers for effective FGFR inhibition.282 Most of these studies are with single agents, although a few are testing in combination with various chemotherapy regimens.
12. MITOTIC/CYCLIN INHIBITORS IN LUNG CANCER
Disrupting cell division has been a cornerstone of cancer drug development. Mitotic inhibitors are among the most widely developed agents in oncology, and have been used in lung cancer treatment for over three decades. These agents bind tubulin and prevent polymerization to microtubules, hence, preventing cell division. Multiple mitotic inhibitors have been developed and many are still standard therapies in lung cancer treatment including paclitaxel, docetaxel, vinorelbine, and etoposide. These agents are routinely combined in platinum regimens in the adjuvant, locally advanced, and metastatic NSCLC; and platinum/etoposide remains the established treatment for limited- and extensive-stage SCLC.
More recent advances in targeting cell cycling have come with the development of cell cycle checkpoint inhibitors. Cell cycle check points are important in maintaining genomic stability and preventing cancer development in normal cells (Figure 5).268 These checkpoints help in cellular surveillance of DNA damage by causing cycle arrest and permitting DNA repair. However, these checkpoints also protect cancer cells from the effects of DNA-damaging agents such as cisplatin/carboplatin and gemcitabine and from the effects of radiation. Cyclin dependent kinases (CDKs) are key regulators of sequential progression through the G1, S, G2, and M phases of the cell cycle. Checkpoint kinase inhibitors disrupt the cancer cell's ability to repair this damage, and have recently shown promising activity as single agents in select patient populations and in combination with DNA-damaging therapies in broader tumor settings.269, 270 Many of these novel agents are being developed in lung cancer treatment.
Figure 5.
Mitotic/Cyclin Inhibitors
12.1 LY2606368
LY2606368 (Eli Lilly) is an oral small molecule selective ATP competitive inhibitor of the checkpoint kinase 1 (CHK1), and to a lesser extent CHK2. CHK1 and 2 regulate DNA damage response by inhibiting CDK1, and preventing entry into mitosis.271 This leads to cell cycle arrest, DNA repair, and apoptosis of damaged cells.272 LY2606368 has been shown to potentiate DNA damaging agents, and has potent antitumor activity as a single agent in preclinical studies.273 LY2606368 is currently in a phase I study in patients with advanced refractory squamous NSCLC, head and neck cancer, and anal cancer (N=150; NCT01115790).
12.2 Palbociclib (PD-0332991)
Palbociclib (Pfizer) is an oral CDK4/6 inhibitor, inhibiting retinoblastoma (Rb) protein phosphorylation in early G1 and disrupting cell cycle progression to S. Palbociclib has recently shown remarkable activity in a randomized phase II trial in patients with advanced hormone positive breast cancer.274 One hundred sixty-five women were randomized 1:1 to letrozole 2.5mg orally daily +/− palbociclib 125mg daily for 3 weeks followed by 1 week off. The primary endpoint was investigator assessed PFS. Palbociclib/letrozole was associated with a significant improvement in PFS compared with letrozole alone (20.2 v. 10.2 months; HR 0.488; 95% CI: 0.32–0.75; 1-sided p=0.0004). The most common toxicities in the palbociclib/letrozole arm were neutropenia, leukopenia, fatigue, and anemia. Palbociclib is in planned investigation in CDK4/6-amplified recurrent squamous lung cancer as part of the NCI-sponsored biomarker driven 'Master' protocol.
12.3 LY2835219
LY2835219 (Eli Lilly) is an oral selective ATP-competitive inhibitor of CDK4/6 which has entered into phase I study in NSCLC. (NCT02079636) Ninety-nine patients will be enrolled across multiple cohorts including combinations with pemetrexed (nonsquamous only), gemcitabine, ramicirumab, and trametinib. Development in other tumors including breast cancer, colorectal cancer, melanoma, glioblastoma multiforme, and mantle cell lymphoma is ongoing.
12.4 AZD1775
TP53 mutations are the most common genomic alterations in lung cancer, occurring in an estimated 51% of squamous lung cancers and 34% of adenocarcinomas. (COSMIC) These mutations render the G1 checkpoint defective, making these cancers more dependent on the S/G(2) cell cycle checkpoint for repair and resistant to DNA-damaging agents. This resistance can be overcome in the presence of S/G(2) inhibitors.271 The WEE1 kinase coordinates cell cycle progression and DNA damage checkpoints. AZD1775 (Astra-Zeneca) is an oral ATP-competitive inhibitor of WEE1 (IC50 5nM; EC50 80nM v. pCDK1Y15).275 WEE1 inhibition leads to unregulated CDK1 (and 2) activity, overriding S/G2 checkpoints leading to mitotic catastrophe and cell death in DNA damaged cells. This activity may be most pronounced in p53-mutated (G1 deficient) cells in combination with platinum and gemcitabine-based regimens. A phase I trial of AZD1775 + cisplatin, + carboplatin, and +gemcitabine in over 180 patients with refractory solid tumors has recently been completed; and early activity and safety have been reported in combination with carboplatin/paclitaxel in patients with p53-mutated platinum-sensitive recurrent ovarian cancer.276 AZD1775 has entered into trials in p53-mutated lung cancer: a first-line randomized phase trial of carboplatin/pemetrexed +/− AZD1775 in patients with nonsquamous NSCLC (NCT02087241); and a second line randomized phase II trial of docetaxel +/− AZD1775 in patients with nonsquamous and squamous histologies (NCT02087176). Trials are also ongoing in p53-mutated platinum-sensitive and -resistant recurrent ovarian cancer.
12.5 Volasertib (PLK-1 ONO01910)
Polo-like kinase 1 (PLK1) is important for cellular recovery from G2/M arrest due to DNA damage. Overexpression of PLK1 leads to chromosomal instability and is seen in many tumors including NSCLC.277 Volasertib (Boehringer-Ingelheim) is an IV PLK1 inhibitor in development in NSCLC. Recent data were presented from a study of 131 patients with recurrent nonsquamous NSCLC randomized 1:1:1 to volasertib 300mg/m2, volasertib 300mg/m2 and pemetrexed 500mg/m2, or pemetrexed alone IV day 1 every 3 weeks.278 The median PFSs (primary endpoint) for these cohorts were: 1.4, 3.3, and 5.3 months, respectively. The ORRs were: 8.1%, 21.3%, and 10.6%, respectively. Grade 3/4 toxicity was primarily limited to fatigue (all arms) and neutropenia (volasertib/pemetrexed, 11%). Development of volasertib is ongoing in acute myeloid leukemia and urothelial cancer.
12.6 Alisertib (MLN8237)
The aurora kinases play important roles in mitosis. Aurora Kinase A promotes mitosis through activation of CDK1, and its overexpression has been linked to taxane resistance. Aurora kinase B is linked to cytokinesis, and its inhibition leads to dysfunctional chromosomal alignment and segregation. Several aurora kinase inhibitors (A and B) are in development.
Alisertib (Millennium) is an oral aurora kinase A inhibitor. A phase I/II trial of alisertib in patients with refractory SCLC, NSCLC, breast cancer, head and neck cancer, and gastroesophageal cancer was recently presented.279 Patients received the recommended phase II dose of 50mg orally BID for 1 week every 3 weeks. The ORR in 23 patients with NSCLC was 4% with a median PFS of 3.1 months. However, in the SCLC (n=47) cohort the ORR was 21% (including 3 pts (ORR 27%) with refractory relapsed disease) with a PFS of 2.8 months. Grade 3/4 toxicities (all patients) included: neutropenia (38%), anemia (10%), stomatitis (8%), and thromobocytopenia (6%). A randomized phase II study of paclitaxel +/− alisertib in patients with relapsed SCLC (NCT02038647), and a trial of alisertib and erlotinib in patients with EGFR-WT NSCLC. (NCT01471964) are in progress. Several other A and B, and pan-aurora, kinase inhibitors are in early development in solid and hematologic cancers.
13. PARP INHIBITORS IN LUNG CANCER
Poly (ADP-ribose) polymerase (PARP) includes a family of 17 proteins that play important roles in DNA repair.280 In addition to its function in DNA repair, PARP proteins also play major roles in a number of other cellular processes such as transcription, epigenetic regulation, mitosis and inflammation, which have all been recognized in recent years. PARP 1 and 2 are considered to be highly relevant for DNA repair. Following single strand DNA damage, PARP is recruited as the first step of the repair process (Figure 6).281 Subsequently, multiple ADP-ribose units are added to the complex in a NAD-dependent manner. This confers a net negative charge that induces conformational changes and attracts a number of key repair proteins such as DNA ligase III, X-ray cross-complementing gene 1, etc., which ultimately work to repair the DNA damage. In situations of catastrophic DNA damage, massive recruitment of PARP leads to depletion of NAD, resulting in necrotic cell death. Thus the extent of PARP activation could be the determinant of successful DNA repair or cell death. Unrepaired single strand damage leads to double strand DNA damage that is repaired by the homologous recombination (HR) repair pathway. In subjects with BRCA 1 or 2 mutations, the HR pathway is deficient and there is an overwhelming reliance on PARP for DNA repair.282
Figure 6.
PARP inhibition: Mechanism of action (reproduced from Oncology Live, 2013, permission requested)
13.1 PARP Inhibition
PARP inhibition was initially studied in cancer in combination with agents that induce DNA repair such as platinum compounds, alkylating agents, and ionizing radiation.283 Suppression of DNA repair with PARP inhibitors in conjunction with these agents results in enhanced anti-cancer activity in several pre-clinical models. In patients with deficient HR pathway, PARP inhibition results in robust anti-cancer activity due to the reliance of these cells on PARP for DNA repair.282 This effect, referred to as ‘synthetic lethality’, has formed the basis for the evaluation of PARP inhibitors as monotherapy in breast and ovarian cancer patients that have BRCA 1 or 2 mutations. In lung cancer, BRCA mutations are rare and hence PARP inhibitors are unlikely to be effective as monotherapy. A small subset of lung cancer patients are known to have ‘functional’ BRCA deficiency, and could be candidates for the monotherapy approach, though this has not been tested in clinical trials. Nearly 10% of non-small cell lung cancers harbor a mutation in ATM gene, a condition with known deficiency in HR.27 Another mechanism referred to as ‘PARP trapping’ has recently been described to account for the anti-cancer effects of PARP inhibition.284 Retention of the PARP inhibitor-DNA complex confers cytotoxicity to cells and the extent of PARP trapping is variable among the presently available PARP inhibitors, contributing to potential differences in efficacy of these agents based on this effect.
A number of novel PARP inhibitors are presently in clinical development for the treatment of cancer. Iniparib, which was initially considered to be a PARP inhibitor, had been tested in phase III studies in breast cancer and squamous cell lung cancer in combination with platinum-based chemotherapy.285 These studies failed to demonstrate survival benefit, and by then it was also clear that the mechanism of action of inparib was not related to PARP inhibition. Recently, the use of olaparib, a potent small molecule inhibitor of PARP, as maintenance therapy in platinum-sensitive ovarian cancer was associated with a significant improvement in PFS compared to placebo (8.4 m vs. 4.8 m, P < 0.001).286 In another phase 2 study, olaparib demonstrated a response rate of nearly 40% in ovarian cancer patients with BRCA mutation.287 Veliparib, a small molecule PARP inhibitor, improved the pathological complete response rate for patients with breast cancer in the neo-adjuvant therapy setting. From these lines of evidence, it is clear that PARP inhibitors represent a novel approach for the treatment of cancer.
13.2 PARP inhibitors under development in lung cancer
13.2.1 Small cell lung cancer
Increasing evidence suggest that PARP inhibition might be a novel strategy for the treatment of SCLC.288 Objective responses have been reported with BMN-673, a highly potent PARP inhibitor when given as monotherapy to patients with SCLC that had progressed on standard chemotherapy. Biological rationale for the sensitivity of SCLC might be due to the higher PARP 1 expression and other DNA repair proteins in SCLC tumor samples. Based on the synergy between alkylating agents and PARP inhibitors, a phase 2 study is presently evaluating the combination of temozolamide in combination with veliparib, a PARP inhibitor, for patients with relapsed/refractory SCLC (NCT01638546). The Eastern Cooperative Oncology Group is conducting a randomized phase 2 study of cisplatin and etoposide with either veliparib or placebo for first line therapy of patients with extensive stage SCLC (EA2511) (NCT01642251). Veliparib is given at a dose of 100 mg twice daily on days 1–7 of each treatment cycle to synchronize with the administration of cisplatin (day 1) and etoposide (days 1–3). This ongoing study will enroll a total of 135 patients with the primary endpoint of comparing median PFS between the two arms.
13.2.2 Non-small cell lung cancer
Since platinum-based chemotherapy is the standard treatment for majority of patients with NSCLC, the use of PARP inhibitors in combination with platinum compounds has been studied extensively in pre-clinical studies. A phase I study of carboplatin, paclitaxel and veliparib in patients with advanced solid organ malignancies noticed good tolerability and promising activity in advanced NSCLC.289 Subsequently, a randomized phase 2 study of carboplatin and paclitaxel with either veliparib or placebo for first line therapy of advanced NSCLC was conducted. Accrual to this study has been completed and the results are awaited (NCT01560104). The same combination is presently being tested in conjunction with radiation therapy for patients with surgically unresectable, locally advanced NSCLC by the Southwest Oncology Group (NCT01386385).
Olaparib, another PARP inhibitor, is also under extensive evaluation in NSCLC. A European study will administer Olaparib in combination with cisplatin and radiotherapy to patients with unresectable stage III NSCLC (NCT01562210). It is also being studied as maintenance therapy for advanced NSCLC following combination chemotherapy in a randomized study (NCT01788332). More recently, a phase 1b/II study has been initiated to evaluate the combination of olaparib with gefitinib in patients with advanced NSCLC that harbor an EGFR mutation (NCT01513174).
14. IMMUNE CHECKPOINT INHIBITORS
Lung cancer has not traditionally been viewed as an immune responsive tumor. Immune checkpoint inhibitors have recently demonstrated promising results in lung cancer patients. In particular inhibitors to cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) and programmed death 1 (PD-1) and programmed death receptor ligand 1 (PD-L1) have shown promise in early studies and are currently in clinical trials in both SCLC and NSCLC. This review provides an update on immune checkpoint inhibitors currently in development in lung cancer patients.
14.1 Anti-CTLA-4 Inhibitors
Ipilimumab is a fully human IgG1 antibody to CTLA4 that inhibits the binding of CTLA4 to its ligands (CD80 or B7-1 and CD86 or B7-2). Inhibition augments T-cell activation and proliferation resulting in T-cell infiltration of tumor cells and tumor regression.290 It is FDA approved for the treatment of melanoma.291 Ipilimumab was evaluated in a randomized phase II trial that compared six cycles of standard chemotherapy (carboplatin and paclitaxel) with two different schedules of ipilimumab in 204 patients with stage IV NSCLC.292 In the concurrent schedule ipilimumab was given with cycle 1 to 4 of chemotherapy followed by two doses of placebo, in the phased schedule placebo was given with the first two cycles of chemotherapy and ipilimumab was added with cycle 3 to 6. Eligible patients were given maintenance ipilimumab or placebo every 12 weeks until progression. The primary endpoint was immune relate progression free survival (irPFS). This endpoint was chosen to better capture the unique pattern of response to immune therapy including regression of index lesions in the face of new lesions and initial progression followed by tumor stabilization or regression.293, 294 The phased arm demonstrated an improvement in irPFS compared to chemotherapy (5.7 vs. 4.6 months, HR 0.72; P=0.05), and OS (12.2 vs. 8. 3 months). The concurrent arm did not result in an improvement in irPFS (5.5 months, HR 0.81; P = 0.81) or OS (9.7 months). There was also a higher WHO best overall response rate in the phased arm compared to chemotherapy or concurrent (32%, 18% and 21% respectively). Treatment related adverse events were similar across treatment groups (concurrent, 41%; phased, 39%; control 31%). However grade 3/4 immune related adverse events were higher in the concurrent (20%) and phased (15%) arms compared to the control (6%). Two treatment related deaths were reported including one in the concurrent arm due to septic shock secondary to epidermal necrolysis, and one death occurred in the control arm as a result of neutropenic sepsis. In a preplanned subset analysis patients with squamous cell carcinoma had a significantly improved irPFS (HR 0.55 (95% CI 0.27–1.12)) and OS (HR 0.4 (95% CI 0.22–1.03) when treated with the phased schedule. This was not observed in nonsquamous NSCLC patients or for any histology treated with the concurrent schedule. Based on these promising results a randomized phase III trial is underway comparing standard chemotherapy with or without phased ipilimumab in patients with squamous cell NSCLC (NCT01285609)
The same trial enrolled 103 patients with extensive stage SCLC and noted an improvement in ifPFS in patients treated with the phased ipilimumab schedule compared to chemotherapy alone (6.4 vs. 5.3 months, HR 0.64, P=0.03) with a non-significant trend towards improvement in RR (57% vs. 49%) and OS (12.9 vs. 9.9 months).295 This was not seen in the concurrent treatment arm (PFS 3.9 months, RR 30% and OS 9.1 months). Treatment-related and immune related grade 3/4 adverse events were more common in ipilimumab-containing arms (concurrent 43% and 21%; phased 50% and 17%; control 30% and 9%). One treatment related death due to hepatotoxicity was seen in the concurrent-treatment group. Based on this data a randomized phase III trial of platinum based chemotherapy (carboplatin or cisplatin and etoposide for four cycles) with or without phased ipilimumab in patients with extensive stage small cell lung cancer is underway (NCT01450761). Tremelimumab is a fully human IgG2 antibody. A phase II trial failed to show an improvement in progression free survival when maintenance tremelimumab was compared to best supportive care in patients with disease control (CR/PR or SD) following four cycles of platinum-based chemotherapy (20.9% vs. 14.3% patients progression free at 3 months).296 Nine (20.5%) of the tremelimumab patients experienced a grade 3/4 AE the most common being diarrhea and colitis (9.1%). Studies with tremelimumab in combination with anti-PD-L1 therapy and gefitinib in patients with NSCLC are ongoing (NCT02000947; NCT02040064).
14.2 Anti-PD-1 antibodies
Nivolumab (BMS-936558), a human monoclonal IgG4 antibody was the first anti-PD-1 antibody to demonstrate activity in NSCLC patients. PD-1 is an inhibitory T-cell receptor that is engaged by it ligands PD-L1 (or B7-H1) and PD-L2 (or B7-DC) predominantly within the tumor microenvironment.297, 298 Promising activity was seen in a dose escalation trial that included 129 NSCLC patients treated with nivolumab 1, 3 or 10 mg/kg IV every 2 weeks in an 8 week cycle.299 A response rate of 17.1% was noted in the NSCLC population with no significant difference between squamous (16.7%) and nonsquamous (17.6%) patients. Drug related adverse events were seen in 53% of patients, 6% of which were grade 3/4 including gastrointestinal, pulmonary (pneumonitis), hepatitis and infusion reactions.300 On subset analysis no significant difference was seen in patients who were EGFR mutation positive or wild type, KRAS positive or wild type.301 There was a difference in response rate between different dose levels; 3% for the 1 mg/kg cohort compared to 24.3% and 20.3% for the 3 mg/kg and 10 mg/kg cohort respectively. Based on this data the 3 mg/kg dose was selected for further study, including a single arm phase II trial of nivolumab in squamous cell lung cancer patients (NCT01721759) and two randomized phase III trials comparing nivolumab to second line chemotherapy (docetaxel) in squamous and nonsquamous NSCLC patients (NCT01673867; NCT01642004). All three trials have completed accrual and results are anticipated. In patients enrolled in the phase I trial with tumor samples available for assessment, PD-L1 expression by immunohistochemistry was associated with a response to therapy, while no responses were observed in patients with tumors that were PD-L1 negative.299 A phase III trial is ongoing comparing first line nivolumab to investigator choice chemotherapy in patients with PD-L1 positive tumors (NCT02041533). Promising results were seen in melanoma patients when nivolumab was combined with ipilimumab300 and a phase I trial is currently evaluating nivolumab alone or in combination with ipilimumab in select tumor types including SCLC (NCT01928394). In addition a phase I trial is ongoing evaluating nivolumab in combination with first line platinum-based chemotherapy in NSCLC patients (NCT01454102). Initial data presented at ASCO in 2013 indicated a fairly high rate of grade 3/4 AEs (49%) for combination nivolumab and chemotherapy.302
MK3475 is a humanized IgG4 anti-PD1 antibody that is also being evaluated in NSCLC patients. Preliminary results from a phase I trial with MK-3475 10 mg/kg administered every 2 or 3 weeks reported a 24% response in the first 38 evaluable patients using immune related response criteria and a 21% response rate using conventional RECIST response criteria.303 This response was higher in patients with tumors that were positive for expression of PD-L1 (67% vs. 4%). Median PFS had not been reached at the time of initial data cut off. Treatment related adverse events, the majority of which were grade 1/2 were noted in 53% of patients including fatigue (16%), rash (16%) and pruritis (16%). Grade 3 pulmonary edema was reported in one patient and two patients experienced grade 2 pneumonitis. Ongoing trials are comparing two different schedules of MK-3475 to standard chemotherapy (docetaxel) as second line therapy in patients with tumors that are positive for PD-L1 expression (NCT01905657). In addition MK-3475 is being combined with standard chemotherapy and immunotherapy in an ongoing phase I trial (NCT02039674; NCT01840579).
14.3 Anti-PD-L1 Antibodies
BMS-936559, a fully human IgG4 molecule, was the first anti-PD-L1 antibody to demonstrate activity in NSCLC patients. A response rate of 10% was observed in 49 patients enrolled in a phase I trial evaluating multiple different dose levels with no significant difference between squamous and nonsquamous NSCLC patients.304 Despite initial promising results this agent is not being further explored in lung cancer patients at this time.
MPDL3280A, is a human IgG1 monoclonal antibody to PD-L1. A phase I trial that included 85 NSCLC treated with MPDL3280A reported a response rate of 23%.305 Preliminary data reported the response rate was higher in tumors that were IHC3 positive (83%), defined as 10% of tumors staining positive for expression of PD-L1 and in former and current smokers (11 of 43) compared to never smokers (1 of 10 ).306 Treatment related adverse events occurred in 66% of patients of which 11% were grade 3/4 including fatigue, nausea, dyspnea and emesis. Trials of MPDL3280A are ongoing in patients with tumors that are positive for expression of PD-L1 are ongoing including a single arm phase II trial of MPDL3280A (NCT01846416; NCT02031458) and a randomized phase III trial comparing MPDL3280A to standard chemotherapy (docetaxel) (NCT02008227). In addition an upcoming phase I trial is combining MPDL3280A with or erlotinib in NSCLC patients (NCT02013219).
MEDI-4736 is a fully human antibody specific for PD-L1. Binding of MEDI-4736 relieves B7-H1 mediated suppression of T cell activation in vitro. An ongoing phase I dose escalation study including patients with NSCLC is evaluating different dose levels of MEDI-4736 including 0.1 mg/kg, 0.3 mg/kg and 1 mg/kg every two or three weeks. Data reported on the first 11 patients enrolled indicated toxicities similar to other agents in this class and responses observed in NSCLC patients.307 A phase Ib trial is evaluating MEDI-4736 in combination with tremelimumab in NSCLC patients (NCT02000947).
15. LUNG CANCER VACCINES
Cancer vaccines are based on immune system stimulation through the use of tumor cell antigens. Once the immune system is activated, it may trigger a response to cells harboring these antigens, potentially leading to elimination of the malignancy.308 The two broad types of vaccines being evaluated in patients with NSCLC are the tumor cell and the antigen-based vaccines. Since the antigens are usually poorly immunogenic by themselves, they are combined with potent adjuvants that stimulate the immune response to the vaccine without intrinsic antigenic effect.309
15.1 Tumor cell vaccines
15.1.1 Belagenpumatucel-L
Belagenpumatucel-L (Lucanix) is an allogeneic tumor cell vaccine made of four irradiated NSCLC cell lines (H460, H520, SKLU1 and RH2) modified with transformed growth factor β2 (TGF-β2) antisense plasmid.310 Antisense gene inhibition with decreased cellular expression of TGF-β2 increases the immunogenicity of the vaccine. In a randomized phase II trial, 75 patients with NSCLC stages II-IV were randomized to one of 3 doses (1.25, 2.5, or 5.0 × 107 cells/injection) of the vaccine administered once every one or two months for a maximum of 16 injections. The treatment was well tolerated and the two high-dose cohorts had a significant improvement in OS compared to low-dose. In the phase III STOP trial, 532 patients with NSCLC stage IIIA to IV were randomized to Belagenpumatucel or placebo after frontline therapy.311 The study did not meet the primary endpoint with a median OS of 20.3 and 17.3 months in the vaccine and placebo arms respectively (HR 0.94, p = 0.594). Among the 490 patients with stage IIIB or IV who were randomized within 12 weeks from completion of frontline therapy, there was a 7.4 month improvement in OS for the vaccine arm, which did not reach statistical significance (20.7 vs 13.4 months; HR 0.75, p = 0.083). In the subset of 99 patients with stage IIIB or IV non-adenocarcinoma, the median OS was significantly higher for the vaccine arm (19.9 vs 12.3 months; HR0.55, p = 0.036). Therefore, although the study did not meet the endpoint, the authors suggested that selected subset analyses support the continued development of belagenpumatucel-L in NSCLC.
15.1.2 Tergenpumatucel-L
Tergenpumatucel-L (HyperAcute-Lung) consists of three allogeneic lung tumor cell lines (derived from adenocarcinoma, squamous cell carcinoma and large cell carcinoma) that were engineered to express the α-galactosyltransferase (α-GT) enzyme, which is one of the major causes of hyperacute rejection induced with porcine xenografts transplanted into baboons.312 In a phase II trial, 28 patients with advanced NSCLC received Tergenpumatucel-L 300 million cells per injection every 2 weeks for 8 doses.313 The treatment was well tolerated without serious adverse events. Eight patients (29%) achieved SD for 4 or more months, with 5 out of 16 (31%) responding to subsequent therapy. The median and 1-year OS were 11.3 months and 46% respectively. An ongoing phase III study is comparing Tergenpumatucel-L to docetaxel in patient with previously treated NSCLC.
15.2 Antigen-associated vaccines
15.2.1 MAGE-A3
The melanoma-associated antigen-A3 (MAGE-A3) is an antigen with expression limited to non-malignant cells, except for placental trophoblasts and testicular germ cells. MAGE-A3 is expressed in approximately 35% of patients with NSCLC. MAGE-A3 vaccine is composed of the protein plus the adjuvant AS15. In a phase II trial, patients with completely resected MAGE-A3 positive stage IB or II NSCLC were randomized to the vaccine (122 patients) administered in 13 doses over 27 months or placebo (60).314 Although the treatment was well tolerated, there were no statistically significant differences in disease-free interval (DFI), disease-free survival (DFS) or OS. Nevertheless, the trend favoring the vaccine arm for DFI (HR 0.75), DFS (HR 0.76) and OS (HR 0.81) led to the large phase III trial MAGRIT, where patients with resected stage IB to IIIA NSCLC and MAGE-A3 positive tumors were randomized to placebo or vaccine after adjuvant chemotherapy. The press release from GlaxoSmithKline on March 30, 2014 indicated that the trial enrolled 2,312 patients worldwide and did not meet the primary endpoint of extending DFS.
15.2.2 MUC-1
Liposomal BLP-25 (Tecemotide, Stimuvax) is a peptide-based vaccine consisting of a synthetic MUC-1 lipopeptide combined with the adjuvant monphosphoryl lipid A and three lipids forming a liposomal product. In a phase II study, 171 patients with stage IIIB or IV NSCLC and no progressive disease (PD) after first line therapy were randomized to best supportive care (BSC) or vaccine with 1000 mcg weekly for 8 weeks followed by administrations every 6 weeks until tumor progression.315 The vaccine was preceded by one dose of cyclophosphamide 300 mg/m2. This low-dose cyclophosphamide, administered 3 days prior to the immunotherapy does not have significant anti-tumor activity in NSCLC and was used to increase the immune response. The study did not meet the primary endpoint with median OS increasing from 13 months in the BSC arm to 17.4 months in the vaccine group (p = 0.66). The greatest benefit for the vaccine was in the subset analysis of patients with locoregional stage IIIB disease, where the posthoc analysis showed that both median (not reached vs 13.3 months) and 2-year OS (60% vs 36.7%) favored the experimental arm. The Stimulating Targeted Antigen Responses To NSCLC (START) trial was a large international randomized double-blind clinical study that randomized patients with stage III NSCLC who did not have PD after chemoradiotherapy, to Tecemotide or placebo.316 Following the primary treatment, 829 and 410 patients were randomized to tecemotide and placebo respectively. The study did not meet the primary endpoint of improving OS, with the median OS increasing from 22.3 months in the placebo to 25.6 months in the tecemotide arm (HR 0.88, p = 0.12). Subset analysis of patients receiving concurrent chemoradiotherapy showed an improved median OS for the 538 patients receiving tecemotide compared to the 268 patients randomized to placebo (30.8 vs 20.6 months, HR 0.78, p = 0.016). A randomized phase III trial comparing tecemotide to placebo in patients with stage III NSCLC treated with concurrent chemoradiotherapy (START2) started in March 2014. A phase III trial (INSPIRE) with an almost identical design is being conducted in Asia.
TG4010 is a vaccine composed of the modified vaccinia virus Ankara (MVA) containing the sequence for the MUC-1 antigen and interleukin-2 (IL-2). In a phase II trial, two schedules of cisplatin plus vinorelbine and TG4010 were evaluated, including concurrent therapy upfront and TG4010 followed by the combination at progression.317 Since only 2 out of the initial 21 patients in the sequential arm achieved SD for more than 6 months, this strategy did not meet criteria by the two-stage Simon design for further evaluation. In the concurrent arm, 13 out of 37 evaluable patients (35%) achieved partial response (PR), with a median OS of 12.7 months and 1-year OS of 53%. In the phase IIB trial, 148 patients with stage IIIB with malignant pleural effusion or stage IV NSCLC were randomized to cisplatin plus gemcitabine alone or in combination with TG4010.318 The primary endpoint of the study of PFS at 6 months was met, with a significant prolongation in the vaccine arm compared to chemotherapy alone (43.2 vs 30%, p = 0.01). The experimental arm was also associated with increased in response rate (41.9 vs 28.4%) and median OS (23.3 vs 12.5 months). A confirmatory phase IIB/III trial (TIME) started in January 2012 and allows a chemotherapy choice among multiple platinum-based doublets.
16. OPTIMAL TRIAL DESIGN IN THE ERA OF GENOMICS
Advancements in next-generation sequencing technologies have resulted in a dramatic shift in the clinical trials paradigm such that cancer, once defined by many pathologically defined tumor types, is considered to be a disease of the genome consisting of copious small molecular subsets. This has motivated tailoring therapy with molecularly targeted agents and resulted in reexamination of clinical trials conduct in light of the rarity of certain genetic aberrations, the desire to bring new drugs to market more quickly, and financial resources. Here, we outline some current issues with the design of clinical trials with respect to the bench-to-bedside approach of drug development.
16.1 Early Drug Development
National and international efforts such as TCGA and the International Cancer Genomics Consortium (ICGC) have catalogued genetic aberrations of dozens of tumor types across thousands of candidate genes, resulting in massive public data sets and innumerable hypotheses for new therapeutic targets.319–321 When paralleled by the advancement and reduction in costs for the associated technologies, as well as the scientific successes of targeted agents such as imatinib and crizotinib in phase I trials, the number of phase I studies enrolling patients by molecular abnormality is increasing, as is the size of their expansion cohorts, even though there is little statistical design literature to support this approach.12, 321–327 The expansion cohort has gradually morphed from an opportunity to learn more about the safety of an novel agent to one in which efficacy data is becoming of increasing importance despite a general lacking of any expectation of statistical design for them. Often, the total sample size of the expansion cohort may exceed the sample size anticipated in the phase II setting, where one would otherwise formally test a pre-specified hypothesis with clearly stated type I and type II error rates. The problem with not incorporating a trial design in this setting is that any expansion cohort may be deemed a success from being subjected to many subset analyses by histopathology, genetic mutation, and/or outcomes. Statistically, this type of “sampling to a foregone conclusion” will result in false positive findings; as this practice becomes more common and omics-based tests are more likely to impact this setting, discussions about whether these cohorts truly serve the “phase I intent” should be revisited. Consideration of unambiguous rules for stopping and study success should also be given to expansion cohorts in light of the historically low response rate on phase I trials and the goal of minimizing exposure to ineffective or toxic drugs.
16.2 Phase II and Phase III Studies
In order to prevent premature advancement of genomic tests for guiding treatment decisions, one of the significant recent advancements in the design of oncology clinical trials has been the development of a 30-point checklist to determine the readiness of omics-based tests for guiding patient care in clinical trials by the National Cancer Institute.328 The criteria apply to any trial in which the investigational use of a laboratory test will impact therapy, and cover a wide range of topics from establishing standards for sample collection to acquiring strong evidence in support of the test to feasibility, ethics, and legal issues. It is important to note that several of the checklist criteria also apply to studies of single biomarkers, or panels of biomarkers, measured by conventional methods as opposed to high throughput methods.
Assuming that the criteria from the checklist described above are met, the next step is determining the optimal trial design for evaluation of a therapy in the phase II or phase III setting. This choice of design may vary depending on the situation, but the fundamental statistical principles for power and type I error rate considerations still apply for each phase of development. An enrichment design is appropriate when there is strong evidence that a molecularly targeted agent improves outcomes among patients diagnosed with a cancer harboring a particular biomarker; this type of trial enrolls only those patients who test positive for the marker of interest, and in this setting the biomarker is referred to as a selection marker.329, 330 The efficiency of the enrichment design depends on proportion of patients with the marker of interest as well as the level of efficacy among patients without the marker of interest. Results obtained from these types of trials may not necessarily be generalizable to populations of patients with different tumor types characterized by the same marker.
If there is evidence that a therapy may benefit the marker positive and marker negative patients, one can use marker status as a stratification factor in a randomized trial to ensure that the treatment assignment is equal within marker subsets. If the goal is to demonstrate that a new agent has a dramatically different effect on outcome in one marker group than in another, thee it would also be appropriate to power and test for a marker by treatment interaction – this is statistically the only way in which one may declare a candidate marker as “predictive.” It is not appropriate to declare a marker as predictive simply by observing differential relative outcomes between the two groups of patients. If this latter situation is likely the case, then another design option may be powering the study for an overall treatment effect as well as for tests of efficacy within each of the marker subgroups. With this type of design, treatment assignment depends on marker status, so it is critical that test results be returned within a reasonable timeframe so that randomization may take place, and the planned marker subgroup analyses should be specified in the protocol a priori. In the event that the marker analysis must be done retrospectively, for example due to feasibility issues or issues with assay development, one may still be able to obtain meaningful results in favor of prediction.331, 332
At this time, randomized designs remain the gold standard and are being implemented to validate the clinical utility of a single biomarker but implementation of these studies can be challenging for two reasons: first, the rarity of some tumors can dramatically hinder study accrual and make for longer study durations and second, at this time we often proceed with designing a study under the assumption that the molecularly targeted population is characterized by the same response to standard of care as the entire population when establishing a null hypothesis for the control group. The latter may not actually be the case, and in the event that a genetic aberration confers better outcomes than a design had planned, a randomized study may be underpowered to detect the improvement in outcome for which it was designed. To counter these concerns, non-randomized designs may appropriate in some settings, but come with the caveat that response or duration of response are really the only reliable efficacy endpoints and are generally more common in earlier phases of drug development, with confirmatory experience to follow after registration since drugs often look promising in preliminary studies and do not always translate to improvements in clinical outcomes.333
Predictive oncology has also spurred the research community to reevaluate the target effect sizes incorporated in statistical designs. The bar is much higher now. The dramatic improvement in efficacy with drugs like crizotinib and erlotinib in targeted populations has demonstrated that large effect sizes are possible, and that the relatively resource-intense approach of designing studies to detect small differences that may not be clinically meaningful does not parallel the goals of rapid discovery and efficiency of the cancer genome era.
16.3 Platform Studies
The era of genomics has also motivated the oncology community to re-examine the way that phase II and III studies are conducted such that “platform designs” are quickly becoming the new standard. These trials enroll thousands of patients to a single protocol for genomic screening and treatment assignment to a sub-study based on the genetic characteristics of their disease. Examples of such efforts currently under development are the ALCHEMIST, SWOG 1400, M-PACT and MATCH trials. Though each of these differs in terms of the statistical designs and endpoints employed by each of the trials encapsulated by the overarching platform, it is believed that these now serve as the new model for trial conduct and will result in more rapid drug discovery as well as more definitive trials. These studies also have the advantage of profiling all the tumors in a standard fashion in a single protocol, but come with some hurdles as well, such as securing drug supply across multiple sponsors and some uncertainty about the ability to accrue a sufficient number of patients with each aberration of interest.
16.4 Summary
Some research areas that are likely to influence further the design and conduct of clinical trials in the era of genomics include studies of intratumoral heterogeneity, epigenetics, mechanisms of resistance and clonal evolution. Presumably more “trials of n=1” will surface, but it is important to recall that “the pleural of anecdote is not data” and that, while playing a role in hypothesis generation, these types of experiences are not comparable to prospectively designed studies. Moving forward many of the fundamental principles of trial design, such adequately powering a study and controlling the false positive rate, will remain even as our designs change. With national and international collaborations that carefully consider all aspects of the research process, transformative clinical trials will continue to impact patient care.
Table 1.
A Tabulated Summary of Targeted and Biologic Therapies for Non- Small Cell Lung Cancer
In this sixth annual update of the Table, the current status of targeted drugs in clinical development for lung cancer is detailed. Only compounds that have entered clinical trials at the time of writing are included. In this version, we have attempted to make this reference tool dynamic and allow it to evolve as the information evolves. To facilitate this we have attached a hyperlink with each category. Clicking on the hyperlink will take the reader to the clinicaltrials.gov website for each compound and update the reader on the current status of the ongoing clinical trials. We have also ‘de-listed’ some of the drugs whose development has been discontinued in lung cancer from this version of the Table. Drugs whose development has been discontinued in the past year included to update the reader as to their current status.
As in the previous updates, the compounds are grouped by their mechanism of action. Under each class they are listed in the order of their phase of clinical development, with those in the latest phase of development being listed first. The categories are listed alphabetically, except for the first three categories (EGFR and VEGFR inhibitors and ALK inhibitors) since drug(s) from each of these category are approved for the treatment of patients with NSCLC.
The five new categories added in the previous update have been maintained in this current update and consist of immunomodulatory antibodies, SMACmimetics, antisense oligonucleotides, therapeutic antibody engineering and therapeutic viruses. These new categories are listed at the end of the table. Also at the end of the table are drugs that do not fall into a specific category. These are listed under ‘miscellaneous therapeutic agents’.
In the last column, the commonly reported toxicities are listed. This list of toxicities is not intended to be comprehensive but only the prototypic or most commonly seen ‘class effect’ toxicities are noted. The toxicity column has been left blank for compounds very early in development for which mature toxicity data are not yet available. The phase of the trial in also listed in the last but one column. The phase of development in lung cancer has been specified only if it differs from the overall phase of development of the agent. Compounds still in phase I development are also included. However, only those compounds enrolling lung cancer patients are listed. When available, the generic name, trade name(s) and other accepted name(s) or numbers used to refer to an agent are also listed.
| Hyperlink | Trial Sponsor(s) | Generic Name Trade Name Other Name(s) |
Type | Target(s) | Current Phase of Development |
Prototypic Side Effects |
|---|---|---|---|---|---|---|
| EPIDERMAL GROWTH FACTOR RECEPTOR (EGFR) INHIBITORS (HER1, 2, and pan-inhibitors) | ||||||
| http://clinicaltrials.gov/ct2/results?term=cetuximab+lung+cancer&Search=Search | ImClone/Eli Lilly/ Bristol-Myers/ Squibb |
Cetuximab/ Erbitux/ IMC-C225 |
Chimeric MoAb | EGFR | FDA approved -head and neck cancer -colorectal cancer Ph III studies for NSCLC have been completed and reported. |
Rash and other skin toxicities, nail changes, diarrhea, infusion reaction, headache,hypomagnesemia |
| http://clinicaltrials.gov/ct2/results?term=erlotinib+and+lung+cancer&Search=Search | Genentech / OSI Pharmaceuticals |
Erlotinib/ Tarceva OSI-774 |
Reversible small molecule TKI |
EGFR | FDA approved -advanced NSCLC
Gemcitabine for advanced pancreatic cancer |
Skin toxicity, diarrhea, |
| http://clinicaltrials.gov/ct2/results?term=afatinib+and+lung+cancer&Search=Search | Boehringer Ingelheim |
Afatinib Gilotrif BIBW-2992 |
Irreversible small molecule TKI |
EGFR, HER2 | Approved for the first- line treatment of patients with L858R and Exon 19 EGFR mutations. |
Rash, diarrhea, fatigue |
| http://clinicaltrials.gov/ct2/results?term=gefitinib+and+lung+cancer&Search=Search | AstraZeneca | Gefitinib/ Iressa ZD 1839 |
Reversible small molecule TKI |
EGFR | Available only as part of a special program called the Iressa Access Program in the US. Approved for use in patients with EGFR mutations in Europe and Japan. |
Skin toxicity, diarrhea, interstitial lung disease |
| http://clinicaltrials.gov/ct2/results?term=lapatinib+and+lung+cancer&Search=Search | GlaxoSmithKline | Lapatinib/ Tykerb GW 572016 |
Reversible small molecule TKI |
EGFR, HER2 (erb-B2) |
FDA approved for HER2-overexpressing advanced breast cancer Ph II for NSCLC |
Diarrhea, nausea, vomiting, dermatologic (palmar-plantar erythrodysesthesia and rash), fatigue, hepatotoxicity, |
| http://clinicaltrials.gov/ct2/results?term=panitumumab+lung+cancer&Search=Search | Amgen | Panitumumab/ Vectibix ABX-EGF MAb |
Human IgG2 MoAb | EGFR | FDA approved for colorectal cancer Ph II for NSCLC |
Hypomagnesemia, paronychia, fatigue, nausea, diarrhea, infusion reaction |
| http://clinicaltrials.gov/ct2/results?term=trastuzumab+and+lung+cancer&Search=Search | Genentech | Trastuzumab Herceptin |
Humanized MoAb | HER2 | FDA approved for HER2-overexpressing breast cancer Ph II for NSCLC (completed) |
Cardiomyopathy, infusion reactions, diarrhea |
| http://clinicaltrials.gov/ct2/results?term=icotinib+and+lung+cancer&Search=Search | Zhejiang Beta Pharma Inc. |
Icotinib Conmana (China) BPI-2009H |
Small molecule TKI | EGFR | Approved by the SFDA in China and currently in Phase IV development |
Rash, diarrhea |
| http://clinicaltrials.gov/ct2/results?term=necitumumab+lung+cancer&Search=Search | Eli Lilly/ImClone | Necitumumab NA IMC-11F8 |
Human IgG1 MoAb | EGFR | Ph III completed. Data to be presented at American Society of Clinical Oncology meeting in June 2014. |
Thromboembolic events |
| http://clinicaltrials.gov/ct2/results?term=dacomitinib+and+lung+cancer&Search=Search | Pfizer | Dacomitinib NA PF299804 |
Irreversible small molecule TKI |
Pan-HER | Ph III (ARCHER 1009 and BR26 were completed and did not meet their primary endpoints. ARCHER 1050 is currently ongoing and is expected to be completed in 2015) |
Diarrhea, acne, rash |
| https://clinicaltrials.gov/ct2/results?term=Sym004&Search=Search | Merck/Symphoge n |
NA NA Sym004 |
A mixture of two monoclonal antibodies directed against nonoverlapping epitopes. |
EGFR extracellular domain III |
Phase I/II | Rash and diarrhea |
| http://clinicaltrials.gov/ct2/results?term=pertuzumab+lung+cancer&Search=Search | Genentech/Roche | Pertuzumab Perjeta rhuMAb 2C4 |
Humanized murine MoAb |
Prevents dimerization of HER2 with other HER receptors |
FDA approved for HER2-overexpressing metastatic breast cancer in combination with trastuzumab and docetaxel. Currently no ongoing studies in Lung Cancer. Phase II studies completed. |
Fatigue, diarrhea, LVEF decrease |
| http://clinicaltrials.gov/ct2/results?term=neratinib+lung+cancer&Search=Search | Pfizer (Wyeth) | Neratinib NA HKI-272 |
Irreversible small molecule TKI |
EGFR, HER2 | Ph III (Breast Cancer) Ph II for NSCLC completed |
Diarrhea, asthenia, rash |
| http://clinicaltrials.gov/ct2/results?term=nimotuzumab+lung+cancer&Search=Search | YM BioSciences | Nimotuzumab TheraCIM h-R3 |
Humanized MoAb | EGFR | Approved in Thailand and Myanmar for relapsed high-grade Glioma Ph II for NSCLC |
Rash, diarrhea |
| http://clinicaltrials.gov/ct2/results?term=BMS-690514+lung+cancer&Search=Search | Bristol-Myers Squibb |
NA NA BMS-690514/ EVR1 |
Small molecule TKI | Pan-HER VEGFR2 |
Ph II completed. Currently there are no ongoing studies. |
Diarrhea, rash, arterial hypertension, pulmonary embolism, angioedema |
| http://clinicaltrials.gov/ct2/results?term=pelitinib+and+lung+cancer&Search=Search | Pfizer (Wyeth) | Pelitinib NA EKB-569 |
Irreversible small molecule TKI |
EGFR, HER2, HER4 |
Ph II completed. No ongoing studies currently. |
Diarrhea, rash, nausea, asthenia |
| http://clinicaltrials.gov/ct2/results?term=RO5083945+lung+cancer&Search=Search | Roche | NA NA RO5083945/ GA201 |
Glyco-engineered anti-EGFR IgG1 MoAb |
EGFR (also improved antibody dependent cellular cytotoxicity |
Ph II. Now closed to accrual. |
Infusion reactions, rash, hypomagnesaemia |
| http://clinicaltrials.gov/ct2/results?term=U3-1287+AMG888+lung+cancer&Search=Search | Daiichi Sankyo | NA NA U3-1287/ AMG888 |
Human MoAb | HER3 | Ph Ib/II. Closed to accrual. |
Rash, anemia, diarrhea |
| http://clinicaltrials.gov/ct2/results?term=MM121+lung+cancer&Search=Search | Merrimack Pharmaceuticals |
NA NA MM121/SAR256212 |
Human MoAb | HER3 | Ph I/II | |
| http://clinicaltrials.gov/ct2/results?term=CO+1686+lung+cancer&Search=Search | Clovis | NA NA CO 1686 |
Irreversible oral small molecule inhibitor |
Mutant forms of EGFR including T790M |
Ph II | |
| https://clinicaltrials.gov/ct2/results?term=AZD9291&Search=Search | AstraZeneca | NA NA AZD 9291 |
Irreversible oral small molecule inhibitor |
Mutant forms of EGFR including T790M |
PhII | |
| http://clinicaltrials.gov/ct2/results?term=azd8931&Search=Search | AstraZeneca | Momelotinib NA AZD8931 |
Reversible small molecule inhibitor |
EGFR, HER2, HER3 |
Ph II No ongoing studies for lung cancer |
|
| http://clinicaltrials.gov/ct2/results?term=MEHD7945+A&Search=Search | Genentech | NA NA MEHD7945 A |
Humanized dual action IgG1 Moab |
EGFR, HER3 | Ph II Ph I for lung cancer currently not recruiting |
|
| http://clinicaltrials.gov/ct2/results?term=AV-203+and+cancer&Search=Search | AVEO Pharmaceuticals |
NA NA AV 203 |
Humanized Moab | HER3 | Ph I | |
| http://clinicaltrials.gov/ct2/results?term=ARRY+380+cancer&Search=Search | Array BioPharma | NA NA ARRY 380 |
Reversible small molecule inhibitor |
HER2 | Ph I (completed) | Nausea, rash, fatigue |
| http://clinicaltrials.gov/ct2/results?term=BMS-599626+cancer&Search=Search | Bristol-Myers Squibb/ Ambit Biosciences |
NA NA BMS-599626 |
Reversible small molecule inhibitor |
Pan-HER (EGFR, HER2, HER4) |
Ph I (completed) | Diarrhea, nausea, rash, musculoskeletal cramps |
| http://clinicaltrials.gov/ct2/results?term=MM151+cancer&Search=Search | Merrimack Pharmaceuticals |
NA NA MM151 |
Combination of three Human MoAb |
EGFR | Ph I | |
| http://clinicaltrials.gov/ct2/results?term=MM-111+cancer&Search=Search | Merrimack Pharmaceuticals |
NA NA MM111 |
Bi-specific antibody fusion protien |
HER2-HER3 heterodimer |
Ph I/II Phase I for lung cancer |
|
| http://clinicaltrials.gov/ct2/results?term=Zalutumumab+cancer&Search=Search | Genmab | Zalutumumab NA HuMax-EGFr |
Human MoAb | EGFR | Not recruiting patients currently. |
Rash, fatigue, pyrexia |
| VASCULAR ENDOTHELIAL GROWTH FACTOR (VEGF) and VEGF RECEPTOR (VEGFR) INHIBITORS | ||||||
| http://clinicaltrials.gov/ct2/results?term=bevacizumab+lung+cancer&Search=Search | Genentech | Bevacizumab Avastin |
Humanized MoAb | VEGF-A | FDA approved -metastatic HER2 negative breast -metastatic colorectal -metastatic NSCLC -Phase III testing on going in the adjuvant setting (ECOG 1505) |
Hypertension, proteinuria, thrombosis, hemorrhage, gastrointestinal perforation |
| http://clinicaltrials.gov/ct2/results?term=sorafenib+lung+cancer&Search=Search | Bayer | Sorafenib Nexavar BAY43-9006 |
Small molecule TKI | VEGFR-2,3, PDGFR β, KIT, RAF/MEK, FLT-3 |
FDA approved -unresectable hepatocellular cancer -advanced renal cell carcinoma Ph III terminated, phase II ongoing for NSCLC. Being evaluated in KRAS positive NSCLC. |
Rash/desquamatio n, hand-foot skin reaction, diarrhea, fatigue, hypertension |
| http://clinicaltrials.gov/ct2/results?term=Sunitinib+lung+cancer&Search=Search | Pfizer | Sunitinib Sutent SU 011248 |
Small molecule TKI | VEGFR-1,2,3, PDGFR β, KIT, FLT-3, RET |
FDA approved -gastrointestinal stromal tumor -advanced renal cell carcinoma Ph III for NSCLC. Being evaluated in the maintenance setting for NSCLC and SCLC. |
Hypertension, rash, stomatitis, diarrhea, hypothyroidism, hand-foot syndrome |
| http://clinicaltrials.gov/ct2/results?term=pazopanib+lung+cancer&Search=Search | GlaxoSmithKline | Pazopanib Votrient GW786034 |
Small molecule TKI | VEGFR-1,2,3, PDGFR α, β, KIT |
FDA approved for advanced renal cell carcinoma Ph III for NSCLC |
Nausea, hypertension, diarrhea, fatigue, transaminase elevation, vomiting, hair depigmentation. |
| http://clinicaltrials.gov/ct2/results?term=vandetanib | AstraZeneca | Vandetanib Zactima ZD6474 |
Small molecule TKI | VEGFR-2, 3, EGFR, RET |
FDA approved for -advanced, unresectable medullary thyroid cancer Ph III studies in NSCLC completed (lung indication not being pursued) |
Hypertension, diarrhea, rash |
| http://clinicaltrials.gov/ct2/results?term=Aflibercept+lung+cancer&Search=Search | Sanofi-Aventis | Aflibercept Zaltrap VEGF-TRAP/ AVE0005 |
Fusion protein of extracellular domain portions from VEGFR-1 & VEGFR-2 combined with Fc of human IgG |
VEGF, Placental Growth Factor (PIGF) |
Ph III (VITAL- failed to meet primary end- point of OS)). Currently not accruing lung cancer patients. |
Hypertension, dysphonia, epistaxis, proteinuria, headache, diarrhrea, |
| http://clinicaltrials.gov/ct2/results?term=cediranib+lung+cancer | AstraZeneca | Cediranib Recentin AZD2171 |
Small molecule TKI | VEGFR-1,2,3, PDGFR β, KIT |
Ph III Currently no active studies for lung cancer |
Diarrhea, dysphonia, hypertension |
| http://clinicaltrials.gov/ct2/results?term=motesanib+lung+cancer&Search=Search | Amgen | Motesanib NA AMG706 |
Small molecule TKI | VEGFR-1,2,3, PDGFR β, KIT, RET |
Ph III (NSCLC study failed to improve survival). Currently no active studies for lung cancer |
Nausea, diarrhea, fatigue, hypertension |
| http://clinicaltrials.gov/ct2/results?term=BIBF1120+lung+cancer&Search=Search | Boehringer Ingelheim |
Nintedanib Vargatef BIBF 1120 |
Small molecule TKI | VEGFR-1,2,3, PDGFR-α, β, FGFR-1,2,3, Src kinases |
Ph III completed. Phase III LUME-lung 1 showed a survival benefit. Submitted to the EMEA for approval. |
Nausea, vomiting, elevated liver enzymes, diarrhea |
| http://clinicaltrials.gov/ct2/results?term=Ramucirumab+lung+cancer&Search=Search | ImClone/ Eli Lilly | Ramucirumab Cyramza IMC-1121B |
Human MoAb | VEGFR-2 | Approved for Gastric cancer. Phase III study (REVEL) completed. Primary endpoint of overall survival was met in the second-line treatment of advanced NSCLC in combination with docetaxel. |
Hypertension, abdominal pain, nausea, anorexia, headache, proteinuria, deep venous thrombosis (DVT). |
| http://clinicaltrials.gov/ct2/results?term=axitinib+and+lung+cancer&Search=Search | Pfizer | Axitinib Inlyta AG013736 |
Small molecule TKI | VEGFR-1,2,3, PDGFR β, KIT |
Not recruiting NSCLC patients. Phase II study for carcinoid tumors ongoing. |
Diarrhea, hypertension, fatigue, nausea, hand-foot syndrome, proteinuria |
| http://clinicaltrials.gov/ct2/results?term=vatalanib+lung+cancer&Search=Search | Novartis/ Bayer Schering |
Vatalanib NA PTK787/ZK 222584 |
Small molecule TKI | VEGFR-1,2, PDGFR β, KIT |
Ph III Ph II for lung cancer |
Hypertension, diarrhea, nausea, fatigue, dizziness |
| http://clinicaltrials.gov/ct2/results?term=tivozanib+cancer&Search=Search | AVEO Pharmaceuticals |
Tivozanib Tivopath AV-951 |
Small molecule inhibitor |
Pan VEGFR- 1,2,3 |
Ph III completed. Improvement in Overall Survival was not seen. US FDA did not approve tivozanib for lung cancer. |
Diarrhea, dysphonia, asthenia, hypertension |
| http://clinicaltrials.gov/ct2/results?term=linifanib+lung+cancer&Search=Search | Abbott (Abbvie)/Genente ch |
Linifanib NA ABT-869 |
Small molecule TKI | VEGFR, PDGFR |
Ph III Ph II for NSCLC |
Fatigue, hypertension, proteinuria, hand foot skin reaction |
| http://clinicaltrials.gov/ct2/results?term=cabozantinib+lung+cancer&Search=Search | Exelixis/ Bristol Myers Squibb |
Cabozantinib Cometriq XL184 |
Small molecule TKI | VEGFR-2, MET, RET |
Ph III Ph II for NSCLC. Also being evaluated in patients KIF5B/RET translocations. |
Diarrhea, mucositis, anorexia, vomiting, hand- foot skin reaction, hypertension, elevation of liver enzymes |
| http://clinicaltrials.gov/ct2/results?term=Regorafenib&Search=Search | Bayor | Regorafenib Stivarga BAY73-4506 |
Diphenylurea multikinase inhibitor |
VEGFR-1,2,3, TIE2, PDGFRβ, FGFR, KIT, RET, RAF |
Ph III Ph I for lung cancer |
Hand-foot skin reaction, extremity pain, hypothyroidism, rash |
| http://clinicaltrials.gov/ct2/results?term=lenvatinib&Search=Search | Eisai Limited | Lenvatinib NA E7080 |
Small molecule TKI | VEGFR-2 (also 1,3), FGFR-2, RET |
Ph III Ph I for lung cancer. Also being evaluated in patients KIF5B/RET translocations. |
Hypertension, fatigue, proteinuria, anorexia, diarrhea, dysphonia |
| http://clinicaltrials.gov/ct2/results?term=XL647+cancer&Search=Search | Exelixis | NA NA XL647 |
Irreversible small molecule TKI |
VEGFR-2, EGFR, HER2, EphB4 |
Ph II completed. Currently no active ongoing studies. |
Diarrhea, rash, fatigue, nausea, hypertension, anorexia |
| http://clinicaltrials.gov/ct2/results?term=CT-322+cancer&Search=Search | Adnexus (Bristol- Myers Squibb) |
NA NA CT-322 |
Adnectin (Fibronectin based small protein) |
VEGFR-2 | Ph II. | Proteinuria, hypertension |
| http://clinicaltrials.gov/ct2/results?term=foretinib+cancer&Search=Search | Exelixis/ GlaxoSmithKline |
Foretinib NA GSK1363089 / XL880 |
Small molecule TKI | VEGFR-2, MET, Ron |
Ph II | Hypertension, fatigue, nausea, diarrhea, night blindness |
| http://clinicaltrials.gov/ct2/results?term=AEE+788+cancer&Search=Search | Novartis | NA NA AEE 788 |
Irreversible small molecule TKI |
VEGFR-2, EGFR, HER2 |
Ph I/II. Currently not recruiting patients. |
Fatigue, diarrhea, nausea, rash |
| http://clinicaltrials.gov/ct2/results?term=xl820+cancer&Search=Search | Exelixis | NA NA XL820 |
Small molecule TKI | VEGFR-2, PDGFR β, KIT |
Ph II No active lung trials |
Nausea, fatigue, rash |
| http://clinicaltrials.gov/ct2/results?term=xl999+and+cancer&Search=Search | Exelixis | NA NA XL999 |
Small molecule TKI | VEGFR, PDGFR, FLT- 3, Src, FGFR |
Ph II (terminated due to safety concerns) |
Cardiac toxicity, diarrhea, asthenia, hypersensitivity |
| http://clinicaltrials.gov/ct2/show/NCT00952497?term=telatinib+cancer&rank=1 | ACT Biotech Inc. | Telatinib NA BAY57-9352 |
Small molecule TKI | VEGFR-2,3, PDGFR-β and KIT |
Ph I (completed) | Hypertension, hoarseness, anorexia, diarrhea |
| http://clinicaltrials.gov/ct2/results?term=ombrabulin+cancer | Sanofi-Aventis | Ombrabulin NA AVE8062 |
Combretastatin A-4 derivative | tubulin- binding agent that targets the immature neovasculature of tumors |
Ph II (completed). Currently no active studies accruing lung cancer patients. |
|
| http://clinicaltrials.gov/ct2/results?term=megf0444a+AND+cancer | Genentech | NA NA MEGF0444A/RG7414 |
humanized antibody against EGFL7 (Epidermal Growth Factor Domain-Like 7) |
Targets EGFL7. a vascular- restricted secreted protein present in the tracks that surround tumor blood vessels |
Ph I completed. Currently not accruing lung cancer patients. |
|
| ANAPLASTIC LYMPHOMA KINASE (ALK) & ROS1 INHIBITORS. | ||||||
| http://clinicaltrials.gov/ct2/results?term=crizotinib+lung+cancer&Search=Search | Pfizer | Crizotinib Xalkori PF-02341066 |
Dual small molecule ATP competitive inhibitor |
Anaplastic lymphoma kinase (ALK), c-Met, ROS1 |
FDA approved for ALK+ locally advanced or metastatic NSCLC |
Nausea, diarrhea, visual disturbances, alanine aminotransferase elevation, fatigue |
| http://clinicaltrials.gov/ct2/results?term=X-396+cancer&Search=Search | Xcovery | NA NA X-396 |
Small molecule inhibitor |
ALK | Ph I | |
| http://clinicaltrials.gov/ct2/results?term=LDK378+lung+cancer&Search=Search | Novartis | Ceritinib Zykadia LDK378 |
Small molecule inhibitor |
ALK Translocations |
Approved by the US FDA for the treatment for patients with ALK- positive metastatic non- small cell lung cancer (NSCLC) following treatment with crizotinib |
Nausea, vomiting, diarrea. Notably visual disturbances were not seen. |
| http://clinicaltrials.gov/ct2/results?term=AP26113+cancer&Search=Search | Ariad Pharmeceuticals |
NA NA AP26113 |
Small molecule inhibitor |
ALK/EGFR | Ph I/II | |
| http://clinicaltrials.gov/ct2/results?term=ch5424802 | Chugai Pharmaceuticals |
NA NA CHS424802/AF802 |
Small molecule inhibitor |
ALK | Ph I/II | |
| BCL-2 INHIBITORS | ||||||
| http://clinicaltrials.gov/ct2/results?term=ABT-263&Search=Search | Abbott(Abbvie)& Genentech |
Navitoclax NA ABT-263 |
Small molecule inhibitor (ABT-263 is orally bioavailable) |
Bad-like BH3 mimetic |
Ph II for small cell lung cancer (SCLC). Not accruing patients currently. |
Diarrhea, back pain, thrombocytopenia |
| http://clinicaltrials.gov/ct2/results?term=G3139+cancer&pg=1 | Genta | Oblimerson Genasense G3139 |
Antisense oligo- deoxyribonucleotide |
Bcl-2 | Ph II/III. Not recruiting patients currently |
Fever, elevated liver enzymes |
| http://clinicaltrials.gov/ct2/results?term=Obatoclax&Search=Search | GeminX | Obatoclax NA GX15-070 |
Small molecule inhibitor |
Pan Bcl-2 | Ph I/II. Not recruiting patients currently. |
Neurotoxicity, cytopenias |
| http://clinicaltrials.gov/ct2/results?term=AT101+and+cancer&Search=Search | Ascenta | NA NA AT101 |
Negative enantiomer of gossypol |
Pan Bcl-2 | Ph II (NSCLC studies terminated, SCLC ongoing) |
Gastrointestinal side effects |
| BCR-ABL/SRC TYROSINE KINASE / STAT INHIBITORS | ||||||
| http://clinicaltrials.gov/ct2/results?term=dasatinib+and+lung+cancer&Search=Search | Bristol-Myers Squibb |
Dasatinib Sprycel BMS-354825 |
Small molecule TKI of SRC-family |
Src, BCR- ABL, KIT, PDGFR, FMS or colony stimulating factor 1 receptor CSF1R |
FDA approved for chronic myelogenous leukemia Ph II for NSCLC. Also being studied in NSCLC patients with DDR2 mutations and inactivating BRAF mutations. |
Fluid retention, pleural effusion, diarrhea, prolonged QTc, myelosuppression, rash |
| http://clinicaltrials.gov/ct2/results?term=imatinib+and+lung+cancer&pg=1 | Novartis | Imatinib Gleevec STI-571 |
Small molecule TKI | KIT, PDGFR, BCR-ABL fusion protein |
FDA approved for -gastro-intestinal stromal tumor -dermato-fibrosarcoma protuberans -philadelphia chromosome positive chronic myelogenous leukemia Ph II for NSCLC |
Fluid retention, diarrhea, myelosuppression, rash |
| http://clinicaltrials.gov/ct2/results?term=saracatinib | AstraZeneca | Saracatinib NA AZD 0530 |
Small molecule TKI of SRC-family binds the active conformation of the ATP binding pocket |
Src, BCR- ABL (Inhibits Src kinase mediated osteoclast resorption) |
Ph II. Currently not accruing patients. |
Leukopenia, febrile neutropenia, asthenia |
| http://clinicaltrials.gov/ct2/results?term=bosutinib&Search=Search | Pfizer (Wyeth) | Bosutinib NA SKI-606 |
4-anilino-3- quinolinecarbonitrile dual Src/Abl kinase inhibitor |
Src, ABL | Ph II Ph I for lung cancer. Completed |
Diarrhea, anorexia, nausea |
| http://clinicaltrials.gov/ct2/results?term=KX2-391&Search=Search | Kinex Pharmaceuticals |
NA NA KX2-391 |
Small molecule TKI targeting the substrate binding site |
c-Src | Ph II Ph I for lung cancer |
Hypokalemia, anemia, elevated AST, fatigue, dyspnea, fever, vomiting, constipation, hematuria, lymphopenia |
| http://clinicaltrials.gov/ct2/results?term=XL228+cancer&Search=Search | Exelixis | NA NA XL228 |
Multi-targeted TKI | Src, ABL, IGF-1R, AURORA, FGFR1-3 |
Ph I (closed) | Nausea, neutropenia, fatigue, hypoglycemia |
| http://clinicaltrials.gov/ct2/results?term=OPB+51602+cancer&Search=Search | Otsuka Beijing Research Institute |
NA NA OPB 51602 |
inhibitor of signal transducer and activator of transcription 3 |
STAT 3 | Ph I | |
| BRAF inhibitors | ||||||
| http://clinicaltrials.gov/ct2/results?term=Vemurafenib+cancer&Search=Search | Plexxikon/Roche | Vemurafenib Zelboraf PLX4032/RG7204 |
Small molecule inhibitor |
BRAF (V600E) mutation) |
FDA approved for metastatic melanoma Being evaluated in BRAFV600E cancers including lung cancer. |
Diarrhea, rash, fatigue, skin squamous cell carcinoma |
| http://clinicaltrials.gov/ct2/results?term=dabrafenib+and+cancer&Search=Search | GlaxoSmithKline | Dabrafenib Tafinlar GSK2118436 |
Small molecule inhibitor |
Mutant BRAF kinase |
FDA approved for Melanoma. Being evaluated in BRAFV600E cancers including lung cancer. |
Pyrexia, rash, skin squamous cell carcinoma, diarrhea |
| http://clinicaltrials.gov/ct2/results?term=LGX818+cancer&Search=Search | Novartis | NA NA LGX818 |
Small molecule inhibitor |
BRAF kinase inhibitor |
Ph I/II in BRAF mutant or dependent tumors. Currently not being evaluated in lung cancer. |
|
| http://clinicaltrials.gov/ct2/results?term=ArQ736+cancer&Search=Search | ArQule | NA NA ArQ736 |
Small molecule inhibitor |
Pan-Raf inhibitor |
Ph I completed in patients with BRAF or Nras mutations. |
|
| EPIGENETIC MODULATORS OF GENE EXPRESSION OR PROTEIN DEGRADATION | ||||||
| Heat Shock Protein (HSP)-90 Inhibitors | ||||||
| http://clinicaltrials.gov/ct2/results?term=IPI-504+cancer&pg=1 | Infinity | Retaspimycin NA IPI-504 |
Water soluble geldanamycin derivative |
HSP-90 | Ph III Ph II for NSCLC (activity in ALK translocated patients). Also being evaluated in KRAS mutant NSCLC |
Fatigue, nausea, diarrhea, renal failure, liver failure |
| http://clinicaltrials.gov/ct2/results?term=STA-9090+lung+cancer&Search=Search | Synta Pharmaceuticals |
Ganetespib NA STA-9090 |
Small molecule inhibitor |
HSP-90 | Ph II/III. Currently being evaluated in ALK positive disease. |
Diarrhea, anemia, fatigue, abdominal pain, elevated alkaline phosphatase, insomnia |
| http://clinicaltrials.gov/ct2/results?term=AUY922+cancer&Search=Search | Novartis | NA NA AUY922 |
Isoxazole based compound (non- geldanamycin) |
HSP90 | Ph II | Diarrhea, nausea, fatigue, visual symptoms, vomiting |
| http://clinicaltrials.gov/ct2/results?term=Alvespimycin+cancer&Search=Search | Bristol-Myers Squibb/Kosan Biosciences |
Alvespimycin NA KOS-1022/ 17-DMAG |
Benzoquinone antineoplastic antibiotic |
HSP-90 | Ph II Ph I for lung cancer |
Diarrhea, fatigue, headache, joint pain |
| http://clinicaltrials.gov/ct2/results?term=Tanespimycin+cancer&Search=Search | Bristol-Myers Squibb/ Kosan Biosciences |
Tanespimycin NA KOS-953/ 17-AAG |
Benzoquinone antineoplastic antibiotic |
HSP-90 | Development halted by company (Ph III & Ph I for lung cancer) |
Fatigue, lymphopenia |
| http://clinicaltrials.gov/ct2/results?term=DS+2248+cancer&Search=Search | Daiichi Sankyo | NA NA DS 2248 |
Benzoquinone antineoplastic antibiotic |
HSP-90 | Ph I | |
| Histone Deacetylase (HDAC) Inhibitors | ||||||
| http://clinicaltrials.gov/ct2/results?term=vorinostat+lung+cancer&pg=1 | Merck | Vorinostat Zolinza MK 0683/ SAHA |
Hydroxamic acid- type |
Class I, II and IV | FDA approved cutaneous T-cell Lymphoma Ph III in NSCLC terminated Ph II in mesothelioma completed. |
Diarrhea, nausea, fatigue, thrombocytopenia, muscle spasms |
| http://clinicaltrials.gov/ct2/results?term=Romidepsin+cancer&Search=Search | Astellas/ Gloucester Pharmaceuticals |
Romidepsin Istodax FK228 |
Cyclic tetrapeptide (Depsipeptide) | Class I specific | FDA approved cutaneous T-cell Lymphoma Ph I/II for NSCLC |
Fatigue, rash, thrombocytopenia, nausea |
| http://clinicaltrials.gov/ct2/results?term=entinostat+lung+cancer&Search=Search | Syndax Pharmaceuticals |
Entinostat NA MS-275/ SNDX-275 |
Benzamide derivative | Class I specific | Ph II in E-cadherin positive NSCLC. |
Pancytopenia, hypophosphatemia, nausea, fatigue |
| http://clinicaltrials.gov/ct2/results?term=Belinostat+lung+cancer&Search=Search | CuraGen/ Topotarget |
Belinostat NA PXD 101 |
Hydroxamic acid- type |
Class I & II isoforms |
Ph I/II | Nausea, emesis, fatigue |
| http://clinicaltrials.gov/ct2/results?term=pivanex+cancer&Search=Search | Titan | Pivanex (pivaloyloxymethyl butyrate) NA AN-9 |
Aliphatic acid | Class I and IIa specific |
Ph II (completed) | Fatigue, nausea, dysgeusia |
| http://clinicaltrials.gov/ct2/results?term=Valproic+acid+lung+cancer&Search=Search | Valproic acid Depakote |
Aliphatic acid | Class I and IIa specific |
Ph II | Anemia, neurological toxicity, nausea, hepatic toxicity |
|
| http://clinicaltrials.gov/ct2/results?term=Sodium+phenylbutyrate+lung+cancer&Search=Search | Sodium phenylbutyrate | Aliphatic acid | Class I and IIa specific |
Ph II (completed) | Neuro-cortical toxicity, hypokalemia, hyponatremia, hyperuricemia, nausea |
|
| http://clinicaltrials.gov/ct2/results?term=Panobinostat+lung+canc er&Search=Search | Novartis | Panobinostat Faridak LBH589 |
Hydroxamic acid- type |
Pan-HDAC (all isoforms) |
Ph II Ph I for lung cancer |
Rash, QT interval prolongation, nausea, diarrhea, hypokalemia, thrombocytopenia |
| http://clinicaltrials.gov/ct2/results?term=SB939+cancer&Search=Search | S*BIO | NA NA SB939 |
Small molecule HDAC inhibitor |
HDAC | Ph II Ph I for lung (no ongoing trials) |
Fatigue, troponin elevation, QTc prolongation |
| http://clinicaltrials.gov/ct2/results?term=CUDC-101+cancer&Search=Search | Curis | NA NA CUDC-101 |
Hydroxamic acid | HDAC, HER2, EGFR |
Ph Ib | Fatigue, increased creatinine, increased hepatic enzymes |
| http://clinicaltrials.gov/ct2/results?term=PCI-24781+cancer&Search=Search | Pharmacyclics | NA NA PCI-24781 |
Cyclic tetrapeptide | Class I & II | Ph I/II | |
| http://clinicaltrials.gov/ct2/results?term=FK-228+lung+cancer&Search=Search | Gloucestor Pharmaceuticals |
NA NA FK-228 |
Cyclic tetrapeptide (Depsipeptide) |
Class I | Ph IIb Ph I for lung |
|
| http://clinicaltrials.gov/ct2/results?term=CHR-3996+cancer&Search=Search | Chroma Therapeutics |
NA NA CHR-3996 |
Small molecule inhibitor |
Class I HDAC isoforms |
Ph I (completed) | Fatigue, nausea, vomiting |
| http://clinicaltrials.gov/ct2/results?term=Givinostat+cancer&Search=Search | Italformaco | Givinostat NA ITF2357 |
Hydroxamic acid derivative |
Class I & II | Currently being studied in hematologic malignancies in Ph II |
|
| PROTEASOME INHIBITORS | ||||||
| http://clinicaltrials.gov/ct2/results?term=bortezomib+and+lung+cancer&Search=Search | Millennium Pharmaceuticals (Takeda Oncology) |
Bortezomib Velcade PS-341 |
Reversible inhibitor | 26S proteasome and client proteins (i.e. p27, p53, NFkB, Bcl-2, Bax) |
FDA approved for -multiple myeloma -mantle cell lymphoma Ph II for NSCLC. Being explored in KRAS mutant patients. |
Asthenia, nausea, diarrhea, constipation, peripheral neuropathy, hypotension, thrombocytopenia |
| http://clinicaltrials.gov/ct2/results?term=carfilzomib+and+lung+cancer&Search=Search | Onyx Pharmaceuticals |
Carfilzomib NA PR-171 |
Irreversible inhibitor | 20S proteasome subunit |
Ph II. Currently not recruiting patients. Ph I for lung cancer |
Pancytopenia, peripheral neuropathy |
| http://clinicaltrials.gov/ct2/results?term=mln9708+AND+cancer | Millennium Pharmaceuticals (Takeda Oncology) |
NA NA MLN9708 |
Reversible inhibitor | 20S proteasome subunit |
Ph I/II Ph I for lung cancer |
Anorexia, dehydration, fatigue, nausea, peripheral sensory neuropathy, macular rash, renal failure and thrombocytopenia |
| http://clinicaltrials.gov/ct2/results?term=NPI-0052+cancer&Search=Search | Nereus Pharmaceuticals |
Salinosporamide A NA NPI-0052 |
Irreversible inhibitor | 20S catalytic core subunit of the proteasome |
Ph I for solid tumors ongoing, |
Fatigue, nausea, neurological toxicity |
| http://clinicaltrials.gov/ct2/results?term=CEP-18770+cancer&Search=Search | Cephalon and Ethical Oncology Science |
NA NA CEP-18770 |
Slowly reversible | Inhibits chymotrypsin like activity of proteasome |
Ph I for solid tumors completed |
|
| FIBROBLAST GROWTH FACTOR RECEPTOR (FGFR) INHIBITORS | ||||||
| http://clinicaltrials.gov/ct2/results?term=brivanib+and+cancer&Search=Search | Bristol-Myers Squibb |
Brivanib NA BMS-582664 |
Small molecule TKI | FGFR and VEGFR-2 |
Ph III Ph II for NSCLC completed. |
Hypertension, fatigue |
| http://clinicaltrials.gov/ct2/results?term=Dovitinib+and+cancer&Search=Search | Novartis | Dovitinib NA TKI-258 |
Small molecule inhibitor |
FGFR-1,2,3, PDGFR, VEGFR-2 |
Ph II Ph II for lung cancer |
Hypertension, anorexia, nausea, vomiting, fatigue, headache |
| http://clinicaltrials.gov/ct2/results?term=GSK3052230+and+cancer&Search=Search | GSK/Five Prime therapeutics |
GSK3052230 FP-1039 |
Soluble fusion protein consisting of a portion of the FGFR1 linked to Fc portion of IgG1 |
FGF ligand trap (multiple FGF’s) |
Ph I in patients with FGFR deregulated patients. |
Neutropenia, bowel perforation, urticaria, atrial fibrillation |
| http://clinicaltrials.gov/ct2/results?term=AZD4547+and+cancer&Search=Search | AstraZeneca | NA NA AZD4547 |
Small molecule inhibitor |
FGFR1,2,3 | Ph II | |
| http://clinicaltrials.gov/ct2/results?term=Ponatinib+and+cancer&Search=Search | ARIAD | Ponatinib NA AP24534 |
Small Molecule inhibitor |
Pan-FGFR inhibitor Pan-BCR ABL inhibitor RET |
Phase II CML NSCLC study on going in patients with RET translocations |
low platelet counts, headache, nausea, joint pain, fatigue, anemia, increased lipase, muscle spasms, rash, pancreatitis |
| http://clinicaltrials.gov/ct2/results?term=TSU-68+and+cancer&Search=Search | Taiho Pharmaceutical |
Orantinib TSU-68 SU6668 |
Small molecule inhibitor |
FGFR, PDGFR, VEGFR |
Ph I/II No active trials for lung |
Fatigue, AST/ALT elevation, diarrhea |
| http://clinicaltrials.gov/ct2/results?term=BGJ398+cancer&Search=Search | Novartis | NA NA BGJ398 |
Small molecule inhibitor |
FGFR1,2,3 | Ph I in solid tumor patients with FGFR amplifications. |
|
| HEDGEHOG ANTAGONISTS | ||||||
| http://clinicaltrials.gov/ct2/results?term=Vismodegib+and+cancer&Search=Search | Genentech | Vismodegib NA GDC-0449 |
Small molecule inhibitor |
Smoothened receptor in the sonic hedgehog pathway |
Ph II study completed in SCLC. Currently no studies on going for NSCLC. |
Dysgeusia, hyponatremia, fatigue, muscle spasms |
| http://clinicaltrials.gov/ct2/results?term=LDE+225+and+cancer&Search=Search | Novartis | NA NA LDE 225 |
Small molecule inhibitor |
Smoothened receptor in the hedgehog pathway |
Ph II Ph I for lung cancer |
Fatigue, nausea, anorexia, muscle cramps, dysgeusia |
| http://clinicaltrials.gov/ct2/results?term=IPI-926+and+cancer&Search=Search | Infinity | NA NA IPI-926 |
Small molecule inhibitor |
Smoothened receptor in the hedgehog pathway |
Ph II Ph I for solid tumors including lung cancer.(completed) |
Fatigue, nausea, transaminitis |
| http://clinicaltrials.gov/ct2/results?term=PF04449913+cancer&Search=Search | Pfizer | NA NA PF04449913 |
Small molecule inhibitor |
Smoothened receptor in the hedgehog pathway |
Ph I | |
| http://clinicaltrials.gov/ct2/results?term=LY2940680+cancer&Search=Search | Eli Lilly | NA NA LY2940680 |
Small molecule inhibitor |
Smoothened receptor in the hedgehog pathway |
Ph I SCLC | |
| HORMONE THERAPY | ||||||
| http://clinicaltrials.gov/ct2/results?term=Fulvestrant+lung+cancer&Search=Search | AstraZeneca | Fulvestrant Faslodex ICI 182780 |
Blocks estrogen activity through receptor |
Estrogen Receptor (ER) |
FDA approved for hormone receptor positive breast cancer Ph II for post- menopausal women with NSCLC |
Hot flashes, injection site reaction, headache, gastrointestinal disturbances, back pain |
| http://clinicaltrials.gov/ct2/results?term=Anastrazole+and+lung+cancer&Search=Search | AstraZeneca | Anastrazole Arimidex |
Decreases estrogen in post-menopausal women |
Aromatase inhibitor |
FDA approved for hormone receptor positive breast cancer Ph II for post- menopausal women with NSCLC has been terminated. |
Hot flashes, joint disorders, osteoporosis, nausea, mood changes, hypertension |
| http://clinicaltrials.gov/ct2/results?term=enobosarm+AND+cancer | GTx Inc | Enobosarm Osterine GTx-024 |
A selective androgen receptor modulator (SARM) |
aryl propionamides |
Phase III Currently no studies on going for lung cancer |
hair growth /virilization, prostatic hyperplasia, elevated red blood cell counts, decrease in HDL cholesterol, liver function abnormalities |
| HYPOXIA ACTIVATED DRUGS | ||||||
| http://clinicaltrials.gov/ct2/results?term=TH-302+AND+CANCER&Search=Search | Threshold Pharmaceuticals |
NA NA TH-302 |
Tumor selective hypoxia activated prodrug |
2- nitroimidazole moiety is triggered by hypoxic conditions to release DNA- alkylating dibromo isophosphoram ide mustard |
Ph II Ph I for NSCLC. Currently not recruiting patients. |
Skin lesions, mucositis, fatigue, nausea, |
| http://clinicaltrials.gov/ct2/results?term=PR-104+cancer&Search=Search | Proacta | NA NA PR-104 |
Tumor selective hypoxia activated pre-prodrug |
Converted to a pro-drug which is reduced under hypoxic conditions to a hydroxylamine metabolite, PR-104H, which is a cytotoxic nitrogen mustard alkylating agent. |
Ph II (Ph II in NSCLC & SCLC terminated) |
Cytopenias, nausea, vomiting, fatigue |
| HYPOXIA INDUCIBLE FACTOR-1α INHIBITORS | ||||||
| http://clinicaltrials.gov/ct2/results?term=PX-478+AND+CANCER&Search=Search | Oncothyreon | PX-478 | Small molecule inhibitor |
HIF-1α | Ph I | Anemia, fatigue, nausea, elevated AST/ALT |
| http://clinicaltrials.gov/ct2/results?term=EZN-2968+AND+CANCER&Search=Search | Eon Pharmaceuticals |
EZN-2968 | Hypoxia-inducible factor-1α (HIF-1α) messenger ribonucleic acid (mRNA) antagonist |
HIF-1α | Ph I in solid tumors have been completed. |
Vomiting, fatigue |
| IMMUNOMODULATORY AGENTS | ||||||
| http://clinicaltrials.gov/ct2/results?term=Lenalidomide+lung+cancer&Search=Search | Celgene | Lenalidomide Revlimid CC-5013 |
Immunomodul atory, anti- inflammatory, antiangiogenic |
FDA approved for -multiple myeloma, -myelodysplastic syndrome. Ph II completed for NSCLC |
Myelosuppression, rash, thrombosis |
|
| http://clinicaltrials.gov/ct2/results?term=Thalidomide+lung+cancer&Search=Search | Celgene | Thalidomide Thalomid |
Immunomodul atory, anti- inflammatory, antiangiogenic |
FDA approved for - multiple myeloma Ph II for NSCLC. Currently no active trials. |
Somnolence, peripheral neuropathy, dizziness, neutropenia, thrombosis, rash |
|
| IMMUNOMODULATORY ANTIBODIES | ||||||
| http://clinicaltrials.gov/ct2/results?term=Ipilimumab+and+lung+cancer&Search=Search | Bristol-Myers Squibb |
Ipilimumab Yervoy MDX 010 |
IgG1 Human MoAb | Cytotoxic T- lymphocyte antigen-4 (CTLA-4) |
FDA approved -metastatic melanoma Ph III trials ongoing in squamous NSCLC and phase II in SCLC. |
Rash, diarrhea (autoimmune colitis), hypothyroidism, hypophisitis, hepatitis |
| http://clinicaltrials.gov/ct2/results?term=tremelimumab+AND+cancer | MedImmune | Tremelimumab/Ticilim umab NA CP675,206 |
IgG2 monoclonal antibody | Cytotoxic T- lymphocyte antigen-4 (CTLA-4) |
Ph II (Malignant Mesothelioma). Phase I in lung cancer. |
|
| http://clinicaltrials.gov/ct2/results?term=BMS-936559&Search=Search | Bristol-Meyers Squibb |
NA BMS-936559 MDX- 1105 |
Human IgG4 MoAb | Inhibitor of programmed death-1 Ligand (PD-L1) |
Ph I completed. Currently not accruing patients. |
Rash, diarrhea, fatigue, hypothyroidism, hypophisitis, hepatitis |
| http://clinicaltrials.gov/ct2/results?term=BMS936558+and+cancer&Search=Search | Bristol-Meyers Squibb |
Nivolumab NA MDX- 1106/ BMS936558/ ONO4538 |
Human IgG4 MoAb | Inhibitor of programmed death-1 (PD1) A receptor expressed on activated T cells, and may suppress antitumor immunity |
Ph III in NSCLC | Rash, lymphopeni, arthralgia, myalgia |
| http://clinicaltrials.gov/ct2/results?term=AMP-224+cancer&Search=Search | GSK/ Amplimmune |
NA NA AMP-224 |
Fc-fusion protein | Targets PD- L2, which binds to PD-1 |
Ph 1 completed. | |
| http://clinicaltrials.gov/ct2/results?term=BMS-663513+cancer&Search=Search | Bristol-Myers Squibb |
Urelumab NA BMS-663513 |
Humanized MoAb | Agonist of CD-137, aTNF receptor |
Phase I in solid tumors | Neutropenia, elevated liver enzymes, rash, pruritus, diarrhea |
| INHIBITOR OF APOPTOSIS PROTEINS (IAPs) ANTAGONIST | ||||||
| http://clinicaltrials.gov/ct2/results?term=HGS+1029+and+cancer&Search=Search | Human Genome Sciences/ Aegera |
NA NA HGS 1029/ AEG 40826 |
Small molecule Smac mimetic |
IAPs antagonist |
Ph I completed | Nausea, anorexia, diarrhea, fatirue, elevated amylase and lipase, supra- ventricular tachycardia |
| http://clinicaltrials.gov/ct2/results?term=TL32711+and+cancer&Search=Search | Tetralogic | Birinapant NA TL32711 |
Small molecule Smac mimetic |
IAPs antagonist |
Ph I/II. Phase I completed. Currently no ongoing studies for lung cancer |
|
| http://clinicaltrials.gov/ct2/results?term=AT-406+and+cancer&Search=Search | Ascenta Therapeutics |
NA NA AT-406 |
Small molecule Smac (second mitochondria-derived activator of caspases) mimetic |
Multi-IAP antagonist (XIAP, c- IAP1, c-IAP2, and ML-IAP) |
Ph I | |
| http://clinicaltrials.gov/ct2/results?term=GDC0917+and+cancer&Search=Search | Genentech | NA NA GDC0917 |
Small molecule peptide Smac mimetic |
IAPs antagonist |
Ph I completed | |
| http://clinicaltrials.gov/ct2/results?term=LCL161+and+cancer&Search=Search | Novartis | NA NA LCL161 |
Small molecule Smac mimetic |
IAPs antagonist |
Ph II Phase I completed in lung cancer. |
|
| INSULIN GROWTH FACTOR-1 RECEPTOR (IGF-1R) INHIBITOR | ||||||
| http://clinicaltrials.gov/ct2/results?term=Figitumumab&Search=Search | Pfizer | Figitumumab CP-751871 |
IgG2 type human MoAb |
IGF-1R | Ph III terminated (further development halted |
Cardiac toxicity, hyperglycemia, asthenia, anorexia, pneumonia, dehydration, early death. |
| http://clinicaltrials.gov/ct2/results?term=OSI-906&Search=Search | OSI Pharmaceuticals/ Astellas |
Linsitinib NA OSI-906/ASP-7487 |
Small molecule inhibitor |
IGF-1R and insulin receptor (IR) |
Ph II completed in NSCLC Not accruing patients |
Nausea, vomiting, fatigue, hyperglycemia, elevated liver enzymes |
| http://clinicaltrials.gov/ct2/results?term=AMG479+cancer&Search=Search | Amgen | NA NA AMG-479 |
IgG1 type human MoAb |
IGF-1R | Ph III Ph I/II for lung was terminated) |
Thrombocytopeni a, neutropenia, hyperglycemia, fatigue, rash, elevated LFT’s, asymptomatic TSH increase |
| http://clinicaltrials.gov/ct2/results?term=Cixutumumab+and+cancer&Search=Search | ImClone | Cixutumumab NA IMC-A12 |
IgG1 type human MoAb |
IGF-1R | Ph III completed. Development has been discontinued. |
Pruritus, rash, anemia, hyperglycemia, infusion-related reaction |
| http://clinicaltrials.gov/ct2/results?term=Dalotuzumab+and+lung+cancer&Search=Search | Merck | Dalotuzumab NA MK-0646 |
IgG1 type humanized MoAb |
IGF-1R | Ph II completed. Currently not recruiting patients. |
Fatigue, hyperglycemia, nausea, constipation, diarrhea |
| http://clinicaltrials.gov/ct2/results?term=BIIB022+cancer&Search=Search | Biogen Idec | BIIB022 | Human nonglycosylated IgG4 MoAb |
IGF-1R | Ph II Ph I for NSCLC |
Hypertension, fatigue, dyspnea, QTc prolongation |
| http://clinicaltrials.gov/ct2/results?term=BMS-754807&Search=Search | Bristol-Myers Squibb |
NA NA BMS-754807 |
Small molecule reversible inhibitor |
IGF-1R and IR | Ph I completed. Currently not recruiting patients. |
Fatigue, hyperglycemia |
| http://clinicaltrials.gov/ct2/results?term=AVE1642+cancer&Search=Search | Sanofi-Aventis | NA NA AVE1642 |
Humanized MoAb | IGF-1R | Ph I/II (No active trials. Company discontinued development) |
Hyperglycemia, asthenia, hypersensitivity |
| http://clinicaltrials.gov/ct2/results?term=R1507&Search=Search | Genmab & Roche | Robatumumab NA R1507 |
IgG1 type human MoAb |
IGF-1R | Ph II (Development is halted) |
Fatigue, anorexia, weight loss |
| INTEGRINS | ||||||
| http://clinicaltrials.gov/ct2/results?term=EMD121974+cancer&Search=Search | Merck Serono | Cilengitide NA EMD121974 |
Cyclic peptide | αvβ3 and αv β5 integrin |
Ph III Ph I/II for NSCLC |
Lymphopenia, thrombocytopenia, neutropenia, fatigue, nausea, anorexia |
| http://clinicaltrials.gov/ct2/results?term=Volociximab+cancer&Search=Search | PDL BioPharma & Biogen Idec |
Volociximab NA M200 |
Chimeric MoAb | α 5 β 1 integrin |
Ph II | Fatigue, nausea, constipation, diarrhea, arthralgia |
| http://clinicaltrials.gov/ct2/results?term=PF-04605412+cancer&Search=Search | Pfizer | NA NA PF-04605412 |
Human IgG1 MOAb | α 5 β 1 integrin |
Ph I terminated. Currently there are no on going studies. |
|
| http://clinicaltrials.gov/ct2/results?term=Vitaxin+cancer&Search=Search | MedImmune | Vitaxin NA MEDI-522 |
Humanized IgG1 MoAb |
α 5 β 1 integrin |
Ph I completed. | Chills, fever, nausea |
| MAMMALIAN TARGET OF RAPAMYCIN (mTOR) INHIBITORS | ||||||
| http://clinicaltrials.gov/ct2/results?term=Temsirolimus+lung+cancer&Search=Search | Pfizer (Wyeth) | Temsirolimus Torisel CCI-779 |
Ester analog of rapamycin |
mTORC1 | FDA approved -advanced renal cell carcinoma Ph II for NSCLC |
Fatigue, rash, asthenia, hyperglycemia, hyperlipemia, hypophosphatemia, myelosuppression, nausea, diarrhea |
| http://clinicaltrials.gov/ct2/results?term=Everolimus+lung+cancer&Search=Search | Novartis | Everolimus Afinitor RAD001 |
Derivative of the natural macrocyclic lactone sirolimus |
mTORC1 | FDA approved -advanced renal cell carcinoma Ph II for NSCLC |
Stomatitis, asthenia, pneumonitis, fatigue, infections, diarrhea, neutropenia |
| http://clinicaltrials.gov/ct2/results?term=Ridaforolimus+lung+cancer&Search=Search | Merck/Ariad | Ridaforolimus Taltorvic AP-23573 |
Small molecule serine/threonine kinase inhibitor |
mTOR | Ph II for NSCLC terminated. |
Fatigue, anorexia, mucositis |
| http://clinicaltrials.gov/ct2/results?term=Sirolimus+and+lung+cancer&Search=Search | Generic drug with multiple manufactures |
Sirolimus Rapamune Rapamycin |
A macrolide derived from Streptomyces hygroscopicus |
mTORC1 | Ph I/II | Cytopenias, hypoalbuminemia, hyperglycemia, hypercholesterole mia, hypertriglyceride mia |
| http://clinicaltrials.gov/ct2/results?term=AZD8055+cancer&Search=Search | AstraZeneca | NA NA AZD 8055 |
ATP competitive small molecule inhibitor |
mTOR C1,2 | Ph I/II completed. | Transaminitis, |
| http://clinicaltrials.gov/ct2/results?term=OSI+027+cancer&Search=Search | OSI Pharmaceuticals |
NA NA OSI 027 |
Small molecule inhibitor |
mTORC1,2 | Ph II completed. | |
| http://clinicaltrials.gov/ct2/results?term=BEZ+235+and+cancer&Search=Search | Novartis | NA NA BEZ 235 |
Small molecule inhibitor |
PI3K/ mTORC1 |
Ph I/II | |
| c-MET / HEPATOCYTE GROWTH FACTOR RECEPTOR (HGFR) PATHWAY INHIBITORS | ||||||
| http://clinicaltrials.gov/ct2/results?term=Tivantinib+lung+cancer&Search=Search | ArQule | Tivantinib NA ARQ197 |
Small molecule inhibitor |
c-MET/ HGFR | Ph III discontinued. Did not meet its primary endpoint. |
Hepatotoxicity |
| http://clinicaltrials.gov/ct2/results?term=Onartuzumab+lung+cancer&Search=Search | Roche/Genentech | Onartuzumab NA MetMab/RG3638 |
Humanized monovalent MoAb |
c-MET | Ph III in met positive patients discontinued for lack of benefit. |
Peripheral edema |
| http://clinicaltrials.gov/ct2/results?term=Rilotumumab+cancer&Search=Search | Amgen | Rilotumumab NA AMG 102 |
Human IgG2 MoAb | HGF (ligand) | Phase III in Gastric cancer and gastro- esophageal junction malignancies. Ph I/II |
Fatigue, constipation, anorexia, nausea, dyspnea |
| http://clinicaltrials.gov/ct2/results?term=Ficlatuzumab+and+cancer&Search=Search | AVEO Pharmaceuticals |
Ficlatuzumab NA AV-299/SCH900105 |
Humanized IgG1 MoAb |
HGF (ligand) | Randomized Ph II | Fatigue, peripheral edema, headache, diarrhea |
| http://clinicaltrials.gov/ct2/results?term=Amuvatinib+cancer&Search=Search | Astex Pharmaceuticals// |
Amuvatinib NA MP-470 |
Small molecule inhibitor |
KIT, c-MET, RET, PDGFR, FLT3 |
Ph II (SCLC). Currently not recruiting patients. |
|
| http://clinicaltrials.gov/ct2/results?term=MGCD265+and+cancer&Search=Search | MethylGene | NA NA MGCD265 |
Small molecule inhibitor |
c-MET, VEGFR1,2,3, Ron, Tie-2 |
Ph I/II | |
| http://clinicaltrials.gov/ct2/results?term=MK2461+and+cancer&Search=Search | Merck | NA NA MK2461 |
Small molecule inhibitor |
c-MET | Ph I/II completed | |
| http://clinicaltrials.gov/ct2/results?term=B MS777607+and+canc er&Search=Search | Bristol-Myers Squibb |
NA NA BMS777607 |
Small molecule inhibitor |
c-MET | Ph I completed. | |
| http://clinicaltrials.gov/ct2/results?term=INCB28060&Search=Search | Incyte | NA NA INCB28060 |
Small molecule inhibitor |
c-MET | Ph I completed | |
| http://clinicaltrials.gov/ct2/results?term=AMG+208&Search=Search | Amgen | NA NA AMG 208 |
Small molecule inhibitor |
c-MET | Ph I not recruiting patients |
|
| http://clinicaltrials.gov/ct2/results?term=LY2875358&Search=Search | Eli Lilly | NA NA LY2875358 |
Humanized IgG4 MoAb |
c-MET | Ph II | |
| http://clinicaltrials.gov/ct2/results?term=PF-4217903&Search=Search | Pfizer | NA NA PF-4217903 |
Small molecule inhibitor |
c-MET/ HGFR | Ph I (Terminated) | |
| http://clinicaltrials.gov/ct2/results?term=JNJ38877605&Search=Search | Johnson & Johnson |
NA NA JNJ38877605 |
Small molecule inhibitor |
c-MET | Ph I (Terminated) | Elevation in Creatinine |
| http://clinicaltrials.gov/ct2/results?term=JNJ38877605&Search=Search | SGX Pharmaceuticals |
NA NA |
Selective small molecule inhibitor |
Met | Ph I (Terminated) | Nephrotoxicity, fatigue, pyrexia, nausea, vomiting |
| MITOGEN ACTIVATED PROTEIN/EXTRACELLULAR SIGNAL-REGULATED KINASE (MEK) INHIBITORS | ||||||
| http://clinicaltrials.gov/ct2/results?term=Trametinib&Search=Search | GlaxoSmithKline | Trametinib Mekinist GSK1120212 |
Allosteric small molecule inhibitor |
MEK 1/2 | FDA approved in Melanoma in combination with dabrafenib. Phase II in lung cancer. |
Rash, diarrhea, central serous retinopathy |
| http://clinicaltrials.gov/ct2/results?term=Selumetinib+lung+cancer&Search=Search | AstraZeneca/ Array BioPharma |
Selumetinib NA AZD6244/ ARRY142880 |
Allosteric inhibitor | MEK 1 & 2 | Phase III Ph II completed in NSCLC (Ph I ongoing in KRAS & BRAF mutants) |
Rash, diarrhea, nausea, emesis |
| http://clinicaltrials.gov/ct2/results?term=AS+703026&Search=Search | Merck KGaA/ EMD Serono |
Pimasertib NA AS 703026/ MSC1936369B |
Non-competitive small molecule inhibitor |
MEK 1/2 | Ph I/II | Asthenia, diarrhea, constipation, rash, nausea, vomiting |
| http://clinicaltrials.gov/ct2/results?term=RDEA119%2F+&Search=Search | Ardea Biosciences | NA NA RDEA119/ BAY 869766 |
Allosteric inhibitor | MEK 1/2 | Ph I/II | Rash, diarrhea, nausea, vomiting, fatigue, and peripheral edema |
| http://clinicaltrials.gov/ct2/results?term=MEK162&Search=Search | Novartis | NA NA MEK162 |
Small molecule inhibitor |
MEK | Phase II Phase I in Lung Cancer |
|
| http://clinicaltrials.gov/ct2/results?term=PD325901&Search=Search | Pfizer | NA NA PD325901 |
Small molecule inhibitor |
MEK 1/2 | Ph I completed | Ocular toxicity, neurological toxicity |
| http://clinicaltrials.gov/ct2/results?term=AZD8330&Search=Search | AstraZeneca/ Array BioPharma |
NA NA AZD8330 |
Small molecule inhibitor |
MEK 1 | Ph I completed | |
| http://clinicaltrials.gov/ct2/results?term=GDC-0973&Search=Search | Genentech | Cobemetinib NA GDC-0973/ XL518 |
Small molecule inhibitor |
MEK 1 | Ph III Ph II lung cancer |
|
| INHIBITORS OF MITOSIS | ||||||
| Aurora Kinase Inhibitors | ||||||
| http://clinicaltrials.gov/ct2/results?term=AZD+1152&Search=Search | AstraZeneca | NA NA AZD 1152 |
Small molecule inhibitor |
Aurora B | Ph II/III (heme focus) Ph I in solid tumors terminated |
Neutropenia |
| http://clinicaltrials.gov/ct2/results?term=MLN+8237&Search=Search | Millennium Pharmaceuticals (Takeda Oncology) |
Alisertib NA MLN 8237 |
Small molecule serine/threonine protein kinase inhibitor |
Aurora A | Ph I/II | Myelosuppression, mucositis, nausea, fatigue |
| http://clinicaltrials.gov/ct2/results?term=AT9283&Search=Search | Astex | NA NA AT9283 |
Multi-targeted kinase inhibitor |
Aurora A and B, JAK 2 and 3 Tyk2, RSK2, Ret, Mer, Yes and GSK3 beta |
Ph II (multiple myeloma) Ph I (completed) |
Neutropenia |
| http://clinicaltrials.gov/ct2/results?term=Danusertib&Search=Search | Nerviano | Danusertib NA PHA739358 |
Small-molecule 3- aminopyrazole inhibitor |
Pan-aurora (aurora B dominant) |
Ph II (prostate and heme) |
|
| http://clinicaltrials.gov/ct2/results?term=ENMD-2076&Search=Search | Entremed | NA NA ENMD-2076 |
Multi-targeted kinase inhibitor |
Aurora kinase A, VEGFR, Flt-3, FGFR3 |
Ph II (ovarian) Ph I (multiple myeloma) |
|
| http://clinicaltrials.gov/ct2/results?term=MK5108&Search=Search | Merck | NA NA MK5108 |
Small molecule inhibitor |
Aurora A kinase |
Ph I (completed) | Cytopenias |
| http://clinicaltrials.gov/ct2/results?term=GSK1070916&Search=Search | GlaxoSmithKline | NA NA GSK1070916 |
ATP competitive inhibitor |
Aurora kinase B & C |
Ph I (completed) | |
| http://clinicaltrials.gov/ct2/results?term=AS703569&Search=Search | Merck KGaA/ EMD Serono |
NA NA AS703569 |
Multi-targeted kinase inhibitor |
Aurora kinase- A & B, KIT, BTK, LYN, ABL, Akt, and Flt-3 |
Ph I (completed) | |
| http://clinicaltrials.gov/ct2/results?term=PF-03814735&Search=Search | Pfizer | NA NA PF-03814735 |
ATP-competitive, reversible inhibitor |
Aurora A & B | Ph I (completed) | Dairrhea, nausea, anorexia |
| http://clinicaltrials.gov/ct2/results?term=SNS-314&Search=Search | Sunesis | NA NA SNS-314 |
ATP competitive selective inhibitor |
Pan-aurora kinase inhibitor (A, B & C) |
Ph I (completed) | Nausea, fatigue, constipation |
| http://clinicaltrials.gov/ct2/results?term=AMG900&Search=Search | Amgen | NA NA AMG900 |
Small molecule inhibitor |
Pan-aurora kinase (A, B and C) |
Ph I | |
| http://clinicaltrials.gov/ct2/results?term=TAK901&Search=Search | Millennium Pharmaceuticals (Takeda Oncology) |
NA NA TAK901 |
Small molecule inhibitor |
Aurora kinase A |
Ph I (completed) | |
| http://clinicaltrials.gov/ct2/results?term=MK0457&Search=Search | Merck | Tozasertib NA MK0457 |
Small molecule serine/threonine protein kinase inhibitor |
Aurora kinase family | Ph II (halted due to cardiac risk) | Neutropenia, nausea, mucositis |
| http://clinicaltrials.gov/ct2/results?term=CYC116&Search=Search | Cyclacel | NA NA CYC116 |
Small molecule inhibitor |
Pan-aurora | Ph I (terminated) | |
| Checkpoint Kinase (Chk) Inhibitor | ||||||
| http://clinicaltrials.gov/ct2/results?term=LY2603618&Search=Search | Eli Lilly / Array BioPharma |
NA NA LY2603618 |
Inhibitor of Chk, potentiating DNA targeting therapies |
Chk 1 | Ph II (not recruiting patients) |
|
| http://clinicaltrials.gov/ct2/results?term=AZD7762&Search=Search | AstraZeneca | NA NA AZD7762 |
ATP-competitive inhibitor of Chk, potentiating DNA targeting therapies |
Chk1/2 | Ph I (completed) | |
| http://clinicaltrials.gov/ct2/results?term=SCH900776&Search=Search | Schering-Plough | NA NA SCH900776 |
Inhibitor of Chk, potentiating DNA targeting therapies |
Chk 1 | Ph II in liquid tumors. Phase I completed in solid tumors. |
|
| Kinesin Protein Inhibitors | ||||||
| http://clinicaltrials.gov/ct2/results?term=SB-715992&Search=Search | GlaxoSmithKline | Ispinesib NA SB-715992 |
KSP inhibitor | Mitotic kinesin spindle protein Eg5 |
Ph II | Neutropenia, fatigue, anemia, elevated creatinine, lymphopenia, hyperglycemia (no neuropathy like Taxanes) |
| http://clinicaltrials.gov/ct2/results?term=LY-2523355&Search=Search | Eli Lilly/ Kyowa Hakko Kirin |
NA NA LY-2523355 |
Allosteric inhibitor of Eg5 |
Mitotic kinesin spindle protein Eg5 |
Ph II (NSCLC) (completed) |
Neutropenia, fatigue, nausea, rash |
| http://clinicaltrials.gov/ct2/results?term=SB-743921&Search=Search | GlaxoSmithKline | NA NA SB-743921 |
KSP inhibitor | Mitotic kinesin spindle protein Eg5 |
Ph I (completed) | Neutropenia, hypophosphatemia, transaminitis |
| http://clinicaltrials.gov/ct2/results?term=GSK-923295&Search=Search | GlaxoSmithKline | NA NA GSK-923295 |
KSP inhibitor | Centromere- linked kinesin- like motor protein CENP- E (kinesin 7) |
Ph I (completed) | Fatigue, vomiting, hyponatemia, transaminase elevation |
| http://clinicaltrials.gov/ct2/results?term=ARRY-520&Search=Search | Array BioPharma | NA NA ARRY-520 |
KSP inhibitor | Mitotic kinesin spindle protein Eg5 |
Ph I | Cytopenias, nausea, vomiting, fatigue, hyponatremia |
| http://clinicaltrials.gov/ct2/results?term=MK0731&Search=Search | Merck | NA NA MK0731 |
KSP inhibitor | Mitotic kinesin spindle protein Eg5 |
Ph I (completed) | Neutropenia, diarrhea, nausea, mucositis, anorexia |
| http://clinicaltrials.gov/ct2/results?term=ARQ-621&Search=Search | ArQule | NA NA ARQ-621 |
Allosteric inhibitor of Eg5 |
Mitotic kinesin spindle protein Eg5 |
Ph I (completed) | |
| http://clinicaltrials.gov/ct2/results?term=AZD4877&Search=Search | AstraZeneca | NA NA AZD4877 |
KSP inhibitor | Mitotic kinesin spindle protein Eg5 |
Ph II (discontinued from development) |
Neutropenia |
| Polo-Like Kinase Inhibitor | ||||||
| http://clinicaltrials.gov/ct2/results?term=BI-2536&Search=Search | Boehringer Ingelheim |
NA NA BI-2536 |
ATP competitive small molecule inhibitor |
Plk1 (serine threonine kinase) |
Ph II (completed in SCLC) |
Neutropenia, fatigue, nausea |
| http://clinicaltrials.gov/ct2/results?term=BI-6727&Search=Search | Boehringer Ingelheim |
Volasertib NA BI-6727 |
Dihydropteridinone derivative (binds to ATP-binding pocket) |
Plk1 (serine threonine kinase) |
Ph II (completed) | Anemia, neutropenia, thrombocytopenia, fatigue |
| http://clinicaltrials.gov/ct2/results?term=GSK461364&Search=Search | GlaxoSmithKline | NA NA GSK461364 |
ATP competitive inhibitor |
Plk1 (serine threonine kinase) |
Ph I (completed) | Fatigue, anemia, abdominal pain |
| http://clinicaltrials.gov/ct2/results?term=NMS1286937&Search=Search | Nerviano Medical Sciences |
NA NA NMS1286937 |
Small molecule inhibitor |
Plk1 (serine threonine kinase) |
Ph I (completed) | |
| NOTCH PATHWAY INHIBITORS | ||||||
| http://clinicaltrials.gov/ct2/results?term=RO+4929097&Search=Search | Roche | NA NA RO 4929097 |
Gamma secretase inhibitor |
γ secretase inhibitor (Pan- notch) |
Ph II | Asthenia, nausea, diarrhea, hypophosphatemia, pruritus. |
| http://clinicaltrials.gov/ct2/results?term=MK0752&Search=Search | Merck | NA NA MK0752 |
Gamma secretase inhibitor |
γ secretase inhibitor (Pan- notch |
Ph I/II Ph I in lung cancer |
Abdominal cramps, diarrhea, nausea, fatigue |
| http://clinicaltrials.gov/ct2/results?term=PF03084014&Search=Search | Pfizer | NA NA PF03084014 |
Gamma secretase inhibitor |
γ secretase inhibitor (Pan- notch) |
Ph I (completed) | Gastrointestinal toxicity (reduced with steroids) |
| http://clinicaltrials.gov/ct2/results?term=REGN421&Search=Search | Regeneron | NA NA REGN421 |
Human MoAb | Delta-4 ligand | Ph I | |
| OSTEOCLAST FUNCTION MODIFIERS | ||||||
| http://clinicaltrials.gov/ct2/results?term=zoledronate+and+lung+cancer&Search=Search | Novartis | Zoledronate Zometa NA |
Farnesyl pyrophosphate |
Osteoclast inhibitor |
FDA approved for -osteoporosis -cancer related bone metastases (multiple myeloma and solid tumors) |
Hypocalcemia, osteonecrosis of jaw, renal toxicity |
| http://clinicaltrials.gov/ct2/results?term=Denosumab+lung+cancer&Search=Search | Amgen | Denosumab Xgeva/ Prolia AMG-162 |
Fully human monoclonal antibody |
RANK ligand inhibitor (transmembrane protein important for osteoclast activity and survival) |
FDA approved for -osteoporosis -prevention of skeletal- related events (SREs) in patients with bone metastases from solid tumors |
Hypocalcemia, osteonecrosis of jaw, serious infections, skin reactions |
| http://clinicaltrials.gov/ct2/results?term=etidronate+AND+cancer | Procter & Gamble Pharmaceuticals |
Etidronate Didronel |
Bisphosphonate-ATP requiring small molecule |
Osteoclast inhibitor |
FDA approved for symptomatic Paget’s disease, hypercalcemia from cancer Ph III for bone metastasis |
Esophagitis, arthralgias, hypersensitivity reactions |
| http://clinicaltrials.gov/ct2/results?term=Alendronate+cancer&Search=Search | Novartis | Alendronate Fosamax |
Nitrogen containing bisphosphonate targets farnesyl pyrophosphate synthase |
Osteoclast inhibitor |
FDA approved for osteoporosis Ph III for bone metastasis |
Esophagitis, osteonecrosis of jaw, delayed healing, hypersensitivity reaction, bone/muscle pains |
| http://clinicaltrials.gov/ct2/results?term=MK-0822+and+cancer&Search=Search | Merck | Odanacatib NA MK-0822 |
Anti-cathepsin K, (decreases bone resorption) |
Cathepsin K (osteoclast specific enzyme) |
Ph III for osteoporosis No open cancer specific trials |
|
| http://clinicaltrials.gov/ct2/results?term=AAE-581&Search=Search | Novartis | Balicatib NA AAE-581 |
Anti-cathepsin K | Ph II for osteoporosis (completed) No open cancer trials |
||
| PI-3K/AKT INHIBITORS | ||||||
| AKT Inhibitors | ||||||
| http://clinicaltrials.gov/ct2/results?term=MK2206+lung+&Search=Search | Merck | NA NA MK2206 |
Non-ATP competitive allosteric Akt |
Akt | Ph II | Rash, mucositis, gastrointestinal toxicity |
| http://clinicaltrials.gov/ct2/results?term=AG+1343+lung&Search=Search | Agouron Pharmaceuticals |
Nelfinavir Viracept AG 1343 |
Protease inhibitor | Akt | Ph II in combination radiotherapy trials. |
Diarrhea, rash, fatigue, leukopenia |
| http://clinicaltrials.gov/ct2/results?term=perifosine+lung&Search=Search | Keryx/ AOI Pharmaceuticals |
Perifosine NA KRX-0401 |
Alkylphospholipid | Akt | Ph I/II (NSCLC trial suspended) |
Nausea, vomiting, diarrhea, fatigue |
| Phosphatidylinositol-3-kinase (PI-3K) Inhibitors | ||||||
| http://clinicaltrials.gov/ct2/results?term=BEZ235&Search=Search | Novartis | Dactolisib NA BEZ235 |
Small molecule inhibitor |
PI-3K (pan- class 1), mTOR complexes 1/2 |
Ph I/II | Nausea, emesis, diarrhea, fatigue, anemia |
| http://clinicaltrials.gov/ct2/results?term=BKM120+lung+cancer&Search=Search | Novartis | Buparlisib NA BKM120 |
Small molecule ATP competitive inhibitor |
PI-3K (pan- class 1) |
Ph II | Rash, hyperglycemia, altered mood, pruritus |
| http://clinicaltrials.gov/ct2/results?term=Rigosertib+lung+cancer&Search=Search | Onconova Therapeutics |
Rigosertib Estybon ON01910 |
Non-ATP competitive small molecule inhibitor |
PI-3K, inhibitor Downregulates Cyclin D1, induces NOXA, BIM and JNK |
Ph III Phase II in squamous tumors including Lung Cancer |
Fatigue, anorexia |
| http://clinicaltrials.gov/ct2/results?term=XL147&Search=Search | Exelixis | NA NA XL147 |
Small molecule inhibitor |
PI-3K (class 1 isoforms) |
Ph I completed | Rash, arterial thrombosis, transaminitis, hyperglycemia |
| http://clinicaltrials.gov/ct2/results?term=BGT226&Search=Search | Novartis | NA NA BGT226 |
Dual small molecule inhibitor |
PI-3K and mTOR |
Ph I (completed) | |
| http://clinicaltrials.gov/ct2/results?term=PX-866&Search=Search | Oncothyreon | NA NA PX-866 |
Irreversible small molecule inhibitor (Wortmanin analog) |
PI-3K, lowers p-mTOR, p-S6 ribosomal protein |
Ph I/II | Diarrhea, nausea, vomiting |
| http://clinicaltrials.gov/ct2/results?term=GDC-0941&Search=Search | Genentech | Pictilisib NA GDC-0941 |
Small molecule inhibitor |
PI-3K (class 1 isoforms) |
Ph II Squamous NSCLC |
Nausea, fatigue, diarrhea, dysgeusia, headache, pleural effusion |
| http://clinicaltrials.gov/ct2/results?term=PF-04691502&Search=Search | Pfizer | NA NA PF-04691502 |
Dual molecule inhibitor |
PI-3K and mTOR |
Ph II Ph I for lung cancer |
|
| http://clinicaltrials.gov/ct2/results?term=XL765&Search=Search | Exelixis | NA NA XL765 |
Dual selective oral inhibitor |
PI-3K (Class 1) and mTOR |
Ph I (completed) | Nausea, diarrhea, transaminitis, rash, anorexia, fatigue |
| http://clinicaltrials.gov/ct2/results?term=BAY80-6946&Search=Search | Bayor | NA NA BAY80-6946 |
Highly selective reversible inhibitor |
Pan-class I PI- 3K |
Ph II Ph I (NSCLC) |
Fatigue, nausea, diarrhea, mucositis, dysgeusia, anemia |
| PLATELET DERIVED GROWTH FACTOR α (PDGFRα) INHIBITORS | ||||||
| http://clinicaltrials.gov/ct2/results?term=Ramucirumab&Search=Search | Imclone LLC | Ramucirumab NA IMC-3G3 |
Human IgG1 MoAb | PDGFRα | Ph II | Fatigue |
| http://clinicaltrials.gov/ct2/results?term=MEDI-575&Search=Search | MedImmune | NA NA MEDI-575 |
MoAb | PDGFRα | Ph II (currently not accruing patients) |
|
| POLY(ADP-RIBOSE) POLYMERASE (PARP) INHIBITORS | ||||||
| http://clinicaltrials.gov/ct2/results?term=BSI-201&Search=Search | Sanofi-Aventis (BiPar Sciences) |
Iniparib NA BSI-201 |
Small lipophilic molecule inhibitor |
PARP-1 | Ph III (squamous) (ECLIPSE) (completed, no benefit noted and further development discontinued) |
Nausea, fatigue |
| http://clinicaltrials.gov/ct2/results?term=Rucaparib&Search=Search | Pfizer | Rucaparib NA AG014699/ PF- 01367338 |
Small molecule inhibitor |
PARP-1 | Ph II Ph I for NSCLC |
Fatigue, thrombocytopenia, hypo- phosphatemia, lymphopenia |
| http://clinicaltrials.gov/ct2/results?term=Veliparib&Search=Search | Abbott/AbbVie | Veliparib NA ABT 888 |
Small molecule inhibitor |
PARP-1, 2 | Ph II | Fatigue, neutropenia (with chemotherapy) |
| http://clinicaltrials.gov/ct2/show/NCT01788332?term=AZD2281&rank=29 | AstraZeneca | Olaparib NA AZD2281 |
Small molecule inhibitor |
PARP | Ph II | Nausea, fatigue, anemia |
| http://clinicaltrials.gov/ct2/results?term=MK4827&Search=Search | Merck | Niraparib NA MK4827 |
Small molecule inhibitor |
PARP -1. 2 | Ph I Phase III Breast cancer |
Fatigue, nausea, myelosuppression |
| http://clinicaltrials.gov/ct2/results?term=BMN673&Search=Search | Biomarin | NA NA BMN673 |
Small molecule inhibitor |
PARP -1. 2 | Phase III Breast Cancer Ph I solid tumors including SCLC |
|
| SMAC (Second mitochondria-derived activator of caspase) mimetics | ||||||
| http://clinicaltrials.gov/ct2/results?term=TL32711&Search=Search | Tetralogic | Birinapant NA TL32711 |
small molecule Smac mimetic |
antagonizes inhibitors of apoptosis proteins (IAPs) |
Ph I | |
| http://clinicaltrials.gov/ct2/results?term=AT-406&Search=Search | Ascenta Therapeutics |
NA NA AT-406 |
small molecule Smac mimetic | antagonizes inhibitors of apoptosis proteins (IAPs) |
Ph I | |
| SURVIVIN INHIBITORS | ||||||
| http://clinicaltrials.gov/ct2/results?term=LY2181308&Search=Search | Isis Pharmaceuticals & Eli Lilly |
NA NA LY2181308 |
Anti-sense oligonucleotide |
Blocks survivin |
Ph II | PTT prolongation, headache, lymphopenia, fever, fatigue, nausea |
| http://clinicaltrials.gov/ct2/results?term=YM155&Search=Search | Astellas | NA NA YM155 |
Small molecule inhibitor |
Suppresses survivin |
Ph II | Hypertension, neutropenia, fatigue, nausea, stomatitis, fever. |
| TELOMERASE INHIBITORS | ||||||
| http://clinicaltrials.gov/ct2/results?term=GRN163L&Search=Search | Geron Corporation |
Imetelstat NA GRN163L |
Competitive telomerase RNA template antagonist |
Telomerase | Ph I (completed) | PTT prolongation, gastrointestinal side effects, fatigue, anemia, GGT elevation, peripheral neuropathy |
| http://clinicaltrials.gov/ct2/results?term=KML001&Search=Search | University of Maryland |
Sodium metaarsenite NA KML001 |
Oral arsenic agent | Telomerase | Ph I | |
| TNF RELATED APOPTOSIS INDUCING LIGAND (TRAIL) RECEPTOR AGONISTS | ||||||
| http://clinicaltrials.gov/ct2/results?term=PRO95780&Search=Search | Genentech | Apomab / Drozitumab NA PRO95780 |
Human IgG1 MoAb | TNF related apoptosis inducing ligand receptor 2 (TRAIL- R2)/Death receptor 5 (DR5) |
Ph II (no active studies) | Neutropenia, elevated liver enzymes, supra- ventricular tachycardia, pulmonary embolism |
| http://clinicaltrials.gov/ct2/results?term=HGS-ETR1&Search=Search | Human Genome Sciences |
Mapatumumab NA HGS-ETR1 |
Human MoAb | TRAIL- R1/DR4 |
Ph I (completed) | Fatigue, nausea, hypotension, transaminitis. |
| http://clinicaltrials.gov/ct2/results?term=HGS-ETR2&Search=Search | Human Genome Sciences |
Lexatumumab NA HGS-ETR2 |
Human MoAb | TRAIL-R2/ DR5 |
Ph I completed in pediatric solid tumors. No currently active studies in Lung Cancer |
Fatigue, transaminitis. |
| http://clinicaltrials.gov/ct2/results?term=AMG-655+&Search=Search | Amgen | Conatumumab NA AMG-655 |
Human MoAb | TRAIL-R2/ DR5 |
Ph II. Open label extension study in multiple solid tumors currently ongoing. |
Fever, fatigue, gastrointestinal toxicity |
| http://clinicaltrials.gov/ct2/results?term=AMG951+&Search=Search | Amgen/ Genentech |
Dulanermin NA AMG951 (rhApo2L/TRAIL) |
Recombinant human MoAb |
DR4 and DR5 | Ph II (completed) | Arthralgia, myalgias, nausea, transaminitis |
| VASCULAR DISRUPTING AGENTS (VDA) | ||||||
| http://clinicaltrials.gov/ct2/results?term=ASA404&Search=Search | Antisoma & Novartis |
Vadimezan NA ASA404 |
Small molecule VDA | Induces tumor necrosis alpha (TNF-alpha), serotonin and nitric oxide |
Ph III (development halted as no benefit seen in NSCLC study) |
|
| http://clinicaltrials.gov/ct2/results?term=NGR-hTNF&Search=Search | MolMed | NA NA NGR-hTNF |
CNGRC peptide- TNF α conjugate (NGR-hTNF is a first-in-class compound based on the combination of a tumour homing peptide (NGR) with the human Tumour Necrosis Factor (hTNF) |
Membrane- bound metalloproteas e CD13 expressed on endothelial cells |
Ph III Mesothelioma, Phase II SCLC (completed), Phase II NSCLC (completed) |
Infusion related Chills |
| http://clinicaltrials.gov/ct2/results?term=BNC105P&Search=Search | Bionomics Limited |
NA NA BNC105P |
VDA | Inhibits tubulin polymerization and acts as a VDA |
Ph II (mesothelioma in Australia) Ph I (completed) |
Fatigue, rash, infusion reaction |
| http://clinicaltrials.gov/ct2/results?term=Combretastatin-A4+phosphate&Search=Search | OxiGENE | Combretastatin-A4 phosphate/ CA4P Zybrestat NA |
Small molecule VDA | Reversible tubulin depolymerizin g agent. Disrupts E- cadherin |
Ph II (completed) | Hypertension, reversible cardiac ischemia, pulmonary embolism |
| http://clinicaltrials.gov/ct2/results?term=NPI-2358&Search=Search | Nereus Pharmaceuticals |
Plinabulin NA NPI-2358 |
Small molecule VDA | Disrupts the endothelial tubulin cytoskeleton |
Ph I/II (completed) | Nausea, vomiting, diarrhea, fatigue, fever, tumor pain |
| http://clinicaltrials.gov/ct2/results?term=OXi4503&Search=Search | OXiGENE | Combretastatin A1 diphosphate NA OXi4503 |
Small molecule VDA | Tubulin- binding agent |
Ph I (completed) | Hypertension, tumor pain, fatigue, cytopenias, nausea |
| http://www.medicinova.com/html/research_cancer.html | MediciNova | NA NA MN-029 |
Binds reversibly to the colchicine- binding site on tubulin. |
Disrupts the endothelial tubulin cytoskeleton |
Ph I (completed) | Nausea, vomiting, hypotension, fatigue, diarrhea |
| http://clinicaltrials.gov/ct2/results?term=ZD6126&Search=Search | AstraZeneca | NA NA ZD6126 |
Small molecule VDA | Disrupts the endothelial tubulin cytoskeleton |
Ph I (terminated) | Anorexia, constipation, dyspnea, fatigue |
| VACCINES | ||||||
| http://clinicaltrials.gov/ct2/results?term=Belagenpumatucel-L&Search=Search | NovaRx | Belagenpumatucel-L Lucanix |
Transforming growth factor beta2 (TGF- beta2) antisense gene-modified allogeneic whole tumor cell vaccine |
Cytotoxic T lymphocyte (CTL) response against NSCLC cells |
Ph III (completed). Primary endpoint of Overall Survival was not met. In a predefined subset analyses, improved survival was noted in patients with Squamous Cell Carcinoma |
Mild reactions -local (pain, redness, swelling) -systemic (fever, fatigue, muscle pain) |
| http://clinicaltrials.gov/ct2/results?term=Stimuvax&Search=Search | Merck KGaA/ EMD Serono |
BLP25 Liposome vaccine Stimuvax |
Synthetic peptide derived from the mucin 1 (Muc-1) antigen |
Cytotoxic T lymphocyte (CTL) response against Muc-1- expressing tumor cells |
Ph III. No survival benefit noted with 75% of the planned patients events occurring. |
Cough, fatigue, dyspnea |
| http://clinicaltrials.gov/ct2/results?term=MAGE-A3&Search=Search | GlaxoSmithKline | NA NA GSK1572932A/ MAGE-A3 |
Antigen-Specific Cancer Immunotherapeutic |
Cytotoxic T lymphocyte (CTL) response against MAGE-A3 positive tumors |
Ph III (MAGRIT). Completed. Co-primary endpoints note met. No clear subset likely to benefit identified. |
Mild reactions -local (pain, redness, swelling) -systemic (fever, fatigue, muscle pain) |
| http://clinicaltrials.gov/ct2/results?term=GSK+249553&Search=Search | GlaxoSmithKline | Astuprotimut-R NA GSK 249553 |
Antigen-Specific Cancer Immunotherapeutic |
MAGE-3 positive tumors |
Ph III | Mild Grade 1 or 2 local or systemic reactions |
| http://clinicaltrials.gov/ct2/results?term=PANVAC-VF&Search=Search | Therion Biologics | PANVAC-VF NA NA |
Delivered through two viral vectors-- recombinant vaccinia and recombinant fowlpox—containing transgenes to Muc-1 and carcinoembryonic antigen |
EpthelialMuc- 1 and carcinoembryo nic antigen |
Ph III Ph I for lung cancer |
Injection site reaction |
| http://clinicaltrials.gov/ct2/results?term=Montanide+&Search=Search | Bioven Sdn. Bhd (bioven) |
Recombinant human EGF-rP64K/Montanide ISA 51 NA |
rEGF linked to the Neisseria meningitidis-derived recombinant immunogenic carrier protein P64k (rP64K) and mixed with immunoadjuvant Montanide ISA 51 |
Antibody- mediated inhibition of endogenous EGF binding to its receptor, epithelial growth factor receptor (EGFR |
Ph II. Also recruiting mesothelioma patients. |
|
| http://clinicaltrials.gov/ct2/results?term=TG+4010+&Search=Search | Transgene | NA NA TG 4010 (MVA-MUC1-IL2) |
Modified vaccinia Ankara virus vector with Muc1 and IL-2 sequences |
Cytotoxic T lymphocyte (CTL) response against Muc1 |
Ph II/III | Influenza-like symptoms |
| http://clinicaltrials.gov/ct2/results?term=HSPPC-96&Search=Search | Antigenics | Oncophage Vitespen HSPPC-96 |
Autologous tumor vaccine made by extractingheat shock protein gp96 and its associated peptides |
Autologous tumor cells |
Ph III (completed or terminated) Ph II feasibility study completed in NSCLC |
|
| http://clinicaltrials.gov/ct2/results?term=CeaVac+Monoclonal+antibody+3H1+antiidiotype+vaccine&Search=Search | Titan Pharmaceuticals |
CeaVac Monoclonal antibody 3H1 anti- idiotype vaccine NA |
Recombinant MoAb that mimics a specific epitope of the carcinoembryonic antigen (CEA) |
Tumors that express CEA |
Ph III (colorectal- completed) Ph II completed for NSCLC |
|
| http://clinicaltrials.gov/ct2/results?term=AG3340&Search=Search | Agouron Pharmac euticals/ Pfizer |
Prinomastat NA AG3340 |
Small molecule, nonpeptidic, hydroxymate matrix metalloproteinase (MMP) inhibitor |
MMPs 2, 3, 9, 13, and 14 |
Ph III completed showed no benefit (no active trials) |
|
| http://clinicaltrials.gov/ct2/results?term=1650-G+Vaccine&Search=Search | University of Kentucky |
NA NA 1650-G Vaccine |
Allogenic cellular vaccine |
CTL response against NSCLC cells |
Ph II (completed) | |
| http://clinicaltrials.gov/ct2/results?term=CG8123+%28GVAX%29&Search=Search | Cell Genesys | NA NA CG8123 (GVAX) |
GM-CSF gene- modified autologous whole tumor vaccine |
Cytotoxic T lymphocyte (CTL) response against tumor cells |
Ph II (terminated) | |
| http://clinicaltrials.gov/ct2/results?term=L-Vax&Search=Search | AVAX Technologies |
NA NA L-Vax |
DNP (dinitrophenyl)- modified autologous NSCLC vaccine |
CTL response against NSCLC cells |
Ph I/II (Study has been suspended) |
|
| http://clinicaltrials.gov/ct2/results?term=Monoclonal+antibody+11D10+anti-idiotype+vaccine&Search=Search | NCI/RTOG/SWO G |
Monoclonal antibody 11D10 anti-idiotype vaccine |
MoAb against an idiotype that mimics a human milk fat globule (HMFG) membrane epitope |
Cells expressing HMFG membrane epitope |
Ph II (NSCLC completed) (Limited-Stage SCLC) |
|
| http://clinicaltrials.gov/ct2/results?term=EP-2101&Search=Search | Epimmune | NA NA EP-2101 |
Multi-epitope DNA vaccine, emulsified in montanide ISA-51 |
Tumors expressing CEA, HER2/neu, p53, and MAGE 2/3 |
Ph I (completed) | |
| http://clinicaltrials.gov/ct2/results?term=Mutant+p53+peptide+pulsed+dendritic+cell&Search=Search | NCI | NA NA Mutant p53 peptide pulsed dendritic cell |
Autologous dendritic cells pulsed with a mutant p53 peptide |
Tumor cells expressing mutant p53 |
Ph II (completed) | |
| http://clinicaltrials.gov/ct2/results?term=INGN-225&Search=Search | Introgen Therapuetics |
Ad.p53-DC NA INGN-225 |
Autologous dendritic cells transduced with a recombinant adenovirus encoding p53 peptide |
Tumor cells expressing mutant p53 |
Ph II (SCLC) | |
| http://clinicaltrials.gov/ct2/show/NCT00019006?term=Ras+peptide+cancer+vaccine&rank=1 | NCI | Ras peptide cancer vaccine NA |
Mutated K- Ras |
Ph II (completed) | ||
| http://clinicaltrials.gov/ct2/results?term=S-3304&Search=Search | Shionogi | NA NA S-3304 |
Non-cytotoxic inhibitor of matrix metalloproteinase (MMP) |
MMP-2 MMP- 9 |
Ph I/II | Gastrointestinal toxicities, including dairrhea |
| http://clinicaltrials.gov/ct2/results?term=CEA+%286D%29&Search=Search | NCI | Recombinant fowl pox or vaccinia- CEA(6D)/TRICOM NA |
Recombinant fowl pox/ vaccinia virus vector encoding carcinoembryonic antigen (CEA) and a triad of costimulatory Molecules (B7-1, ICAM-1 and LFA-3) (TRICOM) |
CEA- expressing tumors |
Ph II (terminated) | |
| http://clinicaltrials.gov/ct2/results?term=CCL21&Search=Search | NCI | NA NA CCL21 |
Adenovirus CCL21 gene modified autologous dendritic cells |
Ph II | ||
| http://clinicaltrials.gov/ct2/results?term=DRibble+vaccine&Search=Search | Providence Health Services / The Wayne D. Kuni and Joan E. Kuni Foundation |
DRibble vaccine NA |
Autologous tumor vaccine |
Autologous tumor cells |
Ph II | |
| http://clinicaltrials.gov/ct2/results?term=Ad100-gp96Ig-HLA+A1&Search=Search | University of Miami, Sylvester Cancer Center |
NA NA Ad100-gp96Ig-HLA A1 |
Irradiated non-small cell lung cancer cells, manipulated to express and secrete heat shock protein gp96-Ig fusion protein |
CTL response against NSCLC cells |
Ph I | |
| http://clinicaltrials.gov/ct2/results?term=MAGE-12+peptide+vaccine+&Search=Search | NCI | MAGE-12 peptide vaccine (emulsified in Montanide ISA-51) NA |
Peptide vaccine | MAGE-12 Antigen positive tumors |
Ph I (completed) (all solid tumors- completed) |
|
| http://clinicaltrials.gov/ct2/results?term=pVAX%2FL523S+&Search=Search | Corixa Corporation |
pVAX/L523S and Ad/L523S NA |
Recombinant DNA and Adenovirus Expressing L523S Protein |
Ph I (no updates available) |
||
| http://clinicaltrials.gov/ct2/results?term=Semi-allogeneic+human+fibroblasts+%28MRC-5%29+transfected&Search=Search | University of Pittsburgh |
Semi-allogeneic human fibroblasts (MRC-5) transfected NA |
Fibroblasts Transfected With DNA From Autologous Tumor |
Autologous Tumor Cells |
Ph I | |
| http://clinicaltrials.gov/ct2/results?term=WT-1+%28Wilm’s+tumor%29+analog+peptide+vaccine&Search=Search | Memorial Sloan Kettering Cancer Center |
WT-1 (Wilm’s tumor) analog peptide vaccine NA |
Peptide vaccine | Wilm’s tumor gene expressing tumors |
Ph II for mesothelioma. Ph1 completed for NSCLC |
|
| ANTI-SENSE OLIGONUCLEOTIDES (ASO) | ||||||
| file://localhost/ttp/::clinicaltrials.gov:ct2:results%3Fterm=Oblimerson+&Search=Search | Genta | Oblimerson Genasense G3139 |
Antisense oligo- deoxyribonucleotide |
Bcl-2 | Currently not under development for lung cancer. |
Fever, elevated liver enzymes |
| http://clinicaltrials.gov/ct2/results?term=Custirsen&Search=Search | OncGenex | Custirsen NA OGX 011 |
Anti-sense oligonucleotide |
Clusterin | Ph III | |
| http://clinicaltrials.gov/ct2/results?term=LY2181308&Search=Search | Isis Pharmaceuticals & Eli Lilly |
NA NA LY2181308 |
Anti-sense oligonucleotide |
Blocks survivin |
Ph II (completed) | PTT prolongation, headache, lymphopenia, fever, fatigue, nausea |
| http://clinicaltrials.gov/ct2/results?term=ISIS+2503&Search=Search | Isis Pharmaceuticals |
NA NA ISIS 2503 |
Anti-sense oligonucleotide |
H-ras | Currently no studies for Lung Cancer |
|
| http://clinicaltrials.gov/ct2/results?term=ISIS+5132&Search=Search | Isis Pharmeceuticals |
NA NA ISIS 5132 |
Anti-sense oligonucleotide against c-Raf kinase mRNA expression |
Raf-1 | Currently no studies for Lung Cancer |
Mild hematologic toxicity, asthenia, fever |
| THERAPEUTIC ANTIBODY ENGINEERING (Novel targets, antibody-drug conjugates, antibody fragments) | ||||||
| http://clinicaltrials.gov/ct2/results?term=Catumaxomab&Search=Search | Trion Pharma | Catumaxomab Removab |
Rat-murine hybrid monoclonal antibody binding to EpCAM and CD3 antibody |
EpCAM/ CD3 | Ph III (Ph I studies in solid tumors terminated) -Approved in Europe for malignant ascitis |
Fever, Nausea, Vomiting |
| http://clinicaltrials.gov/ct2/results?term=GC-1008&Search=Search | Genzyme | Fresolimumab NA GC-1008 |
Human IgG4 MoAb | Pan- neutralizing TGF- β |
Ph II (mesothelioma) | |
| http://clinicaltrials.gov/ct2/results?term=Bavituximab&Search=Search | Peregrine Pharmaceuticals |
Bavituximab Tarvacin UNII-Q16CT95N25 3G4 |
Chimeric IgG1 MoAb |
Membrane phosphatidylse rine complexed with β2- glycoprotein I on tumor vasculature |
Ph II | Náusea, fatigue, headache, alopecia, anemia, hypertension |
| http://clinicaltrials.gov/ct2/results?term=ALD518&Search=Search | Alder Pharmaceuticals |
ALD518 | Anti-IL-6 antibody | To treat anemia, cachexia and fatigue |
Ph II (completed) | |
| http://clinicaltrials.gov/ct2/results?term=ACE-011&Search=Search | Acceleron and Celgene |
Sotatercept NA ACE-011 |
Fully human soluble activin receptor type 2A IgG-Fc fusion protein |
Activin antagonist (Increases hemoglobin and also increases bone mineral density) |
Ph II (All solid tumor studies terminated) |
Headache, paresthesia, dizziness, fatigue, hypertension |
| http://clinicaltrials.gov/ct2/results?term=Sonepcizumab%2F+&Search=Search | Lpath/ Merck- Sereno |
Sonepcizumab/ ASONEP |
Humanized MoAb | Shingosine-1- phosphate |
Ph I (completed) | Infusion reaction |
| http://clinicaltrials.gov/ct2/show/NCT00635596?term=MT110&rank=1 | Micromet AG | MT110 | EpCAM/ CD3 bispecific antibody construct (BiTE) |
EpCAM/ CD3 | Ph I (not recruiting participants) |
Fever, elevated liver enzymes |
| http://clinicaltrials.gov/ct2/results?term=CVX-045&Search=Search | Pfizer | CVX-045 | Human MoAb | Thrombospond in-1 Mimetic |
Ph I (completed) | Fatigue, gastrointestinal upset, dyspnea, headache, dizziness, anemia |
| http://clinicaltrials.gov/ct2/results?term=AGS-22M6E&Search=Search | Astellas Pharmaceuticals |
NA NA AGS-22M6E |
Monoclonal antibody-drug conjugate |
anti-Nectin-4 monoclonal antibody- conjugated to the cytotoxic agent monomethyl auristatin E |
Ph I | |
| THERAPEUTIC VIRUSES | ||||||
| http://clinicaltrials.gov/ct2/results?term=Seneca+Valley+virus-001+&Search=Search | NCI | NA NA Seneca Valley virus- 001 (NTX-010) |
Picornavirus | Replication competent anti-cancer virus |
Ph II (SCLC) (completed accrual) |
|
| http://clinicaltrials.gov/ct2/show/NCT01708993?term=Reolysin&rank=3 | Oncolytics Biotech |
NA Reolysin NA |
oncolytic reovirus | replicates selectively in cells with activated Ras pathway causing cell lysis |
Ph II | Chills, fever, headache, runny nose, fatigue and myelosuppression |
| MISCELLANEOUS THERAPEUTIC AGENTS | ||||||
| http://clinicaltrials.gov/ct2/results?term=Iloprost+CANCER&Search=Search | Actelion | Iloprost Ventavis (inhaled) ACT-213105 |
Oral PGI2 (prostacyclin) analogue |
Multiple effects including activation of PPAR (peroxisome proliferators activated receptor |
FDA approved for pulmonary arterial hypertension Ph II completed for lung cancer prevention |
Headache, flushing |
| http://clinicaltrials.gov/ct2/results?term=TLK286&Search=Search | Telik | Canfosfamide hydrochloride Telcyta TLK286 |
Cancer cell-activated chemotherapeutic |
Activated by glutathione S- transferase P1- 1 (overexpressed in many tumors) |
Ph III (completed) | Nausea, Vomiting, Fatigue, Microscopic Hematuria, Anemia |
| http://clinicaltrials.gov/ct2/results?term=Isotretinoin+cancer&Search=Search | NCI | Isotretinoin Several brand names includingAccutane Roaccutane |
Retinoid | Exact mechanism of action unknown. Isotretinoin may down- regulate the telomerase enzyme, inhibiting "cellular immortalization and tumorigenesis |
Ph II (completed) | Dryness of the skin, Acne flare and skin rash, Raised liver enzyme, Hyperlipedemia, Birth defects |
| http://clinicaltrials.gov/ct2/results?term=endostatin&Search=Search | Simcere pharmaceuticals |
Endostar NA YH-16 |
Recombinant human endostatin |
Angiogenesis inhibitors |
Ph III/ IV. Phase II study in stage III NSCLC. |
Pneumonia, pulmonary embolism |
| http://clinicaltrials.gov/ct2/show/NCT01748825?term=MK-1775&rank=3 | Merck | MK-1775 | Sensitizes p53 negative cells to DNA damage by abrogation of G2 checkpoint |
WEE1 inhibitor |
Ph I/II. | |
| http://clinicaltrials.gov/ct2/results?term=CHR-2797&Search=Search | Chroma Therapeutics |
Tosedostat CHR-2797 |
Small molecule aminopeptidase inhibitor |
M1 family of aminopeptidases |
Ph I/II. All studies have either completed accrual, terminated or suspended |
|
| http://clinicaltrials.gov/ct2/results?term=PF-3512676&Search=Search | Pfizer | NA NA PF-3512676/CPG 7909 |
Synthetic agonist of Toll-like receptor 9 (TLR9) |
TLR9 | Ph III terminated due to lack of efficacy |
Sepsis |
| http://clinicaltrials.gov/ct2/results?term=PF-00562271&Search=Search | Pfizer | PF-00562271 | Reversible focal adhesion kinase (FAK) inhibitor |
FAK | Ph I (completed) | Headache, nausea, peripheral neuropathy, diarrhea, fatigue, edema |
| http://clinicaltrials.gov/ct2/results?term=ZD4054&Search=Search | AstraZeneca | Zibotentan NA ZD4054 |
Endothelin A Receptor (ETAR) antagonist |
ETAR | Ph II | |
| http://clinicaltrials.gov/ct2/results?term=AZD+6918&Search=Search | AstraZeneca | AZD 6918 | Small molecule inhibitor |
Tropomyosin- related kinases (Trk) |
Ph I (Development discontinued) |
|
| http://clinicaltrials.gov/ct2/results?term=Eribulin+mesylate+lung+cancer&Search=Search | Eisai Inc. | Eribulin mesylate E7389 |
Non-taxane microtubule dynamics inhibitor |
Ph II (completed) | Fatigue, cytopenias |
|
| http://clinicaltrials.gov/ct2/results?term=Triapine+lung+cancer&Search=Search | Vion Pharmaceuticals, NCI |
Triapine | Ribonucleotide reductase inhibitor (enhancing the activity of gemcitabine) |
Ph II (completed) | ||
| http://clinicaltrials.gov/ct2/results?term=Epofolate&Search=Search | Bristol-Meyers Squibb |
Epofolate BMS-753493 |
Folate conjugate of epothilone analog BMS-748265 |
Ph I (terminated) | Fatigue, nausea, elevated liver enzymes, diarrhea |
|
| http://clinicaltrials.gov/ct2/results?term=Efatutazone&Search=Search | Daiichi Sankyo | Efatutazone NA CS 7017/ RS5444 |
PPARγ agonist | Peroxisome proliferator- activated receptor (PPAR)- γ |
Ph I | Fluid retention |
| http://clinicaltrials.gov/ct2/results?term=PD++0332991+lung+cancer&Search=Search | Pfizer/Onyx | NA NA PD 0332991 |
Small Molecule inhibitor |
CDK 4/6 inhibitor |
Ph II | |
| http://clinicaltrials.gov/ct2/results?term=Celecoxib+lung+cancer&Search=Search | Pfizer | Celecoxib Celebrex or Celebra for arthritis; Onsenal for polyps |
Eicosanoid | Cyclooxygena se-2 inhibitor |
Ph II | |
| http://clinicaltrials.gov/ct2/results?term=apricoxib | Tragara Pharmaceuticals |
Aprecoxib CS-706. TG01 Capoxigem |
A benzenesulfonamide non-steroidal anti- inflammatory drug |
Small molecule selective Cox- 2 inhibitor |
Phase II (completed) | |
| http://clinicaltrials.gov/ct2/results?term=QBI-139&Search=Search | Quintessence Biosciences |
QBI-139 | Variant of human pancreatic ribonuclease 1 |
Causes destruction of RNA |
Ph I | |
| http://clinicaltrials.gov/ct2/results?term=CVX-060&Search=Search | Pfizer | CVX-060 | Anti-Angiogenic COVX-Body |
A Selective Angiopoietin-2 (ANG-2) Binding |
Ph I (completed) | Fatigue, proteinuria |
| http://clinicaltrials.gov/ct2/results?term=AZD1480&Search=Search | AstraZeneca | NA NA AZD1480 |
Small molecule inhibitor |
JAK2 kinase | Ph I (terminated in solid tumors) |
|
| http://clinicaltrials.gov/ct2/results?term=GSAO&Search=Search | NCI | 4-(N-(S- glutathionylacetylamino ) phenylarsonous acid (GSAO) |
Synthetic tripeptide trivalent arsenical |
Angiogenesis inhibitor that targets the mitcohondria o actively dividing but not quiescent endothelial cells arresting their proliferation and causing apoptosis |
Ph I (terminated) | |
| http://clinicaltrials.gov/ct2/results?term=chol-FUS1&Search=Search | Introgen Therapeutics |
DOTAP: chol-FUS1 | Lipid based nanoparticles |
Fus1 tumor suppressor gene |
Ph I (completed). Phase II initiated in NSCLC. |
Fever, hypophosphatemia |
| http://clinicaltrials.gov/ct2/results?term=LY+2510924&Search=Search | Eli Lilly | NA NA LY 2510924 |
Peptide Antagonist | Antagonizes the CXCR4 peptide. |
Ph II (completed enrollment in ES-SCLC |
|
NSCLC – Non-small cell lung cancer. SCLC – Small cell lung cancer, ES-SCLC – Extensive stage small cell lung cancer. Ph1 – Phase I. Ph II – Phase II. Ph III – Phase III.
Acknowledgement
RG thanks Johanna Duke for editorial assistance.
References
- 1.Lawrence MS, Stojanov P, Polak P, et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature. 2013;499:214–218. doi: 10.1038/nature12213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paez JG, Janne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science (New York, NY) 2004;304:1497–1500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 3.Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. The New England journal of medicine. 2004;350:2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 4.Pao W, Miller V, Zakowski M, et al. EGF receptor gene mutations are common in lung cancers from "never smokers" and are associated with sensitivity of tumors to gefitinib and erlotinib. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:13306–13311. doi: 10.1073/pnas.0405220101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mano H. Non-solid oncogenes in solid tumors: EML4-ALK fusion genes in lung cancer. Cancer science. 2008;99:2349–2355. doi: 10.1111/j.1349-7006.2008.00972.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. The New England journal of medicine. 2009;361:947–957. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 7.Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol. 2010;11:121–128. doi: 10.1016/S1470-2045(09)70364-X. [DOI] [PubMed] [Google Scholar]
- 8.Sequist LV, Waltman BA, Dias-Santagata D, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Science translational medicine. 2011;3:75ra26. doi: 10.1126/scitranslmed.3002003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou W, Ercan D, Chen L, et al. Novel mutant-selective EGFR kinase inhibitors against EGFR T790M. Nature. 2009;462:1070–1074. doi: 10.1038/nature08622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Network T. Nature. 2014 doi: 10.1038/nature11903. [DOI] [PubMed] [Google Scholar]
- 11.Shaw AT, Kim DW, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. The New England journal of medicine. 2013;368:2385–2394. doi: 10.1056/NEJMoa1214886. [DOI] [PubMed] [Google Scholar]
- 12.Kwak EL, Bang YJ, Camidge DR, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. The New England journal of medicine. 2010;363:1693–1703. doi: 10.1056/NEJMoa1006448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Imielinski M, Berger AH, Hammerman PS, et al. Mapping the hallmarks of lung adenocarcinoma with massively parallel sequencing. Cell. 2012;150:1107–1120. doi: 10.1016/j.cell.2012.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.(CLCGP) CLCGP, (NGM) NGM. A genomics-based classification of human lung tumors. Science translational medicine. 2013;5:209ra153. doi: 10.1126/scitranslmed.3006802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Janne PA, Shaw AT, Pereira JR, et al. Selumetinib plus docetaxel for KRAS-mutant advanced non-small-cell lung cancer: a randomised, multicentre, placebo-controlled, phase 2 study. The lancet oncology. 2013;14:38–47. doi: 10.1016/S1470-2045(12)70489-8. [DOI] [PubMed] [Google Scholar]
- 16.Dutt A, Ramos AH, Hammerman PS, et al. Inhibitor-sensitive FGFR1 amplification in human non-small cell lung cancer. PloS one. 2011;6:e20351. doi: 10.1371/journal.pone.0020351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Network T. Comprehensive genomic characterization of squamous cell lung cancers. Nature. 2012;489:519–525. doi: 10.1038/nature11404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weiss J, Sos ML, Seidel D, et al. Frequent and focal FGFR1 amplification associates with therapeutically tractable FGFR1 dependency in squamous cell lung cancer. Science translational medicine. 2010;2:62ra93. doi: 10.1126/scitranslmed.3001451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Malchers F, Dietlein F, Schottle J, et al. Cell-autonomous and non-cell-autonomous mechanisms of transformation by amplified FGFR1 in lung cancer. Cancer discovery. 2014;4:246–257. doi: 10.1158/2159-8290.CD-13-0323. [DOI] [PubMed] [Google Scholar]
- 20.Kim Y, Hammerman PS, Kim J, et al. Integrative and comparative genomic analysis of lung squamous cell carcinomas in East Asian patients. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2014;32:121–128. doi: 10.1200/JCO.2013.50.8556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Majewski IJ, Mittempergher L, Davidson NM, et al. Identification of recurrent FGFR3 fusion genes in lung cancer through kinome-centred RNA sequencing. The Journal of pathology. 2013;230:270–276. doi: 10.1002/path.4209. [DOI] [PubMed] [Google Scholar]
- 22.Wu YM, Su F, Kalyana-Sundaram S, et al. Identification of targetable FGFR gene fusions in diverse cancers. Cancer discovery. 2013;3:636–647. doi: 10.1158/2159-8290.CD-13-0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rudin CM, Durinck S, Stawiski EW, et al. Comprehensive genomic analysis identifies SOX2 as a frequently amplified gene in small-cell lung cancer. Nature genetics. 2012;44:1111–1116. doi: 10.1038/ng.2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peifer M, Fernandez-Cuesta L, Sos ML, et al. Integrative genome analyses identify key somatic driver mutations of small-cell lung cancer. Nature genetics. 2012;44:1104–1110. doi: 10.1038/ng.2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baylin SB, Jones PA. A decade of exploring the cancer epigenome - biological and translational implications. Nature reviews Cancer. 2011;11:726–734. doi: 10.1038/nrc3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heyn H, Mendez-Gonzalez J, Esteller M. Epigenetic profiling joins personalized cancer medicine. Expert Rev Mol Diagn. 2013;13:473–479. doi: 10.1586/erm.13.36. [DOI] [PubMed] [Google Scholar]
- 27.Network CGAR. Comprehensive genomic characterization of squamous cell lung cancers. Nature. 2012;489:519–525. doi: 10.1038/nature11404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Azad N, Zahnow CA, Rudin CM, et al. The future of epigenetic therapy in solid tumours--lessons from the past. Nature reviews Clinical oncology. 2013;10:256–266. doi: 10.1038/nrclinonc.2013.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kandoth C, McLellan MD, Vandin F, et al. Mutational landscape and significance across 12 major cancer types. Nature. 2013;502:333–339. doi: 10.1038/nature12634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Turcan S, Rohle D, Goenka A, et al. IDH1 mutation is sufficient to establish the glioma hypermethylator phenotype. Nature. 2012;483:479–483. doi: 10.1038/nature10866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hughes LA, Melotte V, de Schrijver J, et al. The CpG island methylator phenotype: what's in a name? Cancer research. 2013;73:5858–5868. doi: 10.1158/0008-5472.CAN-12-4306. [DOI] [PubMed] [Google Scholar]
- 32.Gilbert J, Gore SD, Herman JG, et al. The clinical application of targeting cancer through histone acetylation and hypomethylation. Clinical cancer research : an official journal of the American Association for Cancer Research. 2004;10:4589–4596. doi: 10.1158/1078-0432.CCR-03-0297. [DOI] [PubMed] [Google Scholar]
- 33.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 34.Berman BP, Weisenberger DJ, Aman JF, et al. Regions of focal DNA hypermethylation and long-range hypomethylation in colorectal cancer coincide with nuclear lamina-associated domains. Nature genetics. 2012;44:40–46. doi: 10.1038/ng.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Suva ML, Riggi N, Bernstein BE. Epigenetic reprogramming in cancer. Science (New York, NY) 2013;339:1567–1570. doi: 10.1126/science.1230184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tsai HC, Li H, Van Neste L, et al. Transient low doses of DNA-demethylating agents exert durable antitumor effects on hematological and epithelial tumor cells. Cancer cell. 2012;21:430–446. doi: 10.1016/j.ccr.2011.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sharma SV, Lee DY, Li B, et al. A chromatin-mediated reversible drug-tolerant state in cancer cell subpopulations. Cell. 2010;141:69–80. doi: 10.1016/j.cell.2010.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Juergens RA, Wrangle J, Vendetti FP, et al. Combination epigenetic therapy has efficacy in patients with refractory advanced non-small cell lung cancer. Cancer discovery. 2011;1:598–607. doi: 10.1158/2159-8290.CD-11-0214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wrangle J, Wang W, Koch A, et al. Alterations of immune response of Non-Small Cell Lung Cancer with Azacytidine. Oncotarget. 2013;4:2067–2079. doi: 10.18632/oncotarget.1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39:1–10. doi: 10.1016/j.immuni.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 41.Filippakopoulos P, Qi J, Picaud S, et al. Selective inhibition of BET bromodomains. Nature. 2010;468:1067–1073. doi: 10.1038/nature09504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Delmore JE, Issa GC, Lemieux ME, et al. BET bromodomain inhibition as a therapeutic strategy to target c-Myc. Cell. 2011;146:904–917. doi: 10.1016/j.cell.2011.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mirguet O, Gosmini R, Toum J, et al. Discovery of epigenetic regulator I-BET762: lead optimization to afford a clinical candidate inhibitor of the BET bromodomains. Journal of medicinal chemistry. 2013;56:7501–7515. doi: 10.1021/jm401088k. [DOI] [PubMed] [Google Scholar]
- 44.Keedy VL, Temin S, Somerfield MR, et al. American Society of Clinical Oncology provisional clinical opinion: epidermal growth factor receptor (EGFR) Mutation testing for patients with advanced non-small-cell lung cancer considering first-line EGFR tyrosine kinase inhibitor therapy. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29:2121–2127. doi: 10.1200/JCO.2010.31.8923. [DOI] [PubMed] [Google Scholar]
- 45.Sharma SV, Bell DW, Settleman J, et al. Epidermal growth factor receptor mutations in lung cancer. Nature reviews Cancer. 2007;7:169–181. doi: 10.1038/nrc2088. [DOI] [PubMed] [Google Scholar]
- 46.Inoue A, Suzuki T, Fukuhara T, et al. Prospective phase II study of gefitinib for chemotherapy-naive patients with advanced non-small-cell lung cancer with epidermal growth factor receptor gene mutations. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2006;24:3340–3346. doi: 10.1200/JCO.2005.05.4692. [DOI] [PubMed] [Google Scholar]
- 47.Asahina H, Yamazaki K, Kinoshita I, et al. A phase II trial of gefitinib as first-line therapy for advanced non-small cell lung cancer with epidermal growth factor receptor mutations. British journal of cancer. 2006;95:998–1004. doi: 10.1038/sj.bjc.6603393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sequist LV, Martins RG, Spigel D, et al. First-line gefitinib in patients with advanced non-small-cell lung cancer harboring somatic EGFR mutations. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26:2442–2449. doi: 10.1200/JCO.2007.14.8494. [DOI] [PubMed] [Google Scholar]
- 49.Thongprasert S, Duffield E, Saijo N, et al. Health-Related Quality-of-Life in a Randomized Phase III First-Line Study of Gefitinib Versus Carboplatin/Paclitaxel in Clinically Selected Patients from Asia with Advanced NSCLC (IPASS) Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2011;6:1872–1880. doi: 10.1097/JTO.0b013e31822adaf7. [DOI] [PubMed] [Google Scholar]
- 50.Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362:2380–2388. doi: 10.1056/NEJMoa0909530. [DOI] [PubMed] [Google Scholar]
- 51.Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. The lancet oncology. 2011;12:735–742. doi: 10.1016/S1470-2045(11)70184-X. [DOI] [PubMed] [Google Scholar]
- 52.Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. The lancet oncology. 2012;13:239–246. doi: 10.1016/S1470-2045(11)70393-X. [DOI] [PubMed] [Google Scholar]
- 53.Sequist LV, Yang JC, Yamamoto N, et al. Phase III Study of Afatinib or Cisplatin Plus Pemetrexed in Patients With Metastatic Lung Adenocarcinoma With EGFR Mutations. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013 doi: 10.1200/JCO.2012.44.2806. [DOI] [PubMed] [Google Scholar]
- 54.Sequist LV, Yang JC, Yamamoto N, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013;31:3327–3334. doi: 10.1200/JCO.2012.44.2806. [DOI] [PubMed] [Google Scholar]
- 55.Wu YL, Zhou C, Hu CP, et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. The Lancet Oncology. 2014;15:213–222. doi: 10.1016/S1470-2045(13)70604-1. [DOI] [PubMed] [Google Scholar]
- 56.Yang JC, Sequist LV, Schuler M, et al. Overall survival in patients with advanced NSCLC harboring common (Del19/L858R) EGFR mutations: analysis of two large, open-label phase III studies of afatinib vs chemotherapy, LUX-Lung 3 and LUX-Lung 6. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2014;32 (suppl; abstr 8004). [Google Scholar]
- 57.NCCN Clinical Practice Guidelines: NSCLC version 4. 2014 Available at http://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf.
- 58.Yasuda H, Kobayashi S, Costa DB. EGFR exon 20 insertion mutations in non-small-cell lung cancer: preclinical data and clinical implications. Lancet Oncol. 2012;13:e23–e31. doi: 10.1016/S1470-2045(11)70129-2. [DOI] [PubMed] [Google Scholar]
- 59.Moran T, Sequist LV. Timing of epidermal growth factor receptor tyrosine kinase inhibitor therapy in patients with lung cancer with EGFR mutations. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30:3330–3336. doi: 10.1200/JCO.2012.43.1858. [DOI] [PubMed] [Google Scholar]
- 60.Chaft JE, Oxnard GR, Sima CS, et al. Disease flare after tyrosine kinase inhibitor discontinuation in patients with EGFR-mutant lung cancer and acquired resistance to erlotinib or gefitinib: implications for clinical trial design. Clinical cancer research : an official journal of the American Association for Cancer Research. 2011;17:6298–6303. doi: 10.1158/1078-0432.CCR-11-1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Weickhardt AJ, Scheier B, Burke JM, et al. Local ablative therapy of oligoprogressive disease prolongs disease control by tyrosine kinase inhibitors in oncogene-addicted non-small-cell lung cancer. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2012;7:1807–1814. doi: 10.1097/JTO.0b013e3182745948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yu HA, Sima CS, Huang J, et al. Local therapy with continued EGFR tyrosine kinase inhibitor therapy as a treatment strategy in EGFR-mutant advanced lung cancers that have developed acquired resistance to EGFR tyrosine kinase inhibitors. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2013;8:346–351. doi: 10.1097/JTO.0b013e31827e1f83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nishie K, Kawaguchi T, Tamiya A, et al. Epidermal growth factor receptor tyrosine kinase inhibitors beyond progressive disease: a retrospective analysis for Japanese patients with activating EGFR mutations. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2012;7:1722–1727. doi: 10.1097/JTO.0b013e31826913f7. [DOI] [PubMed] [Google Scholar]
- 64.Nishino M, Dahlberg SE, Cardarella S, et al. Volumetric tumor growth in advanced non-small cell lung cancer patients with EGFR mutations during EGFR-tyrosine kinase inhibitor therapy: developing criteria to continue therapy beyond RECIST progression. Cancer. 2013;119:3761–3768. doi: 10.1002/cncr.28290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Goldberg SB, Oxnard GR, Digumarthy S, et al. Chemotherapy with Erlotinib or chemotherapy alone in advanced non-small cell lung cancer with acquired resistance to EGFR tyrosine kinase inhibitors. The oncologist. 2013;18:1214–1220. doi: 10.1634/theoncologist.2013-0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yu HA, Arcila ME, Rekhtman N, et al. Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Clinical cancer research : an official journal of the American Association for Cancer Research. 2013;19:2240–2247. doi: 10.1158/1078-0432.CCR-12-2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sequist LV, Soria JC, Gadgeel SM, et al. First-in-human evaluation of CO-1686, an irreversible, highly selective tyrosine kinase inhibitor of mutations of EGFR (activating and T790M) Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2014;32(suppl) abstr 8010. [Google Scholar]
- 68.Janne PA, Ramalingam S, Yang JC, et al. Clinical activity of the mutant-selective EGFR inhibitor AZD9291 in patients (pts) with EGFR inhibitor–resistant non-small cell lung cancer (NSCLC) Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2014;32 (suppl: abstr 8009) [Google Scholar]
- 69.Kwak EL, Sordella R, Bell DW, et al. Irreversible inhibitors of the EGF receptor may circumvent acquired resistance to gefitinib. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:7665–7670. doi: 10.1073/pnas.0502860102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sequist LV, Besse B, Lynch TJ, et al. Neratinib, an irreversible pan-ErbB receptor tyrosine kinase inhibitor: results of a phase II trial in patients with advanced non-small-cell lung cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28:3076–3083. doi: 10.1200/JCO.2009.27.9414. [DOI] [PubMed] [Google Scholar]
- 71.Miller VA, Hirsh V, Cadranel J, et al. Afatinib versus placebo for patients with advanced, metastatic non-small-cell lung cancer after failure of erlotinib, gefitinib, or both, and one or two lines of chemotherapy (LUX-Lung 1): a phase 2b/3 randomised trial. The lancet oncology. 2012;13:528–538. doi: 10.1016/S1470-2045(12)70087-6. [DOI] [PubMed] [Google Scholar]
- 72.Reckamp KL, Giaccone G, Camidge DR, et al. A phase 2 trial of dacomitinib (PF-00299804), an oral, irreversible pan-HER (human epidermal growth factor receptor) inhibitor, in patients with advanced non-small cell lung cancer after failure of prior chemotherapy and erlotinib. Cancer. 2014;120:1145–1154. doi: 10.1002/cncr.28561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Janjigian YY, Smit E, Horn L, et al. Activity of afatinib/cetuximab in patients with EGFR mutant non-small cell lung cancer and acquires resistance to EGFR inhibitors. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2012;23(suppl 9) [Google Scholar]
- 74.Regales L, Gong Y, Shen R, et al. Dual targeting of EGFR can overcome a major drug resistance mutation in mouse models of EGFR mutant lung cancer. The Journal of clinical investigation. 2009;119:3000–3010. doi: 10.1172/JCI38746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pennell NA, Neal JW, Chaft JE, et al. SELECT: A multicenter phase II trial of adjuvant erlotinib in resected early-stage EGFR mutation-positive NSCLC. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2014;32 (suppl; abstr 7514) [Google Scholar]
- 76.Kelly K, Altorki NK, Eberhardt WEE, et al. A randomized, double-blind phase 3 trial of adjuvant erlotinib (E) versus placebo (P) following complete tumor resection with or without adjuvant chemotherapy in patients (pts) with stage IB-IIIA EGFR positive (IHC/FISH) non-small cell lung cancer (NSCLC): RADIANT results. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2014;32 doi: 10.1200/JCO.2015.61.8918. (suppl; abstr 7501). [DOI] [PubMed] [Google Scholar]
- 77.Herter-Sprie GS, Greulich H, Wong KK. Activating Mutations in ERBB2 and Their Impact on Diagnostics and Treatment. Frontiers in oncology. 2013;3:86. doi: 10.3389/fonc.2013.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Stephens P, Hunter C, Bignell G, et al. Lung cancer: intragenic ERBB2 kinase mutations in tumours. Nature. 2004;431:525–526. doi: 10.1038/431525b. [DOI] [PubMed] [Google Scholar]
- 79.Tomizawa K, Suda K, Onozato R, et al. Prognostic and predictive implications of HER2/ERBB2/neu gene mutations in lung cancers. Lung Cancer. 2011;74:139–144. doi: 10.1016/j.lungcan.2011.01.014. [DOI] [PubMed] [Google Scholar]
- 80.Arcila ME, Chaft JE, Nafa K, et al. Prevalence, clinicopathologic associations, and molecular spectrum of ERBB2 (HER2) tyrosine kinase mutations in lung adenocarcinomas. Clinical cancer research : an official journal of the American Association for Cancer Research. 2012;18:4910–4918. doi: 10.1158/1078-0432.CCR-12-0912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mazieres J, Peters S, Lepage B, et al. Lung cancer that harbors an HER2 mutation: epidemiologic characteristics and therapeutic perspectives. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013;31:1997–2003. doi: 10.1200/JCO.2012.45.6095. [DOI] [PubMed] [Google Scholar]
- 82.Greulich H, Kaplan B, Mertins P, et al. Functional analysis of receptor tyrosine kinase mutations in lung cancer identifies oncogenic extracellular domain mutations of ERBB2. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:14476–14481. doi: 10.1073/pnas.1203201109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kan Z, Jaiswal BS, Stinson J, et al. Diverse somatic mutation patterns and pathway alterations in human cancers. Nature. 2010;466:869–873. doi: 10.1038/nature09208. [DOI] [PubMed] [Google Scholar]
- 84.Sergina NV, Rausch M, Wang D, et al. Escape from HER-family tyrosine kinase inhibitor therapy by the kinase-inactive HER3. Nature. 2007;445:437–441. doi: 10.1038/nature05474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Engelman JA, Zejnullahu K, Mitsudomi T, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science (New York, NY) 2007;316:1039–1043. doi: 10.1126/science.1141478. [DOI] [PubMed] [Google Scholar]
- 86.Li D, Ambrogio L, Shimamura T, et al. BIBW2992, an irreversible EGFR/HER2 inhibitor highly effective in preclinical lung cancer models. Oncogene. 2008;27:4702–4711. doi: 10.1038/onc.2008.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.De Greve J, Teugels E, Geers C, et al. Clinical activity of afatinib (BIBW 2992) in patients with lung adenocarcinoma with mutations in the kinase domain of HER2/neu. Lung Cancer. 2012;76:123–127. doi: 10.1016/j.lungcan.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 88.Kris MGZ, Jänne P, Kim D, Martins R, Mok TSK, O'Connell J, Ou S, Taylor I, Zhang I. Dacomitinib (Pf-00299804), an Irreversible Pan-HER Tyrosine Kinase Inhibitor, for First-Line Treatment of EGFR-Mutant or HER2-Mutant or -Amplified Lung Cancers. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2012;23:ix401–ix402. [Google Scholar]
- 89.Perera SA, Li D, Shimamura T, et al. HER2YVMA drives rapid development of adenosquamous lung tumors in mice that are sensitive to BIBW2992 and rapamycin combination therapy. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:474–479. doi: 10.1073/pnas.0808930106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gandhi L, Bahleda R, Tolaney SM, et al. Phase I study of neratinib in combination with temsirolimus in patients with human epidermal growth factor receptor 2-dependent and other solid tumors. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2014;32:68–75. doi: 10.1200/JCO.2012.47.2787. [DOI] [PubMed] [Google Scholar]
- 91.McDonagh CF, Huhalov A, Harms BD, et al. Antitumor activity of a novel bispecific antibody that targets the ErbB2/ErbB3 oncogenic unit and inhibits heregulin-induced activation of ErbB3. Molecular cancer therapeutics. 2012;11:582–593. doi: 10.1158/1535-7163.MCT-11-0820. [DOI] [PubMed] [Google Scholar]
- 92.Huang S, Li C, Armstrong EA, et al. Dual targeting of EGFR and HER3 with MEHD7945A overcomes acquired resistance to EGFR inhibitors and radiation. Cancer research. 2013;73:824–833. doi: 10.1158/0008-5472.CAN-12-1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cervantes-Ruiperez AJD, Hidalgo M, Messersmith WA, Blumenschein GR, Baselga J, Perez DR, Dienstmann R, Calles A, Jimeno A, Sanabria S, Littman C, Amler LC, Pirzkall A, Tabernero J. A phase I study of MEHD7945A (MEHD), a first-in-class HER3/EGFR dual-action antibody, in patients (pts) with refractory/recurrent epithelial tumors: Expansion cohorts. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;(suppl) abstr 2568. [Google Scholar]
- 94.Sequist LVMM, Rixe O, Jackman DM, Andreas K, Pearlberg J, Moyo VM, Harb WA. Targeting EGFR and ERBB3 in lung cancer patients: Clinical outcomes in a phase I trial of MM-121 in combination with erlotinib. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;(suppl) abstr 7556. [Google Scholar]
- 95.Camidge DR, Bang YJ, Kwak EL, et al. Activity and safety of crizotinib in patients with ALK-positive non-small-cell lung cancer: updated results from a phase 1 study. The lancet oncology. 2012;13:1011–1019. doi: 10.1016/S1470-2045(12)70344-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pfizer I, editor. XALKORI [package insert] New York, NY: 2013. [Google Scholar]
- 97.Soda M, Choi YL, Enomoto M, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature. 2007;448:561–566. doi: 10.1038/nature05945. [DOI] [PubMed] [Google Scholar]
- 98.Shepherd FA, Dancey J, Ramlau R, et al. Prospective randomized trial of docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2000;18:2095–2103. doi: 10.1200/JCO.2000.18.10.2095. [DOI] [PubMed] [Google Scholar]
- 99.Hanna N, Shepherd FA, Fossella FV, et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2004;22:1589–1597. doi: 10.1200/JCO.2004.08.163. [DOI] [PubMed] [Google Scholar]
- 100.Scagliotti G, Hanna N, Fossella F, et al. The differential efficacy of pemetrexed according to NSCLC histology: a review of two Phase III studies. The oncologist. 2009;14:253–263. doi: 10.1634/theoncologist.2008-0232. [DOI] [PubMed] [Google Scholar]
- 101.Camidge DR, Kono SA, Lu X, et al. Anaplastic lymphoma kinase gene rearrangements in non-small cell lung cancer are associated with prolonged progression-free survival on pemetrexed. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2011;6:774–780. doi: 10.1097/JTO.0b013e31820cf053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lee JO, Kim TM, Lee SH, et al. Anaplastic lymphoma kinase translocation: a predictive biomarker of pemetrexed in patients with non-small cell lung cancer. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2011;6:1474–1480. doi: 10.1097/JTO.0b013e3182208fc2. [DOI] [PubMed] [Google Scholar]
- 103.Sakamoto H, Tsukaguchi T, Hiroshima S, et al. CH5424802, a selective ALK inhibitor capable of blocking the resistant gatekeeper mutant. Cancer cell. 2011;19:679–690. doi: 10.1016/j.ccr.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 104.Seto T, Kiura K, Nishio M, et al. CH5424802 (RO5424802) for patients with ALK-rearranged advanced non-small-cell lung cancer (AF-001JP study): a single-arm, open-label, phase 1–2 study. The lancet oncology. 2013;14:590–598. doi: 10.1016/S1470-2045(13)70142-6. [DOI] [PubMed] [Google Scholar]
- 105.Marsilje TH, Pei W, Chen B, et al. Synthesis, structure-activity relationships, and in vivo efficacy of the novel potent and selective anaplastic lymphoma kinase (ALK) inhibitor 5-chloro-N2-(2-isopropoxy-5-methyl-4-(piperidin-4-yl)phenyl)-N4-(2-(isopropylsulf onyl)phenyl)pyrimidine-2,4-diamine (LDK378) currently in phase 1 and phase 2 clinical trials. Journal of medicinal chemistry. 2013;56:5675–5690. doi: 10.1021/jm400402q. [DOI] [PubMed] [Google Scholar]
- 106.Shaw AT, Kim DW, Mehra R, et al. Ceritinib in ALK-rearranged non-small-cell lung cancer. The New England journal of medicine. 2014;370:1189–1197. doi: 10.1056/NEJMoa1311107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gainor JF, Ou SH, Logan J, et al. The central nervous system as a sanctuary site in ALK-positive non-small-cell lung cancer. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2013;8:1570–1573. doi: 10.1097/JTO.0000000000000029. [DOI] [PubMed] [Google Scholar]
- 108.Sholl LM, Sun H, Butaney M, et al. ROS1 immunohistochemistry for detection of ROS1-rearranged lung adenocarcinomas. Am J Surg Pathol. 2013;37:1441–1449. doi: 10.1097/PAS.0b013e3182960fa7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sasaki H, Shimizu S, Tani Y, et al. RET expression and detection of KIF5B/RET gene rearrangements in Japanese lung cancer. Cancer Med. 2012;1:68–75. doi: 10.1002/cam4.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Terry J, De Luca A, Leung S, et al. Immunohistochemical expression of neurotrophic tyrosine kinase receptors 1 and 2 in lung carcinoma: potential discriminators between squamous and nonsquamous subtypes. Arch Pathol Lab Med. 2011;135:433–439. doi: 10.5858/2010-0038-OA.1. [DOI] [PubMed] [Google Scholar]
- 111.Takeuchi K, Soda M, Togashi Y, et al. RET, ROS1 and ALK fusions in lung cancer. Nat Med. 2012;18:378–381. doi: 10.1038/nm.2658. [DOI] [PubMed] [Google Scholar]
- 112.Vaishnavi A, Capelletti M, Le AT, et al. Oncogenic and drug-sensitive NTRK1 rearrangements in lung cancer. Nat Med. 2013;19:1469–1472. doi: 10.1038/nm.3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Davies KD, Doebele RC. Molecular pathways: ROS1 fusion proteins in cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2013;19:4040–4045. doi: 10.1158/1078-0432.CCR-12-2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Davies KD, Le AT, Theodoro MF, et al. Identifying and targeting ROS1 gene fusions in non-small cell lung cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2012;18:4570–4579. doi: 10.1158/1078-0432.CCR-12-0550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Matsubara D, Kanai Y, Ishikawa S, et al. Identification of CCDC6-RET fusion in the human lung adenocarcinoma cell line, LC-2/ad. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2012;7:1872–1876. doi: 10.1097/JTO.0b013e3182721ed1. [DOI] [PubMed] [Google Scholar]
- 116.Wang R, Hu H, Pan Y, et al. RET fusions define a unique molecular and clinicopathologic subtype of non-small-cell lung cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30:4352–4359. doi: 10.1200/JCO.2012.44.1477. [DOI] [PubMed] [Google Scholar]
- 117.Rimkunas VM, Crosby KE, Li D, et al. Analysis of receptor tyrosine kinase ROS1-positive tumors in non-small cell lung cancer: identification of a FIG-ROS1 fusion. Clinical cancer research : an official journal of the American Association for Cancer Research. 2012;18:4449–4457. doi: 10.1158/1078-0432.CCR-11-3351. [DOI] [PubMed] [Google Scholar]
- 118.Warth A, Muley T, Dienemann H, et al. ROS1 Expression and Translocations in Non-Small Cell Lung Cancer: Clinicopathological Analysis of 1478 Cases. Histopathology. 2014 doi: 10.1111/his.12379. [DOI] [PubMed] [Google Scholar]
- 119.Aisner DL, Nguyen TT, Paskulin DD, et al. ROS1 and ALK fusions in colorectal cancer, with evidence of intratumoral heterogeneity for molecular drivers. Mol Cancer Res. 2014;12:111–118. doi: 10.1158/1541-7786.MCR-13-0479-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Alrifai D, Popat S, Ahmed M, et al. A rare case of squamous cell carcinoma of the lung harbouring ALK and BRAF activating mutations. Lung Cancer. 2013;80:339–340. doi: 10.1016/j.lungcan.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 121.Yang JJ, Zhang XC, Su J, et al. Lung Cancers with Concomitant EGFR Mutations and ALK Rearrangements: Diverse Responses to EGFR-TKI and Crizotinib in Relation to Diverse Receptors Phosphorylation. Clinical cancer research : an official journal of the American Association for Cancer Research. 2014;20:1383–1392. doi: 10.1158/1078-0432.CCR-13-0699. [DOI] [PubMed] [Google Scholar]
- 122.Doebele RC, Pilling AB, Aisner DL, et al. Mechanisms of resistance to crizotinib in patients with ALK gene rearranged non-small cell lung cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2012;18:1472–1482. doi: 10.1158/1078-0432.CCR-11-2906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Seo JS, Ju YS, Lee WC, et al. The transcriptional landscape and mutational profile of lung adenocarcinoma. Genome Res. 2012 doi: 10.1101/gr.145144.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bergethon K, Shaw AT, Ou SH, et al. ROS1 rearrangements define a unique molecular class of lung cancers. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30:863–870. doi: 10.1200/JCO.2011.35.6345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Cai W, Su C, Li X, et al. KIF5B-RET fusions in Chinese patients with non-small cell lung cancer. Cancer. 2013;119:1486–1494. doi: 10.1002/cncr.27940. [DOI] [PubMed] [Google Scholar]
- 126.Fernandez-Cuesta L, Peifer M, Lu X, et al. Cross-entity mutation analysis of lung neuroendocrine tumors sheds light into their molecular origin and identifies new therapeutic targets. Proceedings of the 105th Annual Meeting of the American Association for Cancer Research; 2014 Apr 5–9; San Diego, CA Philadelphia (PA): AACR. 2014. Abstract nr 1531. [Google Scholar]
- 127.Lee J, Lee SE, Kang SY, et al. Identification of ROS1 rearrangement in gastric adenocarcinoma. Cancer. 2013;119:1627–1635. doi: 10.1002/cncr.27967. [DOI] [PubMed] [Google Scholar]
- 128.Wiesner T, He J, Yelensky R, et al. Kinase fusions are frequent in Spitz tumours and spitzoid melanomas. Nat Commun. 2014;5:3116. doi: 10.1038/ncomms4116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zitzelsberger H, Bauer V, Thomas G, et al. Molecular rearrangements in papillary thyroid carcinomas. Clin Chim Acta. 2010;411:301–308. doi: 10.1016/j.cca.2009.11.028. [DOI] [PubMed] [Google Scholar]
- 130.Bossi D, Carlomagno F, Pallavicini I, et al. Functional characterization of a novel FGFR1OP-RET rearrangement in hematopoietic malignancies. Mol Oncol. 2014;8:221–231. doi: 10.1016/j.molonc.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Martin-Zanca D, Mitra G, Long LK, et al. Molecular characterization of the human trk oncogene. Cold Spring Harb Symp Quant Biol. 1986;51 Pt 2:983–992. doi: 10.1101/sqb.1986.051.01.112. [DOI] [PubMed] [Google Scholar]
- 132.Kim J, Cho HJ, Cho GH, et al. Recurrent fusion of NTRK1 in glioblastoma multiforme. Proceedings of the 104th Annual Meeting of the American Association for Cancer Research; 2013 Apr 6–10; Washington, DC Philadelphia (PA) AACR. 2013. 2013 Abstract nr 1798. [Google Scholar]
- 133.Ross JS, Wang K, Gay L, et al. New Routes to Targeted Therapy of Intrahepatic Cholangiocarcinomas Revealed by Next-Generation Sequencing. The oncologist. 2014;19:235–242. doi: 10.1634/theoncologist.2013-0352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Eguchi M, Eguchi-Ishimae M, Tojo A, et al. Fusion of ETV6 to neurotrophin-3 receptor TRKC in acute myeloid leukemia with t(12;15)(p13;q25) Blood. 1999;93:1355–1363. [PubMed] [Google Scholar]
- 135.Jones DT, Hutter B, Jager N, et al. Recurrent somatic alterations of FGFR1 and NTRK2 in pilocytic astrocytoma. Nature genetics. 2013;45:927–932. doi: 10.1038/ng.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Tognon C, Knezevich SR, Huntsman D, et al. Expression of the ETV6-NTRK3 gene fusion as a primary event in human secretory breast carcinoma. Cancer cell. 2002;2:367–376. doi: 10.1016/s1535-6108(02)00180-0. [DOI] [PubMed] [Google Scholar]
- 137.Wai DH, Knezevich SR, Lucas T, et al. The ETV6-NTRK3 gene fusion encodes a chimeric protein tyrosine kinase that transforms NIH3T3 cells. Oncogene. 2000;19:906–915. doi: 10.1038/sj.onc.1203396. [DOI] [PubMed] [Google Scholar]
- 138.Ou SHI, Bang YJ, Camidge DR, et al. Efficacy and safety of crizotinib in patients with advanced ROS1-rearranged non-small cell lung cancer (NSCLC) J Clin Oncol. 2013;31 (suppl; abstr 8032). [Google Scholar]
- 139.Davare MA, Saborowski A, Eide CA, et al. Foretinib is a potent inhibitor of oncogenic ROS1 fusion proteins. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:19519–19524. doi: 10.1073/pnas.1319583110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zou HY, Engstrom LD, Li Q, et al. PF-06463922, a novel ROS1/ALK inhibitor, demonstrates sub-nanomolar potency against oncogenic ROS1 fusions and capable of blocking the resistant ROS1G2032R mutant in preclinical tumor models. Mol Cancer Ther; Proceedings of the 2013 AACR-NCI-EORTC International Conference on Molecular Targets and Cancer Therapeutics; 2013 Oct 19–23; Boston, Massachusetts Philadelphia (PA): AACR. 2013. Abstract nr A277. [Google Scholar]
- 141.Drilon A, Wang L, Hasanovic A, et al. Response to Cabozantinib in Patients with RET Fusion-Positive Lung Adenocarcinomas. Cancer discovery. 2013;3:630–635. doi: 10.1158/2159-8290.CD-13-0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Gautschi O, Zander T, Keller FA, et al. A patient with lung adenocarcinoma and RET fusion treated with vandetanib. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2013;8:e43–e44. doi: 10.1097/JTO.0b013e31828a4d07. [DOI] [PubMed] [Google Scholar]
- 143.Varella-Garcia M, Xu LG, Mahale S, et al. RET rearrangements detected by FISH in ‘pan-negative’ lung adenocarcinoma. American Society of Clinical Oncology Annual Meeting. 2013 abstract 8024, accepted 2013. [Google Scholar]
- 144.Okamoto K, Kodama K, Takase K, et al. Antitumor activities of the targeted multi-tyrosine kinase inhibitor lenvatinib (E7080) against RET gene fusion-driven tumor models. Cancer Lett. 2013;340:97–103. doi: 10.1016/j.canlet.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 145.Gozgit JM, Wong MJ, Zhu X, et al. Ponatinib, a potent pan-BCR-ABL inhibitor, retains activity against gatekeeper mutants of FLT3, RET, KIT, PDGFRα/β and FGFR1. Cancer research. 2012;72(Supplement 1) [Google Scholar]
- 146.Mologni L, Redaelli S, Morandi A, et al. Ponatinib is a potent inhibitor of wild-type and drug-resistant gatekeeper mutant RET kinase. Mol Cell Endocrinol. 2013;377:1–6. doi: 10.1016/j.mce.2013.06.025. [DOI] [PubMed] [Google Scholar]
- 147.Weiss GJ, Sachdev JC, Infante JR, et al. TSR-011, A Potent ALK Inhibitor with Clinical Activity in Phase I/IIa Development. Journal of Thoracic Oncology. 2013;8:S618. [Google Scholar]
- 148.Indo Y, Tsuruta M, Hayashida Y, et al. Mutations in the TRKA/NGF receptor gene in patients with congenital insensitivity to pain with anhidrosis. Nature genetics. 1996;13:485–488. doi: 10.1038/ng0896-485. [DOI] [PubMed] [Google Scholar]
- 149.Weickhardt AJ, Scheier B, Burke JM, et al. Local ablative therapy of oligoprogressive disease prolongs disease control by tyrosine kinase inhibitors in oncogene-addicted non-small-cell lung cancer. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2012;7:1807–1814. doi: 10.1097/JTO.0b013e3182745948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Weiss GJ, Hidalgo M, Borad MJ, et al. Phase I study of the safety, tolerability and pharmacokinetics of PHA-848125AC, a dual tropomyosin receptor kinase A and cyclin-dependent kinase inhibitor, in patients with advanced solid malignancies. Invest New Drugs. 2012;30:2334–2343. doi: 10.1007/s10637-011-9774-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Qiao X, Hefti F, Knusel B, et al. Selective failure of brain-derived neurotrophic factor mRNA expression in the cerebellum of stargazer, a mutant mouse with ataxia. J Neurosci. 1996;16:640–648. doi: 10.1523/JNEUROSCI.16-02-00640.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Awad MM, Katayama R, McTigue M, et al. Acquired resistance to crizotinib from a mutation in CD74-ROS1. The New England journal of medicine. 2013;368:2395–2401. doi: 10.1056/NEJMoa1215530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Katayama R, Shaw AT, Khan TM, et al. Mechanisms of acquired crizotinib resistance in ALK-rearranged lung Cancers. Science translational medicine. 2012;4:120ra117. doi: 10.1126/scitranslmed.3003316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Doebele RC, Aisner DL, Le AT, et al. Analysis of resistance mechanisms to ALK kinase inhibitors in ALK+ NSCLC patients. J Clin Oncol. 2012;30 (suppl; abstr 7504) 2012. [Google Scholar]
- 155.Heuckmann JM, Holzel M, Sos ML, et al. ALK mutations conferring differential resistance to structurally diverse ALK inhibitors. Clinical cancer research : an official journal of the American Association for Cancer Research. 2011;17:7394–7401. doi: 10.1158/1078-0432.CCR-11-1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Squillace RM, Anjum R, Miller D, et al. AP26113 possesses pan-inhibitory activity versus crizotinib-resistant ALK mutants and oncogenic ROS1 fusions. Cancer research. 2013;73 abstr 5655. [Google Scholar]
- 157.Davies KD, Mahale S, Astling DP, et al. Resistance to ROS1 inhibition mediated by EGFR pathway activation in non-small cell lung cancer. PloS one. 2013;8:e82236. doi: 10.1371/journal.pone.0082236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Oxnard GR, Binder A, Janne PA. New targetable oncogenes in non-small-cell lung cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013;31:1097–1104. doi: 10.1200/JCO.2012.42.9829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Brose MS, Volpe P, Feldman M, et al. BRAF and RAS mutations in human lung cancer and melanoma. Cancer research. 2002;62:6997–7000. [PubMed] [Google Scholar]
- 160.Homet B, Ribas A. New drug targets in metastatic melanoma. The Journal of pathology. 2014;232:134–141. doi: 10.1002/path.4259. [DOI] [PubMed] [Google Scholar]
- 161.Prahallad A, Sun C, Huang S, et al. Unresponsiveness of colon cancer to BRAF(V600E) inhibition through feedback activation of EGFR. Nature. 2012;483:100–103. doi: 10.1038/nature10868. [DOI] [PubMed] [Google Scholar]
- 162.Naoki K, Chen TH, Richards WG, et al. Missense mutations of the BRAF gene in human lung adenocarcinoma. Cancer research. 2002;62:7001–7003. [PubMed] [Google Scholar]
- 163.Paik PK, Arcila ME, Fara M, et al. Clinical characteristics of patients with lung adenocarcinomas harboring BRAF mutations. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29:2046–2051. doi: 10.1200/JCO.2010.33.1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Marchetti A, Felicioni L, Malatesta S, et al. Clinical features and outcome of patients with non-small-cell lung cancer harboring BRAF mutations. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29:3574–3579. doi: 10.1200/JCO.2011.35.9638. [DOI] [PubMed] [Google Scholar]
- 165.Cardarella S, Ogino A, Nishino M, et al. Clinical, pathologic, and biologic features associated with BRAF mutations in non-small cell lung cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2013;19:4532–4540. doi: 10.1158/1078-0432.CCR-13-0657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Kinno T, Tsuta K, Shiraishi K, et al. Clinicopathological features of nonsmall cell lung carcinomas with BRAF mutations. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2014;25:138–142. doi: 10.1093/annonc/mdt495. [DOI] [PubMed] [Google Scholar]
- 167.Peters S, Michielin O, Zimmermann S. Dramatic response induced by vemurafenib in a BRAF V600E-mutated lung adenocarcinoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013;31:e341–344. doi: 10.1200/JCO.2012.47.6143. [DOI] [PubMed] [Google Scholar]
- 168.Planchard DMJ, Riely GJ. Interim results of phase II study BRF113928 of dabrafenib in BRAF V600E mutation-position non-small cell lung cancer (NSCLC) patients. Journal of Clinical Oncology. 2013;31 [Google Scholar]
- 169.Sen B, Peng S, Tang X, et al. Kinase-impaired BRAF mutations in lung cancer confer sensitivity to dasatinib. Science translational medicine. 2012;4:136ra170. doi: 10.1126/scitranslmed.3003513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kranenburg O. The KRAS oncogene: past, present, and future. Biochimica et biophysica acta. 2005;1756:81–82. doi: 10.1016/j.bbcan.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 171.Bos JL. ras oncogenes in human cancer: a review. Cancer research. 1989;49:4682–4689. [PubMed] [Google Scholar]
- 172.Chetty R, Govender D. Gene of the month: KRAS. Journal of clinical pathology. 2013;66:548–550. doi: 10.1136/jclinpath-2013-201663. [DOI] [PubMed] [Google Scholar]
- 173.Shepherd FA, Domerg C, Hainaut P, et al. Pooled analysis of the prognostic and predictive effects of KRAS mutation status and KRAS mutation subtype in early-stage resected non-small-cell lung cancer in four trials of adjuvant chemotherapy. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013;31:2173–2181. doi: 10.1200/JCO.2012.48.1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Garassino MC, Marabese M, Rusconi P, et al. Different types of K-Ras mutations could affect drug sensitivity and tumour behaviour in non-small-cell lung cancer. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2011;22:235–237. doi: 10.1093/annonc/mdq680. [DOI] [PubMed] [Google Scholar]
- 175.Karachaliou N, Mayo C, Costa C, et al. KRAS mutations in lung cancer. Clinical lung cancer. 2013;14:205–214. doi: 10.1016/j.cllc.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 176.Nelson MA, Wymer J, Clements N., Jr Detection of K-ras gene mutations in non-neoplastic lung tissue and lung cancers. Cancer Lett. 1996;103:115–121. doi: 10.1016/0304-3835(96)04202-4. [DOI] [PubMed] [Google Scholar]
- 177.Ding L, Getz G, Wheeler DA, et al. Somatic mutations affect key pathways in lung adenocarcinoma. Nature. 2008;455:1069–1075. doi: 10.1038/nature07423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Suzuki Y, Orita M, Shiraishi M, et al. Detection of ras gene mutations in human lung cancers by single-strand conformation polymorphism analysis of polymerase chain reaction products. Oncogene. 1990;5:1037–1043. [PubMed] [Google Scholar]
- 179.Mitsudomi T, Viallet J, Mulshine JL, et al. Mutations of ras genes distinguish a subset of non-small-cell lung cancer cell lines from small-cell lung cancer cell lines. Oncogene. 1991;6:1353–1362. [PubMed] [Google Scholar]
- 180.Rodenhuis S, Slebos RJ. Clinical significance of ras oncogene activation in human lung cancer. Cancer research. 1992;52:2665s–2669s. [PubMed] [Google Scholar]
- 181.Mao C, Qiu LX, Liao RY, et al. KRAS mutations and resistance to EGFR-TKIs treatment in patients with non-small cell lung cancer: a meta-analysis of 22 studies. Lung Cancer. 2010;69:272–278. doi: 10.1016/j.lungcan.2009.11.020. [DOI] [PubMed] [Google Scholar]
- 182.Mascaux C, Iannino N, Martin B, et al. The role of RAS oncogene in survival of patients with lung cancer: a systematic review of the literature with meta-analysis. British journal of cancer. 2005;92:131–139. doi: 10.1038/sj.bjc.6602258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Gatzemeier U, Paz-Ares L, Rodrigues Pereira J, et al. Molecular and clinical biomarkers of cetuximab efficacy: data from the phase III FLEX study in non-small cell lung cancer (NSCLC) Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2009;4:S324. (abstract B322.323). [Google Scholar]
- 184.Ostrem JM, Peters U, Sos ML, et al. K-Ras(G12C) inhibitors allosterically control GTP affinity and effector interactions. Nature. 2013;503:548–551. doi: 10.1038/nature12796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Lim SM, Westover KD, Ficarro SB, et al. Therapeutic Targeting of Oncogenic K-Ras by a Covalent Catalytic Site Inhibitor. Angewandte Chemie. 2013 doi: 10.1002/anie.201307387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Fiordalisi JJ, Johnson RL, 2nd, Weinbaum CA, et al. High affinity for farnesyltransferase and alternative prenylation contribute individually to K-Ras4B resistance to farnesyltransferase inhibitors. The Journal of biological chemistry. 2003;278:41718–41727. doi: 10.1074/jbc.M305733200. [DOI] [PubMed] [Google Scholar]
- 187.Lobell RB, Liu D, Buser CA, et al. Preclinical and clinical pharmacodynamic assessment of L-778,123, a dual inhibitor of farnesyl:protein transferase and geranylgeranyl:protein transferase type-I. Molecular cancer therapeutics. 2002;1:747–758. [PubMed] [Google Scholar]
- 188.Kim ES, Herbst RS, Wistuba II, et al. The BATTLE trial: personalizing therapy for lung cancer. Cancer discovery. 2011;1:44–53. doi: 10.1158/2159-8274.CD-10-0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Heidorn SJ, Milagre C, Whittaker S, et al. Kinase-dead BRAF and oncogenic RAS cooperate to drive tumor progression through CRAF. Cell. 2010;140:209–221. doi: 10.1016/j.cell.2009.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Poulikakos PI, Zhang C, Bollag G, et al. RAF inhibitors transactivate RAF dimers and ERK signalling in cells with wild-type BRAF. Nature. 2010;464:427–430. doi: 10.1038/nature08902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Su F, Viros A, Milagre C, et al. RAS mutations in cutaneous squamous-cell carcinomas in patients treated with BRAF inhibitors. The New England journal of medicine. 2012;366:207–215. doi: 10.1056/NEJMoa1105358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Konstantinidou G, Ramadori G, Torti F, et al. RHOA-FAK Is a Required Signaling Axis for the Maintenance of KRAS-Driven Lung Adenocarcinomas. Cancer discovery. 2013;3:444–457. doi: 10.1158/2159-8290.CD-12-0388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Sequist LV, von Pawel J, Garmey EG, et al. Randomized phase II study of erlotinib plus tivantinib versus erlotinib plus placebo in previously treated non-small-cell lung cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29:3307–3315. doi: 10.1200/JCO.2010.34.0570. [DOI] [PubMed] [Google Scholar]
- 194.Westcott PM, To MD. The genetics and biology of KRAS in lung cancer. Chinese journal of cancer. 2013;32:63–70. doi: 10.5732/cjc.012.10098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Engelman JA. Targeting PI3K signalling in cancer: opportunities, challenges and limitations. Nature reviews Cancer. 2009;9:550–562. doi: 10.1038/nrc2664. [DOI] [PubMed] [Google Scholar]
- 196.Courtney KD, Corcoran RB, Engelman JA. The PI3K pathway as drug target in human cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28:1075–1083. doi: 10.1200/JCO.2009.25.3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Heavey S, O'Byrne KJ, Gately K. Strategies for co-targeting the PI3K/AKT/mTOR pathway in NSCLC. Cancer treatment reviews. 2014;40:445–456. doi: 10.1016/j.ctrv.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 198.Yamamoto H, Shigematsu H, Nomura M, et al. PIK3CA mutations and copy number gains in human lung cancers. Cancer research. 2008;68:6913–6921. doi: 10.1158/0008-5472.CAN-07-5084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Okudela K, Suzuki M, Kageyama S, et al. PIK3CA mutation and amplification in human lung cancer. Pathology international. 2007;57:664–671. doi: 10.1111/j.1440-1827.2007.02155.x. [DOI] [PubMed] [Google Scholar]
- 200.Kawano O, Sasaki H, Okuda K, et al. PIK3CA gene amplification in Japanese non-small cell lung cancer. Lung Cancer. 2007;58:159–160. doi: 10.1016/j.lungcan.2007.06.020. [DOI] [PubMed] [Google Scholar]
- 201.Samuels Y, Wang Z, Bardelli A, et al. High frequency of mutations of the PIK3CA gene in human cancers. Science (New York, NY) 2004;304:554. doi: 10.1126/science.1096502. [DOI] [PubMed] [Google Scholar]
- 202.Kawano O, Sasaki H, Endo K, et al. PIK3CA mutation status in Japanese lung cancer patients. Lung Cancer. 2006;54:209–215. doi: 10.1016/j.lungcan.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 203.Janku F, Wheler JJ, Naing A, et al. PIK3CA mutations in advanced cancers: characteristics and outcomes. Oncotarget. 2012;3:1566–1575. doi: 10.18632/oncotarget.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Wang L, Hu H, Pan Y, et al. PIK3CA mutations frequently coexist with EGFR/KRAS mutations in non-small cell lung cancer and suggest poor prognosis in EGFR/KRAS wildtype subgroup. PloS one. 2014;9:e88291. doi: 10.1371/journal.pone.0088291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Chaft JE, Arcila ME, Paik PK, et al. Coexistence of PIK3CA and other oncogene mutations in lung adenocarcinoma-rationale for comprehensive mutation profiling. Molecular cancer therapeutics. 2012;11:485–491. doi: 10.1158/1535-7163.MCT-11-0692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Hollander MC, Blumenthal GM, Dennis PA. PTEN loss in the continuum of common cancers, rare syndromes and mouse models. Nature reviews Cancer. 2011;11:289–301. doi: 10.1038/nrc3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Jin G, Kim MJ, Jeon HS, et al. PTEN mutations and relationship to EGFR, ERBB2, KRAS, and TP53 mutations in non-small cell lung cancers. Lung Cancer. 2010;69:279–283. doi: 10.1016/j.lungcan.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 208.Marsit CJ, Zheng S, Aldape K, et al. PTEN expression in non-small-cell lung cancer: evaluating its relation to tumor characteristics, allelic loss, and epigenetic alteration. Human pathology. 2005;36:768–776. doi: 10.1016/j.humpath.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 209.Carpten JD, Faber AL, Horn C, et al. A transforming mutation in the pleckstrin homology domain of AKT1 in cancer. Nature. 2007;448:439–444. doi: 10.1038/nature05933. [DOI] [PubMed] [Google Scholar]
- 210.Malanga D, Scrima M, De Marco C, et al. Activating E17K mutation in the gene encoding the protein kinase AKT1 in a subset of squamous cell carcinoma of the lung. Cell cycle (Georgetown, Tex) 2008;7:665–669. doi: 10.4161/cc.7.5.5485. [DOI] [PubMed] [Google Scholar]
- 211.Rodon J, Dienstmann R, Serra V, et al. Development of PI3K inhibitors: lessons learned from early clinical trials. Nature reviews Clinical oncology. 2013;10:143–153. doi: 10.1038/nrclinonc.2013.10. [DOI] [PubMed] [Google Scholar]
- 212.Sadiq AA, Salgia R. Inhibition of MET receptor tyrosine kinase and its ligand hepatocyte growth factor. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2012;7:S372–374. doi: 10.1097/JTO.0b013e31826df03e. [DOI] [PubMed] [Google Scholar]
- 213.Gelsomino F, Facchinetti F, Haspinger ER, et al. Targeting the MET gene for the treatment of non-small-cell lung cancer. Critical reviews in oncology/hematology. 2014;89:284–299. doi: 10.1016/j.critrevonc.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 214.Maroun CR, Rowlands T. The Met receptor tyrosine kinase: A key player in oncogenesis and drug resistance. Pharmacology & therapeutics. 2013 doi: 10.1016/j.pharmthera.2013.12.014. [DOI] [PubMed] [Google Scholar]
- 215.Ponzetto C, Bardelli A, Zhen Z, et al. A multifunctional docking site mediates signaling and transformation by the hepatocyte growth factor/scatter factor receptor family. Cell. 1994;77:261–271. doi: 10.1016/0092-8674(94)90318-2. [DOI] [PubMed] [Google Scholar]
- 216.Furge KA, Zhang YW, Vande Woude GF. Met receptor tyrosine kinase: enhanced signaling through adapter proteins. Oncogene. 2000;19:5582–5589. doi: 10.1038/sj.onc.1203859. [DOI] [PubMed] [Google Scholar]
- 217.Stamos J, Lazarus RA, Yao X, et al. Crystal structure of the HGF beta-chain in complex with the Sema domain of the Met receptor. The EMBO journal. 2004;23:2325–2335. doi: 10.1038/sj.emboj.7600243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Guo A, Villen J, Kornhauser J, et al. Signaling networks assembled by oncogenic EGFR and c-Met. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:692–697. doi: 10.1073/pnas.0707270105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Tan YH, Krishnaswamy S, Nandi S, et al. CBL is frequently altered in lung cancers: its relationship to mutations in MET and EGFR tyrosine kinases. PloS one. 2010;5:e8972. doi: 10.1371/journal.pone.0008972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Ma PC, Jagadeeswaran R, Jagadeesh S, et al. Functional expression and mutations of c-Met and its therapeutic inhibition with SU11274 and small interfering RNA in non-small cell lung cancer. Cancer research. 2005;65:1479–1488. doi: 10.1158/0008-5472.CAN-04-2650. [DOI] [PubMed] [Google Scholar]
- 221.Park S, Choi YL, Sung CO, et al. High MET copy number and MET overexpression: poor outcome in non-small cell lung cancer patients. Histology and histopathology. 2012;27:197–207. doi: 10.14670/HH-27.197. [DOI] [PubMed] [Google Scholar]
- 222.Bean J, Brennan C, Shih JY, et al. MET amplification occurs with or without T790M mutations in EGFR mutant lung tumors with acquired resistance to gefitinib or erlotinib. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:20932–20937. doi: 10.1073/pnas.0710370104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Lutterbach B, Zeng Q, Davis LJ, et al. Lung cancer cell lines harboring MET gene amplification are dependent on Met for growth and survival. Cancer research. 2007;67:2081–2088. doi: 10.1158/0008-5472.CAN-06-3495. [DOI] [PubMed] [Google Scholar]
- 224.Kong-Beltran M, Seshagiri S, Zha J, et al. Somatic mutations lead to an oncogenic deletion of met in lung cancer. Cancer research. 2006;66:283–289. doi: 10.1158/0008-5472.CAN-05-2749. [DOI] [PubMed] [Google Scholar]
- 225.Ma PC, Tretiakova MS, MacKinnon AC, et al. Expression and mutational analysis of MET in human solid cancers. Genes, chromosomes & cancer. 2008;47:1025–1037. doi: 10.1002/gcc.20604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Ludovini V, Bianconi F, Pistola L, et al. Optimization of patient selection for EGFR-TKIs in advanced non-small cell lung cancer by combined analysis of KRAS, PIK3CA, MET, and non-sensitizing EGFR mutations. Cancer chemotherapy and pharmacology. 2012;69:1289–1299. doi: 10.1007/s00280-012-1829-7. [DOI] [PubMed] [Google Scholar]
- 227.Tang Z, Du R, Jiang S, et al. Dual MET-EGFR combinatorial inhibition against T790M-EGFR-mediated erlotinib-resistant lung cancer. British journal of cancer. 2008;99:911–922. doi: 10.1038/sj.bjc.6604559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Stabile LP, Rothstein ME, Keohavong P, et al. Targeting of Both the c-Met and EGFR Pathways Results in Additive Inhibition of Lung Tumorigenesis in Transgenic Mice. Cancers. 2010;2:2153–2170. doi: 10.3390/cancers2042153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Kawada I, Hasina R, Arif Q, et al. Dramatic Antitumor Effects of the Dual MET/RON Small-Molecule Inhibitor LY2801653 in Non-Small Cell Lung Cancer. Cancer research. 2014;74:884–895. doi: 10.1158/0008-5472.CAN-12-3583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Burgess TL, Sun J, Meyer S, et al. Biochemical characterization of AMG 102: a neutralizing, fully human monoclonal antibody to human and nonhuman primate hepatocyte growth factor. Molecular cancer therapeutics. 2010;9:400–409. doi: 10.1158/1535-7163.MCT-09-0824. [DOI] [PubMed] [Google Scholar]
- 231.Jun HT, Sun J, Rex K, et al. AMG 102, a fully human anti-hepatocyte growth factor/scatter factor neutralizing antibody, enhances the efficacy of temozolomide or docetaxel in U-87 MG cells and xenografts. Clinical cancer research : an official journal of the American Association for Cancer Research. 2007;13:6735–6742. doi: 10.1158/1078-0432.CCR-06-2969. [DOI] [PubMed] [Google Scholar]
- 232.Gordon MS, Sweeney CJ, Mendelson DS, et al. Safety, Pharmacokinetics, and Pharmacodynamics of AMG 102, a Fully Human Hepatocyte Growth Factor-Neutralizing Monoclonal Antibody, in a First-in-Human Study of Patients with Advanced Solid Tumors. Clin Cancer Res. 2010;16:699–710. doi: 10.1158/1078-0432.CCR-09-1365. [DOI] [PubMed] [Google Scholar]
- 233.D'Arcangelo M, Cappuzzo F. Focus on the potential role of ficlatuzumab in the treatment of non-small cell lung cancer. Biologics : targets & therapy. 2013;7:61–68. doi: 10.2147/BTT.S28908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Tan E, Park K, Lim WT, et al. Phase Ib study of ficlatuzumab (formerly AV-299), an anti-hepatocyte growth factor (HGF) monoclonal antibody (MAb) in combination with gefitinib (G) in Asian patients (pts) with NSCLC. ASCO Meeting Abstracts. 2011;29:7571-. [Google Scholar]
- 235.Mok T, Park K, Geater S, et al. A Randomized Phase 2 Study with Exploratory Biomarker Analysis of Ficlatuzumab A Humanized Hepatocyte Growth Factor (HGF) Inhibitor MAB in Combination with Gefitinib (G) Versus G in Asian Patients (PTS) with Lung Adenocarcinoma (LA) Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2012;23(suppl 9):ix389–ix399. abstr 1198P. [Google Scholar]
- 236.Jin H, Yang R, Zheng Z, et al. MetMAb, the one-armed 5D5 anti-c-Met antibody, inhibits orthotopic pancreatic tumor growth and improves survival. Cancer research. 2008;68:4360–4368. doi: 10.1158/0008-5472.CAN-07-5960. [DOI] [PubMed] [Google Scholar]
- 237.Salgia R, Patel P, Bothos J, et al. Phase I Dose-Escalation Study of Onartuzumab as a Single Agent and in Combination with Bevacizumab in Patients with Advanced Solid Malignancies. Clinical cancer research : an official journal of the American Association for Cancer Research. 2014 doi: 10.1158/1078-0432.CCR-13-2070. [DOI] [PubMed] [Google Scholar]
- 238.Zeng W, Peek V, Wortinger M, et al. LY2875358, a bivalent MET antibody with anti-tumor activity through blocking HGF as well as inducing degradation of MET, differentiates from a one-armed 5D5 MET antibody. Cancer research. 2013;73(8 Supplement):5465. [Google Scholar]
- 239.Wortinger MA, Peek V, Zeng W, et al. Abstract 2738: c-Met antibody LY2875358 (LA480) has pre-clinical enhanced efficacy with gastric cancer standard-of-care in vitro and in vivo. Cancer Res. 2012;72:2738-. [Google Scholar]
- 240.Zeng W, Peek V, Wortinger M, et al. Abstract 5465: LY2875358, a bivalent MET antibody with anti-tumor activity through blocking HGF as well as inducing degradation of MET, differentiates from a one-armed 5D5 MET antibody. Cancer Res. 2013;73:5465-. [Google Scholar]
- 241.Goldman JW, Rosen LS, Algazi AP, et al. First-in-human dose escalation study of LY2875358 (LY), a bivalent MET antibody, as monotherapy and in combination with erlotinib (E) in patients with advanced cancer. ASCO Meeting Abstracts. 2013;31:8093-. [Google Scholar]
- 242.Spigel DR, Ervin TJ, Ramlau RA, et al. Randomized phase II trial of Onartuzumab in combination with erlotinib in patients with advanced non-small-cell lung cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013;31:4105–4114. doi: 10.1200/JCO.2012.47.4189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Ou SH, Kwak EL, Siwak-Tapp C, et al. Activity of crizotinib (PF02341066), a dual mesenchymal-epithelial transition (MET) and anaplastic lymphoma kinase (ALK) inhibitor, in a non-small cell lung cancer patient with de novo MET amplification. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2011;6:942–946. doi: 10.1097/JTO.0b013e31821528d3. [DOI] [PubMed] [Google Scholar]
- 244.Hellerstedt BA, Edelman G, Vogelzang NJ, et al. Activity of cabozantinib (XL184) in metastatic NSCLC: Results from a phase II randomized discontinuation trial (RDT) ASCO Meeting Abstracts. 2012;30:7514-. [Google Scholar]
- 245.Wakelee HA, Gettinger S, Engelman JA, et al. A phase Ib/II study of XL184 (BMS 907351) with and without erlotinib (E) in patients (pts) with non-small cell lung cancer (NSCLC) Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28 [Google Scholar]
- 246.Basilico C, Pennacchietti S, Vigna E, et al. Tivantinib (ARQ197) displays cytotoxic activity that is independent of its ability to bind MET. Clinical cancer research : an official journal of the American Association for Cancer Research. 2013;19:2381–2392. doi: 10.1158/1078-0432.CCR-12-3459. [DOI] [PubMed] [Google Scholar]
- 247.Katayama R, Aoyama A, Yamori T, et al. Cytotoxic activity of tivantinib (ARQ 197) is not due solely to c-MET inhibition. Cancer research. 2013;73:3087–3096. doi: 10.1158/0008-5472.CAN-12-3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Michieli P, Di Nicolantonio F. Targeted therapies: Tivantinib--a cytotoxic drug in MET inhibitor's clothes? Nature reviews Clinical oncology. 2013;10:372–374. doi: 10.1038/nrclinonc.2013.86. [DOI] [PubMed] [Google Scholar]
- 249.Mason I. Initiation to end point: the multiple roles of fibroblast growth factors in neural development. Nature reviews Neuroscience. 2007;8:583–596. doi: 10.1038/nrn2189. [DOI] [PubMed] [Google Scholar]
- 250.Haugsten EM, Wiedlocha A, Olsnes S, et al. Roles of fibroblast growth factor receptors in carcinogenesis. Mol Cancer Res. 2010;8:1439–1452. doi: 10.1158/1541-7786.MCR-10-0168. [DOI] [PubMed] [Google Scholar]
- 251.Acevedo VD, Ittmann M, Spencer DM. Paths of FGFR-driven tumorigenesis. Cell cycle (Georgetown, Tex) 2009;8:580–588. doi: 10.4161/cc.8.4.7657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Turner N, Grose R. Fibroblast growth factor signalling: from development to cancer. Nature reviews Cancer. 2010;10:116–129. doi: 10.1038/nrc2780. [DOI] [PubMed] [Google Scholar]
- 253.Wesche J, Haglund K, Haugsten EM. Fibroblast growth factors and their receptors in cancer. The Biochemical journal. 2011;437:199–213. doi: 10.1042/BJ20101603. [DOI] [PubMed] [Google Scholar]
- 254.Dieci MV, Arnedos M, Andre F, et al. Fibroblast growth factor receptor inhibitors as a cancer treatment: from a biologic rationale to medical perspectives. Cancer discovery. 2013;3:264–279. doi: 10.1158/2159-8290.CD-12-0362. [DOI] [PubMed] [Google Scholar]
- 255.Bass AJ, Watanabe H, Mermel CH, et al. SOX2 is an amplified lineage-survival oncogene in lung and esophageal squamous cell carcinomas. Nature genetics. 2009;41:1238–1242. doi: 10.1038/ng.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Heist RS, Mino-Kenudson M, Sequist LV, et al. FGFR1 amplification in squamous cell carcinoma of the lung. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2012;7:1775–1780. doi: 10.1097/JTO.0b013e31826aed28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Craddock KJ, Ludkovski O, Sykes J, et al. Prognostic value of fibroblast growth factor receptor 1 gene locus amplification in resected lung squamous cell carcinoma. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2013;8:1371–1377. doi: 10.1097/JTO.0b013e3182a46fe9. [DOI] [PubMed] [Google Scholar]
- 258.Kim HR, Kim DJ, Kang DR, et al. Fibroblast growth factor receptor 1 gene amplification is associated with poor survival and cigarette smoking dosage in patients with resected squamous cell lung cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013;31:731–737. doi: 10.1200/JCO.2012.43.8622. [DOI] [PubMed] [Google Scholar]
- 259.Gadgeel SM, Chen W, Cote ML, et al. Fibroblast growth factor receptor 1 amplification in non-small cell lung cancer by quantitative real-time PCR. PloS one. 2013;8:e79820. doi: 10.1371/journal.pone.0079820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Tran TN, Selinger CI, Kohonen-Corish MR, et al. Fibroblast growth factor receptor 1 (FGFR1) copy number is an independent prognostic factor in non-small cell lung cancer. Lung Cancer. 2013;81:462–467. doi: 10.1016/j.lungcan.2013.05.015. [DOI] [PubMed] [Google Scholar]
- 261.Dienstmann R, Rodon J, Prat A, et al. Genomic aberrations in the FGFR pathway: opportunities for targeted therapies in solid tumors. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2014;25:552–563. doi: 10.1093/annonc/mdt419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Williams SV, Hurst CD, Knowles MA. Oncogenic FGFR3 gene fusions in bladder cancer. Human molecular genetics. 2013;22:795–803. doi: 10.1093/hmg/dds486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Singh D, Chan JM, Zoppoli P, et al. Transforming fusions of FGFR and TACC genes in human glioblastoma. Science (New York, NY) 2012;337:1231–1235. doi: 10.1126/science.1220834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Parker BC, Annala MJ, Cogdell DE, et al. The tumorigenic FGFR3-TACC3 gene fusion escapes miR-99a regulation in glioblastoma. The Journal of clinical investigation. 2013;123:855–865. doi: 10.1172/JCI67144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Liao RG, Jung J, Tchaicha J, et al. Inhibitor-sensitive FGFR2 and FGFR3 mutations in lung squamous cell carcinoma. Cancer research. 2013;73:5195–5205. doi: 10.1158/0008-5472.CAN-12-3950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Wolf JLPM, Camidge RD, Perez JM, Tabernero J, Hidalgo M, Schuler M, Tian GG, Soria JC, Delord JP, Campone M, Bachelot T, van der Noll R, Ringeisen FP, Nogova L, Sequist LV, Schellens JHM. A phase I dose escalation study of NVP-BGJ398, a selective pan FGFR inhibitor in genetically preselected advanced solid tumors. Canc Res; Proceedings of the 103rd Annual Meeting of the American Association for Cancer Research; 2012 Mar 31-Apr 4; Chicago, IL. Philadelphia (PA): AACR. 2012. p. 72. [abstact] [Google Scholar]
- 267.Andre FRM, Dean E, Varga A, van der Noll R, Stockman PK, Ghiorghiu D, Kilgour E, Smith PD, Macpherson M, Lawrence P, Hastie A, Schellens JHM. Results of a phase I study of AZD4547, an inhibitor of fibroblast growth factor receptor (FGFR), in patients with advanced solid tumors. Canc Res; Proceedings of the 104th Annual Meeting of the American Association for Cancer Research; 2013 Apr 6–10; Washington, DC. Philadelphia (PA): AACR. 2013. p. 73. [Google Scholar]
- 268.Johnson N, Shapiro GI. Cyclin-dependent kinases (cdks) and the DNA damage response: rationale for cdk inhibitor-chemotherapy combinations as an anticancer strategy for solid tumors. Expert Opin Ther Targets. 2010;14:1199–1212. doi: 10.1517/14728222.2010.525221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Harper JW, Elledge SJ. The DNA damage response: ten years after. Mol Cell. 2007;28:739–745. doi: 10.1016/j.molcel.2007.11.015. [DOI] [PubMed] [Google Scholar]
- 270.Xu H, Cheung IY, Wei XX, et al. Checkpoint kinase inhibitor synergizes with DNA-damaging agents in G1 checkpoint-defective neuroblastoma. International journal of cancer Journal international du cancer. 2011;129:1953–1962. doi: 10.1002/ijc.25842. [DOI] [PubMed] [Google Scholar]
- 271.Enomoto M, Goto H, Tomono Y, et al. Novel positive feedback loop between Cdk1 and Chk1 in the nucleus during G2/M transition. The Journal of biological chemistry. 2009;284:34223–34230. doi: 10.1074/jbc.C109.051540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Chini CC, Chen J. Claspin, a regulator of Chk1 in DNA replication stress pathway. DNA Repair (Amst) 2004;3:1033–1037. doi: 10.1016/j.dnarep.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 273.W W, C B, AK B, et al. Abstract 1776: Antitumor activity of Chk1 inhibitor LY2606368 as a single agent in SW1990 human pancreas orthotopic tumor model. Proceedings: AACR 103rd Annual Meeting; Chicago. 2012. [Google Scholar]
- 274.RS F, JP C, I L, et al. Final results of a randomized Phase II study of PD 0332991, a cyclin-dependent kinase (CDK)-4/6 inhibitor, in combination with letrozole vs letrozole alone for first-line treatment of ER+/HER2− advanced breast cancer (PALOMA-1; TRIO-18) San Diego: AACR; 2014. p. CT101. [Google Scholar]
- 275.Guertin AD, Li J, Liu Y, et al. Preclinical evaluation of the WEE1 inhibitor MK-1775 as single-agent anticancer therapy. Molecular cancer therapeutics. 2013;12:1442–1452. doi: 10.1158/1535-7163.MCT-13-0025. [DOI] [PubMed] [Google Scholar]
- 276.I B, KN M, R S-F, et al. Targeting p53 mutant ovarian cancer: Phase I results of the WEE1 inhibitor MK-1775 with carboplatin plus paclitaxel in patients (pts) with platinum-sensitive, p53-mutant ovarian cancer (OC). 2013 ASCO Annual Meeting; Chicago. 2013. p. 5518. [Google Scholar]
- 277.Trenz K, Errico A, Costanzo V. Plx1 is required for chromosomal DNA replication under stressful conditions. The EMBO journal. 2008;27:876–885. doi: 10.1038/emboj.2008.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.P E. Trial of BI 6727 (Volasertib) Monotherapy and BI 6727 in Combination With Pemetrexed Compared to Pemetrexed Monotherapy in Advanced NSCLC. Sydney: WLCC; 2013. p. A2307. [Google Scholar]
- 279.Melichar B, Adenis A, Havel L, et al. Phase (Ph) I/II study of investigational Aurora A kinase (AAK) inhibitor MLN8237 (alisertib): Updated ph II results in patients (pts) with small cell lung cancer (SCLC), non-SCLC (NSCLC), breast cancer (BrC), head and neck squamous cell carcinoma (HNSCC), and gastroesophageal cancer (GE) ASCO Meeting Abstracts. 2013;31:605. [Google Scholar]
- 280.Krishnakumar R, Kraus WL. The PARP side of the nucleus: molecular actions, physiological outcomes, and clinical targets. Mol Cell. 2010;39:8–24. doi: 10.1016/j.molcel.2010.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Ellisen LW. PARP inhibitors in cancer therapy: promise, progress, and puzzles. Cancer cell. 2011;19:165–167. doi: 10.1016/j.ccr.2011.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Do K, Chen AP. Molecular pathways: targeting PARP in cancer treatment. Clinical cancer research : an official journal of the American Association for Cancer Research. 2013;19:977–984. doi: 10.1158/1078-0432.CCR-12-0163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Donawho CK, Luo Y, Luo Y, et al. ABT-888, an orally active poly(ADP-ribose) polymerase inhibitor that potentiates DNA-damaging agents in preclinical tumor models. Clinical cancer research : an official journal of the American Association for Cancer Research. 2007;13:2728–2737. doi: 10.1158/1078-0432.CCR-06-3039. [DOI] [PubMed] [Google Scholar]
- 284.Murai J, Huang SY, Das BB, et al. Trapping of PARP1 and PARP2 by Clinical PARP Inhibitors. Cancer research. 2012;72:5588–5599. doi: 10.1158/0008-5472.CAN-12-2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.O'Shaughnessy J, Osborne C, Pippen JE, et al. Iniparib plus chemotherapy in metastatic triple-negative breast cancer. The New England journal of medicine. 2011;364:205–214. doi: 10.1056/NEJMoa1011418. [DOI] [PubMed] [Google Scholar]
- 286.Ledermann J, Harter P, Gourley C, et al. Olaparib maintenance therapy in platinum-sensitive relapsed ovarian cancer. The New England journal of medicine. 2012;366:1382–1392. doi: 10.1056/NEJMoa1105535. [DOI] [PubMed] [Google Scholar]
- 287.Gelmon KA, Tischkowitz M, Mackay H, et al. Olaparib in patients with recurrent high-grade serous or poorly differentiated ovarian carcinoma or triple-negative breast cancer: a phase 2, multicentre, open-label, non-randomised study. The lancet oncology. 2011;12:852–861. doi: 10.1016/S1470-2045(11)70214-5. [DOI] [PubMed] [Google Scholar]
- 288.Byers LA, Wang J, Nilsson MB, et al. Proteomic profiling identifies dysregulated pathways in small cell lung cancer and novel therapeutic targets including PARP1. Cancer discovery. 2012;2:798–811. doi: 10.1158/2159-8290.CD-12-0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Appleman L, Beumar J, Jiang Y, et al. A phase I study of veliparib (ABT-888) in combination with carboplatin and paclitaxel in advanced solid malignancies. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30 Abstract # 3049. [Google Scholar]
- 290.Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science (New York, NY) 1996;271:1734–1736. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- 291.Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. The New England journal of medicine. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Lynch TJ, Bondarenko I, Luft A, et al. Ipilimumab in combination with paclitaxel and carboplatin as first-line treatment in stage IIIB/IV non-small-cell lung cancer: results from a randomized, double-blind, multicenter phase II study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30:2046–2054. doi: 10.1200/JCO.2011.38.4032. [DOI] [PubMed] [Google Scholar]
- 293.Hoos A, Eggermont AM, Janetzki S, et al. Improved endpoints for cancer immunotherapy trials. Journal of the National Cancer Institute. 2010;102:1388–1397. doi: 10.1093/jnci/djq310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Wolchok JD, Hoos A, O'Day S, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clinical cancer research : an official journal of the American Association for Cancer Research. 2009;15:7412–7420. doi: 10.1158/1078-0432.CCR-09-1624. [DOI] [PubMed] [Google Scholar]
- 295.Reck M, Bondarenko I, Luft A, et al. Ipilimumab in combination with paclitaxel and carboplatin as first-line therapy in extensive-disease-small-cell lung cancer: results from a randomized, double-blind, multicenter phase 2 trial. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2013;24:75–83. doi: 10.1093/annonc/mds213. [DOI] [PubMed] [Google Scholar]
- 296.Zatloukal P, Heo DS, Park K, et al. Randomized phase II clinical trial comparing tremelimumab (CP-675,206) with best supportive care (BSC) following first-line platinum-based therapy in patients (pts) with advanced non-small cell lung cancer (NSCLC) ASCO Meeting Abstracts. 2009;27:8071. [Google Scholar]
- 297.Ribas A. Tumor immunotherapy directed at PD-1. The New England journal of medicine. 2012;366:2517–2519. doi: 10.1056/NEJMe1205943. [DOI] [PubMed] [Google Scholar]
- 298.Topalian SL, Drake CG, Pardoll DM. Targeting the PD-1/B7-H1(PD-L1) pathway to activate anti-tumor immunity. Current opinion in immunology. 2012;24:207–212. doi: 10.1016/j.coi.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. The New England journal of medicine. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab plus ipilimumab in advanced melanoma. The New England journal of medicine. 2013;369:122–133. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Brahmer JR, Horn L, Antonia SJ, et al. Nivolumab (anti-PD-1; BMS-936558; ONO-4538) in patients with non-small cell lung cancer (NSCLC): overall survival and long-term safety in a phase 1 trial. World Conference on Lung Cancer. 2013 [Google Scholar]
- 302.Rizvi NA, Antonia SJ, Chow LQM, et al. A phase I study of nivolumab (anti-PD-1; BMS-936558, ONO-4538) plus platinum-based doublet chemotherapy (PT-doublet) in chemotherapy-naive non-small cell lung cancer (NSCLC) patients (pts) ASCO Meeting Abstracts. 2013;31:8072. [Google Scholar]
- 303.Garon EB, Balmanoukian A, Hamid O, et al. Preliminary clinical safety and activity of MK-3475 monotherapy for the treatment of previously treated patients with non-small cell lung cancer (NSCLC. World Conference on Lung Cancer. 2013 [Google Scholar]
- 304.Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. The New England journal of medicine. 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Soria JCCC, Bahleda R, et al. European Cancer Congress. Amsterdam; 2013. Clinical activity, safety, and biomarkers of PD-L1 blockade in non-small cell lung cancer; additional analyses from a clinical study of the engineered antibody MPDL2380A (Anti-PD-L1) (Abstract 3408). [Google Scholar]
- 306.Horn L, Herbst RS, Spigel DR, et al. An analysis of the relationship of clinical activity to baseline EGFR status, PD-L1 expression and prior treatment history in patients with non-small cell lung cancer (NSCLC) following PD-L1 blockade with MPDL3280A (anti-PDL1) World Conference on Lung Cancer. 2013 [Google Scholar]
- 307.Khleif S, Lutzky J, Segal NH, et al. MEDI4736, an anti-PD-L1 antibody with modified Fc domain: Preclinical evaluation and early clinical results from a phase 1 study in patients with advanced solid tumors. European Society of Medical Oncology Annual Meeting. 2013 [Google Scholar]
- 308.Goldman B, DeFrancesco L. The cancer vaccine roller coaster. Nature biotechnology. 2009;27:129–139. doi: 10.1038/nbt0209-129. [DOI] [PubMed] [Google Scholar]
- 309.Mellstedt H, Vansteenkiste J, Thatcher N. Vaccines for the treatment of non-small cell lung cancer: investigational approaches and clinical experience. Lung Cancer. 2011;73:11–17. doi: 10.1016/j.lungcan.2011.02.023. [DOI] [PubMed] [Google Scholar]
- 310.Nemunaitis J, Dillman RO, Schwarzenberger PO, et al. Phase II study of belagenpumatucel-L, a transforming growth factor beta-2 antisense gene-modified allogeneic tumor cell vaccine in non-small-cell lung cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2006;24:4721–4730. doi: 10.1200/JCO.2005.05.5335. [DOI] [PubMed] [Google Scholar]
- 311.Giaconne GBL, Nemunaitis J, et al. A phase III study of belagenpumatucel-L therapeutic tumor cell vaccine for non-small-cell lung cancer (NSCLC) 2013 [Google Scholar]
- 312.Pruitt SK, Kirk AD, Bollinger RR, et al. The effect of soluble complement receptor type 1 on hyperacute rejection of porcine xenografts. Transplantation. 1994;57:363–370. doi: 10.1097/00007890-199402150-00009. [DOI] [PubMed] [Google Scholar]
- 313.Morris JC, Rossi GR, Harold N, et al. Potential chemo-sensitization effect of tergenpumatucel-L immunotherapy in treated patients with advanced non-small cell lung cancer (NSCLC) ASCO Meeting Abstracts. 2013;31:8094. [Google Scholar]
- 314.Vansteenkiste J, Zielinski M, Linder A, et al. Adjuvant MAGE-A3 Immunotherapy in Resected Non-Small-Cell Lung Cancer: Phase II Randomized Study Results. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013;31:2396–2403. doi: 10.1200/JCO.2012.43.7103. [DOI] [PubMed] [Google Scholar]
- 315.Butts C, Murray N, Maksymiuk A, et al. Randomized phase IIB trial of BLP25 liposome vaccine in stage IIIB and IV non-small-cell lung cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2005;23:6674–6681. doi: 10.1200/JCO.2005.13.011. [DOI] [PubMed] [Google Scholar]
- 316.Butts C, Socinski MA, Mitchell PL, et al. Tecemotide (L-BLP25) versus placebo after chemoradiotherapy for stage III non-small-cell lung cancer (START): a randomised, double-blind, phase 3 trial. The lancet oncology. 2014;15:59–68. doi: 10.1016/S1470-2045(13)70510-2. [DOI] [PubMed] [Google Scholar]
- 317.Ramlau R, Quoix E, Rolski J, et al. A phase II study of Tg4010 (Mva-Muc1-Il2) in association with chemotherapy in patients with stage III/IV Non-small cell lung cancer. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2008;3:735–744. doi: 10.1097/JTO.0b013e31817c6b4f. [DOI] [PubMed] [Google Scholar]
- 318.Quoix E, Ramlau R, Westeel V, et al. Therapeutic vaccination with TG4010 and first-line chemotherapy in advanced non-small-cell lung cancer: a controlled phase 2B trial. The lancet oncology. 2011;12:1125–1133. doi: 10.1016/S1470-2045(11)70259-5. [DOI] [PubMed] [Google Scholar]
- 319.Hudson TJ, Anderson W, Artez A, et al. International network of cancer genome projects. Nature. 2010;464:993–998. doi: 10.1038/nature08987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Ledford H. Big science: The cancer genome challenge. Nature. 2010;464:972–974. doi: 10.1038/464972a. [DOI] [PubMed] [Google Scholar]
- 321.Druker BJ, Sawyers CL, Kantarjian H, et al. Activity of a specific inhibitor of the BCR-ABL tyrosine kinase in the blast crisis of chronic myeloid leukemia and acute lymphoblastic leukemia with the Philadelphia chromosome. The New England journal of medicine. 2001;344:1038–1042. doi: 10.1056/NEJM200104053441402. [DOI] [PubMed] [Google Scholar]
- 322.Druker BJ, Talpaz M, Resta DJ, et al. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. The New England journal of medicine. 2001;344:1031–1037. doi: 10.1056/NEJM200104053441401. [DOI] [PubMed] [Google Scholar]
- 323.Ivy SP, Siu LL, Garrett-Mayer E, et al. Approaches to phase 1 clinical trial design focused on safety, efficiency, and selected patient populations: a report from the clinical trial design task force of the national cancer institute investigational drug steering committee. Clinical cancer research : an official journal of the American Association for Cancer Research. 2010;16:1726–1736. doi: 10.1158/1078-0432.CCR-09-1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Dowlati A, Manda S, Gibbons J, et al. Multi-institutional phase I trials of anticancer agents. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26:1926–1931. doi: 10.1200/JCO.2007.13.3793. [DOI] [PubMed] [Google Scholar]
- 325.Manji A, Brana I, Amir E, et al. Evolution of clinical trial design in early drug development: systematic review of expansion cohort use in single-agent phase I cancer trials. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013;31:4260–4267. doi: 10.1200/JCO.2012.47.4957. [DOI] [PubMed] [Google Scholar]
- 326.Wetterstrand KA. DNA Sequencing Costs: Data from the NHGRI Large-Scale Genome Sequencing Program [Google Scholar]
- 327.Metzker ML. Sequencing technologies - the next generation. Nature reviews Genetics. 2010;11:31–46. doi: 10.1038/nrg2626. [DOI] [PubMed] [Google Scholar]
- 328.McShane LM, Cavenagh MM, Lively TG, et al. Criteria for the use of omics-based predictors in clinical trials. Nature. 2013;502:317–320. doi: 10.1038/nature12564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Freidlin B, McShane LM, Korn EL. Randomized clinical trials with biomarkers: design issues. Journal of the National Cancer Institute. 2010;102:152–160. doi: 10.1093/jnci/djp477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Polley MY, Freidlin B, Korn EL, et al. Statistical and practical considerations for clinical evaluation of predictive biomarkers. Journal of the National Cancer Institute. 2013;105:1677–1683. doi: 10.1093/jnci/djt282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Taube SE, Clark GM, Dancey JE, et al. A perspective on challenges and issues in biomarker development and drug and biomarker codevelopment. Journal of the National Cancer Institute. 2009;101:1453–1463. doi: 10.1093/jnci/djp334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Simon RM, Paik S, Hayes DF. Use of archived specimens in evaluation of prognostic and predictive biomarkers. Journal of the National Cancer Institute. 2009;101:1446–1452. doi: 10.1093/jnci/djp335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Simon R, Roychowdhury S. Implementing personalized cancer genomics in clinical trials. Nature reviews Drug discovery. 2013;12:358–369. doi: 10.1038/nrd3979. [DOI] [PubMed] [Google Scholar]